Introduction
The complex metabolic policies of biological organisms are very difficult to explain, but one possibility that may be a previously overlooked strong influencing factor is that much of the complexity might relate to maintaining deuterium homeostasis. Deuterium is a natural heavy isotope of hydrogen, the most common atom in the universe. While hydrogen contains just one proton and one electron, deuterium also contains a neutron, and this makes it about twice as heavy as hydrogen, changing its biophysical and biochemical properties in significant ways, compared to hydrogen. Wherever there is a hydrogen atom in a molecule, there is a small probability that it could be a deuterium atom. Deuterium is present in sea water at 156 parts per million, which sounds small, but, because hydrogen is so common, this translates into a level in the blood that is at least five times as high as the amount of calcium, atom for atom [
1].
Most of the energy supplied to human cells is produced by ATP synthase (ATPase) in the mitochondria via the synthesis of ATP from ADP. Mitochondrial ATP production depends upon the universal cofactor nicotinamide adenine dinucleotide (NAD). In multiple steps in the citric acid cycle in the mitochondrial matrix, NAD
+ gets reduced to NADH by gaining two electrons and one proton from various substrates. NADH then provides protons to the intermembrane space to build up a proton gradient, while providing electrons to the electron transport chain in the inner membrane. Ultimately, the protons leave the intermembrane space to return to the matrix, exiting via ATPase pumps and providing the motive force to rotate the pump and fuel the production of ATP, while reducing oxygen to metabolic water [
2].
F
0F
1 ATPase (F-ATPase) is a molecular motor positioned in the inner membrane of the mitochondrial intermembrane space that uses proton motive force as protons cross the membrane to generate the energy needed to phosphorylate ATP, while producing water from oxygen molecules. Protons moving through the ATPase pumps need to dissociate rapidly from Asp61 in F
0 as an essential part of the catalytic reaction. Deuterons bind more tightly to organic molecules, and dissociate more slowly, resulting in a stutter [
1,
3].
The γ c-terminal helix of the ATPase molecular motor is the tip of the rotor that drives ATP synthesis. A hydrogen-deuterium exchange experiment showed that this helix accumulates significantly more deuterium from heavy water than other parts of the enzyme complex. Rotation of the γ rotor caused greatly enhanced deuteration in this c-terminal helix. The rotor tip is also prone to get stuck and unfold, being much less stable than other helices in the protein. Deuterium weakens hydrogen bonds, and their breakage likely causes unfolding, inducing a stutter in the motor rotation and possibly ultimately completely disabling the enzyme [
4].
Repeated stutters caused by deuterons can be highly disruptive of the ATPase function, leading to inefficiencies in ATP production and increases in reactive oxygen species (ROS) [
1,
3].
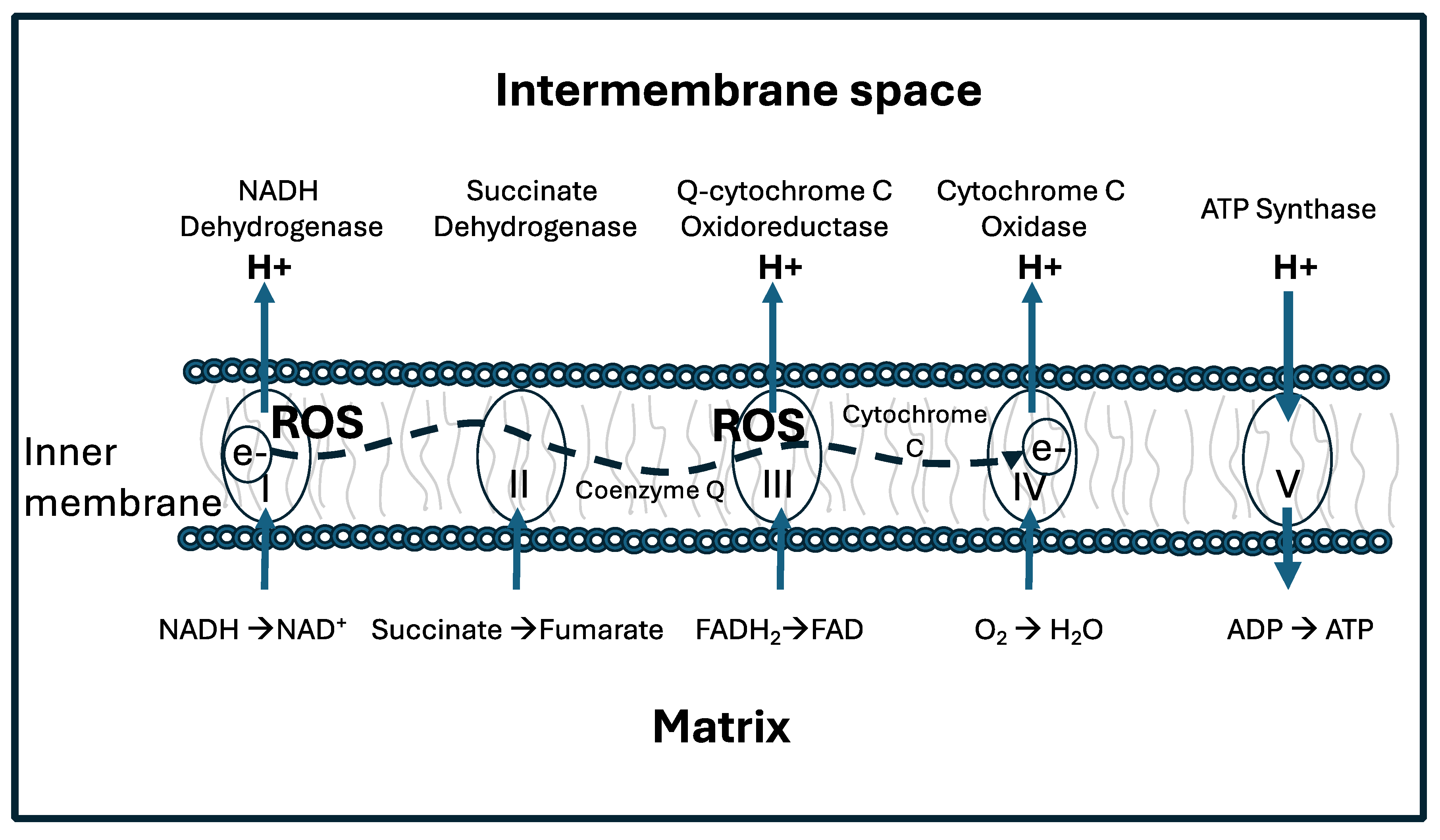
Figure 1 shows a schematic representation of the electron transport chain (ETC), including the delivery of protons to the intermembrane space by Complex I-IV, the reduction of oxygen to metabolic water by Complex IV, and the synthesis of ATP in Complex V. When ATP synthesis gets stalled by deuterons, Complex I and III in particular can release reactive oxygen species (ROS), most notably the superoxide anion (O
2*-).
Mitochondrial dysfunction is a core feature of many chronic diseases, including neurodegenerative and neurodevelopmental diseases, metabolic diseases, autoimmune diseases and cancer [
5,
6]. It therefore behooves the cell to minimize the number of deuterium atoms in the mitochondrial water, to the extent that this is possible.
The cell likely also needs to minimize deuterium concentrations in the DNA molecules in the nucleus. The two strands of DNA are held together by hydrogen bonds between the two bases on opposite strands. Deuterium, being heavier, binds more strongly in covalent bonds, weakening its bond strength in ionic bonds [
1,
7]. Human stem cells grown in vitro on deuterium-enriched medium show a reduction in population doubling time, reflecting a slowing down of the cell cycle [
8].
Over many decades, researchers have determined through cleverly designed experiments that different enzymes are compartmentalized into different organelles within the cell, and complex transport mechanisms are required to deliver both substrate and enzyme to and from the appropriate compartment [
9]. A good example is malic enzyme, which converts malate to pyruvate, releasing carbon dioxide and reducing NAD(P)+ to NAD(P)H [
10]. The malate-aspartate shuttle transports malate from the cytoplasm to the mitochondrial matrix, where mitochondrial malic enzyme can then convert it to pyruvate, while reducing NAD
+ to NADH. NADH then provides protons to fuel the ATPase pumps, while pyruvate can feed into the citric acid cycle. Rather remarkably, NADH is unable to cross the mitochondrial membrane, so malate serves as a transport molecule to indirectly deliver NADH to the mitochondria. There are three different isoforms of malic enzyme, one in the cytoplasm that reduces NADP+ to NADPH, and two in the mitochondria, one using NAD
+ as a cofactor and the other using NADP+. It is highly likely that deuterium plays a significant role in influencing these complex maneuvers.
Because deuterium has different physical and chemical properties from hydrogen, it is often the case that an enzyme that extracts a hydrogen atom from a substrate may show significantly different kinetics if that hydrogen is replaced by deuterium. Researchers have defined a deuterium kinetic isotope effect (KIE) as a measure of the change in the rate of a reaction when deuterium replaces hydrogen, compared to the reaction when hydrogen is present. Generally, the KIE is greater than 1.0, meaning that deuterium weakens the enzyme’s kinetics. Some enzymes have a remarkable ability to reject deuterium, with KIEs over 100 [
11].
