Submitted:
17 June 2024
Posted:
18 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
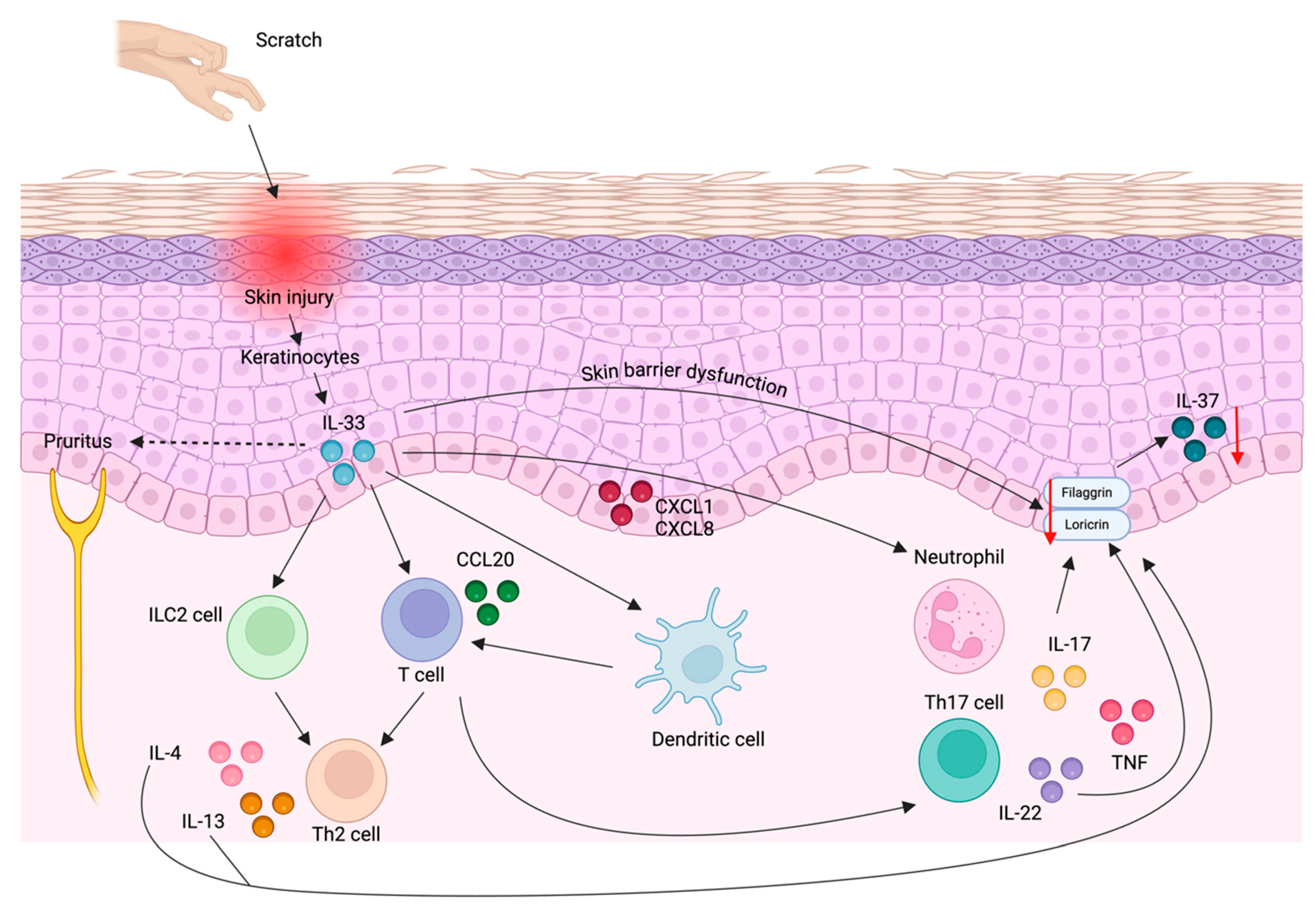
3. Atopic Dermatitis and Psoriasis Pathogenesis
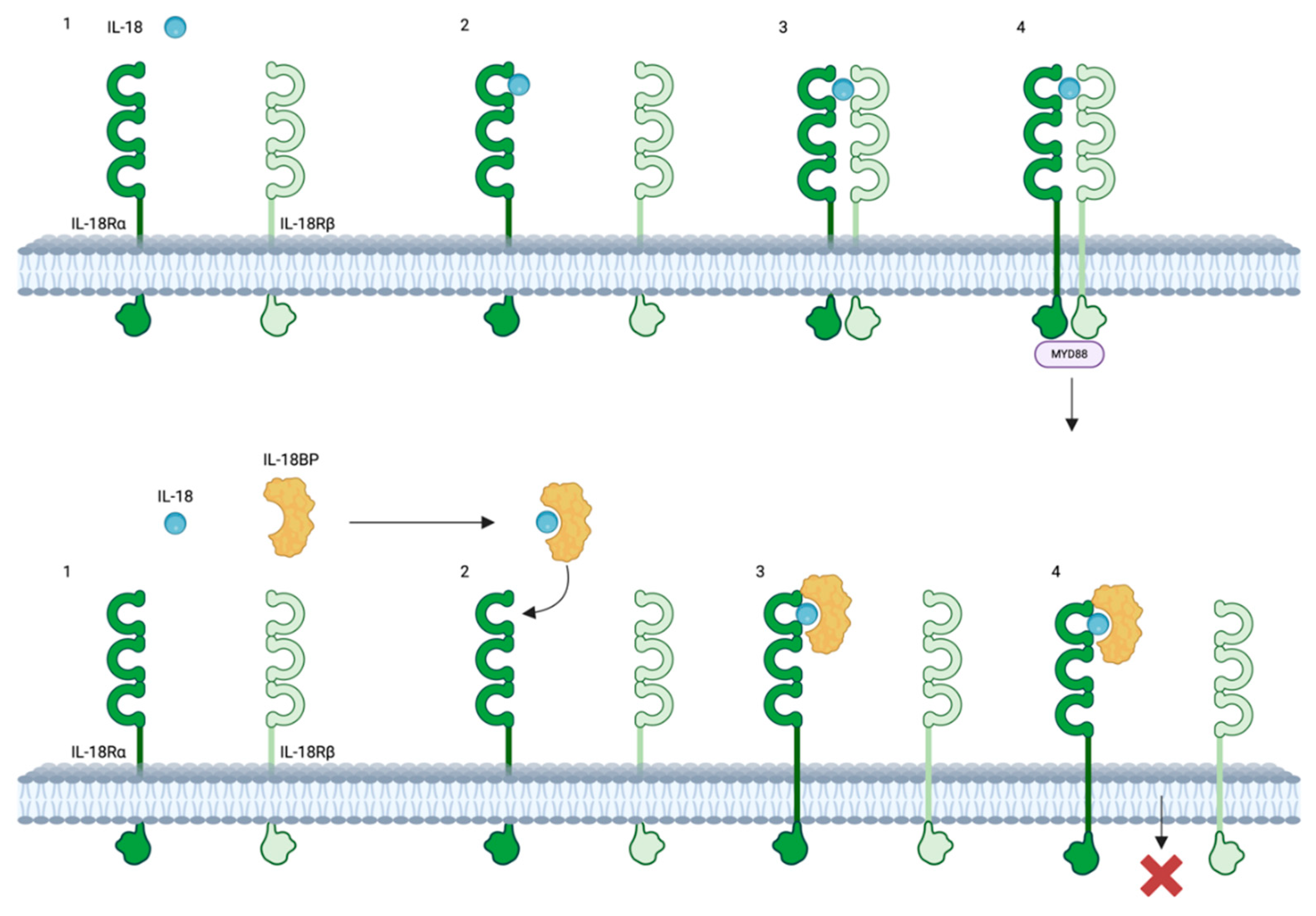
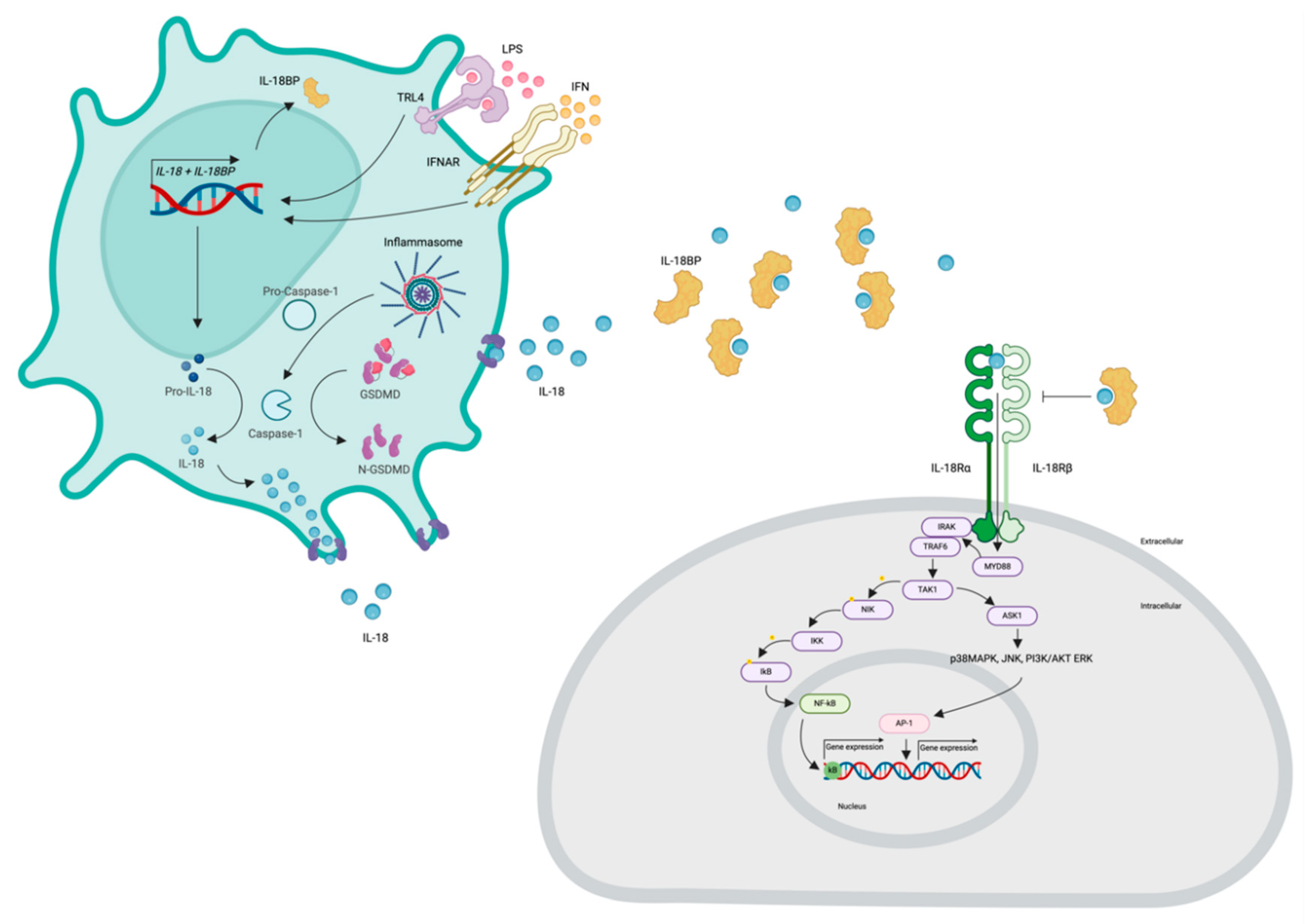
4. Biology of IL-18
4.1. IL-18 in Keratinocytes
4.2. IL-18 in Immune Cells
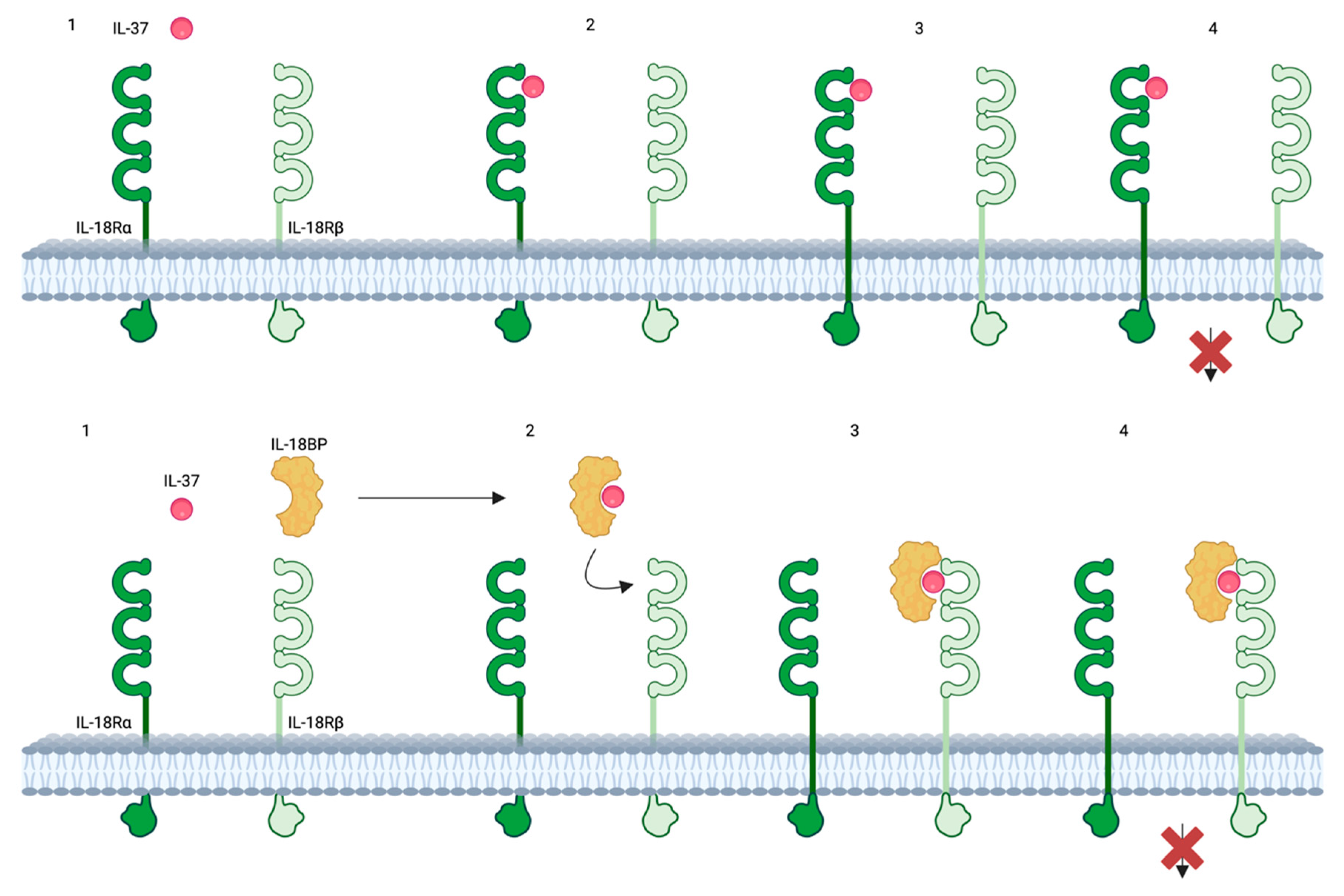
5. Biology of IL-37
5.1. IL-37 in the Skin
5.2. IL-37 in Immune Cells
6. IL-18 and IL-37 in Inflammatory Skin Diseases
6.1. IL-18 and IL-37 as Therapeutic Targets
6.2. IL-18 as a Therapeutic Target
6.3. Tadekinig Alfa
6.4. CMK-389
6.5. AMP18P1RA/IL-18bp-Fc-IL-1ra
6.6. IL-37 as a Therapeutic Target
7. Conclusions
8. Future Directions
Author Contributions
Conflicts of interest
References
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The Family of the Interleukin-1 Receptors. Immunol Rev 2018, 281, 197–232. [CrossRef]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol Rev 2018, 281, 8–27. [CrossRef]
- Matarazzo, L.; Hernandez Santana, Y.E.; Walsh, P.T.; Fallon, P.G. The IL-1 Cytokine Family as Custodians of Barrier Immunity. Cytokine 2022, 154, 155890. [CrossRef]
- Nowarski, R.; Jackson, R.; Gagliani, N.; de Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.D.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [CrossRef]
- Pechkovsky, D. V; Goldmann, T.; Vollmer, E.; Müller-Quernheim, J.; Zissel, G. Interleukin-18 Expression by Alveolar Epithelial Cells Type II in Tuberculosis and Sarcoidosis. FEMS Immunol Med Microbiol 2006, 46, 30–38. [CrossRef]
- Companjen, A.R.; van der Velden, V.H.; Vooys, A.; Debets, R.; Benner, R.; Prens, E.P. Human Keratinocytes Are Major Producers of IL-18: Predominant Expression of the Unprocessed Form. Eur Cytokine Netw 2000, 11, 383–390.
