You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Prognostic Value of Tertiary Lymphoid Structures in Epithelial Ovarian Carcinoma
Altmetrics
Downloads
111
Views
30
Comments
0
This version is not peer-reviewed
The Tumor Microenvironment, Immuno-Oncology, and Immune Checkpoint: Implications for Current and Emergent Immunotherapies, 2nd Edition
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pathological Assessment
2.3. Diagnostic Criteria for TLSs and TILs
2.4. Diagnostic Criteria for PD-L1
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Tomsová, M.; Melichar, B.; Sedláková, I.; Steiner, I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol. Oncol. 2008, 108, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Raspollini, M.R.; Castiglione, F.; Rossi Degl’Innocenti, D.; Amunni, G.; Villanucci, A.; Garbini, F.; Baroni, G.; Taddei, G.L. Tumour-infiltrating gamma/Delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann. Oncol. 2005, 16, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian Cancer. Proc. Natl Acad. Sci. U. S. A. 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Zahn, L.M. Effects of the tumor microenvironment. Science. 2017, 355, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lauss, M.; Donia, M.; Svane, I.M.; Jönsson, G. B cells and tertiary lymphoid structures: Friends or foes in cancer immunotherapy? Clin. Cancer Res. 2022, 28, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer. 2019, 19, 307–325. [Google Scholar] [CrossRef]
- Ukita, M.; Hamanishi, J.; Yoshitomi, H.; Yamanoi, K.; Takamatsu, S.; Ueda, A.; Suzuki, H.; Hosoe, Y.; Furutake, Y.; Taki, M.; et al. CXCL13-producing CD4+ T cells accumulate in the early phase of tertiary lymphoid structures in ovarian Cancer. JCI Insight. 2022, 7, e157215. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, X.; Zou, L.H.; Guo, S.Q. Tertiary lymphoid structures are associated with a favorable prognosis in high-grade serous ovarian cancer patients. Reprod. Sci. 2023, 30, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Qiao, S.; Li, M.; Han, X.; Wei, X.; Pang, Y.; Mao, H. The gene signature of tertiary lymphoid structures within ovarian cancer predicts the prognosis and immunotherapy benefit. Front. Genet. 2023, 13, 1090640. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian Cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Siliņa, K.; Soltermann, A.; Attar, F.M.; Casanova, R.; Uckeley, Z.M.; Thut, H.; Wandres, M.; Isajevs, S.; Cheng, P.; Curioni-Fontecedro, A.; et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 2018, 78, 1308–1320. [Google Scholar] [CrossRef]
