1. Introduction
Primary Ciliary Dyskinesia (PCD) is a rare autosomal recessive genetic disease with an estimated prevalence of 1:7,500 to 1:15,000 individuals [
1,
2]. The functional role of the motile cilia is exemplified by the spectrum of phenotypical manifestations seen in PCD [
3]. The clinical observations of PCD ultimately stem from the functional impairment of cilia, leading to ciliary dyskinesia [
4]. Common clinical features include a year-round wet cough, neonatal respiratory distress, recurrent respiratory tract infections leading to bronchiectasis, persistent rhinosinusitis, recurrent otitis media, infertility, as well as organ laterality defects depending on the affected gene [
5]. Despite the early onset of symptoms, PCD diagnosis is often delayed, resulting in underdiagnosis, and missed opportunities for early intervention [
6]. This delay can be partially attributed to the spectrum of clinical presentations of the disorder, and the concomitant presence of other chronic respiratory diseases, posing challenges in accurate identification and leading to potential diagnostic gaps [
7].
Presently, there is no gold standard test for PCD [
8]. ATS and ERS guidelines agree PCD diagnosis can be confirmed with hallmark ultrastructural defects or a positive genetic test. However, neither Transmission Electron Microscopy (TEM) or genetic testing alone have good sensitivity [
9]. For example, it is estimated that Transmission Electron Microscopy use in identification of hallmark ciliary ultrastructure is non-diagnostic in 30% of PCD cases [
10]. In addition, genetic sequencing testing is estimated to be non-diagnosable in 20-30% of cases [
11]. Moreover, issues arise in following an algorithm for an accurate diagnosis due to several factors, including limited access and expertise required for recommended PCD diagnostic tests, especially in underdeveloped countries [
12]. Specialized PCD diagnostic centers in the United States and Europe employ algorithms that incorporate multiple diagnostic tools such as measuring nasal nitric oxide (nNO), genetic sequencing, immunofluorescence, and high-speed video microscopy analysis (HSVA) [
13,
14]. However, the availability of these diagnostic tools is limited worldwide, and their operation requires specialized expertise [
15]. To date, diagnosing PCD remains a complex task that requires the integration of multiple modalities and a collaborative effort from a multidisciplinary team [
13].
Among the available diagnostic tools, HSVA is the only method that enables direct observation of ciliary motion and assessment of ciliary function [
5]. In HSVA, the ciliary beat frequency (CBF) and ciliary beat pattern (CBP) are key observable variables for PCD evaluation [
16]. HSVA and CBF measurement are key diagnostic tools, but manual counting is tedious and subjective [
17,
18]. Software tools like CiliarMove and Cilialyzer have emerged to automate CBF analysis from HSVA [
17,
19,
20,
21]. The first commercially available software, Sisson-Ammons Video Analysis (SAVA) system, was developed in 2003 [
18]. The development of freely available open-source software followed, which notably included the software named CiliaFA in 2012, but more recently, CiliarMove in 2021, and Cilialyzer in 2022 [
17,
20,
21]. All the software applications mentioned above have individually demonstrated the capability to accurately calculate CBF, aligning with manually determined values [
17,
18,
20,
21]. Among the available open-source software platforms, CiliarMove and Cilialyzer, have been validated independently in recent literature for their ability to perform such analyses [
20,
21].
Table 1 provides a comparative analysis of the two most recent open-source software tools, CiliarMove and Cilialyzer.
Despite establishing clinical utility, knowledge regarding performance analysis across software platforms is limited. When considering the origin of such software, it is reasonable to expect further developments and improvements. A software automating analysis sounds promising when coupled with a disorder requiring diagnostic standardization. Thus, as the technology develops, analysis across software platforms could provide useful information aiding HSVA standardization. Our study presents a comparison of the manual count, Cilialyzer, and CiliarMove open-source software ability to compute CBF from healthy control and PCD patients with a confirmed diagnosis of the same
RSPH4A (c.921+3_921+6delAAGT (intronic)) founder mutation [
22].