- Wittmann, M.; Macdonald, A.; Renne, J. IL-18 and Skin Inflammation. Autoimmun Rev 2009, 9, 45–48. [CrossRef]
- Landy, E.; Carol, H.; Ring, A.; Canna, S. Biological and Clinical Roles of IL-18 in Inflammatory Diseases. Nat Rev Rheumatol 2024, 20, 33–47. [CrossRef]
- Cavalli, G.; Dinarello, C.A. Suppression of Inflammation and Acquired Immunity by IL-37. Immunol Rev 2018, 281, 179–190. [CrossRef]
- Osborne, D.G.; Domenico, J.; Fujita, M. Expression of IL-37 Induces a Regulatory T-Cell-like Phenotype and Function in Jurkat Cells. Cells 2022, 11. [CrossRef]
- Carrascosa-Carrillo, J.M.; Aterido, A.; Li, T.; Guillén, Y.; Martinez, S.; Marsal, S.; Julià, A. Toward Precision Medicine in Atopic Dermatitis Using Molecular-Based Approaches. Actas Dermosifiliogr 2023. [CrossRef]
- Guo, Y.; Luo, L.; Zhu, J.; Li, C. Multi-Omics Research Strategies for Psoriasis and Atopic Dermatitis. Int J Mol Sci 2023, 24.
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int J Mol Sci 2021, 22. [CrossRef]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv Exp Med Biol 2017, 1027, 21–37. [CrossRef]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic Dermatitis. Nat Rev Dis Primers 2018, 4, 1. [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. The Lancet 2020, 396, 345–360. [CrossRef]
- Oyoshi, M.K.; He, R.; Kumar, L.; Yoon, J.; Geha, R.S. Cellular and Molecular Mechanisms in Atopic Dermatitis. Adv Immunol 2009, 102, 135–226. [CrossRef]
- Çetinarslan, T.; Kümper, L.; Fölster-Holst, R. The Immunological and Structural Epidermal Barrier Dysfunction and Skin Microbiome in Atopic Dermatitis-an Update. Front Mol Biosci 2023, 10, 1159404. [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int J Mol Sci 2019, 20.
- Ni, X.; Lai, Y. Crosstalk between Keratinocytes and Immune Cells in Inflammatory Skin Diseases. Exploration of Immunology 2021, 418–431. [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of Psoriasis. Annu Rev Immunol 2014, 32, 227–255. [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [CrossRef]
- Nestle, F.O.; Kaplan, D.H.; Barker, J. Psoriasis. N Engl J Med 2009, 361, 496–509. [CrossRef]
- Ben Abdallah, H.; Johansen, C.; Iversen, L. Key Signaling Pathways in Psoriasis: Recent Insights from Antipsoriatic Therapeutics. Psoriasis: Targets and Therapy 2021, Volume 11, 83–97. [CrossRef]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis Pathogenesis and the Development of Novel Targeted Immune Therapies. J Allergy Clin Immunol 2017, 140, 645–653. [CrossRef]
- Ganguly, D.; Chamilos, G.; Lande, R.; Gregorio, J.; Meller, S.; Facchinetti, V.; Homey, B.; Barrat, F.J.; Zal, T.; Gilliet, M. Self-RNA-Antimicrobial Peptide Complexes Activate Human Dendritic Cells through TLR7 and TLR8. J Exp Med 2009, 206, 1983–1994. [CrossRef]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.-H.; Homey, B.; Cao, W.; Wang, Y.-H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid Dendritic Cells Sense Self-DNA Coupled with Antimicrobial Peptide. Nature 2007, 449, 564–569. [CrossRef]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.-H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A Distinct Lineage of CD4 T Cells Regulates Tissue Inflammation by Producing Interleukin 17. Nat Immunol 2005, 6, 1133–1141. [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-Producing CD4+ Effector T Cells Develop via a Lineage Distinct from the T Helper Type 1 and 2 Lineages. Nat Immunol 2005, 6, 1123–1132. [CrossRef]
- Nakajima, A.; Matsuki, T.; Komine, M.; Asahina, A.; Horai, R.; Nakae, S.; Ishigame, H.; Kakuta, S.; Saijo, S.; Iwakura, Y. TNF, but Not IL-6 and IL-17, Is Crucial for the Development of T Cell-Independent Psoriasis-like Dermatitis in Il1rn-/- Mice. J Immunol 2010, 185, 1887–1893. [CrossRef]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C.; Chamilos, G.; Feldmeyer, L.; Marinari, B.; Chon, S.; et al. The Antimicrobial Peptide LL37 Is a T-Cell Autoantigen in Psoriasis. Nat Commun 2014, 5, 5621. [CrossRef]
- Aggarwal, S.; Ghilardi, N.; Xie, M.-H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 Promotes a Distinct CD4 T Cell Activation State Characterized by the Production of Interleukin-17. J Biol Chem 2003, 278, 1910–1914. [CrossRef]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [CrossRef]
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.; Wang, T.; Zheng, J.; et al. Pivotal Role of Dermal IL-17-Producing Γδ T Cells in Skin Inflammation. Immunity 2011, 35, 596–610. [CrossRef]
- Bielecki, P.; Riesenfeld, S.J.; Hütter, J.-C.; Torlai Triglia, E.; Kowalczyk, M.S.; Ricardo-Gonzalez, R.R.; Lian, M.; Amezcua Vesely, M.C.; Kroehling, L.; Xu, H.; et al. Skin-Resident Innate Lymphoid Cells Converge on a Pathogenic Effector State. Nature 2021, 592, 128–132. [CrossRef]
- Matos, T.R.; O’Malley, J.T.; Lowry, E.L.; Hamm, D.; Kirsch, I.R.; Robins, H.S.; Kupper, T.S.; Krueger, J.G.; Clark, R.A. Clinically Resolved Psoriatic Lesions Contain Psoriasis-Specific IL-17-Producing Aβ T Cell Clones. J Clin Invest 2017, 127, 4031–4041. [CrossRef]
- Blauvelt, A.; Chiricozzi, A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin Rev Allergy Immunol 2018, 55, 379–390. [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [CrossRef]
- Chiricozzi, A.; Guttman-Yassky, E.; Suárez-Fariñas, M.; Nograles, K.E.; Tian, S.; Cardinale, I.; Chimenti, S.; Krueger, J.G. Integrative Responses to IL-17 and TNF-α in Human Keratinocytes Account for Key Inflammatory Pathogenic Circuits in Psoriasis. J Invest Dermatol 2011, 131, 677–687. [CrossRef]
- Nolan, K.F.; Greaves, D.R.; Waldmann, H. The Human Interleukin 18 Gene IL18 Maps to 11q22.2-Q22.3, Closely Linked to the DRD2 Gene Locus and Distinct from Mapped IDDM Loci. Genomics 1998, 51, 161–163. [CrossRef]
- Chen, G.; Deutsch, G.H.; Schulert, G.S.; Zheng, H.; Jang, S.; Trapnell, B.; Lee, P.Y.; Macaubas, C.; Ho, K.; Schneider, C.; et al. Identification of Distinct Inflammatory Programs and Biomarkers in Systemic Juvenile Idiopathic Arthritis and Related Lung Disease by Serum Proteome Analysis. Arthritis Rheumatol 2022, 74, 1271–1283. [CrossRef]
- Mostafavi, S.; Yoshida, H.; Moodley, D.; LeBoité, H.; Rothamel, K.; Raj, T.; Ye, C.J.; Chevrier, N.; Zhang, S.-Y.; Feng, T.; et al. Parsing the Interferon Transcriptional Network and Its Disease Associations. Cell 2016, 164, 564–578. [CrossRef]
- Heng, T.S.P.; Painter, M.W.; Immunological Genome Project Consortium The Immunological Genome Project: Networks of Gene Expression in Immune Cells. Nat Immunol 2008, 9, 1091–1094. [CrossRef]
- Wang, X.; Wang, L.; Wen, X.; Zhang, L.; Jiang, X.; He, G. Interleukin-18 and IL-18BP in Inflammatory Dermatological Diseases. Front Immunol 2023, 14, 955369. [CrossRef]
- Ghayur, T.; Banerjee, S.; Hugunin, M.; Butler, D.; Herzog, L.; Carter, A.; Quintal, L.; Sekut, L.; Talanian, R.; Paskind, M.; et al. Caspase-1 Processes IFN-Gamma-Inducing Factor and Regulates LPS-Induced IFN-Gamma Production. Nature 1997, 386, 619–623. [CrossRef]
- Fantuzzi, G.; Puren, A.J.; Harding, M.W.; Livingston, D.J.; Dinarello, C.A. Interleukin-18 Regulation of Interferon Gamma Production and Cell Proliferation as Shown in Interleukin-1beta-Converting Enzyme (Caspase-1)-Deficient Mice. Blood 1998, 91, 2118–2125.
