1. Introduction
Floral fragrances are secondary metabolic compounds within low molecular weight, polarity, boiling point and high fat solubility gradually secreted by odor glands in the process of plant adaptation [
1]. Acting as natural ingredients synthesized by multiple plants in their principal habitat, floral fragrance is possessing significant biological functions such as disease and pest resistance, as well as attraction of insect pollinators [
2]. Furthermore, specific floral constituents such as linalool possess potent aromatic properties and find extensive applications in the fields of cosmetics, perfumery, culinary spices, and food additives [
3,
4,
5,
6]. Concurrently, variety of floral constituents, including linalool and geraniol, demonstrate a wide range of biological activities encompassing antibacterial, anti-inflammatory, anticancer, antidepressant properties among others. Consequently, their application is widely prevalent in the pharmaceutical field [
7,
8,
9,
10]. Currently, more than 1700 components have been identified in plants as constituents of floral fragrances, primarily classified into terpenoids, benzene rings/phenylpropanoids, and aliphatic derivatives [
11,
12]. Among these substances, terpenoids emerge as the predominant constituents of floral volatiles [
13].
The biosynthesis of terpenoids is intricately regulated by plant growth and development, circadian rhythm, and tissue specificity [
14,
15,
16,
17,
18]. Additionally, these compounds are synthesized in specific plant tissues and released into the environment during precise developmental or growth stages. For instance, certain plants exhibit nocturnal flowers that emit abundant volatiles, thereby adapting to the behavior of nocturnal insects; this specific synthesis and release mechanism facilitates enhanced adaptation of the plant to its ecological niche [
19]. Terpenoid synthases, positioned at the pivotal points of terpenoid synthesis metabolism, possess the ability to enzymatically convert FPP or GPP into a diverse repertoire encompassing monoterpenes, sesquiterpenes, and other associated compounds [
20,
21]. The synthesis and release of terpenoids often exhibit a positive correlation with the transcriptional levels of their corresponding terpenoid synthase genes [
22]. The predominant volatile compound in
A. thaliana is caryophyllene, accounting for approximately 90% of the total volatiles. To date, a total of 32 terpenoid synthase genes have been successfully cloned from
A. thaliana, with
TPS11 and
TPS21 demonstrating pronounced expression levels and widespread involvement in the biosynthesis of caryophyllene and specific sesquiterpenes. Equally, several individuals, such as TPS2, TPS3, TPS14, and TPS23 are also responsible for the production of certain volatile monoterpenes [
23]. The release of linalool and nerolidol exhibited a positive correlation with the expression of corresponding TPS genes in
Snapdragons [
24]. Additionally, the
HcTPS2 gene in white ginger exhibited specific expression exclusively within floral organs [
25]. Therefore, it can be inferred that terpene synthase plays a pivotal role in the biosynthesis of terpenoids and serves as a crucial determinant of floral composition.
Currently, the transcriptional regulation of plant volatile terpenoids involves a repertoire of 8 distinct types of transcription factors, comprising AP2/ERF, WRKY, NAC, bZIP, SRS, SBP, MYB and bHLH family member [
26]. In
A. thaliana, AtMYB21 and AtMYB24 possess positive regulatory on flower development, wherein the expression levels of
AtTPS11 and
AtTPS21 gradually increase during flower development to facilitate the release of sesquiterpene caryophyllene [
27,
28,
29]; AtMYC2 occupy dominant role in the development of flower stamens and exhibits the ability to specifically recognize promoters of
AtTPS11 and
AtTPS21 genes, thereby directly regulating their expression and facilitating the release of sesquiterpene caryophyllene [
30,
31]. However, the synthesis and transcriptional regulation of terpenoids represent a highly intricate process that has hitherto been exclusively investigated in
A. thaliana; nevertheless, the precise underlying mechanism remains elusive. Moreover, the investigation of transcription factors derived from flowers, which are pivotal organs for volatile emission, is lacking in scientific literature. Therefore, it is imperative to identify a species exhibiting a robust floral fragrance in order to conduct comprehensive investigations aimed at elucidating the transcriptional regulatory mechanisms governing volatile terpenoid production in plant flowers.
Freesia hybrida, a bulbous herbaceous flower belonging to the iris family, showcases lavish blooms and exquisite floral aesthetics, making it an ideal specimen for investigating the intricate realm of botanical fragrance and floral metabolism [
32,
33]. In this study, FhMYB108, a transcription factors involved in regulating the expression of terpenoid synthase genes was screened with the transcription database of
Red River®, a breed of
Freesia hybrida. Moreover, the molecular mechanism underlying FhMYB108-mediated regulation of volatile terpenoid synthesis was further elucidated. This study establishes a theoretical framework to elucidate the molecular mechanism underlying plant floral development, thereby providing a robust foundation for enhancing plant and flower traits.
2. Results
2.1. Composition and Release Regulations of Volatile Compounds in F. hybrida
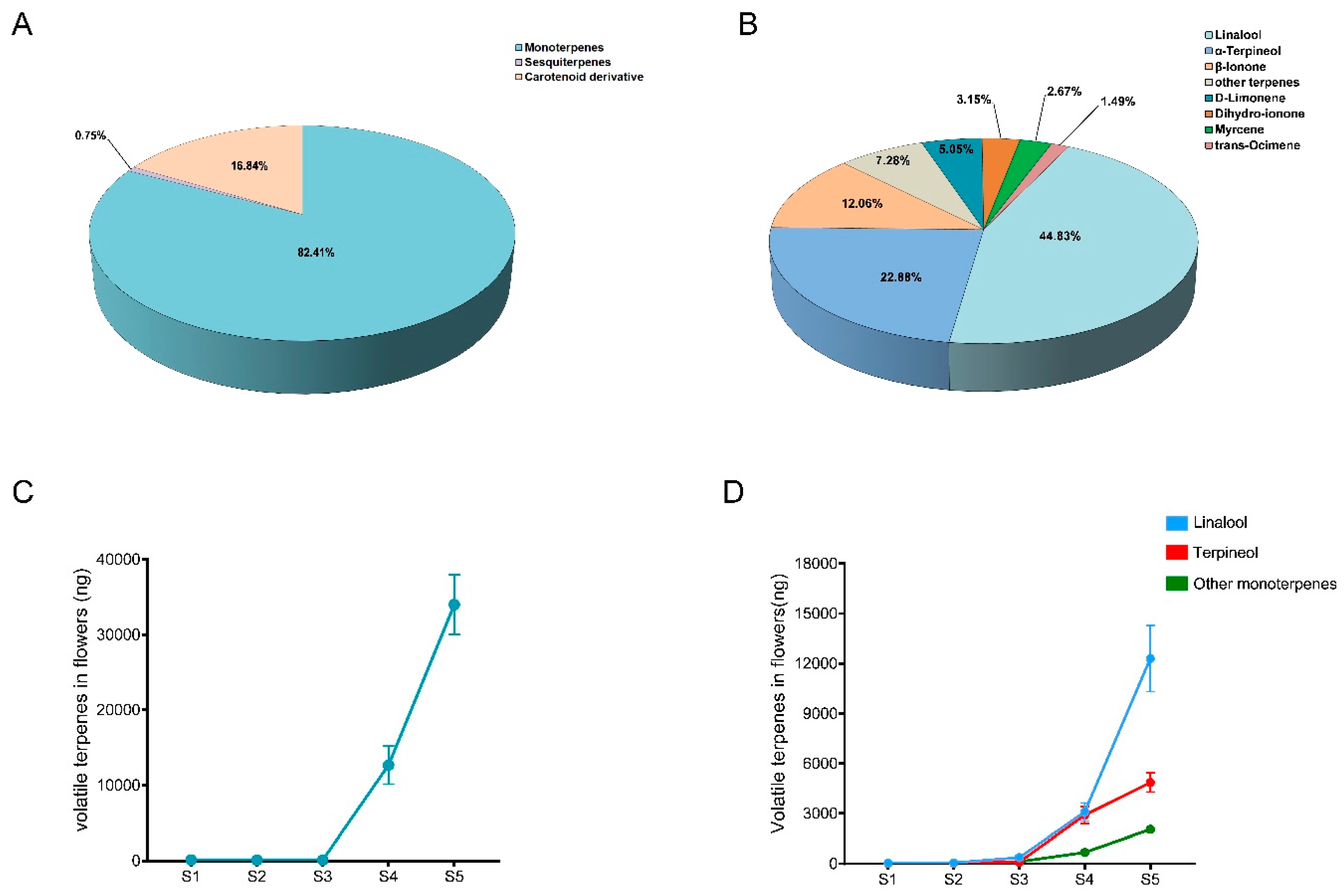
The blossoming of flowers is accompanied by the release of a multitude of volatile compounds, serving as chemical signals to attract insects for pollination purposes. GC-MS analysis revealed that the volatile compounds emitted during the flowers blooming phase of
Red River® were predominantly composed of 18 monoterpenes, 5 sesquiterpenoids, and 3 carotenoid derivatives (
Table 1). Among them, the monoterpenes constitute 82.41% of the content, while carotenoid derivatives account for 16.84%, and sesquiterpenes contribute only a negligible 0.75% (
Figure 1A). The results of further qualitative analysis revealed that the volatile compounds identified in the sample were primarily composed of linalool (44.83%), followed by terpineol (22.88%). Additionally, a certain quantity of limonene (D-limonene) (5.05%), ionone (β-Ionone) (12.66%), dihydroionone (dihydro-πIonone) (3.15%), myrcene (2.66%), and basil ((E)-ocimene) were also detected (
Figure 1B).
