1. Introduction
Epithelial ovarian cancer (EOC) is the fifth leading cause of cancer-related deaths in women, with an estimated 22,530 new cases and 13,980 deaths reported in the US in 2019 [
1]. The main treatments for advanced EOC are surgical cytoreduction and primary debulking surgery (PDS), followed by platinum-based systemic chemotherapy. Additionally, neoadjuvant chemotherapy (NAC) followed by interval debulking surgery (IDS) is a treatment modality for patients with inoperable EOC. Despite presenting a good response to initial chemotherapy, approximately 70% of EOCs recur, with recurrent EOC having a poor prognosis.
The accumulation of gene mutations causes the expression of tumor antigens, which trigger immune responses against cancer cells. The stromal tissue and infiltrated immune cells surrounding the tumor cells are defined as the tumor microenvironment (TME), while the lymphocyte infiltration in the TME is recognized as tumor-infiltrating lymphocytes (TILs), which mainly comprise T cells. TILs are a prognostic factor in ovarian cancers, with CD3+ tumor-infiltrating T cells being correlated with improved progression-free survival (PFS) and overall survival (OS) [
2,
3,
4]. Moreover, higher CD8/4 and CD8/Treg cell ratios were also associated with improved OS [
5]. However, cancer cells can escape immune surveillance through various mechanisms, including immune checkpoints such as PD-1 and PD-L1. Immune checkpoints are inhibitory receptors on immune cell membranes, and the expression of immune checkpoint receptors negatively regulates immune responses. In the TME, the expression of immune checkpoint receptors becomes dysregulated, evading an antitumor immune response [
6], and the expression of PD-L1 ligands on tumor cells suppresses lymphocyte infiltration [
7].
In addition to lymph nodes and the spleen, the accumulation of B cells with follicular structures in tumor tissues has recently been shown as important for antitumor immunity [
8]. Such accumulation of B cells with follicular structures in intratumoral tissues is called a tertiary lymphoid structure (TLS), which contains plasma cells, follicular helper T cells, follicular dendritic cells, and germinal centers [
9]. The relationship between TLS and prognosis in patients with EOC has also been investigated. TLS is associated with good prognosis in ovarian high-grade serous carcinoma (HGSC) [
10,
11,
12,
13]. Furthermore, the presence of TLS is correlated with inflammatory cell infiltration [
11]; a high density of CD138+ plasma cells in TLS is associated with favorable prognosis [
10]; and the TLS is matured by CXCL13 and is correlated with PFS [
13]. However, although chemotherapy can lead to tumor inflammatory cell infiltration and necrosis, which may affect the immune system in the TME, the prognostic value of TLS has not been compared between cases of PDS and NAC-IDS, and the significance of TLS in non-HGSC histologic types has also not been studied. Therefore, we examined TLS in tumor tissues collected during PDS and IDS for patients with EOC, including those with non-HGSC histology, with the aim to evaluate its prognostic significance.
2. Materials and Methods
2.1. Patients
This retrospective study evaluated the correlation between TLS and prognosis in patients with EOC treated at a hospital in Japan. The study was approved by the hospital Institutional Review Board (Approval number: 2020-414) and was conducted according to the Declaration of Helsinki.
We reviewed patients with newly diagnosed stage III-IV ovarian, tubal, and peritoneal carcinomas who underwent surgical treatment and chemotherapy in our hospital between April 1, 2017 and March 31, 2020. All patients were pathologically diagnosed and classified according to the International Federation of Gynecology and Obstetrics (FIGO) 2014 classification.
The following clinical data were collected from electronic medical records: age, clinical history, history of the disease, clinical stage, tumor markers, presence or absence of BRCA1/2 mutations, type of surgical treatment and chemotherapy, date of treatment initiation and last administration of chemotherapy, number of chemotherapy cycles, response to chemotherapy, presence or absence of recurrence and the date of diagnosis if present, presence or absence of death, and date of death or last confirmed survival. The data cutoff date was March 31, 2020. The clinical efficacy of NAC on tumor shrinkage was assessed in patients who received NAC followed by IDS according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.