- Kulangara, K.; Zhang, N.; Corigliano, E.; Guerrero, L.; Waldroup, S.; Jaiswal, D.; Ms, M.J.; Shah, S.; Hanks, D.; Wang, J.; et al. Clinical utility of the combined positive score for programmed death Ligand-1 expression and the approval of Pembrolizumab for treatment of gastric Cancer. Arch. Pathol. Lab. Med. 2019, 143, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Maehara, N.; Hirota, A.; Eguchi, A.; Yasuda, K.; Taniguchi, K.; Nishijima, A.; Matsuhashi, N.; Shiga, Y.; Ishii, R.; et al. Two independent modes of kidney stone suppression achieved by AIM/CD5L and KIM-1. Commun. Biol. 2022, 5, 783. [Google Scholar] [CrossRef] [PubMed]
- Dieu-Nosjean, M.C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; de Chaisemartin, L.; et al. Long-term survival for patients with non-small-cell lung Cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008, 26, 4410–4417. [Google Scholar] [CrossRef] [PubMed]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, N.; Ino, Y.; Yamazaki-Itoh, R.; Kanai, Y.; Kosuge, T.; Shimada, K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic Cancer. Br. J. Cancer. 2015, 112, 1782–1790. [Google Scholar] [CrossRef]
- Goc, J.; Germain, C.; Vo-Bourgais, T.K.; Lupo, A.; Klein, C.; Knockaert, S.; de Chaisemartin, L.; Ouakrim, H.; Becht, E.; Alifano, M.; et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014, 74, 705–715. [Google Scholar] [CrossRef]
- Song, I.H.; Heo, S.H.; Bang, W.S.; Park, H.S.; Park, I.A.; Kim, Y.A.; Park, S.Y.; Roh, J.; Gong, G.; Lee, H.J. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast Cancer. Cancer Res. Treat. 2017, 49, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Lee, S.J.; Ahn, J.; Park, W.Y.; Shin, D.H.; Lee, C.H.; Kwon, H.; Jeong, Y.J.; Ahn, H.Y.; I, H.; et al. The prognostic significance of tumor-infiltrating lymphocytes assessment with hematoxylin and eosin sections in resected primary lung adenocarcinoma. PLOS ONE. 2019, 14, e0224430. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, Z.; Zhang, L.; Zou, Y.; Li, J.; Yan, W.; Li, C.; Liu, N.; Wu, H. Overcoming Resistance to Immune Checkpoint Therapy in PTEN-null Prostate Cancer by Intermittent anti-PI3 Kα/β/δ treatment. Nat. Commun. 2022, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus Nivolumab in Locoregionally advanced urothelial Cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ito, M.; Ohmura, H.; Hanamura, F.; Nakano, M.; Tsuchihashi, K.; Nagai, S.; Ariyama, H.; Kusaba, H.; Yamamoto, H.; et al. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal Cancer. Oncoimmunology. 2020, 9, 1724763. [Google Scholar] [CrossRef]
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.J.; Rochaix, P.; Girard, J.P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast Cancer. Cancer Res. 2011, 71, 5678–5687. [Google Scholar] [CrossRef] [PubMed]