By monitoring the emission of volatile compounds at different stages of flower blooming in
F. hybrida, it becomes evident that the quantity of released volatile substances gradually increases as fragrant orchid flowers unfold (
Figure 1C). The release levels of linalool and terpineol gradually increase concomitant with floral blooming, culminating during the peak blooming stage (S5 stage) (
Figure 1D). Moreover, the release of other terpenoids was also in accordance with this principle (
Table 2). The results reveal the unequivocal dominance of linalool among the volatile compounds present in
F. hybrida, thereby highlighting its significant biological implications for this species.
2.2. Relevant Regulatory Molecules of Linalool
Currently, eight terpene synthase genes (
FhTPS1, FhTPS2, FhTPS3, FhTPS4, FhTPS5, FhTPS6, FhTPS7, and
FhTPS8) have been identified in
F. hybrida [
34]. Among them, it has been discovered that
FhTPS1 can utilize GPP as a substrate to exclusively generate linalool, which potentially serves as the predominant source of volatile compounds in
F. hybrida flowers. To quantify the expression of
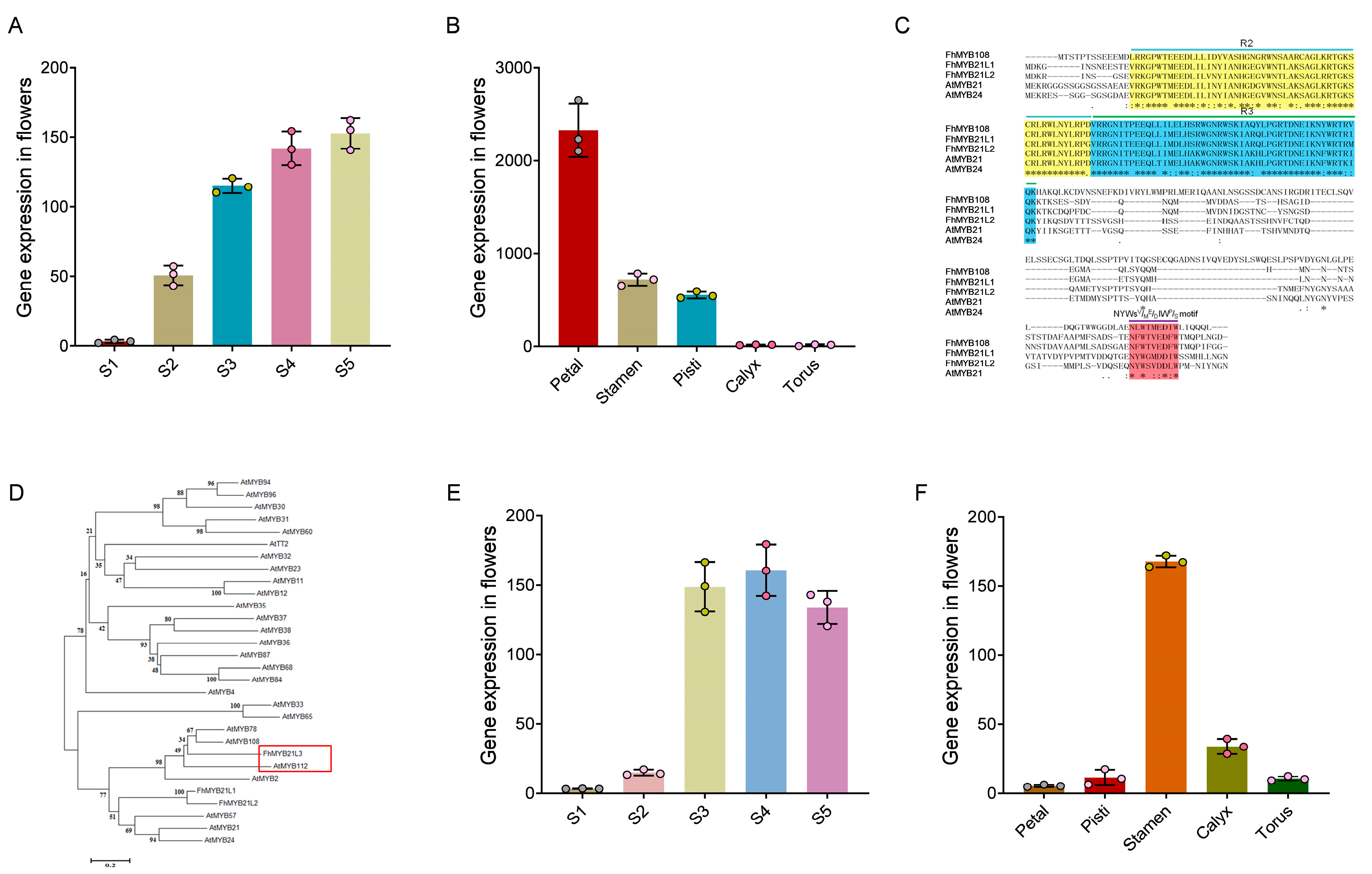
FhTPS1 across diverse developmental stages and floral organs, including petals, pistils, sepals, receptacle and stamens, our findings revealed a robust expression pattern of
FhTPS1 in petals, pistils and stamens; conversely, its expression was significantly lower in sepals and receptacle (
Figure 2A). The crucial aspect lies in the remarkable resemblance between its expression level and the release pattern of linalool, which exhibits a gradual increase that coincides with flower blooming (
Figure 2B). This observation strongly implies that
FhTPS1 catalysis may be solely responsible for the production of linalool in
F. hybrida flowers.
Three full-length cDNA sequences encoding MYB transcription factors
FhMYB21L1 (591 bp),
FhMYB21L2 (591 bp) and
FhMYB108 (789 bp) were obtained from
F. hybrida by homologous comparison of
AtMYB21 and
AtMYB24 transcription factors in
A. thaliana, which have the function of regulating terpenoid metabolism. The regulatory function of
FhMYB21L1 and
FhMYB21L2 in terpenoid metabolism has been experimentally validated, whereas the role of
FhMYB108 remains unreported [
35]. The FhMYB108 showed two characteristic R2R3-MYB transcription factor domains, which are known to bind to MYB elements (
Figure 2C). Additionally, phylogenetic analysis revealed that FhMYB108 and other MYB-type transcription factors implicated in terpenoid metabolism regulation form a distinct cluster, suggesting a potential role for FhMYB108 in the regulation of terpenoid metabolism (
Figure 2D).
The expression patterns of
FhMYB108 were observed to be downregulated during the green bud stage (S1) and the red-green interphase stage (S2), while being upregulated during the red bud stage (S3), early opening stage (S4), and full opening stage (S5) of flower development (
Figure 2E). The regular expression pattern is consistent with both the expression pattern of
FhTPS1 and the release pattern of linalool, suggesting that
FhMYB108 potentially serves as a regulator for
FhTPS1 expression. Moreover,
FhMYB108 exhibits negligible expression levels in petals, pistils, and receptacle, while displaying prominent expression in stamens (
Figure 2F). This implies that
FhMYB108 may primarily regulate linalool synthesis in flower stamens, serving as a potential marker for nectary localization and attracting pollinating insects.
