Submitted:
17 June 2024
Posted:
20 June 2024
You are already at the latest version
Abstract
Keywords:
1. Why Nanovaccines?
2. How Particle Size and Positive Charge Drive the Immune Response?
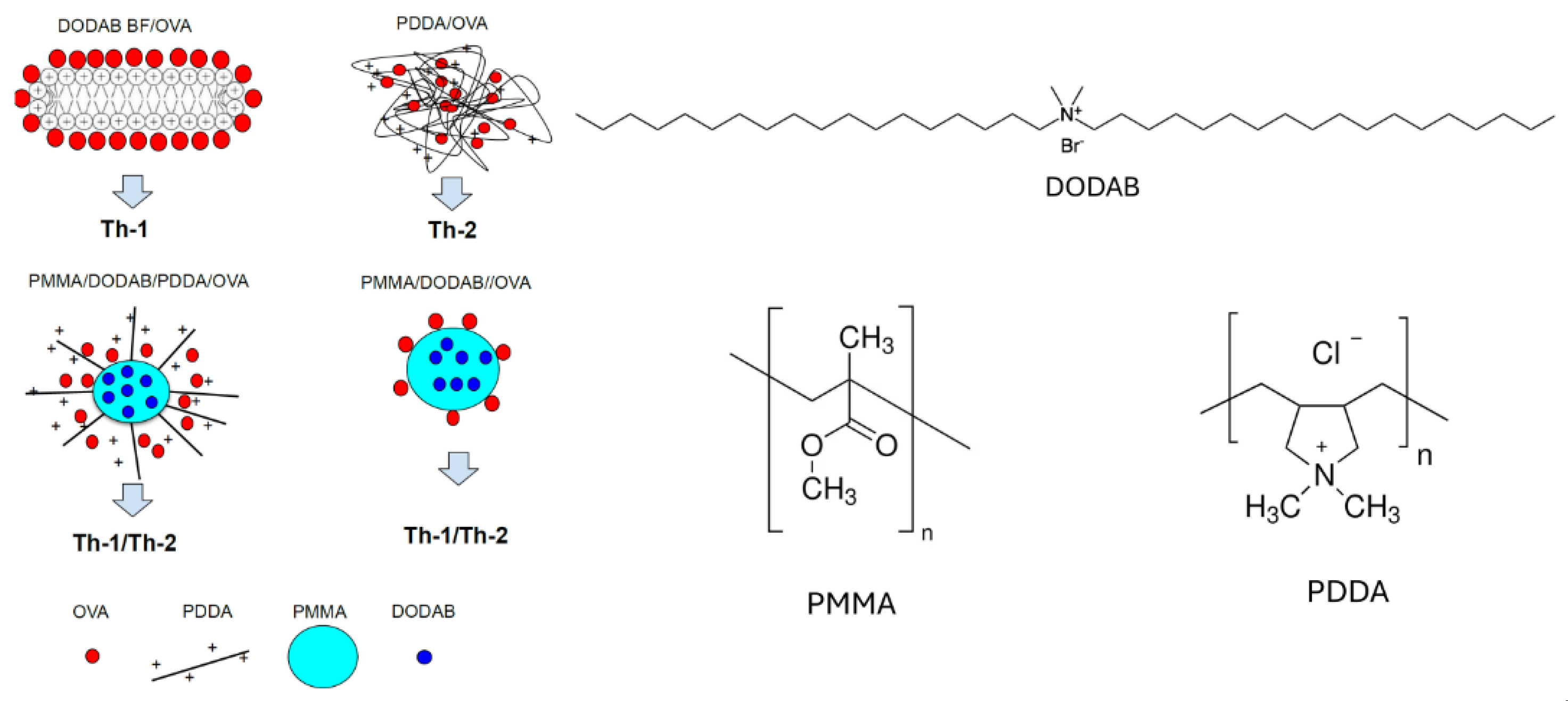
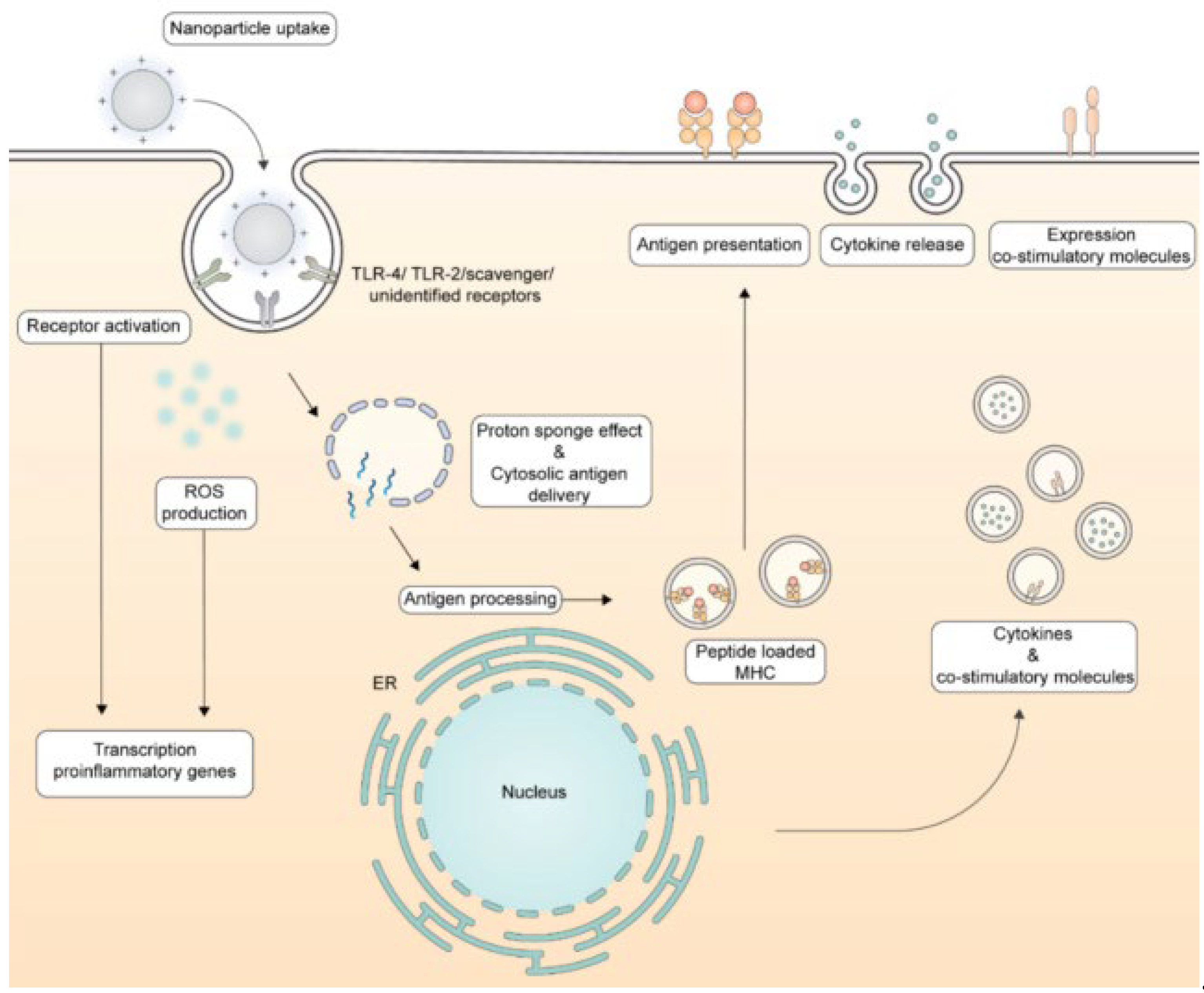
3. Cationic Nanostructures in Vaccine Design Against Infections
4. Cationic Nanostructures in Vaccine Design against Cancer
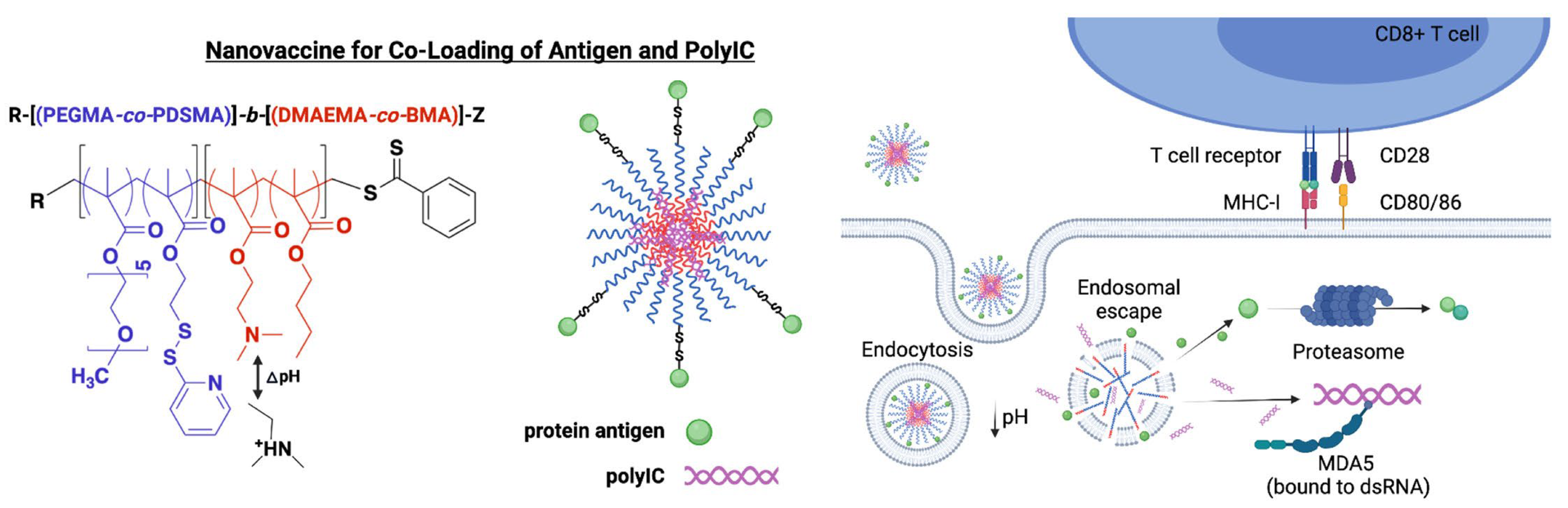
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 9th ed.; Elsevier Ltd: Philadelphia, PA, USA, 2018; p. 608. ISBN 978-0-323-47978-3. [Google Scholar]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles Target Distinct Dendritic Cell Populations According to Their Size. Eur J Immunol 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Fifis, T.; Mottram, P.; Bogdanoska, V.; Hanley, J.; Plebanski, M. Short Peptide Sequences Containing MHC Class I and/or Class II Epitopes Linked to Nano-Beads Induce Strong Immunity and Inhibition of Growth of Antigen-Specific Tumour Challenge in Mice. Vaccine 2004, 23, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines against Tumors. The Journal of Immunology 2004, 173, 3148–3154. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen Recognition and Development of Particulate Vaccines: Does Size Matter? Methods 2006, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Wilson, K.L.; Goubier, A.; Heyerick, A.; Plebanski, M. Design of Peptide-Based Nanovaccines Targeting Leading Antigens From Gynecological Cancers to Induce HLA-A2.1 Restricted CD8(+) T Cell Responses. Frontiers in immunology 2018, 9, 2968. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Ugozzoli, M.; Briones, M.; Kazzaz, J.; Soenawan, E.; O’Hagan, D.T. The Effect of CTAB Concentration in Cationic PLG Microparticles on DNA Adsorption and in Vivo Performance. Pharm Res 2003, 20, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Briones, M.; Ott, G.; O’Hagan, D. Cationic Microparticles: A Potent Delivery System for DNA Vaccines. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Denis-Mize, K.S.; Dupuis, M.; MacKichan, M.L.; Singh, M.; Doe, B.; O’Hagan, D.; Ulmer, J.B.; Donnelly, J.J.; McDonald, D.M.; Ott, G. Plasmid DNA Adsorbed onto Cationic Microparticles Mediates Target Gene Expression and Antigen Presentation by Dendritic Cells. Gene Therapy 2000, 7, 2105–2112. [Google Scholar] [CrossRef]
- Xiang, S.D.; Selomulya, C.; Ho, J.; Apostolopoulos, V.; Plebanski, M. Delivery of DNA Vaccines: An Overview on the Use of Biodegradable Polymeric and Magnetic Nanoparticles. WIREs Nanomed Nanobiotechnol 2010, 2, 205–218. [Google Scholar] [CrossRef]
- Nazarizadeh, A.; Staudacher, A.H.; Wittwer, N.L.; Turnbull, T.; Brown, M.P.; Kempson, I. Aluminium Nanoparticles as Efficient Adjuvants Compared to Their Microparticle Counterparts: Current Progress and Perspectives. IJMS 2022, 23, 4707. [Google Scholar] [CrossRef]