At least half of all enzyme-catalyzed reactions involve hydrogen transfer. Furthermore, many hydrogen transfer reactions involve some amount of quantum mechanical hydrogen tunneling. Protons, being lighter, are much more capable of tunneling than are deuterons [
12], and therefore these reactions tend to have a high deuterium KIE [
13]. Flavoproteins are a large class of enzymes that exploit proton tunneling mediated by the bound flavin, such as flavin adenine dinucleotide (FAD). They tend to have a KIE for deuterium that ranges from 3.5 to 10 and can be as high as 25 [
14]. Most dehydrogenases are flavoproteins, and therefore they are capable of depleting deuterium in the product of their reaction.
One more example should suffice to drive this point home. There are three primary isoforms of the enyzme isocitrate dehydrogenase (IDH), two in the mitochondria and one in the cytoplasm. Highly significant is IDH3 in the mitochondria, which uses NAD
+ as a cofactor and which is a critical participant in the citric acid cycle. IDH2, also in the mitochondria, reduces NADP+ to NADPH, which is essential for catalyzing the reduction of oxidized glutathione, GSSG, to two glutathione molecules via glutathione reductase. Glutathione is an essential antioxidant in the mitochondria. The third isoform is in the cytoplasm, and it too uses NADP+ as a cofactor [
15].
Both of the mitochondrial isoforms have a high deuterium KIE, so the isocitrate that they leave behind is enriched in deuterium. This unmetabolized pool of isocitrate is actively transported to the cytoplasm, where it is converted to alpha-ketoglutarate by IDH3, which has no ability to select hydrogen over deuterium. As a consequence, the protons carried by NADPH produced by IDH3 in the cytoplasm are relatively enriched in deuterium, whereas the protons carried by NADH and NADPH in the mitochondria are deuterium depleted (deupleted) [
16].
NADPH oxidase (NOX) is a large family of enzymes that are a major source of reactive oxygen species (ROS) in the cell. NOX2 is rapidly activated in neutrophils, macrophages, and dendritic cells when they encounter a pathogen, and it releases ROS into the extracellular space and the phagosomal lumen, to facilitate the destruction of the pathogen [
17]. NOX2 operates at the plasma membrane, and it transfers an electron from intracellular NADPH across the membrane, to generate the highly reactive molecule, O
*-, in the extracellular space. At the same time, it also transfers two protons to the extracellular space, acidifying the water there. The protons can react with the superoxide to produce hydrogen peroxide, which will be enriched in deuterium if it came from an NADPH molecule produced through IDH3. Thus, by exporting deuterium-rich protons, the cell is reducing its internal deuterium burden.
Overall, a pattern emerges that suggests that the cells are able to somewhat independently control deuterium levels in the water in different compartments so as to optimize metabolic health. To a first approximation, it appears that the cell strategizes to maintain deupleted water in the major organelles, most especially the mitochondria, but also the endoplasmic reticulum (ER), the peroxisome, the lysosomes and the nucleus. In parallel, as we will argue later on, the cell likely sequesters deuterium in the collagen molecules and gelled water in the extracellular matrix, particularly in the bone [
18]. Deuterium doping actually strengthens bone, whereas deuterium is toxic to the mitochondrial ATPase pumps, so such a strategy is consistent with metabolic needs.
In this paper, we will carefully examine several aspects of disease from the perspective of impaired deuterium homeostasis, and we find that the need to maintain different concentrations of deuterium in different compartments may begin to explain why cells behave the way they do. Every effort is made to maintain low deuterium levels in mitochondrial NADH, because this is the molecule that delivers protons to the intermembrane space. We will argue that the amino acid proline has a unique capability to sequester deuterium, and that this capability is exploited by the cells to trap proline in the extracellular space while maintaining low levels of deuterium in the cell interior, in order to protect mitochondria and DNA from deuterium toxicity. Finally, we will make a case for the idea that cancer cells not only thrive in the presence of excess deuterium, but also carry out a program that can ameliorate deuterium toxicity in the organism as a whole, beginning with the immune cells that infiltrate the tumor microenvironment.
Redox Reactions and the “Redox Triangle”
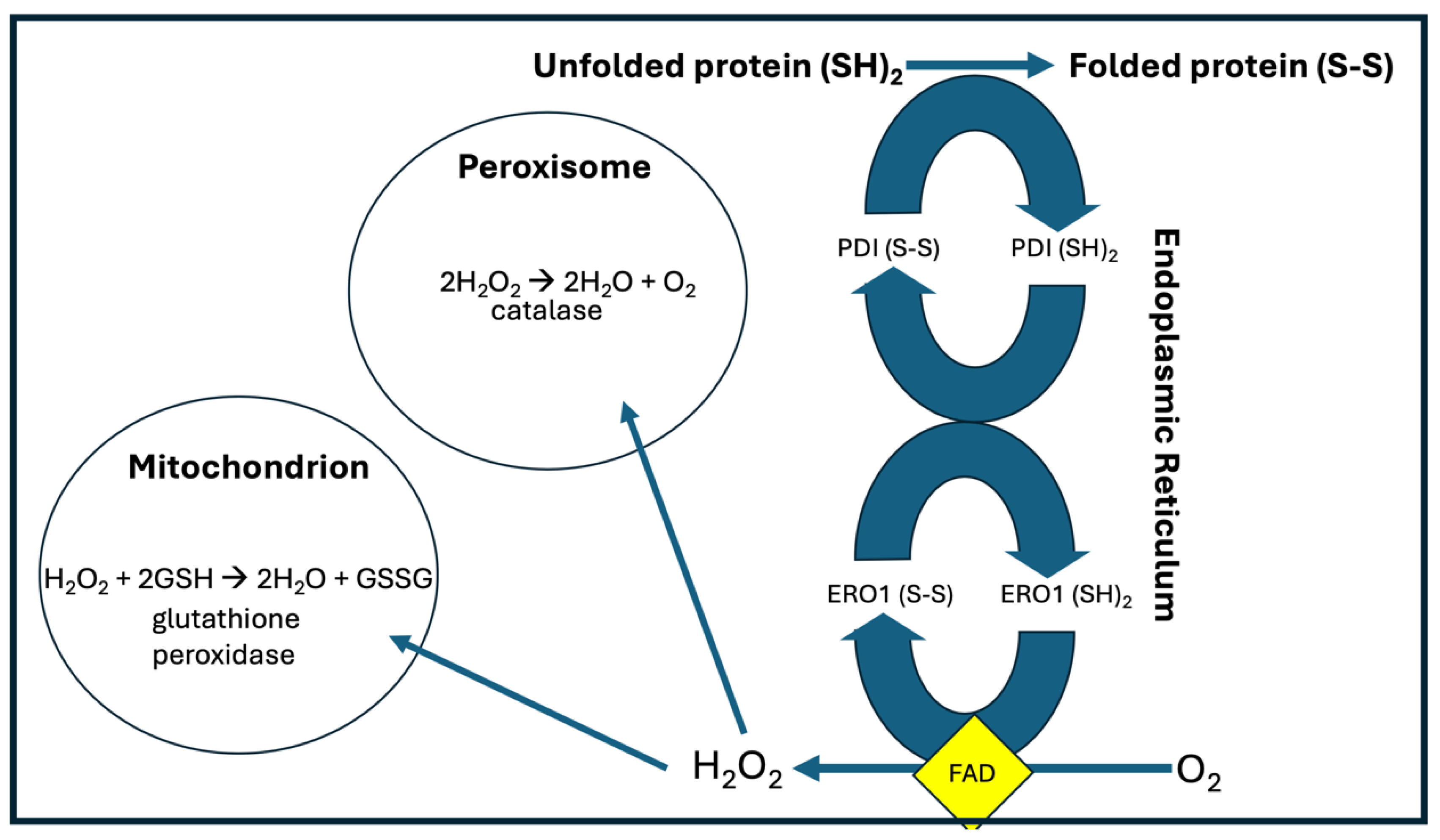
Protein Disulfide Isomerase (PDI) is an essential protein in the ER, which assists in protein folding by repeatedly forming cysteine disulfide links between pairs of cysteine molecules in proteins undergoing folding. It works in conjunction with ER oxidoreductin 1 (ERO1), which reoxidizes PDI, in order to allow it to once again catalyze a disulfide bond formation between two new cysteine residues. ERO1 passes its electrons to molecular oxygen via FAD, ultimately producing hydrogen peroxide (H
2O
2). Together these two enzymes gather protons from cysteine residues and use them to reduce oxygen to deupleted H
2O
2 [
21].