2.2. Pathological Assessment
Specimens were collected from patients who underwent PDS, IDS, or biopsy. Surgical specimens from patients treated with PDS were analyzed. Of these tissue specimens, sections in which tumor cells and immune cells could be appropriately observed were selected after discussion between the investigator (T.N.) and a pathologist (T.T.). In patients who underwent NAC followed by IDS, histological analyses were performed using IDS samples because pretreatment biopsy specimens could not be evaluated owing to insufficient tumor volume. The following histopathological findings were extracted: pathological stage, final diagnosis, tumor size and extent, presence or absence of vascular invasion, evaluation of surgical margins, and evaluation of pathological residual cancer cells.
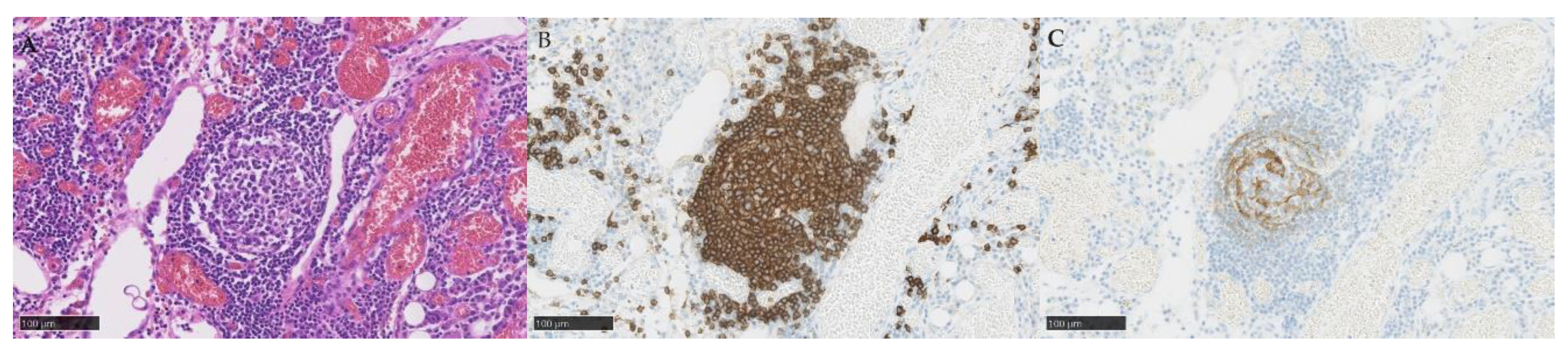
TLSs and TILs were diagnosed by hematoxylin and eosin (HE) staining and immunohistochemistry staining (IHC) with CD3 antibody (790-4341, Roche, Switzerland), CD4 (790-4423, Roche, Switzerland), CD8 (790-4460, Roche, Switzerland), CD20 (760-2591, Roche, Switzerland), CD21 (760-4245, Roche, Switzerland), CD25 (760-4439, Roche, Switzerland), and FOXP3 (ab20034, Abcam, UK). TILs and TLSs were counted manually by an investigator (T. N.) and confirmed by a pathologist (T. T.).
2.3. Diagnostic Criteria for TLSs and TILs
The TLS was defined as a cluster of CD20+ cells with the presence of CD21+ cells in the embryonic center. Three independent sites (20× objective field of view, 5 mm
2 total sites) that contained the most intratumoral TLS were selected [
14]. TILs were defined as the infiltration of CD4+ or CD8+ T cells. Three independent sites (20× objective field of view, total site 5 mm
2) with the most tumor infiltration by lymphocytic cells, excluding the TLS, were selected. CD4+ and CD8+ cells, as well as CD3+ and FOXP3+ cells, were examined. The numbers of total lymphocytes and CD3+, CD4+, CD8+, and FOXP3+ cells at the selected sites were counted. In addition, the CD8/CD4 ratio and CD8/FOXP-3 ratio/mm
2 were calculated [
3,
4,
5]. The CD3+ cells, CD8/CD4 ratio, and CD8/FOXP-3 ratio/mm
2 were divided into two groups based on high (≥ median) or low (< median) expression.
