1. Introduction
The fruit’s surface serves as a habitat for microorganisms, and grapes, like other fruits, possess a distinct chemical composition. The chemical composition of grapes is characterized by a high content of simple sugars and a low pH, which provides an environment conducive to the growth of yeasts and bacteria that facilitate sugar fermentation. Clusters of grapes are extensively populated by various yeast species, including Hanseniaspora, Candida, Metschnikowia, Pichia, Rhodotorula, Torulaspora, Aureobasidium, as well as bacteria such as Lactobacillus, Acinetobacter, Pseudomonas, Leuconostoc, Sphingomonas, Lactococcus, Bacillus, Arthrobacter, and others (Francesca et al., 2014; Kántor et al., 2017). Additionally, there are mould fungi and microorganisms (zymogenes) from other habitats (Kačániováet al., 2018).
The microbiological composition of grapes is particularly important in the production of natural wines, where fermentation occurs spontaneously through a consortium of indigenous yeast and bacterial strains. Consequently, the unique secondary bouquet of wines is largely dependent on the microbiological composition of the grapes. Under conditions of elevated humidity and temperature, filamentous fungi most often belonging to the genera Alternaria, Botrytis, Cladosporium, Fusarium, Cladosporium, Rhizopus, and Mucor tend to grow (Tournas and Katsoudas, 2005).Fungal activity depends on weather conditions; in dry growing seasons, where the grapes remain dry, fungi do not develop but persist in spore form. Certain species of mould fungi are toxigenic (for example, from the genera Aspergillus, Penicillium, and Fusarium) and pose a potential risk to consumers of fruits, juices, or wines. The most common contaminants of fruits are ochratoxins, patulin, aflatoxins, fumonisin, and alternariol (Fernández-Cruz et al., 2010). The question arises as to whether limited chemical protection, as practiced in organic cultivation, affects the growth of toxigenic fungi and whether it carries risks related to mycotoxins. The increasing demand for organic food in developed countries is increasing due to consumer awareness. Cancer, allergies of unknown origin, and fertility issues are increasingly attributed to the contamination of crops with pesticides and industrial chemicals in the air and water (Anand and Sati, 2013; Quirós-Alcalá et al., 2018; Weitekamp et al., 2021). This growing demand for organic food is reflected in the expanding acreage dedicated to organic cultivation. Data shows that over the past two decades (2001–2021), the total area under organic cultivation and land undergoing conversion has increased fivefold, reaching 1.3 million hectares. In a single year, from 2020 to 2021, there was a global increase of 1.7% in this area. The organic grape area doubled between 2010 and 2020 and continues to increase every year (Willer et al. 2023).
However, in unfavorable weather conditions, fruit in organic cultivation is susceptible to mould growth and potential mycotoxin contamination (Ochmian et al., 2020). The sulfur and copper compounds allowed in organic cultivation may not always provide sufficient protection. Therefore, in conventionally grown fruit, it is pesticide residues, whereas in organic farming, it is mycotoxins that may (but not necessarily) pose a risk.
Another critical aspect concerns the nutritional value and health-promoting properties of organically and conventionally grown fruits. Particular attention is given to polyphenolic compounds, which are considered to be known for their health-promoting qualities as antioxidants with positive effects on the human body (Cory et al., 2018).These compounds exhibit well-documented antioxidant, antimutagenic (Fahmi et al., 2013; Flamini et al., 2013), cardioprotective, anticancer, antiaging, antimicrobial, and anti-inflammatory properties (Xia et al., 2010; Toaldoet al., 2016). Furthermore, these compounds have an impact on the taste and aroma qualities of both the raw material and the final product, such as wine (Jiang et al., 2022). The composition of polyphenolic compounds in fruit particularly depends on grape cultivars, cultural and agronomic practices (Fenollet al., 2009; Lutz et al., 2011), and climate conditions (Koundouraset al., 2006). Grape maturity is also a very important parameter, as ripening leads to both quantitative and qualitative modifications in these compounds (Kuhn et al., 2013). The most important polyphenols identified in grapes are anthocyanins, flavanols (also called flavan-3-ols), flavonols, and phenolic acids (Chedeaet al., 2010). Dark-skinned fruits, in particular, are rich in these compounds, especially anthocyanins. Moreover, the cultivation method can also influence the technological parameters of the fruit, including the content of basic substances that influence wine taste and the compounds responsible for the proper fermentation of must (Lytraet al., 2020). Organic grapes are characterized by higher total acidity, lower pH, higher content of total polyphenols, including anthocyanins, phenolic acids, and flavonols, and lower volatile acidity compared to conventionally produced grapes (Vrčeket al., 2011; Laureatiet al., 2014). Frequently, organically grown fruits are smaller in size compared to conventionally grown ones, which results in a concentration of compounds within the fruit, often increased by unfavorable growing conditions. Such compounds include polyphenols, which influence the plant’s defense response. Additionally, the organic farming system necessitates the use of organic fertilizers, which have a beneficial effect on soil structure by increasing humus and nitrogen content (Hepperly et al., 2006). Consequently, such fruits contain higher nitrogen content (higher yeast assimilable nitrogen- YAN), which significantly influences the fermentation process by which yeast nutrient doses can be reduced (Sweet and Schreiner, 2010).
Therefore, the aim of the research is to obtain information about the impact of plant protection methods and farming practices (organic and conventional) on the quality of fruit, grape must, and wine. An important aspect of this research is to disseminate and publicize knowledge about the risks and benefits associated with the consumption of fruits and fruit preserves grown using these distinct approaches.
2. Materials and Methods
2.1. Characteristics of the Area of Research and Plant Material
Vitis vinifera L. cultivar Solaris was used for the study (Fot. 1). Fruit was taken from an organic and conventional plantation located near Szczecin in north-western Poland (53°21′09.8″ N 14°26′23.3″ E). In the area of Szczecin and in the nearby northern region, minimal temperatures range from -12 °C to -15 °C, which corresponds to values typical of zone 7B. The average temperature during the growing season (April-October) between 1951 and 2019 was 14.3 °C, and rainfall was approximately 350 mm (Figiel-Kroczyńska et al., 2021).
Figure 1.
Fruit of the Solaris cultivar on an organic plantation (Fot. I. Ochmian).
Figure 1.
Fruit of the Solaris cultivar on an organic plantation (Fot. I. Ochmian).
During 2020-2022, major changes in the weather were observed. In July and August, temperatures exceeded 30 °C for several days and did not fall below 20 °C at night—a phenomenon of tropical nights. There were prolonged periods of drought—2020: 35 days without rainfall; 2021—32 days without rainfall; 2022—46 days without rainfall. This period was followed by heavy/intense rainfall exceeding 100 mm per day. September was characterised by weather typical of the period and similar to that of many years (data obtained from the Meteorological Experimental Station in Lipnik, Poland 53°20′35″N 14°58′10″E).
2.2. Cultivation Scheme
Plants were planted in 2016 at a spacing of 1.01 x 2.28 m (Fot. 2). Pruning is carried out in January-February. The plants were pruned with a Guyot (one arm) training system and vertically positioned with eight shoots with two clusters per each. On both plantations in the rows, weeds are removed with a mechanical weeder. In the inter-rows, mixtures are used to improve the soil structure and enrich it with organic matter (lucerne, red clover, oil radish). During the growing season, shoots and excess leaves from the cluster zone are removed mechanically.
Figure 2.
Organic vineyard plantation (Fot. I. Ochmian).
Figure 2.
Organic vineyard plantation (Fot. I. Ochmian).
During the growing season (May-October), the following was applied for crop protection (in pure component per hectare):
In the organic plantation:
sulphur—12.5 kg ha-1,
copper (copper oxychloride and copper hydroxide)—1.75 kg ha-1
potassium carbonate—17.5 kg ha-1,
potassium grey soap—4 kg ha-1.
In a conventional plantation:
metalaxyl-M (a compound of the phenylacetamide group 3.8%) and mancozeb (a compound of the dithiocarbamate group 64%)—2.25 kg ha-1;
cyflufenamid (a compound of the phenylacetamide group 5.32%)—0.3 kg ha-1;
cyprodinil (a compound of the anilinopyrimidine group 37.5%) and fludioxonil (a compound of the phenylpyrrole group 25%)—1.2 kg ha-1.
Collection samples from the organic and conventional field (three containers of 5 kg fruit each) were taken in late September/early October, in sterile containers. Each bulk sample collected from 10 vine bushes came from a different location within the field. After transport to the laboratory, juice was pressed from the fruit and analyses were performed. Mycotoxins were also determined in the pomace. And the copper and sulphur content was additionally checked in the wine as well (results in preparation for publication).
2.3. Bacteria and Fungi Microbiome Analysis
One gram of each samples (grape must) were grinding and homogenized using TissueLyser LT (Qiagen, Germany). DNA was isolated from prepared material with a QIAampPowerFecal DNA Kit (Qiagen, Germany) and concentrations for each sample were quantified by fluorometric quantitation using a Quantus™ Fluorometer (Promega, Germany).
The gene fragments were amplified with the PCR primers recommended for the Illumina technique. Primers ITS3F (GCATCGATGAAGAACGCAGC) and ITS4R (TCCTCCGCTTATTGATATGC) for fungal ITS library. 341F (CCTACGGGNGGCWGCAG) and 805R (GACTACHVGGGTATCTAATCC) for bacterial 16S rRNA libraries were employed. The 16S rRNA and ITS genes fragment was amplified with the PCR primers recommended for the Illumina method. The primers were designed by adding Illumina adapter overhang nucleotide sequences to the PCR primers provided by Klindworth et al. (2013). Amplicons were indexed using a Nextera® XT Index Kit according to the manufacturer’s instructions. DNA was sequenced in Illumina MiSeq in 2 × 250 paired-end mode. The results of the sequencing were saved in FASTQ files and uploaded to the MetaGenome Rapid Annotation Subsystems Technology (MG-RAST) server for analysis (Meyer et al. 2008). Each file underwent quality control (QC) which included quality filtering (removing sequences with ≥5 ambiguous base pairs) and length filtering (removing sequences with a length ≥2 standard deviations from the mean).
