Submitted:
16 June 2024
Posted:
18 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Proteins Involved in Vitamin B12 Metabolism
2.1. Gastrointestinal Vitamin B12 Transport
2.1.1. Haptocorrin (HC) and Intrinsic Factor (IF)
2.1.2. Cubam Receptor
2.1.3. Multidrug Resistant Protein 1 (MRP1)
2.2. Proteins involved in Vitamin B12 Blood Transport
2.2.1. Transcobalamin II (TCN2)
2.3. Proteins Involved in Intracellular Vitamin B12 Transport
2.3.1. CD320 Receptor
2.3.2. ATP Binding Cassette Subfamily D Member (ABCD4) and Lysosomal Cobalamin Transport Escort Protein (LMBD1)
2.3.3. Methylmalonic Aciduria and Homocystinuria Type C Protein (MMACHC)
2.3.4. Methylmalonic Aciduria and Homocystinuria Type D Protein (MMADHC)
2.4. Vitamin B12-Dependent Intracellular Enzymes
2.4.1. Methionine Synthase (MTR)
2.4.2. Methylmalonyl-CoA Mutase (MMUT)
3. Vitamin B12 Deficiency
3.1. Epidemiology and Symptoms of Vitamin B12 Deficiency
3.2. Diagnosis and Treatment of Vitamin B12 Deficiency
3.3. Long-Distance Consequences of Vitamin B12 Deficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Folkers, K., D.D., Ed.; J.W.& S. History of Vitamin B12: Pernicious Anemia to Crystalline Cyanocobalamin. In Vitamin B12; NY, USA, 1982; Vol. I.
- Randaccio, L.; Geremia, S.; Demitri, N.; Wuerges, J. Vitamin B12: Unique Metalorganic Compounds and the Most Complex Vitamins. Molecules 2010, 15, 3228–3259. [Google Scholar] [CrossRef]
- Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, D.C., 1998; ISBN 978-0-309-06554-2.
- Obeid, R.; Fedosov, S.N.; Nexo, E. Cobalamin Coenzyme Forms Are Not Likely to Be Superior to Cyano- and Hydroxyl-cobalamin in Prevention or Treatment of Cobalamin Deficiency. Mol. Nutr. Food Res. 2015, 59, 1364–1372. [Google Scholar] [CrossRef]
- Herrmann, W.; Obeid, R. Cobalamin Deficiency. In; 2012; pp. 301–322.
- Santos, F.; Vera, J.L.; Lamosa, P.; de Valdez, G.F.; de Vos, W.M.; Santos, H.; Sesma, F.; Hugenholtz, J. Pseudovitamin Is the Corrinoid Produced by Lactobacillus Reuteri CRL1098 under Anaerobic Conditions. FEBS Lett. 2007, 581, 4865–4870. [Google Scholar] [CrossRef] [PubMed]
- Latner, A.L.; Raine, L. Effect of Analogues on the Uptake of Vitamin B12 by the Intact Rat. Nature 1957, 180, 1197–1198. [Google Scholar] [CrossRef] [PubMed]
- Bito, T.; Bito, M.; Hirooka, T.; Okamoto, N.; Harada, N.; Yamaji, R.; Nakano, Y.; Inui, H.; Watanabe, F. Biological Activity of Pseudovitamin B12 on Cobalamin-Dependent Methylmalonyl-CoA Mutase and Methionine Synthase in Mammalian Cultured COS-7 Cells. Molecules 2020, 25, 3268. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, F. Vitamin B12 Sources and Bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 Deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Gherasim, C.; Lofgren, M.; Banerjee, R. Navigating the B12 Road: Assimilation, Delivery, and Disorders of Cobalamin. J. Biol. Chem. 2013, 288, 13186–13193. [Google Scholar] [CrossRef]
- O’Leary, F.; Samman, S. Vitamin B12 in Health and Disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef]
- Kozyraki, R.; Cases, O. Vitamin B12 Absorption: Mammalian Physiology and Acquired and Inherited Disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Wuerges, J.; Geremia, S.; Randaccio, L. Structural Study on Ligand Specificity of Human Vitamin B12 Transporters. Biochem. J. 2007, 403, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.S.; Gordon, M.M.; Chen, Z.; Rajashankar, K.R.; Ealick, S.E.; Alpers, D.H.; Sukumar, N. Crystal Structure of Human Intrinsic Factor: Cobalamin Complex at 2.6-Å Resolution. Proc. Natl. Acad. Sci. USA 2007, 104, 17311–17316. [Google Scholar] [CrossRef] [PubMed]
- Alpers, D.H.; Russell-Jones, G. Gastric Intrinsic Factor: The Gastric and Small Intestinal Stages of Cobalamin Absorption. A Personal Journey. Biochimie 2013, 95, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, J.E.; Gordon, M.M.; Taggart, R.T.; Mohandas, T.K.; Alpers, D.H. Human Gastric Intrinsic Factor: Characterization of CDNA and Genomic Clones and Localization to Human Chromosome 11. Genomics 1991, 10, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Coleman, P.S.; Dupont, B. The Biochemical and Genetic Basis for the Microheterogeneity of Human R-Type Vitamin B12 Binding Proteins. Blood 1982, 59, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Furger, E.; Frei, D.C.; Schibli, R.; Fischer, E.; Prota, A.E. Structural Basis for Universal Corrinoid Recognition by the Cobalamin Transport Protein Haptocorrin. J. Biol. Chem. 2013, 288, 25466–25476. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Rasmussen, M.R.; Andersen, C.B.F.; Nexø, E.; Moestrup, S.K. Vitamin B12 Transport from Food to the Body’s Cells—A Sophisticated, Multistep Pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef]
- Allen, R.H.; Seetharam, B.; Podell, E.; Alpers, D.H. Effect of Proteolytic Enzymes on the Binding of Cobalamin to R Protein and Intrinsic Factor. J. Clin. Investig. 1978, 61, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.T.; Warwick, R.R.G.; Simpson, J.D.; Shearman, D.J.C. Does Malabsorption Of Vitamin B12 Occur In Chronic Pancreatitis ? Lancet 1972, 300, 241–243. [Google Scholar] [CrossRef]
- Guéant, J.-L.; Champigneulle, B.; Gaucher, P.; Nicolas, J.-P. Malabsorption of Vitamin B12 in Pancreatic Insufficiency of the Adult and of the Child. Pancreas 1990, 5, 559–567. [Google Scholar] [CrossRef]
- Forsmark, C.E. Diagnosis and Management of Exocrine Pancreatic Insufficiency. Curr. Treat. Options Gastroenterol. 2018, 16, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Hygum, K.; Lildballe, D.L.; Greibe, E.H.; Morkbak, A.L.; Poulsen, S.S.; Sorensen, B.S.; Petersen, T.E.; Nexo, E. Mouse Transcobalamin Has Features Resembling Both Human Transcobalamin and Haptocorrin. PLoS ONE 2011, 6, e20638. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N.; Fedosova, N.U.; Berglund, L.; Moestrup, S.K.; Nexø, E.; Petersen, T.E. Assembly of the Intrinsic Factor Domains and Oligomerization of the Protein in the Presence of Cobalamin. Biochemistry 2004, 43, 15095–15102. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N.; Fedosova, N.U.; Kräutler, B.; Nexø, E.; Petersen, T.E. Mechanisms of Discrimination between Cobalamins and Their Natural Analogues during Their Binding to the Specific B 12 -Transporting Proteins. Biochemistry 2007, 46, 6446–6458. [Google Scholar] [CrossRef] [PubMed]
- Ayesh, M.H.; Jadalah, K.; Al Awadi, E.; Alawneh, K.; Khassawneh, B. Association between Vitamin B12 Level and Anti-Parietal Cells and Anti-Intrinsic Factor Antibodies among Adult Jordanian Patients with Helicobacter Pylori Infection. Braz. J. Infect. Dis. 2013, 17, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Kulnigg-Dabsch, S. Autoimmune Gastritis. Wien. Med. Wochenschr. 2016, 166, 424–430. [Google Scholar] [CrossRef]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.-H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune Gastritis. Nat. Rev. Dis. Primers 2020, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Htut, T.W.; Thein, K.Z.; Oo, T.H. Pernicious Anemia: Pathophysiology and Diagnostic Difficulties. J. Evid. Based Med. 2021, 14, 161–169. [Google Scholar] [CrossRef]
- Qudsiya Z, J.O.D. Subacute Combined Degeneration of the Spinal Cord. StatPearls Publishing 2022.