2. Materials and Methods
2.1. Subjects
The study included confirmed PCD patients (n=12) with bi-allelic pathogenic RSPH4A (c.921+3_921+6del (intronic)) founder mutation and six healthy controls (n=6). The healthy controls were not smokers, and none exhibited any clinical symptoms associated with PCD, asthma, or chronic respiratory illness as per past medical history. At the time of nasal biopsy, all subjects from the PCD cohort and healthy controls were at their baseline status of health for more than two weeks, free of any upper respiratory track infection.
2.2. Sample Collection and Preparation
Nasal ciliated epithelial samples were obtained via cytology brushing (Puritan Sterile Cytology Brushes). The nasal cytology brush was briefly inserted in one nostril and gently moved into and out of the inferior nasal turbinate ten times while performing internal rotation of the brush and slightly pressing on the lateral wall of the inferior turbinate [
23]. Once brushing was completed, sample preparation began immediately. The nasal cytology brush was twisted in 3 mL of PneumaCult™-Ex Plus Medium to release any tissue that remained adherent to the bristles. The sample required to be placed in a sufficient volume of media, permitting full immersion of the brush. Samples were washed in 5 mL of D-PBS without calcium and magnesium to remove mucus and debris. The sample would then be centrifuged at 400xg for seven minutes to pellet the epithelial cells. The nasal brushings were then resuspended in 500μL of PneumaCult™-Ex Plus Medium and incubated at 37° Celsius for 30 minutes prior to HSVA at 500 fps under 60x magnification. After this period, 100μL of the cell suspension was plated in a glass slide for analysis.
2.3. Highspeed Video Microscopy Analysis (HSVA)
The observation of samples was conducted using the Nikon Eclipse Ti2 inverted microscope with a long working distance 60X objective lens. An AOS PROMON U750 monochrome high-speed camera was attached to the microscope to capture high-speed video recordings. The camera had a light sensitivity grade of ISO 3600 and a sensor size of 4.8 μm pixel. This setup recorded samples at a frame rate of 500 frames per second (fps). The AOS Imaging Software Version 4 was used to process the footage. A microscope air table was employed in conjunction with the HSVA to minimize movement during the recording. The optimal resolution setting for HSVA recorded at 500 fps was recommended by the AOS software in the camera suite and set at 880 x 637 pixels. Following the guidelines of the European Respiratory Society (ERS) [
24], 2,200 frames were recorded for five seconds at the specified resolution and under 60x. The recorded HSVA footage was saved as unprocessed image data (RAW) files. During the analysis, the region of interest (ROI) was not selected if there was mucus or debris on the surface interface of the cell cluster. To ensure representative footage of the ciliary population, an ROI was only selected if 10-15 equally distant adjacent ciliated cells were present. A thermal plate (F/TS2R & TI2 Stage) for Nikon Eclipse Ti2 Inverted microscope was used to maintain extended observation of motile cilia at 37°C to minimize variability in CBF. Intact cell clusters were chosen over single cells to obtain a more representative sample [
25]. All the HSVA was completed in less than 30 minutes per sample.
2.4. Manual Method for CBF Count
CBF was counted manually from 10 beat cycles, which was followed by CiliarMove and Cilialyzer in a standardized workflow. The manual method for CBF counting was performed as previously described in the literature using the following formula: Manual CBF (Hz) = (fps / number of frames elapsed for 10 full ciliary beats) * 10 (conversion per beat cycle) [
17,
21]. When performing the manual CBF count, we paused the recordings at a point where the cilia were closest to the maximal bend. We adjusted the footage frame by frame until most of the cilia were fully bent; at this point, we marked the starting frame for reference. We counted the number of full ciliary beats, including the forward and backstroke, from the starting to the end frame. We then recorded the number of frames that elapsed while observing ten complete ciliary beats for the given cycle.