2.4. Must Quality
The total Soluble Solid Content-SSC (°Bx) in samples was measured at 20 °C by digital refractometer (PAL-1, Atago, Japan). Acidity was determined by titration of aqueous extract with 0.1 N NaOH to an end point with pH 8.1 (Elmetron CX-732, Zabrze, Poland), according to the PN-90/A-75101/04 standard.
The must’s turbidity was measured using a Lovibond TB211IR working on the principle of measuring scattered light in the 400–600 nm range.
YAN was quantified using the enzymatic method, readings were taken in an automatic wine analyser using the spectrophotometric method.
The DPPH (1,1-diphenyl-2-picrylhydrazyl) assay was conducted according to the method of Yen and Chen (1995). The FRAP (ferric-reducing antioxidant power) assay was conducted according to the method ofBenzie and Strain (1996). The antioxidant capacity is expressed as millimoles of Trolox per 100 g DW. Measurements in the DPPH and FRAP assays used a UV-2401 PC spectrophotometer.
Polyphenolic compounds were analyzed using UPLC-PDA-MS/MS Waters ACQUITY system (Waters, Milford, MA, USA) consisting of a binary pump manager, sample manager, column manager, photodiode array (PDA) detector, and quadrupole mass spectrometer with electrospray ionization (ESI) (Mijowskaet al., 2016).
All tests were performed in three replications.
2.5. Mycotoxin Detection
Detection of mycotoxins in grape must and pomace
Trichothecenes and Zearalenone Analysis. A 12.5 g sample was mixed with 50 mL ACN:H2O (80:20, v/v) for 1 hour, then centrifuged at 5000 rpm for 10 min. The supernatant was subjected to a clean-up process using a Bond Elut® Mycotoxin column (Agilent, USA). An aliquot of 40 µL internal standard solution (13C-ZAN; c = 1000 µg L-1) was added to 4 mL of the extract, and then 2 mL of the purified extract was combined with 50 µL internal standards solution (13C-DON; c = 2500 µg L-1; 13C-T2; c = 250 µg L-1 and 13C-HT2; c = 250 µg L-1). This mixture was evaporated to dryness using nitrogen at 45 °C. Subsequently, 495 µL of MeOH:H2O (1:4) was added and the sample was reconstituted. Mycotoxins were determined using HPLC with MS/MS detection on a Shimadzu Nexera coupled to API4000 mass spectrometer, equipped with a Gemini-NX C18 chromatographic column, employing a gradient elution using 1% CH3COOH in H2O (mobile phase A) and MeOH (mobile phase B) with the addition of 5 mM CH3COONH4 to both mobile phases.
Patulin Analysis. A 5 g sample was mixed with 20 mL ACN:H2O (80:20, v/v) for 1 hour, followed by centrifugation at 5000 rpm for 10 min. The supernatant was clean-up using a push-through-type SPE column, MycoSep 228 AflaPat (Romer, Austria). The purified eluate (4 mL) was combined with 20 µL of isotopic 13C-labeled PAT (13C-PAT; c = 50 µg L-1) and evaporated to dryness under a gentle stream of nitrogen at 45 °C. The residue was then redissolved in 1 mL of a mobile phase mixture, MeOH:H2O (3:7, v/v), and filtered prior to analysis. Detection was carried out using HPLC Nexera coupled with a 5500 QTrap mass detector, and a Gemini C18 column for separation.
Fumonisins Analysis. A 25 g sample was homogenized with 100 ml ACN:H2O (50:50) for 3 min. The extract was filtered, and the pH was adjusted to 6-9. Three milliliters of the filtrate were mixed with 8 ml of MeOH:H2O (75:25), and this mixture was applied to a conditioned MultiSep 211 Fum column (Romer Labs, Tulln, Austria). The column was washed with 8 ml of MeOH:H2O (75:25) and 3 ml of methanol. Toxins were eluted using 10 ml of MeOH: CH3COOH (99:1). The eluate was collected and evaporated to dryness with nitrogen. Then, 1 ml of ACN:H2O 1:1 solution was added to the vial, and the sample was mixed. Detection was achieved using HPLC Nexera, API4000 mass spectrometer, and a Gemini-NX C18 column.
Ochratoxin A Analysis. A sample portion (12.5 g) was homogenized with 50 ml ACN:H2O (60:40) for 2 min. After filtration, a 5 ml aliquot of supernatant was added to 55 ml PBS solution, and the mixture was filtered again. A total of 48 ml of the diluted extract was applied to an Ochraprep column (Rhone Diagnostic, Glasgow, UK). The column was washed with 20 ml of H2O and dried with air. OTA was eluted using 1.5 ml of MeOH:CH3COOH (98:2). The eluate was collected, and 1.5 ml of H2O was passed through the column, followed by mixing of the sample. Detection was performed using HPLC with fluorescence detection.
Aflatoxins Analysis. To 25 g of the sample, 2.5 g of NaCl were added and homogenized with 50 ml of MeOH:H2O (80:20, v/v) for 1 min. After filtration, 10 ml of the extract was added to 40 ml of H2O, shaken, and filtered again. A total of 10 ml of the diluted extract was applied to an AflaTest column (Vicam, Watertown, USA). The column was washed twice with 10 ml of H2O. Aflatoxins were eluted using 1 ml of MeOH. The eluate was collected, and 1 ml of H2O was added before mixing the sample. Aflatoxins were determined using HPLC with FLD preceded by post-column derivatization.
Detection of the presence of genes encoding ochratoxins, aflatoxins and patulin in must
To prepare the sample for the isolation of total genetic material, 10 ml of the test material in liquid form was concentrated by centrifugation (7000 rcf for 20 minutes), and the supernatant was decanted. The pellet, which consisted of the concentrated material, was then homogenized in 2 ml of sterile deionized water. For DNA isolation, 250 µl of the homogenate was collected, and the remaining material was dried (using a drying scale—Radwag, Poland) to determine the dry weight. DNA isolation was performed by lysing the collected material in tubes containing a lysis buffer and glass beads. Lysis was carried out for 10 minutes at a frequency of 50 oscillations per second (using a TissueLyser LT from Qiagen). Subsequently, total DNA was isolated using the Food-Extract DNA Purification Kit (EURx) according to the manufacturer’s instructions. The isolated DNA was used for qPCR reactions to quantitatively determine mycotoxin-producing fungi.
Standards for qPCR reactions were prepared by incorporating amplification products (OTA—Penicillium verrucosum, AFLA, and patulin—a sample of degraded food) into a plasmid using the TOPO™ TA Cloning™ Kit, with pCR™ 2.1-TOPO™ (Thermo Fisher Scientific/Invitrogen). The copy number of genes was calculated using a calculator available at DNA calculator.
qPCR reactions for patulin and AFLA were performed following the methodology described by Rodrigez and others (2011a, 2011b, 2012). AFLA: F-omt GGCCGCCGCTTTGATCTAGG, R-omt ACCACGACCGCCGCC, OMTprobe [HEX]-CCACTGGTAGAGGAGATGT-[BHQ1]. Patulin: F-idhtrb GGCATCCATCATCGT, R-idhtrb CTGTTCCTCCACCCA, IDHprobe [FAM]-CCGAAGGGCATCCG-[TAMRA]. Ochratoxin: F-npstr GCCGCCCTCTGTCATTCCAAG, R-npstr GCCATCTCCAAACTCAAGCGTG 5185 NPSprobe [Cy5]-CGGCCGACCTCGGGAGAGA-[BHQ2].
The reaction mixtures were prepared using a master mix buffer (Maxima Probe 2X, ThermoFisher Scientific), 720 nM of each F-idhtrb and R-idhtrb primer and IDHprobe, 480 nM of each F-npstr and R-npstr primer and 600 nM of NPSprobe, and 80 nM of F-omt primer and 160 nM of each R-omt primer and OMTprobe. Each reaction included 1 μL of DNA template from each of the mycotoxin-producing strains (3 μL of total DNA template) in a final volume of 25 μL. The reactions were carried out using the RotorGene Q platform (Qiagen).
The qPCR program included an initial incubation at 50 °C for 2 minutes to activate the uracil-N-glycosylase (UNG) enzyme, followed by an incubation at 95 °C for 10 minutes to denature the UNG enzyme. The cycling phase included 40 cycles at 95 °C for 30 seconds and 58 °C for 2 minutes.
2.6. Detection of Pesticide Residues
The analysis was carried out on must samples prepared from grapes. Each bulk sample was analysed for the detection of 280 active substances and their metabolites most commonly used in horticultural crops. Gas-tandem chromatography-mass spectrometry (according to EN 15662:2018-06-GC-MS/MS) was used to detect pesticide active substances. Analyses were performed in triplicate. This set of tests is performed as standard for the control of organically grown fruit at The National Institute of Horticultural Research (Link 1). The contents of Cu, and S were determined after mineralization in HNO3and HClO4at a ratio of plants of 3:1 (IUNG, 1990). The Cu and S contents were measured with flame atomic absorption spectroscopy (iCE 3000 Series).
2.7. Statistical Analysis
Statistical analyses were performed with Statistica 12.5 (StatSoft Polska, Cracow, Poland). The t-Student test was used to compare two means. Fisher’s exact test was used to test statistical significance in contingency table analysis. Statistical methods of data analysis are provided in the descriptions or titles of graphs and tables. The dominance structure was determined based on the following scale: eudominantsabove 10.0% of all individuals in thecompared taxonomic group, dominants: 5.1%–10.0%, subdominants: 2.1%–5.0%, recedents: 1.1%–2.0%, subrecedentsbelow1.0%. The ecological indicators describing the relative size of a fungal community, dominance, diversity and evenness were calculatedwith the use of standard formulas. Principal componentanalysis (PCA) was performed and visualized in XLSTAT software (Lumivero, 2023).
3. Results
3.1. The Grape Microbiome
3.1.1. Bacteria
Different methods of grape cultivation (conventional and organic) influenced the composition of the grape bacterial profile, particularly in terms of the quantitative representation of identified taxa (
Table 1). In most cases, similar types of bacteria were detected in both conventionally and organically grown fruits; however, their respective quantitative contributions varied depending on the cultivation method. It is worth noting the disparity in the number of reads for all the identified bacterial sequences (
Table 1).