- Larsen, C.; Etzerodt, A.; Madsen, M.; Skjødt, K.; Moestrup, S.K.; Andersen, C.B.F. Structural Assembly of the Megadalton-Sized Receptor for Intestinal Vitamin B12 Uptake and Kidney Protein Reabsorption. Nat. Commun. 2018, 9, 5204. [Google Scholar] [CrossRef]
- Pedersen, G.A.; Chakraborty, S.; Steinhauser, A.L.; Traub, L.M.; Madsen, M. AMN Directs Endocytosis of the Intrinsic Factor-Vitamin B12 Receptor Cubam by Engaging ARH or Dab2. Traffic 2010, 11, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Strope, S.; Rivi, R.; Metzger, T.; Manova, K.; Lacy, E. Mouse Amnionless, Which Is Required for Primitive Streak Assembly,Mediates Cell-Surface Localization and Endocytic Function of Cubilin on Visceral Endoderm and Kidney Proximal Tubules. Development 2004, 131, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Perea-Gomez, A.; Cases, O.; Lelièvre, V.; Pulina, M.V.; Collignon, J.; Hadjantonakis, A.-K.; Kozyraki, R. Loss of Cubilin, the Intrinsic Factor-Vitamin B12 Receptor, Impairs Visceral Endoderm Endocytosis and Endodermal Patterning in the Mouse. Sci. Rep. 2019, 9, 10168. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.I.; Verroust, P.J.; Nielsen, R. Receptor-Mediated Endocytosis in Renal Proximal Tubule. Pflug. Arch. 2009, 458, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.F.; Madsen, M.; Storm, T.; Moestrup, S.K.; Andersen, G.R. Structural Basis for Receptor Recognition of Vitamin-B12–Intrinsic Factor Complexes. Nature 2010, 464, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Amsellem, S.; Gburek, J.; Hamard, G.; Nielsen, R.; Willnow, T.E.; Devuyst, O.; Nexo, E.; Verroust, P.J.; Christensen, E.I.; Kozyraki, R. Cubilin Is Essential for Albumin Reabsorption in the Renal Proximal Tubule. J. Am. Soc. Nephrol. 2010, 21, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.C.; Madsen, M.; Højrup, P.; Christensen, E.I.; Tanner, S.M.; de la Chapelle, A.; He, Q.; Moestrup, S.K. The Functional Cobalamin (Vitamin B12)–Intrinsic Factor Receptor Is a Novel Complex of Cubilin and Amnionless. Blood 2004, 103, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.L.; Andersen, R.K.; Hager, H.; Madsen, M. Lack of Megalin Expression in Adult Human Terminal Ileum Suggests Megalin-Independent Cubilin/Amnionless Activity during Vitamin B 12 Absorption. Physiol. Rep. 2014, 2, e12086. [Google Scholar] [CrossRef] [PubMed]
- Gräsbeck, R. Imerslund-Gräsbeck Syndrome (Selective Vitamin B12 Malabsorption with Proteinuria). Orphanet J. Rare Dis. 2006, 1, 17. [Google Scholar] [CrossRef]
- Storm, T.; Zeitz, C.; Cases, O.; Amsellem, S.; Verroust, P.J.; Madsen, M.; Benoist, J.-F.; Passemard, S.; Lebon, S.; Jønsson, I.M.; et al. Detailed Investigations of Proximal Tubular Function in Imerslund-Gräsbeck Syndrome. BMC Med. Genet. 2013, 14, 111. [Google Scholar] [CrossRef]
- Ercan, Z.; Demir, M.E.; Ulas, T.; Ingec, M.; Horoz, M. A Long-Term Follow-up of an Imerslund-Grasbeck Syndrome Patient with Proteinuria. Nefrologia 2013, 33, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Pannérec, A.; Migliavacca, E.; De Castro, A.; Michaud, J.; Karaz, S.; Goulet, L.; Rezzi, S.; Ng, T.P.; Bosco, N.; Larbi, A.; et al. Vitamin B12 Deficiency and Impaired Expression of Amnionless during Aging. J. Cachexia Sarcopenia Muscle 2018, 9, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P.C. Multidrug Resistance Protein 1 (MRP1, ABCC1), a “Multitasking” ATP-Binding Cassette (ABC) Transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Bhardwaj, G.; Gerlach, J.; Mackie, J.; Grant, C.; Almquist, K.; Stewart, A.; Kurz, E.; Duncan, A.; Deeley, R. Overexpression of a Transporter Gene in a Multidrug-Resistant Human Lung Cancer Cell Line. Science (1979) 1992, 258, 1650–1654. [Google Scholar] [CrossRef] [PubMed]
- Bakos, É.; Homolya, L. Portrait of Multifaceted Transporter, the Multidrug Resistance-Associated Protein 1 (MRP1/ABCC1). Pflug. Arch. 2007, 453, 621–641. [Google Scholar] [CrossRef] [PubMed]
- Flens, M.J.; Zaman, G.J.; van der Valk, P.; Izquierdo, M.A.; Schroeijers, A.B.; Scheffer, G.L.; van der Groep, P.; de Haas, M.; Meijer, C.J.; Scheper, R.J. Tissue Distribution of the Multidrug Resistance Protein. Am. J. Pathol. 1996, 148, 1237–1247. [Google Scholar] [PubMed]
- Peng, K.-C.; Cluzeaud, F.; Bens, M.; Duong Van Huyen, J.-P.; Wioland, M.A.; Lacave, R.; Vandewalle, A. Tissue and Cell Distribution of the Multidrug Resistance-Associated Protein (MRP) in Mouse Intestine and Kidney. J. Histochem. Cytochem. 1999, 47, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, A.; Simon, S.M. Subcellular Localization and Activity of Multidrug Resistance Proteins. Mol. Biol. Cell 2003, 14, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Beedholm-Ebsen, R.; van de Wetering, K.; Hardlei, T.; Nexø, E.; Borst, P.; Moestrup, S.K. Identification of Multidrug Resistance Protein 1 (MRP1/ABCC1) as a Molecular Gate for Cellular Export of Cobalamin. Blood 2010, 115, 1632–1639. [Google Scholar] [CrossRef]
- Seetharam, B.; Yammani, R.R. Cobalamin Transport Proteins and Their Cell-Surface Receptors. Expert. Rev. Mol. Med. 2003, 5, 1–18. [Google Scholar] [CrossRef]
- Shah, N.P.; Beech, C.M.; Sturm, A.C.; Tanner, S.M. Investigation of the ABC Transporter MRP1 in Selected Patients with Presumed Defects in Vitamin B12 Absorption. Blood 2011, 117, 4397–4398. [Google Scholar] [CrossRef] [PubMed]
- Quadros, E. V.; Rothenberg, S.P.; Pan, Y.C.; Stein, S. Purification and Molecular Characterization of Human Transcobalamin II. J. Biol. Chem. 1986, 261, 15455–15460. [Google Scholar] [CrossRef] [PubMed]
- Quadros, E.V.; Rothenberg, S.P.; Jaffe, E.A. Endothelial Cells from Human Umbilical Vein Secrete Functional Transcobalamin II. Am. J. Physiol. -Cell Physiol. 1989, 256, C296–C303. [Google Scholar] [CrossRef] [PubMed]
- Quadros, E.V.; Regec, A.L.; Khan, K.M.F.; Quadros, E.; Rothenberg, S.P. Transcobalamin II Synthesized in the Intestinal Villi Facilitates Transfer of Cobalamin to the Portal Blood. Am. J. Physiol. -Gastrointest. Liver Physiol. 1999, 277, G161–G166. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Seetharam, S.; Seetharam, B. Genomic Structure of Human Transcobalamin II: Comparison to Human Intrinsic Factor and Transcobalamin I. Biochem. Biophys. Res. Commun. 1995, 208, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Wuerges, J.; Garau, G.; Geremia, S.; Fedosov, S.N.; Petersen, T.E.; Randaccio, L. Structural Basis for Mammalian Vitamin B 12 Transport by Transcobalamin. Proc. Natl. Acad. Sci. 2006, 103, 4386–4391. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Morkbak, A.L.; Munz, W.; Nexo, E.; Herrmann, W. The Cobalamin-Binding Proteins Transcobalamin and Haptocorrin in Maternal and Cord Blood Sera at Birth. Clin. Chem. 2006, 52, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.L.; Schneider, R.J.; Mehlman, C.S.; Allen, R.H. Human Plasma R-Type Vitamin B12-Binding Proteins. II. The Role of Transcobalamin I, Transcobalamin III, and the Normal Granulocyte Vitamin B12-Binding Protein in the Plasma Transport of Vitamin B12. J. Biol. Chem. 1975, 250, 7707–7713. [Google Scholar] [CrossRef] [PubMed]
- Sugihara, T.; Koda, M.; Okamoto, T.; Miyoshi, K.; Matono, T.; Oyama, K.; Hosho, K.; Okano, J.-I.; Isomoto, H.; Murawaki, Y. Falsely Elevated Serum Vitamin B12 Levels Were Associated with the Severity and Prognosis of Chronic Viral Liver Disease. Yonago Acta Med. 2017, 60, 31–39. [Google Scholar]