- Gu, Y.; Kuida, K.; Tsutsui, H.; Ku, G.; Hsiao, K.; Fleming, M.A.; Hayashi, N.; Higashino, K.; Okamura, H.; Nakanishi, K.; et al. Activation of Interferon-Gamma Inducing Factor Mediated by Interleukin-1beta Converting Enzyme. Science 1997, 275, 206–209. [CrossRef]
- Levy, M.; Thaiss, C.A.; Zeevi, D.; Dohnalová, L.; Zilberman-Schapira, G.; Mahdi, J.A.; David, E.; Savidor, A.; Korem, T.; Herzig, Y.; et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell 2015, 163, 1428–1443. [CrossRef]
- Canna, S.W.; de Jesus, A.A.; Gouni, S.; Brooks, S.R.; Marrero, B.; Liu, Y.; DiMattia, M.A.; Zaal, K.J.M.; Sanchez, G.A.M.; Kim, H.; et al. An Activating NLRC4 Inflammasome Mutation Causes Autoinflammation with Recurrent Macrophage Activation Syndrome. Nat Genet 2014, 46, 1140–1146. [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-Associated Uric Acid Crystals Activate the NALP3 Inflammasome. Nature 2006, 440, 237–241. [CrossRef]
- Bossaller, L.; Chiang, P.-I.; Schmidt-Lauber, C.; Ganesan, S.; Kaiser, W.J.; Rathinam, V.A.K.; Mocarski, E.S.; Subramanian, D.; Green, D.R.; Silverman, N.; et al. Cutting Edge: FAS (CD95) Mediates Noncanonical IL-1β and IL-18 Maturation via Caspase-8 in an RIP3-Independent Manner. J Immunol 2012, 189, 5508–5512. [CrossRef]
- Akeda, T.; Yamanaka, K.; Tsuda, K.; Omoto, Y.; Gabazza, E.C.; Mizutani, H. CD8+ T Cell Granzyme B Activates Keratinocyte Endogenous IL-18. Arch Dermatol Res 2014, 306, 125–130. [CrossRef]
- Heilig, R.; Dick, M.S.; Sborgi, L.; Meunier, E.; Hiller, S.; Broz, P. The Gasdermin-D Pore Acts as a Conduit for IL-1β Secretion in Mice. Eur J Immunol 2018, 48, 584–592. [CrossRef]
- He, W.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D Is an Executor of Pyroptosis and Required for Interleukin-1β Secretion. Cell Res 2015, 25, 1285–1298. [CrossRef]
- Tapia, V.S.; Daniels, M.J.D.; Palazón-Riquelme, P.; Dewhurst, M.; Luheshi, N.M.; Rivers-Auty, J.; Green, J.; Redondo-Castro, E.; Kaldis, P.; Lopez-Castejon, G.; et al. The Three Cytokines IL-1β, IL-18, and IL-1α Share Related but Distinct Secretory Routes. J Biol Chem 2019, 294, 8325–8335. [CrossRef]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 Regulates Both Th1 and Th2 Responses. Annu Rev Immunol 2001, 19, 423–474. [CrossRef]
- Hosohara, K.; Ueda, H.; Kashiwamura, S.-I.; Yano, T.; Ogura, T.; Marukawa, S.; Okamura, H. Interleukin-18 Induces Acute Biphasic Reduction in the Levels of Circulating Leukocytes in Mice. Clin Diagn Lab Immunol 2002, 9, 777–783. [CrossRef]
- Novick, D.; Kim, S.H.; Fantuzzi, G.; Reznikov, L.L.; Dinarello, C.A.; Rubinstein, M. Interleukin-18 Binding Protein: A Novel Modulator of the Th1 Cytokine Response. Immunity 1999, 10, 127–136. [CrossRef]
- Ha, C.T.; Li, X.; Fu, D.; Xiao, M. Circulating IL-18 Binding Protein (IL-18BP) and IL-18 as Dual Biomarkers of Total-Body Irradiation in Mice. Radiat Res 2016, 185, 375–383. [CrossRef]
- Bufler, P.; Azam, T.; Gamboni-Robertson, F.; Reznikov, L.L.; Kumar, S.; Dinarello, C.A.; Kim, S.-H. A Complex of the IL-1 Homologue IL-1F7b and IL-18-Binding Protein Reduces IL-18 Activity. Proc Natl Acad Sci U S A 2002, 99, 13723–13728. [CrossRef]
- Prencipe, G.; Bracaglia, C.; De Benedetti, F. Interleukin-18 in Pediatric Rheumatic Diseases. Curr Opin Rheumatol 2019, 31, 421–427. [CrossRef]
- Harel, M.; Girard-Guyonvarc’h, C.; Rodriguez, E.; Palmer, G.; Gabay, C. Production of IL-18 Binding Protein by Radiosensitive and Radioresistant Cells in CpG-Induced Macrophage Activation Syndrome. J Immunol 2020, 205, 1167–1175. [CrossRef]
- Kaser, A.; Novick, D.; Rubinstein, M.; Siegmund, B.; Enrich, B.; Koch, R.O.; Vogel, W.; Kim, S.H.; Dinarello, C.A.; Tilg, H. Interferon-Alpha Induces Interleukin-18 Binding Protein in Chronic Hepatitis C Patients. Clin Exp Immunol 2002, 129, 332–338. [CrossRef]
- Michels, M.; de Mast, Q.; Netea, M.G.; Joosten, L.A.; Dinarello, C.A.; Rudiman, P.I.F.; Sinarta, S.; Wisaksana, R.; Alisjahbana, B.; van der Ven, A.J.A.M. Normal Free Interleukin-18 (IL-18) Plasma Levels in Dengue Virus Infection and the Need to Measure Both Total IL-18 and IL-18 Binding Protein Levels. Clin Vaccine Immunol 2015, 22, 650–655. [CrossRef]
- Chirathaworn, C.; Poovorawan, Y.; Lertmaharit, S.; Wuttirattanakowit, N. Cytokine Levels in Patients with Chikungunya Virus Infection. Asian Pac J Trop Med 2013, 6, 631–634. [CrossRef]
- Hoshino, K.; Tsutsui, H.; Kawai, T.; Takeda, K.; Nakanishi, K.; Takeda, Y.; Akira, S. Cutting Edge: Generation of IL-18 Receptor-Deficient Mice: Evidence for IL-1 Receptor-Related Protein as an Essential IL-18 Binding Receptor. J Immunol 1999, 162, 5041–5044.