- Stillman, Z.S.; Decker, G.E.; Dworzak, M.R.; Bloch, E.D.; Fromen, C.A. Aluminum-Based Metal–Organic Framework Nanoparticles as Pulmonary Vaccine Adjuvants. J Nanobiotechnol 2023, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Raponi, A.; Brewer, J.M.; Garside, P.; Laera, D. Nanoalum Adjuvanted Vaccines: Small Details Make a Big Difference. Seminars in Immunology 2021, 56, 101544. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Supramolecular Nanostructures for Vaccines. Biomimetics (Basel) 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Betancourt, Y.; Távora, B. de C.L.F.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Biocompatible Lipid Polymer Cationic Nanoparticles for Antigen Presentation. Polymers 2021, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Betancourt, Y.; Araujo, P.M.; Távora, B. de C.L.F.; Pereira, D.R.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Cationic and Biocompatible Polymer/Lipid Nanoparticles as Immunoadjuvants. Pharmaceutics 2021, 13, 1859. [Google Scholar] [CrossRef]
- Lincopan, N.; Carmona-Ribeiro, A.M. Protein Assembly onto Cationic Supported Bilayers. Journal of Nanoscience and Nanotechnology 2009, 9, 3578–3586. [Google Scholar] [CrossRef] [PubMed]
- Lincopan, N.; Espíndola, N.M.; Vaz, A.J.; Costa, M.H.B. da; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Novel Immunoadjuvants Based on Cationic Lipid: Preparation, Characterization and Activity in Vivo. Vaccine 2009, 27, 5760–5771. [Google Scholar] [CrossRef] [PubMed]
- Lincopan, N.; Espíndola, N.; Vaz, A.; Carmonaribeiro, A. Cationic Supported Lipid Bilayers for Antigen Presentation. International journal of pharmaceutics 2007, 340, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Betancourt, Y.; Távora, B. de C.L.F.; Colombini, M.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants. Vaccines 2020, 8, 105. [Google Scholar] [CrossRef]
- Rozenfeld, J.H.K.; Silva, S.R.; Ranéia, P.A.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Stable Assemblies of Cationic Bilayer Fragments and CpG Oligonucleotide with Enhanced Immunoadjuvant Activity in Vivo. J Control Release 2012, 160, 367–373. [Google Scholar] [CrossRef]
- Lincopan, N.; Santana, M.R.; Faquim-Mauro, E.; da Costa, M.H.B.; Carmona-Ribeiro, A.M. Silica-Based Cationic Bilayers as Immunoadjuvants. BMC Biotechnol 2009, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Biomimetic Systems in Nanomedicine. In Handbook of Nanobiomedical Research: Fundamentals, Applications and Recent Developments; 2014; pp. 401–456 ISBN 978-981-4520-64-5.
- Carmona-Ribeiro, A.M. The Versatile Dioctadecyldimethylammonium Bromide. In Application and Characterization of Surfactants; Najjar, R., Ed.; IntechOpen, 2017; pp. 157–182 ISBN 978-953-51-3326-1.
