Submitted:
17 June 2024
Posted:
20 June 2024
You are already at the latest version
Abstract
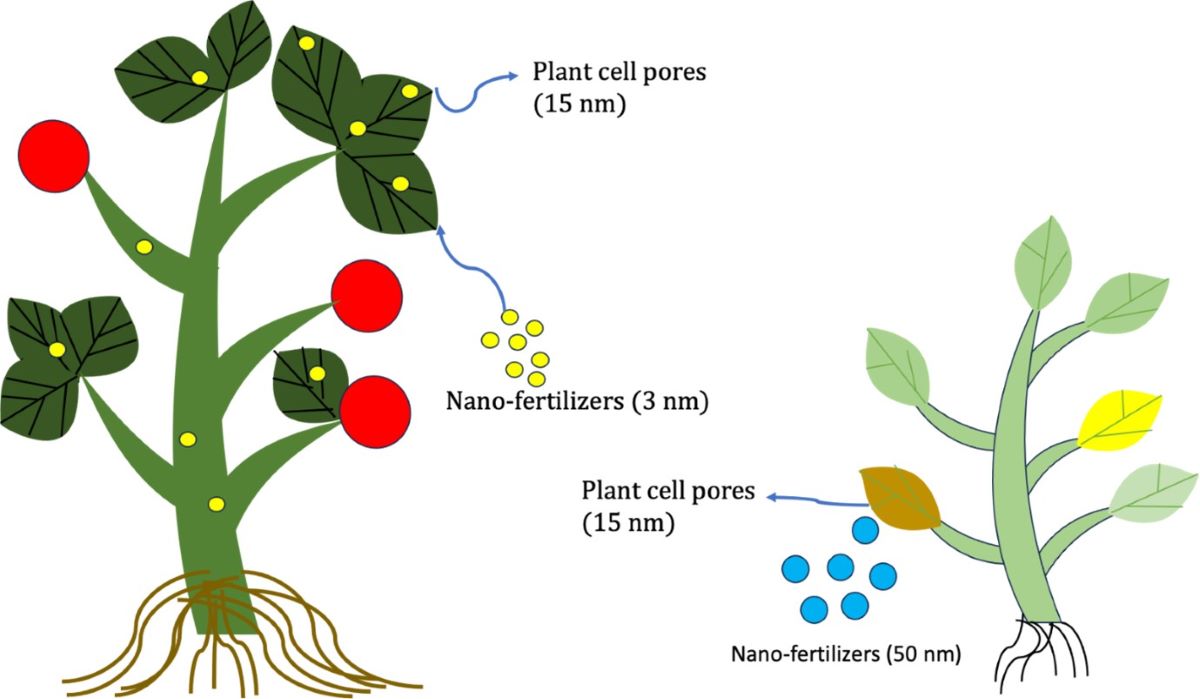
Keywords:
1. Introduction
Search Strategy
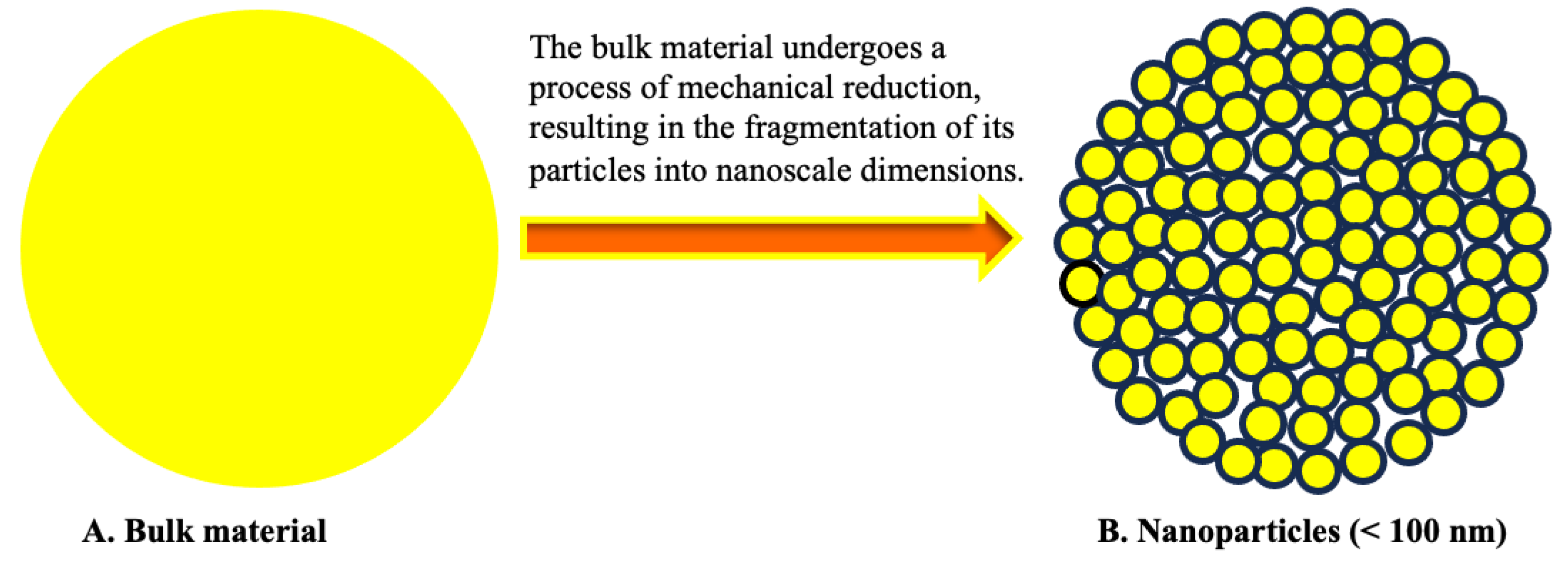
2.1. The Particle Size of Nanoparticles
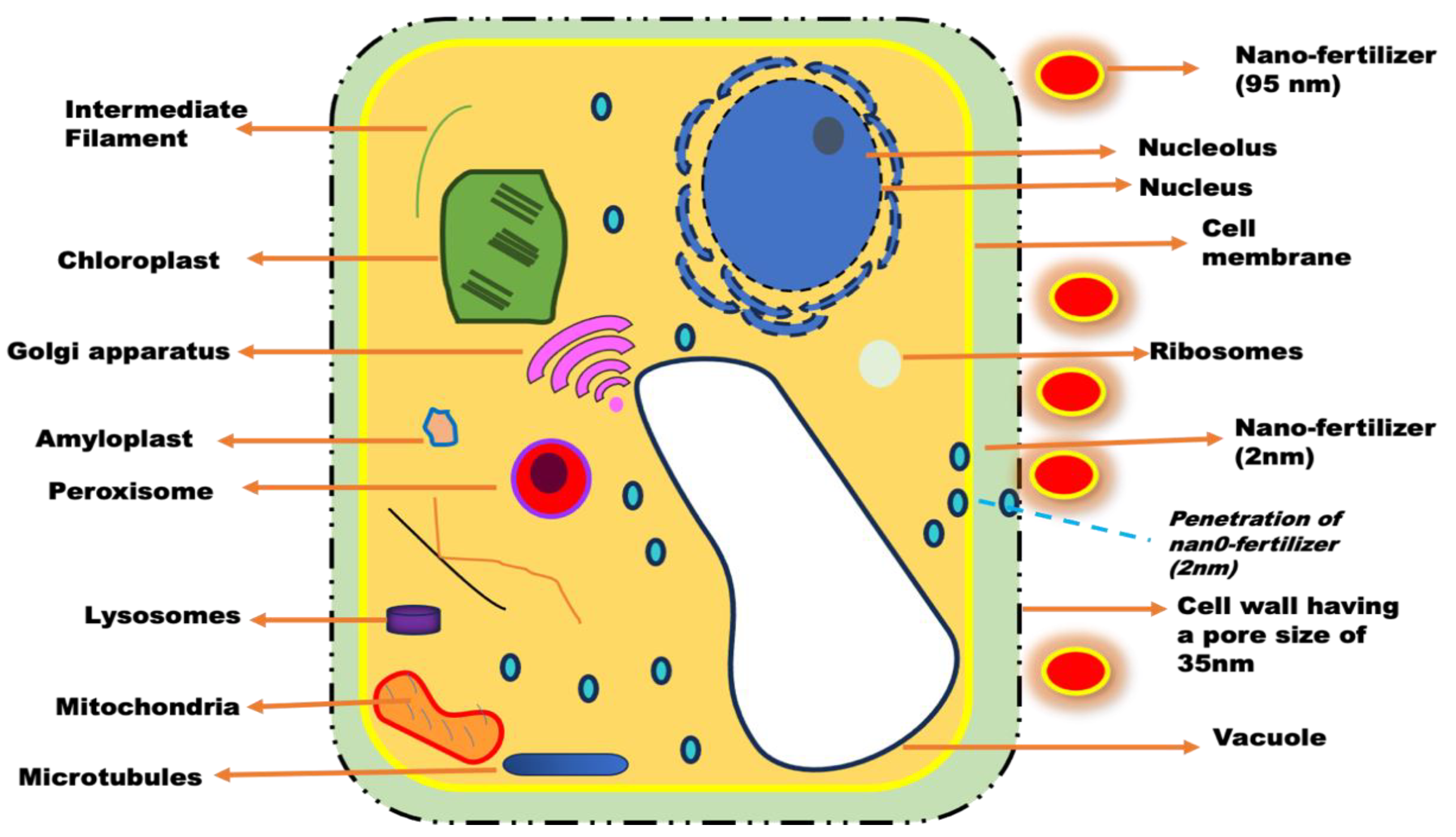
2.2. The Surface area-to-Volume Ratio of a Nanoparticle
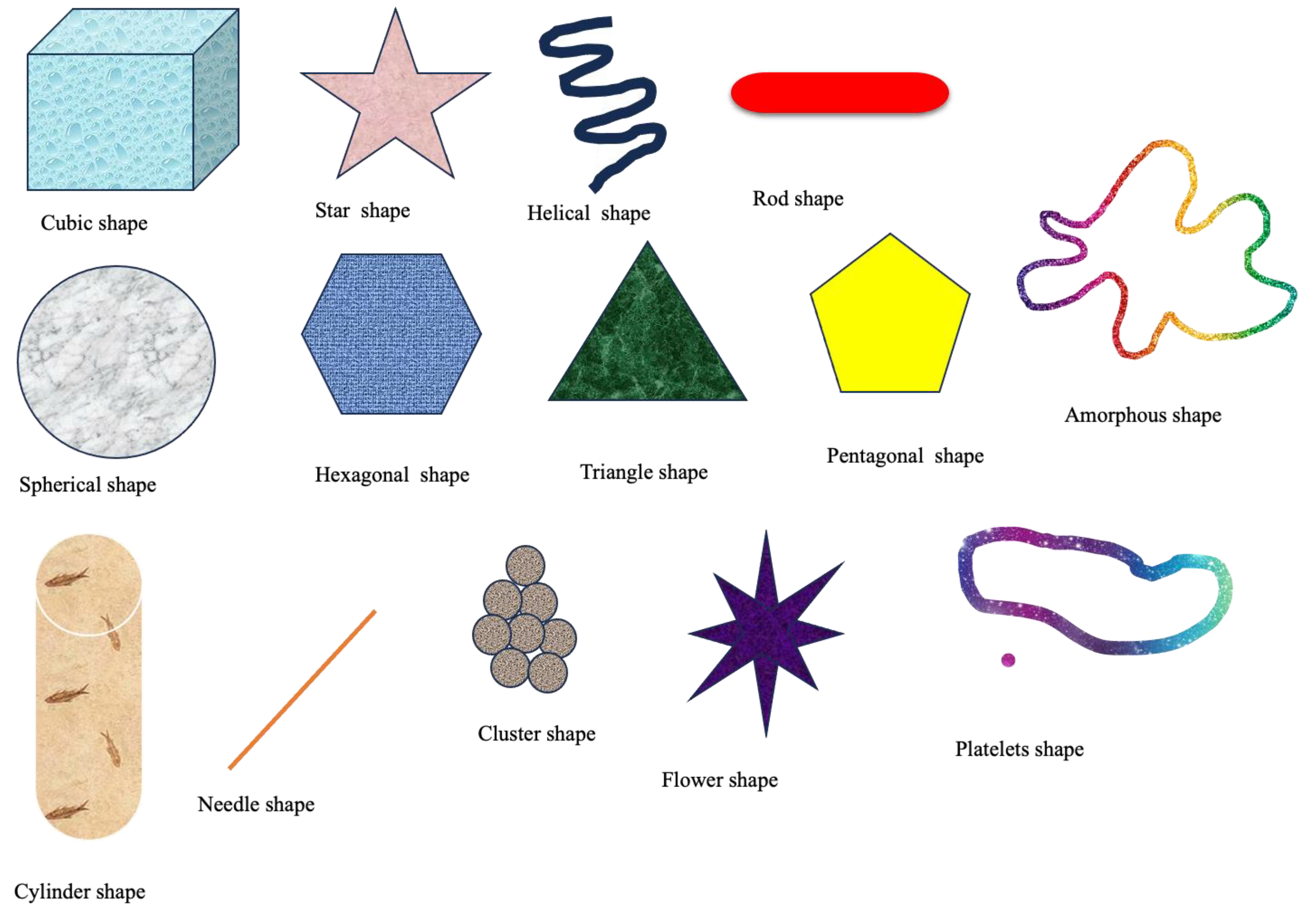
2.3. Shape of Nanoparticles
| Crop | Nanoparticle type | Concentration | Nanoparticle shape | Germination (%) |
Plant development | Reference |
|---|---|---|---|---|---|---|
| Lentil | AuNPs | 5 ppm | Spherical | There was no significant difference observed. | Plant height= 17.90 cm. Number of leaves= 14.33. Biomass production = 6.70 gm |
[76] |
| 10 ppm | No significant difference observed | Plant height= 23.23 cm Number of leaves= 17.67 High biomass production = 8.20 gm. |
||||
| 25 ppm | 26.7 | Plant height = 15.10 cm Number of leaves= 13.33 Biomass production= 5.57 gm |
||||
| 50 ppm | 53.3 | Plant height = 12.90 cm Number of leaves= 10.33 Biomass production= 3.80 (gm) |
||||
| 100 ppm | 66.7 | Plant height = 10.77 cm Number of leaves= 8.00 Biomass production= 2.77 gm |
||||
| Phaseolus vulgaris | AgNPs | 15 mg L−1 | Spherical | 100 | Moderate effect observed for all studied parameters | [77] |
| 30 mg L−1 | 100 | Moderate effect observed for all studied parameters | ||||
| 60 mg L−1 | 100 | Higher shoot growth Higher plant height High number of leaves |
||||
| 120 mg L−1 | 93.33 | Higher root growth observed High root length |
||||
| 240 mg L−1 | 80 | Lower shoot and root growth | ||||
| 480 mg L−1 | 73.33 | Lower shoot and root growth Lower root length Less number of leaves Lower plant height |
||||
| Green pea | AgNPs | 20 mg/L | Spherical | 98 | High root length of 20 cm High root fresh weight Lower root deformation |
[78] |
| 40 mg/L | 96 | Lower root fresh weight | ||||
| 80 mg/L | 87 | Moderate effect for studied parameters | ||||
| 160 mg/L | 85 | Lower root length of 10 cm Lower root fresh weight High root deformation |
||||
| Blackgram | ZnONPs | 100 mg/L | Spherical | 67 | Lower shoot length Lower root length |
[79] |
| 200 mg/L | 68 | Moderate shoot and root length | ||||