2.5. Software Analysis
The Cilialyzer (v1.2.1-b3098cb) and CiliarMove (v219) software were applied to the same set of recordings provided by the PCD patients and healthy controls. A reference picture of the ROI marked with a cursor was applied to ensure consistent analysis of the same ROI. For CBF computation using CiliarMove, the RAW format video files required one conversion step to be transformed into the tag image file format (TIFF) sequence file. After the file conversion, the image sequence was uploaded for analysis. Before analyzing the frequency, the ROI was manually selected using the cropping function. For Cilialyzer, the RAW format video files were converted into sequence images in portable network graphics (PNG) format and pre-processed as stated by the Cilialyzer manual [
20]. The pre-processing methods included initial manual image rotation, selecting ROI, image stabilization, cropping margins, and motion extraction. The CBF was then calculated by both CiliarMove and Cilialyzer, with the results displayed on a frequency distribution histogram and a Power spectrum graph, respectively. These steps were followed to ensure accurate and consistent analysis of the CBF using Cilialyzer and CiliarMove for the respective software platforms.
2.6. Statistical Analysis
Descriptive statistics, including median and interquartile ranges (IQRs), were calculated to summarize the data, providing measures of central tendency and dispersion. The normality of the data was assessed using the Kolmogorov-Smirnov test. Non-parametric data of CBF measurements between the PCD and healthy control groups were compared using the Mann-Whitney U test. Non-parametric Mann-Whitney U test compared CBF between PCD and control for each method. The U score was calculated using the Mann-Whitney U test, which measures the rank sum of the smaller group in relation to all possible pairwise comparisons. A significance level of α = 0.05 was set to determine statistical significance, and all reported p-values were two-tailed. A p-value less than 0.05 indicated a statistically significant difference in CBF median measurements between the two groups. Based on the finite population correction for the available 25 patients with PCD in Puerto Rico with the founder genetic mutation, the sample size calculations indicate that 12 participants in the PCD group and six healthy controls are sufficient to detect significant differences in CBF between groups. The adjusted sample sizes ensure adequate statistical power to validate the performance of CiliarMove and Cilialyzer software compared to the manual count method. These sample sizes were determined to be adequate for detecting meaningful differences in CBF with a high degree of confidence (p-value < 0.0001), ensuring the robustness and reliability of our findings. All statistical analyses were performed using the statistical software package GraphPad Prism version 10.0.0 for Windows, developed by GraphPad Software, San Diego, CA, USA [