- Agger, E.M.; Rosenkrands, I.; Hansen, J.; Brahimi, K.; Vandahl, B.S.; Aagaard, C.; Werninghaus, K.; Kirschning, C.; Lang, R.; Christensen, D.; et al. Cationic Liposomes Formulated with Synthetic Mycobacterial Cordfactor (CAF01): A Versatile Adjuvant for Vaccines with Different Immunological Requirements. PloS one 2008, 3, e3116. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, L.R.; Quintilio, W.; Costa, M.H.; Carmona-Ribeiro, A.M. Interactions between Cationic Liposomes and an Antigenic Protein: The Physical Chemistry of the Immunoadjuvant Action. Journal of Lipid Research 1997, 38, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Hilgers, L.A.; Snippe, H. DDA as an Immunological Adjuvant. Res Immunol 1992, 143, 494–503. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Santos, F.A.; Lincopan, N.; De Gaspari, E. Evaluation of Intranasal and Subcutaneous Route of Immunization in Neonatal Mice Using DODAB-BF as Adjuvant with Outer Membrane Vesicles of Neisseria Meningitis B. Immunobiology 2018, 223, 750–760. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, A.F.; De Gaspari, E. Dioctadecyldimethylammonium Bromide (DODAB-BF) as a New Adjuvant for Maternal-Fetal Immunization in Mice against Neisseria Meningitidis: Evaluation of Humoral Response. Pathogens and disease 2018, 76. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of Cationic Liposomes Based on Dimethyldioctadecylammonium and Synthetic Cord Factor from M. Tuberculosis (Trehalose 6,6’-Dibehenate)-a Novel Adjuvant Inducing Both Strong CMI and Antibody Responses. Biochimica et biophysica acta 2005, 1718, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; Van Den Bogaart, G. Modulation of Immune Responses by Particle Size and Shape. Front. Immunol. 2021, 11, 607945. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, E.B.; Rosetti, A.S.; Lincopan, N.; De Gaspari, E. Neisseria Lactamica Antigens Complexed with a Novel Cationic Adjuvant. Human Vaccines & Immunotherapeutics 2013, 9, 572–581. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Influence of Particle Size on Drug Delivery to Rat Alveolar Macrophages Following Pulmonary Administration of Ciprofloxacin Incorporated into Liposomes. Journal of Drug Targeting 2006, 14, 557–566. [Google Scholar] [CrossRef]
- Epstein-Barash, H.; Gutman, D.; Markovsky, E.; Mishan-Eisenberg, G.; Koroukhov, N.; Szebeni, J.; Golomb, G. Physicochemical Parameters Affecting Liposomal Bisphosphonates Bioactivity for Restenosis Therapy: Internalization, Cell Inhibition, Activation of Cytokines and Complement, and Mechanism of Cell Death. Journal of Controlled Release 2010, 146, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Ortiz, Z.G.; Means, T.K. The Role of Dendritic Cells in the Innate Recognition of Pathogenic Fungi ( A. Fumigatus, C. Neoformans and C. Albicans ). Virulence 2012, 3, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Benne, N.; Van Duijn, J.; Kuiper, J.; Jiskoot, W.; Slütter, B. Orchestrating Immune Responses: How Size, Shape and Rigidity Affect the Immunogenicity of Particulate Vaccines. Journal of Controlled Release 2016, 234, 124–134. [Google Scholar] [CrossRef]
- Mottram, P.L.; Leong, D.; Crimeen-Irwin, B.; Gloster, S.; Xiang, S.D.; Meanger, J.; Ghildyal, R.; Vardaxis, N.; Plebanski, M. Type 1 and 2 Immunity Following Vaccination Is Influenced by Nanoparticle Size: Formulation of a Model Vaccine for Respiratory Syncytial Virus. Molecular pharmaceutics 2007, 4, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, V.; Panda, A.K. Interactions of Antigen-Loaded Polylactide Particles with Macrophages and Their Correlation with the Immune Response. Biomaterials 2007, 28, 5344–5357. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sloat, B.R.; Yanasarn, N.; Cui, Z. Relationship between the Size of Nanoparticles and Their Adjuvant Activity: Data from a Study with an Improved Experimental Design. European Journal of Pharmaceutics and Biopharmaceutics 2011, 78, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Heung, L.J.; Wiesner, D.L.; Wang, K.; Rivera, A.; Hohl, T.M. Immunity to Fungi in the Lung. Seminars in Immunology 2023, 66, 101728. [Google Scholar] [CrossRef] [PubMed]
- Heung, L.J. Monocytes and the Host Response to Fungal Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, H.-J.; Hang, T.; Yu, Y.; Liu, G.; He, G.; Xiao, S.; Yang, B.; Yang, C.; Liu, F.; et al. Physical Activation of Innate Immunity by Spiky Particles. Nature Nanotech 2018, 13, 1078–1086. [Google Scholar] [CrossRef]
- Cong, V.T.; Houng, J.L.; Kavallaris, M.; Chen, X.; Tilley, R.D.; Gooding, J.J. How Can We Use the Endocytosis Pathways to Design Nanoparticle Drug-Delivery Vehicles to Target Cancer Cells over Healthy Cells? Chem. Soc. Rev. 2022, 51, 7531–7559. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. IJMS 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of Nanomedicines. Journal of Controlled Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem Soc Rev 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Sousa De Almeida, M.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding Nanoparticle Endocytosis to Improve Targeting Strategies in Nanomedicine. Chem. Soc. Rev. 2021, 2021 50, 5397–5434. [Google Scholar] [CrossRef]
- Zhu, G.H.; Gray, A.B.C.; Patra, H.K. Nanomedicine: Controlling Nanoparticle Clearance for Translational Success. Trends in Pharmacological Sciences 2022, 43, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Ito, A. Tailoring Inorganic Nanoadjuvants towards Next-Generation Vaccines. Chem. Soc. Rev. 2018, 47, 4954–4980. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Sun, X. Engineering Nanoparticulate Vaccines for Enhancing Antigen Cross-Presentation. Current Opinion in Biotechnology 2020, 66, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Liao, Y.; Zuo, Q.; Liu, N.; Liu, Z. MnO 2 Nanoparticles as a Minimalist Multimode Vaccine Adjuvant/Delivery System to Regulate Antigen Presenting Cells for Tumor Immunotherapy. J. Mater. Chem. B 2022, 10, 3474–3490. [Google Scholar] [CrossRef] [PubMed]
- Breslin, J.W.; Yang, Y.; Scallan, J.P.; Sweat, R.S.; Adderley, S.P.; Murfee, W.L. Lymphatic Vessel Network Structure and Physiology. In Comprehensive Physiology; Prakash, Y.S., Ed.; Wiley, 2018; pp. 207–299 ISBN 978-0-470-65071-4.
- Tang, Y.; Liu, B.; Zhang, Y.; Liu, Y.; Huang, Y.; Fan, W. Interactions between Nanoparticles and Lymphatic Systems: Mechanisms and Applications in Drug Delivery. Advanced Drug Delivery Reviews 2024, 209, 115304. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, L.; Zhu, Z.; Deng, F.; Zhang, W.; Wang, F.; Zeng, P.; Shi, H.; Wang, T.; Chen, Y.; et al. A Manganese-Based Nanodriver Coordinates Tumor Prevention and Suppression through STING Activation in Glioblastoma. Adv Healthcare Materials 2024, 2400421. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid Nanoparticle-Mediated Lymph Node–Targeting Delivery of mRNA Cancer Vaccine Elicits Robust CD8 + T Cell Response. Proc. Natl. Acad. Sci. U.S.A. 2022, 119, e2207841119. [Google Scholar] [CrossRef] [PubMed]
- Pizzuto, M.; Bigey, P.; Lachages, A.-M.; Hoffmann, C.; Ruysschaert, J.-M.; Escriou, V.; Lonez, C. Cationic Lipids as One-Component Vaccine Adjuvants: A Promising Alternative to Alum. Journal of controlled release : official journal of the Controlled Release Society 2018, 287, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wilmar, A.; Lonez, C.; Vermeersch, M.; Andrianne, M.; Perez-Morga, D.; Ruysschaert, J.-M.; Vandenbranden, M.; Leo, O.; Temmerman, S.T. The Cationic Lipid, diC14 Amidine, Extends the Adjuvant Properties of Aluminum Salts through a TLR-4- and Caspase-1-Independent Mechanism. Vaccine 2012, 30, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.-M. Cationic Lipid Nanocarriers Activate Toll-like Receptor 2 and NLRP3 Inflammasome Pathways. Nanomedicine: Nanotechnology, Biology and Medicine 2014, 10, 775–782. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Lipid Bilayer Fragments and Disks in Drug Delivery. Curr Med Chem 2006, 13, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Synthetic Amphiphile Vesicles. Chem. Soc. Rev. 1992, 21, 209–214. [Google Scholar] [CrossRef]
- Naves, A.F.; Palombo, R.R.; Carrasco, L.D.M.; Carmona-Ribeiro, A.M. Antimicrobial Particles from Emulsion Polymerization of Methyl Methacrylate in the Presence of Quaternary Ammonium Surfactants. Langmuir 2013, 29, 9677–9684. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, L.D. de M.; Bertolucci, R.J.; Ribeiro, R.T.; Sampaio, J.L.M.; Carmona-Ribeiro, A.M. Cationic Nanostructures against Foodborne Pathogens. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Bilayer Vesicles and Liposomes as Interface Agents. Chem. Soc. Rev. 2001, 30, 241–247. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Biomimetic Nanomaterials from the Assembly of Polymers, Lipids, and Surfactants. Surfactants and Detergents 2019. [Google Scholar] [CrossRef]
- Ribeiro, R.T.; Galvão, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal Dispersions and Coatings from Hybrid Nanoparticles of Poly (Methyl Methacrylate), Poly (Diallyl Dimethyl Ammonium) Chloride, Lipids, and Surfactants. International journal of molecular sciences 2019, 20, 6150. [Google Scholar] [CrossRef] [PubMed]
- Thangsunan, P.; Kitiyodom, S.; Srisapoome, P.; Pirarat, N.; Yata, T.; Thangsunan, P.; Boonrungsiman, S.; Bunnoy, A.; Rodkhum, C. Novel Development of Cationic Surfactant-Based Mucoadhesive Nanovaccine for Direct Immersion Vaccination against Francisella Noatunensis Subsp. Orientalis in Red Tilapia (Oreochromis Sp.). Fish & Shellfish Immunology 2022, 127, 1051–1060. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Lipid-Based Biomimetics in Drug and Vaccine Delivery. In Biomimetics Learning from Nature; IntechOpen, 2010 ISBN 978-953-307-025-4.