- Thorpe, S.J.; Rigsby, P.; Roberts, G.; Lee, A.; Hamilton, M.; Craig, D. An International Standard for Holotranscobalamin (HoloTC): International Collaborative Study to Assign a HoloTC Value to the International Standard for Vitamin B12 and Serum Folate. Clin. Chem. Lab. Med. (CCLM) 2016, 54, 1467–1472. [Google Scholar] [CrossRef]
- Knoepfel, C.; Michel Blanco, M.; Nydegger, U.; Risch, L.; Renz, H.; Risch, M. Failure of the Holotranscobalamin Assay in Vitamin B12-Deficient Patients. LaboratoriumsMedizin 2018, 42, 141–147. [Google Scholar] [CrossRef]
- Golding, P.H. Experimental Vitamin B12 Deficiency in a Human Subject: A Longitudinal Investigation of the Performance of the Holotranscobalamin (HoloTC, Active-B12) Immunoassay. Springerplus 2016, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Golding, P.H. Holotranscobalamin (HoloTC, Active-B12) and Herbert’s Model for the Development of Vitamin B12 Deficiency: A Review and Alternative Hypothesis. Springerplus 2016, 5, 668. [Google Scholar] [CrossRef]
- Trakadis, Y.J.; Alfares, A.; Bodamer, O.A.; Buyukavci, M.; Christodoulou, J.; Connor, P.; Glamuzina, E.; Gonzalez-Fernandez, F.; Bibi, H.; Echenne, B.; et al. Update on Transcobalamin Deficiency: Clinical Presentation, Treatment and Outcome. J. Inherit. Metab. Dis. 2014, 37, 461–473. [Google Scholar] [CrossRef]
- Nissen, P.H.; Nordwall, M.; Hoffmann-Lücke, E.; Sorensen, B.S.; Nexo, E. Transcobalamin Deficiency Caused by Compound Heterozygosity for Two Novel Mutations in the TCN2 Gene: A Study of Two Affected Siblings, Their Brother, and Their Parents. J. Inherit. Metab. Dis. 2010, 33, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.A.M.; Alfaifi, J.; Alqahtani, Y.A.M.; Aljabri, M.F.; Kamal, N.M.; Althopaity, J.; Althobaiti, K.A.; Almalki, A.M.; Abosabie, S.A.S.; Abosabie, S.A.; et al. “Progressive Myoclonic Ataxia and Developmental/Epileptic Encephalopathy Associated with a Novel Homozygous Mutation in <scp> TCN 2 </Scp> Gene. ” Mol. Genet. Genom. Med. 2024, 12. [Google Scholar] [CrossRef]
- Quadros, E.V. Advances in the Understanding of Cobalamin Assimilation and Metabolism. Br. J. Haematol. 2010, 148, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-J.; Choi, J.-S.; Park, C.-H.; Yoon, S.-O.; Jeoung, D.-I.; Kim, Y.-M.; Choe, J.-S. Expression of CD320 in Human B Cells in Addition to Follicular Dendritic Cells. BMB Rep. 2008, 41, 863–867. [Google Scholar] [CrossRef]
- Jiang, W.; Nakayama, Y.; Sequeira, J.M.; Quadros, E.V. Mapping the Functional Domains of TCblR/ CD320 , the Receptor for Cellular Uptake of Transcobalamin-bound Cobalamin. FASEB J. 2013, 27, 2988–2994. [Google Scholar] [CrossRef]
- Arendt, J.F.B.; Quadros, E.V.; Nexo, E. Soluble Transcobalamin Receptor, SCD320, Is Present in Human Serum and Relates to Serum Cobalamin – Establishment and Validation of an ELISA. Clin. Chem. Lab. Med. 2012, 50. [Google Scholar] [CrossRef]
- Youngdahl-Turner, P.; Mellman, I.S.; Allen, R.H.; Rosenberg, L.E. Protein Mediated Vitamin Uptake. Exp. Cell Res. 1979, 118, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Woo, J.-S.; Schmitz, J.; Prinz, B.; Root, K.; Chen, F.; Bloch, J.S.; Zenobi, R.; Locher, K.P. Structural Basis of Transcobalamin Recognition by Human CD320 Receptor. Nat. Commun. 2016, 7, 12100. [Google Scholar] [CrossRef] [PubMed]
- Gick, G.G.; Arora, K.; Sequeira, J.M.; Nakayama, Y.; Lai, S.-C.; Quadros, E.V. Cellular Uptake of Vitamin B12: Role and Fate of TCblR/CD320, the Transcobalamin Receptor. Exp. Cell Res. 2020, 396, 112256. [Google Scholar] [CrossRef]
- Quadros, E.V.; Jacobsen, D.W. The Dynamics of Cobalamin Utilization in L-1210 Mouse Leukemia Cells: A Model of Cellular Cobalamin Metabolism. Biochim. Et Biophys. Acta (BBA) - General. Subj. 1995, 1244, 395–403. [Google Scholar] [CrossRef]
- Bernard, D.J.; Pangilinan, F.J.; Cheng, J.; Molloy, A.M.; Brody, L.C. Mice Lacking the Transcobalamin-Vitamin B12 Receptor, CD320, Suffer from Anemia and Reproductive Deficits When Fed Vitamin B12-Deficient Diet. Hum. Mol. Genet. 2018, 27, 3627–3640. [Google Scholar] [CrossRef] [PubMed]
- Westerman; Evans; Metz Neutrophil Hypersegmentation in Iron Deficiency Anaemia: A Case-Control Study. Br. J. Haematol. 1999, 107, 512–515. [CrossRef] [PubMed]
- Arora, K.; Sequeira, J.M.; Hernández, A.I.; Alarcon, J.M.; Quadros, E.V. Behavioral Alterations Are Associated with Vitamin B12 Deficiency in the Transcobalamin Receptor/CD320 KO Mouse. PLoS ONE 2017, 12, e0177156. [Google Scholar] [CrossRef] [PubMed]
- Fernàndez-Roig, S.; Lai, S.-C.; Murphy, M.M.; Fernandez-Ballart, J.; Quadros, E. V Vitamin B12 Deficiency in the Brain Leads to DNA Hypomethylation in the TCblR/CD320 Knockout Mouse. Nutr Metab (Lond) 2012, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Kurnat-Thoma, E.L.; Pangilinan, F.; Matteini, A.M.; Wong, B.; Pepper, G.A.; Stabler, S.P.; Guralnik, J.M.; Brody, L.C. Association of Transcobalamin II ( TCN2 ) and Transcobalamin II-Receptor ( TCblR ) Genetic Variations With Cobalamin Deficiency Parameters in Elderly Women. Biol. Res. Nurs. 2015, 17, 444–454. [Google Scholar] [CrossRef]
- Quadros, E.V.; Lai, S.-C.; Nakayama, Y.; Sequeira, J.M.; Hannibal, L.; Wang, S.; Jacobsen, D.W.; Fedosov, S.; Wright, E.; Gallagher, R.C.; et al. Positive Newborn Screen for Methylmalonic Aciduria Identifies the First Mutation in TCblR/CD320, the Gene for Cellular Uptake of Transcobalamin-Bound Vitamin B12. Hum. Mutat. 2010, 31, 924–929. [Google Scholar] [CrossRef]
- Coelho, D.; Kim, J.C.; Miousse, I.R.; Fung, S.; du Moulin, M.; Buers, I.; Suormala, T.; Burda, P.; Frapolli, M.; Stucki, M.; et al. Mutations in ABCD4 Cause a New Inborn Error of Vitamin B12 Metabolism. Nat. Genet. 2012, 44, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Seeger, M.A.; van Veen, H.W. Molecular Basis of Multidrug Transport by ABC Transporters. Biochim. Et Biophys. Acta (BBA) - Proteins Proteom. 2009, 1794, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Feng, Z.; Hou, W.-T.; Jiang, Y.-L.; Wang, L.; Sun, L.; Zhou, C.-Z.; Chen, Y. Cryo-EM Structure of Human Lysosomal Cobalamin Exporter ABCD4. Cell Res. 2019, 29, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Deme, J.C.; Hancock, M.A.; Xia, X.; Shintre, C.A.; Plesa, M.; Kim, J.C.; Carpenter, E.P.; Rosenblatt, D.S.; Coulton, J.W. Purification and Interaction Analyses of Two Human Lysosomal Vitamin B 12 Transporters: LMBD1 and ABCD4. Mol. Membr. Biol. 2014, 31, 250–261. [Google Scholar] [CrossRef]
- Rutsch, F.; Gailus, S.; Miousse, I.R.; Suormala, T.; Sagné, C.; Toliat, M.R.; Nürnberg, G.; Wittkampf, T.; Buers, I.; Sharifi, A.; et al. Identification of a Putative Lysosomal Cobalamin Exporter Altered in the CblF Defect of Vitamin B12 Metabolism. Nat. Genet. 2009, 41, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, K.; Okamoto, T.; Morita, M.; Imanaka, T. Translocation of the ABC Transporter ABCD4 from the Endoplasmic Reticulum to Lysosomes Requires the Escort Protein LMBD1. Sci. Rep. 2016, 6, 30183. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Rosenblatt, D.S. Lessons in Biology from Patients with Inherited Disorders of Vitamin B12 and Folate Metabolism. Biochimie 2016, 126, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.L.; C.N.; A.D.; V.C.P. Disorders of Intracellular Cobalamin Metabolism 2021.