| 300 mg/L | 69 | Moderate shoot and root length | ||||
| 400 mg/L | 70 | Moderate shoot and root length | ||||
| 500 mg/L | 72 | Moderate shoot and root length | ||||
| 600 mg/L | 74 | Higher shoot length Higher root length |
||||
| Wheat | ZnONPs | 10 mg/L | Spherical | 78 | Lower plant fresh biomass Lower leave length |
[80] |
| 25 mg/L | 80 | Moderate results for all parameters studied | ||||
| 50 mg/L | 80 | Higher fresh biomass Higher number of roots Higher leave length |
||||
| 100 mg/L | 80 | Moderate results for all parameters studied | ||||
| Brassica oleracea var italic | ZnONPs | 50 µg/L | Spherical | 87.5 | Lower plant height = 16.6 cm | [81] |
| 100 µg/L | 100 | - | ||||
| 200 µg/L | 87.5 | Higher root length | ||||
| 400 µg/L | 87.5 | Plant height= 19.8 cm | ||||
| 800 µg/L | 87.5 | Plant height = 20 cm Higher number of leaves =8.66 Higher leaf area= 62.48 cm² Higher root length= 57.44 cm |
||||
| 1000 µg/L | 87.5 | Higher plant height= 20.33 cm | ||||
| green gram Vigna radiata | ZnONPs | 100 mg/L | Rod | Lower germination% compared to the other concentration Lower germination% compared to the other concentration |
- | [82] |
| 200 mg/L | Higher germination% compared to the other concentration | Higher shoot length =16 cm Higher root length =6 cm |
||||
| 300 mg/L | Lower germination% compared to the other concentration | - | ||||
| 400 mg/L | Lower germination% compared to the other concentration | - | ||||
| Groundnut | ZnONPs | 500 (mg /kg 1) | Rod | 58 | Lower shoot length =18.40 cm Lower root length =15.67 cm |
[83] |
| 750 (mg /kg 1) | 63 | Shoot length =19.88 cm Root length 17.98 cm |
||||
| 1000 (mg /kg 1) | 75 | Higher shoot length =20.98cm | ||||
| 1250 (mg /kg 1) | 71 | shoot length =20.28 cm Root length =17.98 cm |
2.4. Agglomeration
2.5. Crystalline Structure
2.6. Nutritional Value of Nano-Fertilizers
Conclusions
Recommendations
- Cytotoxicity studies should be conducted prior to the application of nano-fertilizers, as several researchers have expressed concerns about the potential toxicity of nanoparticles due to their small particle size and large surface area, which can lead to increased reactivity. The effects of accidentally ingesting the residue of nanoparticles from plants remain unknown.