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic Antimicrobial Polymers and Their Assemblies. Int J Mol Sci 2013, 14, 9906–9946. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Hoekstra, D.; Zuhorn, I.S. Mechanism of Polyplex- and Lipoplex-Mediated Delivery of Nucleic Acids: Real-Time Visualization of Transient Membrane Destabilization without Endosomal Lysis. ACS Nano 2013, 7, 3767–3777. [Google Scholar] [CrossRef]
- Simón-Vázquez, R.; Peleteiro, M.; González-Fernández, Á. Polymeric Nanostructure Vaccines: Applications and Challenges. Expert Opinion on Drug Delivery 2020, 17, 1007–1023. [Google Scholar] [CrossRef]
- Thompson, K.D.; Rodkhum, C.; Bunnoy, A.; Thangsunan, P.; Kitiyodom, S.; Sukkarun, P.; Yostawornkul, J.; Yata, T.; Pirarat, N. Addressing Nanovaccine Strategies for Tilapia. Vaccines 2023, 11, 1356. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Araújo, P.M. Antimicrobial Polymer−Based Assemblies: A Review. International Journal of Molecular Sciences 2021, 22, 5424. [Google Scholar] [CrossRef] [PubMed]
- Rosa, H.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Interactions between Bacteriophage DNA and Cationic Biomimetic Particles. J. Phys. Chem. B 2008, 112, 16422–16430. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; de Moraes Lessa, M. Interactions between Bilayer Membranes and Latex. Colloids and Surfaces A: Physicochemical and Engineering Aspects 1999, 153, 355–361. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Pérez-Betancourt, Y. Cationic Nanostructures for Vaccines Design. Biomimetics (Basel, Switzerland) 2020, 5. [Google Scholar] [CrossRef]
- Choi, K.C.; Lee, D.H.; Lee, J.W.; Lee, J.S.; Lee, Y.K.; Choi, M.J.; Jeong, H.Y.; Kim, M.W.; Lee, C.-G.; Park, Y.S. Novel Lipid Nanoparticles Stable and Efficient for mRNA Transfection to Antigen-Presenting Cells. IJMS 2024, 25, 1388. [Google Scholar] [CrossRef] [PubMed]
- Anderluzzi, G.; Schmidt, S.T.; Cunliffe, R.; Woods, S.; Roberts, C.W.; Veggi, D.; Ferlenghi, I.; O’Hagan, D.T.; Baudner, B.C.; Perrie, Y. Rational Design of Adjuvants for Subunit Vaccines: The Format of Cationic Adjuvants Affects the Induction of Antigen-Specific Antibody Responses. Journal of Controlled Release 2021, 330, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Heuts, J.; Jiskoot, W.; Ossendorp, F.; Van Der Maaden, K. Cationic Nanoparticle-Based Cancer Vaccines. Pharmaceutics 2021, 13, 596. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; Midmore, B.R. Synthetic Bilayer Adsorption onto Polystyrene Microspheres. Langmuir 1992, 8, 801–806. [Google Scholar] [CrossRef]
- Liu, L.; Cao, F.; Liu, X.; Wang, H.; Zhang, C.; Sun, H.; Wang, C.; Leng, X.; Song, C.; Kong, D.; et al. Hyaluronic Acid-Modified Cationic Lipid–PLGA Hybrid Nanoparticles as a Nanovaccine Induce Robust Humoral and Cellular Immune Responses. ACS Appl. Mater. Interfaces 2016, 8, 11969–11979. [Google Scholar] [CrossRef] [PubMed]
- Galvão, C.N.; Sanches, L.M.; Mathiazzi, B.I.; Ribeiro, R.T.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Antimicrobial Coatings from Hybrid Nanoparticles of Biocompatible and Antimicrobial Polymers. International Journal of Molecular Sciences 2018, 19, 2965. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Alexander-Katz, A. Cell Membranes Open “Doors” for Cationic Nanoparticles/Biomolecules: Insights into Uptake Kinetics. ACS Nano 2013, 7, 10799–10808. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, F.; Gartlan, K.H.; Harandi, A.M.; Brinckmann, S.A.; Coccia, M.; Hillson, W.R.; Kok, W.L.; Cole, S.; Ho, L.-P.; Lambe, T.; et al. Polyethyleneimine Is a Potent Mucosal Adjuvant for Viral Glycoprotein Antigens. Nature Biotechnology 2012, 30, 883–888. [Google Scholar] [CrossRef]
- Kedmi, R.; Ben-Arie, N.; Peer, D. The Systemic Toxicity of Positively Charged Lipid Nanoparticles and the Role of Toll-like Receptor 4 in Immune Activation. Biomaterials 2010, 31, 6867–6875. [Google Scholar] [CrossRef]
- Liao, X.; Liu, Y.; Zheng, J.; Zhao, X.; Cui, L.; Hu, S.; Xia, T.; Si, S. Diverse Pathways of Engineered Nanoparticle-Induced NLRP3 Inflammasome Activation. Nanomaterials 2022, 12, 3908. [Google Scholar] [CrossRef]
- Li, T.; He, J.; Horvath, G.; Próchnicki, T.; Latz, E.; Takeoka, S. Lysine-Containing Cationic Liposomes Activate the NLRP3 Inflammasome: Effect of a Spacer between the Head Group and the Hydrophobic Moieties of the Lipids. Nanomedicine: Nanotechnology, Biology and Medicine 2018, 14, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Tracey, S.R.; Smyth, P.; Herron, U.M.; Burrows, J.F.; Porter, A.J.; Barelle, C.J.; Scott, C.J. Development of a Cationic Polyethyleneimine-Poly(Lactic- Co -Glycolic Acid) Nanoparticle System for Enhanced Intracellular Delivery of Biologics. RSC Adv. 2023, 13, 33721–33735. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In Vitro Cytotoxicity Testing of Polycations: Influence of Polymer Structure on Cell Viability and Hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Pizzuto, M.; Gangloff, M.; Scherman, D.; Gay, N.J.; Escriou, V.; Ruysschaert, J.-M.; Lonez, C. Toll-like Receptor 2 Promiscuity Is Responsible for the Immunostimulatory Activity of Nucleic Acid Nanocarriers. Journal of Controlled Release 2017, 247, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Cationic Nanostructures for Vaccines. In Immune Response Activation; Duc, G.H.T., Ed.; InTech, 2014 ISBN 978-953-51-1374-4.