Reduction/oxidation (redox) reactions govern nearly all aspects of life. They typically produce reactive oxygen species as “by-products,” which need to be metabolized quickly to water to avoid damage to the cellular organelles. A typical pathway involves reducing oxygen to superoxide, which is then converted to H
2O
2 by an enzyme such as superoxide dismutase (SOD), and then ultimately further reduced to water, often by the antioxidant enzymes catalase and/or glutathione peroxidase (GPx). Many of the enzymes involved are flavoproteins, which use FAD as a cofactor to temporarily house the protons, and which often involve proton tunneling which then results in a high deuterium KIE [
22]. It is likely that a primary outcome of these reactions is to produce deupleted metabolic water to minimize the amount of deuterium in the organelles.
It is thought that enzymes that regulate the redox state assemble at a “redox triangle” formed by the ER, peroxisomes and mitochondria. The protein-folding process where protons are scooped up from cysteine residues and used to reduce oxygen to H
2O
2 is an important source of ROS, which can then be transferred to the peroxisomes, where catalase reduces two molecules of H
2O
2 to two water molecules, releasing oxygen, and on to the mitochondria, where GPx reduces a single molecule of H
2O
2 to two water molecules, pulling protons off of the cysteines of glutathione [
23]. Those protons can be expected to be deupleted, because they are resupplied from NADPH via the mitochondrial enzyme glutathione reductase, a flavoprotein [
24]. These pathways are illustrated schematically in
Figure 2.
Proline
Due to its cyclic structure, the amino acid proline has many peculiar properties compared to other amino acids. In fact, proline is technically an imino acid rather than an amino acid. As a consequence, it requires specialized enzymes to break the bond between proline residues and their left neighbors [
25]. Proline is unusual in several respects. It is the only coding amino acid where the side chain circles back around and reattaches to the nitrogen atom, forming a ring. It is distributed highly unevenly among proteins, with particularly high proline representation in several extracellular proteins, most notably, collagen.
Proline’s pyrrolidine ring provides stearic restraints that reduce the energy difference between cisand trans- conformers involving its peptide bonds with the preceding residue in a peptide chain. Whereas all the other amino acids are in the cis- conformation less than 0.5% of the time, proline residues can be in cis- confirmation up to 10% of the time [
26]. The cis- configuration is favored even more when the proline residue is preceded by an aromatic amino acid, especially tyrosine [
27]. The rate of Xaa-Pro cis/trans isomerism is a rate-limiting step in protein folding [
28].
There is a class of enzymes called peptidyl prolyl isomerase (PPIase) that specializes in accelerating the rate at which proline residues flip between the cis- and trans- isomers. Various isoforms of PPIase are present in several different compartments in the cell, and they have remarkable abilities to regulate many cellular functions, independent of their isomerase capabilities, affecting many aspects of cellular policy [
29]. PPIases in the endoplasmic reticulum (ER) play an essential role in protein folding. PIN1 (PPIase, NIMA-interacting 1) is overexpressed in many cancer cells and it facilitates proliferation and metastasis [
30]. We will have more to say about PPIases later in this paper.
There is evidence in the research literature that proline residues have a unique ability to capture and sequester deuterium. In a seminal paper published in 1943, the authors synthesized proline molecules doped with 17% deuterium enrichment, and boiled the proline in water buffered with 20% hydrochloric acid for 72 hours. Despite this very aggressive effort to allow the deuterium bound to the proline molecules to exchange with protons from the water, the percent deuterium in the proline molecules was reduced by only 0.8% to 16.2% following this treatment. The authors stated that proline uniquely among all the amino acids has this special property to trap and retain deuterium [
31].
A study on seals published in 2022 revealed that the bone collagen of seals is remarkably enriched in deuterium, having twice the normal amounts. This deuterium doping led to significant strengthening of the bones, which allowed the seals to resist crushing at the extreme water pressure of deep dives [
18]. The authors conducted several experiments aimed at understanding exactly how the seals achieved this deuterium enrichment, but none of the proposed hypotheses was confirmed experimentally. They concluded that the mechanism by which the seals achieved this enrichment remained a mystery.
4.1. Cyclic Peptides
Proline and small-molecule derivatives of proline have been shown to act as potent catalysts of organic transformations, and this feature has been attributed in part to cis/trans isomerization. As we will explain below, the proton attached to the alpha carbon of proline appears to be extremely labile under certain conditions, which would allow it to readily exchange with deuterium from the water. Once the carbon atom binds to deuterium, it retains it indefinitely, and this property is likely highly exploited by biological organisms.
Cyclic peptides are short sequences of amino acids that are joined at both ends, and they have been shown to have remarkable therapeutic value in treating cancer. They show a resistance to hydrolysis by exopeptidases due to the lack of both amino and carboxyl termini [
32]. Thus, their cyclic structure protects them from proteolysis. Interestingly, they are more readily taken up by cancer cells than by other cells. They are typically enriched in glycine, proline and the aromatic amino acids, tryptophan, tyrosine and phenylalanine. They can influence metabolism by binding to receptors and displacing other ligands. Cyclic peptides have been identified as powerful therapeutics to treat cancer, including breast, lung, liver, colon, and prostate cancers [
33]. It is conceivable that their therapeutic value is due to their powerful ability to sequester deuterium in the proline residues.
Cyclic dipeptides called diketopiperazines (DKPs) are an interesting class of molecules defined as a two amino acid sequence with amide bonds on both ends. A study on multiple DKPs configured from several different pairs of amino acids found that the specific dipeptide, cyclic-glycine-proline (cGP) was by far the most reactive in deuterium hydrogen exchange, and protons attached to the alpha carbon of proline stood out as the most labile above all the other protons in this molecule, accumulating deuterium at an accelerated rate in highly deuterated water [
28]. cGP is produced naturally from the N-terminal tripeptide of the endogenous hormone insulin-like growth factor-1 (IGF-1), and it circulates freely in the circulation. cGP has shown promise as a therapy to treat various conditions associated with aging [
34].
During the metabolism of IGF-1, the terminal tripeptide (GPE), also known as glypromate, is released. This tripeptide has shown promise pharmacologically as a potential treatment for Parkinson’s, Alzheimer’s, and Huntington’s disease [
35,
36], and the pharmaceutical industry is exploring the use of several drugs that are derived from glypromate for potential benefit as neuroprotective agents [
36]. It is conceivable that part of the beneficial action of these short peptides has to do with their ability to sequester deuterium.
4.2. Cyclophilins
Cyclosporin A is a natural cyclic peptide metabolite found in the fungus Tolypocladium inflatum. It is widely used therapeutically for immune suppression during transplant therapy. However, it can be toxic to the liver and kidneys, and there is an increased risk to lymphoma due to severe immune suppression [
37,
38]. Cyclosporin A’s effects are due to suppression of a fascinating highly conserved class of enzymes called cyclophilins (CyPs), most especially CyPA and CyPD [
39]. CyPs are a ubiquitous family of proteins present in all prokaryotes and eukaryotes. CyPs are PPIases, although it is not understood how this enzymatic activity influences their function. CyPA plays an important role in protein folding, protein trafficking and T-cell activation. It can be secreted from cells in response to hypoxia, infection, and oxidative stress. Extracellular CyPA stimulates pro-inflammatory signaling in endothelial cells and vascular smooth muscle cells. CyPA is upregulated in cancer, and it promotes malignant transformation and metastasis [
40]. It is possible that the PPIase activity of CyPA helps to facilitate deuterium capture by proline molecules, which are also highly abundant in cancer cells [
41]. CyPA is expressed predominantly in the cytoplasm, but it can also be released into the circulation under stressful conditions, where it induces an inflammatory response [
40].
CyPD is localized to the mitochondria, where it has fascinating roles to influence mitochondrial function [
42]. While the outer mitochondrial membrane is freely permeable to small molecules, the inner membrane is very restrictive, and a mitochondrial permeability transition pore (mPTP) tightly regulates transport across this membrane [
2]. CyPD has a remarkable ability to open the mPTP when it is acetylated on a lysine residue [
43]. Hyperacetylated CyPD is a feature of heart failure. Short-term opening of the MPTP enables the efflux of calcium ions from the mitochondrial matrix, but long-term opening triggers an irreversible cascade leading to cell death. mPTP opening plays a key role in many diseases [
44].
Loss of CyPD protects from ischemic injury but promotes heart failure. CypD-/- mice have a decreased ability to oxidize fats in the heart [
45]. Transient opening of the pore allows easy entry of NAD
+ across the inner mitochondrial membrane, boosting its levels in the matrix. Abundant NAD
+ supports the activity of the sirtuin deacetylase SIRT3, which deacetylates multiple proteins in the matrix, including CyPD, but consumes NAD
+ in the process [
46].
Importantly, unlike NAD
+, neither NADH nor NADPH can be transferred between the cytoplasm and the mitochondria, so these two compartments maintain separate pools of the reduced form. By requiring the mitochondria to produce their own NADH, it can be assured that the proton is deupleted and that the ATPase pumps are protected. Instead of importing NADH, the cell shuttles small organic molecules that carry reducing equivalents across the mitochondrial membrane, for example, through the malate-aspartate and glycerol-3-phosphate shuttles [
2]. We discussed in the introduction how the cell can maintain deupleted NADPH in the mitochondria and deuterium enriched NADPH at the plasma membrane through separate IDH reductases in the mitochondria and at the cell membrane, with only the mitochondrial IDH having a high deuterium KIE.