- Further studies should focus on investigating the impact of different nanoparticle shapes on nutrient uptake and plant growth. It is important to determine the most suitable nanoparticle shape for different plant species in order to enhance the effectiveness of nano-fertilizers and improve overall plant performance. Additionally, researchers should aim to synthesize nano-fertilizers with specific shapes designed to meet the requirements of different plant species.
- There is scarce information about what happens when nanoparticles enter plant cells or tissues, making it uncertain whether they aggregate into agglomerates. Researchers should develop sensors to monitor nanoparticle behavior once inside plant cells or tissues. This will assist in tailoring the properties of nano-fertilizers to enhance their efficacy.
References
- Verma, K.K.; Song, X.-P.; Joshi, A.; Tian, D.-D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.-R. Recent Trends in Nano-Fertilizers for Sustainable Agriculture under Climate Change for Global Food Security. Nanomaterials 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Yadav D, Dey A, Upadhyay PK. Nanofertilizers In Sustainable Agriculture: Benefits and Drawbacks. Souvenir&. 2021 Dec 13.
- Abdalla, Z.F.; El-Sawy, S.; El-Bassiony, A.E.M.; Jun, H.; Shedeed, S.; Okasha, A.M.; Bayoumi, Y.; El-Ramady, H.; Prokisch, J. Smart Fertilizers vs. Nano-fertilizers: A Pictorial Overview. Environ. Biodivers. Soil Secur. 2022, 6, 191–204. [Google Scholar] [CrossRef]
- Jakhar, A.M.; Aziz, I.; Kaleri, A.R.; Hasnain, M.; Haider, G.; Ma, J.; Abideen, Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. NanoImpact 2022, 27, 100411. [Google Scholar] [CrossRef] [PubMed]
- Zohra E, Ikram M, Raja NI, Omar AA, Mohamed AH, Zahedi SM, Abbas A. Nanomaterials as Nano-Fertilizers. InBiotic Stress Management of Crop Plants using Nanomaterials 2023 May 18 (pp. 35-51).
- Kumar Y, Singh T, Raliya R, Tiwari KN. Nano fertilizers for sustainable crop production, higher nutrient use efficiency and enhanced profitability. Indian Journal of Fertilisers. 2021 Nov;17(11):1206-14.