- Yew, N.S.; Wang, K.X.; Przybylska, M.; Bagley, R.G.; Stedman, M.; Marshall, J.; Scheule, R.K.; Cheng, S.H. Contribution of Plasmid DNA to Inflammation in the Lung after Administration of Cationic Lipid:pDNA Complexes. Human Gene Therapy 1999, 10, 223–234. [Google Scholar] [CrossRef]
- Carvalho, L.A.; Carmona-Ribeiro, A.M. Interactions between Cationic Vesicles and Serum Proteins. Langmuir 1998, 14, 6077–6081. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Strand, S.P.; Tribet, C.; Jaeger, W.; Dubin, P.L. Effect of Polyelectrolyte Structure on Protein-Polyelectrolyte Coacervates: Coacervates of Bovine Serum Albumin with Poly(Diallyldimethylammonium Chloride) versus Chitosan. Biomacromolecules 2007, 8, 3568–3577. [Google Scholar] [CrossRef]
- Li, S.; Tseng, W.-C.; Stolz, D.B.; Wu, S.-P.; Watkins, S.C.; Huang, L. Dynamic Changes in the Characteristics of Cationic Lipidic Vectors after Exposure to Mouse Serum: Implications for Intravenous Lipofection. Gene Ther 1999, 6, 585–594. [Google Scholar] [CrossRef]
- Li, W.; Szoka, F.C. Lipid-Based Nanoparticles for Nucleic Acid Delivery. Pharm Res 2007, 24, 438–449. [Google Scholar] [CrossRef]
- G. Lima, E.; R. Gomes, L.; M. Carmona-Ribeiro, A. Stable Indomethacin Dispersions in Water from Drug, Ethanol, Cationic Lipid and Carboxymethyl-Cellulose. Pharmaceutical Nanotechnology 2016, 4, 126–135. [Google Scholar] [CrossRef]
- Barbassa, L.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Supramolecular Assemblies of Rifampicin and Cationic Bilayers: Preparation, Characterization and Micobactericidal Activity. BMC Biotechnol 2011, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, M.; Malamas, A.; Shin, T.; Jin, E.; Sun, Y.; Lu, Z.-R. Multifunctional Cationic Lipid-Based Nanoparticles Facilitate Endosomal Escape and Reduction-Triggered Cytosolic siRNA Release. Mol. Pharmaceutics 2014, 11, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Marchitto, J.; Nguyen, T.D.T.; Marasini, R.; Celia, C.; Aryal, S. pH-Responsive Cationic Liposome for Endosomal Escape Mediated Drug Delivery. Colloids and Surfaces B: Biointerfaces 2020, 188, 110804. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.I.; in ’t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J.; Huis In ’t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Frontiers in Immunology 2018, 9, 2874. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Y.; Hou, L.; Qiao, X.; Zhang, Y.; Cheng, H.; Lu, H.; Chen, J.; Du, L.; Zheng, Q.; et al. Increases in Cellular Immune Responses Due to Positive Effect of CVC1302-Induced Lysosomal Escape in Mice. Vaccines 2023, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, C.; Wang, W.; Liu, X.; Deng, H. Biomimetic Noncationic Lipid Nanoparticles for mRNA Delivery. Proc. Natl. Acad. Sci. U.S.A. 2023, 120, e2311276120. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Wafa, E.I.; Geary, S.M.; Ebeid, K.; Alhaj-Suliman, S.O.; Salem, A.K. Cationic Nanoparticles Enhance T Cell Tumor Infiltration and Antitumor Immune Responses to a Melanoma Vaccine. Sci. Adv. 2022, 8, eabk3150. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Qi, Y.; Wang, M.; Yu, N.; Nan, F.; Zhang, H.; Tian, M.; Li, C.; Lu, H.; Jin, N. mRNA Vaccines Encoding the HA Protein of Influenza A H1N1 Virus Delivered by Cationic Lipid Nanoparticles Induce Protective Immune Responses in Mice. Vaccines 2020, 8. [Google Scholar] [CrossRef]
- Wusiman, A.; Gu, P.; Liu, Z.; Xu, S.; Zhang, Y.; Hu, Y.; Liu, J.; Wang, D.; Huang, X. Cationic Polymer Modified PLGA Nanoparticles Encapsulating Alhagi Honey Polysaccharides as a Vaccine Delivery System for Ovalbumin to Improve Immune Responses. International journal of nanomedicine 2019, 14, 3221–3234. [Google Scholar] [CrossRef]
- Ebrahimian, M.; Hashemi, M.; Maleki, M.; Hashemitabar, G.; Abnous, K.; Ramezani, M.; Haghparast, A. Co-Delivery of Dual Toll-like Receptor Agonists and Antigen in Poly(Lactic-Co-Glycolic) Acid/Polyethylenimine Cationic Hybrid Nanoparticles Promote Efficient in Vivo Immune Responses. Frontiers in immunology 2017, 8, 1077–1077. [Google Scholar] [CrossRef]
- Rapuano, R.; Carmona-Ribeiro, A.M. Physical Adsorption of Bilayer Membranes on Silica. Journal of Colloid and Interface Science 1997, 193, 104–111. [Google Scholar] [CrossRef]
- Moura, S.P.; Carmona-Ribeiro, A.M. Cationic Bilayer Fragments on Silica at Low Ionic Strength: Competitive Adsorption and Colloid Stability. Langmuir 2003, 19, 6664–6667. [Google Scholar] [CrossRef]
- Ribeiro, R.T.; Braga, V.H.A.; Carmona-Ribeiro, A.M. Biomimetic Cationic Nanoparticles Based on Silica: Optimizing Bilayer Deposition from Lipid Films. Biomimetics 2017, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.D.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Antimicrobial Particles from Cationic Lipid and Polyelectrolytes. Langmuir 2010, 26, 12300–12306. [Google Scholar] [CrossRef] [PubMed]
- de Melo Carrasco, L.D.; Sampaio, J.L.M.; Carmona-Ribeiro, A.M. Supramolecular Cationic Assemblies against Multidrug-Resistant Microorganisms: Activity and Mechanism of Action. Int J Mol Sci 2015, 16, 6337–6352. [Google Scholar] [CrossRef]
- Pereira, E.M.A.; Kosaka, P.M.; Rosa, H.; Vieira, D.B.; Kawano, Y.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Hybrid Materials from Intermolecular Associations between Cationic Lipid and Polymers. J. Phys. Chem. B 2008, 112, 9301–9310. [Google Scholar] [CrossRef]
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The Antimicrobial Activity of Free and Immobilized Poly (Diallyldimethylammonium) Chloride in Nanoparticles of Poly (Methylmethacrylate). Journal of Nanobiotechnology 2015, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; Castuma, C.E.; Sesso, A.; Schreier, S. Bilayer Structure and Stability in Dihexadecyl Phosphate Dispersions. J. Phys. Chem. 1991, 95, 5361–5366. [Google Scholar] [CrossRef]
- Andersson, M.; Hammarstroem, L.; Edwards, K. Effect of Bilayer Phase Transitions on Vesicle Structure, and Its Influence on the Kinetics of Viologen Reduction. J. Phys. Chem. 1995, 99, 14531–14538. [Google Scholar] [CrossRef]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Synthetic Bilayer Fragments for Solubilization of Amphotericin B. Journal of Colloid and Interface Science 2001, 244, 427–431. [Google Scholar] [CrossRef]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic Nanoparticles for Delivery of Amphotericin B: Preparation, Characterization and Activity in Vitro. Journal of Nanobiotechnology 2008, 6, 6. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Biomimetic Nanoparticles: Preparation, Characterization and Biomedical Applications. Int J Nanomedicine 2010, 5, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Biomimetic Particles in Drug and Vaccine Delivery. Journal of Liposome Research 2007, 17, 165–172. [Google Scholar] [CrossRef]
- Yong, C.Y.; Liew, W.P.P.; Ong, H.K.; Poh, C.L. Development of Virus-like Particles-based Vaccines against Coronaviruses. Biotechnology Progress 2022, 38, e3292. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.A.J. Recombinant DNA Technology and DNA Sequencing. Essays in Biochemistry 2019, 63, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2022, 12, 805695. [Google Scholar] [CrossRef]
- Cohen, S.N.; Chang, A.C.Y.; Boyer, H.W.; Helling, R.B. Construction of Biologically Functional Bacterial Plasmids In Vitro. Proc. Natl. Acad. Sci. U.S.A. 1973, 70, 3240–3244. [Google Scholar] [CrossRef]
- Nielsen, J. Production of Biopharmaceutical Proteins by Yeast: Advances through Metabolic Engineering. Bioengineered 2013, 4, 207–211. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. International Journal of Pharmaceutics 2021, 601, 120586. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update Post COVID -19 Vaccines. Bioengineering & Transla Med 2021, 6, e10246. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Suys, E.J.A.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From Influenza to COVID-19: Lipid Nanoparticle mRNA Vaccines at the Frontiers of Infectious Diseases. Acta Biomaterialia 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.-Q.; Ho, W.; Li, F.; Gao, M.; Bai, X.; Xu, X. Enzyme-Catalyzed One-Step Synthesis of Ionizable Cationic Lipids for Lipid Nanoparticle-Based mRNA COVID-19 Vaccines. ACS Nano 2022, 16, 18936–18950. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Srivastava, N.; Mishra, G.; Dhama, K.; Kumar, S.; Puri, B.; Saxena, S.K. Recombinant Vaccines for COVID-19. Human Vaccines & Immunotherapeutics 2020, 16, 2905–2912. [Google Scholar] [CrossRef]
- Global Tuberculosis Report 2023 Available online:. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (accessed on 16 May 2024).