- Kim, J.C.; Lee, N.-C.; Hwu, P.W.-L.; Chien, Y.-H.; Fahiminiya, S.; Majewski, J.; Watkins, D.; Rosenblatt, D.S. Late Onset of Symptoms in an Atypical Patient with the CblJ Inborn Error of Vitamin B12 Metabolism: Diagnosis and Novel Mutation Revealed by Exome Sequencing. Mol. Genet. Metab. 2012, 107, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Miousse, I.R.; Watkins, D.; Rosenblatt, D.S. Novel Splice Site Mutations and a Large Deletion in Three Patients with the CblF Inborn Error of Vitamin B12 Metabolism. Mol. Genet. Metab. 2011, 102, 505–507. [Google Scholar] [CrossRef]
- Alfadhel, M.; Lillquist, Y.P.; Davis, C.; Junker, A.K.; Stockler-Ipsiroglu, S. Eighteen-year Follow-up of a Patient with Cobalamin F Disease (CblF): Report and Review. Am. J. Med. Genet. A 2011, 155, 2571–2577. [Google Scholar] [CrossRef]
- Lerner-Ellis, J.P.; Anastasio, N.; Liu, J.; Coelho, D.; Suormala, T.; Stucki, M.; Loewy, A.D.; Gurd, S.; Grundberg, E.; Morel, C.F.; et al. Spectrum of Mutations in MMACHC , Allelic Expression, and Evidence for Genotype–Phenotype Correlations. Hum. Mutat. 2009, 30, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Lerner-Ellis, J.P.; Tirone, J.C.; Pawelek, P.D.; Doré, C.; Atkinson, J.L.; Watkins, D.; Morel, C.F.; Fujiwara, T.M.; Moras, E.; Hosack, A.R.; et al. Identification of the Gene Responsible for Methylmalonic Aciduria and Homocystinuria, CblC Type. Nat. Genet. 2006, 38, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Guéant, J.-L.; Chéry, C.; Oussalah, A.; Nadaf, J.; Coelho, D.; Josse, T.; Flayac, J.; Robert, A.; Koscinski, I.; Gastin, I.; et al. A PRDX1 Mutant Allele Causes a MMACHC Secondary Epimutation in CblC Patients. Nat. Commun. 2018, 9, 67. [Google Scholar] [CrossRef]
- Kim, J.; Gherasim, C.; Banerjee, R. Decyanation of Vitamin B12 by a Trafficking Chaperone. Proc. Natl. Acad. Sci. U S A 2008, 105, 14551–14554. [Google Scholar] [CrossRef] [PubMed]
- Bassila, C.; Ghemrawi, R.; Flayac, J.; Froese, D.S.; Baumgartner, M.R.; Guéant, J.-L.; Coelho, D. Methionine Synthase and Methionine Synthase Reductase Interact with MMACHC and with MMADHC. Biochim. Et Biophys. Acta (BBA) - Mol. Basis Dis. 2017, 1863, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, S.; Brezzi, B.; Bracciamà, V.; Barreca, A.; Nozza, P.; Vaisitti, T.; Amoroso, A.; Deaglio, S.; Manganaro, M.; Porta, F.; et al. Adult-Onset CblC Deficiency: A Challenging Diagnosis Involving Different Adult Clinical Specialists. Orphanet J. Rare Dis. 2022, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Sorin, M.; Watkins, D.; Gilfix, B.M.; Rosenblatt, D.S. Methionine Dependence in Tumor Cells: The Potential Role of Cobalamin and MMACHC. Mol. Genet. Metab. 2021, 132, 155–161. [Google Scholar] [CrossRef]
- Coelho, D.; Suormala, T.; Stucki, M.; Lerner-Ellis, J.P.; Rosenblatt, D.S.; Newbold, R.F.; Baumgartner, M.R.; Fowler, B. Gene Identification for the CblD Defect of Vitamin B 12 Metabolism. New Engl. J. Med. 2008, 358, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Pupavac, M.; Garcia, M.A.M.; Rosenblatt, D.S.; Jerome-Majewska, L.A. Expression of Mmachc and Mmadhc during Mouse Organogenesis. Mol. Genet. Metab. 2011, 103, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Stucki, M.; Coelho, D.; Suormala, T.; Burda, P.; Fowler, B.; Baumgartner, M.R. Molecular Mechanisms Leading to Three Different Phenotypes in the CblD Defect of Intracellular Cobalamin Metabolism. Hum. Mol. Genet. 2012, 21, 1410–1418. [Google Scholar] [CrossRef]
- Plesa, M.; Kim, J.; Paquette, S.G.; Gagnon, H.; Ng-Thow-Hing, C.; Gibbs, B.F.; Hancock, M.A.; Rosenblatt, D.S.; Coulton, J.W. Interaction between MMACHC and MMADHC, Two Human Proteins Participating in Intracellular Vitamin B12 Metabolism. Mol. Genet. Metab. 2011, 102, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Deme, J.C.; Miousse, I.R.; Plesa, M.; Kim, J.C.; Hancock, M.A.; Mah, W.; Rosenblatt, D.S.; Coulton, J.W. Structural Features of Recombinant MMADHC Isoforms and Their Interactions with MMACHC, Proteins of Mammalian Vitamin B12 Metabolism. Mol. Genet. Metab. 2012, 107, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Kopec, J.; Fitzpatrick, F.; Schuller, M.; McCorvie, T.J.; Chalk, R.; Plessl, T.; Fettelschoss, V.; Fowler, B.; Baumgartner, M.R.; et al. Structural Insights into the MMACHC-MMADHC Protein Complex Involved in Vitamin B12 Trafficking. J. Biol. Chem. 2015, 290, 29167–29177. [Google Scholar] [CrossRef] [PubMed]
- Suormala, T.; Baumgartner, M.R.; Coelho, D.; Zavadakova, P.; Kožich, V.; Koch, H.G.; Berghaüser, M.; Wraith, J.E.; Burlina, A.; Sewell, A.; et al. The CblD Defect Causes Either Isolated or Combined Deficiency of Methylcobalamin and Adenosylcobalamin Synthesis. J. Biol. Chem. 2004, 279, 42742–42749. [Google Scholar] [CrossRef] [PubMed]
- Parini, R.; Furlan, F.; Brambilla, A.; Codazzi, D.; Vedovati, S.; Corbetta, C.; Fedeli, T.; Merinero, B.; Pérez, B.; Ugarte, M. Severe Neonatal Metabolic Decompensation in Methylmalonic Acidemia Caused by CblD Defect. In; 2013; pp. 133–137.