www.graphpad.com].
3. Results
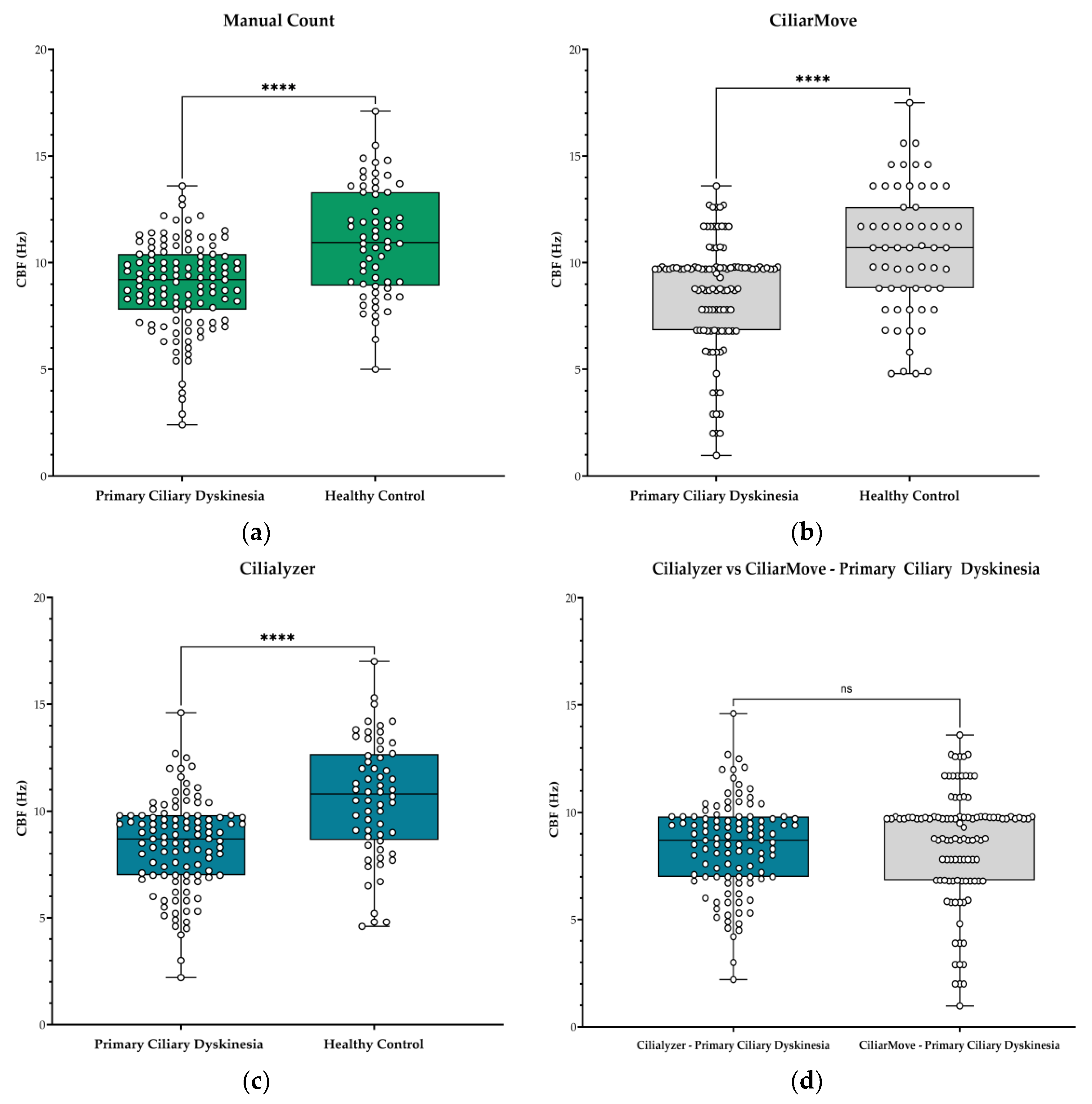
This study included 115 high-speed microscopy recordings from twelve patients (
n=12) with PCD and 60 videos from healthy controls (
n=6). Each recording underwent analysis using three different CBF computational methods, resulting in 175 CBF observation points. Median CBF was 9.2 Hz in PCD patients versus 10.9 Hz in the healthy control by manual counting. CiliarMove measured median CBF of 9.7 Hz and 10.7 Hz in PCD and control groups, respectively. Cilialyzer showed the largest difference with medians of 8.7 Hz for PCD and 10.8 Hz in the control group. The median difference in CBF between PCD and healthy control was 1.7 Hz for Manual Count, 1.0 Hz for CiliarMove, and 2.1 Hz for Cilialyzer. (
Table 2). All methods gave significantly lower CBF in PCD patients compared to the healthy control (p-value <0.001).
Figure 1.
CBF values in hertz (Hz) from patients with Primary Ciliary Dyskinesia (PCD) and healthy controls. Box and whisker plot represented with median and IQR values: (a) Manual count CBF values from PCD cohort and healthy control; (b) CiliarMove, and (c) Cilialyzer. (d) A comparison between Cilialyzer and CiliarMove using recordings from the PCD patient. Level of statistical significance: * p<0.05; ** p<0.01; ***p<0.001; **** p<0.0001 and ns = not significant.
Figure 1.
CBF values in hertz (Hz) from patients with Primary Ciliary Dyskinesia (PCD) and healthy controls. Box and whisker plot represented with median and IQR values: (a) Manual count CBF values from PCD cohort and healthy control; (b) CiliarMove, and (c) Cilialyzer. (d) A comparison between Cilialyzer and CiliarMove using recordings from the PCD patient. Level of statistical significance: * p<0.05; ** p<0.01; ***p<0.001; **** p<0.0001 and ns = not significant.
4. Discussion
This study compared the CBF between individuals diagnosed with PCD and healthy controls. We evaluated CBF data using two software tools (Cilialyzer and CiliarMove) and manual method counting. Utilizing the Mann-Whitney U test for statistical analysis, we identified significant differences in CBF values between the PCD patients and healthy controls for all three methods. Cilia samples in PCD exhibited a slower frequency than a significantly faster beating frequency in samples from the healthy controls. Both software compute CBF through the power spectral density means of each pixel’s variations in light intensity, as previously demonstrated [
17,
21]. All CBF values obtained corresponded well with the manual count. When evaluating the performance of the two software tools, Cilialyzer had the greatest actual CBF median difference between groups. These results indicate that all methods successfully differentiated CBF between PCD patients from healthy controls.