2.4. Diagnostic Criteria for PD-L1
PD-L1 positivity was diagnosed using the PD-L1 IHC 22C3 Pharm Dako (Agilent, USA) assay [
15]. The combined positive score (CPS) was defined as the number of PD-L1–stained cells (including tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells and multiplied by 100, with a score cutoff of ≥1. Cells in which the cell membrane was partially or wholly PD-L1 stained were judged as positive. Stained cells were defined as CPS positive if more than 1% of the cells were stained and CPS negative if less than 1% were stained.
2.5. Statistical Analysis
PFS was defined as the time from treatment initiation to disease progression or death from any cause. Progressive disease was determined based on clinical symptoms or radiographic findings and not solely on CA-125 elevation. OS was measured from the date of treatment initiation to the date of death from any cause. The frequency of TIL and TLS expression and presence or absence of PD-L1 expression were classified, and PFS and OS were examined using the Kaplan–Meier method with the log-rank test. The correlation between TLS and PD-L1 expression was analyzed using Pearson’s chi-squared test. All statistical analyses were performed using Bell Curve for Excel version 4.01 (Social Survey Research Information Co., Ltd., Japan) [
16] and graphing was performed using Microsoft Excel 2019 (Microsoft Co., Ltd., US). A p value of <0.05 was considered statistically significant.
3. Results
A total of 30 patients with advanced ovarian or peritoneal cancers were included in this study; among them, 16 patients were treated with PDS and 14 patients were treated with NAC followed by IDS. Two patients underwent biopsy only and did not undergo IDS. Because biopsy samples from these two patients were insufficient for pathological evaluation, both were excluded from the study (
Figure S1). Ultimately, 28 patients were included. The patient characteristics and clinical outcomes are shown in
Table 1. Overall, 35 patients (85.7%) had ovarian cancer and 4 (14.3%) patients had peritoneal cancer. Only 1 patient had a germline
BRCA1 mutation, and 12 (42.9%) patients were not tested for
BRCA1/2 mutations. The histological type was HGSC in 21 patients (75.0%), endometrioid carcinoma in 3 patients (10.7%), and other types in 4 patients (14.2%). Overall, 24 patients (85.7%) received dose-dense paclitaxel and carboplatin (TC) chemotherapy and 2 patients (7.1%) received triweekly TC. All patients completed the scheduled six cycles of chemotherapy. Among the patients who received PDS, 11 (73.3%) patients achieved complete surgery with no residual gross tumor, while 4 (26.7%) patients had residual disease with PDS treatment. Among the patients who received NAC, 1 (7.6%) and 11 (92.3%) patients achieved complete and partial responses, respectively, and 1 (7.6%) patient developed progressive disease after NAC. In total, 10 patients (84.6%) underwent complete surgery, and 3 patients (20%) had residual disease after IDS.
The results of the pathological analyses are presented in
Table 2. A total of 28 specimens were obtained, among which 3 specimens were excluded because of insufficient diagnosis, resulting in 25 evaluable specimens. Of the 25 specimens, 15 specimens were obtained during PDS and 10 specimens during NAC. A total of 9 specimens (36.0%) were obtained from the ovary, 13 (52.0%) specimens from the omentum, and 3 (12.0%) specimens from the peritoneum. The presence of TLS was confirmed in 13 (52.0%) specimens, and the median number of TLS per 5 mm
2 total sites was 1 (range, 0–10). Typical histopathological images of TLS are shown in
Figure 1. The median number of TLS was one, and the number of TLS did not differ by histological type. The presence or absence of TLS (at least one was present) did not differ by histological type. In TIL analysis, the median number of CD3+ cells was 227 per mm
2 and 296 per mm
2, the median CD8/4 ratios were 0.660 and 1.71, and the median CD8/FOXP-3 ratios were 7.02 and 7.06 for the PDS and IDS groups, respectively. In total, 13 (52.0%) specimens were PD-L1 positive and 12 (48.0%) specimens were negative. CPS did not differ by histological type.