In the case of bacteria originating from organic cultivation, nearly three times more sequences were observed than those detected on conventionally grown fruits. Nineteen additional bacterial taxa were found on organic fruits (
Table 1). Bacteria of the genus Sphingomonas were the most abundant, regardless of the cultivation method. These dominant bacteria constituted approximately 22.5% of the total identified prokaryotic population. The next most prevalent group of bacteria in both cases (fruits from organic and conventional fields) belonged to the genus Massilia (15.7% and 18%, respectively). Notably, there was a statistically significant increase in the presence of these bacteria on fruits treated with conventional pesticides. These two genera of bacteria clearly dominated, regardless of the cultivation method, impacting similar ecological parameters such as dominance, diversity, and evenness, with respect to the bacterial populations in both cultivation types (
Table 1). Bacteria classified into other genera represented a significantly lower percentage. Furthermore, they accounted for significantly fewer representative taxa (ranging from 1.7% to 6.1% of the entire bacterial pool). For instance, Hymenobacter was more abundant in fruits from fields protected by conventional pesticides. In contrast, bacteria from the genera Pseudomonas, Variovorax, Rhizobium, Brevundimonas, and Pedobacter were more abundant in fruits from organic cultivation. The remaining groups were even less represented in the overall bacterial pool (
Table 2). In six instances, there was a lack of trace amounts of bacterial representatives from a particular taxon on fruits from one field. This observation mainly concerned less representative taxa on the grape clusters, constituting less than 1% of the total bacterial population. With one exception, the genus Gluconobacter fairly densely populated fruit from conventional cultivation (5.4% of all bacteria). In organic cultivation, the presence of these bacteria was noted at 0.2%. Similarly, bacteria from the genera Erwinia and Serratia were present in conventionally grown fruits, but their quantities were low, approximately 1.6% (Serratia) and 0.8% (Erwinia), while they were either not detected or found in very minimal amounts on organic fruits (0.2% for Serratia).
3.1.2. Fungi
Considering the total number of sequence reads (16 390 and 17 125) and the number of differing taxa (33 and 35 OTUs), it can be inferred that the farming system did not exert a significant influence on the qualitative and quantitative composition of grape-inhabiting fungi. However, for a more comprehensive analysis of the research findings, differences in ecological parameters should be specified (
Table 1). It is worth noting that the dominance of several taxa was more pronounced in the case of grapes from conventional cultivation. At the same time, greater diversity and evenness within fungal taxa were observed in organic cultivation (
Table 1). The most dominant fungi, inhabiting the fruits regardless of the farming system, were Erysiphe and Aureobasidium. The difference lay in the fact that fungi belonging to the Erysiphe genus constituted nearly 40% of all identified fungal taxa on fruits from conventional cultivation, which is approximately 18% more than on organic fruits. In the case of fungi from the genus Aureobasidium, they were abundant in both organic and conventionally grown fruits (accounting for 29% of all fungi). The subsequent most abundant taxa, irrespective of the type of cultivation, included genera such as Alternaria, Mycosphaerella, Botryotinia, Cladosporium, Dissoconium, and Penicillium. However, the genera Botryotinia, Mucor, Hanseniaspora, Mycosphaerella, and Dissoconium were significantly more abundant in organic fruits than in conventionally protected ones. Hanseniaspora and Mucor were virtually absent from fruits protected with synthetic pesticides. On the other hand, the genus Penicillium was less abundant, by approximately 50% in organic fruits (
Table 3).
3.2. Residues of Active Substances of Pesticides in Must
In organic grape production, sulfur (S) and copper (Cu) used in vineyard management typically originate from specific permitted organic inputs. In both the must and wine, the organic crop exhibited higher levels of Cu when compared to the conventional crop. In the must, the organic crop registered 3.14 mg kg-1 of Cu, whereas the conventional crop showed 2.08 mg kg-1. Similarly, in the wine, the organic crop contained 0.56 mg kg-1 of Cu, while the conventional crop had 0.44 mg kg-1.
The analysis revealed significant disparities in S levels between the organic and conventional crops, both in the must and the resulting wine. The organic crop displayed higher S concentrations compared to the conventional crop. Specifically, in the must, the organic crop displayed a concentration of 17.4 mg kg
-1 of S, whereas the conventional crop had 3.2 mg kg
-1. Likewise, in the wine, the organic crop had 6.7 mg kg
-1 of S, in contrast to the conventional crop which had 2.8 mg kg
-1 (
Table 4). Importantly, no pesticides unauthorized for use on organic plantations were detected in the must from organic fruit.
However, it is worth noting that high levels of synthetic pesticides were found in two out of the three pooled samples obtained from the conventionally cultivated field. The content of two substances (cyprodinil and fludioxonil) exceeded the legal limit by more than four times (
Table 5).
3.3. Mycotoxins
3.3.1. Mycotoxins in Must (Chemical Analysis)
No mycotoxins (aflatoxins, trichothecenes, zearalenone, patulin, fumonisins, ochratoxin A) were detected in any of the must and grape marc.
3.3.2. Load of Mycotoxin-Producing Fungi (Molecular Analysis)
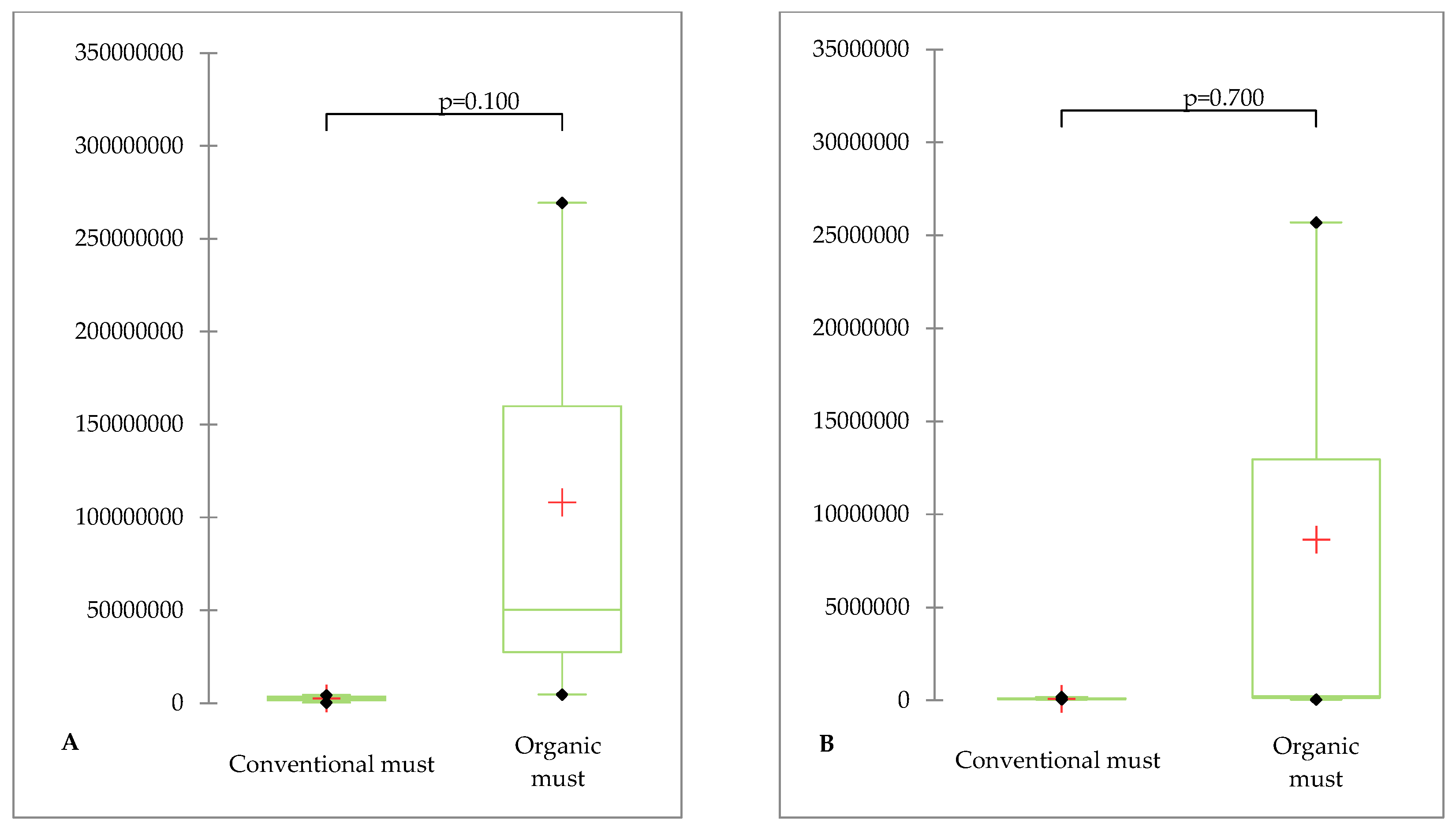
As per the Mann–Whitney test, it was noted that the presence of fungi with the capability to produce aflatoxins and ochratoxin was higher in the case of organic cultivation (
Table 6). However, these differences were not statistically significant (
Figure 1). This lack of significance was due to variations in results observed within the field. In the case of results from the ecological field, the parameter value was influenced by the sampling point.
3.4. Chemical and Health-Promoting Constituents in Musts
The results revealed significant differences in the content of polyphenolic compounds and antioxidant activity between the two cultivation methods. Across all the parameters measured, the organic cultivation method consistently exhibited higher levels in comparison to the conventional method. Polyphenolic compounds, including hydroxycinnamic acids and their derivatives (such as GRP, caftaric acid, coutaric acid, fertaric acid), as well as flavan-3-ols (such as procyanidin dimer B1, (+)-catechin, procyanidin dimer B2, and (−)-epicatechin), were all significantly higher in the must derived from organic cultivation. The predominant compound in the musts was the acid constituting 61% of all acids, and (−)-epicatechins constituting 75% of all flavan-3-ols (
Table 7). Furthermore, the total content of flavan-3-ols, encompassing various monomers, oligomers, and polymers, was also significantly higher in the organic must. Moreover, the highest increase of approximately 32% was recorded in the case of (+)-catechin after organic cultivation. Among phenolic acids, the highest increase was recorded for caftaric acid, which displayed a 15% difference compared to conventional cultivation. In contrast, (−)-epicatechin showed only a 9% variation between products grown in different cultivation systems. On the other hand, the cultivation system had no statistically significant impact on the gallic acid content. Furthermore, the organic cultivation method demonstrated superior antiradical activity and reducing power compared to the conventional method, as indicated by the DPPH and FRAP assays.