4.3. Proline and Collagen
An obvious way to reduce the deuterium load in the organelles is to trap deuterium outside the cell, if such a strategy is feasible. In this section, we will argue that proline may play a critical role in trapping deuterium and sequestering it in the extracellular space. Collagen is the most common protein in the body, making up as much as 30% of the body’s proteins. It is a major constituent of bones, joints, skin, and cartilage, and it is found in horses’ hooves and birds’ feathers. Collagen is a structural protein that is secreted into the extracellular environment. Glycine, proline, and hydroxyproline (derived from proline) are the dominant amino acids in collagen, and together they represent 57% of the total amino acids in collagen. Collagen is essential for maintaining the normal structure and strength of connective tissue [
47].
After synthesis, collagen gets folded into its triple-helix structure in the ER and then secreted into the extracellular space. Collagen molecules self-assemble into interconnecting fibrils that form the scaffold for all tissues. They are the largest constituent of the fascia, the connective tissue that holds the organs, blood vessels, bones, muscles and nerve fibers in place. Collagen molecules bind to glycosaminoglycan chains of heparan sulfate to form the extracellular matrix, and the sulfate anions help to maintain gelled water, also known as “structured water” or “exclusion zone” (EZ) water, which excludes most solutes [
48].
Prof. Gerald Pollack has conducted many experiments demonstrating the ability of EZ water near hydrophilic surfaces such as hydrogels and biological tissues to create a battery, whereby the gel becomes negatively charged and protons gather at the interface between the gelled water and the adjacent fluid water. Pollack wrote in 2010: “Recent observations have shown an unexpected feature of water adjacent to hydrophilic surfaces: the presence of a wide interfacial zone that excludes solutes. The exclusion zone is charged, while the water beyond is oppositely charged, yielding a battery-like feature.” [
49]. It seems clear that protons would be excreted from the gel much more readily than deuterons, given that deuterons bind more strongly to oxygen in the water molecule. Photons from sunlight separate charge, splitting water molecules and facilitating proton expulsion [
49].
Nafion is a sulfonated tetrafluoroethylene-based fluoropolymer that has been used in experiments to study the properties of EZ water. Its effects on water may be an appropriate synthetic model for the sulfonated glycocalyx [
50]. Deuterium adsorbs competitively on the sulfonic groups in Nafion compared to hydrogen. The polymer fibers unwind into the bulk of the adjoining water upon swelling. These unwound fibers form a vastly extended brush type border, projecting out from the membrane surface, reminiscent of the brush-like fibers that form in the endothelial glycocalyx [
51]. A remarkable experiment demonstrated that, if the water next to a hydrophilic Nafion surface is replaced with deupleted water (3 ppm), the fibrils no longer form [
52]. It is possible that deuterium enrichment in the gelled water promotes the formation of a rich network of fibrils that are essential for the many functions of the glycocalyx.
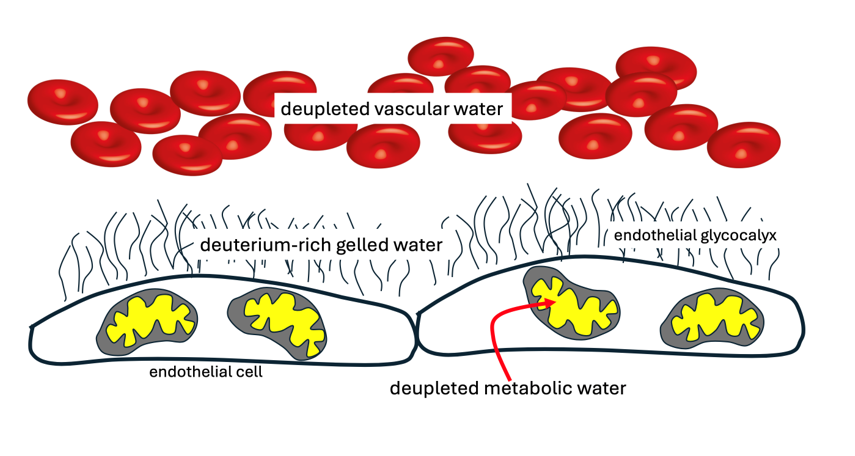
A thin (impaired) endothelial glycocalyx is associated with an increased risk to cardiovascular disease, as shown by elegant experiments where the thickness of the glycocalyx in sublingual microvessels was measured through Darkfield Imaging. Six hundred patients were tracked for six years following this measurement, and those with a thin glycocalyx had a significantly increased risk to major adverse cardiac events, with a hazard ratio of 6.44 (p = 0.011) [
53]. It is possible that a primary consequence of a thin glycocalyx is an inability to supply the blood with a sufficient number of deupleted protons, ultimately resulting in excess deuterium in the mitochondria, systemically. This defect could be due to a decrease in the amount of heparan sulfate in the glycocalyx and/or insufficient sulfation of the heparan sulfate proteoglycans, reducing the size of the EZ water pool [
54].
Water is heavily involved in the self-assembly of collagen molecules. The attractive forces of the water molecules surround the collagen network and tightly bind to it, creating a well-ordered hydrogen shell that controls its properties. Water forms bridges that contribute to the stability of the triple helix. It creates a repulsive interaction between collagen molecules, since the water bridges must be reorganized in order for the collagen molecules to get close to each other [
55]. If the water is replaced by heavy water, the assembly of collagen occurs ten times faster. This is due to the lowering of the energy penalty incurred by displacing the water bridges, which enhances the initial nucleation rate [
55]. It is plausible that the enrichment in deuterium as a consequence of the battery formation accelerates collagen cross-link formation and assembly in the extracellular space.
There are other structural proteins besides collagen that are enriched in proline. The stratum corneum is the outermost layer of the epidermis. Many of the structural proteins of the cornified envelope are very rich in proline content. For example, nearly 40% of the amino acids in the 73-amino acid envelope protein small proline rich protein 2G (SPRR2G) are proline residues [
56].
While this is speculative, it is possible that proline doping with deuterium in collagen molecules takes place in the ER, during protein folding, and that PPIase plays a catalytic role. This process would also result in deupletion of the water-based medium in the ER, reducing deuterium levels in proteins with low proline content. A special property of proline is the fact that it is the only amino acid that has both cis- and trans- isomers. PPIase greatly accelerates (by a factor of 1000) the rate at which proline residues flip between the cis- and trans- isomers [
57]. This causes rapid fire movements in the peptide backbone, switching from bent to straight back and forth in rapid succession. Such flipping would introduce kinetic energy into the system, mobilizing protons, deuterons, hydroxyl groups and deutroxyl groups and facilitating the exchange of hydrogen for deuterium between the proline residues and the water molecules during isomerization.
We theorize that, whenever a proline residue picks up a deuterium atom at the alpha carbon, it resists further exchange with hydrogen, thus permanently sequestering the deuterium atom within the collagen molecule. PPIase is highly expressed in the ER, and it plays an essential role in facilitating the folding of collagen into its triple-helix structure [
58]. If, as we propose, proline can sequester deuterium, it is logical that the high density of proline in collagen can result in a greater ability to trap deuterium outside the cell, which would be a powerful mechanism for reducing deuterium levels in the organelles.
4.4. Collagen Recycling
During extracellular matrix remodelling, collagen is constantly being broken down and resynthesized. Since collagen is very rich in proline, and since proline is resistant to cleavage from its left neighbor, the breakdown of collagen results in a large number of dipeptides and tripeptides terminating in proline.
Enzymes called matrix metalloproteinases (MMPs) specialize in breaking down collagen in the extracellular matrix. These chop the long collagen molecule up into smaller pieces that are then further metabolized by prolylendopeptidase, which produces a large number of tripeptides consisting of proline-glycine-proline (pro-gly-pro) sequences, a very common but proteolytic-resistant sequence in collagen. Pro-gly-pro is an inflammatory agent that induces migration of neutrophils to the site. A final enzyme, leukotriene A4 hydrolase (LTA4H) can resolve the inflammatory response by breaking down the pro-gly-pro tripeptides. However, exaggerated activity of LTA4H is believed to contribute to the pathogenesis of many diseases, including sepsis, cystic fibrosis, asthma, emphysema and chronic obstructive pulmonary disease (COPD) [
59] (and references therein).
A key protein involved in the final stage of collagen metabolism is prolidase, a di- and tripeptidase that specifically cleaves [Xaa]-Xaa-Pro sequences, resulting in a free proline molecule that can then participate in cellular metabolism or be reassembled into new collagen molecules. Prolidase is expressed in all three domains of life, archaea, bacteria, and eukaryotes, attesting to its importance in metabolism. It is essential not just for the recycling of collagen but also for the digestion of proline-rich proteins [
60]. Prolidase deficiency is a very rare genetic disease with severe manifestations, including multiple skin lesions on the legs and feet, mental retardation, and a weakened immune system, leading to frequent respiratory infections [
61]. This makes it clear that the complete degradation of collagen molecules to release free proline is an essential step in collagen recycling.