Image stabilization and motion extraction features may have improved signal quantification; Schneiter, et al. demonstrated the importance of image stabilization in a freshly excised group of nasal epithelial cells exhibiting a superposed quasi-periodic motion on the ciliary function within the ROI [
20]. This resulted in an inaccurate reading of the average power spectral density, which compromised both the display of the CBF-bandwidth, and the actual CBF peak value. The average power spectral density was corrected by applying the image stabilization feature. This feature allows Cilialyzer to remove the undesirable sample motion through the use of its Python package ’pyStackReg’[
26], which results in the alignment of consecutive images in the sequence to remain in place. The other notable pre-processing method offered by Cilialyzer is the “motion extraction” feature, which is performed via a computational function termed “mean image subtraction” previously described in the literature [
27,
28]. Motion extraction removes the static background of an image, which was demonstrated to enhance the visualization of the cilia along with their movement in the selected ROI. These methods bolster the quantitative objective analysis, ensuring its correctness, and also improve the visual assessment of manual count by highlighting the observable cilia [
20]. The clinical value of automated software use in CBF analysis has been supported in prior literature, demonstrating improved efficiency and decreased subjectivity with the use of Sisson-Ammons Video Analysis (SAVA) compared to the manual count method for CBF measurement [
29]. Furthermore, the findings from a double-blind, placebo-controlled, multicenter trial, demonstrated clinical application and validation when using CiliarMove for CBF analysis in cystic fibrosis receiving nebulized gene therapy [
30].
A limitation of this study was the small sample size. Considering, the relatively strict objective analytic nature of investigating software, we hoped to avoid any misleading data from a small sample size through providing a relatively stable CBF to be measured in both cohorts and an adequate amount of sample biopsies analyzed (>100) to support statistical analysis of the Mann-Whitney U test. Using a shared PCD founder mutation and a group of healthy control ciliated epithelial cells for analyze, we attempted to apply a consistent method to extract data. Theoretically, if both high and low-frequency data sets were consistent, a closer measure of the ability to differentiate CBF could be analyzed. Also of note, the study was not matched for age or gender. However, age and CBF correlation appear to be a controversial subject matter as it’s been noted to have been investigated in insufficient studies to confirm a correlation between the two variables. Despite this, some evidence supports that individuals aged above 40 years demonstrate a slower CBF and decreased mucociliary clearance [
31,
32,
33,
34].
Another limitation was the differences in ROI between CBF measurement methods. Despite our efforts to obtain an ROI of relative similarity through a reference image, this was not pixel-per-pixel accurate. In addition, the different file formats used to analyze the sequences may have influenced the results. It would be interesting to perform a comparative analysis with these factors matched in a study design.
Our study did not complete a comparative software analysis with different PCD genotypes. Certain PCD genotypes are known to have relatively higher or lower CBF, and thus the comparative performance of Cilialyzer and CiliarMove in such cases remains unclear. In such instances, CiliarMove’s ability to measure CBF relative to the manual count method was supported in the study performed by Sampio P
et al. [
21], which included PCD subjects with a genetic mutation in
DNAH11 and
DNHA5 displaying a faster and slower CBF respectively. The mean CBF value calculated from the manual count method and CiliarMove, using 10 ROIs per patient, was comparable across these 3 patients (Manual Count Hz/CiliarMove Hz: 14.30/14.34, 5.74/5.70, 5.83/5.71). Further directions should include the comparative efficacy of software analyzing different genetic PCD variants with known CBF. Additional research on cilia analysis software should be undertaken when new developments arise, as such feedback could be conducive to further improvements.