- Vella, G.; Guelfi, S.; Bergers, G. High endothelial venules: A vascular perspective on tertiary lymphoid structures in Cancer. Front. Immunol. 2021, 12, 736670. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Nabet, B.Y.; Müller, S.; Koeppen, H.; Zou, W.; Giltnane, J.; Au-Yeung, A.; Srivats, S.; Cheng, J.H.; Takahashi, C.; et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung Cancer. Cancer Cell. 2022, 40, 289–300.e4. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, B.; Liu, Y.; Wang, Z. Tertiary lymphoid structure signatures are associated with survival and immunotherapy response in muscle-invasive bladder Cancer. Oncoimmunology. 2021, 10, 1915574. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Matsumoto, K.; Takehara, K.; Kawamura, N.; Hasegawa, K.; Takeshima, N.; Aoki, D.; Kamiura, S.; Arakawa, A.; Kondo, E.; et al. Pembrolizumab monotherapy in Japanese patients with advanced ovarian Cancer: Subgroup analysis from the KEYNOTE-100. Cancer Sci. 2020, 111, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Wu, C.Y.; Chen, M.Y.; Liu, S.X.; Yan, S.M.; Kang, Y.F.; Sun, C.; Grandis, J.R.; Zeng, M.S.; Zhong, Q. PD-1+CXCR5-CD4+ Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J. Immunother. Cancer. 2021, 9. [Google Scholar] [CrossRef]
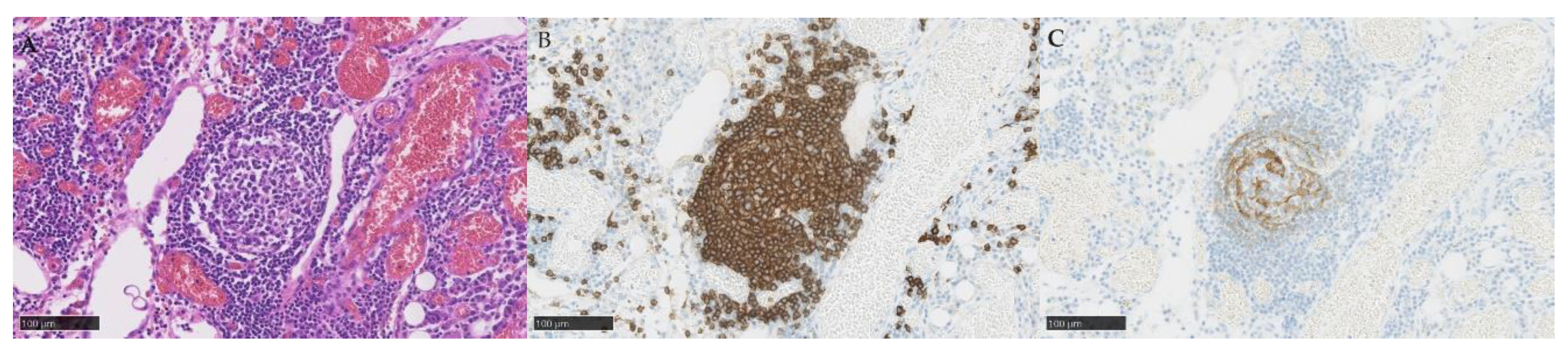
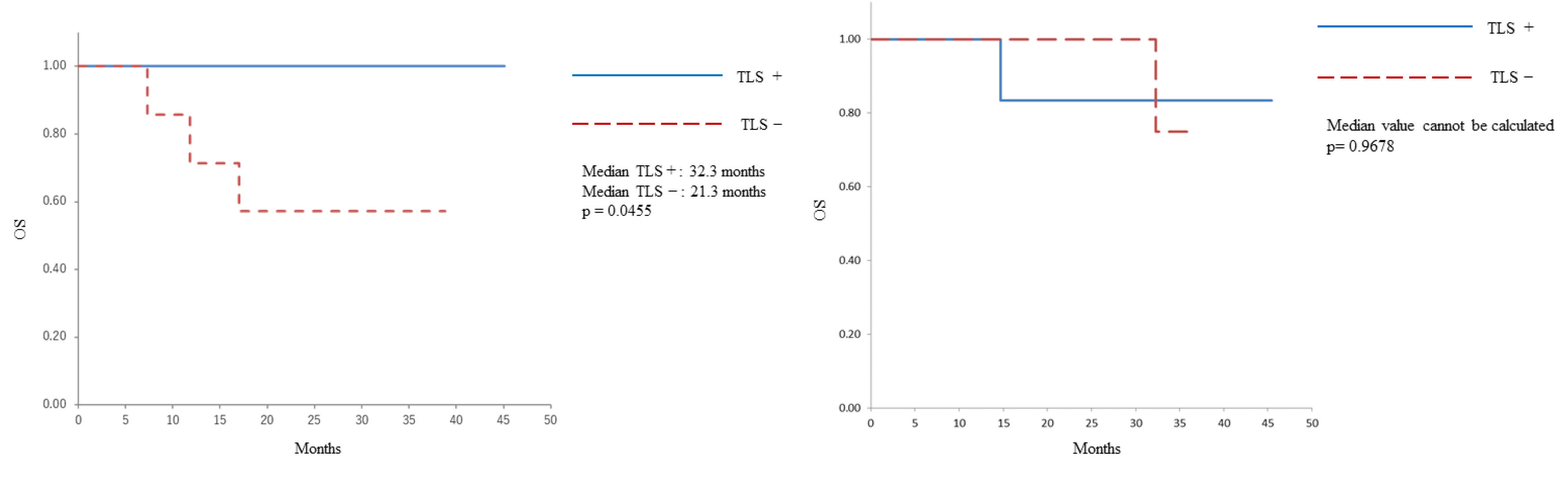
| Characteristic | All patients (n=28) | PDS (n=15) | NAC-IDS (n=13) |
| Age (years), median (range) | 61 (23-80) | 55 (23-80) | 66 (40-74) |
| Primary organ | |||
| Ovary | 24 (85.7) | 12 (80.0) | 12 (92.3) |
| Peritoneum | 4 (14.3) | 3 (20.0) | 1 (7.6) |
| Histological type | |||
| High-grade serous carcinoma | 21 (75.0) | 9 (60.0) | 12 (92.3) |
| Low-grade serous carcinoma | 2 (7.1) | 2 (13.3) | 0 |
| Endometrioid carcinoma | 3 (10.7) | 2 (13.3) | 1 (7.6) |
| Clear cell carcinoma | 2 (7.1) | 2 (13.3) | 0 |
| FIGO (2014) stage | |||
| Stage Ⅲ | 17 (60.7) | 10 (66.6) | 7 (53.8) |
| Stage Ⅳ | 11 (39.3) | 5 (33.3) | 6 (46.1) |
| BRCA mutation status | |||
| BRCA mutation | 1 (3.6) | 0 (0) | 1 (7.6) |
| No BRCA mutation | 15 (53.6) | 10 (66.6) | 5 (38.4) |
| Not tested | 12 (42.9) | 5 (33.3) | 7 (53.8) |
| Chemotherapy treatment | |||
| Dose-dense TC | 24 (85.7) | 12 (80.0) | 12 (92.3) |
| Triweekly TC | 2 (7.1) | 2 (13.3) | 0 |
| Weekly TC | 2 (7.1) | 1 (6.7) | 1 (7.6) |
| Response to NAC | |||
| CR | 1 (7.6) | ||
| PR | 11 (84.6) | ||
| SD | 0 | ||
| PD | 1 (7.6) | ||
| Residual disease | |||
| No evidence of disease (NED) | 21 (75.0) | 11 (73.3) | 10 (76.6) |
| Residual disease | 7 (25.0) | 4 (26.7) | 3 (23.0) |
| Pathological analysis | PDS (n=15) | NAC-IDS (n=10) |
| Analyzed site | ||
| Ovary | 5 (33.3) | 4 (40.0) |
| Omentum | 9 (60.0) | 4 (40.0) |
| Peritoneum | 1 (3.6) | 2 (20.0) |
| TLS | ||
| Number of TLS (5 mm2) | 1 (0-10) | 1 (0-8) |
| Positive | 8 (53.3) | 5 (50) |
| High-grade serous carcinoma | 5 (33.3) | 5 (50) |
| Low-grade serous carcinoma | 2 (7.1) | |
| Clear cell carcinoma | 1 (3.6) | |
| Negative (0) | 7 (46.7) | 5 (50) |
| High-grade serous carcinoma | 4 (26.7) | 4 (40) |
| Endometrioid carcinoma | 2 (7.1) | 1 (10) |