LTA4H is a dual-purpose enzyme in that it converts leukotriene A4 into leukotriene B4 and also clears pro-gly-pro peptides. Inflammation induces upregulation of LTA4H in immune cells, which both increases the production of leukotriene B4 and increases the clearance of pro-gly-pro peptides, in a kind of race. Leukotriene B4, in turn, promotes extravasation of leukocytes into the tissues and the release of reactive oxygen species by the neutrophils, i.e., enhances the inflammatory response [
62]. Thus, LRA4H is a double-edged sword in that it can both reduce (through clearance of pro-gly-pro) and increase (through production of leukotriene A4) inflammation.
4.5. Proline Role in Intrinsically Disordered Proteins
Proteins can be broadly grouped into globular proteins and intrinsically disordered proteins (IDPs). IDPs are typically enriched in proline, relative to globular proteins [
26]. Around 30% of all proteins in eukaryotic organisms are IDPs, and they clearly must have important functions, although their purpose has been relatively obscure, in part because they do not crystallize. Their lack of folded structure is probably crucial to their unknown but surely essential functions. They often participate in critical cellular control mechanisms. As a general rule, they can bind to many target molecules, and many of them become structured upon binding. When left unbound, they are often rapidly broken down [
63].
Tau is an IDP whose amyloidogenic potential is linked to Alzheimer’s disease. Tau hyperphosphorylation increases amyloidogenic potential. PIN1 stimulates dephosphorylation through increased isomerization of proline at pSer-Pro sites [
64]. Only the trans- isoform of proline supports dephosphorylation of pSer-Pro sequences in the tau protein, and the maintenance of the trans- isoform depends on PIN1 activity [
65]. Tau has a proline-rich domain which facilitates binding to actin fibers [
66]. Serine phosphorylation in the proline-rich domain inhibits its binding to microtubules, promoting self-aggregation [
67]. Suppression of PIN1 activity is a characteristic feature of Alzheimer’s disease [
68]. Tau also has enzymatic capability for self-acetylation, and tau acetylation increases its aggregation propensity [
64]. Misfolded phosphorylated tau protein is a significant feature of Alzheimer’s disease. It is intriguing that multiple PPIases, including PIN1, FK506-binding protein (FKBP) 52, FKB51, and FKBP12 interact with and regulate tau biology. Tau phosphorylation is a key aspect of the aggregation and oligomerization of tau [
69].
Tau has a long proline-rich region spanning residues 151-244, and there are several serine and threonine phosphorylation sites within this region. Tau is hyperphosphorylated in the Alzheimer’s brain, and phosphorylation is necessary in order to activate PIN1 and induce proline flipping [
69]. It is possible that serine phosphorylation facilitates deuterium access to the adjacent proline residue, as the phosphate anion can attract deuterons and temporarily bind to them, transferring them to the proline residue during the isomerization step.
Some support for this concept comes from studies on D/H exchange in adenosine monophosphate (AMP) compared to cyclic AMP (cAMP) [
69,
70]. Freitas et al. suggest that the phosphate anion in nucleotide monophosphates “complexes with the exchange reagent and assists H/D exchange at a neighboring site.” [
70]. In the gaseous phase, cyclic AMP and other cyclic nucleotides do not participate in H/D exchange with D
2O, whereas 3’ and 5’ nucleotide monophosphates readily do exchange. This implies that a charged phosphate group near the base is required to catalyze the reaction [
71]. By analogy, the phosphate attached to phosphoserine may play a crucial catalytic role in the H/D exchange that could take place on proline residues catalyzed to isomerize by PIN1. This would be a potent mechanism to allow deuterium to become trapped in proline residues adjacent to phosphorylated serine residues.
α-Synuclein is an IDP that contains five X-Pro bonds. CyPA’s role in neurodegeneration might be due to its ability to accelerate the rate at which proline residues flip between the two conformations. CyPA is highly expressed in neurons, and it has been shown experimentally to bind to α-synuclein. CyPA catalyzes the cis/trans isomerization of P128 of α-synuclein. People with certain mutations in α-synuclein that interfere with its binding to CyPA are at increased risk to Parkinson’s disease, which is associated with the misfolding of α-synuclein. The mutation A53E in α-synuclein causes early-onset PD, and it has been shown experimentally that this mutation strongy attenuates the interaction of α-synuclein with CyPA [
72]. This suggests that disruption of the PPIase function of CyPA towards α-synuclein either through mutations or through post-translational modifications may play an important role in α-synuclein-mediated neurotoxicity. It is possible that CyPA’s ability to greatly accelerate the rate of flipping of the proline residues facilitates mobilization of the water molecules in the solvation shell, protecting α-synuclein from aggregation.
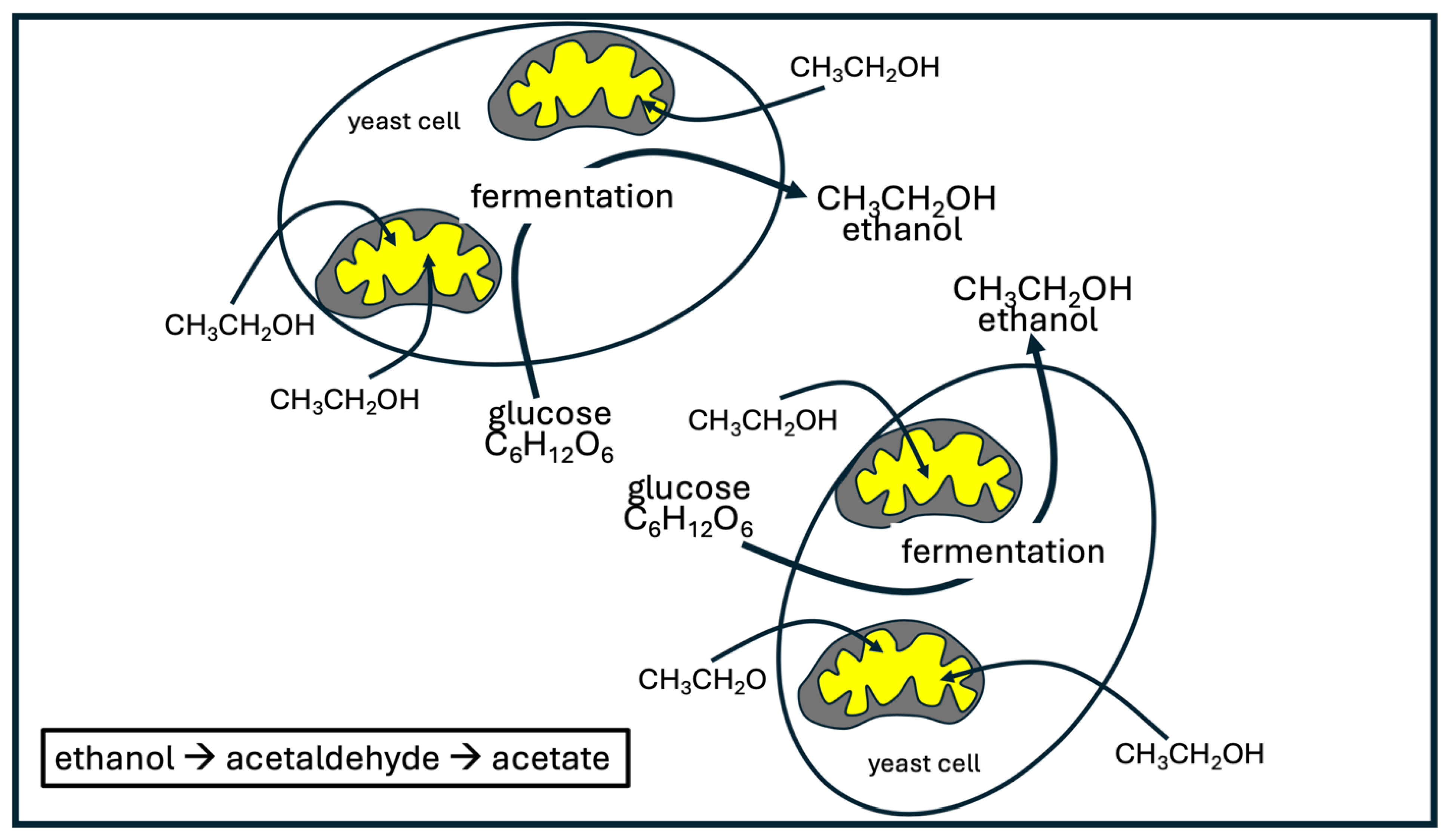
Yeast Overgrowth and Serum Ethanol
An in vivo study on human non-small cell lung cancer showed that lactate is the preferred fuel for the citric acid cycle in the tumor cells. The cells release lactate derived from glucose through glycolysis into the circulation and then retrieve it later for further processing in the mitochondria [
73]. It is likely that the primary purpose of these extra steps is to further reduce the likelihood that the proton that ultimately becomes the hydrogen atom in NADH, produced in the mitochondria, is a deuteron.
