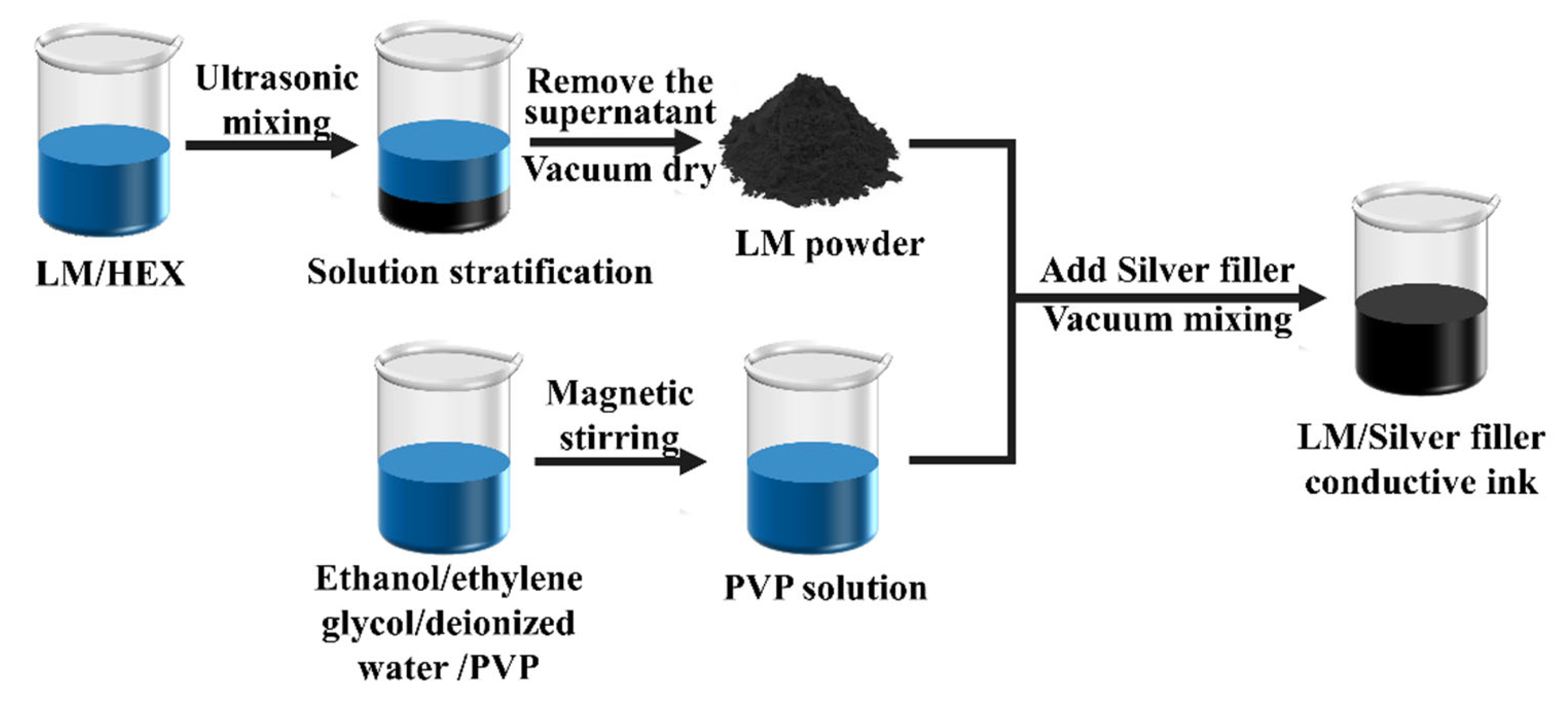
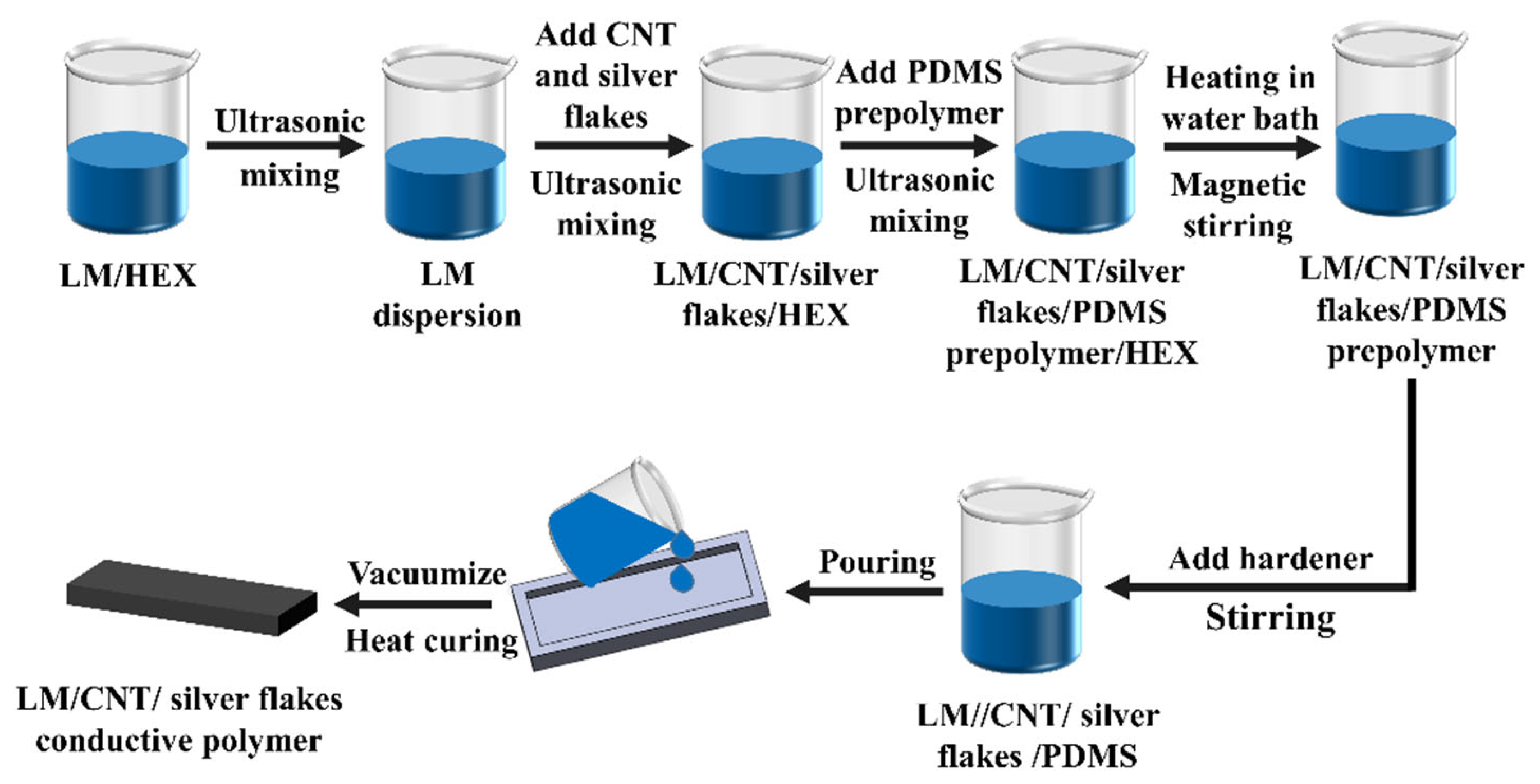
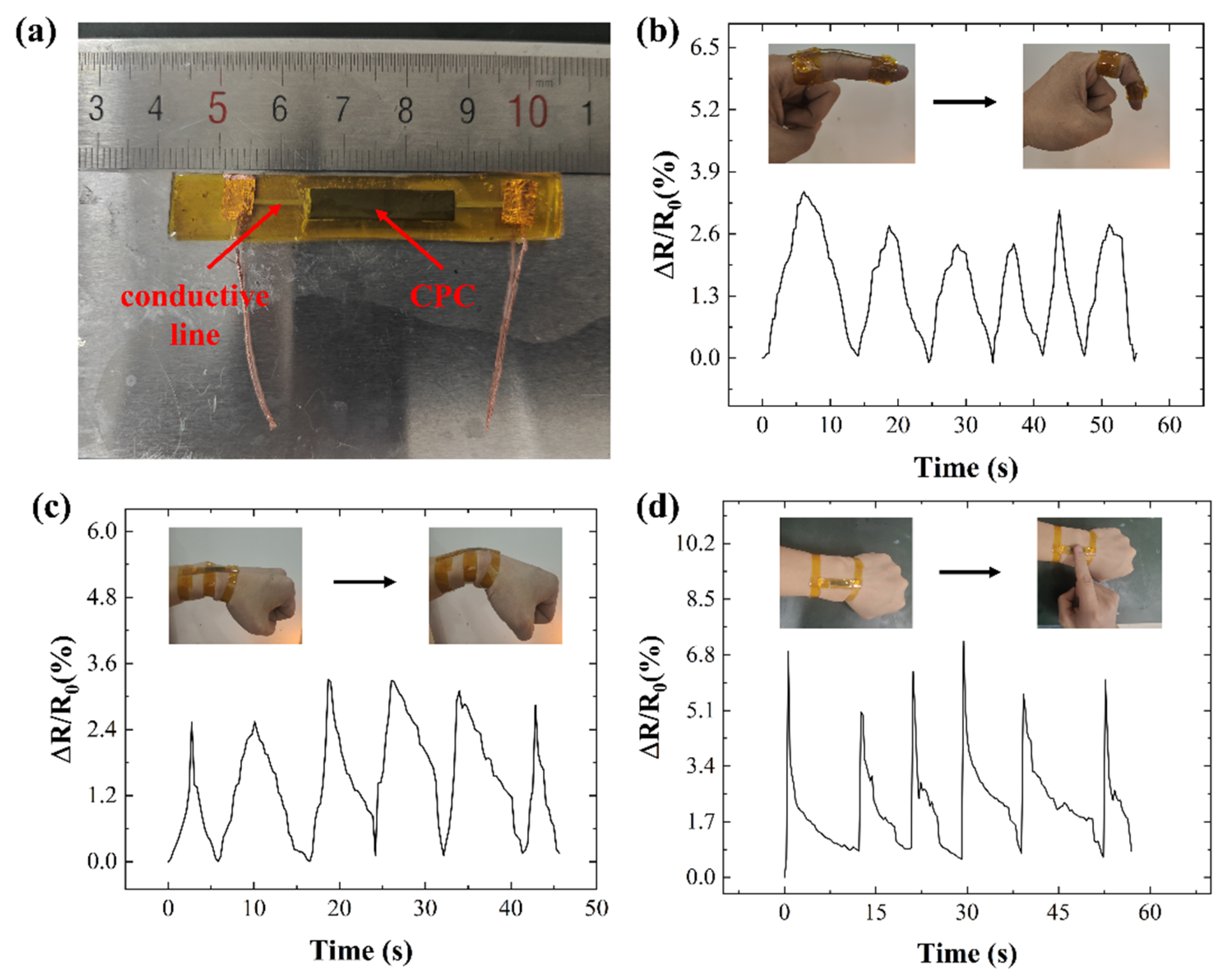
3.1. Influence of Conductive Filler Combinations on Performance of Conductive Inks
Four conductive inks were formulated by varying the type of silver filler, and their initial conductivity after pattern formation was measured. As shown in
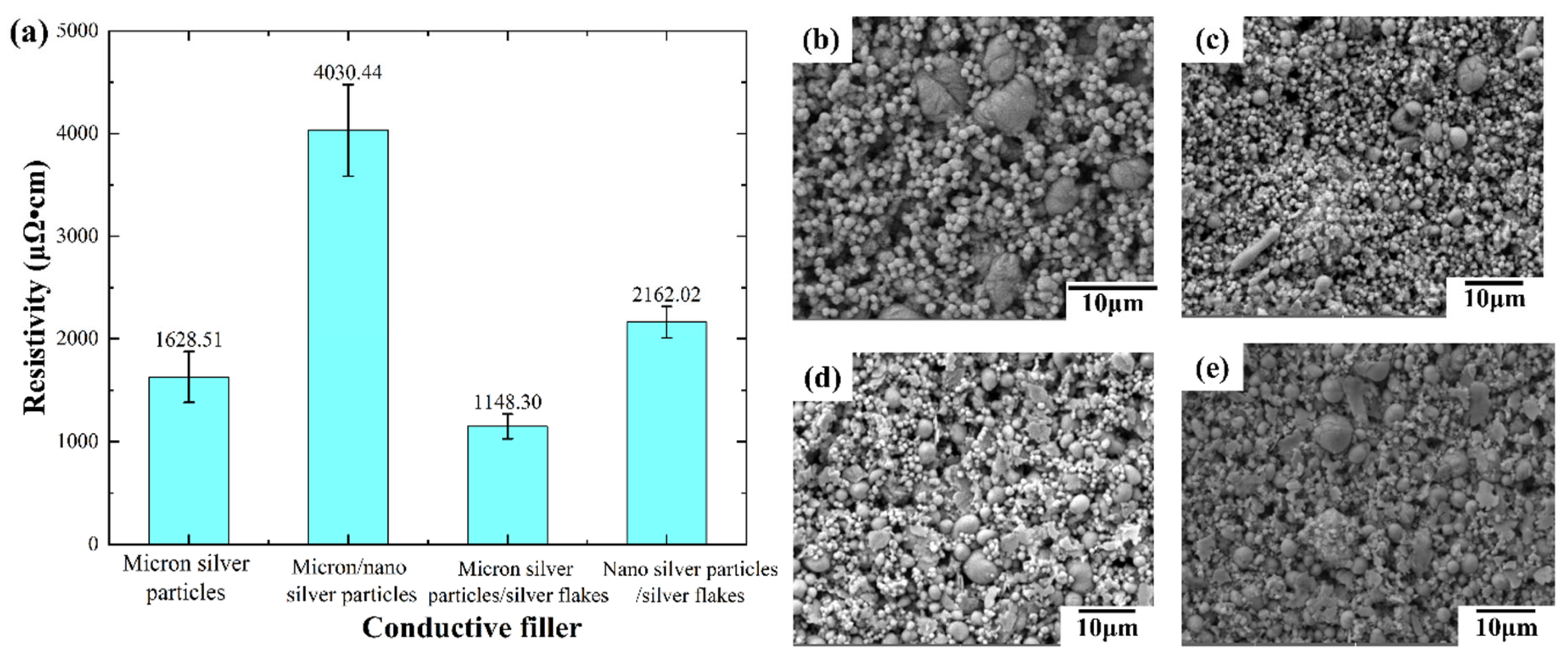
Figure 3(a), the ink containing liquid metal, micron silver particles, and silver flakes exhibits the superior initial conductivity (1148.30 μΩ·cm) without hot-pressing. This surpasses the conductivity of the ink with only liquid metal and micron silver particles, suggesting that silver flakes