- Christensen, E.I.; Birn, H. Megalin and Cubilin: Synergistic Endocytic Receptors in Renal Proximal Tubule. Am. J. Physiol. -Ren. Physiol. 2001, 280, F562–F573. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Liu, M.L.; Hwang, H.Y.; Chen, L.S.; Korenberg, J.; Shane, B. Human Methionine Synthase. CDNA Cloning, Gene Localization, and Expression. J. Biol. Chem. 1997, 272, 3628–3634. [Google Scholar] [CrossRef] [PubMed]
- Drennan, C.L.; Matthews, R.G.; Ludwig, M.L. Cobalamin-Dependent Methionine Synthase: The Structure of a Methylcobalamin-Binding Fragment and Implications for Other B12-Dependent Enzymes. Curr. Opin. Struct. Biol. 1994, 4, 919–929. [Google Scholar] [CrossRef]
- Matthews, R.G.; Sheppard, C.; Goulding, C. Methylenetetrahydrofolate Reductase and Methionine Synthase: Biochemistry and Molecular Biology. Eur. J. Pediatr. 1998, 157, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Guéant, J.-L.; Caillerez-Fofou, M.; Battaglia-Hsu, S.; Alberto, J.-M.; Freund, J.-N.; Dulluc, I.; Adjalla, C.; Maury, F.; Merle, C.; Nicolas, J.-P.; et al. Molecular and Cellular Effects of Vitamin B12 in Brain, Myocardium and Liver through Its Role as Co-Factor of Methionine Synthase. Biochimie 2013, 95, 1033–1040. [Google Scholar] [CrossRef]
- Watkin, D.; Rosenblatt, D.S. Functional Methionine Synthase Deficiency (CblE and CblG): Clinical and Biochemical Heterogeneity. Am. J. Med. Genet. 1989, 34, 427–434. [Google Scholar] [CrossRef]
- Watkins, D.; Ru, M.; Hwang, H.-Y.; Kim, C.D.; Murray, A.; Philip, N.S.; Kim, W.; Legakis, H.; Wai, T.; Hilton, J.F.; et al. Hyperhomocysteinemia Due to Methionine Synthase Deficiency, CblG: Structure of the MTR Gene, Genotype Diversity, and Recognition of a Common Mutation, P1173L. Am. J. Human. Genet. 2002, 71, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Guéant, J.-L.; Guéant-Rodriguez, R.-M.; Kosgei, V.J.; Coelho, D. Causes and Consequences of Impaired Methionine Synthase Activity in Acquired and Inherited Disorders of Vitamin B 12 Metabolism. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Takahashi-Iñiguez, T.; García-Hernandez, E.; Arreguín-Espinosa, R.; Flores, M.E. Role of Vitamin B12 on Methylmalonyl-CoA Mutase Activity. J. Zhejiang Univ. Sci. B 2012, 13, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Mancia, F.; Evans, P.R. Conformational Changes on Substrate Binding to Methylmalonyl CoA Mutase and New Insights into the Free Radical Mechanism. Structure 1998, 6, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, K.; Bailey, H.J.; Burda, P.; Chaikuad, A.; Krysztofinska, E.; Suormala, T.; Bürer, C.; Lutz, S.; Fowler, B.; Froese, D.S.; et al. Genetic, Structural, and Functional Analysis of Pathogenic Variations Causing Methylmalonyl-CoA Epimerase Deficiency. Biochim. Et Biophys. Acta (BBA) - Mol. Basis Dis. 2019, 1865, 1265–1272. [Google Scholar] [CrossRef]
- Pratt, J.M. 988. The Chemistry of Vitamin B12. Part II. Photochemical Reactions. J. Chem. Soc. (Resumed) 1964, 5154. [Google Scholar] [CrossRef]
- Takahashi-Iñiguez, T.; González-Noriega, A.; Michalak, C.; Flores, M.E. Human MMAA Induces the Release of Inactive Cofactor and Restores Methylmalonyl-CoA Mutase Activity through Their Complex Formation. Biochimie 2017, 142, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-Y.; Liu, T.-T.; Yang, Y.-L.; Chang, Y.-C.; Fan, Y.-L.; Lee, S.-F.; Teng, Y.-T.; Chiang, S.-H.; Niu, D.-M.; Lin, S.-J.; et al. Mutation Profile of the MUT Gene in Chinese Methylmalonic Aciduria Patients. In; 2012; pp. 55–64.
- Willard, H.F.; Rosenberg, L.E. Inherited Methylmalonyl CoA Mutase Apoenzyme Deficiency in Human Fibroblasts. J. Clin. Investig. 1980, 65, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, C.; Benoist, J.; Pereira, S.; Callebaut, I.; Koskas, T.; Porquet, D.; Elion, J. Molecular Basis of Methylmalonyl-CoA Mutase Apoenzyme Defect in 40 European Patients Affected by Mut. ° and Mut. – Forms of Methylmalonic Acidemia: Identification of 29 Novel Mutations in the MUT Gene. Hum. Mutat. 2005, 25, 167–176. [Google Scholar] [CrossRef]
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of Vitamin B-12 Nutritional Status in the United States by Using Plasma Methylmalonic Acid and Serum Vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, A.; Yeung, L.F.; Qi, Y.P.; Pfeiffer, C.M.; Crider, K.S. Folate and Vitamin B12 Usual Intake and Biomarker Status by Intake Source in United States Adults Aged ≥19 y: NHANES 2007–2018. Am J Clin Nutr 2023, 118, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Murphy, M.; Solé-Navais, P.; Yajnik, C. Cobalamin Status from Pregnancy to Early Childhood: Lessons from Global Experience. Adv. Nutr. 2017, 8, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, G.; Gütgemann, I.; Kolb, G.; Leischker, A. Klinisch-Hämatologisches Bild Des Vitamin-B12-Mangels Im Alter. Z. Gerontol. Geriatr. 2018, 51, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Rush, E.; Harper, M.; Venn, B. Vitamin B12 Status of Various Ethnic Groups Living in New Zealand: An Analysis of the Adult Nutrition Survey 2008/2009. Nutrients 2018, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Jajoo, S.S.; Zamwar, U.M.; Nagrale, P. Etiology, Clinical Manifestations, Diagnosis, and Treatment of Cobalamin (Vitamin B12) Deficiency. Cureus 2024. [Google Scholar] [CrossRef] [PubMed]
- Dali-Youcef, N.; Andres, E. An Update on Cobalamin Deficiency in Adults. QJM 2009, 102, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Andres, E. Vitamin B12 (Cobalamin) Deficiency in Elderly Patients. Can. Med. Assoc. J. 2004, 171, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton Pump Inhibitor and Histamine 2 Receptor Antagonist Use and Vitamin B 12 Deficiency. JAMA 2013, 310, 2435. [Google Scholar] [CrossRef] [PubMed]
- Green, R. Vitamin B12 Deficiency from the Perspective of a Practicing Hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef]
- Lindenbaum, J.; Healton, E.B.; Savage, D.G.; Brust, J.C.M.; Garrett, T.J.; Podell, E.R.; Margell, P.D.; Stabler, S.P.; Allen, R.H. Neuropsychiatric Disorders Caused by Cobalamin Deficiency in the Absence of Anemia or Macrocytosis. New Engl. J. Med. 1988, 318, 1720–1728. [Google Scholar] [CrossRef]
- Green, R.; Kinsella, L.J. Current Concepts in the Diagnosis of Cobalamin Deficiency. Neurology 1995, 45, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Troen, A.M.; Shea-Budgell, M.; Shukitt-Hale, B.; Smith, D.E.; Selhub, J.; Rosenberg, I.H. B-Vitamin Deficiency Causes Hyperhomocysteinemia and Vascular Cognitive Impairment in Mice. Proc. Natl. Acad. Sci. 2008, 105, 12474–12479. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R. Diagnosis and Management of Clinical and Subclinical Cobalamin Deficiencies: Why Controversies Persist in the Age of Sensitive Metabolic Testing. Biochimie 2013, 95, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R. Subclinical Cobalamin Deficiency. Curr. Opin. Gastroenterol. 2012, 28, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Devalia, V.; Hamilton, M.S.; Molloy, A.M. Guidelines for the Diagnosis and Treatment of Cobalamin and Folate Disorders. Br. J. Haematol. 2014, 166, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R.; Agrawal, Y.P. Failures of Cobalamin Assays in Pernicious Anemia. New Engl. J. Med. 2012, 367, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Garrod, M.G.; Rockwood, A.L.; Kushnir, M.M.; Allen, L.H.; Haan, M.N.; Green, R. Measurement of Total Vitamin B12 and Holotranscobalamin, Singly and in Combination, in Screening for Metabolic Vitamin B12 Deficiency. Clin. Chem. 2006, 52, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Nexo, E.; Hoffmann-Lücke, E. Holotranscobalamin, a Marker of Vitamin B-12 Status: Analytical Aspects and Clinical Utility. Am. J. Clin. Nutr. 2011, 94, 359S–365S. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Pangilinan, F.; Mills, J.L.; Shane, B.; O’Neill, M.B.; McGaughey, D.M.; Velkova, A.; Abaan, H.O.; Ueland, P.M.; McNulty, H.; et al. A Common Polymorphism in HIBCH Influences Methylmalonic Acid Concentrations in Blood Independently of Cobalamin. Am. J. Human. Genet. 2016, 98, 869–882. [Google Scholar] [CrossRef]
- Allen, L.H.; Miller, J.W.; de Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef]
- Hvas, A.-M.; Morkbak, A.L.; Hardlei, T.F.; Nexo, E. The Vitamin B12 Absorption Test, CobaSorb, Identifies Patients Not Requiring Vitamin B12 Injection Therapy. Scand. J. Clin. Lab. Invest. 2011, 71, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Bor, M.V.; Çetin, M.; Aytaç, S.; Altay, C.; Nexo, E. Nonradioactive Vitamin B12 Absorption Test Evaluated in Controls and in Patients with Inherited Malabsorption of Vitamin B12. Clin. Chem. 2005, 51, 2151–2155. [Google Scholar] [CrossRef]
- Hvas, A.-M.; Morkbak, A.L.; Nexo, E. Plasma Holotranscobalamin Compared with Plasma Cobalamins for Assessment of Vitamin B12 Absorption; Optimisation of a Non-Radioactive Vitamin B12 Absorption Test (CobaSorb). Clin. Chim. Acta 2007, 376, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Garrod; Rossow; Calvert; Miller; Green; Buchholz; Allen 14C-Cobalamin Absorption from Endogenously Labeled Chicken Eggs Assessed in Humans Using Accelerator Mass Spectrometry. Nutrients 2019, 11, 2148. [CrossRef]
- Vitamin B 12 Deficiency. New Engl. J. Med. 2013, 368, 2040–2042. [CrossRef]
- Toh, B.-H. Diagnosis and Classification of Autoimmune Gastritis. Autoimmun. Rev. 2014, 13, 459–462. [Google Scholar] [CrossRef]
- Lahner, E.; Norman, G.L.; Severi, C.; Encabo, S.; Shums, Z.; Vannella, L.; Fave, G.D.; Annibale, B. Reassessment of Intrinsic Factor and Parietal Cell Autoantibodies in Atrophic Gastritis With Respect to Cobalamin Deficiency. Am. J. Gastroenterol. 2009, 104, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, C.; Lofgren, M.; Banerjee, R. Navigating the B12 Road: Assimilation, Delivery, and Disorders of Cobalamin. J. Biol. Chem. 2013, 288, 13186–13193. [Google Scholar] [CrossRef]
- Froese, D.S.; Gravel, R.A. Genetic Disorders of Vitamin B 12 Metabolism: Eight Complementation Groups – Eight Genes. Expert. Rev. Mol. Med. 2010, 12, e37. [Google Scholar] [CrossRef]
- British Society for Haematology BSH Guidance on B12 Replacement during the COVID-19 Pandemic.