2.3. FhMYB108 Is a Transcription Factor with Strong Activation Activity
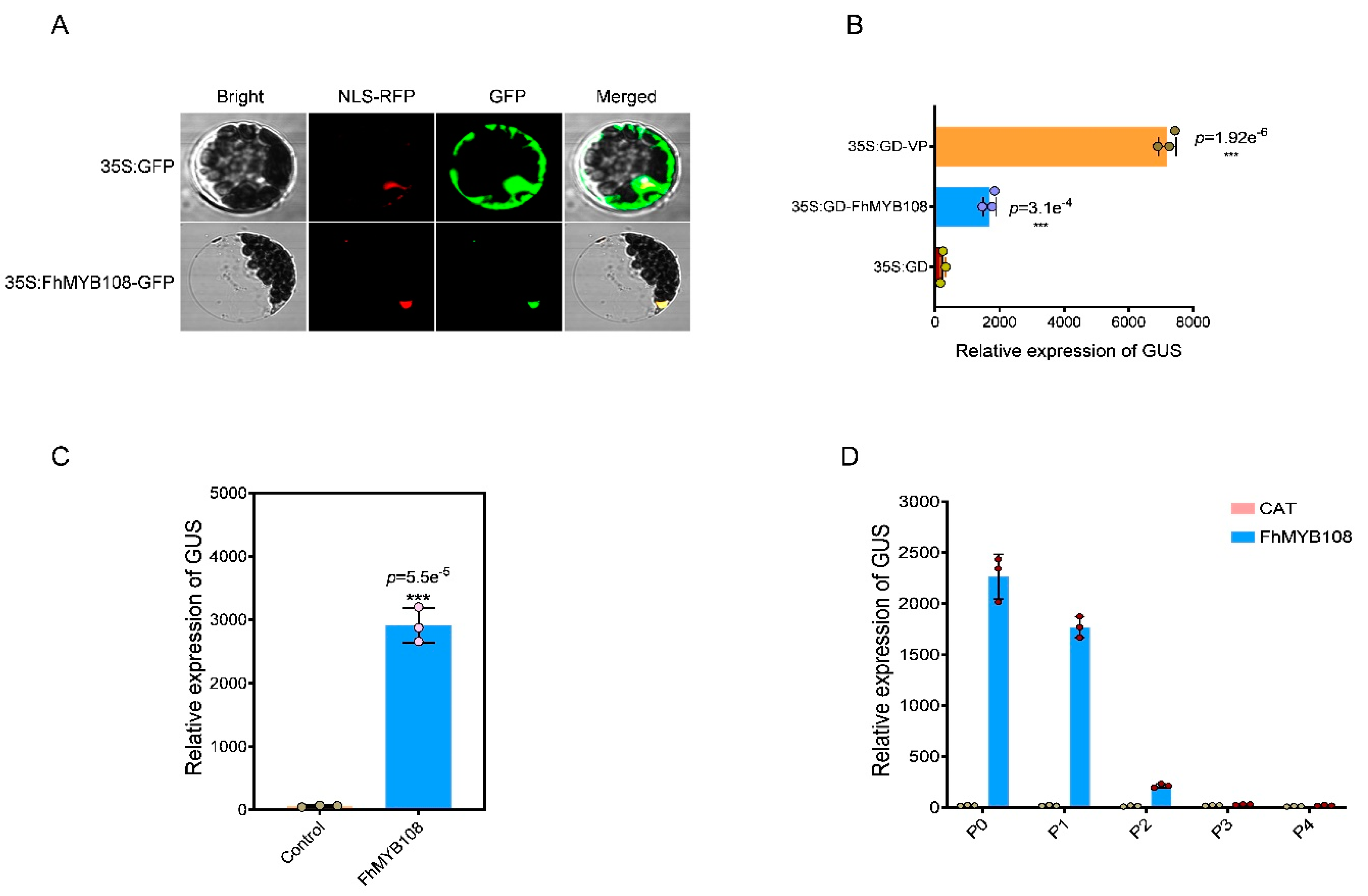
The transient expression vector harboring FhMYB108 fused with a GFP tag was constructed and subsequently transfected into protoplasts. Intense green fluorescence emanated from the nucleus upon fluorescence, indicating nuclear localization of FhMYB108 similar to that of the red NLS nuclear localization signal marker. These findings suggest that FhMYB108 functions as a nuclear-localized transcription factor (
Figure 3A).
GD can interact with Gal4 and subsequently modulate GUS gene expression via GD-associated proteins. Herin, the plasmids pUC19-GD (negative control), pUC19-GD-FhMYB108 and PUC19-GD-VP16 (positive control) harboring the GD label were co-transfected with Gal4: GUS, respectively, followed by subsequent assessment of GUS activity. The robust transcriptional activation potential of FhMYB108 is evident, indicating its role as a proficient transcriptional activator (
Figure 3B).
Additionally, the
TPS1 gene promoter was cloned to generate a HA-pUC19-FhProTPS1: GUS plasmid, which was co-transfected with the transient expression vector HA-pUC19
-FhMYB108 into
A. thaliana protoplasts (HA-pUC19-CAT serving as a negative control). The result suggests that FhMYB108 can significantly enhance GUS activity, indicating the autonomous binding capability of FhMYB108 to the promoter region of
FhTPS1 and its potential for transcriptional activation (
Figure 3C). Moreover, by means of truncation analysis, it was ascertained that FhMYB108 is likely to exert regulatory control over
FhTPS1 expression through its binding affinity towards the -1016~-916 bp region of
FhTPS1 promoter (
Figure 3D).
2.4. The Activation of Sesquiterpene and Monoterpene Synthase Genes by FhMYB108 in Arabidopsis Thaliana
Due to the unavailability of a reliable genetic transformation system in
F. hybrida, we performed transient transformation of FhMYB108 in
A. thaliana protoplasts to validate its regulatory role in terpenoid metabolism. According to the phylogenetic analysis, FhMYB108 exhibits a close evolutionary relationship with MYB21, thereby implying its potential regulatory role in modulating the expression of
A. thaliana sesquiterpene synthase genes
AtTPS11 and
AtTPS21. Additionally, linalool formation is catalyzed by
AtTPS14 in
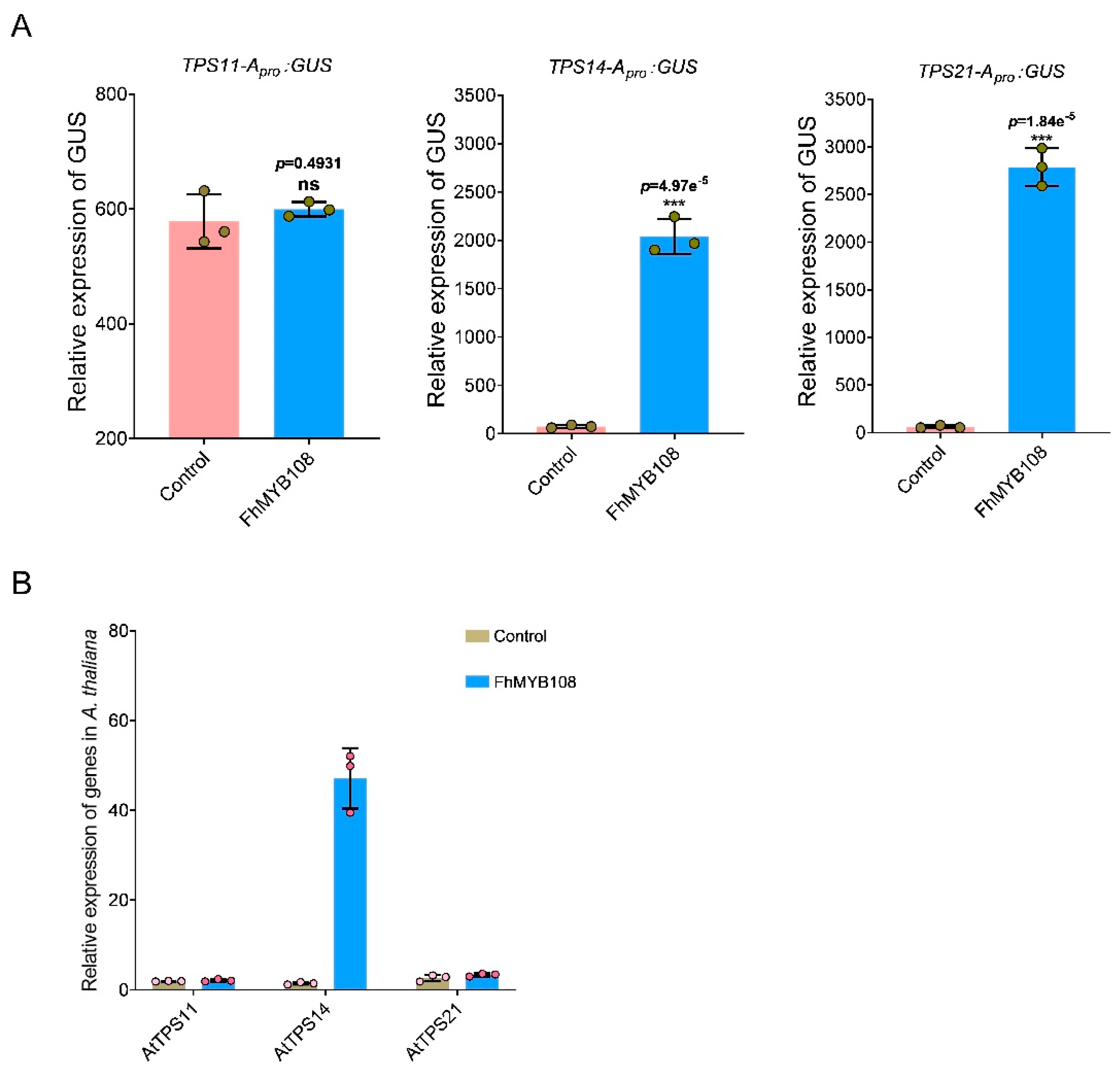
A. thaliana. Therefore, we cloned the promoter sequences of
AtTPS11,
AtTPS14, and
AtTPS21 and subsequently verified the activation potential of FhMYB108 on these promoters (with HA-pUC19-CAT serving as a negative control) in accordance with the previous experiment on transcription factor activation assay. The results demonstrated that co-transfection of FhMYB108 with the promoters of
AtTPS14 and
AtTPS21 led to a significant increase in GUS values, indicating the specific binding ability of FhMYB108 to the promoter regions of AtTPS14 and AtTPS21, thereby activating their transcription (
Figure 4A).