- Tsutsumi, N.; Kimura, T.; Arita, K.; Ariyoshi, M.; Ohnishi, H.; Yamamoto, T.; Zuo, X.; Maenaka, K.; Park, E.Y.; Kondo, N.; et al. The Structural Basis for Receptor Recognition of Human Interleukin-18. Nat Commun 2014, 5, 5340. [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int J Mol Sci 2019, 20. [CrossRef]
- Kim, S.H.; Reznikov, L.L.; Stuyt, R.J.; Selzman, C.H.; Fantuzzi, G.; Hoshino, T.; Young, H.A.; Dinarello, C.A. Functional Reconstitution and Regulation of IL-18 Activity by the IL-18R Beta Chain. J Immunol 2001, 166, 148–154. [CrossRef]
- Wu, C.; Sakorafas, P.; Miller, R.; McCarthy, D.; Scesney, S.; Dixon, R.; Ghayur, T. IL-18 Receptor Beta-Induced Changes in the Presentation of IL-18 Binding Sites Affect Ligand Binding and Signal Transduction. J Immunol 2003, 170, 5571–5577. [CrossRef]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front Immunol 2013, 4, 289. [CrossRef]
- Suzuki, N.; Chen, N.-J.; Millar, D.G.; Suzuki, S.; Horacek, T.; Hara, H.; Bouchard, D.; Nakanishi, K.; Penninger, J.M.; Ohashi, P.S.; et al. IL-1 Receptor-Associated Kinase 4 Is Essential for IL-18-Mediated NK and Th1 Cell Responses. J Immunol 2003, 170, 4031–4035. [CrossRef]
- Kanakaraj, P.; Ngo, K.; Wu, Y.; Angulo, A.; Ghazal, P.; Harris, C.A.; Siekierka, J.J.; Peterson, P.A.; Fung-Leung, W.P. Defective Interleukin (IL)-18-Mediated Natural Killer and T Helper Cell Type 1 Responses in IL-1 Receptor-Associated Kinase (IRAK)-Deficient Mice. J Exp Med 1999, 189, 1129–1138. [CrossRef]
- Carroll, H.P.; Paunovic, V.; Gadina, M. Signalling, Inflammation and Arthritis: Crossed Signals: The Role of Interleukin-15 and -18 in Autoimmunity. Rheumatology 2008, 47, 1269–1277. [CrossRef]
- Puren, A.J.; Fantuzzi, G.; Gu, Y.; Su, M.S.; Dinarello, C.A. Interleukin-18 (IFNgamma-Inducing Factor) Induces IL-8 and IL-1beta via TNFalpha Production from Non-CD14+ Human Blood Mononuclear Cells. J Clin Invest 1998, 101, 711–721. [CrossRef]
- Robinson, D.; Shibuya, K.; Mui, A.; Zonin, F.; Murphy, E.; Sana, T.; Hartley, S.B.; Menon, S.; Kastelein, R.; Bazan, F.; et al. IGIF Does Not Drive Th1 Development but Synergizes with IL-12 for Interferon-Gamma Production and Activates IRAK and NFkappaB. Immunity 1997, 7, 571–581. [CrossRef]
- Murphy, J.E.; Robert, C.; Kupper, T.S. Interleukin-1 and Cutaneous Inflammation: A Crucial Link between Innate and Acquired Immunity. J Invest Dermatol 2000, 114, 602–608. [CrossRef]
- Nakanishi, K.; Yoshimoto, T.; Tsutsui, H.; Okamura, H. Interleukin-18 Is a Unique Cytokine That Stimulates Both Th1 and Th2 Responses Depending on Its Cytokine Milieu. Cytokine Growth Factor Rev 2001, 12, 53–72. [CrossRef]
- Hirooka, Y.; Nozaki, Y. Interleukin-18 in Inflammatory Kidney Disease. Front Med (Lausanne) 2021, 8, 639103. [CrossRef]
- Virtue, A.; Wang, H.; Yang, X. MicroRNAs and Toll-like Receptor/Interleukin-1 Receptor Signaling. J Hematol Oncol 2012, 5, 66. [CrossRef]
- Ricardo-Gonzalez, R.R.; Van Dyken, S.J.; Schneider, C.; Lee, J.; Nussbaum, J.C.; Liang, H.-E.; Vaka, D.; Eckalbar, W.L.; Molofsky, A.B.; Erle, D.J.; et al. Tissue Signals Imprint ILC2 Identity with Anticipatory Function. Nat Immunol 2018, 19, 1093–1099. [CrossRef]
- Leung, B.P.; Culshaw, S.; Gracie, J.A.; Hunter, D.; Canetti, C.A.; Campbell, C.; Cunha, F.; Liew, F.Y.; McInnes, I.B. A Role for IL-18 in Neutrophil Activation. J Immunol 2001, 167, 2879–2886. [CrossRef]
- Yoshimoto, T.; Tsutsui, H.; Tominaga, K.; Hoshino, K.; Okamura, H.; Akira, S.; Paul, W.E.; Nakanishi, K. IL-18, Although Antiallergic When Administered with IL-12, Stimulates IL-4 and Histamine Release by Basophils. Proc Natl Acad Sci U S A 1999, 96, 13962–13966. [CrossRef]
- Gracie, J.A.; Forsey, R.J.; Chan, W.L.; Gilmour, A.; Leung, B.P.; Greer, M.R.; Kennedy, K.; Carter, R.; Wei, X.Q.; Xu, D.; et al. A Proinflammatory Role for IL-18 in Rheumatoid Arthritis. J Clin Invest 1999, 104, 1393–1401. [CrossRef]
- Tucci, M.; Quatraro, C.; Dammacco, F.; Silvestris, F. Increased IL-18 Production by Dendritic Cells in Active Inflammatory Myopathies. Ann N Y Acad Sci 2007, 1107, 184–192. [CrossRef]
- Gutzmer, R.; Langer, K.; Mommert, S.; Wittmann, M.; Kapp, A.; Werfel, T. Human Dendritic Cells Express the IL-18R and Are Chemoattracted to IL-18. J Immunol 2003, 171, 6363–6371. [CrossRef]
- Airoldi, I.; Gri, G.; Marshall, J.D.; Corcione, A.; Facchetti, P.; Guglielmino, R.; Trinchieri, G.; Pistoia, V. Expression and Function of IL-12 and IL-18 Receptors on Human Tonsillar B Cells. J Immunol 2000, 165, 6880–6888. [CrossRef]
- Hu, B.; Ren, J.; Luo, Y.; Keith, B.; Young, R.M.; Scholler, J.; Zhao, Y.; June, C.H. Augmentation of Antitumor Immunity by Human and Mouse CAR T Cells Secreting IL-18. Cell Rep 2017, 20, 3025–3033. [CrossRef]
- Landy, E.; Varghese, J.; Dang, V.; Szymczak-Workman, A.; Kane, L.P.; Canna, S.W. Complementary HLH Susceptibility Factors Converge on CD8 T-Cell Hyperactivation. Blood Adv 2023, 7, 6949–6963. [CrossRef]
- Xu, D.; Trajkovic, V.; Hunter, D.; Leung, B.P.; Schulz, K.; Gracie, J.A.; McInnes, I.B.; Liew, F.Y. IL-18 Induces the Differentiation of Th1 or Th2 Cells Depending upon Cytokine Milieu and Genetic Background. Eur J Immunol 2000, 30, 3147–3156. [CrossRef]
- Chudnovskiy, A.; Mortha, A.; Kana, V.; Kennard, A.; Ramirez, J.D.; Rahman, A.; Remark, R.; Mogno, I.; Ng, R.; Gnjatic, S.; et al. Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome. Cell 2016, 167, 444-456.e14. [CrossRef]
- Hoshino, T.; Yagita, H.; Ortaldo, J.R.; Wiltrout, R.H.; Young, H.A. In Vivo Administration of IL-18 Can Induce IgE Production through Th2 Cytokine Induction and up-Regulation of CD40 Ligand (CD154) Expression on CD4+ T Cells. Eur J Immunol 2000, 30, 1998–2006. [CrossRef]
- Son, Y.I.; Dallal, R.M.; Mailliard, R.B.; Egawa, S.; Jonak, Z.L.; Lotze, M.T. Interleukin-18 (IL-18) Synergizes with IL-2 to Enhance Cytotoxicity, Interferon-Gamma Production, and Expansion of Natural Killer Cells. Cancer Res 2001, 61, 884–888.
- Yoshimoto, T.; Takeda, K.; Tanaka, T.; Ohkusu, K.; Kashiwamura, S.; Okamura, H.; Akira, S.; Nakanishi, K. IL-12 up-Regulates IL-18 Receptor Expression on T Cells, Th1 Cells, and B Cells: Synergism with IL-18 for IFN-Gamma Production. J Immunol 1998, 161, 3400–3407.
- Kanda, N.; Shimizu, T.; Tada, Y.; Watanabe, S. IL-18 Enhances IFN-Gamma-Induced Production of CXCL9, CXCL10, and CXCL11 in Human Keratinocytes. Eur J Immunol 2007, 37, 338–350. [CrossRef]
- Pourcet, B.; Gage, M.C.; León, T.E.; Waddington, K.E.; Pello, O.M.; Steffensen, K.R.; Castrillo, A.; Valledor, A.F.; Pineda-Torra, I. The Nuclear Receptor LXR Modulates Interleukin-18 Levels in Macrophages through Multiple Mechanisms. Sci Rep 2016, 6, 25481. [CrossRef]
- Wang, J.; Zhang, H.; Zheng, W.; Xie, H.; Yan, H.; Lin, X.; He, S. Correlation of IL-18 with Tryptase in Atopic Asthma and Induction of Mast Cell Accumulation by IL-18. Mediators Inflamm 2016, 2016, 4743176. [CrossRef]
- Companjen, A.R.; van der Velden, V.H.; Vooys, A.; Debets, R.; Benner, R.; Prens, E.P. Human Keratinocytes Are Major Producers of IL-18: Predominant Expression of the Unprocessed Form. Eur Cytokine Netw 2000, 11, 383–390.