- Berkins, S.; Schiöth, H.B.; Rukh, G. Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes. Nutrients 2021, 13, 1790. [Google Scholar] [CrossRef]
- Sangle, P.; Sandhu, O.; Aftab, Z.; Anthony, A.T.; Khan, S. Vitamin B12 Supplementation: Preventing Onset and Improving Prognosis of Depression. Cureus 2020. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.R.; Di Matteo, G.; La Rosa, P.; Barbati, S.A.; Mannina, L.; Moreno, S.; Tata, A.M.; Cavallucci, V.; Fidaleo, M. Vitamin B12 Deficiency and the Nervous System: Beyond Metabolic Decompensation—Comparing Biological Models and Gaining New Insights into Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2024, 25, 590. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, M.; Basri, R.; AlRuwaili, R.; Albarrak, A.M.; Ali, N.H. Neurological Implications of Vitamin B12 Deficiency in Diet: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Quan, M.; Li, T.; Jia, J. Serum Homocysteine, Vitamin B12, Folate, and Their Association with Mild Cognitive Impairment and Subtypes of Dementia. J. Alzheimer’s Dis. 2022, 90, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Mander, A.; Ames, D.; Carne, R.; Sanders, K.; Watters, D. Cognitive Impairment and Vitamin B12: A Review. Int. Psychogeriatr. 2012, 24, 541–556. [Google Scholar] [CrossRef]
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the Nervous System: Current Knowledge of the Biochemical Modes of Action and Synergies of Thiamine, Pyridoxine, and Cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Alam, S.F. Role of Homocysteine in the Development of Cardiovascular Disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- COPPOLA, A.; DAVI, G.; DE STEFANO, V.; MANCINI, F.P.; CERBONE, A.M.; DI MINNO, G. Homocysteine, Coagulation, Platelet Function, and Thrombosis. Semin. Thromb. Hemost. 2000, Volume 26, 243–254. [Google Scholar] [CrossRef]
- Ammouri, W.; Tazi, Z.M.; Harmouche, H.; Maamar, M.; Adnaoui, M. Venous Thromboembolism and Hyperhomocysteinemia as First Manifestation of Pernicious Anemia: A Case Series. J. Med. Case Rep. 2017, 11, 250. [Google Scholar] [CrossRef]
- Azzini, E.; Raguzzini, A.; Polito, A. A Brief Review on Vitamin B12 Deficiency Looking at Some Case Study Reports in Adults. Int. J. Mol. Sci. 2021, 22, 9694. [Google Scholar] [CrossRef]
- Ospina-Romero, M.; Cannegieter, S.C.; den Heijer, M.; Doggen, C.J.M.; Rosendaal, F.R.; Lijfering, W.M. Hyperhomocysteinemia and Risk of First Venous Thrombosis: The Influence of (Unmeasured) Confounding Factors. Am. J. Epidemiol. 2018, 187, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Çoker, M.; de Klerk, J.B.C.; Poll-The, B.T.; Huijmans, J.G.M.; Duran, M. Plasma Total Odd-chain Fatty Acids in the Monitoring of Disorders of Propionate, Methylmalonate and Biotin Metabolism. J. Inherit. Metab. Dis. 1996, 19, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Ilter, D.; Low, V.; Endress, J.E.; Fernández-García, J.; Rosenzweig, A.; Schild, T.; Broekaert, D.; Ahmed, A.; Planque, M.; et al. Age-Induced Accumulation of Methylmalonic Acid Promotes Tumour Progression. Nature 2020, 585, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Ilter, D.; Low, V.; Drapela, S.; Schild, T.; Mullarky, E.; Han, J.; Elia, I.; Broekaert, D.; Rosenzweig, A.; et al. Altered Propionate Metabolism Contributes to Tumour Progression and Aggressiveness. Nat. Metab. 2022, 4, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ye, M.; Bai, J.; Liu, P.; Lu, F.; Chen, J.; Yu, P.; Chen, T.; Shi, X.; Tang, Q. Methylmalonic Acid Promotes Colorectal Cancer Progression via Activation of Wnt/β-Catenin Pathway Mediated Epithelial–Mesenchymal Transition. Cancer Cell Int. 2023, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Loedin, A.K.; Speijer, D. Is There a Carcinogenic Risk Attached to Vitamin B 12 Deficient Diets and What Should We Do About It? Reviewing the Facts. Mol. Nutr. Food Res. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R. High Plasma Vitamin B12 and Cancer in Human Studies: A Scoping Review to Judge Causality and Alternative Explanations. Nutrients 2022, 14, 4476. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, G.; Khajeh-Mehrizi, A.; Ranjbar, H. Evaluation of Serum Vitamin B12 Levels in Patients with Colon and Breast Cancer: A Case-Control Study. Int. J. Hematol. Oncol. Stem Cell Res. 2023. [Google Scholar] [CrossRef]
- Zhang, S.M.; Willett, W.C.; Selhub, J.; Hunter, D.J.; Giovannucci, E.L.; Holmes, M.D.; Colditz, G.A.; Hankinson, S.E. Plasma Folate, Vitamin B6, Vitamin B12, Homocysteine, and Risk of Breast Cancer. JNCI J. Natl. Cancer Inst. 2003, 95, 373–380. [Google Scholar] [CrossRef]