3.5. Technological Parameters of Must
Based on the provided data (
Table 8), the results indicate a relationship between the type of crop cultivation (organic or conventional) and several parameters related to the quality and composition of the crop, as well as its subsequent processing into wine. The results are as follows: The organic crop had a higher level of soluble solids (measured as % Brix) in the must (24.8%) in comparison to the conventional crop (22.5%). This indicates that the organic cultivation method may have contributed to a higher sugar content in the grapes, potentially influencing the potential alcohol content of the resulting wine. The organic crop displayed a lower acidity level (7.5 gL
-1) compared to the conventional crop (8.6 gL
-1). This suggests that the organic cultivation method may have resulted in lower levels of organic acids in the grapes, which can have an impact on the taste and overall balance of the wine. The conventional crop had a higher turbidity level in the must (1388 NTU) compared to the organic crop (1116 NTU). Turbidity refers to the cloudiness or haziness of the grape juice, and a lower value indicates a clearer must. The organic crop had a higher level of YAN in the must (147 mgL
-1) in contrast to the conventional crop (117 mgL
-1). YAN represents the amount of nitrogen available to yeast during fermentation, which is essential for yeast growth and fermentation. The higher YAN in the organic crop indicates a potentially better nutrient availability for yeast during fermentation.
3.6. Influence of the Sampling Site in Relation to the Variants
Based on the results of the ANCOVA analysis, it was found that the type of cultivation influenced only a subset of parameters, while the sampling location, including technical replicates, had an impact on seven of them (
Figure 2 and
Figure 3). However, interactions between the sampling location and variant were observed in eight cases, specifically for GRP, dimer B1, dimer B2, acidity, and NTU. The results indicate a trend of increasing values in the first three parameters (GRP, Dimer B1, Dimer B2) for the organic cultivation variant. For acidity and NTU, interactions between the sampling location and variant were also observed. This may suggest a rising trend in these parameters for conventional cultivation, although it could also be related to random variability within the field. The analysis results indicate statistically significant differences between the cultivation types for many chemical parameters of grapes. The statistical significance results for individual features are as follows: caftaric acid, coutaric acid II, fertaric acid, (+)-catechin, BRIX, and HAD had very low p-values (p < 0.001), indicating significant differences between the cultivation types (organic vs. conventional) for these parameters. p-values below 0.001 provide strong evidence of statistical significance. For Gallic acid and YAN, the p-values were lower but still significant (Gallic acid: p = 0.008, YAN: p = 0.048), indicating an impact of the cultivation type on these parameters, although it may be slightly weaker than for the previously mentioned features. For the FRAP parameter, the p-value was 0.057, indicating a lack of statistical significance at the p=0.05 level. However, this result suggests the existence of a trend in the differences between the cultivation types for this parameter, although not strong enough to be considered significant. All models had R2 values exceeding 0.5, indicating a strong influence of the main factors and their interactions on the variability of the results. An R2 value between 0.5 and 1 suggests that the model explains a substantial portion of the variability in the data.
3.7. Relationships between Variables
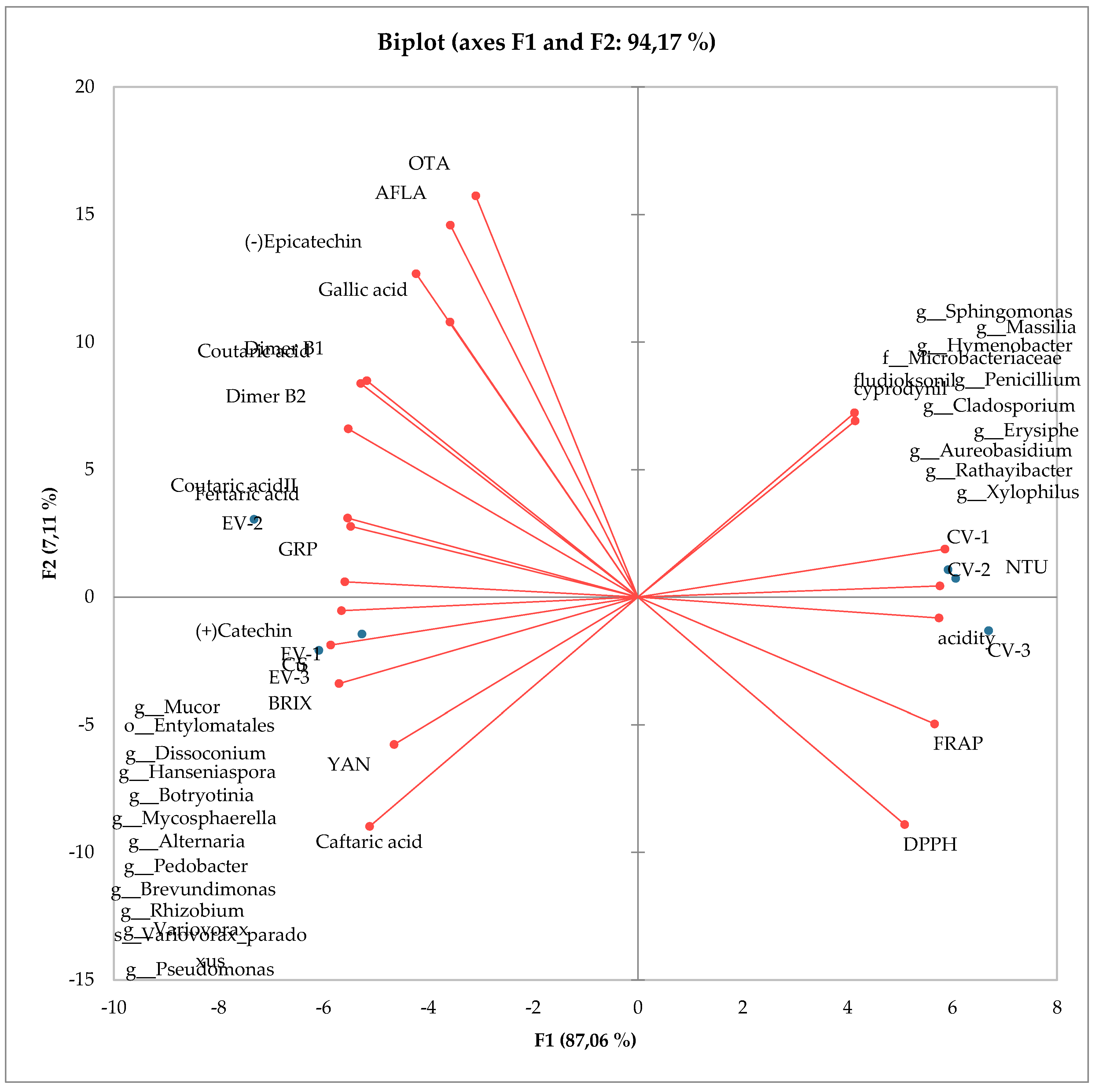
Based on the principal component analysis (PCA), it was evident that this analysis accounted for 94% of the variance, indicating that the selected components explain a significant part of the variability in the data (
Figure 3). The ordination axis F1, which explained 87% of the result’s variability, proved to be decisive. The PCA results delineate a distinct separation of variants based on the cultivation type. The ecological variants (EV) were closely clustered on the left side of the ordination axis F1, while the conventional variants (CV) formed a tight cluster on the right side of the ordination axis F1. This finding suggests that the cultivation type has a significant influence on the overall variability in the data and that there may be distinct differences in the chemical composition between these two cultivation types. Moreover, there was high a within-object similarity, indicating that the features examined within the same variant exhibited similar values. On the other hand, there was a high dissimilarity between variants which indicated that the features differed significantly among different variants. These results confirm that there are clear chemical differences between different grape cultivation variants. Based on a detailed analysis of the associations observed among the variables, the following patterns were noticed: for objects with higher values, which are characteristic of the eco variant and demonstrate strong correlations with each other, the following variables were identified: OTA, AFLA, (−)Epicatechin, gallic acid, dimer B1, coutaric acid, dimer B2, coutaricacid II, fertaric acid, GRP, (+)-catechin, microbiome I, Cu, S, BRIX, YAN, and caftaric acid. This means that these variables are related and have similar values for grapes from organic cultivation. On the other hand, for objects with higher values, which are characteristic of the eco variant and are strongly correlated with each other, the following variables were identified: fludioxonil, cyprodinil, microbiome II, NTU, acidity, FRAP, and DPPH. This indicates that these variables are also associated with each other and have similar values for grapes from organic cultivation. In most cases, variables characteristic of the eco variant were negatively correlated with variables characteristic of the conv variant. However, there were exceptions, such as YAN and caftaric acid, which showed a low negative correlation with DPPH and FRAP, and (−)-epicatechin, gallic acid, AFLA, and OTA, which exhibited a low negative correlation with fludioxonil and cyprodinil. It is also worth noting that there was a strong negative correlation between the remaining variables characteristic of the eco variant and those characteristic of the conv variant. This shows that these two groups of variables differed significantly from each other for grapes from different cultivation types.
Figure 2.
PCA biplot showing relation between the most abundant orders of bacteria, fungi, tested parameters and their influence on ecological (EV 1, 2, 3) and conventional samples (CV 1, 2, 3).
Figure 2.
PCA biplot showing relation between the most abundant orders of bacteria, fungi, tested parameters and their influence on ecological (EV 1, 2, 3) and conventional samples (CV 1, 2, 3).