- Cho, D.; Seung Kang, J.; Hoon Park, J.; Kim, Y.-I.; Hahm, E.; Lee, J.; Yang, Y.; Jeon, J.; Song, H.; Park, H.; et al. The Enhanced IL-18 Production by UVB Irradiation Requires ROI and AP-1 Signaling in Human Keratinocyte Cell Line (HaCaT). Biochem Biophys Res Commun 2002, 298, 289–295. [CrossRef]
- Koizumi, H.; Sato-Matsumura, K.C.; Nakamura, H.; Shida, K.; Kikkawa, S.; Matsumoto, M.; Toyoshima, K.; Seya, T. Distribution of IL-18 and IL-18 Receptor in Human Skin: Various Forms of IL-18 Are Produced in Keratinocytes. Arch Dermatol Res 2001, 293, 325–333. [CrossRef]
- Fenini, G.; Grossi, S.; Contassot, E.; Biedermann, T.; Reichmann, E.; French, L.E.; Beer, H.-D. Genome Editing of Human Primary Keratinocytes by CRISPR/Cas9 Reveals an Essential Role of the NLRP1 Inflammasome in UVB Sensing. J Invest Dermatol 2018, 138, 2644–2652. [CrossRef]
- Flier, J.; Boorsma, D.M.; Bruynzeel, D.P.; Van Beek, P.J.; Stoof, T.J.; Scheper, R.J.; Willemze, R.; Tensen, C.P. The CXCR3 Activating Chemokines IP-10, Mig, and IP-9 Are Expressed in Allergic but Not in Irritant Patch Test Reactions. J Invest Dermatol 1999, 113, 574–578. [CrossRef]
- Kim, H.J.; Song, S.B.; Choi, J.M.; Kim, K.M.; Cho, B.K.; Cho, D.H.; Park, H.J. IL-18 Downregulates Collagen Production in Human Dermal Fibroblasts via the ERK Pathway. J Invest Dermatol 2010, 130, 706–715. [CrossRef]
- Wang, B.; Feliciani, C.; Howell, B.G.; Freed, I.; Cai, Q.; Watanabe, H.; Sauder, D.N. Contribution of Langerhans Cell-Derived IL-18 to Contact Hypersensitivity. J Immunol 2002, 168, 3303–3308. [CrossRef]
- Chang, J.T.; Segal, B.M.; Nakanishi, K.; Okamura, H.; Shevach, E.M. The Costimulatory Effect of IL-18 on the Induction of Antigen-Specific IFN-Gamma Production by Resting T Cells Is IL-12 Dependent and Is Mediated by up-Regulation of the IL-12 Receptor Beta2 Subunit. Eur J Immunol 2000, 30, 1113–1119. [CrossRef]
- Sharaf, N.; Nicklin, M.J.; di Giovine, F.S. Long-Range DNA Interactions at the IL-1/IL-36/IL-37 Gene Cluster (2q13) Are Induced by Activation of Monocytes. Cytokine 2014, 68, 16–22. [CrossRef]
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 Regulatory Pathways in Cancer Progression and Therapy. Immunol Rev 2018, 281, 57–61. [CrossRef]
- Jia, H.; Liu, J.; Han, B. Reviews of Interleukin-37: Functions, Receptors, and Roles in Diseases. Biomed Res Int 2018, 2018, 3058640. [CrossRef]
- Su, Z.; Tao, X. Current Understanding of IL-37 in Human Health and Disease. Front Immunol 2021, 12, 696605. [CrossRef]
- Nold-Petry, C.A.; Lo, C.Y.; Rudloff, I.; Elgass, K.D.; Li, S.; Gantier, M.P.; Lotz-Havla, A.S.; Gersting, S.W.; Cho, S.X.; Lao, J.C.; et al. IL-37 Requires the Receptors IL-18Rα and IL-1R8 (SIGIRR) to Carry out Its Multifaceted Anti-Inflammatory Program upon Innate Signal Transduction. Nat Immunol 2015, 16, 354–365. [CrossRef]
- Pan, Y.; Wen, X.; Hao, D.; Wang, Y.; Wang, L.; He, G.; Jiang, X. The Role of IL-37 in Skin and Connective Tissue Diseases. Biomed Pharmacother 2020, 122, 109705. [CrossRef]
- Mei, Y.; Liu, H. IL-37: An Anti-Inflammatory Cytokine with Antitumor Functions. Cancer Rep (Hoboken) 2019, 2, e1151. [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 Is a Fundamental Inhibitor of Innate Immunity. Nat Immunol 2010, 11, 1014–1022. [CrossRef]
- Rudloff, I.; Cho, S.X.; Lao, J.C.; Ngo, D.; McKenzie, M.; Nold-Petry, C.A.; Nold, M.F. Monocytes and Dendritic Cells Are the Primary Sources of Interleukin 37 in Human Immune Cells. J Leukoc Biol 2017, 101, 901–911. [CrossRef]
- Zhou, J.; Gemperline, D.C.; Turner, M.J.; Oldach, J.; Molignano, J.; Sims, J.T.; Stayrook, K.R. Transcriptomic Analysis of Healthy and Atopic Dermatitis Samples Reveals the Role of IL-37 in Human Skin. Immunohorizons 2021, 5, 830–843. [CrossRef]
- Lachner, J.; Mlitz, V.; Tschachler, E.; Eckhart, L. Epidermal Cornification Is Preceded by the Expression of a Keratinocyte-Specific Set of Pyroptosis-Related Genes. Sci Rep 2017, 7, 17446. [CrossRef]
- Hou, T.; Tsang, M.S.-M.; Chu, I.M.-T.; Kan, L.L.-Y.; Hon, K.-L.; Leung, T.-F.; Lam, C.W.-K.; Wong, C.-K. Skewed Inflammation Is Associated with Aberrant Interleukin-37 Signaling Pathway in Atopic Dermatitis. Allergy 2021, 76, 2102–2114. [CrossRef]
- Guttman-Yassky, E.; Diaz, A.; Pavel, A.B.; Fernandes, M.; Lefferdink, R.; Erickson, T.; Canter, T.; Rangel, S.; Peng, X.; Li, R.; et al. Use of Tape Strips to Detect Immune and Barrier Abnormalities in the Skin of Children With Early-Onset Atopic Dermatitis. JAMA Dermatol 2019, 155, 1358–1370. [CrossRef]
- Fujita, H.; Inoue, Y.; Seto, K.; Komitsu, N.; Aihara, M. Interleukin-37 Is Elevated in Subjects with Atopic Dermatitis. J Dermatol Sci 2013, 69, 173–175. [CrossRef]
- Tsuji, G.; Yamamura, K.; Kawamura, K.; Kido-Nakahara, M.; Ito, T.; Nakahara, T. Regulatory Mechanism of the IL-33-IL-37 Axis via Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int J Mol Sci 2023, 24. [CrossRef]
- Yan, X.; Tsuji, G.; Hashimoto-Hachiya, A.; Furue, M. Galactomyces Ferment Filtrate Potentiates an Anti-Inflammaging System in Keratinocytes. J Clin Med 2022, 11. [CrossRef]
- Mesjasz, A.; Trzeciak, M.; Gleń, J.; Jaskulak, M. Potential Role of IL-37 in Atopic Dermatitis. Cells 2023, 12. [CrossRef]
- Ewald, D.A.; Malajian, D.; Krueger, J.G.; Workman, C.T.; Wang, T.; Tian, S.; Litman, T.; Guttman-Yassky, E.; Suárez-Fariñas, M. Meta-Analysis Derived Atopic Dermatitis (MADAD) Transcriptome Defines a Robust AD Signature Highlighting the Involvement of Atherosclerosis and Lipid Metabolism Pathways. BMC Med Genomics 2015, 8, 60. [CrossRef]
- Schröder, A.; Lunding, L.P.; Zissler, U.M.; Vock, C.; Webering, S.; Ehlers, J.C.; Orinska, Z.; Chaker, A.; Schmidt-Weber, C.B.; Lang, N.J.; et al. IL-37 Regulates Allergic Inflammation by Counterbalancing pro-Inflammatory IL-1 and IL-33. Allergy 2022, 77, 856–869. [CrossRef]