- Wu, W.; Kang, S.; Zhang, D. Association of Vitamin B6, Vitamin B12 and Methionine with Risk of Breast Cancer: A Dose–Response Meta-Analysis. Br. J. Cancer 2013, 109, 1926–1944. [Google Scholar] [CrossRef]
- Matejcic, M.; de Batlle, J.; Ricci, C.; Biessy, C.; Perrier, F.; Huybrechts, I.; Weiderpass, E.; Boutron-Ruault, M.C.; Cadeau, C.; His, M.; et al. Biomarkers of Folate and Vitamin B12 and Breast Cancer Risk: Report from the EPIC Cohort. Int. J. Cancer 2017, 140, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.S.; Gordon, M.M.; Chen, Z.; Rajashankar, K.R.; Ealick, S.E.; Alpers, D.H.; Sukumar, N. Crystal Structure of Human Intrinsic Factor: Cobalamin Complex at 2.6-Å Resolution. Proc. Natl. Acad. Sci. 2007, 104, 17311–17316. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.T.-L.; Lin, C.-L.; Pan, K.-H.; Tzen, K.-Y.; Su, M.-J.; Tsai, C.-T.; Li, Y.-H.; Li, P.-C.; Chiang, F.-T.; Chang, S.C.; et al. Single Allele Lmbrd1 Knockout Results in Cardiac Hypertrophy. J. Formos. Med. Assoc. 2018, 117, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.S.; Kuner, R.; Buness, A.; Ruschhaupt, M.; Merk, S.; Zwermann, L.; Kääb, S.; Kreuzer, E.; Steinbeck, G.; Mansmann, U.; et al. Identification of a Common Gene Expression Signature in Dilated Cardiomyopathy Across Independent Microarray Studies. J. Am. Coll. Cardiol. 2006, 48, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Jonnalagadda, D.; Kihara, Y.; Groves, A.; Ray, M.; Saha, A.; Ellington, C.; Lee-Okada, H.-C.; Furihata, T.; Yokomizo, T.; Quadros, E.V.; et al. FTY720 Requires Vitamin B12-TCN2-CD320 Signaling in Astrocytes to Reduce Disease in an Animal Model of Multiple Sclerosis. Cell Rep. 2023, 42, 113545. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; Henderson, M.; Haber, M.; Norris, M. Role of the MRP1/ABCC1 Multidrug Transporter Protein in Cancer. IUBMB Life 2007, 59, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Mao, C.; Yeh, S.; Xin, J.; Wang, P.; Shi, Q.; Ming, X. Combinatory Therapy of MRP1-Targeted Photoimmunotherapy and Liposomal Doxorubicin Promotes the Antitumor Effect for Chemoresistant Small Cell Lung Cancer. Int. J. Pharm. 2022, 625, 122076. [Google Scholar] [CrossRef] [PubMed]
- Pietz, H.L.; Abbas, A.; Johnson, Z.L.; Oldham, M.L.; Suga, H.; Chen, J. A Macrocyclic Peptide Inhibitor Traps MRP1 in a Catalytically Incompetent Conformation. Proc. Natl. Acad. Sci. 2023, 120. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, K.M.; Haber, M.; Fletcher, J.I. Targeting Multidrug Resistance-Associated Protein 1 (MRP1)-Expressing Cancers: Beyond Pharmacological Inhibition. Drug Resist. Updates 2021, 59, 100795. [Google Scholar] [CrossRef]
- Alhaji, J.H. Vitamin B12 Deficiency in Patients with Diabetes on Metformin: Arab Countries. Nutrients 2022, 14, 2046. [Google Scholar] [CrossRef]
- Bell, D.S.H. Metformin-induced Vitamin B12 Deficiency Can Cause or Worsen Distal Symmetrical, Autonomic and Cardiac Neuropathy in the Patient with Diabetes. Diabetes Obes. Metab. 2022, 24, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-Term Metformin Therapy and Vitamin B12 Deficiency: An Association to Bear in Mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Shaw, S.; Jayatilleke, E.; Spungen, A.M.; Herbert, V. Increased Intake of Calcium Reverses Vitamin B12 Malabsorption Induced by Metformin. Diabetes Care 2000, 23, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Out, M.; Kooy, A.; Lehert, P.; Schalkwijk, C.A.; Stehouwer, C.D.A. Long-Term Treatment with Metformin in Type 2 Diabetes and Methylmalonic Acid: Post Hoc Analysis of a Randomized Controlled 4.3 Year Trial. J. Diabetes Complicat. 2018, 32, 171–178. [Google Scholar] [CrossRef]
- Kovatcheva, M.; Melendez, E.; Chondronasiou, D.; Pietrocola, F.; Bernad, R.; Caballe, A.; Junza, A.; Capellades, J.; Holguín-Horcajo, A.; Prats, N.; et al. Vitamin B12 Is a Limiting Factor for Induced Cellular Plasticity and Tissue Repair. Nat. Metab. 2023, 5, 1911–1930. [Google Scholar] [CrossRef]
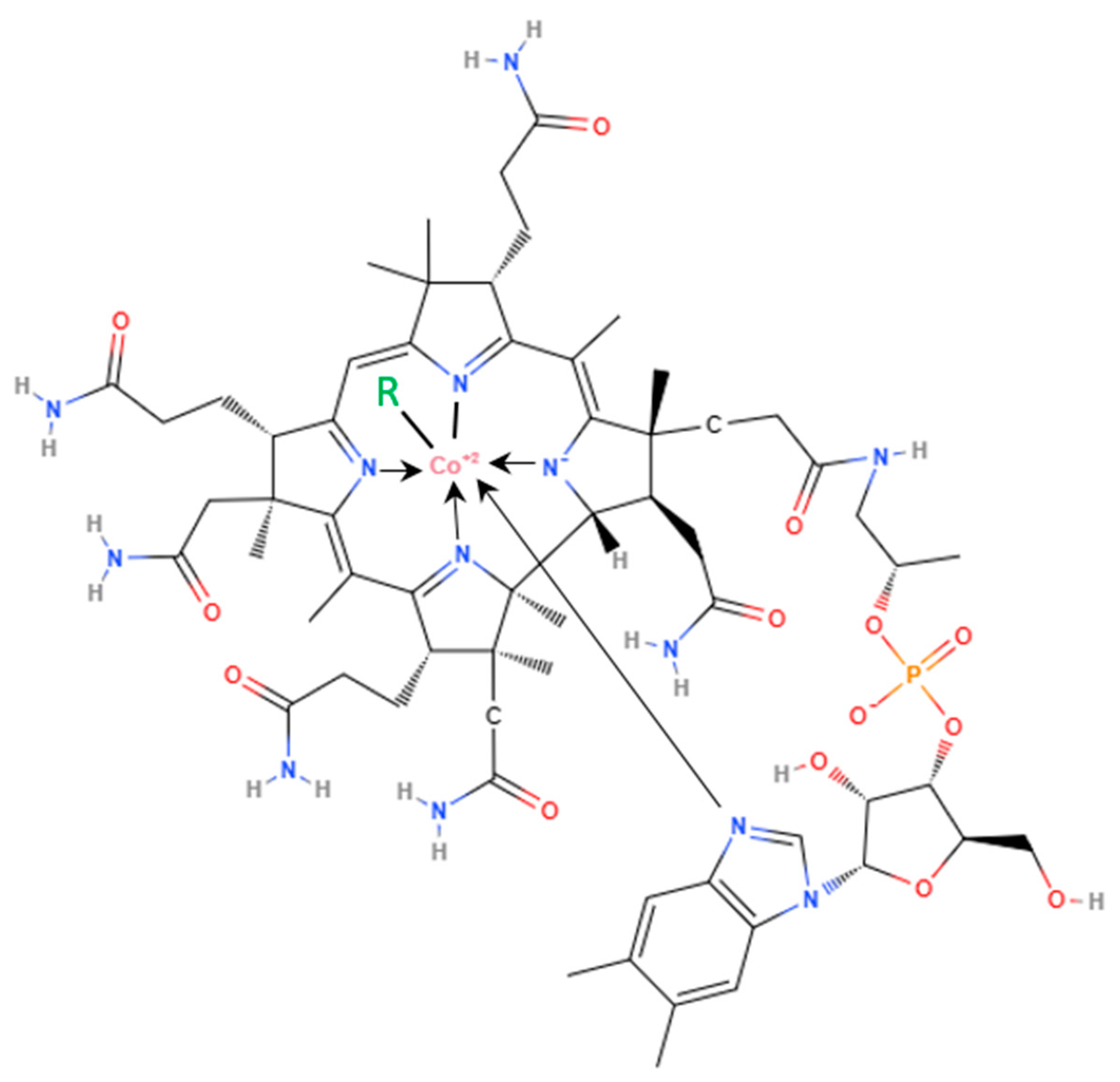
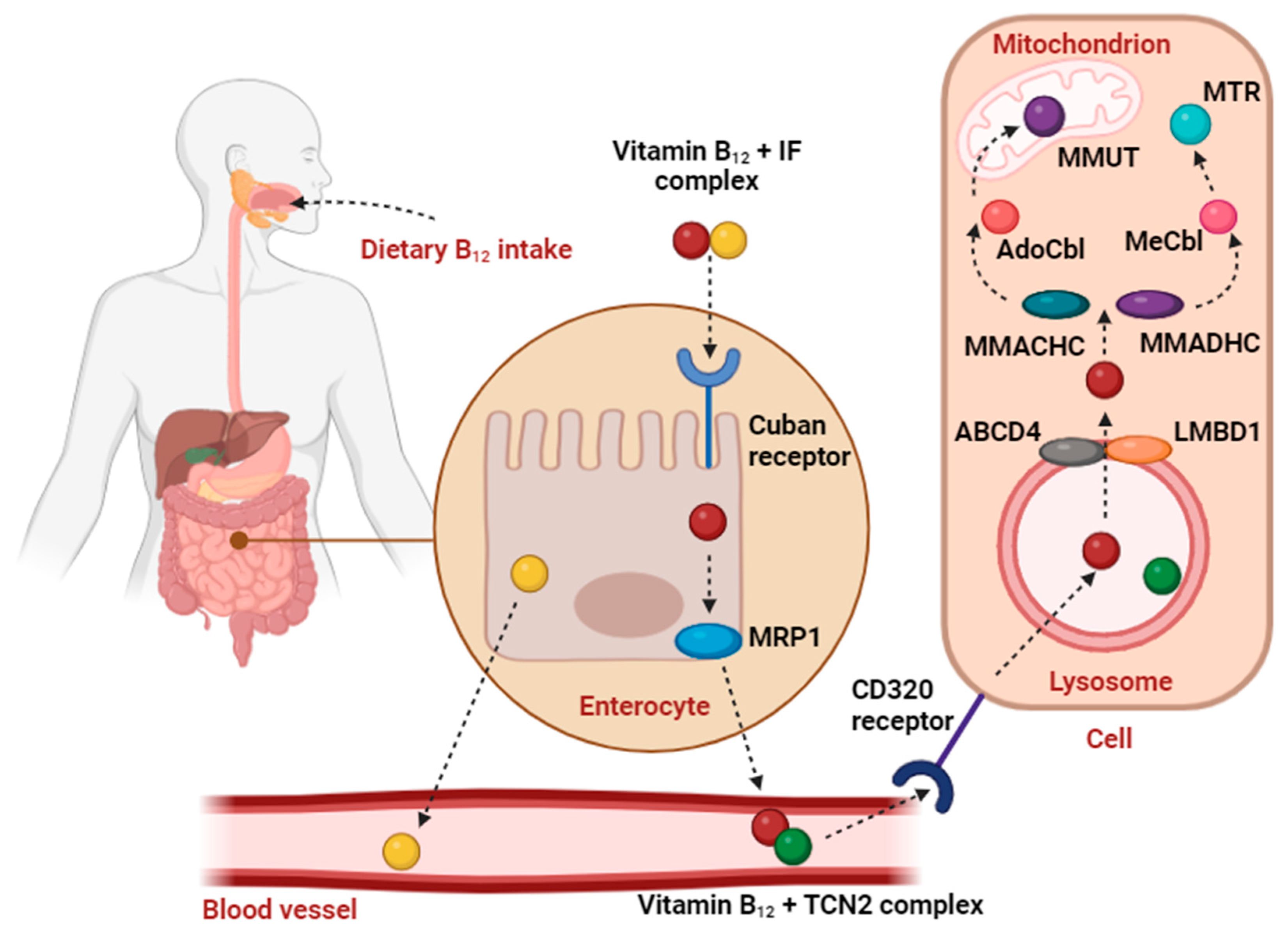
| PROTEIN | SHORTNAME | GENE | SIZE | TYPE | FUNCTION | PATHOLOGY | REF. | |
|---|---|---|---|---|---|---|---|---|
| Haptocorrin, Transcobalamin I | HC, TCN1 | TCN1 | 60 to 70 kDa | Glycoprotein | Binding vitamin B12 in saliva, transferring the vitamin to the stomach; Protection of B12 from hydrolysis in the acidic pH of the stomach. | The inability of HC degradation and release of the vitamin from the vitamin B12-HC complex (caused by deficiency of pancreatic proteases.) may led to vitamin B12 malabsorption | [14,179] | |