4. Discussion
4.1. Influence of the Viticultural System on the Microbiome
Our findings enable a comparison between two cultivation systems. Organic cultivation uses pesticides based on natural active substances such as sulfur and copper, and in conventional cultivation, a number of synthetic pesticides are allowed. In our study, we investigated the differences resulting from the use of the two distinct protection strategies.A significant difference was found regarding the number of reads of all detected bacterial sequences (Reads) on fruit from both types of cultivation. There were almost three times as many bacteria from organically grown fruit. Additionally, there were 19 more bacterial taxa (OTUs) identified on organic fruit. These quantitative differences in bacteria lead to the conclusion that the fungicides used have a substantial impact on the crop. The protection allowed in organic cultivation (pesticides with S, Cu, and potassium compounds) favored a more abundant bacterial presence on the fruit compared to synthetic pesticides. However, it should be noted that the ecological parameters assessing diversity and dominance among bacteria did not show significant differences based on the type of cultivation.The cultivation method has a significant effect on bacteria, especially their quantitative representation of the fruit, and indirectly influences their diversity. Previous studies have indicated the negative effect of fungicides on bacteria (reduction in abundance or elimination) (Gu et al., 2010; Getachew and Abeble, 2021; Ma et al., 2021). Consequently, fungicides may reduce bacterial diversity and the number of bacteria on the fruit, potentially weakening the fruit’s natural biological defense against pathogens.
Such protection may result from antagonistic interactions, such as competition between bacteria and phytopathogenic fungi for habitat (Ma et al., 2021). The genus Sphingomonas are aerobic bacteria belonging to the α-proteobacteria. It is a highly diverse taxon comprising more than 103 species (White et al., 1996). Their characteristic feature is the production of an intense yellow pigment (Yabuuchiet al., 1990). Bacteria of the genus Sphingomonas most abundantly colonized grapes regardless of the type of crop.Therefore, it can be concluded that these bacteria react in the same way (or do not show sensitivity) to both natural and synthetic protectants. Information on the Sphingomonas resistance to pollutants can be found in the works of the authors of the publication. The ability of Sphingomonas strains to biodegrade aromatic hydrocarbons Mueller et al., 1990; Hesham et al., 2014; Zhou et al., 2022), ionic liquids such as commercial imidazolium-, pyridinium-, pyrrolidinium-, ammonium- and phosphonium-based ILs (Abrusciet al., 2011), and pesticides (Ravintheranet al., 2019) has been documented.
These bacteria are widely prevalent in the environment, found in soil and water, and are abundant in plants. They are recognized as part of the plant growth-promoting rhizobacteria (PGPR) and can be located both in the root zone of plants and above the ground (White et al., 1996; Kim et al., 2020; Luo et al., 2020). Similarly, a bacterium belonging to the genus Massilia has been positively identified in the environment (Guo et al., 2019; Fasusiet al., 2021). These bacteria were notably abundant in grapevine fruit grown in both organic and conventional ways (constituting an average of 16.5% of the total pool of bacteria classified as OTUs).Therefore, it can be concluded that these bacteria react similarly to the pesticides used in both cultivation methods, and they do not lose their beneficial role in promoting plant growth. Additionally, these bacteria have the capacity to parasitize fungi, such as Pythium, which helps limit potential yield losses (Ofek et al., 2021). A high percentage of bacteria from the genera Sphingomonas and Massilia was recorded in wine after spontaneous fermentation by Chen et al. (2022).
In contrast, PGPR from the genera Variovorax (including V. paradoxus), Pseudomonas, Rhizobium, and Pedobacter were found to be sensitive to synthetic pesticides, resulting in a significant reduction in their presence on conventionally protected grapes compared to organically grown grapes. An interesting situation arises in the case of bacteria belonging to the genus Brevundimonas, which also displayed sensitivity to pesticides. This bacterium is considered opportunistic, likely due to its isolation from hospital environments (Ryan and Pembroke, 2018). It thus proved difficult to explain the presence of this particular type of bacteria on fruit—almost 4% in organic cultivation and nearly half that amount in conventional cultivation. In 2020, strains of this bacterium were isolated from the soil, specifically from the rhizosphere of potatoes, making it the first description of these strains in soil. The positive role of these bacteria in diazotrophy and the provision of phosphorus to plants was described (Naqqash et al., 2020).Therefore, the detection of this bacterium on grapevines is not longersurprising. Bacteria are not a target of toxic fungicide activity. Nevertheless, the results of the study indicate the coexistence of bacteria in the environment that are both sensitive (described above) and resistant to fungicides (Kecskeméti et al., 2016). In the current study, some bacteria were also found which occupied the habitat of the fruit previously inhabited by susceptible microorganisms (bacteria and fungi).
These included species from the genera Massilia (included in the PGPR) mentioned earlier, Hymenobacter (environmental bacteria, isolated especially from the rhizosphere), Xylophilus (bacterial pathogen of grapevines), and Gluconobacter (associated with causing fruit spoilage and adulteration of wines) (Szegedi and Civerolo, 2011; Kecskeméti et al., 2016). Bacteria of the genus Xylophilus were almost twice as much on conventionally treated grapes. Bacteria of the genus Gluconobacter were less abundant in organic cultivation but clearly present (above 5%OTUs) on fruit that received extensive chemical protection.
Fungicides allowed in both organic and conventional farming had a similar impact on the overall count of fungal sequence reads. The parameter values were similar in both combinations compared (Reads and OTUs). The ecological parameters that describe diversity, evenness in the fungal community, and the dominance index are also worth noting. Based on these data, it can be observed that the fungicides used did not significantly differ in their influence on the number of identified fungi. However, they had a distinct effect on diversity, which was more pronounced compared to the effects seen in the case of bacterial communities (
Table 1). Grapes from both crop types were predominantly colonized by fungi belonging to the genus Erysiphe, with grapevine powdery mildew attributed to the fungus Erysiphenecator.In conventional farming, microorganisms that are sensitive to the pesticide combination used have become extinct or limited. Resistant and also phytopathogenic Erysiphe have gained additional habitat to live in and their numbers have increased. Jones et al. (2014) investigated the phenomenon of Erysiphe necator acquiring resistance to azole fungicides used intensively in grapevine cultivation.In organic cultivation, where a greater biodiversity of fungi and bacteria was found, Erysiphe was about 20% less than on conventionally grown fruit. However, despite the significant contribution of this fungus to the microbiome, no symptoms of powdery mildew were found either on the grapes or on the vine leaves. The fungi thrive especially when sanitary pruning is not performed, the canopy is not thinned, the spring is rainy and the summer is humid (García-Cela et al., 2015; Abarca et al., 2019). The growing season was dry, so although Erysiphe populated the clusters in large numbers, growth did not occur, remaining as spores or at an early stage of development. It is also possible that the abundant fungi of the genus Aureobasidium (in our study they accounted for nearly 30% of the sequence of OTUs in both crop types) limited the proliferation of Erysiphe and other identified phytopathogenic fungi. The yeast Aureobasidium is described as a beneficial microorganism that stimulates plant immunity, including in grapevines (Pinto et al., 2018). Aureobasidiumpollutans occurs abundantly on the grapevine, both in the rhizosphere and phyllosphere, including the fruit and consequently also in the juice affecting the bouquet of natural wine (Onettoet al., 2020). Some fungal genera were present in lower numbers on conventionally protected (CV) grapes (sensitive to pesticides): Botryotinia, Entylomatales, Mycosphaerella (these are mostly phytopathogenic fungi) and also the genus Dissoconium (some beneficial species as they parasitise Erysiphales, Sclerotinia sclerotiorum and other fungi in the phyllosphere) (de Hoog et al., 1991). Some fungi were absent on chemically protected (CV) fruit, although they were present on organic (EV) fruit: Hanseniaspora and Mucor (the most susceptible). In contrast, fungi of the genus Penicilium were almost half as numerous on clusters protected with synthetic fungicides. Regardless of the crop type, the fungi Aureobasidium, Alternaria, Cladosporium and many others with a negligible percentage of the total microbiome were present in similar numbers.
4.2. Influence of Viticultural System (CV and EV) on the Activity of Toxigenic Fungi—Mycotoxin Secretion
No mycotoxins were detected in the grape must or the pomace remaining after pressing. In general, the most commonly detected mycotoxin in grape juices, wines or grapes is ochratoxin A (OTA). Ochratoxin A is secreted by certain fungal species belonging to the genera Aspergillus and Penicilium (Welke, 2019).Fungi of the genus Aspergillus were not identified in the tested material, while Penicilium fungi were found in quite high numbers. Genes encoding ochratoxin A were also found in the juice of grapes (both organic and those protected with synthetic pesticides). This mycotoxin is produced, among others, by species belonging to Penicillium, such as P. verrucosum (Fernández-Cruz et al., 2010). But the presence of ochratoxin A itself was not found in the juice and pomace. Although the genes responsible for the trait are present in the genome of the microorganism, their expression does not always occur. Mycotoxins are produced by the mycelium, which grows under favourable conditions, during the warm and humid growing season.Without mycellium and suitable weather conditions, mycotoxins are not produced (Daou et al. 2021). No fungus was found on the fruit. It is possible that fungi with toxigenic potential remained in spore form. Aflatoxins are produced by fungi of the genus Aspergillus, the presence of which was not confirmed in the material studied. However, genes encoding aflatoxins were found to be present (
Table 6). In view of the fact that only Aspergillus produces aflatoxins, the results were puzzling. We suspect that among the taxonomically unidentified fungi (accounting for 1–0.6% in organic and conventional cultivation, respectively) (
Table 3), it may have been the fungi of the genus Aspergillus, i.e., the ‘owners’ of the genes encoding aflatoxins. It should be clear that there is an increased proportion of genes encoding the tested mycotoxins in the case of organically grown juice (
Table 6). So there is clearly a higher risk of toxin contamination of ‘organic’ must in the case of favourable conditions for the development of toxigenic fungi. In our experiment, fungi did not develop on the clusters which prevented the possible production of mycotoxins. Potentially toxigenic Fusarium were present in negligible numbers and therefore no mycotoxins secreted by these fungi were detected.
Influence of Viticulture Method on the Presence of Pesticide Residues
Copper and sulphur-based fungicides are commonly used in vineyards to control fungal diseases such as grey mould, powdery mildew and powdery mildew (Kraus et al., 2012). However, if fungicides are applied in large quantities (due to unfavourable weather conditions and risk of fungal growth) or are not rinsed off by rain, they can remain on the surface and inside the closely packed clusters of the Solaris cultivar for a long time (Dagostin et al., 2011; La Torre et al., 2018). Higher levels of Cu and very high levels of S were found in the organic must compared to the conventional must. Analysis of the finished wine indicated significantly lower levels of these elements. In contrast, the synthetic pesticides cyprodinil and fludioxonil contaminated must obtained from conventionally grown grapes.The authors of a report on grape contamination by pesticide residues, Golge and Kabak (2018), found exceedances of EU permissible levels in more than 20% of samples from several hundred tested (including high levels of cyprodinil).And nearly 60 per cent of the samples contained pesticides in legally permissible amounts. The presence of pesticide residues in the harvested fruit or in the grape must is often found, even in vineyards following the principles of integrated pest management (IPM) (Česnik et al., 2008).Some of the active substances, for example fludioxonil, should be reassessed for safety due to reports of health risks (Brandhorst and Klein, 2019). The presence of pesticide residues in the must tested may have been influenced by the lack of rainfall during the growing season. Although chemical protection was applied according to guidelines, subsequent doses may have accumulated (Fot. 3).