- Zuma, M.; Arthur, G.; Coopoosamy, R.; Naidoo, K. Incorporating cropping systems with eco-friendly strategies and solutions to mitigate the effects of climate change on crop production. J. Agric. Food Res. 2023, 14. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, Y.K.; Maurya, S.K.; Maurya, S.K.; Maurya, D.K.; Sachan, R.; Gautam, M.K.; Tiwari, A. Efficient Use of Nano-fertilizer for Increasing Productivity and Profitability along with Maintain Sustainability in Rice Crop: A Review. Int. J. Environ. Clim. Chang. 2023, 13, 1358–1368. [Google Scholar] [CrossRef]
- Mejias, J.H.; Salazar, F.; Amaro, L.P.; Hube, S.; Rodriguez, M.; Alfaro, M. Nanofertilizers: A Cutting-Edge Approach to Increase Nitrogen Use Efficiency in Grasslands. Front. Environ. Sci. 2021, 9. [Google Scholar] [CrossRef]
- Rautela I, Dheer PA, Thapliyal PR, Shah DH, Joshi M, Upadhyay S, Gururani P, Sinha VB, Gaurav NA, Sharma MD. Current scenario and future perspectives of nanotechnology in sustainable agriculture and food production. Plant Cell Biotechnol. Mol. Biol. 2021 Mar 1;22:99-121.
- Avila-Quezada, G.D.; Ingle, A.P.; Golińska, P.; Rai, M. Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks. Nanotechnol. Rev. 2022, 11, 2123–2140. [Google Scholar] [CrossRef]
- Mahaletchumi, S. Review on the use of nanotechnology in fertiilzers. Journal of Research Technology and Engineering. 2021;2:60-72.
- Yuvaraj M, Subramanian KS. Novel slow release nanocomposite fertilizers. InNanotechnology and the Environment 2020 Dec 2. IntechOpen.
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, G.; Dhasmana, A.; Chaudhary, P.; Gupta, S.; Gangola, S.; Gupta, A.; Rustagi, S.; Shende, S.S.; Rajput, V.D.; Minkina, T.; et al. A Perspective Review on Green Nanotechnology in Agro-Ecosystems: Opportunities for Sustainable Agricultural Practices & Environmental Remediation. Agriculture 2023, 13, 668. [Google Scholar] [CrossRef]
- Triphati A, Pirzadah TB. Synthesis methods of nanoparticles and their key applications. InSynthesis of bionanomaterials for biomedical applications 2023 Jan 1 (pp. 57-76). Elsevier.
- Alcalá-Alcalá S, Casarrubias-Anacleto JE, Mondragón-Guillén M, Tavira-Montalvan CA, Bonilla-Hernández M, Gómez-Galicia DL, Gosset G, Meneses-Acosta A. Melanin Nanoparticles Obtained from Preformed Recombinant Melanin by Bottom-Up and Top-Down Approaches. Polymers. 2023 May 19;15(10):2381.
- Hajalilou A, Tavakoli M, Parvini E. Insight into the Synthesis of Nanostructured Magnetic Materials.
- Fernandes C, Jathar M, Sawant BK, Warde T. Scale-Up of Nanoparticle Manufacturing Process. InPharmaceutical Process Engineering and Scale-up Principles 2023 Jul 4 (pp. 173-203). Cham: Springer Nature Switzerland.
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Fixen P, Brentrup F, Bruulsema T, Garcia F, Norton R, Zingore S. Nutrient/fertilizer use efficiency: measurement, current situation and trends. Managing water and fertilizer for sustainable agricultural intensification. 2015 Jan;270:1-30.
- Pacheco I, Buzea C. Nanoparticle uptake by plants: beneficial or detrimental?. Phytotoxicity of nanoparticles. 2018:1-61.
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore. 2018; pp. [CrossRef]
- Su, Y.; Ashworth, V.; Kim, C.; Adeleye, A.S.; Rolshausen, P.; Roper, C.; White, J.; Jassby, D. Delivery, uptake, fate, and transport of engineered nanoparticles in plants: a critical review and data analysis. Environ. Sci. Nano 2019, 6, 2311–2331. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical Properties Determine Nanomaterial Cellular Uptake, Transport, and Fate. Accounts Chem. Res. 2012, 46, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Khan I, Awan SA, Rizwan M, Hassan ZU, Akram MA, Tariq R, Brestic M, Xie W. Nanoparticle’s uptake and translocation mechanisms in plants via seed priming, foliar treatment, and root exposure: A review. Environmental Science and Pollution Research. 2022 Dec;29(60):89823-33.
- Findik, F. Nanomaterials and their applications. Periodicals of Engineering and Natural Sciences. 2021 Jun 13;9(3):62-75.
- Ulusoy, U. A Review of Particle Shape Effects on Material Properties for Various Engineering Applications: From Macro to Nanoscale. Minerals 2023, 13, 91. [Google Scholar] [CrossRef]
- Etxeberria, E.; Gonzalez, P.; Bhattacharya, P.; Sharma, P.; Ke, P.C. Determining the Size Exclusion for Nanoparticles in Citrus Leaves. HortScience 2016, 51, 732–737. [Google Scholar] [CrossRef]
- Pérez-De-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5. [Google Scholar] [CrossRef]
- Ghorbanpour M, Bhargava P, Varma A, Choudhary DK, editors. Biogenic nano-particles and their use in agro-ecosystems. Singapore:: Springer; 2020 Mar 20.
- Abbas, Q.; Liu, G.; Yousaf, B.; Ali, M.U.; Ullah, H.; Ahmed, R. Effects of biochar on uptake, acquisition and translocation of silver nanoparticles in rice (Oryza sativa L.) in relation to growth, photosynthetic traits and nutrients displacement. Environ. Pollut. 2019, 250, 728–736. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, J.; Zhao, G.; Wu, W.; Liu, L.; Wang, Q.; Guo, Y. Absorption and Bio-Transformation of Selenium Nanoparticles by Wheat Seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [Google Scholar] [CrossRef]
- Yusefi-Tanha E, Fallah S, Rostamnejadi A, Pokhrel LR. Root system architecture, copper uptake and tissue distribution in soybean (Glycine max (L.) Merr.) grown in copper oxide nanoparticle (CuONP)-amended soil and implications for human nutrition. Plants. 2020 Oct 8;9(10):1326.
- Zhang, Z.; He, X.; Zhang, H.; Ma, Y.; Zhang, P.; Ding, Y.; Zhao, Y. Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 2011, 3, 816–822. [Google Scholar] [CrossRef]
- Kumar Y, Singh T, Raliya R, Tiwari KN. Nano fertilizers for sustainable crop production, higher nutrient use efficiency and enhanced profitability. Indian Journal of Fertilisers. 2021 Nov;17(11):1206-14.
- Tarafdar JC, Xiong Y, Wang WN, Quinl D, Biswas P. Standardization of size, shape and concentration of nanoparticle for plant application. Applied Biological Research. 2012;14(2):138-44.