- Philips, J.A.; Ernst, J.D. Tuberculosis Pathogenesis and Immunity. Annual Review of Pathology: Mechanisms of Disease 2012, 7, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Brewer, T.F. Preventing Tuberculosis with Bacillus Calmette-Guérin Vaccine: A Meta-Analysis of the Literature. Clinical Infectious Diseases 2000, 31, S64–S67. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A. Bacillus Calmette-Guérin (BCG) Revaccination and Protection Against Tuberculosis: A Systematic Review. Cureus 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Szachniewicz, M.M.; Neustrup, M.A.; van Meijgaarden, K.E.; Jiskoot, W.; Bouwstra, J.A.; Haks, M.C.; Geluk, A.; Ottenhoff, T.H.M. Intrinsic Immunogenicity of Liposomes for Tuberculosis Vaccines: Effect of Cationic Lipid and Cholesterol. European Journal of Pharmaceutical Sciences 2024, 195, 106730. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; Ortis, F.; Schumacher, R.I.; Armelin, M.C.S. Interactions between Cationic Vesicles and Cultured Mammalian Cells. Langmuir 1997, 13, 2215–2218. [Google Scholar] [CrossRef]
- Carrasco, L.D.; Sampaio, J.L.M.; Santos, Hadassa; Carmona-Ribeiro, A. Self-Assembled Antibiotic Nanoparticles Against Intracellular Bacteria. DDL 2017, 7, 39–47. [Google Scholar] [CrossRef]
- Carmona Ribeiro, A.M.; Chaimovich, H. Preparation and Characterization of Large Dioctadecyldimethylammonium Chloride Liposomes and Comparison with Small Sonicated Vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes 1983, 733, 172–179. [Google Scholar] [CrossRef]
- Mansury, D.; Ghazvini, K.; Amel Jamehdar, S.; Badiee, A.; Tafaghodi, M.; Nikpoor, A.R.; Amini, Y.; Jaafari, M.R. Enhancement of the Effect of BCG Vaccine against Tuberculosis Using DDA/TDB Liposomes Containing a Fusion Protein of HspX, PPE44, and EsxV. Artificial Cells, Nanomedicine, and Biotechnology 2019, 47, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Billeskov, R.; Doherty, T.M.; Andersen, P. Synergistic Effect of Bacillus Calmette Guerin and a Tuberculosis Subunit Vaccine in Cationic Liposomes: Increased Immunogenicity and Protection1. The Journal of Immunology 2007, 178, 3721–3730. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Du, J.; Wang, X.; Xu, L.; Zhang, Y.; Sun, Q.; Shi, Z.; Xing, Y.; Su, Y.; Wang, S.; et al. Long-Term Immunogenicity and In Vitro Prophylactic Protective Efficacy of M. Tuberculosis Fusion Protein DR2 Combined with Liposomal Adjuvant DIMQ as a Boosting Vaccine for BCG. ACS Infect. Dis. 2023, 9, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Derrick, S.C.; Yang, A.; Parra, M.; Kolibab, K.; Morris, S.L. Effect of Cationic Liposomes on BCG Trafficking and Vaccine-Induced Immune Responses Following a Subcutaneous Immunization in Mice. Vaccine 2015, 33, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; Chaimovich, H. Salt-Induced Aggregation and Fusion of Dioctadecyldimethylammonium Chloride and Sodium Dihexadecylphosphate Vesicles. Biophys J 1986, 50, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Sobral, C.N.C.; Soto, M.A.; Carmona-Ribeiro, A.M. Characterization of DODAB/DPPC Vesicles. Chemistry and Physics of Lipids 2008, 152, 38–45. [Google Scholar] [CrossRef]
- Tian, M.; Zhou, Z.; Tan, S.; Fan, X.; Li, L.; Ullah, N. Formulation in DDA-MPLA-TDB Liposome Enhances the Immunogenicity and Protective Efficacy of a DNA Vaccine against Mycobacterium Tuberculosis Infection. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Agger, E.M.; Rosenkrands, I.; Olsen, A.W.; Hatch, G.; Williams, A.; Kritsch, C.; Lingnau, K.; von Gabain, A.; Andersen, C.S.; Korsholm, K.S.; et al. Protective Immunity to Tuberculosis with Ag85B-ESAT-6 in a Synthetic Cationic Adjuvant System IC31. Vaccine 2006, 24, 5452–5460. [Google Scholar] [CrossRef]
- Gao, Y.; Han, S.; Lu, F.; Liu, Q.; Yang, J.; Wang, W.; Wang, Y.; Zhang, J.; Ju, R.; Shen, X.; et al. Dimethyl-Dioctadecyl-Ammonium Bromide/Poly(Lactic Acid) Nanoadjuvant Enhances the Immunity and Cross-Protection of an NM2e-Based Universal Influenza Vaccine. ACS Nano 2024, 18, 12905–12916. [Google Scholar] [CrossRef] [PubMed]
- Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Hybrid Nanoparticles of Poly (Methyl Methacrylate) and Antimicrobial Quaternary Ammonium Surfactants. Pharmaceutics 2020, 12, 340. [Google Scholar] [CrossRef]
- Henson, T.R.; Richards, K.A.; Gandhapudi, S.K.; Woodward, J.G.; Sant, A.J. R-DOTAP Cationic Lipid Nanoparticles Outperform Squalene-Based Adjuvant Systems in Elicitation of CD4 T Cells after Recombinant Influenza Hemagglutinin Vaccination. Viruses 2023, 15, 538. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.V.; Reece, W.; Gothard, P.; Moorthy, V.; Roberts, M.; Flanagan, K.; Plebanski, M.; Hannan, C.; Hu, J.T.; Anderson, R.; et al. DNA-Based Vaccines for Malaria: A Heterologous Prime-Boost Immunisation Strategy. Developments in biologicals 2000, 104, 171–179. [Google Scholar] [PubMed]
- González-Sanz, M.; Berzosa, P.; Norman, F.F. Updates on Malaria Epidemiology and Prevention Strategies. Curr Infect Dis Rep 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Laurens, M.B. RTS,S/AS01 Vaccine (MosquirixTM): An Overview. Hum Vaccin Immunother 2019, 16, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.Y.; Shehzad, A.; Islam, S.U.; Al-Suhaimi, E.A.; Lee, Y.S. MosquirixTM RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines 2022, 10, 713. [Google Scholar] [CrossRef] [PubMed]
- Fotoran, W.L.; Silva, J.R. da; Glitz, C.; Ferreira, L.C. de S.; Wunderlich, G. Establishment of an Antiplasmodial Vaccine Based on PfRH5-Encoding RNA Replicons Stabilized by Cationic Liposomes. Pharmaceutics 2023, 15, 1223. [Google Scholar] [CrossRef] [PubMed]
- Fotoran, W.L.; Kleiber, N.; Glitz, C.; Wunderlich, G. A DNA Vaccine Encoding Plasmodium Falciparum PfRH5 in Cationic Liposomes for Dermal Tattooing Immunization. Vaccines 2020, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.T.; Khadke, S.; Korsholm, K.S.; Perrie, Y.; Rades, T.; Andersen, P.; Foged, C.; Christensen, D. The Administration Route Is Decisive for the Ability of the Vaccine Adjuvant CAF09 to Induce Antigen-Specific CD8(+) T-Cell Responses: The Immunological Consequences of the Biodistribution Profile. Journal of controlled release : official journal of the Controlled Release Society 2016, 239, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.A.; Christensen, D.; Muñoz, C.; Singh, S.; Locke, E.; Andersen, P.; Zavala, F. Robust Antibody and CD8+ T-Cell Responses Induced by P. Falciparum CSP Adsorbed to Cationic Liposomal Adjuvant CAF09 Confer Sterilizing Immunity against Experimental Rodent Malaria Infection. npj Vaccines 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Monnery, B.D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J.H.G.; Thanou, M. Cytotoxicity of Polycations: Relationship of Molecular Weight and the Hydrolytic Theory of the Mechanism of Toxicity. International journal of pharmaceutics 2017, 521, 249–258. [Google Scholar] [CrossRef]