Figure 3.
Sulphur and copper solution on grape leaves in an organic plantation (Fot. I. Ochmian).
Figure 3.
Sulphur and copper solution on grape leaves in an organic plantation (Fot. I. Ochmian).
Chemical protection of vines starts in spring, after the start of vegetation, and is carried out throughout the growing season, with withdrawal periods. The measures used were aimed at protecting the plants against powdery mildew, powdery mildew and grey mould. These diseases are the biggest problem in grapevine cultivation in our climate. Consumption of grapes with high levels of copper can pose a health risk, especially if they are consumed in large quantities or for long periods of time. Copper toxicity can lead to gastrointestinal problems, liver damage and other health problems (Strausaket al., 2001). Grapes contaminated with copper can adversely affect wine quality. Copper can react with other compounds during fermentation, leading to unpleasant aromas, shortened shelf life and stability problems in the finished wine. They can also affect the colour, aroma and flavour profile of the wine (Clark and Scollary, 2000). These results imply that the organic cultivation approach potentially led to elevated S content in both the grapes and the resultant wine. Sulphur is one of the few synthetic substances allowed in organic farming due to its relatively low toxicity and compatibility with organic principles (Grangeteauet al., 2017; Moine et al., 2023). Sulphur compounds are a commonly used food preservative. For this reason, consumers may unknowingly ingest high doses of it by consuming many preserved products (for example, dried fruit, fruit juices) in a day. Sensitive individuals suffer from allergies, gastrointestinal problems of varying severity, and asthma (Vally and Thompson, 2001).Based on the information provided, it can be concluded that the use of synthetic fungicides had a somewhat limited impact on the typical plant pathogen, Erysiphe sp. Despite the targeted protection against this pathogen, synthetic fungicides were not fully effective. However, the fungicide protection was aimed at controlling graymould (Botrytis cinerea) and some saprotrophic fungi that degrade product quality (e.g., Penicillium spp.). This contrast in efficacy was less noticeable in organic cultivation. It can be inferred that synthetic fungicides were comparatively more effective in preventing the development of these pathogens in conventionally grown grapes. Nevertheless, despite a well-designed fungicide protection strategy for conventional grapes, it was not possible to completely eliminate the presence of phytopathogens. However, there was a notable reduction in infection levels, which approached economic profitability thresholds (as communicated personally). An important observation is that synthetic fungicides had a nonselective impact on beneficial microbiota, leading to a reduction in nonpathogenic bacterial communities. Furthermore, there was a clear decline in the presence of antagonistic fungi, such as Hanseniaspora sp. and Dissoconium sp., which exhibited a negative correlation with the occurrence of the Erysiphe sp. pathogen. Based on the results from the PCA analysis, certain relationships related to predictors of Erysiphe sp. infection severity and other fungal infections become evident. Cu, Sand (+)-catechin show a strong correlation with the severity of grapevine powdery mildew occurrence. This suggests that higher levels of these components may lead to a greater risk of Erysiphe sp. infection. One of the reasons for the higher severity of Botryotinia in organic farming compared to conventional farming (
Table 3) could be the fungicide protection strategy. Synthetic fungicides used in conventional farming possess different properties, such as surface, translaminar, and systemic actions, which increase their effectiveness in protecting plants against phytopathogens. These fungicides operate both preventively, targeting spores present in the air, and interventionally, targeting infection or advanced disease stages and the pathogen’s development within plant tissues. This approach prolongs the duration of active substances. In the case of copper and sulfur applications, pathogens that have already infected plants can continue to develop after the infection phase. Additionally, the penetration of active substances is confined to the sprayed areas, leaving hard-to-reach places vulnerable to infection, particularly in the case of gray mould (Botrytis cinerea).
4.3. Influence of the Wine Farming Method on the Polyphenol Content and on the Functional Characteristics of the Must
The must prepared from organic fruit was characterised by better technological parameters. The fruit had a higher SS content and, consequently, more sugars and lower acidity. These parameters directly influence the type of wine and its taste (Schmit et al., 2013). The must from organic fruit was also characterised by a lower turbidity, allowing the use of lower clarifying agent doses. It also contained a higher amount of nitrogen compounds, which are essential for a proper fermentation process (Ugliano et al., 2007). Grape musts grown under the organic system showed a statistically significantly higher content of polyphenolic compounds compared to the product obtained from conventionally grown fruit. Such observations are also confirmed by other authors (Carbonaro et al., 2002; Asami et al., 2003; Wojdyłoet al., 2013).The concentration and quality of polyphenolic compounds in plant material are strongly influenced by, among other things, fungicide resistance and the quantity and quality of pesticides used for cultivation (Juroszeket al., 2009). Some herbicides interfere with the synthesis of aromatic amino acids and consequently the biosynthesis of polyphenols (Juroszeket al., 2009). Polyphenols present in fruits can inhibit the growth of fungi (Dhalariaet al., 2020). Polyphenols have antimicrobial properties that can help prevent the growth of various microorganisms, including fungi. They can interfere with fungal enzymes, disrupt cell membranes, and inhibit fungal spore germination, thereby reducing fungal colonization of the fruit (Mosele et al., 2015; Fereidoon et al., 2019; Kong et al., 2020). The abundance of polyphenols in fruit from organic plantations may indicate a higher pathogen pressure. Additionally, the microbiome identified on organic fruit also differed significantly from that of conventional fruit. Further research is essential to explore the precise mechanisms underlying the differences in polyphenolic compounds and antioxidant activity between cultivation methods. Furthermore, it is crucial to evaluate the sensory attributes and organoleptic properties of the must obtained from organic cultivation.
The findings suggest that factors such as fertilization, cultivation practices, and the presence of grapevine powdery mildew can impact the lower sugar content (Brix) and nitrogen content (YAN). Reduced levels of highly active polyphenols and higher acidity are associated with the severity of secondary fungal infections, aligning with the increased presence of Penicillium sp. and Cladosporium sp. It is worth noting that a negative correlation exists between microbiota I and the overall antioxidant content (FRAP and DPPH). This suggests that there are other antioxidants, besides the identified polyphenols, that may influence microorganisms, including the pathogenic Botrytis cinerea or saprotrophic Mucor spp.
5. Conclusions
The viticultural system influenced the microorganisms inhabiting the grapes, with a higher taxonomic diversity observed among microorganisms from organic fruit. Bacterial species belonging to the genus Sphingomonas predominated and were equally abundant on fruit from both crops. However, other genera differed in quantity (Massilia, Hymenobacter, Pseudomonas, Variovorax, Rhizobium, and others).In contrast, fungi displayed a different pattern. The largest number of ITS sequences detected in both types of grapes belonged to phytopathogenic fungi of the genus Erysiphe. But there were definitely more of them on conventionally grown fruit. Less abundant fungal genera, such as Aureobasidium, Alternaria, Mycosphaerella, and Botryotinia, were comparable in quantity in both cultivation types.It can be concluded that the presence of numerous antagonistic microorganisms (e.g., species from genera Sphingomonas, Aureobasidium, Pseudomonas, Variovorax, etc.) and possibly drought inhibited powdery mildew, rather than synthetic pesticides. Synthetic pesticides on the grapes contributed to a lower diversity of microorganisms (OTUs, Dominance, Diversity).It is possible that phytopathogenic fungi of the genus Erysiphe were therefore more prevalent on grapes protected with synthetic pesticides. This fact, like the presence of synthetic pesticide residues in the must, definitely worsened the quality and use parameters of the conventional must. The organic must was significantly better in quality. It contained more polyphenols, sugar, and nitrogen, along with lower acidity and turbidity.
High levels of both natural and synthetic pesticide residues in the must pose a risk, with synthetic pesticides, particularly fludioxonil, being more harmful to health. Sulfur and copper levels in the wine were much lower than in the must. Sulfites are typically used to preserve wines, making sulfur common in both conventional and organic wines. True powdery mildew infesting the grapes and leaves was not detected, although Erysiphe and other moulds were found in the composition of the microbiome. This may have been influenced by the weather, low humidity, and lack of rainfall. Therefore, mycotoxins were also not found (a mycelium is needed for the biosynthesis of harmful mycotoxins to occur), although genes encoding aflatoxins and ochratoxins were present in the must, especially in the organic must. For organic wines, mycotoxins are a potential threat (although there were no mycotoxins in our wines, but there were genes encoding them), while for conventional wines pesticides are a real threat as confirmed by the study.
In summary, organic must displayed better quality and use parameters, was free of mycotoxins. However, it contained sulfur and copper; their amounts were reduced during the vinification process to comply with the standards. Conventional must had poorer technological parameters, high pesticide residues, no mycotoxins, and fewer genes encoding them.