- Sabo-Attwood, T.; Unrine, J.M.; Stone, J.W.; Murphy, C.J.; Ghoshroy, S.; Blom, D.; Bertsch, P.M.; Newman, L.A. Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 2011, 6, 353–360. [Google Scholar] [CrossRef]
- Eichert, T.; Kurtz, A.; Steiner, U.; Goldbach, H.E. Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles. Physiol. Plant. 2008, 134, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.; Sabularse, D.; Montezinos, D.; Delmer, D.P. Determination of the Pore Size of Cell Walls of Living Plant Cells. Science 1979, 205, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arabian journal of chemistry. 2019 Nov 1;12(7):908-31.
- Sohrabi, Y.; Kalyani, F.S.; Heydari, M.; Yazdani, M.; Omer, K.M.; Yousefi, A.R. Plant-based nano-fertilizer prepared from Paulownia Tomentosa: fabrication, characterization, and application on Ocimum basilicum. Chem. Biol. Technol. Agric. 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Greco, G.H.; Mazzucchi, S.; Pagani, E.M. Peano on definition of surface area. Rendiconti Lince- - Mat. E Appl. 2016, 27, 251–286. [Google Scholar] [CrossRef]
- Denison E, Cawthray R. The Big Book of Packaging Prototypes: Templates for Innovative Cartons, Packages, and Boxes. RotoVision; 2010.
- Gómez-Tena MP, Gilabert J, Toledo J, Zumaquero E, Machí C. Relationship between the specific surface area parameters determined using different analytical techniques. Proceedings of the XII Foro Global Del Recubrimiento Cerámico, Universitat Jaume I, Castellón, Spain. 2014 Feb 14:17-8.https://www.researchgate.net/profile/Maria-Pilar-Gomez-Tena/publication/260298466_RELATIONSHIP_BETWEEN_THE_SPECIFIC_SURFACE_AREA_PARAMETERS_DETERMINED_USING_DIFFERENT_ANALYTICAL_TECHNIQUES/links/00b7d5386114fad0e6000000/RELATIONSHIP-BETWEEN-THE-SPECIFIC-SURFACE-AREA-PARAMETERS-DETERMINED-USING-DIFFERENT-ANALYTICAL-TECHNIQUES.
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J. Nanobiotechnology 2022, 20, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Andrievski, R.A. Review of thermal stability of nanomaterials. J. Mater. Sci. 2013, 49, 1449–1460. [Google Scholar] [CrossRef]
- Saikia J, Ramakrishnan V. Peptide nanocatalysts. InDe Novo Peptide Design 2023 Jan 1 (pp. 173-206). Academic Press.https://www.sciencedirect.com/science/article/pii/B9780323999175000068.
- Rahale S. Nutrient release pattern of nanofertilizer formulation. PhD (Agri.) Thesis, Tamilnadu Agricultural University, Coimbatore. 2011. [google scholar].
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Nanofertilizers: Types, Delivery and Advantages in Agricultural Sustainability. Agrochemicals 2023, 2, 296–336. [Google Scholar] [CrossRef]
- Naderi MR, Danesh-Shahraki A. Nanofertilizers and their roles in sustainable agriculture.https://www.cabidigitallibrary.org/doi/full/10.5555/20133304426.
- Tarafder, C.; Daizy, M.; Alam, M.; Ali, R.; Islam, J.; Islam, R.; Ahommed, S.; Aly, M.A.S.; Khan, Z.H. Formulation of a Hybrid Nanofertilizer for Slow and Sustainable Release of Micronutrients. ACS Omega 2020, 5, 23960–23966. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanpour M, Bhargava P, Varma A, Choudhary DK, editors. Biogenic nano-particles and their use in agro-ecosystems. Singapore:: Springer; 2020 Mar 20.https://link.springer.com/content/pdf/10.1007/978-981-15-2985-6.pdf.
- Hidayat R, Fadillah G, Chasanah U, Wahyuningsih S, Ramelan AH. Effectiveness of urea nanofertilizer based aminopropyltrimethoxysilane (APTMS)-zeolite as slow release fertilizer system. African Journal of Agricultural Research. 2015 May 13;10(14):1785-8.https://www.academia.edu/download/52651767/article1429362190_Hidayat_20et_20al.pdf.
- Kottegoda N, Munaweera I, Madusanka N, Karunaratne V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Current science. 2011 Jul 10:73-8. https://www.jstor.org/stable/24077865.
- Pereira, E.I.; da Cruz, C.C.T.; Solomon, A.; Le, A.; Cavigelli, M.A.; Ribeiro, C. Novel Slow-Release Nanocomposite Nitrogen Fertilizers: The Impact of Polymers on Nanocomposite Properties and Function. Ind. Eng. Chem. Res. 2015, 54, 3717–3725. [Google Scholar] [CrossRef]
- Al-Juthery, H.W.; Lahmod, N.R.; Al-Taee, R.A. Intelligent, Nano-fertilizers: A New Technology for Improvement Nutrient Use Efficiency (Article Review). IOP Conf. Series: Earth Environ. Sci. 2021, 735. [Google Scholar] [CrossRef]
- Saleem, I.; Maqsood, M.A.; Rehman, M.Z.U.; Aziz, T.; Bhatti, I.A.; Ali, S. Potassium ferrite nanoparticles on DAP to formulate slow release fertilizer with auxiliary nutrients. Ecotoxicol. Environ. Saf. 2021, 215, 112148. [Google Scholar] [CrossRef] [PubMed]
- Nongbet, A.; Mishra, A.K.; Mohanta, Y.K.; Mahanta, S.; Ray, M.K.; Khan, M.; Baek, K.-H.; Chakrabartty, I. Nanofertilizers: A Smart and Sustainable Attribute to Modern Agriculture. Plants 2022, 11, 2587. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilisers: a review of formulation and nutrient release patterns. J. Sci. Food Agric. 2014, 95, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Mohammadi, A.M.; Nojavan, S. Factors Affecting Farmer’s Chemical Fertilizers Consumption and Water Pollution in Northeastern Iran. J. Agric. Sci. 2017, 9. [Google Scholar] [CrossRef]
- Fincheira, P.; Hoffmann, N.; Tortella, G.; Ruiz, A.; Cornejo, P.; Diez, M.C.; Seabra, A.B.; Benavides-Mendoza, A.; Rubilar, O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials 2023, 13, 1978. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, R.; Ferreira, Q.; Rodrigues, G.C.; Oliveira, M. Nanofertilizer Use for Adaptation and Mitigation of the Agriculture/Climate Change Dichotomy Effects. Climate 2023, 11, 129. [Google Scholar] [CrossRef]
- Marciniak, L.; Nowak, M.; Trojanowska, A.; Tylkowski, B.; Jastrzab, R. The Effect of pH on the Size of Silver Nanoparticles Obtained in the Reduction Reaction with Citric and Malic Acids. Materials 2020, 13, 5444. [Google Scholar] [CrossRef] [PubMed]
- Rai P, Jo JN, Wu XF, Yoon JM, Yu YT. Synthesis of well dispersed, regular shape ZnO nanorods: effect of pH, time and temperature. Journal of Nanoscience and Nanotechnology. 2011 Jan 1;11(1):647-51. https://www.ingentaconnect.com/contentone/asp/jnn/2011/00000011/00000001/art00116.