This same principle probably applies for yeast cells populating the gut, except that the glucose is converted to ethanol rather than lactate. A rare condition aptly named “auto-brewery syndrome” happens when a person experiences a massive overgrowth of yeast in the gut, which are producing large quantities of ethanol from glucose via fermentation and releasing it into the gut, from which it is ultimately absorbed into the bloodstream [
74]. The person experiences symptoms of severe inebriation, despite not having consumed any alcohol. Two species of yeast are most noted for causing this condition, namely Saccharomyces and Candida [
75].
Ethanol and lactate have long been viewed as waste products of glycolysis, but this view is rapidly changing. Tracer studies conducted in 2020 on
13C-labeled lactate have shown that this nutrient is taken up by every tissue in the body, even tumor cells [
76]. A study on fermenting yeast cells (Saccharomyces cerevisiae) showed that these cells practice a near complete uncoupling of glycolysis from oxidative phosphorylation. These authors wrote: “Specifically, we show that fermenting budding yeast simultaneously release and uptake ethanol, much as many mammalian cells simultaneously produce and consume circulating lactate.” [
77]. Two dehydrogenase steps take ethanol to acetaldehyde and then acetyl-CoA, which directly feeds into the citric acid cycle. Both steps convert NAD
+ to NADH. Under oxidative stress conditions, an even larger portion of the supply of protons for NADH production in the mitochondria is contributed by externally supplied ethanol, compared to pyruvate derived from glucose metabolism [
77].
Figure 3 illustrates the common but surprising metabolic practice of yeast cells.
Deuterium and Cancer
The growth of cancer cells in vitro is suppressed when their medium is deupleted (e.g., half the amount in seawater), whereas it is accelerated when the medium is deuterium enriched (e.g., twice the amount in seawater) [
78]. This suggests that cancer cells are capable of sensing the amount of deuterium in the medium, and when it is elevated they are triggered through signaling mechanisms to proliferate. It could even be the case that the main reason for their existence is to help restore deuterium homeostasis for the organism as a whole. When the deuterium level in their surrounding medium is low, they commit apoptosis, since deuterium overload is no longer a problem.
In another experiment involving nasopharyngeal carcinoma (NPC) cells, researchers showed that deuterium-depleted water (DDW) with deuterium concentrations at 100, 75, and 50 ppm suppressed the growth and invasiveness of the tumor cells and caused cell cycle arrest. By contrast, DDW promoted the growth of normal control cells [
79].
There is gaining support for the concept of treating cancer with DDW. A double-blind clinical trial showed that administration of DDW caused a 3-7 fold increase in mean survival time in lung cancer patients, and a 2-fold increased survival time in breast cancer [
80]. A case-control study involved pancreatic cancer patients, where both groups received chemotherapy but the treatment group also drank deupleted water (45-65 ppm deuterium tailored down over a three month period). The treated group survived on average for 19.6 months compared to only 6.36 months for the control group [
1]. Exposure of tumor cells to DDW decreases the number of cells in S phase and increases the number in G1 phase, essentially inducing G1-phase cell growth arrest [
81].
Yeast cells and cancer cells have much in common. It seems that both have a responsibility to try to restore deuterium homeostasis to the organism, or perhaps more specifically to the immune cells that infiltrate their microenvironment. Candida albicans is an opportunistic pathogenic fungus, and it tends to infect those with an impaired immune function, including cancer patients [
82]. Ironically, Candida overgrowth is also a risk factor for cancer [
83]. As was discussed previously, Candida overgrowth leads to excess accumulation of ethanol in the blood.
Ethanol is metabolized into acetate in two steps involving ethanol dehydrogenase (producing acetaldehyde) and acetaldehyde dehydrogenase (Aldh), producing acetate. The primary enzyme in the liver for metabolizing acetaldehyde is Aldh2, localized to the mitochondria. Genetic mutations causing deficiencies in Aldh2 increase the risk to hepatic cell carcinoma (HCC) following alcohol-related liver fibrosis. The description of this process by Seo et al. is precise and revealing: “Mechanistic studies revealed that after chronic alcohol exposure, Aldh2-deficient hepatocytes produce a large amount of harmful oxidized mitochondrial DNA via extracellular vesicles, which can be delivered into neighboring HCC cells and subsequently activate multiple oncogenic pathways, promoting HCC.” [
84].
Chronic ethanol consumption increases cancer risk, not only in the liver but also in the digestive tract and breast [
85]. People with genetic defects in Aldh are especially vulnerable to cancer from chronic ethanol exposure, because acetaldehyde lingers long enough to cause DNA damage [
84]. It may be for this reason that frequent episodes of Candida overgrowth are a risk factor for cancer [
83]. Several strains of Candida are transcriptionally active in gastrointestinal tumors and predictive of worse tumor outcomes, but it is likely that their presence is just an indicator, not a cause [
86]. It is also interesting from the standpoint of the potential for systemic deupletion that the development of cancer might alleviate the burden on the Candida fungi to repair the deuterium overload problem, a rather clever design. Chemotherapy can increase the risk of systemic Candidiasis in cancer patients [
87], and this may simply be because the cancer cells can no longer adequately support deuterium detoxification for the host, so Candida species rise to the occasion.
Chronological aging is defined as diminished survival of nondividing cells. In an experiment involving growing yeast cells on a medium with 50% D
2O enrichment, it was found that D
2O actually increased the chronological lifespan of the yeast cells by up to 85%. D
2O substantially suppressed the production of reactive oxygen species, which was thought to be due to increased use of glycolysis and reduced use of oxidative phosphorylation for ATP synthesis. It was hypothesized that aging could be a consequence of the damaging effects of ROS produced primarily by the mitochondria [
88]. Yeast cells can survive in both an aerobic and an anaerobic environment. They are quite capable of surviving long term on glycolysis alone, and, apparently, they can alter their metabolism strategically towards aerobic glycolysis, in the presence of high deuterium exposure, shutting down ATP production by the mitochondria. The same could be said of cancer cells, and both cell types may serve a positive role in the organism through the release of deupleted nutrients such as lactate and ethanol, triggered by elevated deuterium levels in the medium.
6.1. V-ATPase and Microenvironment Proton Deupletion
There are two main types of ATPases in biological organisms: F-ATPase and V-ATPase. We have seen that F-ATPase is the enzyme in the mitochondria that synthesizes ATP from ADP, while reducing oxygen to metabolic water. It relies on deupleted protons crossing the inner membrane of the mitochondria from a proton-dense to a proton-sparse medium as the motive force. V-ATPases (V stands for “vacuole”) have an opposite function. Protons are pumped across a membrane from a proton-sparse medium to a proton-dense medium, and this requires work, which is supplied by converting ATP to ADP. When they are localized to the plasma membrane, they
pump protons into the extracellular space, and when they are localized at the membrane of the endosome/lysosome organelle, they can acidify the endosome to convert it to a lysosome [
89].
A seminal paper published in 1990 involved evaluating the deuterium KIE of yeast V-ATPase acting at the plasma membrane. The authors were surprised to find that the extrusion of H
+ ions was reduced by as much as 90% when the cells were suspended in heavy water. In referring to V-ATPase, these authors wrote: “It thus appears that the binding site for protons (or hydronium ions) to be transported does not accept deuterons (or deuteronium ions) with equal ease or perhaps not at all.” [
90]. In other words, yeast V-ATPase has an extremely high deuterium KIE.
It is significant that human breast cancer cells with high metastatic potential overexpress V-ATPase at the plasma membrane, and they use it presumably to transfer deupleted protons into the extracellular space, thus enriching deuterium levels in the cell’s cytoplasm [
91]. This strange practice is consistent with a theory that the primary role of cancer cells is in some sense to maintain high internal levels of deuterium in order to promote reduced deuterium levels systemically.
Immune cells routinely invade the tumor microenvironment, but there are signaling mechanisms that cause them to stand down, failing to kill the tumor cells, via a process called “tumor immune escape” [
92]. These immune cells can presumably benefit from the deupleted protons released by the tumor cells to help them maintain low deuterium levels in their own organelles, with the ultimate goal of restoring their ability to kill and clear the cancer cells, once they regain health.
6.2. Lactate
While lactate had long been viewed as a waste product of glycolysis, researchers have now come to appreciate the fact that it is actually a valuable nutrient, and that its presence in the microenvironment has powerful signaling capabilities, especially towards immune cells [
93]. Lactate accumulation in the tissue microenvironment is a common feature of both inflammatory disease and cancer [
94]. The Warburg effect is a hallmark characteristic of cancer cells: they produce ATP primarily through glycolysis even in the presence of adequate oxygen for oxidative phosphorylation [
95]. This is similar to the strategy that yeast use in the presence of excess deuterium. Aerobic glycolysis produces lactate under stressful conditions, such as trauma, infection, myocardial infarction, and heart failure [
94].