- Naumenko, E.; Akhatova, F.; Rozhina, E.; Fakhrullin, R. Revisiting the Cytotoxicity of Cationic Polyelectrolytes as a Principal Component in Layer-by-Layer Assembly Fabrication. Pharmaceutics 2021, 13, 1230. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhou, H.; Nian, Q.-G.; Yang, Y.; Qin, C.-F.; Tang, R. Robust Vaccine Formulation Produced by Assembling a Hybrid Coating of Polyethyleneimine–Silica. Chemical Science 2016, 7, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, N.C.; Brinckmann, S.A.; Gartlan, K.H.; Puthia, M.; Svanborg, C.; Krashias, G.; Eisenbarth, S.C.; Flavell, R.A.; Sattentau, Q.J.; Wegmann, F. Polyethyleneimine Is a Potent Systemic Adjuvant for Glycoprotein Antigens. International Immunology 2014, 26, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Eusébio, D.; Paul, M.; Biswas, S.; Cui, Z.; Costa, D.; Sousa, Â. Mannosylated Polyethylenimine-Cholesterol-Based Nanoparticles for Targeted Delivery of Minicircle DNA Vaccine against COVID-19 to Antigen-Presenting Cells. International Journal of Pharmaceutics 2024, 654, 123959. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Liu, Y.; Wu, X.; Ji, Y.; Zhao, Y.; et al. Surface-Engineered Gold Nanorods: Promising DNA Vaccine Adjuvant for HIV-1 Treatment. Nano letters 2012, 12, 2003–2012. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical Applications of Quaternized Chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; Tsai, M.-H.; Yen, H.; Shyu, H.-F.; Cheng, K.; Chen, X.; Chen, C.; Young, J.; Kau, J.-H. A Fucoidan-Quaternary Chitosan Nanoparticle Adjuvant for Anthrax Vaccine as an Alternative to CpG Oligodeoxynucleotides. Carbohydrate Polymers 2020, 229, 115403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, S.; Chen, Y.; Wang, F.; Jiang, W. Preparation and Characterization of Curdlan-Chitosan Conjugate Nanoparticles as Mucosal Adjuvants for Intranasal Influenza H1N1 Subunit Vaccine. International Journal of Biological Macromolecules 2024, 266, 131289. [Google Scholar] [CrossRef]
- Fan, B.; Gu, J.; Deng, B.; Guo, W.; Zhang, S.; Li, L.; Li, B. Positively Charged-Amylose-Entangled Au-Nanoparticles Acting as Protein Carriers and Potential Adjuvants to SARS-CoV-2 Subunit Vaccines. ACS Appl. Mater. Interfaces 2023, 15, 29982–29997. [Google Scholar] [CrossRef]
- Altay Benetti, A.; Tan, E.Y.Z.; Chang, Z.W.; Bae, K.H.; Thwin, M.T.; Muthuramalingam, R.P.K.; Liao, K.-C.; Wan, Y.; Ng, L.F.P.; Renia, L.; et al. Design and Characterization of a New Formulation for the Delivery of COVID-19-mRNA Vaccine to the Nasal Mucosa. Vaccines (Basel) 2024, 12, 409. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yu, W.; Shen, L.; Yan, W.; Qi, J.; Hu, T. Mucosal SARS-CoV-2 Nanoparticle Vaccine Based on Mucosal Adjuvants and Its Immune Effectiveness by Intranasal Administration. ACS Appl. Mater. Interfaces 2023, 15, 35895–35905. [Google Scholar] [CrossRef] [PubMed]
- Pati, R.; Shevtsov, M.; Sonawane, A. Nanoparticle Vaccines Against Infectious Diseases. Front Immunol 2018, 9, 2224–2224. [Google Scholar] [CrossRef] [PubMed]
- Heng, W.T.; Yew, J.S.; Poh, C.L. Nanovaccines against Viral Infectious Diseases. Pharmaceutics 2022, 14, 2554. [Google Scholar] [CrossRef] [PubMed]
- Priyanka; Abusalah, M. A.H.; Chopra, H.; Sharma, A.; Mustafa, S.A.; Choudhary, O.P.; Sharma, M.; Dhawan, M.; Khosla, R.; Loshali, A.; et al. Nanovaccines: A Game Changing Approach in the Fight against Infectious Diseases. Biomedicine & Pharmacotherapy 2023, 167, 115597. [Google Scholar] [CrossRef]
- Singh, A. Eliciting B Cell Immunity against Infectious Diseases Using Nanovaccines. Nat. Nanotechnol. 2021, 16, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sangiao, E.; Holban, A.M.; Gestal, M.C. Advanced Nanobiomaterials: Vaccines, Diagnosis and Treatment of Infectious Diseases. Molecules 2016, 21, 867. [Google Scholar] [CrossRef] [PubMed]
- Look, M.; Bandyopadhyay, A.; Blum, J.S.; Fahmy, T.M. Application of Nanotechnologies for Improved Immune Response against Infectious Diseases in the Developing World. Advanced Drug Delivery Reviews 2010, 62, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Wang, H. Materials-Based Vaccines for Infectious Diseases. WIREs Nanomedicine and Nanobiotechnology 2022, 14, e1824. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Liu, H.; Zhang, S.; Hu, J.; Zhang, L. Delivery of Nanovaccine towards Lymphoid Organs: Recent Strategies in Enhancing Cancer Immunotherapy. J Nanobiotechnol 2021, 19, 389. [Google Scholar] [CrossRef]
- Mao, C.; Gorbet, M.-J.; Singh, A.; Ranjan, A.; Fiering, S. In Situ Vaccination with Nanoparticles for Cancer Immunotherapy: Understanding the Immunology. International Journal of Hyperthermia 2020, 37, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mooney, D.J. Biomaterial-Assisted Targeted Modulation of Immune Cells in Cancer Treatment. Nature Mater 2018, 17, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-X.; Jia, Y.-B.; Huang, Y.-R.; Liu, H.-N.; Sun, X.-M.; Cai, T.; Liu, R.-T.; Xu, Z.P. Efficient Delivery of Clay-Based Nanovaccines to the Mouse Spleen Promotes Potent Anti-Tumor Immunity for Both Prevention and Treatment of Lymphoma. Nano Res. 2021, 14, 1326–1334. [Google Scholar] [CrossRef]
- Den Haan, J.M.M.; Martinez-Pomares, L. Macrophage Heterogeneity in Lymphoid Tissues. Semin Immunopathol 2013, 35, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.J.; Poon, W.; Zhang, Y.-N.; Dai, Q.; Besla, R.; Ding, D.; Ouyang, B.; Li, A.; Chen, J.; Zheng, G.; et al. Effect of Removing Kupffer Cells on Nanoparticle Tumor Delivery. Proc. Natl. Acad. Sci. U.S.A. 2017, 114. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, X.; Ma, H.; Zhang, J.; Yu, D.; Zhao, R.; Yu, S.; Nie, G.; Wang, H. In Situ Transforming RNA Nanovaccines from Polyethylenimine Functionalized Graphene Oxide Hydrogel for Durable Cancer Immunotherapy. Nano Lett. 2021, 21, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, D.; Saw, P.E.; Song, E. Turning Cold Tumors Hot: From Molecular Mechanisms to Clinical Applications. Trends in Immunology 2022, 43, 523–545. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Chapter 1 - Immunoadjuvants for Cancer Immunotherapy. In Nanomedicine in Cancer Immunotherapy; Kesharwani, P., Ed.; Academic Press, 2024; pp. 1–36 ISBN 978-0-443-18770-4.