| Clear cell carcinoma | 1 (3.6) | |
| TILs | ||
| CD3 (mm2) | 227 (4.67-1110) | 296 (9-661) |
| CD8/4 | 0.660 (0.042-3.98) | 1.71 (0.572-13.9) |
| CD8/FOXP3 | 7.02 (1.58-55.2) | 7.06 (4.05-109) |
| PD-L1 | ||
| CPS positive | 8 (53.3) | 5 (50.0) |
| High grade serous carcinoma | 5 (33.3) | 5 (50.0) |
| Endometrioid carcinoma | 1 (6.7) | |
| Cear cell carcinoma | 2 (13.3) | |
| CPS negative | 7 (46.7) | 5 (50.0) |
| High grade serous carcinoma | 4 (40.0) | 4 (40.0) |
| Endometrioid carcinoma | 1 (10.0) | 1 (10.0) |
| Low grade serous carcinoma | 2 (20.0) |
| PFS median (months) | p value | OS median (months) | p value | |
| PDS | ||||
| CD3 (mm2) | ||||
| High | 24.6 | 0.2774 | 27.1 | 0.5652 |
| Low | 17.3 | 29.3 | ||
| CD8/4 | ||||
| High | 17.3 | 0.0845 | 27.1 | 0.1797 |
| Low | 24.6 | 33.5 | ||
| CD8/FOXP3 | ||||
| High | 21.3 | 0.949 | 27.1 | 0.7493 |
| Low | 24.6 | 31.1 | ||
| NAC-IDS | ||||
| CD3 (mm2) | ||||
| High | 22.6 | 0.8234 | 42.1 | 0.9193 |
| Low | 23.7 | 34.5 | ||
| CD8/4 | ||||
| High | 23.9 | 0.634 | 39.3 | 0.9193 |
| Low | 19.8 | 35.2 | ||
| CD8/FOXP3 | ||||
| High | 27.2 | 0.1758 | 45.4 | 0.1287 |
| Low | 18 | 31.1 |
| TLS positive | TLS negative | |
| PD-L1 positive | 10 | 3 |
| PD-L1 negative | 3 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
supplementary.docx (73.02KB )
Submitted:
18 June 2024
Posted:
18 June 2024
You are already at the latest version
supplementary.docx (73.02KB )
This version is not peer-reviewed
The Tumor Microenvironment, Immuno-Oncology, and Immune Checkpoint: Implications for Current and Emergent Immunotherapies, 2nd Edition
Submitted:
18 June 2024
Posted:
18 June 2024
You are already at the latest version
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Pathological Assessment
2.3. Diagnostic Criteria for TLSs and TILs
2.4. Diagnostic Criteria for PD-L1
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Tomsová, M.; Melichar, B.; Sedláková, I.; Steiner, I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol. Oncol. 2008, 108, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Raspollini, M.R.; Castiglione, F.; Rossi Degl’Innocenti, D.; Amunni, G.; Villanucci, A.; Garbini, F.; Baroni, G.; Taddei, G.L. Tumour-infiltrating gamma/Delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann. Oncol. 2005, 16, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian Cancer. Proc. Natl Acad. Sci. U. S. A. 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Zahn, L.M. Effects of the tumor microenvironment. Science. 2017, 355, 1386–1388. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lauss, M.; Donia, M.; Svane, I.M.; Jönsson, G. B cells and tertiary lymphoid structures: Friends or foes in cancer immunotherapy? Clin. Cancer Res. 2022, 28, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Sautès-Fridman, C.; Petitprez, F.; Calderaro, J.; Fridman, W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat. Rev. Cancer. 2019, 19, 307–325. [Google Scholar] [CrossRef]