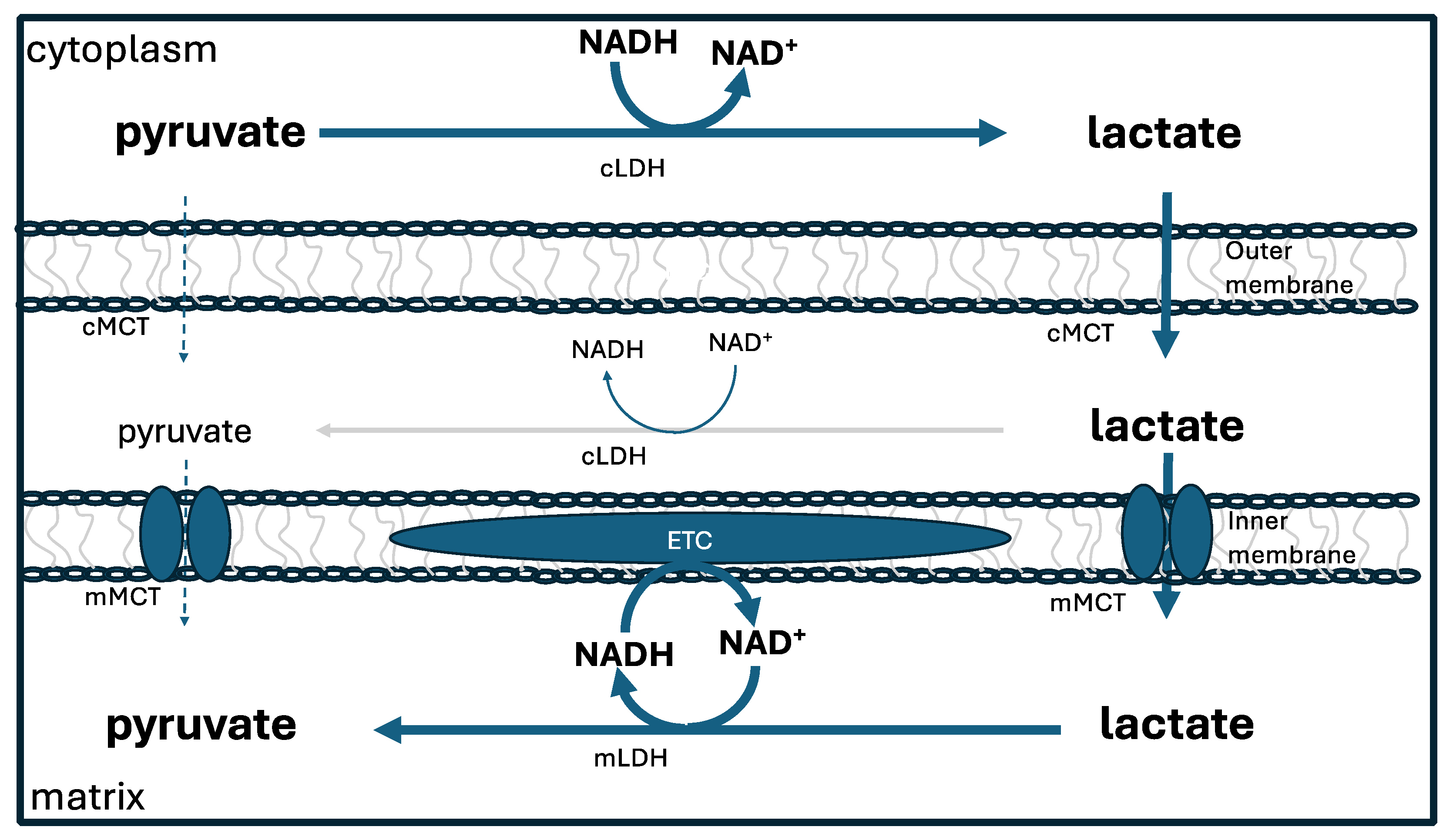
Lactate is a very useful fuel for the mitochondria, because it can easily be converted to pyruvate via lactate dehydrogenase. Glycolysis, taking place in the cytoplasm, converts glucose to pyruvate. This pyruvate molecule can be transported into the mitochondria and used directly to generate large amounts of ATP via the citric acid cycle. However, most of the time this is not what happens. Instead, pyruvate is converted to lactate in the cytoplasm, and then the lactate is transported across the mitochondrial membrane and converted back to pyruvate [
96].
While this appears to be a superfluous step, it is actually a very clever strategy for assuring that the hydrogen atoms in the mitochondrial NADH are deupleted. Cytoplasmic lactate dehydrogenase has a high deuterium KIE ( 3-4) [
97], so it provides lactate with a deupleted proton, which is then delivered to NAD
+ in the mitochondria, to finally produce a further deupleted NADH molecule within the mitochondria, again, most likely, since it is a flavoprotein, with a high deuterium KIE. The extra steps of passing a proton to lactate and then from lactate to NAD
+ provide a simple mechanism for further shedding of deuterium.
Figure 4 depicts the lactate shuttle.
Very frequently, the metabolism of glucose to produce ATP is carried out in two steps in two different cells, with the first cell producing lactate from glucose via glycolysis in the cytoplasm, and the second one metabolizing lactate to CO
2 and water in the mitochondria. A paper published by Xiao et al. in 2022 brings awareness to the fact that lactate, rather than being a waste product, is a very common metabolic intermediate between glycolysis in one cell and the citric acid cycle in another distant cell. These authors wrote: “Indeed, it seems that most carbohydrate oxidation in mammals, rather than occurring by a tissue taking up glucose and fully oxidizing it to carbon dioxide, instead involves carbon flowing through circulating lactate as a metabolic intermediate” [
77].
Cancer cells commonly overproduce lactate and then release it to the circulation, allowing the recipient cell to skip glycolysis and efficiently produce ATP directly from the tumor-provided lactate molecule, a process known as the Warburg effect. Meanwhile, the cancer cell can repurpose its mitochondria towards anabolic pathways, thus avoiding exposure to ROS generated by oxidative phosphorylation [
98].
6.3. Succinate Dehydrogenase and Proline Dehydrogenase
The tumor microenvironment, i.e., the extracellular space encasing the cancer cells, accumulates a unique set of metabolites that are released from the cancer cells, including not only lactate, but also glutamate, fumarate and succinate. These organic molecules collectively are called “oncometabolites,” but they are actually useful fuels for the cells that migrate into the tumor microenvironment, including immune cells, fibroblasts and endothelial cells lining the neovasculature [
99].
Through suppression of oxidative phosphorylation, the tumor accumulates but then disposes of metabolic intermediates of the citric acid cycle, providing them as nutrients to their supporting cells. Succinate dehydrogenase (SDH) is the only enzyme that participates in both the citric acid cycle and oxidative phosphorylation. SDH is often defective in cancer cells, whether through genetic mutations, post-translational modifications or endogenous SDH inhibitors [
100].
Protein acetylation is a post-translational modification that is particularly prevalent in the mitochondrial proteins. SDH has been shown to be suppressed by acetylation, and pre-incubation of mitochondria with NAD
+ could partially prevent this effect [
101]. Sirtuins (SIRTs) are an important class of enzymes that deacetylate proteins. SIRT3 is highly expressed in mitochondria, but its deacetylation activity depends upon adequate availability of NAD
+, which is needed as a cofactor and consumed in the process. Hence, NAD
+ deficiency could lead to inactivation of SDH. When SDH is deficient, succinate accumulates in the mitochondria, and it is transported to the cytoplasm and secreted into the extracellular space. Extracellular succinate promotes tumor growth by inducing tumor angiogenesis [
102].
When SDH is deficient, proline can step up to substitute for succinate to fuel the electron transport chain. Proline dehydrogenase (PDH) is the first step in the metabolism of proline, and it can donate electrons to the electron transport chain to support ATP production [
103]. The activity of enzymes involved in proline synthesis is reduced in senescent stem cells, and proline supplementation has been shown to restore mitochondrial function and offer therapeutic value in slowing the aging process [
104].There is a reversible pathway between proline and glutamate which cyclically converts NADH to NAD
+ while delivering two protons to ubiquinol to form ubiquinone in the inner membrane of the mitochondrial intermembrane space. Those protons will be deupleted because PDH has a deuterium KIE of 5.2 [
105]. Furthermore, the proline that is left behind will be enriched in deuterium. The enriched proline can be put back into the extracellular matrix after it has been incorporated into newly synthesized collagen molecules [
106].
It had long been a mystery as to which protein transports NAD
+ into the mitochondria, but only recently it was conclusively shown to be the elusive protein named solute carrier family 25 member 51 (SLC25A51), also known as Myc-DDK-tagged-Human mitochondrial carrier triple repeat 1 (MCART1), which is localized to the inner membrane of the intermembrane space [
107]. This protein is upregulated in multiple cancers, and it promotes cancer cell proliferation. Loss of MCART1 leads to SIRT3 dysfunction due to insufficient NAD
+, which is a substrate for SIRT3’s deacetylation activity. Pharmacological inhibition of MCART1 causes protein hyperacetylation in the mitochondria. By upregulating MCART1, cancer cells increase mitochondrial NAD
+, which promotes deacetylation and thereby activates various proteins involved in mitochondrial metabolism.