- Park, J.-H.; Gu, L.; Von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable Luminescent Porous Silicon Nanoparticles for in Vivo Applications. Nature Mater 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Zhu, S.; Yao, R.; Li, Y.; Zhao, P.; Ren, C.; Du, X.; Yao, Y. Lysosomal Quality Control of Cell Fate: A Novel Therapeutic Target for Human Diseases. Cell Death Dis 2020, 11, 817. [Google Scholar] [CrossRef]
- Wojnilowicz, M.; Glab, A.; Bertucci, A.; Caruso, F.; Cavalieri, F. Super-Resolution Imaging of Proton Sponge-Triggered Rupture of Endosomes and Cytosolic Release of Small Interfering RNA. ACS Nano 2019, 13, 187–202. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The Possible “Proton Sponge ” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Molecular Therapy 2013, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wen, J.; Wang, W.; Hu, Z.; Ling, C.; Zhao, Z.; Cheng, Y.; Chang, Y.; Xu, M.; Jin, Z.; et al. Peptide-Driven Proton Sponge Nano-Assembly for Imaging and Triggering Lysosome-Regulated Immunogenic Cancer Cell Death. Advanced Materials 2024, 36, 2307679. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Hu, X.; Saeed, M.; Chen, B.; Li, Y.; Yu, H. Overview of Recent Advances in Liposomal Nanoparticle-Based Cancer Immunotherapy. Acta Pharmacol Sin 2019, 40, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Ahmad, J.; Alasmary, M.Y.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A.; Chaubey, P. Emerging Advances in Cationic Liposomal Cancer Nanovaccines: Opportunities and Challenges. Immunotherapy 2021, 13, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, C.; Qi, Z.; Qiu, T.; Zhang, J.; Yang, H. Engineering Customized Nanovaccines for Enhanced Cancer Immunotherapy. Bioactive Materials 2024, 36, 330–357. [Google Scholar] [CrossRef] [PubMed]
- Alfagih, I.M.; Aldosari, B.; AlQuadeib, B.; Almurshedi, A.; Alfagih, M.M. Nanoparticles as Adjuvants and Nanodelivery Systems for mRNA-Based Vaccines. Pharmaceutics 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; Mathiazzi, B.I.; Pérez-Betancourt, Y. Cationic Nanostructures as Adjuvants for Vaccines. In Vaccine Design: Methods and Protocols, Volume 3. Resources for Vaccine Development; Thomas, S., Ed.; Springer US: New York, NY, 2022; ISBN 978-1-07-161892-9. [Google Scholar]
- Fan, Y.-N.; Li, M.; Luo, Y.-L.; Chen, Q.; Wang, L.; Zhang, H.-B.; Shen, S.; Gu, Z.; Wang, J. Cationic Lipid-Assisted Nanoparticles for Delivery of mRNA Cancer Vaccine. Biomater. Sci. 2018, 6, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Xiang, J.; Wang, H.; Zhuang, Q.; Wei, T.; Cao, Z.; Gu, Q.; Liu, Z.; Peng, R. Fluoroalkane Modified Cationic Polymers for Personalized mRNA Cancer Vaccines. Chemical Engineering Journal 2023, 456, 140930. [Google Scholar] [CrossRef]
- Carson, C.S.; Becker, K.W.; Garland, K.M.; Pagendarm, H.M.; Stone, P.T.; Arora, K.; Wang-Bishop, L.; Baljon, J.J.; Cruz, L.D.; Joyce, S.; et al. A Nanovaccine for Enhancing Cellular Immunity via Cytosolic Co-Delivery of Antigen and polyIC RNA. Journal of Controlled Release 2022, 345, 354–370. [Google Scholar] [CrossRef]
- Sultan, H.; Salazar, A.M.; Celis, E. Poly-ICLC, a Multi-Functional Immune Modulator for Treating Cancer. Seminars in Immunology 2020, 49, 101414. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Zhao, Z.; Shan, X.; Wang, Y.; Feng, Z.; Li, B.; Luo, C.; Chen, X.; Sun, J. Self-Adjuvanting Polyguanidine Nanovaccines for Cancer Immunotherapy. ACS Nano 2024, 18, 7136–7147. [Google Scholar] [CrossRef]
- Luo, X.; Chen, X.; Ma, R.; Fu, Z.; Liu, Z.; Su, Q.; Fu, H.; Yang, Y.; Xue, W. Cancer Cell Membrane Proteins-Encapsulated Nanovaccine Enhances Cancer Immunotherapy and Prevention by Provoking Antigen-Specific Cellular Immunity via the Dendritic Cell-Targeted Delivery. Chemical Engineering Journal 2024, 481, 148611. [Google Scholar] [CrossRef]
- Diao, L.; Liu, M. Rethinking Antigen Source: Cancer Vaccines Based on Whole Tumor Cell/Tissue Lysate or Whole Tumor Cell. Advanced Science 2023, 10, 2300121. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, X.; Liu, L.; Zhao, C.; Zhang, J.; Yang, S.; Pan, P.; Huang, Q.; Zhao, X.; Tian, R.; et al. Genetically Engineered Cytomembrane Nanovaccines for Cancer Immunotherapy. Adv Healthcare Materials 2024, 13, 2400068. [Google Scholar] [CrossRef]
- Liu, M.; Feng, Y.; Lu, Y.; Huang, R.; Zhang, Y.; Zhao, Y.; Mo, R. Lymph-Targeted High-Density Lipoprotein-Mimetic Nanovaccine for Multi-Antigenic Personalized Cancer Immunotherapy. Sci. Adv. 2024, 10, eadk2444. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, H.; Yang, X.; Yu, Q.; Wang, H.; Zhang, L.; Zhao, Y.; Zhu, D. Cascade Carrier-Free Nanoparticles Forming In Situ Nanovaccines for Synergistic Photothermal-Immunotherapy of Cancer. Adv Funct Materials 2024, 2401489. [Google Scholar] [CrossRef]
- Yu, X.; Dai, Y.; Zhao, Y.; Qi, S.; Liu, L.; Lu, L.; Luo, Q.; Zhang, Z. Melittin-Lipid Nanoparticles Target to Lymph Nodes and Elicit a Systemic Anti-Tumor Immune Response. Nat Commun 2020, 11, 1110. [Google Scholar] [CrossRef]
- Zhang, H.-Q.; Sun, C.; Xu, N.; Liu, W. The Current Landscape of the Antimicrobial Peptide Melittin and Its Therapeutic Potential. Front. Immunol. 2024, 15, 1326033. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Antimicrobial Peptides and Their Assemblies. Future Pharmacology 2023, 3, 763–788. [Google Scholar] [CrossRef]
- Pérez-Betancourt, Y.; Zaia, R.; Evangelista, M.F.; Ribeiro, R.T.; Roncoleta, B.M.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Characterization and Differential Cytotoxicity of Gramicidin Nanoparticles Combined with Cationic Polymer or Lipid Bilayer. Pharmaceutics 2022, 14, 2053. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Liu, X.; Chai, M.; Diao, L.; Ma, L.; Nie, S.; Xu, M.; Wang, Y.; Mo, F.; et al. Probiotics Formulation and Cancer Nanovaccines Show Synergistic Effect in Immunotherapy and Prevention of Colon Cancer. iScience 2023, 26, 107167. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Su, J.-Y.; Li, Y.-M. Rational Design of T-Cell- and B-Cell-Based Therapeutic Cancer Vaccines. Acc. Chem. Res. 2022, 55, 2660–2671. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Thangaraj, P.; Wang, L.; Cao, Q.; Kim, J.-H. Nanovaccines: An Effective Therapeutic Approach for Cancer Therapy. Biomedicine & Pharmacotherapy 2024, 170, 115992. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