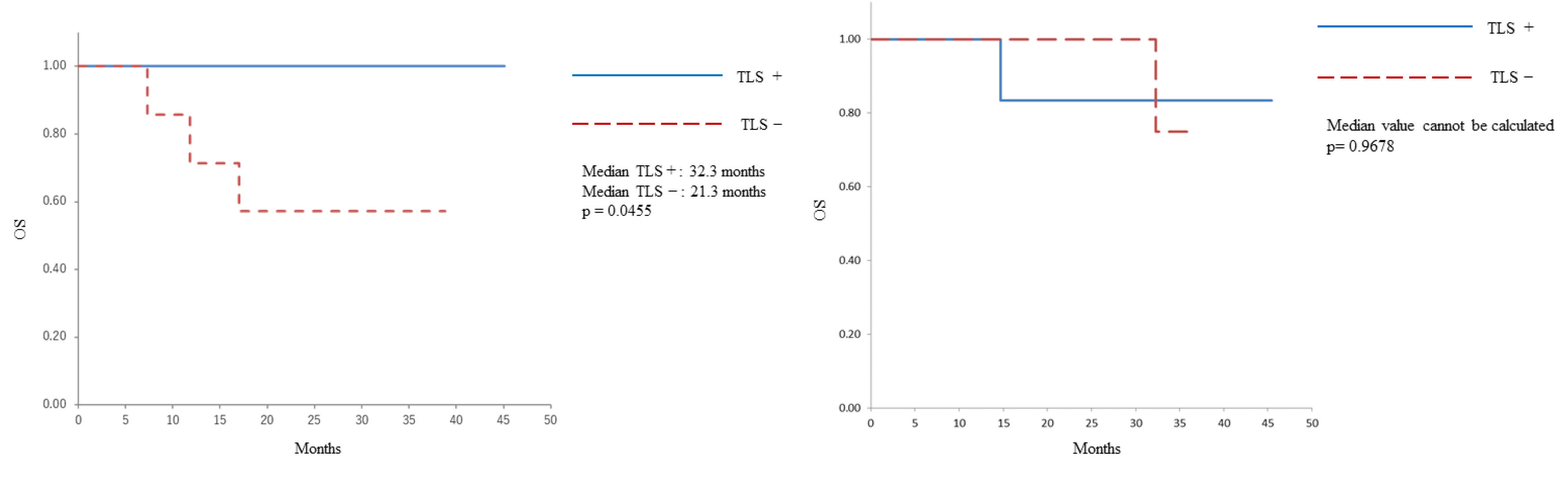
The Kaplan–Meier OS curve is shown in
Figure 1. In patients who underwent PDS, those with a TLS-positive status had a longer OS than those with a TLS-negative status (median: 32.3 months vs 21.3 months, p=0.0455). Conversely, no correlation was identified between the presence of TLS and OS in patients who underwent IDS (median: could not be calculated, p=0.9678).
The Kaplan–Meier curve for the PFS of the patients is shown in
Figure S2. No correlation was found between the presence of TLS and PFS in patients who underwent PDS (median: 31.1 months vs 17.3 months, p=0.3970). Additionally, no correlation was identified between the presence of TLS and PFS in patients who underwent IDS (median: 28.7 months for with TLS vs 16.3 months for median without TLS, p=0.1653).
The correlations between the cytologic characteristics of TILs and OS or PFS were observed, as shown in
Table 3. No molecular characteristics of TILs were correlated with OS or PFS in patients who underwent PDS or NAC-IDS.
The correlations between TLS and PD-L1 positivity are shown in
Table 4. The presence of TLS was associated with PD-L1 positivity (p=0.0094).
4. Discussion
The results of this study indicated that the presence of TLS in chemotherapy-naïve patients with ovarian cancer was significantly associated with longer OS, but not in patients who received NAC, and that the presence of TLS was associated with PD-L1 positivity.