When FhMYB108 was transiently expressed in protoplasts alone, it was observed that only
AtTPS14, which governs linalool biosynthesis, exhibited a significant upregulation in expression compared to the control group; however, no significant alteration in expression was detected for
AtTPS21 (
Figure 4B).
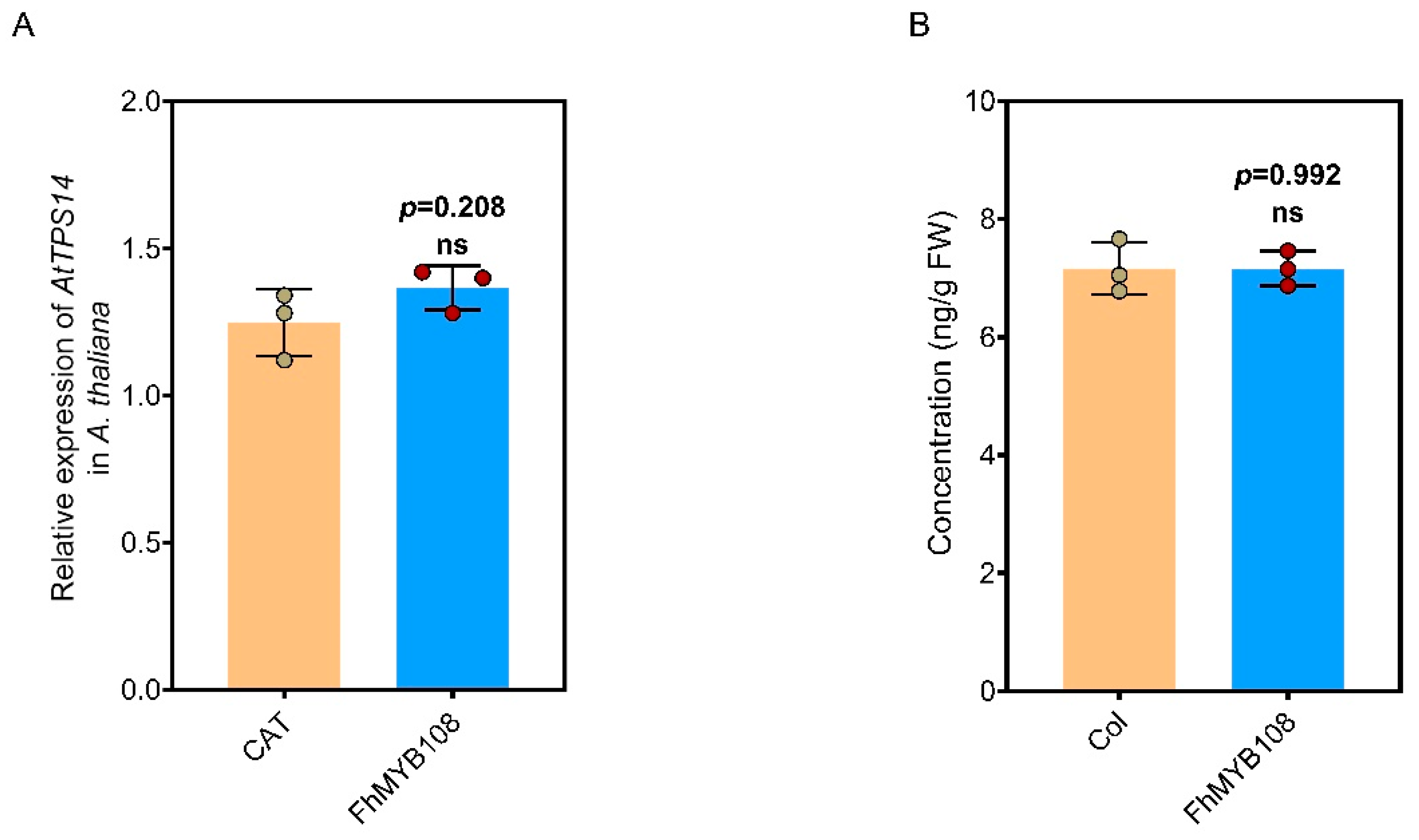
2.5. The Expression of TPS14 and Release of Linalool in FhMYB108 Overexpressing Seedlings
The regulatory role of FhMYB108 was further elucidated by successful expression in A. thaliana through inflorescence transformation. The mRNA extraction was performed to quantify the expression level of
AtTPS14, however, no significant increase in its expression level was observed upon the overexpression of
FhMYB108 (
Figure 5A). Additionally, GC-MS analysis consistently revealed a negligible linalool content with no discernible alterations observed in the transgenic line when compared to the wild type (WT) (
Figure 5B). Based on the transient expression results, we propose the presence of a putative transcription factor in
A. thaliana that potentially interacts with FhMYB108 or competes for binding to the promoter region of
AtTPS14, thereby exerting robust inhibitory effects on
AtTPS14 gene expression.