- Ahmed, M.B.; Ad’hiah, A.H. Reduced Levels of Interleukin-37 in Serum of Patients with Allergic Rhinitis or Asthma. Rev Fr Allergol 2021, 61, 410–414. [CrossRef]
- Shilovskiy, I.P.; Dyneva, M.E.; Kurbacheva, O.M.; Kudlay, D.A.; Khaitov, M.R. The Role of Interleukin-37 in the Pathogenesis of Allergic Diseases. Acta Naturae 2019, 11, 54–64. [CrossRef]
- Wang, J.; Shen, Y.; Li, C.; Liu, C.; Wang, Z.-H.; Li, Y.-S.; Ke, X.; Hu, G.-H. IL-37 Attenuates Allergic Process via STAT6/STAT3 Pathways in Murine Allergic Rhinitis. Int Immunopharmacol 2019, 69, 27–33. [CrossRef]
- Zeng, H.; Zhou, K.; Ye, Z. Biology of Interleukin-37 and Its Role in Autoimmune Diseases (Review). Exp Ther Med 2022, 24, 495. [CrossRef]
- Mao, X.; Zhu, R.; Zhang, F.; Zhong, Y.; Yu, K.; Wei, Y.; Sun, H.; Xu, W.; Luo, Q.; Wang, Y.; et al. IL-37 Plays a Beneficial Role in Patients with Acute Coronary Syndrome. Mediators Inflamm 2019, 2019, 9515346. [CrossRef]
- Ye, L.; Jiang, B.; Deng, J.; Du, J.; Xiong, W.; Guan, Y.; Wen, Z.; Huang, K.; Huang, Z. IL-37 Alleviates Rheumatoid Arthritis by Suppressing IL-17 and IL-17-Triggering Cytokine Production and Limiting Th17 Cell Proliferation. J Immunol 2015, 194, 5110–5119. [CrossRef]
- Wang, D.-W.; Dong, N.; Wu, Y.; Zhu, X.-M.; Wang, C.-T.; Yao, Y.-M. Interleukin-37 Enhances the Suppressive Activity of Naturally Occurring CD4+CD25+ Regulatory T Cells. Sci Rep 2016, 6, 38955. [CrossRef]
- Shuai, X.; Wei-min, L.; Tong, Y.; Dong, N.; Sheng, Z.; Yao, Y. Expression of IL-37 Contributes to the Immunosuppressive Property of Human CD4+CD25+ Regulatory T Cells. Sci Rep 2015, 5, 14478. [CrossRef]
- Hou, T.; Sun, X.; Zhu, J.; Hon, K.-L.; Jiang, P.; Chu, I.M.-T.; Tsang, M.S.-M.; Lam, C.W.-K.; Zeng, H.; Wong, C.-K. IL-37 Ameliorating Allergic Inflammation in Atopic Dermatitis Through Regulating Microbiota and AMPK-MTOR Signaling Pathway-Modulated Autophagy Mechanism. Front Immunol 2020, 11, 752. [CrossRef]
- Zhou, P.; Li, Q.; Su, S.; Dong, W.; Zong, S.; Ma, Q.; Yang, X.; Zuo, D.; Zheng, S.; Meng, X.; et al. Interleukin 37 Suppresses M1 Macrophage Polarization Through Inhibition of the Notch1 and Nuclear Factor Kappa B Pathways. Front Cell Dev Biol 2020, 8, 56. [CrossRef]
- Luo, Y.; Cai, X.; Liu, S.; Wang, S.; Nold-Petry, C.A.; Nold, M.F.; Bufler, P.; Norris, D.; Dinarello, C.A.; Fujita, M. Suppression of Antigen-Specific Adaptive Immunity by IL-37 via Induction of Tolerogenic Dendritic Cells. Proc Natl Acad Sci U S A 2014, 111, 15178–15183. [CrossRef]
- Liu, T.; Liu, J.; Lin, Y.; Que, B.; Chang, C.; Zhang, J.; Liang, Z.; Gao, X.; Liu, S.; Liu, L.; et al. IL-37 Inhibits the Maturation of Dendritic Cells through the IL-1R8-TLR4-NF-ΚB Pathway. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 1338–1349. [CrossRef]
- Liu, F.-T.; Goodarzi, H.; Chen, H.-Y. IgE, Mast Cells, and Eosinophils in Atopic Dermatitis. Clin Rev Allergy Immunol 2011, 41, 298–310. [CrossRef]
- Hou, T.; Tsang, M.S.-M.; Kan, L.L.-Y.; Li, P.; Chu, I.M.-T.; Lam, C.W.-K.; Wong, C.-K. IL-37 Targets TSLP-Primed Basophils to Alleviate Atopic Dermatitis. Int J Mol Sci 2021, 22. [CrossRef]
- Mali, S.S.; Bautista, D.M. Basophils Add Fuel to the Flame of Eczema Itch. Cell 2021, 184, 294–296. [CrossRef]
- Yamanishi, Y.; Mogi, K.; Takahashi, K.; Miyake, K.; Yoshikawa, S.; Karasuyama, H. Skin-Infiltrating Basophils Promote Atopic Dermatitis-like Inflammation via IL-4 Production in Mice. Allergy 2020, 75, 2613–2622. [CrossRef]
- Saluja, R.; Khan, M.; Church, M.K.; Maurer, M. The Role of IL-33 and Mast Cells in Allergy and Inflammation. Clin Transl Allergy 2015, 5, 33. [CrossRef]
- Li, W.; Ding, F.; Zhai, Y.; Tao, W.; Bi, J.; Fan, H.; Yin, N.; Wang, Z. IL-37 Is Protective in Allergic Contact Dermatitis through Mast Cell Inhibition. Int Immunopharmacol 2020, 83, 106476. [CrossRef]
- Kader, H.A.; Azeem, M.; Jwayed, S.A.; Al-Shehhi, A.; Tabassum, A.; Ayoub, M.A.; Hetta, H.F.; Waheed, Y.; Iratni, R.; Al-Dhaheri, A.; et al. Current Insights into Immunology and Novel Therapeutics of Atopic Dermatitis. Cells 2021, 10. [CrossRef]
- Lei, H.; Sun, Y.; Quan, S. IL-37 Relieves Allergic Inflammation by Inhibiting the CCL11 Signaling Pathway in a Mouse Model of Allergic Rhinitis. Exp Ther Med 2020, 20, 3114–3121. [CrossRef]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum Levels of TNF-Alpha, IFN-Gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in Patients with Active Psoriasis and Correlation with Disease Severity. Mediators Inflamm 2005, 2005, 273–279. [CrossRef]
- Forouzandeh, M.; Besen, J.; Keane, R.W.; de Rivero Vaccari, J.P. The Inflammasome Signaling Proteins ASC and IL-18 as Biomarkers of Psoriasis. Front Pharmacol 2020, 11, 1238. [CrossRef]
- Gangemi, S.; Merendino, R.A.; Guarneri, F.; Minciullo, P.L.; DiLorenzo, G.; Pacor, M.; Cannavò, S.P. Serum Levels of Interleukin-18 and s-ICAM-1 in Patients Affected by Psoriasis: Preliminary Considerations. J Eur Acad Dermatol Venereol 2003, 17, 42–46. [CrossRef]
- Flisiak, I.; Klepacki, A.; Chodynicka, B. Plasma and Scales Levels of Interleukin 18 in Comparison with Other Possible Clinical and Laboratory Biomarkers of Psoriasis Activity. Biomarkers 2006, 11, 194–200. [CrossRef]
- Verma, D.; Fekri, S.Z.; Sigurdardottir, G.; Bivik Eding, C.; Sandin, C.; Enerbäck, C. Enhanced Inflammasome Activity in Patients with Psoriasis Promotes Systemic Inflammation. J Invest Dermatol 2021, 141, 586-595.e5. [CrossRef]
- Niu, X.-L.; Huang, Y.; Gao, Y.-L.; Sun, Y.-Z.; Han, Y.; Chen, H.-D.; Gao, X.-H.; Qi, R.-Q. Interleukin-18 Exacerbates Skin Inflammation and Affects Microabscesses and Scale Formation in a Mouse Model of Imiquimod-Induced Psoriasis. Chin Med J (Engl) 2019, 132, 690–698. [CrossRef]