Author Contributions
Conceptualization I.O. and M.B.; methodology, I.O., S.W.P., M.B., M.T. and S.L.-W.; software, I.O. and S.W.P.; validation, I.O., S.W.P., M.B., M.T. and S.L.-W.; formal analysis, I.O., M.B., M.T. and S.L.-W., X.X.; I.O., S.W.P., M.B., M.T. and S.L.-W.; I.O.; I.O. and M.B.; writing—original draft preparation, I.O., S.W.P., M.B., M.T. and S.L.-W.; writing—review and editing, I.O. and M.B.; visualization, M.B. and S.W.P.; supervision, I.O.; project administration, I.O.; funding acquisition, I.O. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by project No. 00020.DDD.6509.00056.2019.16 carried out under the Rural Development Programme—Action WSPÓŁPRACA M16
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abarca, M.L.; Bragulat, M.R.; Castellá, G.; Cabañes, F.J. Impact of some environmental factors on growth and ochratoxin A production by Aspergillus nigerand Aspergillus welwitschiae. Int. J. Food Microbiol. 2019, 291, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Abrusci, A.; Palomar, J.; Pablos, J.L.; Rodriguez, F.; Catalinac, F. Efficient biodegradation of common ionic liquids by Sphingomonaspaucimobilisbacterium. Green Chem. 2011, 13, 709–717. [Google Scholar] [CrossRef]
- Anand, S.P.; Sati, N. Artificial preservatives and their harmful effects: Looking toward nature for safer alternatives. Int. J. Pharm. Sci. Res. 2013, 4, 2496–2501. [Google Scholar]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze‐dried and air‐dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, T.T.; Klein, B.S. Uncertainty surrounding the mechanism and safety of the postharvest fungicide fludioxonil. Food Chem. Toxicol. 2019, 123, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Mattera, M.; Nicoli, S.; Bergamo, P.; Cappelloni, M. Modulation of antioxidant compounds in organic vs conventional fruit (peach, Prunus persica L., and pear, Pyrus communis L.). J. Agric. Food Chem. 2002, 50, 5458–5462. [Google Scholar] [CrossRef] [PubMed]
- Česnik, H.B.; Gregorcic, A.; Cus, F. Pesticide residues in grapes from vineyards included in integrated pest management in Slovenia. Food Addit. Contam. Part A 2008, 25, 438–443. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010, 121, 132–139. [Google Scholar] [CrossRef]
- Chen, H.; Yaqiong, L.; Chen, J.; Fu, X.; Suo, R.; Chitrakarn, B.; Wang, J. Effects of spontaneous fermentation on microbial succession and its correlation with volatile compounds during fermentation of Petit Verdot wine. LWT 2022, 168, 113890. [Google Scholar] [CrossRef]
- Clark, A.C.; Scollary, G.R. Determination of total copper in white wine by stripping potentiometry utilising medium exchange. Anal. Chim. Acta. 2000, 413, 25–32. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Dagostin, S.; Schärer, H.J.; Pertot, I.; Tamm, L. Are there alternatives to copper for controlling grapevine downy mildew in organic viticulture? Crop Prot. 2011, 30, 776–788. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food. 2021, 6, 416–447. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Hijwegen, T.; Batenburg-Van Der Vegte, W.H. A new species of Dissoconium. Mycol. Res. 1991, 95, 679–682. [Google Scholar] [CrossRef]
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- DNA calculator. Available online: https://www.thermofisher.com/pl/en/home/brands/thermo-scientific/molecularbiology/molecular-biology-learning-center/molecular-biology-resource-library/thermo-scientificweb-tools/dna-copy-number-calculator.html.
- Fahmi, A.I.; El-Shehawi, A.M.; Nagaty, M.A. Antioxadant and antimutagenic activities of Taif grape (Vitis vinifera) cultivars. Am. J. Biochem. Biotechnol. 2013, 9, 102–117. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Amoo, A.E.; Babalola, O.O. Characterization of plant growth-promoting rhizobacterial isolates associated with food plants in South Africa. Antonie Leeuwenhoek 2021, 114, 1683–1708. [Google Scholar] [CrossRef] [PubMed]
- Fenoll, J.; Manso, A.; Hellin, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Fereidoon, S.; Vamadevan, V.; Won Young, O.; Han, P. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Fernández-Cruz, M.L.; Mansilla, M.L.; Tadeo, J.L. Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. J. Adv. Res. 2010, 1, 113–122. [Google Scholar] [CrossRef]
- Figiel-Kroczyńska, M.; Ochmian, I.; Lachowicz, S.; Krupa-Małkiewicz, M.; Wróbel, J.; Gamrat, R. Actinidia (mini kiwi) fruit quality in relation to summer cutting. Agronomy 2021, 11, 964. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; de Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Francesca, N.; Sannino, C.; Settanni, L.; Corona, O.; Barone, E.; Moschetti, G. Microbiological and chemical monitoring of Marsala base wine obtained by spontaneous fermentation during large-scale production. Ann. Microbiol. 2014, 64, 1643–1657. [Google Scholar] [CrossRef]
- García-Cela, E.; Crespo-Sempere, A.; Gil- Serna, J.; Porqueres, A.; Marin, S. Fungal diversity, incidence and mycotoxin contamination in grapes from two agro-climatic Spanish regions with emphasis on Aspergillus species. J. Sci. Food Agric. 2015, 95, 1716–1729. [Google Scholar] [CrossRef] [PubMed]
- Getachew, Z.; Abeble, L. Effect of seed treatment using Mancozeb and Ridomil fungicideson Rhizobium strain performance, nodulation and yield ofsoybean (Glycine max L.). J. Agric. Nat. Resour 2021, 4, 86–97. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Pesticide Residues in Table Grapes and Exposure Assessment. J. Agric. Food Chem. 2018, 66, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Grangeteau, C.; David, V.; Herve, A.; Guilloux-Benatier, M.; Rousseaux, S. The sensitivity of yeasts and yeasts-like fungi to copper and sulfur could explain lower yeast biodiversity in organic vineyards. FEMS Yeast Res. 2017, 17, fox092. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Bai, Z.; Jin, B.; Hu, Q.; Wang, H.; Zhuang, G.; Zhang, H. Assessing the impact of fungicide enostroburin application on bacterial community in wheat phyllosphere. J. Environ. Sci. 2010, 22, 134–141. [Google Scholar] [CrossRef]
- Guo, Y.Z.; Ding, X.M.; Yao, L.; Xu, D.M.; Zhao, Y.J.; Feng, F.Y.; Meng, J.Y. Isolation and Identification of Massilia sp. B260 and Its Effect on Seedling Raising. Biotechnol. Bull. 2019, 35, 144–149. [Google Scholar] [CrossRef]
- Hepperly, P.R.; Douds, D.; Seidel, R. The Rodale Institute Farming System Trial 1981 to 2005: Long-term analysis of organic and conventional maize and soybean cropping systems. In Long-term Field Experiments in Organic Farming; Raupp, J., Pekrun, C., Oltmanns, M., Köpke, U., Eds.; Verlag Dr. Köster: Berlin, 2006; pp. 15–31. [Google Scholar]
- Hesham, Ael-L.; Mawad, A.M.; Mostafa, Y.M.; Shoreit, A. Biodegradation ability and catabolic genes of petroleum-degrading Sphingomonaskoreensisstrain ASU-06 isolated from Egyptian oily soil. Biomed Res. Int. 2014, 2014, 127674–127674. [Google Scholar] [CrossRef] [PubMed]
- IUNG (Institute of Soil Science and Plant Cultivation). Fertiliser Recommendations Part I. Limits for Estimating Soil Macro- and Microelement Content. Series P. 44. National Research Institute in Puławy: Puławy, 1990; pp. 26–28. [Google Scholar]
- Jiang, W.W.; Bilogrevic, E.; Parker, M.; Francis, I.L.; Leske, P.; Hayasaka, Y.; Barter, S.; Herderich, M. The effect of pre-veraison smoke exposure of grapes on phenolic compounds and smoky flavour in wine. J. Grape Wine Res. 2022, 9820204. [Google Scholar] [CrossRef]
- Jones, L.; Riaz, S.; Morales-Cruz, A.; Amrine, K.C.; McGuire, B.; Gubler, W.D.; Walker, M.A.; Cantu, D. Adaptive genomic structural variation in the grape powdery mildew pathogen Erysiphe necator. BMC Genomics 2014, 15, 1081. [Google Scholar] [CrossRef] [PubMed]
- Juroszek, P.; Heidi, M.L.; Ray-Yu, Y.; Dolores, R.L.; Chin-Hua, M. Fruit quality and bioactive compounds with antioxidant activity of tomatoes grown on-farm: comparison of organic and conventional management systems. J. Agric. Food Chem. 2009, 57, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Terentjeva, M.; Felsöciová, S.; Ivanišová, E.; Kunová, S.; Žiarovská, J.; Kluz, M.; Hanus, P.; Puchalski, C.; Kántor, A. Bacteria and yeasts isolated from different grape varieties. Potr. S. J. F. Sci. 2018, 12, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Kántor, A.; Mareèek, J.; Ivaniðová, E.; Terentjeva, M.; Kaèániová, M. Microorganisms of grape berries. Proc. Latv. Acad. Sci. Section B. 2017, 71, 502–508. [Google Scholar] [CrossRef]
- Kecskeméti, E.; Berkelmann-Löhnertz, B.; Reineke, A. Are Epiphytic Microbial Communities in the Carposphere of Ripening Grape Clusters (Vitis vinifera L.) Different between Conventional, Organic, and Biodynamic Grapes? PLOS ONE 2016, 11, e0160852. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Park, J.Y.; Balusamy, S.R.; Huo, Y.; Nong, L.K.; Thi Le, H.; Yang, D.C.; Kim, D. Comprehensive Genome Analysis on the Novel Species SphingomonaspanacisDCY99TReveals Insights into Iron Tolerance of Ginseng. Int. J. Mol. Sci. 2020, 21, 2019. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; An, P.; Xu, Z.; Zhang, R.; Qi, J.; Ren, X. New Insights into the Alleviating Role of Melaleuca alternifolia Oil on Metabolites Pathway Disorder of Grapes Caused by Aspergillus niger, Verified by Corresponding Key Genes Expression. Food Chem. 2020, 327, 127083. [Google Scholar] [CrossRef]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.), Effects on wine phenolic and aroma components. J. Agric. Food Chem. 2006, 54, 5077–5086. [Google Scholar] [CrossRef]
- Kraus, C.; Abou-Ammar, R.; Schubert, A.; Fischer, M. WarburgiaugandensisLeaf and Bark Extracts: An Alternative to Copper as Fungicide against Downy Mildew in Organic Viticulture? Plants 2021, 10, 2765. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.H.; Lauvergeat, V.; Gomès, E.; Li, S.H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2013, 65, 4543–4559. [Google Scholar] [CrossRef] [PubMed]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Laureati, M.; Gaeta, D.; Pagliarini, E. Qualitative and sensory evaluation of Sangiovese red wine obtained from organically and conventionally grown grapes. Ital. J. Food Sci. 2014, 26, 355–362. [Google Scholar]
- Link 1. Available online: http://www.inhort.pl/files/laboratoria_akredytowane/badania_skazen/Offer_FSL_18_10_2022.pdf.