TLS and TIL contribute to tumor immunity in the TME and are related to a favorable prognosis in various cancers. Increased numbers of follicular helper T cells, follicular B cells, and mature dendritic cells in the TLS are correlated with longer OS in non-small cell lung cancer, pancreatic ductal cancer, and triple-negative breast cancer [
14,
17,
18,
19,
20,
21,
22]. Additionally, the presence of TLS is related to good responses to immunotherapy in sarcoma, metastatic melanoma, and renal cell carcinoma [
23,
24,
25]. In ovarian cancer, the presence of TLS is also associated with good prognosis in HGSC [
10,
11,
12,
13]. Histological types other than HGSC have not been adequately studied. The addition of the histological types of low-grade serous carcinoma and clear cell carcinoma (CCC), which have not been reported previously, is significant for the analysis. Particularly, CCC is highly frequent in Japan, and its inclusion in the analysis is noteworthy. The present study found no correlation between histological type and the number of TLS. The presence of TLS was associated with longer OS in patients with advanced ovarian cancer, including those with non-HGSC histology. In contrast to previous studies, we found no association between TLS and PFS, which may be because of the limited number of patients in the study and/or the inclusion of many patients with early-stage cancer and non-HGSC histology.
The number of TLS in this study was lower than that in other cancer types, such as lung and breast cancers [
26,
27]. This may be because EOC is considered a “cold tumor” with more limited response to immunotherapy compared to lung or breast cancers. However, previous reports have suggested that TLS was correlated with inflammatory cell infiltration, suggesting that CXCL13 promoted TLS maturation and that TLS promoted CD8+ T cell infiltration in ovarian HGSCs [
10,
11,
12]. Furthermore, TLS is considered to be involved in the systematic development of cellular and humoral immunity. Our current finding that the presence of even one TLS is associated with favorable clinical outcomes in ovarian cancer, a cold tumor, is of great interest.
Previous studies have focused on PDS tissue [
10,
11,
12,
13]. To our best knowledge, this is the first study to examine differences in the prognostic significance of TLS between PDS tumors and NAC-IDS tumors. In contrast to the findings in the PDS group, the presence of TLS in the IDS group did not correlate with prognosis. In non-small cell lung cancer, corticosteroids impair germinal center formation in TLS [
12]. Therefore, corticosteroid administration during ovarian cancer chemotherapy may alter TLS development. The constituent cells of TLS are prognostically important, and their composition may be altered by immunotherapy [
28,
29]. Similarly, TLS maturation may be impaired by NAC in ovarian cancer, which could explain the differential prognostic significance between the PDS and IDS groups in the present study.
This study found that TIL levels were not correlated with prognosis. CD3+ T cells, the CD8/4 cell ratio, and the CD8 /FOXP3 ratio are associated with prognosis in ovarian cancer [
2,
3,
4,
5]. Both TIL and TLS induce lymphocytic infiltration via high endothelial venules (HEVs) and are considered favorable prognostic factors [
21,
30]. However, the prognostic significance of the presence of CD20+ B cells and CD3+ T cells via the HEV has been conflicting [
31]. The mechanism underlying the correlation between TIL and TLS warrants clarification.
The present study revealed a correlation between the TLS and PD-L1 expression. PD-L1 expression is one of the most promising predictive markers of immune checkpoint inhibitor efficacy in multiple cancer types. Further, a positive correlation between TLS density and efficacy of immune checkpoint inhibitors has been demonstrated in non-small-cell lung cancer and bladder cancer [
32,
33]. Although immune checkpoint inhibitors have also received attention in ovarian cancer through the KEYNOTE 100 trial, the modest 7.4–9.9% response rates to pembrolizumab in previously treated recurrent ovarian cancer cases [
34] highlights the need for reliable response-predictive markers. Our results indicate that TLS may also contribute to an antitumor immune function in ovarian cancer, and its significance as a response-predictive marker for immune checkpoint inhibitors needs to be elucidated in future studies.
This study had some limitations. First, it was a single-site study with a small number of patients. Examining the prognostic value of TLS in histological and NAC-IDS patients may have limited statistical power. Second, pretreatment biopsy specimens from patients who received NAC could not be evaluated due to insufficient tumor volume. This limited the interpretation of the lack of prognostic value of TLS in NAC-IDS patients, that is, whether such occurred because of preceding chemotherapy, as discussed above, or because of a high tumor burden preventing PDS. Third, this study was an integrated analysis including histological types, and analysis by each histological type was insufficient. Fourth, although evaluable pathological sections were selected in cooperation with an expert pathologist, the evaluators did not follow certain criteria. The TME and genetic background could vary between sections even within one tumor, and potential bias in sample selection cannot be excluded. Fifth, TLS was evaluated only using HE and IHC; functional analysis using other techniques, such as transcriptome analysis, can deepen our understanding of TLS in the TME [
11,
35].