- Shimoura, N.; Nagai, H.; Fujiwara, S.; Jimbo, H.; Yoshimoto, T.; Nishigori, C. Interleukin (IL)-18, Cooperatively with IL-23, Induces Prominent Inflammation and Enhances Psoriasis-like Epidermal Hyperplasia. Arch Dermatol Res 2017, 309, 315–321. [CrossRef]
- Zhang, W.; Guo, S.; Li, B.; Liu, L.; Ge, R.; Cao, T.; Wang, H.; Gao, T.; Wang, G.; Li, C. Proinflammatory Effect of High-Mobility Group Protein B1 on Keratinocytes: An Autocrine Mechanism Underlying Psoriasis Development. J Pathol 2017, 241, 392–404. [CrossRef]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-Associated Uric Acid Crystals Activate the NALP3 Inflammasome. Nature 2006, 440, 237–241. [CrossRef]
- Kou, K.; Aihara, M.; Matsunaga, T.; Chen, H.; Taguri, M.; Morita, S.; Fujita, H.; Yamaguchi, Y.; Kambara, T.; Ikezawa, Z. Association of Serum Interleukin-18 and Other Biomarkers with Disease Severity in Adults with Atopic Dermatitis. Arch Dermatol Res 2012, 304, 305–312. [CrossRef]
- Lyubchenko, T.; Collins, H.K.; Goleva, E.; Leung, D.Y.M. Skin Tape Sampling Technique Identifies Proinflammatory Cytokines in Atopic Dermatitis Skin. Ann Allergy Asthma Immunol 2021, 126, 46-53.e2. [CrossRef]
- Andersson, A.M.; Sølberg, J.; Koch, A.; Skov, L.; Jakasa, I.; Kezic, S.; Thyssen, J.P. Assessment of Biomarkers in Pediatric Atopic Dermatitis by Tape Strips and Skin Biopsies. Allergy 2022, 77, 1499–1509. [CrossRef]
- Inoue, Y.; Aihara, M.; Kirino, M.; Harada, I.; Komori-Yamaguchi, J.; Yamaguchi, Y.; Nagashima, Y.; Ikezawa, Z. Interleukin-18 Is Elevated in the Horny Layer in Patients with Atopic Dermatitis and Is Associated with Staphylococcus Aureus Colonization. Br J Dermatol 2011, 164, 560–567. [CrossRef]
- McAleer, M.A.; Jakasa, I.; Hurault, G.; Sarvari, P.; McLean, W.H.I.; Tanaka, R.J.; Kezic, S.; Irvine, A.D. Systemic and Stratum Corneum Biomarkers of Severity in Infant Atopic Dermatitis Include Markers of Innate and T Helper Cell-Related Immunity and Angiogenesis. Br J Dermatol 2019, 180, 586–596. [CrossRef]
- Konishi, H.; Tsutsui, H.; Murakami, T.; Yumikura-Futatsugi, S.; Yamanaka, K.-I.; Tanaka, M.; Iwakura, Y.; Suzuki, N.; Takeda, K.; Akira, S.; et al. IL-18 Contributes to the Spontaneous Development of Atopic Dermatitis-like Inflammatory Skin Lesion Independently of IgE/Stat6 under Specific Pathogen-Free Conditions. Proc Natl Acad Sci U S A 2002, 99, 11340–11345. [CrossRef]
- Chen, J.-L.; Niu, X.-L.; Gao, Y.-L.; Ma, L.; Gao, X.-H.; Chen, H.-D.; Qi, R.-Q. IL-18 Knockout Alleviates Atopic Dermatitis-like Skin Lesions Induced by MC903 in a Mouse Model. Int J Mol Med 2020, 46, 880–888. [CrossRef]
- Yoshimoto, T.; Mizutani, H.; Tsutsui, H.; Noben-Trauth, N.; Yamanaka, K.; Tanaka, M.; Izumi, S.; Okamura, H.; Paul, W.E.; Nakanishi, K. IL-18 Induction of IgE: Dependence on CD4+ T Cells, IL-4 and STAT6. Nat Immunol 2000, 1, 132–137. [CrossRef]
- Luo, Y.; Cai, X.; Liu, S.; Wang, S.; Nold-Petry, C.A.; Nold, M.F.; Bufler, P.; Norris, D.; Dinarello, C.A.; Fujita, M. Suppression of Antigen-Specific Adaptive Immunity by IL-37 via Induction of Tolerogenic Dendritic Cells. Proc Natl Acad Sci U S A 2014, 111, 15178–15183. [CrossRef]
- Li, B.; Tsoi, L.C.; Swindell, W.R.; Gudjonsson, J.E.; Tejasvi, T.; Johnston, A.; Ding, J.; Stuart, P.E.; Xing, X.; Kochkodan, J.J.; et al. Transcriptome Analysis of Psoriasis in a Large Case-Control Sample: RNA-Seq Provides Insights into Disease Mechanisms. J Invest Dermatol 2014, 134, 1828–1838. [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int J Mol Sci 2020, 21. [CrossRef]
- Dai, X.; Shiraishi, K.; Muto, J.; Utsunomiya, R.; Mori, H.; Murakami, M.; Sayama, K. Nuclear IL-33 Plays an Important Role in IL-31‒Mediated Downregulation of FLG, Keratin 1, and Keratin 10 by Regulating Signal Transducer and Activator of Transcription 3 Activation in Human Keratinocytes. J Invest Dermatol 2022, 142, 136-144.e3. [CrossRef]
- Dai, X.; Utsunomiya, R.; Shiraishi, K.; Mori, H.; Muto, J.; Murakami, M.; Sayama, K. Nuclear IL-33 Plays an Important Role in the Suppression of FLG, LOR, Keratin 1, and Keratin 10 by IL-4 and IL-13 in Human Keratinocytes. J Invest Dermatol 2021, 141, 2646-2655.e6. [CrossRef]
- Zeng, F.; Chen, H.; Chen, L.; Mao, J.; Cai, S.; Xiao, Y.; Li, J.; Shi, J.; Li, B.; Xu, Y.; et al. An Autocrine Circuit of IL-33 in Keratinocytes Is Involved in the Progression of Psoriasis. J Invest Dermatol 2021, 141, 596-606.e7. [CrossRef]
- Teng, X.; Hu, Z.; Wei, X.; Wang, Z.; Guan, T.; Liu, N.; Liu, X.; Ye, N.; Deng, G.; Luo, C.; et al. IL-37 Ameliorates the Inflammatory Process in Psoriasis by Suppressing Proinflammatory Cytokine Production. J Immunol 2014, 192, 1815–1823. [CrossRef]
- Rønholt, K.; Nielsen, A.L.-L.; Johansen, C.; Vestergaard, C.; Fauerbye, A.; López-Vales, R.; Dinarello, C.A.; Iversen, L. IL-37 Expression Is Downregulated in Lesional Psoriasis Skin. Immunohorizons 2020, 4, 754–761. [CrossRef]
- Krueger, J.; Clark, J.D.; Suárez-Fariñas, M.; Fuentes-Duculan, J.; Cueto, I.; Wang, C.Q.; Tan, H.; Wolk, R.; Rottinghaus, S.T.; Whitley, M.Z.; et al. Tofacitinib Attenuates Pathologic Immune Pathways in Patients with Psoriasis: A Randomized Phase 2 Study. J Allergy Clin Immunol 2016, 137, 1079–1090. [CrossRef]
- Tak, P.P.; Bacchi, M.; Bertolino, M. Pharmacokinetics of IL-18 Binding Protein in Healthy Volunteers and Subjects with Rheumatoid Arthritis or Plaque Psoriasis. Eur J Drug Metab Pharmacokinet 2006, 31, 109–116. [CrossRef]
- Leung K 99mTc-Interleukin-18-Binding Protein-Fc-Interlukin-1 Receptor Antagonist Available online: https://www.ncbi.nlm.nih.gov/books/NBK138562/ (accessed on 12 May 2024).
- Liu, Z.; Wyffels, L.; Barber, C.; Wan, L.; Xu, H.; Hui, M.M.; Furenlid, L.R.; Woolfenden, J.M. Characterization of 99mTc-Labeled Cytokine Ligands for Inflammation Imaging via TNF and IL-1 Pathways. Nucl Med Biol 2012, 39, 905–915. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