Freely available open-source software such as CiliaFA, CilarMove, and Cilialyzer had literature published documenting their efficacy from 2012, 2021, and 2022 respectively [
17,
20,
21]. The first two software’s were developed excluding programming that permitted methodical pre-processing ability. Within one year of CiliarMove, literature was published on Cilialyzer, which almost appears as a radically different functioning freely available type of software. Considering the cost of commercially available software lacking Cilialyzer’s added features, the development of freely available software is well-deserving of recognition. A freely available open-source code coupled with multiple added features, from ROI correction to spatial frequency maps and particle tracking, provides a promising outlook on the capability and accessibility of future development in such technology. However, when using HSVA, multiple parameters are assessed from the recordings which include CBF, as well as ciliary beat pattern (CBP), and mucociliary clearance [
35]. The use of CBF analysis alone in PCD diagnosis is insufficient [
9]. Isolated CBF analysis in PCD diagnosis results in low sensitivity and specificity, partially resolved when combined with CBP rendering higher positive and negative predictive values [
36]. Therefore, it should be emphasized that open-source software with the capability of analyzing CBF, CBP, and other variables would need to be developed to automate ciliary functional analysis fully.
5. Conclusions
Our findings demonstrate that manual count, Cilialyzer, and CiliarMove are effective tools for evaluating CBF in PCD patients, as they could accurately identify lower CBF values compared to healthy controls. However, our results highlight the practicality of utilizing software-based analysis tools in diagnosing and assessing PCD. Each open-source platforms offer a semi-automated and less time-consuming option for evaluating CBF, overcoming the subjectivity and labor-intensive nature of manual counting. The findings of this study contribute to the growing body of evidence supporting the utility of open-source ciliary analysis software in PCD evaluation and offering valuable insights for researchers and clinicians in selecting the appropriate software tool for accurate CBF measurement. However, the performance capability of open-access software in analyzing CBF for other genotypes with relatively higher or lower CBF remains unknown. Further research is warranted to validate these findings across different genetic variants, age groups, and new software platforms. Overall, this study highlights the importance of leveraging technology and software advancements to improve the diagnostic capabilities of PCD.
Author Contributions
Conceptualization, W.D. J-R, Z.J.D., J.M-H. ; methodology, W.D. J-R, Z.J.D., J.M-H.; software, W.D. J-R, Z.J.D., J.M-H.; formal analysis, Z.J.D.; investigation, Z.J.D.; data acquisition, Z.J.D., J.M-H., F.Q., G.G-D., G.R., W.D. J-R.; writing—original draft preparation, Z.J.D., W.D. J-R.; writing—review and editing, Z.J.D., J.M-H., F.Q., G.R., G.G-D., M.J.R-B., W.D. J-R. ; R.A.M., visualization, Z.J.D., J.M-H., G.G-D., M.J.R-B., W.D. J-R.; supervision, J.M-H., F.Q., W.D. J-R.; R.A.M., project administration, J.M-H., F.Q., G.R.; funding acquisition, R.A.M., M.J.R-B., W.D. J-R. All authors have read and agreed to the published version of the manuscript.