- Sajanlal, P.R.; Sreeprasad, T.S.; Samal, A.K.; Pradeep, T. Anisotropic nanomaterials: structure, growth, assembly, and functions. Nano Rev. 2011, 2. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef]
- Sundén B. Hydrogen, batteries and fuel cells. Academic Press; 2019 Jul 2. https://books.google.com/books?hl=en&lr=&id=eCugDwAAQBAJ&oi=fnd&pg=PP1&dq=+Sundén,+B.,+2019.+Hydrogen,+batteries+and+fuel+cells.+Academic+Press.&ots=Xddl8xJjrp&sig=PSghe3cvZP3uaPjgAOWrWoUcuDA.
- Salah, M.; Yehia, S.; Ali, R.T. Cytogenetic effect of some nanostructure polymers prepared via gamma irradiation on Vicia faba plant. Chem. Biol. Technol. Agric. 2022, 9, 1–16. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Flores, A.D.R.; Zhai, R.; et al. Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 2021, 17, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz AR, Al-Othman MR. Gold nanoparticles biosynthesis using zingiber officinale and their impact on the growth and chemical composition of lentil (lens culinaris medic.). Pak. J. Bot. 2019 Apr 1;51(2):443-50. http://mail.pakbs.org/pjbot/papers/1550951878.pdf.
- Verma, D.K.; Patel, S.; Kushwah, K.S. Green biosynthesis of silver nanoparticles and impact on growth, chlorophyll, yield and phytotoxicity of Phaseolus vulgaris L. Vegetos 2020, 33, 648–657. [Google Scholar] [CrossRef]
- Labeeb M, Badr A, Haroun SA, Mattar MZ, El-Kholy AS, El-Mehasseb IM. Ecofriendly synthesis of silver nanoparticles and their effects on early growth and cell division in roots of green pea (Pisum sativum L.). Gesunde Pflanz. 2020 Jun 1;72:113-27. https://www.researchgate.net/profile/Aziza-El-Kholy/publication/337948557_Ecofriendly_Synthesis_of_Silver_Nanoparticles_and_Their_Effects_on_Early_Growth_and_Cell_Division_in_Roots_of_Green_Pea_Pisum_sativum_L_Umweltfreundliche_Synthese_von_Silber-Nanopartikeln_und_ihre_Aus/links/5df795d2a6fdcc283724a04b/Ecofriendly-Synthesis-of-Silver-Nanoparticles-and-Their-Effects-on-Early-Growth-and-Cell-Division-in-Roots-of-Green-Pea-Pisum-sativum-L-Umweltfreundliche-Synthese-von-Silber-Nanopartikeln-und-ihre-Aus.pdf.
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K. Biogenic ZnO and Cu nanoparticles to improve seed germination quality in blackgram (Vigna mungo). Mater. Lett. 2018, 235, 164–167. [Google Scholar] [CrossRef]
- Awasthi, A.; Bansal, S.; Jangir, L.K.; Awasthi, G.; Awasthi, K.K.; Awasthi, K. Effect of ZnO Nanoparticles on Germination of Triticum aestivum Seeds. Macromol. Symp. 2017, 376. [Google Scholar] [CrossRef]
- Awan, S.; Shahzadi, K.; Javad, S.; Tariq, A.; Ahmad, A.; Ilyas, S. A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleracea var italic. J. Saudi Soc. Agric. Sci. 2020, 20, 18–24. [Google Scholar] [CrossRef]
- Suganya, P.; Rajamohan, C.; Mahalingam, P.U. Synthesis and surface modification of Zinc Nano rods using vermiwash of Eudrilus eugeniae and Functionalization to seed germination of green gram Vigna radiata. Mater. Res. Express 2018, 6, 025409. [Google Scholar] [CrossRef]
- Shyla KK, Natarajan N. Customizing zinc oxide, silver and titanium dioxide nanoparticles for enhancing groundnut seed quality. Indian Journal of Science and Technology. 2014 Sep 30:1376-81. https://ischolar.sscldl.in/index.php/indjst/article/view/59479.
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Sharma P, Chauhan NS. Effect on nanoparticles on plant cell morphology, physiology, and metabolism. InThe Impact of Nanoparticles on Agriculture and Soil 2023 Jan 1 (pp. 95-113). Academic Press. https://www.sciencedirect.com/science/article/pii/B978032391703200004X.
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Ceridono, M.; Urbán, P.; Bogni, A.; Ponti, J.; Gioria, S.; Kinsner-Ovaskainen, A. The agglomeration state of nanoparticles can influence the mechanism of their cellular internalisation. J. Nanobiotechnology 2017, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bruinink, A.; Wang, J.; Wick, P. Effect of particle agglomeration in nanotoxicology. Arch. Toxicol. 2015, 89, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Bini, M.; Brancolini, G.; Tozzini, V. Aggregation behavior of nanoparticles: Revisiting the phase diagram of colloids. Front. Mol. Biosci. 2022, 9, 986223. [Google Scholar] [CrossRef] [PubMed]
- Tilley RJ. Crystals and crystal structures. John Wiley & Sons; 2020 Aug 3. https://books.google.com/books?hl=en&lr=&id=VU7iDwAAQBAJ&oi=fnd&pg=PR9&dq=Tilley,+R.J.,+2020.+Crystals+and+crystal+structures.+John+Wiley+%26+Sons.&ots=CK1H94TTe_&sig=sbXXeV0p7Qw6S-3rqBi2IyzYwes.