enhance electrical performance. Conversely, the inclusion of silver nanoparticles significantly reduces conductivity. The resistivity of the ink containing all three fillers (4030.44 μΩ·cm) is more than double that without nanoparticles.
Conductive ink performance is heavily influenced by the content and characteristics (morphology, particle size) of the fillers. When solely relying on micron-sized silver particles for conductivity (no doping with different sized fillers), their contact mode is primarily point-to-point, as illustrated in
Figure 3(b). However, incorporating silver flakes facilitates a transition from point contact to partial surface or line contact (
Figure 3(d)), ultimately improving electrical conductivity.
In contrast, the small size and agglomeration tendency of silver nanoparticles hinder their dispersion within the ink, consequently impeding the formation of a conductive path. This phenomenon, visualized in
Figure 3(c,e), leads to a decrease in overall conductivity. Therefore, the addition of silver nanoparticles in this case has a detrimental effect.
Electrical properties were then measured for the remaining three conductive lines, each containing different silver fillers, after hot pressing and sintering at 210 °C and 2.5 MPa for 20 minutes (
Figure 4(a)). Hot pressing and sintering significantly improved the electrical conductivity of all lines. Compared to the untreated state, the resistivity of the line containing liquid metal, micron silver particles, and silver nanoparticles decreased from 4030.44 μΩ·cm to 1458.22 μΩ·cm, representing nearly a twofold increase. Similar improvements were observed for lines containing other filler combinations. To further investigate this effect, we examined the hot-pressed conductive lines using a scanning electron microscope (SEM). Compared to untreated lines, the hot-pressed lines exhibited smoother surfaces with significantly reduced surface pores and improved contact between conductive fillers (
Figure 4(a)). These changes directly contributed to the enhanced electrical properties.