| Intrinsic Factor | IF | GIF or CBLIF | Two domains of approx. 30 kDa and 20 kDa (399 amino acid residues) | Glycoprotein | Binding vitamin B12 in the duodenum. | The lack of IF in the condition of H. pylori infection or AIG leads to long-lasting vitamin B12 deficiency due to the inability to deliver vitamin B12 to the cells within the ileum. | [14,15,20,179,179] | |
|
Cubam receptor |
Cubilin | CUBN | CUBN | 460 kDa | Multiligand, apical membrane receptor |
Interaction with IF; Docking to transmembrane protein AMN; Forming with AMN a receptor specific for IF-vitamin B12 internalization. | Disrupting this Cubam results in malabsorption of vitamin B12 and the development of Imerslund-Gräsbeck syndrome (IGS), a rare autosomal recessive disorder that appears in childhood, characterized by megaloblastic anemia | [33,34,37,38] |
| Type-1 transmembrane protein Amnionless | AMN | AMN | 45 kDa | Clathrin-mediated internalization; Anchoring Cubilin; Forming with CUBN a receptor specific for IF-vitamin B12 internalization. |
||||
|
Multidrug Resistant Protein 1 |
MRP1, ABCC1 | ABCC1 | 190 kDa | ATP-binding cassette (ABC) transporters family | Effluxing vitamin B12 from the intestinal epithelium to the blood circulation. | Mutations of gene ABCC1 were detected in some patients with vitamin B12 malabsorption. | [46,47,52,54] |
|
| Transcobalamin II | TCN2 | TCN2 | 43 kDa | β-globulin protein | Binding vitamin B12 in the bloodstream; Distribution of vitamin B12 into all cells in the body. | Mutations in the gene can lead to intracellular cobalamin depletion, causing a rare multisystemic disorder characterized by autosomal recessive inheritance. | [55,59,60,67,68,69] |
|
| Receptor for cluster of differentiation 320, Receptor for cobalamin-saturated transcobalamin | CD320, TCblR | TCblR or CD320 | 29 kDa (282 amino acids) (observed 58 kDa) | Low-density Lipoprotein Receptor protein family | Endocytosis of TCN2-saturated cobalamin. | In humans, very few cases of CD320 receptor defects have been confirmed to be the direct reason for lowered B12 levels in serum and increased MMA concentration in newborns. | [70,71,72,74,75,82,83] |
|
| ATP-binding cassette sub-family D member 4 | ABCD4 | ABCD4 | 68.6 kDa | Exporter protein | Transport free vitamin B12 into the cytoplasm. | Mutations are related to disease groups cblJ and cblF. At the cellular level, both errors are marked with a decreased function of MMUT and MTR and an accumulation of free vitamin B12 in the lysosomes. | [84,84,84,87,88,88,88,89,91,91,94] | |
| Lysosomal cobalamin transport escort protein | LMBD1 | LMBDR1 | 61.4 kDa | Adaptor protein, chaperone | Mediates the translocation of ABCD4 from ER to the lysosomes. | |||
| Methylmalonic aciduria and homocystinuria type C protein | MMACHC | MMACHC | 31.7 kDa (282 amino acids) | Chaperone, enzyme | Potentially receiving the Cbl cargo upon its release from the lysosomal compartment; Catalyzing the reductive decyanation of cyanocobalamin. | Mutations result in a rare genetic disorder referred to as methylmalonic aciduria and homocystinuria type C (cblC); deficiencies in both B12-dependent enzymatic functions MTR and MMUT - accumulation of both MMA and Hcy in serum. | [95,96,97,98,100] | |
| Methylmalonic aciduria and homocystinuria type D protein | MMADHC | MMADHC | 32.9 kDa (296 amino acids) | Probably enzyme | Play a role in the production of the two active cobalamin forms, MeCbl and AdoCb. | The cblD complementation group can be linked to isolated methylmalonic aciduria (cblD-MMA), isolated homocystinuria (cblD-HC), or a combination of both methylmalonic aciduria and homocystinuria (cblD-MMA/HC). | [102,103,104,107,108,109] |
|
| Methionine synthase | MTR, MS | MTR | 140 kDa | Cytoplasmic enzyme | Vitamin B12-dependent transfer of methyl group from 5-methyltetrahydrofolate to homocysteine with the production of tetrahydrofolate and methionine. | Missense mutation or deletion of three base pairs was identified in the cblG group of patients with cobalamin metabolism disorder and methylcobalamin deficiency cblE type, rare autosomal diseases associated with homocysteinemia, homocystinuria, and hypomethioninemia. | [83,84,85,87,88,110,115,116] |
|
| Methylmalonyl-CoA mutase | MMUT, MCM, MUT | MMUT | 78 kDa | Mitochondrial enzyme | Vitamin B12-dependent conversion methylmalonyl-CoA to succinyl-CoA. | Mutation leads to methylmalonic acidemia. | [89,90,91,93,120,122] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