- Haydar, S.; Ghosh, D.; Roy, S. Slow and controlled release nanofertilizers as an efficient tool for sustainable agriculture: Recent understanding and concerns. Plant Nano Biol. 2024, 7. [Google Scholar] [CrossRef]
- Martins, P.C.; Latorres, J.M.; Martins, V.G. Impact of starch nanocrystals on the physicochemical, thermal and structural characteristics of starch-based films. LWT 2022, 156. [Google Scholar] [CrossRef]
- Cahyono, O.; Minardi, S. Effect of Fast Dissolved Phosphorus Fertilizer on the Growth, Seed Product, and Phosphorus Uptake Efficiency of Soybean (Glycine max L.). AGRIVITA J. Agric. Sci. 2022, 44, 21–30. [Google Scholar] [CrossRef]
- Kalia, A.; Sharma, S.P.; Kaur, H. Nanoscale Fertilizers: Harnessing Boons for Enhanced Nutrient Use Efficiency and Crop Productivity. In Applications of Nanotechnology for Green Synthesis; Springer: Cham, Switzerland, 2019; pp. 191–208. [Google Scholar]
- Carmona, F.J.; Dal Sasso, G.; Bertolotti, F.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Pedersen, J.S.; Masciocchi, N.; Guagliardi, A. The role of nanoparticle structure and morphology in the dissolution kinetics and nutrient release of nitrate-doped calcium phosphate nanofertilizers. Sci. Rep. 2020, 10, 12396. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-De-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering Biomimetic Calcium Phosphate Nanoparticles: A Green Synthesis of Slow-Release Multinutrient (NPK) Nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- Jayanudin; Lestari, R. S.D. FERTILIZER ENCAPSULATION TO IMPROVE THE NUTRIENTS USE EFFICIENCY OF PLANT THROUGH SLOW/CONTROLLED RELEASE TO ENSURE FOOD SECURITY. Rasayan J. Chem. 2020, 13, 1074–1082. [Google Scholar] [CrossRef]
- Elsabagh, S.S.; Elkhatib, E.A.; Rashad, M. Novel nano-fertilizers derived from drinking water industry waste for sustained release of macronutrients: performance, kinetics and sorption mechanisms. Sci. Rep. 2024, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Carmona, F.J.; Guagliardi, A.; Masciocchi, N. Nanosized Calcium Phosphates as Novel Macronutrient Nano-Fertilizers. Nanomaterials 2022, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Sakhno, Y.; Degli Esposti, L.; Adamiano, A.; Borgatta, J.; Cahill, M.; Vaidya, S.; White, J.C.; Iafisco, M.; Jaisi, D.P. Citrate-Stabilized Amorphous Calcium Phosphate Nanoparticles Doped with Micronutrients as a Highly Efficient Nanofertilizer for Environmental Sustainability. ACS Agric. Sci. Technol. 2023, 3, 845–854. [Google Scholar] [CrossRef]
- Rehana, M.R.; Gladis, R.; Joseph, B. Controlled Release of Nutrients for Soil Productivity- A Review. Curr. J. Appl. Sci. Technol. 2022, 34–46. [Google Scholar] [CrossRef]
- Wesołowska M, Rymarczyk J, Góra R, Baranowski P, Sławiński C, Klimczyk M, Supryn G, Schimmelpfennig L. New slow-release fertilizers-economic, legal and practical aspects: a Review. International Agrophysics. 2021;35(1):11-24. https://bibliotekanauki.pl/articles/2083050.