Funding
The National Institute of Health: Award Number: HCTRECD R25MD007607 from the National Institute on Minority Health and Health Disparities. The authors acknowledge support from the RCMI Specialized Center for Health Disparities at PHSU, funded by MD007579. Research reported in this publication was partially supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20 GM103475. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review of Ponce Health Sciences University, protocol code: 2301128951, approval date: 5/4/2023
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are available upon request through the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- W. B. Hannah et al., “The global prevalence and ethnic heterogeneity of primary ciliary dyskinesia gene variants: a genetic database analysis,” Lancet Respir Med, vol. 10, no. 5, pp. 459–468, May 2022. [CrossRef]
- M. R. Knowles, L. A. Daniels, S. D. Davis, M. A. Zariwala, and M. W. Leigh, “Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease,” Am J Respir Crit Care Med, vol. 188, no. 8, pp. 913–922, Oct. 2013. [CrossRef]
- M. G. O’Connor, R. Mosquera, H. Metjian, M. Marmor, K. N. Olivier, and A. J. Shapiro, “Primary Ciliary Dyskinesia,” CHEST Pulmonary, vol. 1, no. 1, p. 100004, Jun. 2023. [CrossRef]
- J. C. Nawroth, A. M. Van Der Does, A. Ryan, and E. Kanso, “Multiscale mechanics of mucociliary clearance in the lung,” Philos Trans R Soc Lond B Biol Sci, vol. 375, no. 1792, Feb. 2020. [CrossRef]
- A.J. Shapiro et al., “Diagnosis, monitoring, and treatment of primary ciliary dyskinesia: PCD foundation consensus recommendations based on state of the art review,” Pediatr Pulmonol, vol. 51, no. 2, pp. 115–132, Feb. 2016. [CrossRef]
- C. E. Kuehni et al., “Factors influencing age at diagnosis of primary ciliary dyskinesia in European children,” European Respiratory Journal, vol. 36, no. 6, pp. 1248–1258, Dec. 2010. [CrossRef]
- N. Bricmont, M. Alexandru, B. Louis, J. F. Papon, and C. Kempeneers, “Ciliary Videomicroscopy: A Long Beat from the European Respiratory Society Guidelines to the Recognition as a Confirmatory Test for Primary Ciliary Dyskinesia,” Diagnostics (Basel), vol. 11, no. 9, Sep. 2021. [CrossRef]
- J. S. Lucas and M. W. Leigh, “Diagnosis of primary ciliary dyskinesia: searching for a gold standard,” European Respiratory Journal, vol. 44, no. 6, pp. 1418–1422, Dec. 2014. [CrossRef]
- A. Shoemark, S. Dell, A. Shapiro, and J. S. Lucas, “ERS and ATS diagnostic guidelines for primary ciliary dyskinesia: similarities and differences in approach to diagnosis,” European Respiratory Journal, vol. 54, no. 3, Sep. 2019. [CrossRef]
- P. Kouis, P. K. Yiallouros, N. Middleton, J. S. Evans, K. Kyriacou, and S. I. Papatheodorou, “Prevalence of primary ciliary dyskinesia in consecutive referrals of suspect cases and the transmission electron microscopy detection rate: a systematic review and meta-analysis,” Pediatric Research 2017 81:3, vol. 81, no. 3, pp. 398–405, Dec. 2016. [CrossRef]
- M. A. Zariwala, M. R. Knowles, and M. W. Leigh, “Primary Ciliary Dyskinesia,” GeneReviews®, Dec. 2019, Accessed: Aug. 18, 2023. [Online]. Available: http://europepmc.org/books/NBK1122.
- N. Rumman, C. Jackson, S. Collins, P. Goggin, J. Coles, and J. S. Lucas, “Diagnosis of primary ciliary dyskinesia: potential options for resource-limited countries,” European Respiratory Review, vol. 26, no. 143, Mar. 2017. [CrossRef]
- J. S. Lucas et al., “European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia,” European Respiratory Journal, vol. 49, no. 1, Jan. 2017. [CrossRef]
- A.J. Shapiro et al., “Diagnosis of primary ciliary dyskinesia: An official American thoracic society clinical practice guideline,” Am J Respir Crit Care Med, vol. 197, no. 12, pp. e24–e39, Jun. 2018. [CrossRef]
- J. S. Lucas, T. Paff, P. Goggin, and E. Haarman, “Diagnostic Methods in Primary Ciliary Dyskinesia,” Paediatr Respir Rev, vol. 18, pp. 8–17, Mar. 2016. [CrossRef]
- A. Bush et al., “Primary ciliary dyskinesia: current state of the art,” Arch Dis Child, vol. 92, no. 12, pp. 1136–1140, Dec. 2007. [CrossRef]
- C. M. Smith et al., “ciliaFA: a research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software,” Cilia, vol. 1, Aug. 2012. [CrossRef]
- J. H. Sisson, J. A. Stoner, B. A. Ammons, and T. A. Wyatt, “All-digital image capture and whole-field analysis of ciliary beat frequency,” J Microsc, vol. 211, no. 2, pp. 103–111, Aug. 2003. [CrossRef]
- M. Armengot et al., “Development and validation of a method of cilia motility analysis for the early diagnosis of primary ciliary dyskinesia,” Acta Otorrinolaringol Esp, vol. 63, no. 1, pp. 1–8, Jan. 2012. [CrossRef]
- M. Schneiter, S. A. Tschanz, L. Müller, and M. Frenz, “The Cilialyzer – a freely available open-source software for a standardised identification of impaired mucociliary activity facilitating the diagnostic testing for PCD,” bioRxiv, p. 2022.09.04.506514, Sep. 2022. [CrossRef]
- P. Sampaio et al., “Ciliarmove: New software for evaluating ciliary beat frequency helps find novel mutations by a portuguese multidisciplinary team on primary ciliary dyskinesia,” ERJ Open Res, vol. 7, no. 1, pp. 1–12, 2021. [CrossRef]
- W. De Jesús-Rojas et al., “The RSPH4A Gene in Primary Ciliary Dyskinesia,” Int J Mol Sci, vol. 24, no. 3, Feb. 2023. [CrossRef]
- L. Müller et al., “A Comprehensive Approach for the Diagnosis of Primary Ciliary Dyskinesia-Experiences from the First 100 Patients of the PCD-UNIBE Diagnostic Center,” Diagnostics (Basel), vol. 11, no. 9, Sep. 2021. [CrossRef]
- J. S. Lucas et al., “European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia”. [CrossRef]
- C. L. Jackson and M. Bottier, “Methods for the assessment of human airway ciliary function,” Eur Respir J, vol. 60, no. 1, Jul. 2022. [CrossRef]
- P. Thévenaz, U. E. Ruttimann, and M. Unser, “A pyramid approach to subpixel registration based on intensity,” IEEE Transactions on Image Processing, vol. 7, no. 1, pp. 27–41, 1998. [CrossRef]
- E. Puybareau et al., “Automating the measurement of physiological parameters: A case study in the image analysis of cilia motion,” in 2016 IEEE International Conference on Image Processing (ICIP), IEEE, Sep. 2016, pp. 1240–1244. [CrossRef]
- E. Puybareau et al., “A regionalized automated measurement of ciliary beating frequency,” in 2015 IEEE 12th International Symposium on Biomedical Imaging (ISBI), IEEE, Apr. 2015, pp. 528–531. [CrossRef]
- J. H. Sisson, J. A. Stoner, B. A. Ammons, and T. A. Wyatt, “All-digital image capture and whole-field analysis of ciliary beat frequency,” J Microsc, vol. 211, no. 2, pp. 103–111, Aug. 2003. [CrossRef]
- E. W. Alton et al., “A randomised, double-blind, placebo-controlled trial of repeated nebulisation of non-viral cystic fibrosis transmembrane conductance regulator ( CFTR) gene therapy in patients with cystic fibrosis,” Efficacy and Mechanism Evaluation, vol. 3, no. 5, pp. 1–210, Jul. 2016. [CrossRef]
- M. Jorissen, T. Willems, and B. Van Der Schueren, “Nasal ciliary beat frequency is age independent,” Laryngoscope, vol. 108, no. 7, pp. 1042–1047, Jul. 1998. [CrossRef]
- J. C. Ho et al., “The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia,” Am J Respir Crit Care Med, vol. 163, no. 4, pp. 983–988, 2001. [CrossRef]
- K. L. Bailey et al., “Aging causes a slowing in ciliary beat frequency, mediated by PKCε,” Am J Physiol Lung Cell Mol Physiol, vol. 306, no. 6, Mar. 2014. [CrossRef]
- M. Svartengren, R. Falk, and K. Philipson, “Long-term clearance from small airways decreases with age,” Eur Respir J, vol. 26, no. 4, pp. 609–615, Oct. 2005. [CrossRef]
- B. Rubbo et al., “Accuracy of High-Speed Video Analysis to Diagnose Primary Ciliary Dyskinesia,” 2019. [CrossRef]
- W. A. Stannard, M. A. Chilvers, A. R. Rutman, C. D. Williams, and C. O’Callaghan, “Diagnostic testing of patients suspected of primary ciliary dyskinesia,” Am J Respir Crit Care Med, vol. 181, no. 4, pp. 307–314, Feb. 2010. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).