- Ukita, M.; Hamanishi, J.; Yoshitomi, H.; Yamanoi, K.; Takamatsu, S.; Ueda, A.; Suzuki, H.; Hosoe, Y.; Furutake, Y.; Taki, M.; et al. CXCL13-producing CD4+ T cells accumulate in the early phase of tertiary lymphoid structures in ovarian Cancer. JCI Insight. 2022, 7, e157215. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, X.; Zou, L.H.; Guo, S.Q. Tertiary lymphoid structures are associated with a favorable prognosis in high-grade serous ovarian cancer patients. Reprod. Sci. 2023, 30, 2468–2480. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Qiao, S.; Li, M.; Han, X.; Wei, X.; Pang, Y.; Mao, H. The gene signature of tertiary lymphoid structures within ovarian cancer predicts the prognosis and immunotherapy benefit. Front. Genet. 2023, 13, 1090640. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian Cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Siliņa, K.; Soltermann, A.; Attar, F.M.; Casanova, R.; Uckeley, Z.M.; Thut, H.; Wandres, M.; Isajevs, S.; Cheng, P.; Curioni-Fontecedro, A.; et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 2018, 78, 1308–1320. [Google Scholar] [CrossRef]
- Kulangara, K.; Zhang, N.; Corigliano, E.; Guerrero, L.; Waldroup, S.; Jaiswal, D.; Ms, M.J.; Shah, S.; Hanks, D.; Wang, J.; et al. Clinical utility of the combined positive score for programmed death Ligand-1 expression and the approval of Pembrolizumab for treatment of gastric Cancer. Arch. Pathol. Lab. Med. 2019, 143, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Maehara, N.; Hirota, A.; Eguchi, A.; Yasuda, K.; Taniguchi, K.; Nishijima, A.; Matsuhashi, N.; Shiga, Y.; Ishii, R.; et al. Two independent modes of kidney stone suppression achieved by AIM/CD5L and KIM-1. Commun. Biol. 2022, 5, 783. [Google Scholar] [CrossRef] [PubMed]
- Dieu-Nosjean, M.C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; de Chaisemartin, L.; et al. Long-term survival for patients with non-small-cell lung Cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008, 26, 4410–4417. [Google Scholar] [CrossRef] [PubMed]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, N.; Ino, Y.; Yamazaki-Itoh, R.; Kanai, Y.; Kosuge, T.; Shimada, K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic Cancer. Br. J. Cancer. 2015, 112, 1782–1790. [Google Scholar] [CrossRef]
- Goc, J.; Germain, C.; Vo-Bourgais, T.K.; Lupo, A.; Klein, C.; Knockaert, S.; de Chaisemartin, L.; Ouakrim, H.; Becht, E.; Alifano, M.; et al. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res. 2014, 74, 705–715. [Google Scholar] [CrossRef]