5. Conclusions
The prognostic impact of TLS, specifically in pre-chemotherapy specimens, and the positive correlation between TLS and PD-L1 positivity indicate the potential of TLS as a meaningful biomarker for understanding tumor immunity in the TME and developing immunotherapy for ovarian cancer.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org., Figure S1: Flow diagram of patients with PDS or with NAC-IDS therapy; Figure S2: Progression-free survival of patients with or without TLS.
Author Contributions
Conceptualization, Takehiro Nakao and Kenichi Harano; methodology, Takehiro Nakao and Kenichi Harano; software, X.X.; validation, Masashi Wakabayashi and Reiko Watanabe; formal analysis, Masashi Wakabayashi, Tetsuro Taki and Reiko Watanabe; investigation, Takehiro Nakao; writing—original draft preparation, Takehiro Nakao; writing—review and editing, Kenichi Harano and Toru Mukohara; visualization, Takehiro Nakao.; supervision, Toru Mukohara; project administration, Kenichi Harano. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of National Cancer Center Hospital East (protocol code 2020-414, December 21, 2020).
Informed Consent Statement
Informed consent was obtained in the form of opt-out on the website. Those who rejected were excluded.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Tomsová, M.; Melichar, B.; Sedláková, I.; Steiner, I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol. Oncol. 2008, 108, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Raspollini, M.R.; Castiglione, F.; Rossi Degl’Innocenti, D.; Amunni, G.; Villanucci, A.; Garbini, F.; Baroni, G.; Taddei, G.L. Tumour-infiltrating gamma/Delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann. Oncol. 2005, 16, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian Cancer. Proc. Natl Acad. Sci. U. S. A. 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Zahn, L.M. Effects of the tumor microenvironment. Science. 2017, 355, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lauss, M.; Donia, M.; Svane, I.M.; Jönsson, G. B cells and tertiary lymphoid structures: Friends or foes in cancer immunotherapy? Clin. Cancer Res. 2022, 28, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer. 2019, 19, 307–325. [Google Scholar] [CrossRef]
- Ukita, M.; Hamanishi, J.; Yoshitomi, H.; Yamanoi, K.; Takamatsu, S.; Ueda, A.; Suzuki, H.; Hosoe, Y.; Furutake, Y.; Taki, M.; et al. CXCL13-producing CD4+ T cells accumulate in the early phase of tertiary lymphoid structures in ovarian Cancer. JCI Insight. 2022, 7, e157215. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, X.; Zou, L.H.; Guo, S.Q. Tertiary lymphoid structures are associated with a favorable prognosis in high-grade serous ovarian cancer patients. Reprod. Sci. 2023, 30, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Qiao, S.; Li, M.; Han, X.; Wei, X.; Pang, Y.; Mao, H. The gene signature of tertiary lymphoid structures within ovarian cancer predicts the prognosis and immunotherapy benefit. Front. Genet. 2023, 13, 1090640. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian Cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Siliņa, K.; Soltermann, A.; Attar, F.M.; Casanova, R.; Uckeley, Z.M.; Thut, H.; Wandres, M.; Isajevs, S.; Cheng, P.; Curioni-Fontecedro, A.; et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 2018, 78, 1308–1320. [Google Scholar] [CrossRef]
- Kulangara, K.; Zhang, N.; Corigliano, E.; Guerrero, L.; Waldroup, S.; Jaiswal, D.; Ms, M.J.; Shah, S.; Hanks, D.; Wang, J.; et al. Clinical utility of the combined positive score for programmed death Ligand-1 expression and the approval of Pembrolizumab for treatment of gastric Cancer. Arch. Pathol. Lab. Med. 2019, 143, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Maehara, N.; Hirota, A.; Eguchi, A.; Yasuda, K.; Taniguchi, K.; Nishijima, A.; Matsuhashi, N.; Shiga, Y.; Ishii, R.; et al. Two independent modes of kidney stone suppression achieved by AIM/CD5L and KIM-1. Commun. Biol. 2022, 5, 783. [Google Scholar] [CrossRef] [PubMed]
- Dieu-Nosjean, M.C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; de Chaisemartin, L.; et al. Long-term survival for patients with non-small-cell lung Cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008, 26, 4410–4417. [Google Scholar] [CrossRef] [PubMed]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, N.; Ino, Y.; Yamazaki-Itoh, R.; Kanai, Y.; Kosuge, T.; Shimada, K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic Cancer. Br. J. Cancer. 2015, 112, 1782–1790. [Google Scholar] [CrossRef]
- Goc, J.; Germain, C.; Vo-Bourgais, T.K.; Lupo, A.; Klein, C.; Knockaert, S.; de Chaisemartin, L.; Ouakrim, H.; Becht, E.; Alifano, M.; et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014, 74, 705–715. [Google Scholar] [CrossRef]
- Song, I.H.; Heo, S.H.; Bang, W.S.; Park, H.S.; Park, I.A.; Kim, Y.A.; Park, S.Y.; Roh, J.; Gong, G.; Lee, H.J. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast Cancer. Cancer Res. Treat. 2017, 49, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Lee, S.J.; Ahn, J.; Park, W.Y.; Shin, D.H.; Lee, C.H.; Kwon, H.; Jeong, Y.J.; Ahn, H.Y.; I, H.; et al. The prognostic significance of tumor-infiltrating lymphocytes assessment with hematoxylin and eosin sections in resected primary lung adenocarcinoma. PLOS ONE. 2019, 14, e0224430. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, Z.; Zhang, L.; Zou, Y.; Li, J.; Yan, W.; Li, C.; Liu, N.; Wu, H. Overcoming Resistance to Immune Checkpoint Therapy in PTEN-null Prostate Cancer by Intermittent anti-PI3 Kα/β/δ treatment. Nat. Commun. 2022, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus Nivolumab in Locoregionally advanced urothelial Cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ito, M.; Ohmura, H.; Hanamura, F.; Nakano, M.; Tsuchihashi, K.; Nagai, S.; Ariyama, H.; Kusaba, H.; Yamamoto, H.; et al. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal Cancer. Oncoimmunology. 2020, 9, 1724763. [Google Scholar] [CrossRef]
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.J.; Rochaix, P.; Girard, J.P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast Cancer. Cancer Res. 2011, 71, 5678–5687. [Google Scholar] [CrossRef] [PubMed]
- Vella, G.; Guelfi, S.; Bergers, G. High endothelial venules: A vascular perspective on tertiary lymphoid structures in Cancer. Front. Immunol. 2021, 12, 736670. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Nabet, B.Y.; Müller, S.; Koeppen, H.; Zou, W.; Giltnane, J.; Au-Yeung, A.; Srivats, S.; Cheng, J.H.; Takahashi, C.; et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung Cancer. Cancer Cell. 2022, 40, 289–300.e4. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, B.; Liu, Y.; Wang, Z. Tertiary lymphoid structure signatures are associated with survival and immunotherapy response in muscle-invasive bladder Cancer. Oncoimmunology. 2021, 10, 1915574. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Matsumoto, K.; Takehara, K.; Kawamura, N.; Hasegawa, K.; Takeshima, N.; Aoki, D.; Kamiura, S.; Arakawa, A.; Kondo, E.; et al. Pembrolizumab monotherapy in Japanese patients with advanced ovarian Cancer: Subgroup analysis from the KEYNOTE-100. Cancer Sci. 2020, 111, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Wu, C.Y.; Chen, M.Y.; Liu, S.X.; Yan, S.M.; Kang, Y.F.; Sun, C.; Grandis, J.R.; Zeng, M.S.; Zhong, Q. PD-1+CXCR5-CD4+ Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J. Immunother. Cancer. 2021, 9. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).