- Chaudhary, I.J.; Neeraj, A.; Siddiqui, M.A.; Singh, V. Nutrient Management Technologies and the Role of Organic Matrix-Based Slow-Release Biofertilizers for Agricultural Sustainability: A Review. Agric. Rev. 2020, 41, 1–13. [Google Scholar] [CrossRef]
- Nagargade M, Tyagi V, Kumar D, Shukla SK, Pathak AD. Nanofertilizers: Importance in Nutrient Management. InNanotechnology in Agriculture and Environmental Science 2022 Nov 17 (pp. 69-80). CRC Press. https://www.taylorfrancis.com/chapters/edit/10.1201/9781003323945-6/nanofertilizers-importance-nutrient-management-mona-nagargade-vishal-tyagi-dileep-kumar-sk-shukla-ad-pathak.
- Yomso J, Menon S. Impact of nanofertilizers on growth and yield parameters of rice crop; A Review. J. Pharm. Innov. 2021;10:249-53. https://www.thepharmajournal.com/archives/2021/vol10issue6/PartD/10-5-213-129.
- Cheng, B.; Wang, C.; Yue, L.; Chen, F.; Cao, X.; Lan, Q.; Liu, T.; Wang, Z. Selenium nanomaterials improve the quality of lettuce (Lactuca sativa L.) by modulating root growth, nutrient availability, and photosynthesis. NanoImpact 2023, 29, 100449. [Google Scholar] [CrossRef]
- Rahman, H.; Hasan, N.; Nigar, S.; Ma, F.; Aly, M.A.S.; Khan, Z.H. Synthesis and Characterization of a Mixed Nanofertilizer Influencing the Nutrient Use Efficiency, Productivity, and Nutritive Value of Tomato Fruits. ACS Omega 2021, 6, 27112–27120. [Google Scholar] [CrossRef]
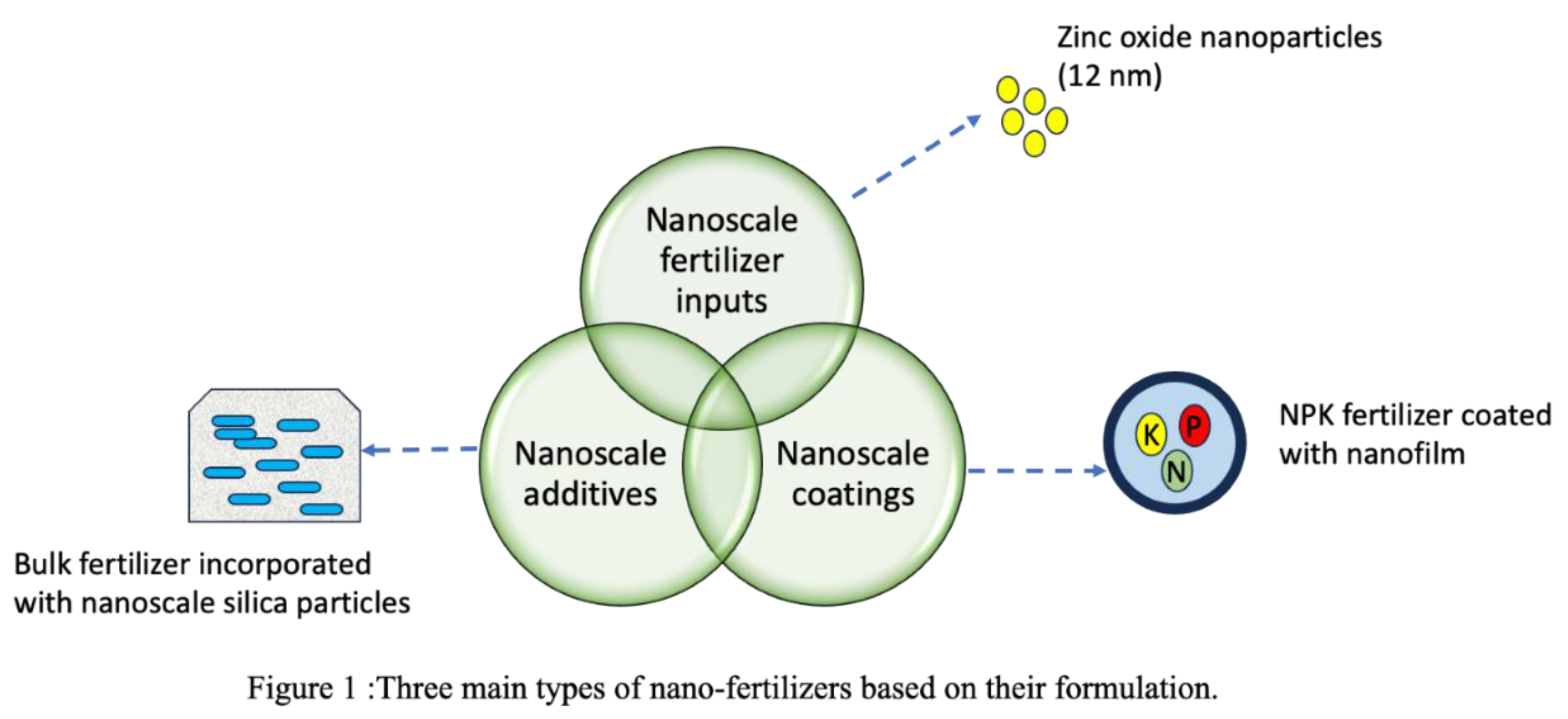
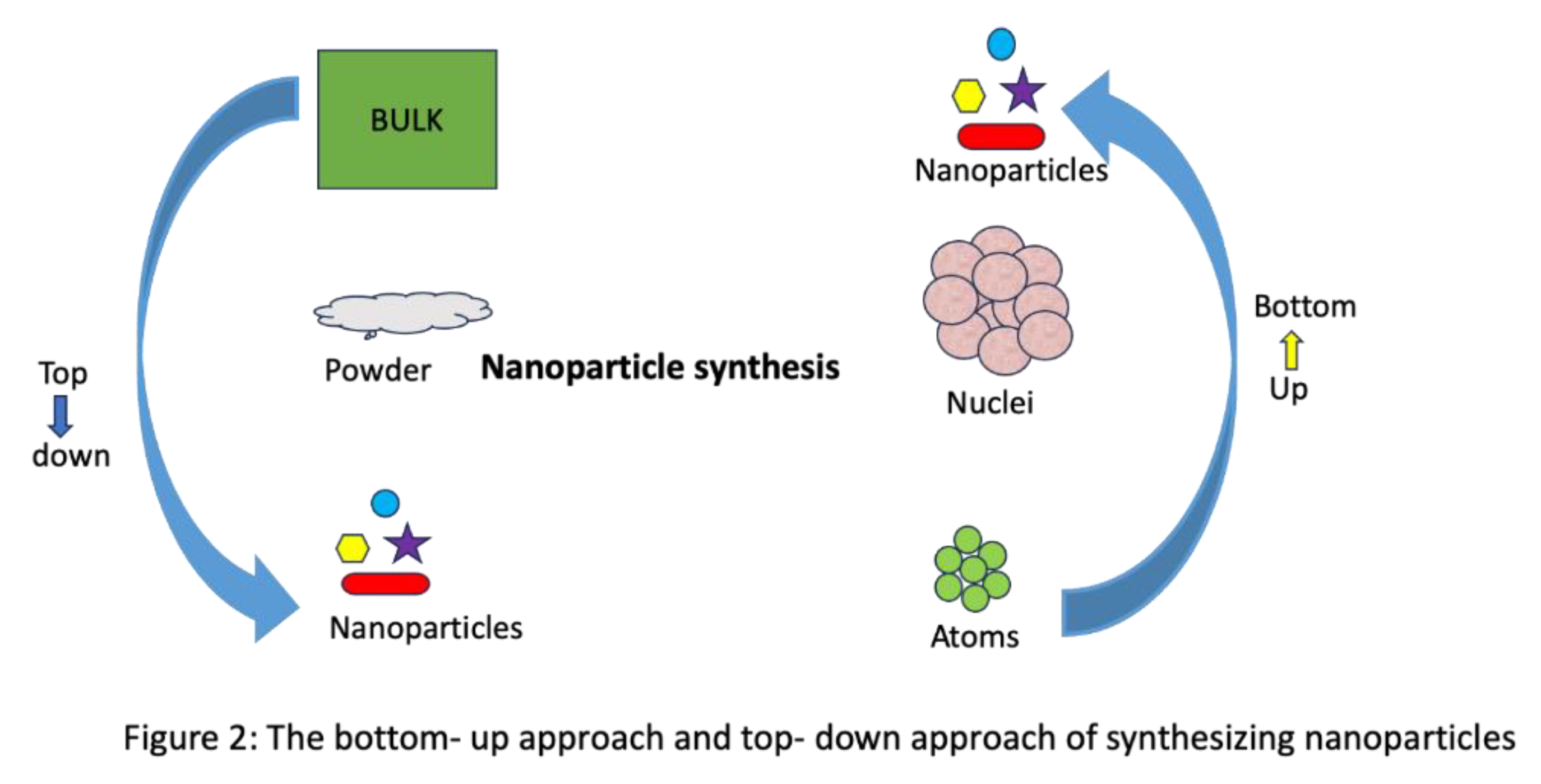
| Crop type | Nanoparticle type | Nanoparticle size | Effect on nutrient uptake | Reference |
|---|---|---|---|---|
| Watermelon | AgNPs | 20 nm |
|
[38] |
| 60 nm |
|
|||
| Nicotiana xanthi | AgNPs | 3.5 nm |
|
[39] |
| 18 nm |
|
|||
| Soybean | CuONPs | 25 nm |
|
[35] |
| 50 nm |
|
|||
| Wheat | SeNPs | 40 nm |
|
[34] |
| 140 nm |
|
|||
| Cucumber | Ceria NPs | 7 nm |
|
[36] |
| 25 nm |
|
|||
| Allium porrum | water-suspended fluorescent polystyrene NPs | 43 nm |
|
[40] |
| 1100 nm |
|
| Type of fertilizers | Nanoparticle material release time | Bulk material release time | Reference |
|---|---|---|---|
| Nitrogen-based fertilizer | 1000 hours (about 1 and a half months) | 500 hours | [50] |
| Nitrate nitrogen fertilizer | Exceeded 50 days | 10-12 days | [54] |
| APTMS-modified zeolite | 120 minutes (2 hours) | 10 minutes | [55] |
| urea- hydroxyapatite fertilizer | 60 days | 30 days | [56] |
| Urea-loaded polycaprolactone nanocomposite | > 90 hours | < 25 hours | [57] |
| Phosphate fertilizer | 40-50 days | 10-12 days | [58] |
| DAP | 60 days | 15 days | [59] |
| Control-release fertilizers | Properties | Reference |
|---|---|---|
| 1. Slow-release fertilizer |
|
[66] |
| 2. Quick release fertilizer |
|
[12] |
| 3. Specific-release fertilizer |
|
[13] |
| 4. Moisture release fertilizer |
|
[12,13] |
| 5. Heat release fertilizer |
|
[12] |
| 6. pH release fertilizer |
|
[13] |
| 7. Ultrasound release |
|
[66] |
| 8. Magnetic release |
|
[12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