- Song, I.H.; Heo, S.H.; Bang, W.S.; Park, H.S.; Park, I.A.; Kim, Y.A.; Park, S.Y.; Roh, J.; Gong, G.; Lee, H.J. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast Cancer. Cancer Res. Treat. 2017, 49, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Lee, S.J.; Ahn, J.; Park, W.Y.; Shin, D.H.; Lee, C.H.; Kwon, H.; Jeong, Y.J.; Ahn, H.Y.; I, H.; et al. The prognostic significance of tumor-infiltrating lymphocytes assessment with hematoxylin and eosin sections in resected primary lung adenocarcinoma. PLOS ONE. 2019, 14, e0224430. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020, 577, 556–560. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, Z.; Zhang, L.; Zou, Y.; Li, J.; Yan, W.; Li, C.; Liu, N.; Wu, H. Overcoming Resistance to Immune Checkpoint Therapy in PTEN-null Prostate Cancer by Intermittent anti-PI3 Kα/β/δ treatment. Nat. Commun. 2022, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus Nivolumab in Locoregionally advanced urothelial Cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Ito, M.; Ohmura, H.; Hanamura, F.; Nakano, M.; Tsuchihashi, K.; Nagai, S.; Ariyama, H.; Kusaba, H.; Yamamoto, H.; et al. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal Cancer. Oncoimmunology. 2020, 9, 1724763. [Google Scholar] [CrossRef]
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.J.; Rochaix, P.; Girard, J.P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast Cancer. Cancer Res. 2011, 71, 5678–5687. [Google Scholar] [CrossRef] [PubMed]
- Vella, G.; Guelfi, S.; Bergers, G. High endothelial venules: A vascular perspective on tertiary lymphoid structures in Cancer. Front. Immunol. 2021, 12, 736670. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Nabet, B.Y.; Müller, S.; Koeppen, H.; Zou, W.; Giltnane, J.; Au-Yeung, A.; Srivats, S.; Cheng, J.H.; Takahashi, C.; et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung Cancer. Cancer Cell. 2022, 40, 289–300.e4. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, B.; Liu, Y.; Wang, Z. Tertiary lymphoid structure signatures are associated with survival and immunotherapy response in muscle-invasive bladder Cancer. Oncoimmunology. 2021, 10, 1915574. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Matsumoto, K.; Takehara, K.; Kawamura, N.; Hasegawa, K.; Takeshima, N.; Aoki, D.; Kamiura, S.; Arakawa, A.; Kondo, E.; et al. Pembrolizumab monotherapy in Japanese patients with advanced ovarian Cancer: Subgroup analysis from the KEYNOTE-100. Cancer Sci. 2020, 111, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Wu, C.Y.; Chen, M.Y.; Liu, S.X.; Yan, S.M.; Kang, Y.F.; Sun, C.; Grandis, J.R.; Zeng, M.S.; Zhong, Q. PD-1+CXCR5-CD4+ Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J. Immunother. Cancer. 2021, 9. [Google Scholar] [CrossRef]


| Characteristic | All patients (n=28) | PDS (n=15) | NAC-IDS (n=13) |
| Age (years), median (range) | 61 (23-80) | 55 (23-80) | 66 (40-74) |
| Primary organ | |||
| Ovary | 24 (85.7) | 12 (80.0) | 12 (92.3) |
| Peritoneum | 4 (14.3) | 3 (20.0) | 1 (7.6) |
| Histological type | |||