One of those enzymes that is activated through deacetylation is P5CS, the key enzyme in proline biosynthesis from glutamate. As a result, cancer cells synthesize abundant amounts of proline in their mitochondria [
108]. Not only do tumor cells upregulate proline synthesis, but also the fibroblasts that are associated with tumor cells in the tumor microenvironment produce excessive amounts of proline from glutamine in order to synthesize large quantities of collagen to support the build-up of a deuterium-enriched extracellular matrix in the tumor [
109].
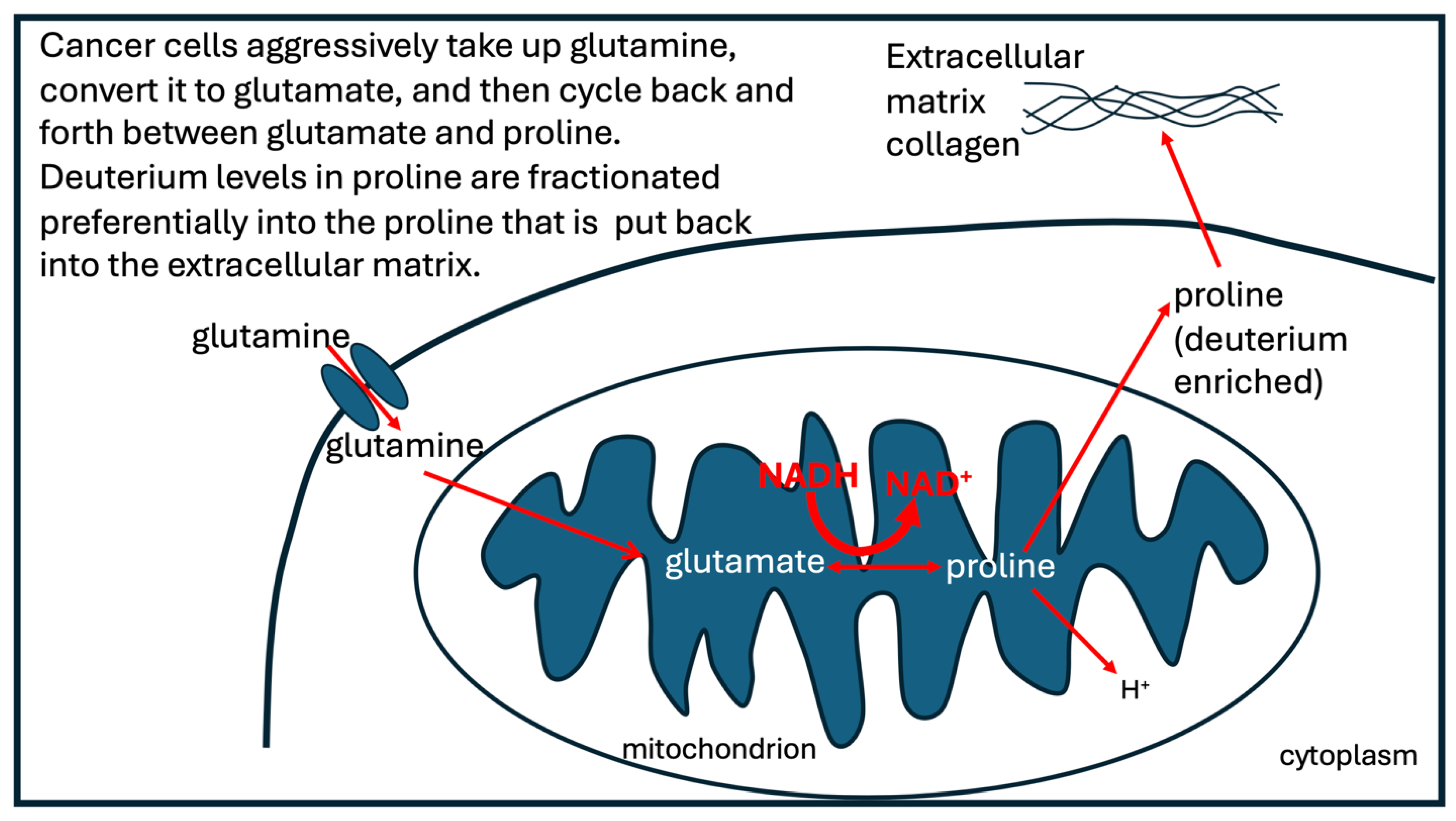
The process that takes place in cancer cells that cycles between glutamate and proline, delivering deupleted protons to the ATPase pumps in the mitochondria, while enriching deuterium levels in the extracellular matrix collagen, is depicted schematically in
Figure 5.
Figure 5.
Illustration of the activities that take place in tumor cells to accommodate a loss in succinate dehydrogenase, by replacing its role with proline. The proline that is left behind after proline dehydrogenase delivers protons to the quinone in the inner membrane is used to synthesize deuterium-rich collagen molecules that are added to the extracellular matrix pool.
Figure 5.
Illustration of the activities that take place in tumor cells to accommodate a loss in succinate dehydrogenase, by replacing its role with proline. The proline that is left behind after proline dehydrogenase delivers protons to the quinone in the inner membrane is used to synthesize deuterium-rich collagen molecules that are added to the extracellular matrix pool.
6.4. Epidermal Growth Factor and Cancer
The epidermal growth factor receptor (EGFR) plays a critical role in cancer and malignancy. Its activity enhances tumor growth, invasion, and metastasis [
110]. HER2 (human EGFR-2) is upregulated in many chemotherapy-resistant cancers, particularly breast cancer. HER2-positive breast cancer is generally associated with poor outcomes and higher mortality rates compared to other breast cancer subtypes [
111].
Prolidase is a high-affinity ligand to HER2. It binds to the extracellular domain of HER2 and causes its internalization and degradation. It inhibits growth, invasion and migration of cancer cells that overexpress HER2 [
112]. The fact that it can free up proline to both fuel the mitochondria with deupleted protons and promote trapping of deuterium in the extracellular matrix may give it license to suppress tumor growth.
6.5. NOX4 and DDW
NOX4 is a member of the NOX family that localizes to the inner membrane of the mitochondrial intermembrane space. Remarkably, it binds to ATP and such binding suppresses its activity. When ATP production is low (an indicator of mitochondrial dysfunction), NOX4 becomes liberated and starts producing large quantities of superoxide, derived from mitochondrial NADPH, according to the following equation:
Superoxide dismutase (SOD), in turn, can combine protons with superoxide to produce hydrogen peroxide:
This superoxide will be deupleted if the H
+ came from mitochondrial NADPH, and either catalase or glutathione peroxidase (important antioxidants) can convert it to DDW, thus reducing the levels of deuterium in the mitochondrial water. This in turn can help restore the health of the mitochondria. NOX4 is upregulated in cancer cells, and it seems to play a protective role in preventing apoptosis [
113]. Critically, the concept that superoxide can be a source of deupleted water may be key to answering the question of why inflammation has beneficial effects. On the other hand, if antioxidant defenses are inadequate, the cell will likely be vulnerable to cellular death from excessive production of oxygen radicals.
Conclusion
In this paper, we have developed a theory that deuterium, a natural heavy isotope of hydrogen, plays a central role in metabolism, and that disrupted deuterium homeostasis is a major driver behind human disease. We have shown how cellular metabolism utilizes specialized enzymes such as isomerases and flavoproteins to sequester deuterium in the extracellular space and deplete deuterium levels in the cellular organelles. Deuterium doping in the bones increases their strength, whereas deuterium is toxic to the ATPase pumps in the mitochondria. Mitochondrial dysfunction is a core feature of many human diseases and might even be characterized as a primary feature of aging. We have shown how yeast cells and cancer cells alike practice certain metabolic policies utilizing aerobic glycolysis to synthesize large quantities of deupleted nutrients (ethanol in the case of yeast cells and lactate in cancer cells) which are released into the extracellular space and shared with the host cells, ameliorating their deuterium overload problem. Cancer cells repurpose their mitochondria towards anabolic synthesis of substrates to support proliferation. They also release many deupleted nutrients that can help infiltrating immune cells recover from deuterium toxicity.
The amino acid proline has unique properties that make it ideal for trapping deuterium, and we argue that peptidyl prolyl isomerases facilitate deuterium trapping in proline residues of proline-rich proteins, which are especially common as structural proteins in the extracellular space. The endothelial glycocalyx supports deuterium trapping in the gelled water maintained by the highly sulfated heparan sulfate molecules found there. The so-called exclusion zone (EZ) water in the glycocalyx releases protons preferentially into the circulation, generally leaving behind the deuterons, which strengthen the gel and the collagen matrices. Deficiencies in sulfation in the glycocalyx lead to weakening of the battery maintained by the gelled water, ultimately resulting in higher levels of deuterium circulating in the blood. It is possible although still speculative that proline-rich intrinsically disordered proteins (IDPs) fractionate deuterium out of the medium and sequester it in the “misfolded” oligomers and fibrils that are a feature of neurodegenerative diseases such as Alzheimer’s.
Some aspects of this paper are admittedly speculative, but speculation is sometimes essential to stimulate ideas that can lead to the design of carefully constructed research experiments to either verify or disprove the hypotheses presented. There is no doubt that more research is needed in the budding field of deutenomics.