- Lumivero. 2023. XLSTAT statistical and data analysis solution. New York, USA. Available online: https://www.xlstat.com/en (accessed on 10 August 2023).
- Luo, Y.; Zhou, M.; Zhao, Q.; Wang, F.; Gao, J.; Sheng, H.; An, L. Complete genome sequence of Sphingomonassp. Cra20, a drought resistant and plant growth promoting rhizobacteria. Genomics 2020, 112, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Jorquera, K.; Cancino, B.; Ruby, R.; Henriquez, C. Phenolics and antioxidant capacity of table grape (Vitis vinifera L.) cultivars grown in Chile. J. Food Sci. 2011, 76, 1088–1093. [Google Scholar] [CrossRef]
- Lytra, G.; Miot-Sertier, C.; Moine, V.; Coulon, J.; Barbe, J.C. Influence of mustyeastassimilable nitrogen content on fruity aroma variation during malolactic fermentation in red wine. Food Res. Int. 2020, 135, 109294. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Gao, X.; Nan, J.; Zhang, T.; Xie, X.; Cai, Q. Fungicides alter the distribution and diversity of bacterial and fungal communities in ginseng fields. Bioengineered 2021, 12, 8043–8056. [Google Scholar] [CrossRef]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.M.; Kubal, M.; Paczian, T.; Rodriguez, A.; Stevens, R.; Wilke, A.; Wilkening, J.; Edwards, R.A. The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics 2008, 9, 386. [Google Scholar] [CrossRef]
- Mijowska, K.; Ochmian, I.; Oszmiański, J. Impact of cluster zone leaf removal on grapes cv. Regent polyphenol content by the UPLC-PDA/MS method. Molecules 2016, 21, 1688. [Google Scholar] [CrossRef]
- Moine, A.; Pugliese, M.; Monchiero, M.; Gribaudo, I.; Gullino, M.L.; Pagliarani, C.; Gambino, G. Effects of fungicide application on physiological and molecular responses of grapevine (Vitis vinifera L.): a comparison between copper and sulphur fungicides applied alone and in combination with novel fungicides. Pest Manag. Sci. 2023, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mosele, J.I.; Macià, A.; Motilva, M.-J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: a review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.G.; Chapman, P.J.; Blattmann, B.O.; Pritchard, P.H. Isolation and characterization of a fluoranthene-utilizing strain of Pseudomonas paucimobilis. AEM 1990, 56, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Naqqash, T.; Imran, A.; Hameed, S.; Shahid, M.; Majeed, A.; Iqbal, J.; Hanif, M.K.; Ejaz, S.; Malik, K.A. First report of diazotrophic Brevundimonasspp. as growth enhancer and root colonizer of potato. Sci. Rep. 2020, 10, 12893. [Google Scholar] [CrossRef] [PubMed]
- Ochmian, I.; Błaszak, M.; Lachowicz, S.; Piwowarczyk, R. The impact of cultivation systems on the nutritional and phytochemical content, and microbiological contamination of highbush blueberry. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ofek, M.; Hadar, Y.; Minz, D. Ecology of root colonizing Massilia(Oxalobacteraceae). PLoS One 2012, 7, e40117. [Google Scholar] [CrossRef] [PubMed]
- Onetto, C.A.; Borneman, A.; Schmidt, S. Investigating the effects of Aureobasidium pullulans on grape juice composition and fermentation. Food Microbiol. 2020, 90, 103451. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Custódio, V.; Nunes, M.; Songy, A.; Rabenoelina, F.; Courteaux, B.; Clément, C.; Gomes, A.C.; Fontaine, F. Understand the Potential Role of Aureobasidium pullulans, a Resident Microorganism From Grapevine, to Prevent the Infection Caused by Diplodiaseriata. Front Microbiol. 2018, 9, 3047. [Google Scholar] [CrossRef]
- PN-EN 15662:2018-06. Polish Committee for Standardisation. Fruit and Vegetable Preparations—Sample preparation and Physicochemical Test Methods. Polish Committee for Standardisation: Warszawa, Poland, 2001.
- Przemieniecki, S.W.; Damszel, M.; Kurowski, T.P.; Mastalerz, J.; Kotlarz, K. Identification, ecological evaluation and phylogenetic analysis of non-symbiotic endophytic fungi colonizing timothy grass and perennial ryegrass grown in adjacent plots. Grass Forage Sci. 2019, 1–11. [Google Scholar] [CrossRef]
- Quirós-Alcalá, L.; Hansel, N.N.; McCormack, M.C.; Matsui, E.C. Paraben exposures and asthma-related outcomes among children from the US general population. J. Allergy Clin. Immunol. 2018, 14, 948–956. [Google Scholar] [CrossRef]
- Ravintheran, S.K.; Sivaprakasam, S.; Loke, S.; Lee, S.Y.; Manickam, R.; Yahya, A.; Croft, L.; Millard, A.; Parimannan, S.; Rajandas, H. Complete genome sequence of Sphingomonas paucimobilisAIMST S2, a xenobiotic-degrading bacterium. Sci. Data. 2019, 6, 280. [Google Scholar] [CrossRef]
- Rodríguez, A.; Luque, M.I.; Andrade, M.J.; Rodríguez, M.; Asensio, M.A.; Córdoba, J.J. Development of real-time PCR methods to quantify patulin-producing molds in food products. Food Microbiol. 2011, 28, 1190–1199. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Andrade, M.J.; Córdoba, J.J. Development of a multiplex real-time PCR to quantify aflatoxin, ochratoxin A and patulin producing molds in foods. Int. J. Food Microbiol. 2012, 155, 10–18. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Luque, M.I.; Justesen, A.F.; Córdoba, J.J. Quantification of ochratoxin A-producing molds in food products by SYBR Green and TaqMan real-time PCR methods. Int. J. Food Microbiol. 2011, 149, 226–235. [Google Scholar] [CrossRef]
- Ryan, M.P.; Pembroke, J.T. Brevundimonasspp: Emerging global opportunistic pathogens. Virulence 2018, 9, 480–493. [Google Scholar] [CrossRef]
- Schmit, T.M.; Rickard, B.J.; Taber, J. Consumer valuation of environmentally friendly production practices in wines considering asymmetric information and sensory effects. J. Agr. Econ. 2013, 64, 483–504. [Google Scholar] [CrossRef]
- Strausak, D.; Mercer, J.F.; Dieter, H.H.; Stremmel, W.; Multhaup, G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res. Bull. 2001, 55, 175–185. [Google Scholar] [CrossRef]
- Sweet, R.M.; Schreiner, R.P. Alleyway cover crops have little influence on Pinot noir grapevines (Vitis vinifera L.) in two western Oregon vineyards. AJEV 2010, 61, 240–252. [Google Scholar] [CrossRef]
- Szegedi, E.; Civerolo, L. Bacterial diseases of grape vine. Int. J. Hortic. Sci. 2011, 17, 45–49. [Google Scholar] [CrossRef]
- Toaldo, I.M.; van Camp, J.; Gonzales, G.B.; Kamiloglu, S.; Bordignon-Luiz, M.T.; Smagghe, G.; Raes, K.; Capanoglu, E.; Grootaert, C. Resveratrol improves TNF-α-induced endothelial dysfunction in a coculture model of a Caco-2 with an endothelial cell line. J. Nutr. Biochem. 2016, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Tournas, V.H.; Katsoudas, E. Mould and yeast flora in fresh berries, grapes and citrus fruits. Int. J. Food Microbiol. 2005, 15, 11–17. [Google Scholar] [CrossRef]
- Ugliano, M.; Henschke, P.A.; Herderich, M.J.; Pretorius, I.S. Nitrogen management is critical for wine flavour and style. Aust. New Zeal. Wine Ind. J. 2007, 22, 24–30, https://www.researchgate.net/publication/275832110_Nitrogen_management_is_critical_for_wine_flavour_and_style. [Google Scholar]
- Vally, H.; Thompson, P.J. Role of sulfite additives in wine induced asthma: Single dose and cumulative dose studies. Thorax 2001, 56, 763–769. [Google Scholar] [CrossRef]
- Vrček, I.V.; Bojić, M.; Žuntar, I.; Mendaš, G.; Medić-Šarić, M. Phenol content, antioxidant activity and metal composition of Croatian wines deriving from organically and conventionally grown grapes. Food Chem. 2011, 124, 354–361. [Google Scholar] [CrossRef]
- Weitekamp, C.A.; Hofmann, H.A. Effects of air pollution exposure on social behavior: a synthesis and call for research. Environ. Health. 2021, 20, 72. [Google Scholar] [CrossRef]
- Welke, J.E. Fungal and mycotoxin problems in grape juice and wine industries. Curr. Opin. Food Sci. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- White, D.C.; Sutton, S.D.; Ringelberg, D.B. The genus Sphingomonas: Physiology and ecology. Curr. Opin. Biotechnol. 1996, 7, 301–306. [Google Scholar] [CrossRef]
- Willer, H.; Schlatter, B.; Trávníček, J. The World og Organic Agriculture. Statistics and Emerrging Trends 2023. FiBL, IFOAM https://www.fibl.org/fileadmin/documents/shop/1254-organic-world-2023.pdf. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Milczarek, M.; Wietrzyk, J. Phenolic profile, antioxidant and antiproliferative activity of black and red currants (Ribes spp.) from organic and conventional cultivation. Int. J. Food Sci. Technol. 2013, 48, 715–726. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, E.; Yano, I.; Oyaizu, H.; Hashimoto, Y.; Ezaki, T.; Yamamoto, H. Proposals of Sphingomonaspaucimobilisgen. nov.& comb. nov., Sphingomonasparapaucimobilissp. nov., Sphingomonasyanoikuyaesp. nov., Sphingomonasadhaesivasp. nov., Sphingomonascapsulata comb. nov.& two genospecies of the genus Sphingomonas. Microbiol. Immunol. 1990, 34, 99–119. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Z.; Wang, J.; Zhao, Y.; Hu, B. SphingomonasRelies on Chemotaxis to Degrade Polycyclic Aromatic Hydrocarbons and Maintain Dominance in Coking Sites. Microorganisms 2022, 10, 1109. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).