| High-grade serous carcinoma | 21 (75.0) | 9 (60.0) | 12 (92.3) |
| Low-grade serous carcinoma | 2 (7.1) | 2 (13.3) | 0 |
| Endometrioid carcinoma | 3 (10.7) | 2 (13.3) | 1 (7.6) |
| Clear cell carcinoma | 2 (7.1) | 2 (13.3) | 0 |
| FIGO (2014) stage | |||
| Stage Ⅲ | 17 (60.7) | 10 (66.6) | 7 (53.8) |
| Stage Ⅳ | 11 (39.3) | 5 (33.3) | 6 (46.1) |
| BRCA mutation status | |||
| BRCA mutation | 1 (3.6) | 0 (0) | 1 (7.6) |
| No BRCA mutation | 15 (53.6) | 10 (66.6) | 5 (38.4) |
| Not tested | 12 (42.9) | 5 (33.3) | 7 (53.8) |
| Chemotherapy treatment | |||
| Dose-dense TC | 24 (85.7) | 12 (80.0) | 12 (92.3) |
| Triweekly TC | 2 (7.1) | 2 (13.3) | 0 |
| Weekly TC | 2 (7.1) | 1 (6.7) | 1 (7.6) |
| Response to NAC | |||
| CR | 1 (7.6) | ||
| PR | 11 (84.6) | ||
| SD | 0 | ||
| PD | 1 (7.6) | ||
| Residual disease | |||
| No evidence of disease (NED) | 21 (75.0) | 11 (73.3) | 10 (76.6) |
| Residual disease | 7 (25.0) | 4 (26.7) | 3 (23.0) |
| Pathological analysis | PDS (n=15) | NAC-IDS (n=10) |
| Analyzed site | ||
| Ovary | 5 (33.3) | 4 (40.0) |
| Omentum | 9 (60.0) | 4 (40.0) |
| Peritoneum | 1 (3.6) | 2 (20.0) |
| TLS | ||
| Number of TLS (5 mm2) | 1 (0-10) | 1 (0-8) |
| Positive | 8 (53.3) | 5 (50) |
| High-grade serous carcinoma | 5 (33.3) | 5 (50) |
| Low-grade serous carcinoma | 2 (7.1) | |
| Clear cell carcinoma | 1 (3.6) | |
| Negative (0) | 7 (46.7) | 5 (50) |
| High-grade serous carcinoma | 4 (26.7) | 4 (40) |
| Endometrioid carcinoma | 2 (7.1) | 1 (10) |
| Clear cell carcinoma | 1 (3.6) | |
| TILs | ||
| CD3 (mm2) | 227 (4.67-1110) | 296 (9-661) |
| CD8/4 | 0.660 (0.042-3.98) | 1.71 (0.572-13.9) |
| CD8/FOXP3 | 7.02 (1.58-55.2) | 7.06 (4.05-109) |
| PD-L1 | ||
| CPS positive | 8 (53.3) | 5 (50.0) |
| High grade serous carcinoma | 5 (33.3) | 5 (50.0) |
| Endometrioid carcinoma | 1 (6.7) | |
| Cear cell carcinoma | 2 (13.3) | |
| CPS negative | 7 (46.7) | 5 (50.0) |
| High grade serous carcinoma | 4 (40.0) | 4 (40.0) |
| Endometrioid carcinoma | 1 (10.0) | 1 (10.0) |
| Low grade serous carcinoma | 2 (20.0) |
| PFS median (months) | p value | OS median (months) | p value | |
| PDS | ||||
| CD3 (mm2) | ||||
| High | 24.6 | 0.2774 | 27.1 | 0.5652 |
| Low | 17.3 | 29.3 | ||
| CD8/4 | ||||
| High | 17.3 | 0.0845 | 27.1 | 0.1797 |
| Low | 24.6 | 33.5 | ||
| CD8/FOXP3 | ||||
| High | 21.3 | 0.949 | 27.1 | 0.7493 |
| Low | 24.6 | 31.1 | ||
| NAC-IDS | ||||
| CD3 (mm2) | ||||
| High | 22.6 | 0.8234 | 42.1 | 0.9193 |
| Low | 23.7 | 34.5 | ||
| CD8/4 | ||||
| High | 23.9 | 0.634 | 39.3 | 0.9193 |
| Low | 19.8 | 35.2 | ||
| CD8/FOXP3 | ||||
| High | 27.2 | 0.1758 | 45.4 | 0.1287 |
| Low | 18 | 31.1 |
| TLS positive | TLS negative | |
| PD-L1 positive | 10 | 3 |
| PD-L1 negative | 3 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Alexandros Lalos
et al.
Cancers,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated