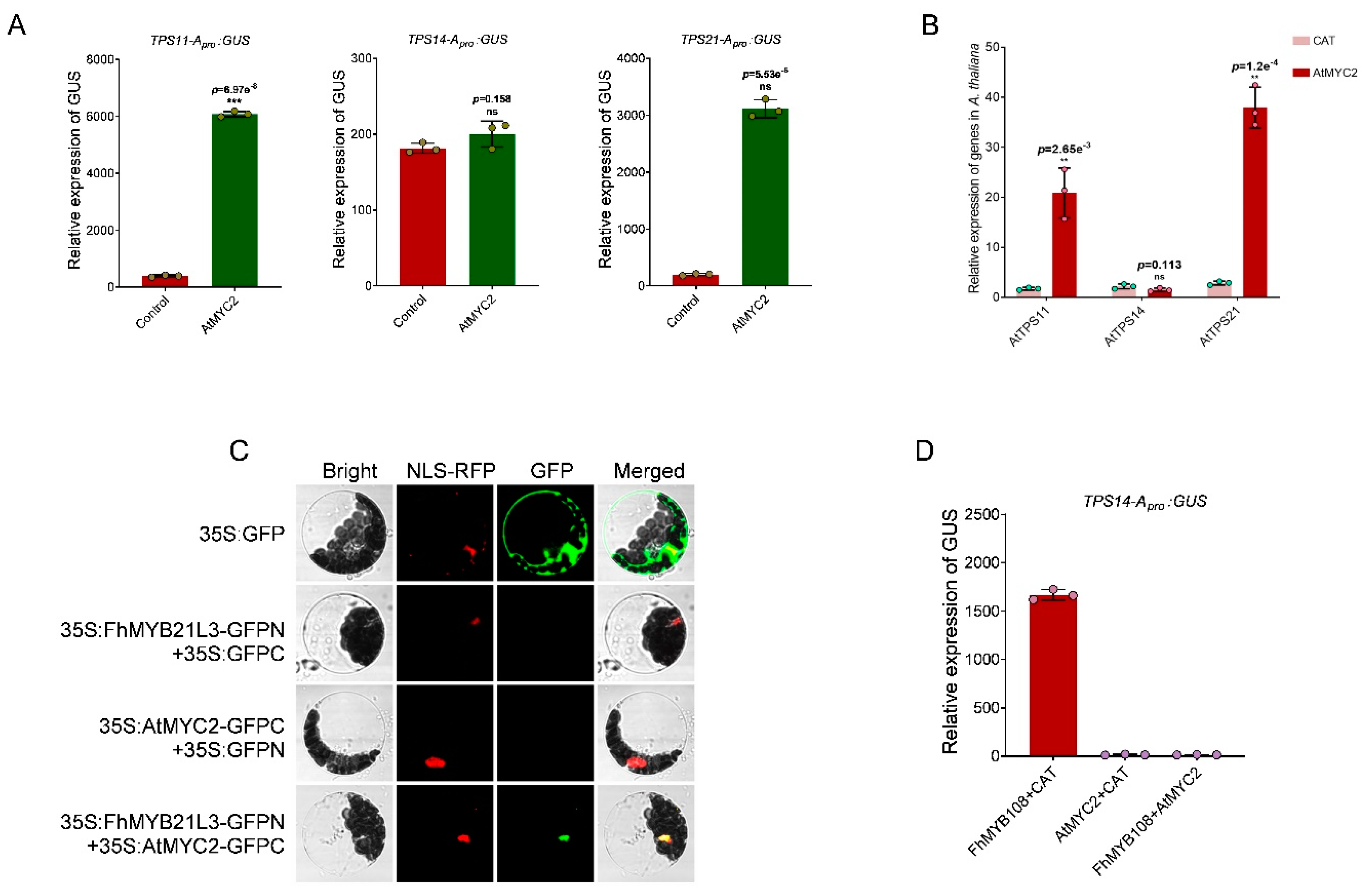
2.6. The Expression of the AtTPS14 Gene by FhMYB108 and AtMYC2 in Arabidopsis
In
A. thaliana, besides MYB21 and MYB24, which have been demonstrated to modulate terpene metabolism, an additional bHLH-type transcription factor (AtMYC2) has also been identified as a regulator of terpene biosynthesis via the jasmonic acid (JA) pathway according to research reports [
36]. AtMYC2 plays a role in regulating sesquiterpenoid anabolism, which is highly expressed in
Arabidopsis flowers and has been reported to interact with MYB21. The reported results showed that the content of sesquiterpene caryophylene in flower volatiles of
Arabidopsis thaliana was more than 90%, while the content of linalool was very low, suggesting that AtMYC2 may not promote the accumulation of linalool. Based on these results, we can make the following bold assumptions: (1) AtMYC2 cannot activate the expression of linalool synthase gene AtTPS14; (2) AtMYC2 may interact with FhMYB108 to inhibit the activation of FhMYB108 on
AtTPS14 in Arabidopsis. That is to say, the absence of any discernible impact on linalool content resulting from
FhMYB108 overexpression can plausibly be attributed to the involvement of AtMYC2. By conducting transcription factor activation experiments, we have observed that AtMYC2 exhibits a strong binding affinity towards the promoters of
TPS11 and
TPS21, while it does not demonstrate any discernible binding capability towards the promoters of
AtTPS14 (
Figure 6A). In contrast, transient expression of
AtMYC2 in protoplasts resulted in significant upregulation of
TPS11 and
TPS21, while no alteration was observed for the
AtTPS14 gene, implying that AtMYC2 lacks the ability to positively regulate linalool synthase gene expression (
Figure 6B).
Additionally, the interaction between FhMYB108 and AtMYC2 was confirmed via a double fluorescence complementation experiment in this study. The results demonstrated that the fluorescence signal was exclusively observed upon co-transfection of FHMYB108-GFPN and ATMYC2-GFPC, thereby indicating a potential occurrence of an interaction between FhMYB108 and AtMYC2. Furthermore, FhMYB108 and AtMYC2 were individually or co-transferred with AtProTPS14: GUS into the protoplasts of A. thaliana leaves to measure the binding changes. The results demonstrate that FhMYB108 exerts significant transcriptional activation on the TPS14 promoter, whereas AtMYC2 fails to activate transcription of the TPS14 promoter. Moreover, co-expression of AtMYC2 and FhMYB108 strongly attenuates the transcriptional activation capacity of FhMYB108.
3. Discussion
To enhance their chances of survival and reproductive success, plants typically release a significant amount of volatile small molecule compounds, particularly terpenoids, as chemical signaling agents to establish connections between plants or between plants and animals. These compounds play pivotal roles in crucial processes such as pollination, induction of ovulation, and even direct or indirect involvement in plant defense [
37,
38,
39]. Therefore, revealing the composition of plant volatile terpenoids is crucial for comprehending the physiological functions and potential practical applications of these compounds.
The release of terpenoids demonstrates spatiotemporal specificity, with peak emission observed during the period of full flower bloom or fruit ripening. In this study, the
Red River® cultivar of
F. hybrida at various stages of flower opening were selected as the experimental materials to investigate the expression pattern of the terpenoid synthase gene (
FhTPS1), which plays a crucial role in regulating the biosynthesis of its major volatile compound (linalool). The results demonstrated a gradual increase in the expression of FhTPS1 in
F. hybrida during flower blooming, mirroring the pattern observed in
Clarkia Breweri [
40]. The presence of this regular expression suggests the existence of a regulatory system in
F. hybrida that governs the expression of the linalool synthase gene, indicating a sophisticated mechanism for controlling secondary metabolism in this plant species.
Currently, there is a lack of a systematic approach in the research on the transcriptional regulation of terpenoids. On one hand, the existing studies lack coherence and fail to elucidate the synergistic relationships among multiple transcription factors. On the other hand, most investigations have predominantly focused on specific cash crops such as tobacco and tomato, neglecting more significant flower plants. Within multiple transcription factors reported, only AtMYB21 and AtMYC2 exhibit dual regulatory functions in both terpenoid metabolism and development (specifically stamen formation) in A. thaliana. Previous investigations on anthocyanins and proanthocyanidins in F. hybrida have highlighted the potential significance of MYB transcription factors in secondary metabolism, thereby prompting our focus on elucidating the role of MYB transcription factors in the synthesis and regulation of linalool.
By employing AtMYB21 as a bait for comparative transcriptome analysis in F. hybrida, we successfully identified FhMYB108, a transcription factor with the potential to regulate terpenoid metabolism. FhMYB108 and AtMYB21 are phylogenetically grouped together, implying potential functional similarities between them. Furthermore, experimental evidence obtained from transcription factor activation assays has substantiated the specific binding capability of FhMYB108 to the promoter regions of AtTPS11 and AtTPS21 (which are known targets of AtMYB21), thereby effectively inducing their transcriptional activity. Meanwhile, FhMYB108 exhibits a comparable expression pattern to FhTPS1 and can specifically bind to the -916-1016 region of TPS1 promoter. Furthermore, FhMYB108 demonstrates its regulatory role in monoterpene biosynthesis by activating the monoterpene synthase gene TPS14 in A. thaliana, providing additional evidence.
The results of promoter activation and transient overexpression demonstrate the capacity of FhMYB108 to induce the expression of linalool synthase genes in F. hybrida and A. thaliana. However, this phenomenon was not observed upon stable expression in A. thaliana. Relevantly, FhMYB108 demonstrated the capacity to induce transcription of AtTPS11 and AtTPS21; however, no discernible enhancement in gene expression or metabolite levels was observed in FhMYB108 overexpressed Arabidopsis. Through comprehensive analysis, it was determined that AtMYC2, a highly expressed transcription factor found in both the leaves and flowers of A. thaliana, played a pivotal role in driving these observed changes. Notably, this transcription factor not only plays a crucial role in the terpenoid metabolism of A. thaliana but also exerts regulatory control over its growth and development. In stable lines, the highly expressed AtMYC2 would interact with the FhMYB108 transcription factor, thereby suppressing the activation of TPS14 by FhMYB108 and subsequently inhibiting the biosynthesis of linalool and other related metabolites.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
F. hybrida cultivar Red River® was grown in a greenhouse at 20℃-25℃ with light/dark period of 16/8h. Flowers of Red River® from different individuals were sampled at different florescence were also collected for volatiles measure, RNA extraction and gene expression assay.
A. thaliana (Columbia-0) were used in this study. After undergoing vernalization at 4℃ for a duration of 2-3 days, the specimen was subsequently transplanted into soil and cultivated under controlled conditions at room temperature with a photoperiod of 16 hours light followed by 8 hours darkness. Fresh and tender leaves cultivated for duration of 3-4 weeks were utilized for the preparation of A. thaliana protoplasts, whereas A. thaliana seedlings grown for a period of 6-8 weeks are suitable for transgenic functional validation.
4.2. Volatiles Collection and Analysis
The volatiles within F. hybrida were collected by using solid phase headspace micro extraction. Briefly, the fresh flowers at various developmental stages were meticulously arranged within a sealed glass enclosure, while the polydimethylsiloxane (PDMS) solid phase microextraction needle was carefully inserted 2 cm above the flower through the rubber pad positioned atop the glass cover. The entire apparatus was placed within a controlled environment chamber, simulating an artificial climate incubator, where the volatile compounds emitted by F. hybrida flowers were collected at a temperature of 25℃ for a duration of 2 hour. Then, the extracted needles were subjected to gas chromatography-mass spectrometry (GC-MS) analysis, and the resulting mass spectrum was compared with the standard spectrum (NIST2008) for qualitative identification of volatile components.
4.3. Quantitative Real-Time PCR
RNA was isolated with TRIzol reagents from relevant samples previously obtained. First-strand cDNA was synthesized from 1 μg of total RNA using the RevertAid First Strand cDNA Synthesis Kit (cat. no. K1621; Thermo Fisher, USA). Then, qPCR (qRT-PCR) was carried out by SYBR Green. All primers used for qRT-PCR were designed by using the National Center for Biotechnology Information (NCBI) online software Primer-BLAST (
Table S1), and the 18S rRNA was used as internal controls to normalize gene expression.
4.4. Sequence Analysis and Phylogenetic Analysis
The
A. thaliana AtMYB21 was employed as a bait sequence for conducting BLAST analysis to identify potential MYB transcription factors involved in the transcriptional regulation of terpene synthases in
F. hybrida. Then, the candidate genes were cloned with specific primers. Homologous proteins from different organisms were identified using the BLAST algorithm on NCBI (
https://blast.ncbi.nlm.nih.gov). Phylogenetic analysis was carried out with the program MEGA542 using the neighbor-joining method and the phylogenetic tree was then displayed using the iTOL online service (
https://itol.embl.de/). Sequence alignment was generated by the MEGA5 software and then was visualized using the DNAMAN.
4.5. Subcellular Localization and BiFC Assays
For subcellular localization assay, genes were cloned into the HA-pUC19-GFP (p35S: GFP) vector and these recombinant plasmids were then introduced into the protoplasts of
A. thaliana by PEG-mediated transformation as previously reported [
41]. For BiFC assay, genes were cloned into HA-pUC19–GFPN vector carrying N-terminal GFP and HA-pUC19-GFPC vector carrying C-terminal GFP, respectively, and these recombinant plasmids were also introduced into the protoplasts of Arabidopsis with PEG-mediated transformation. Fluorescence in subcellular localization and BiFC assays was observed under a confocal fluorescence microscope at 20 hpi.
4.6. Transcriptional Activity Analysis
To assess the transcriptional activation activity of FhMYB108, a transient transformation vector with GD label was constructed under the control of 35S promoter. Subsequently, the concentrated pUC19-GD (Negative control), pUC19-GD-FhMYB108, and pUC19-GD-VP16 (positive control) plasmids were co-transfected into A. thaliana protoplasts along with Gal4: GUS, respectively. The GUS activity was assessed following incubation at 22℃ in darkness for 21~23 hours. In order to investigate the activation of a specific gene by the transcription factor FhMYB108, the gene promoter was cloned into the HA-pUC19: GUS vector and co-transformed it with pUC19-FhMYB108 as mentioned above.
4.7. Overexpression
To further validate the regulatory role of FhMYB108 in linalool metabolism, a plant transformation overexpression vector was constructed and subsequently introduced into wild-type A. thaliana (Col) via Agrobacterium-mediated pollen tube channel method. In short, the FhMYB108 sequence was amplified using cDNA as a template, seamlessly cloned and ligated into the pBI121 vector, and subsequently transformed into Agrobacterium. Suspend Agrobacterium in a 5% sucrose solution in a beaker supplemented with surfactants. Then, submerge the flowers of mature, non-podded wild-type A. thaliana in a beaker, ensuring they are positioned below the liquid surface for a duration of 3 minutes. Labeling plants and placing treated ones into a water-filled tray, shield from light overnight, and proceed with normal conditions cultivation following day.
4.8. Statistical Analysis
The GraphPad Prism was used to conduct statistical analysis. The mean ± standard deviation is reported based on the results obtained from a minimum of three independent experiments. The Student's t-test was employed to assess differences between groups, with a significance level of P<0.05 indicating statistical significance.
5. Conclusions
In this study, a MYB type transcription factor with regulatory function in terpenoid metabolism was cloned within Red River®. The mechanism of its regulation on linalool synthesis was subsequently validated in both F. hybrida and A. thaliana. Simultaneously, it was revealed that AtMYC2 can interact with FhMYB108 and exerts a negative regulatory role in linalool synthesis. This study unveils the synergistic impact of MYB and bHLH transcription factors on terpenoid regulation, thereby establishing a fundamental basis for further enhancement of plant terpenoid metabolism regulatory networks.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1 Primers used in this study.
Author Contributions
Conceptualization, W.J. and Z.Y.; formal analysis, Q.L. and C.C.; investigation, X.L.; data curation, F.M.; writing—original draft preparation, Z.Y. and Y.L.; writing—review and editing, K.X. and Y.W.; funding acquisition, K.X and Z.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Henan Province (222300420395), Henan Province Science and technology research project (222102110057), The Foundation of Henan Science and Technology Committee (242102110238).
Institutional Review Board Statement
Ethical review and approval were waived for this study, because it did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
We would like to thank Xiang Gao Research Group of the Key Laboratory of Molecular Epigenetics of MOE and Institute of Genetics and Cytology, Northeast Normal University, for their great assistance in the design and execution of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Muhlemann, J.K., Klempien, A., Dudareva, N. Floral volatiles: From biosynthesis to function. Plant Cell Environ. 2014, 37, 1936-49. [CrossRef]
- Dudareva, N., Klempien, A., Muhlemann, J. K., Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [CrossRef]
- Aprotosoaie, A.C., Hăncianu, M., Costache, I., Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Frag J. 2014, 29, 193-219. [CrossRef]
- Amiri, P., Shahpiri, A., Asadollahi, M. A., Momenbeik, F., Partow, S. Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol let. 2016, 38, 503–508. [CrossRef]
- Lapczynski, A., Letizia, C.S., Api, A.M. Addendum to Fragrance material review on linalool. Food Chem Toxicol. 2008, 46, Suppl 11, S190–S192. [CrossRef]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol. 2006, 9(3): 297-304. [CrossRef]
- Cho, M., So, I., Chun, J.N., Jeon, J.H. The antitumor effects of geraniol: Modulation of cancer hallmark pathways (Review). Int J Oncol. 2016, 48, 1772–1782. [CrossRef]
- Herman, A., Tambor, K., Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr Microbiol, 2016, 72, 165–172. [CrossRef]
- Sobral, M. V., Xavier, A. L., Lima, T. C., & de Sousa, D. P. (2014). Antitumor activity of monoterpenes found in essential oils. The ScientificWorld Journal. 2014, 953451. [CrossRef]
- Guzmán-Gutiérrez, S.L., Gómez-Cansino, R., García-Zebadúa, J.C., Jiménez-Pérez, N.C., Reyes-Chilpa, R. Antidepressant activity of Litsea glaucescens essential oil: Identification of β-pinene and linalool as active principles. J Ethnopharmacol, 2012, 143, 673–679. [CrossRef]
- Knudsen, J.T., Eriksson, R., Gershenzon, J., Ståhl, B. Diversity and distribution of floral scent. Bot Rev. 2006, 72, 1-120. [CrossRef]
- Pichersky, E., Noel, J.P., Dudareva, N. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science. 2006, 311, 808–811. [CrossRef]
- Yonekura-Sakakibara, K., Saito, K. Functional genomics for plant natural product biosynthesis. Nat Prod Rep. 2009, 26, 1466–1487. [CrossRef]
- Tang, D.Y., Guo, J., Song, Y.F., Li, L., Zhao, L.M., Shen, D.Y. GHz pulse train generation in fiber lasers by cavity induced modulation instability. Plant Physiol. 2014, 6, 610-614. [CrossRef]
- Turner, G.W., Gershenzon, J., Croteau, R.B. Distribution of peltate glandular trichomes on developing leaves of peppermint. Plant physiol. 2000, 124, 655–664. [CrossRef]
- Lu, S., Xu, R., Jia, J.W., Pang, J., Matsuda, S.P., Chen, X.Y. Cloning and functional characterization of a beta-pinene synthase from Artemisia annua that shows a circadian pattern of expression. Plant Physiol. 2002, 130, 477–486. [CrossRef]
- Loughrin, J.H., Manukian, A., Heath, R.R., Turlings, T.C., Tumlinson, J.H. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. PNAS. 1994, 91, 11836–11840. [CrossRef]
- Jørgensen, K., Rasmussen, A.V., Morant, M., Nielsen, A.H., Bjarnholt, N., Zagrobelny, M., Bak, S., Møller, B.L. Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol. 2005, 8, 280–291. [CrossRef]
- Yu, F., Utsumi, R. Diversity, regulation, and genetic manipulation of plant mono- and sesquiterpenoid biosynthesis. CMLS. 2009, 66, 3043–3052. [CrossRef]
- Degenhardt, J., Köllner, T.G., Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009, 70, 1621–1637. [CrossRef]
- Chen, F., Tholl, D., Bohlmann, J., Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [CrossRef]
- Nagegowda D. A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS letters. 2010, 584, 2965–2973. [CrossRef]
- Tholl, D., Lee, S. Terpene Specialized Metabolism in Arabidopsis thaliana. The arabidopsis book. 2011, 9, e0143. [CrossRef]
- Nagegowda, D.A., Gutensohn, M., Wilkerson, C.G., Dudareva, N. Two nearly identical terpene synthases catalyze the formation of nerolidol and linalool in snapdragon flowers. Plant J. 2008, 55, 224–239. [CrossRef]
- Li, R., Fan, Y. Molecular Cloning and Expression Analysis of a Terpene Synthase Gene, HcTPS2, in Hedychium coronarium. Plant Mol Biol Rep. 2011, 29, 35-42.
- Jin, J., Zhang, H., Kong, L., Gao, G., Luo, J. PlantTFDB 3.0: A portal for the functional and evolutionary study of plant transcription factors. Nucleic acids res. 2014, 42, 1182–1187. [CrossRef]
- Reeves, P.H., Ellis, C.M., Ploense, S.E., Wu, M.F., Yadav, V., Tholl, D., Chételat, A., Haupt, I., Kennerley, B.J., Hodgens, C., Farmer, E.E., Nagpal, P., Reed, J.W. A regulatory network for coordinated flower maturation. PLoS genet. 2012, 8, e1002506. [CrossRef]
- Cheng, H., Song, S., Xiao, L., Soo, H.M., Cheng, Z., Xie, D., Peng, J. Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS genet. 2009, 5, e1000440. [CrossRef]
- Mandaokar, A., Thines, B., Shin, B., Lange, B. M., Choi, G., Koo, Y.J., Yoo, Y.J., Choi, Y.D., Choi, G., Browse, J. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 2006, 46, 984–1008. [CrossRef]
- Qi, T., Huang, H., Song, S., Xie, D. Regulation of Jasmonate-Mediated Stamen Development and Seed Production by a bHLH-MYB Complex in Arabidopsis. Plant cell. 2015, 27, 1620–1633. [CrossRef]
- Hong, G.J., Xue, X.Y., Mao, Y.B., Wang, L.J., Chen, X.Y. Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant cell. 2012, 24, 2635–2648. [CrossRef]
- Uwagaki, Y., Matsuda, E., Komaki, M., Murahama, M., Hamada, T. Agrobacterium-mediated transformation and regeneration of freesia×hybrida. Plant Biotechnol. 2015, 32, 165-168. [CrossRef]
- Ao, M., Liu, B., Wang, L. Volatile compound in cut and un-cut flowers of tetraploid Freesia hybrida. Nat Prod res. 2013, 27, 37–40. [CrossRef]
- Gao, F., Liu, B., Li, M., Gao, X., Fang, Q., Liu, C., Ding, H., Wang, L., Gao, X. Identification and characterization of terpene synthase genes accounting for volatile terpene emissions in flowers of Freesia x hybrida. J Exp Bot. 2018, 69, 4249–4265. [CrossRef]
- Yang, Z., Li, Y., Gao, F., Jin, W., Li, S., Kimani, S., Yang, S., Bao, T., Gao, X., Wang, L. MYB21 interacts with MYC2 to control the expression of terpene synthase genes in flowers of Freesia hybrida and Arabidopsis thaliana. J Exp Bot. 2020, 71, 4140–4158. [CrossRef]
- Bu, Q., Jiang, H., Li, C.B., Zhai, Q., Zhang, J., Wu, X., Sun, J., Xie, Q., Li, C. Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses. Cell Res. 2008, 18, 756–767. [CrossRef]
- Holopainen, J.K., Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [CrossRef]
- Galliot, C., Stuurman, J., Kuhlemeier, C. The genetic dissection of floral pollination syndromes. Current Opin Plant Biol. 2006, 9, 78–82. [CrossRef]
- Klahre, U., Gurba, A., Hermann, K., Saxenhofer, M., Bossolini, E., Guerin, P.M., Kuhlemeier, C. Pollinator choice in Petunia depends on two major genetic Loci for floral scent production. Curr boil. 2011, 21, 730–739. [CrossRef]
- Dudareva, N., Cseke, L., Blanc, V. M., Pichersky, E. Evolution of floral scent in Clarkia: Novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell. 1996, 8, 1137–1148. [CrossRef]
- Shen, J., Fu, J., Ma, J., Wang, X., Gao, C., Zhuang, C., Wan, J., Jiang, L. Isolation, culture, and transient transformation of plant protoplasts. Curr Protoc Cell Biol. 2014, 63, 2.8.1-17. [CrossRef]
Figure 1.
Diverse volatile terpenoids and corresponding volatilization regulations in F. hybrida. (A) The relative proportions of monoterpenes, sesquiterpenes, and carotenoid derivatives in the volatile compounds emitted by Red River® flowers. (B) The proportion of the main volatile monomer substance in Red River® flowers. (C) The release levels of volatiles in different blooming stages of Red River®. (D) The release levels of linalool, terpineol and other monoterpenes in different flowering stage.
Figure 1.
Diverse volatile terpenoids and corresponding volatilization regulations in F. hybrida. (A) The relative proportions of monoterpenes, sesquiterpenes, and carotenoid derivatives in the volatile compounds emitted by Red River® flowers. (B) The proportion of the main volatile monomer substance in Red River® flowers. (C) The release levels of volatiles in different blooming stages of Red River®. (D) The release levels of linalool, terpineol and other monoterpenes in different flowering stage.
Figure 2.
Analysis of the differential expression patterns of FhTPS1 and FhMYB108 in floral tissues. (A) Expression level of FhTPS1 in different tissues. (B) Expression level of FhTPS1 in different blooming stages of Red River®. (C) Amino acid sequence alignment diagram between FhMYB108 and its homologous proteins. (D) Phylogenetic analysis of FhMYB108. (E) and (F) Spatial and temporal expression pattern of FhMYB108 in Red River® flowers. The data were represented by means ± standard deviations with three replicates.
Figure 2.
Analysis of the differential expression patterns of FhTPS1 and FhMYB108 in floral tissues. (A) Expression level of FhTPS1 in different tissues. (B) Expression level of FhTPS1 in different blooming stages of Red River®. (C) Amino acid sequence alignment diagram between FhMYB108 and its homologous proteins. (D) Phylogenetic analysis of FhMYB108. (E) and (F) Spatial and temporal expression pattern of FhMYB108 in Red River® flowers. The data were represented by means ± standard deviations with three replicates.
Figure 3.
Transcriptional activity analysis of FhMYB108. (A) Localization of FhMYB108 in cells. (B) Measurement of GUS reporter gene expression. (C) Binding assay of FhMYB108 to TPS1 promoter. (D) The activation validation of FhMYB108 to truncated promoter of TPS1. The data were represented by means ± standard deviations with three replicates, and significant difference by Duncan’s multiple ranges represented with different letters, *p<0.05, **p<0.01, ***p<0.001.
Figure 3.
Transcriptional activity analysis of FhMYB108. (A) Localization of FhMYB108 in cells. (B) Measurement of GUS reporter gene expression. (C) Binding assay of FhMYB108 to TPS1 promoter. (D) The activation validation of FhMYB108 to truncated promoter of TPS1. The data were represented by means ± standard deviations with three replicates, and significant difference by Duncan’s multiple ranges represented with different letters, *p<0.05, **p<0.01, ***p<0.001.
Figure 4.
Regulation of gene expression by transient expression of FhMYB108 in A. thaliana. (A) Promoter activation assay in A. thaliana (B) The expression level of terpene synthase genes in FhMYB108 overexpressed A. thaliana. The data were represented by means ± standard deviations with three replicates, and significant difference by Duncan’s multiple ranges represented with different letters, *p<0.05, **p<0.01, ***p<0.001.
Figure 4.
Regulation of gene expression by transient expression of FhMYB108 in A. thaliana. (A) Promoter activation assay in A. thaliana (B) The expression level of terpene synthase genes in FhMYB108 overexpressed A. thaliana. The data were represented by means ± standard deviations with three replicates, and significant difference by Duncan’s multiple ranges represented with different letters, *p<0.05, **p<0.01, ***p<0.001.
Figure 5.
TPS14 expression and linalool release in FhMYB108 overexpressed A. thaliana. (A) Quantification the expression level of AtTPS14 upon the overexpression of FhMYB108 in A. thaliana. (B) linalool content in overexpressed seedlings. The data were represented by means ± standard deviations with three replicates, and significant difference by Duncan’s multiple ranges represented with different letters, *p<0.05, **p<0.01, ***p<0.001.
Figure 5.
TPS14 expression and linalool release in FhMYB108 overexpressed A. thaliana. (A) Quantification the expression level of AtTPS14 upon the overexpression of FhMYB108 in A. thaliana. (B) linalool content in overexpressed seedlings. The data were represented by means ± standard deviations with three replicates, and significant difference by Duncan’s multiple ranges represented with different letters, *p<0.05, **p<0.01, ***p<0.001.
Figure 6.
Interaction between FhMYB108 and AtMYC2. (A) The transcriptional activation assay of AtMYC2. (B) The expression of terpene synthase genes in AtMYC2 overexpressed A. thaliana. (C) The interaction between FhMYB108 and AtMYC2 verified by BiFC. (D) FhMYB108 and AtMYC2 jointly regulate the transcription of AtTPS14. The data were represented by means ± standard deviations with three replicates, and significant difference by Duncan’s multiple ranges represented with different letters, *p<0.05, **p<0.01, ***p<0.001.
Figure 6.
Interaction between FhMYB108 and AtMYC2. (A) The transcriptional activation assay of AtMYC2. (B) The expression of terpene synthase genes in AtMYC2 overexpressed A. thaliana. (C) The interaction between FhMYB108 and AtMYC2 verified by BiFC. (D) FhMYB108 and AtMYC2 jointly regulate the transcription of AtTPS14. The data were represented by means ± standard deviations with three replicates, and significant difference by Duncan’s multiple ranges represented with different letters, *p<0.05, **p<0.01, ***p<0.001.
Table 1.
Qualitative and quantitative analysis of volatile terpenes in Red River® flowers.
Table 1.
Qualitative and quantitative analysis of volatile terpenes in Red River® flowers.
| Categories |
RT(min) |
Compounds |
Formula |
Relative contents |
| Monoterpenes |
5.036 |
α-Pinene |
C10H16
|
0.25% |
| 6.089 |
Sabinene |
C10H16
|
0.42% |
| 6.182 |
β-pinene |
C10H16
|
0.15% |
| 6.476 |
Dehydrocineole |
C10H16O |
0.06% |
| 6.662 |
Myrcene |
C10H16
|
2.67% |
| 7.413 |
α-Terpinene |
C10H16
|
0.89% |
| 7.707 |
Eucalyptol |
C10H18O |
0.69% |
| 7.865 |
D-Limonene |
C10H16
|
5.05% |
| 8.138 |
trans-Ocimene |
C10H16
|
1.49% |
| 8.458 |
Terpinene |
C10H16
|
0.27% |
| 8.985 |
cis-Linaloloxide |
C10H18O2
|
0.28% |
| 9.418 |
Furanoid linalool oxide |
C10H18O2
|
0.24% |
| 9.629 |
Terpinolene |
C10H16
|
0.39% |
| 9.922 |
Linalool |
C10H18O |
44.83% |
| 12.028 |
4-Terpineol |
C10H18O |
0.32% |
| 12.407 |
α-Terpineol |
C10H18O |
22.88% |
| 12.726 |
Decanal |
C10H20O |
0.31% |
| 12.872 |
β-Cyclocitral |
C10H16O |
0.38% |
| |
|
|
|
|
| Sesquiterpenes |
16.077 |
Cycloisosativene |
C15H24
|
0.16% |
| 16.212 |
Copaene |
C15H24
|
0.04% |
| 17.163 |
Farnesene |
C15H24
|
0.11% |
| 17.627 |
g-Gurjunene |
C15H24
|
0.19% |
| 18.867 |
Nerodilol |
C15H26O |
0.24% |
| |
|
|
|
|
| Carotenoid derivatives |
16.875 |
Dihydro-ionone |
C13H22O |
3.15% |
| 17.525 |
β-Ionone |
C13H20O |
12.06% |
| 16.699 |
Ionone |
C13H20O |
0.14% |
Table 2.
Quantitative of volatile compound release from Red River® flowers at different stages of blooming (ng g-1 FW).
Table 2.
Quantitative of volatile compound release from Red River® flowers at different stages of blooming (ng g-1 FW).
| Compounds |
S1 |
S2 |
S3 |
S4 |
S5 |
| α-Pinene |
n.d |
n.d |
n.d |
23.58±2.16 |
30.87±4.52 |
| β-pinene |
n.d |
n.d |
n.d |
40.99±4.85 |
24.86±3.10 |
| Dehydrocineole |
n.d |
n.d |
n.d |
n.d |
20.83±1.48 |
| Myrcene |
n.d |
n.d |
n.d |
59.02±4.87 |
72.65±4.88 |
| α-Terpinene |
n.d |
n.d |
n.d |
6.89±2.01 |
38.15±3.75 |
| D-Limonene |
n.d |
n.d |
15.89±4.02 |
42.89±4.17 |
155.21±8.55 |
| trans-Ocimene |
n.d |
n.d |
48.56±3.41 |
120.36±10.25 |
635.1±26.55 |
| Cis-ocimene |
n.d |
n.d |
24.26±3.88 |
189.25±10.52 |
745.12±20.78 |
| cis-Linaloloxide |
n.d |
n.d |
n.d |
70.68±7.45 |
147.29±18.26 |
| Terpinolene |
n.d |
n.d |
n.d |
8.88±6.02 |
46.21±5.12 |
| Linalool |
n.d |
29.32±2.06 |
158.36±7.69 |
6845.48±204.36 |
17963.14±364.88 |
| 4-Terpineol |
n.d |
n.d |
n.d |
24.84±2.86 |
34.29±5.14 |
| α-Terpineol |
8.26±2.06 |
18.26±3.07 |
135.98±8.66 |
6889.21±59.64 |
8795.66±276.11 |
| Cycloisosativene |
n.d |
n.d |
n.d |
10.25±1.87 |
33.26±3.55 |
| γ-Gurjunene |
n.d |
n.d |
n.d |
22.02±4.20 |
73.58±2.55 |
| Nerodilol |
n.d |
n.d |
n.d |
21.55±3.47 |
37.98±5.64 |
| Dihydro-ionone |
n.d |
n.d |
3.02±2.55 |
588.21±60.54 |
908.23±40.88 |
| β-Ionone |
n.d |
n.d |
8.22±4.20 |
2701.84±105.32 |
4429.14±207.44 |
| Ionone |
n.d |
n.d |
n.d |
n.d |
65.88±6.45 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).