Interestingly, for the line containing liquid metal, micron silver particles, and silver nanoparticles, block-like features were observed on the surface after hot pressing (
Figure 4(b)). We hypothesize that this is due to the melting of silver nanoparticles and the formation of sintering necks under the applied pressure and temperature (210 °C, 2.5 MPa). Additionally, the pressure likely disrupts the oxide film on the liquid metal, causing spilled liquid metal to connect the sintering necks. This unique micro-morphology significantly improves the electrical conductivity. Energy-dispersive X-ray spectroscopy (EDS) analysis of these surface blocks revealed their primary composition as Ag elements, with the connecting parts containing Ga, In, Sn, and other elements from the liquid metal, supporting our hypothesis. Notably, this phenomenon was not observed in lines containing silver flakes (
Figure 4(c,d)). Under pressure, the silver flakes likely intermix with the liquid metal, leading to a smoother and more compact surface structure, ultimately enhancing conductivity.
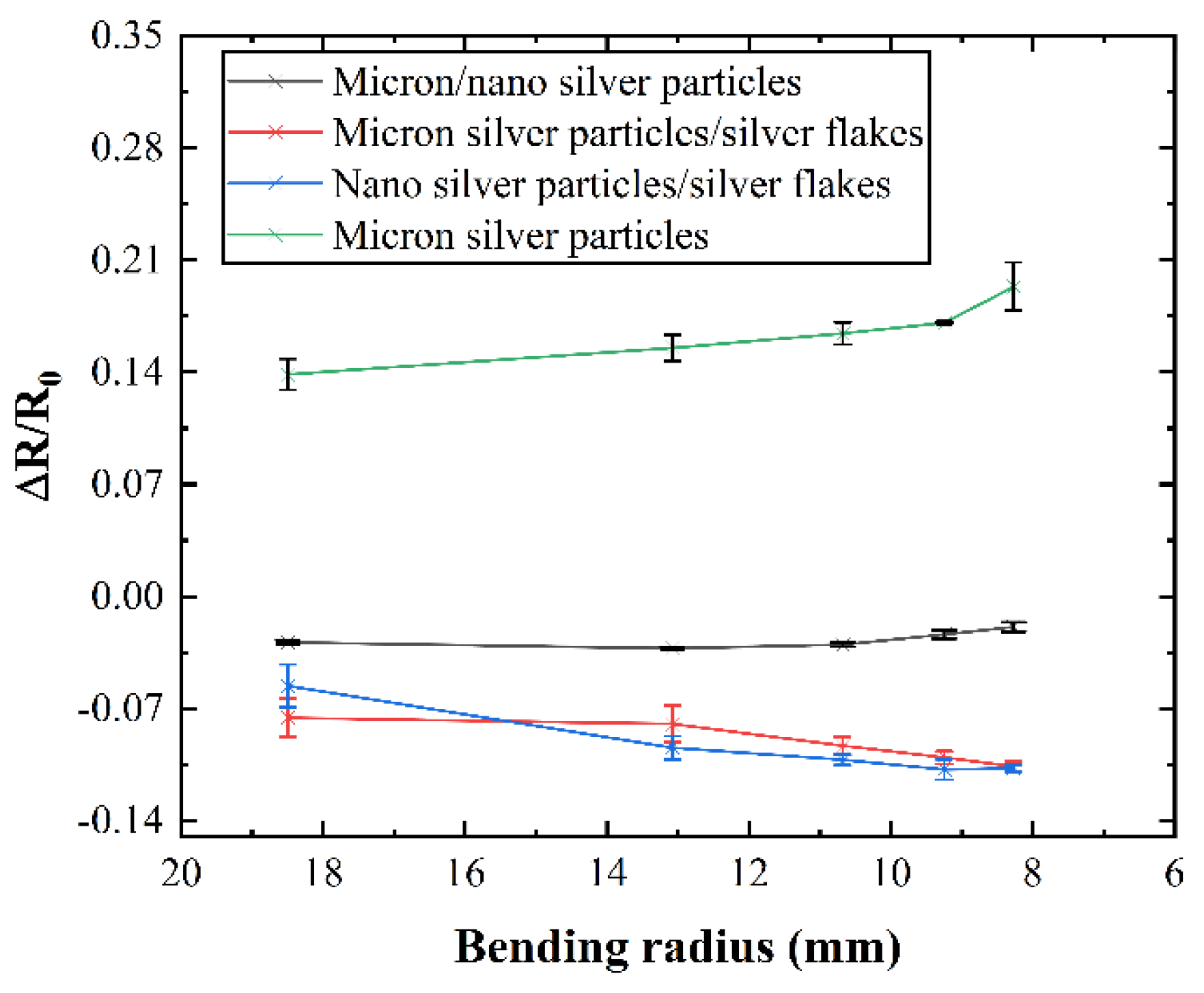
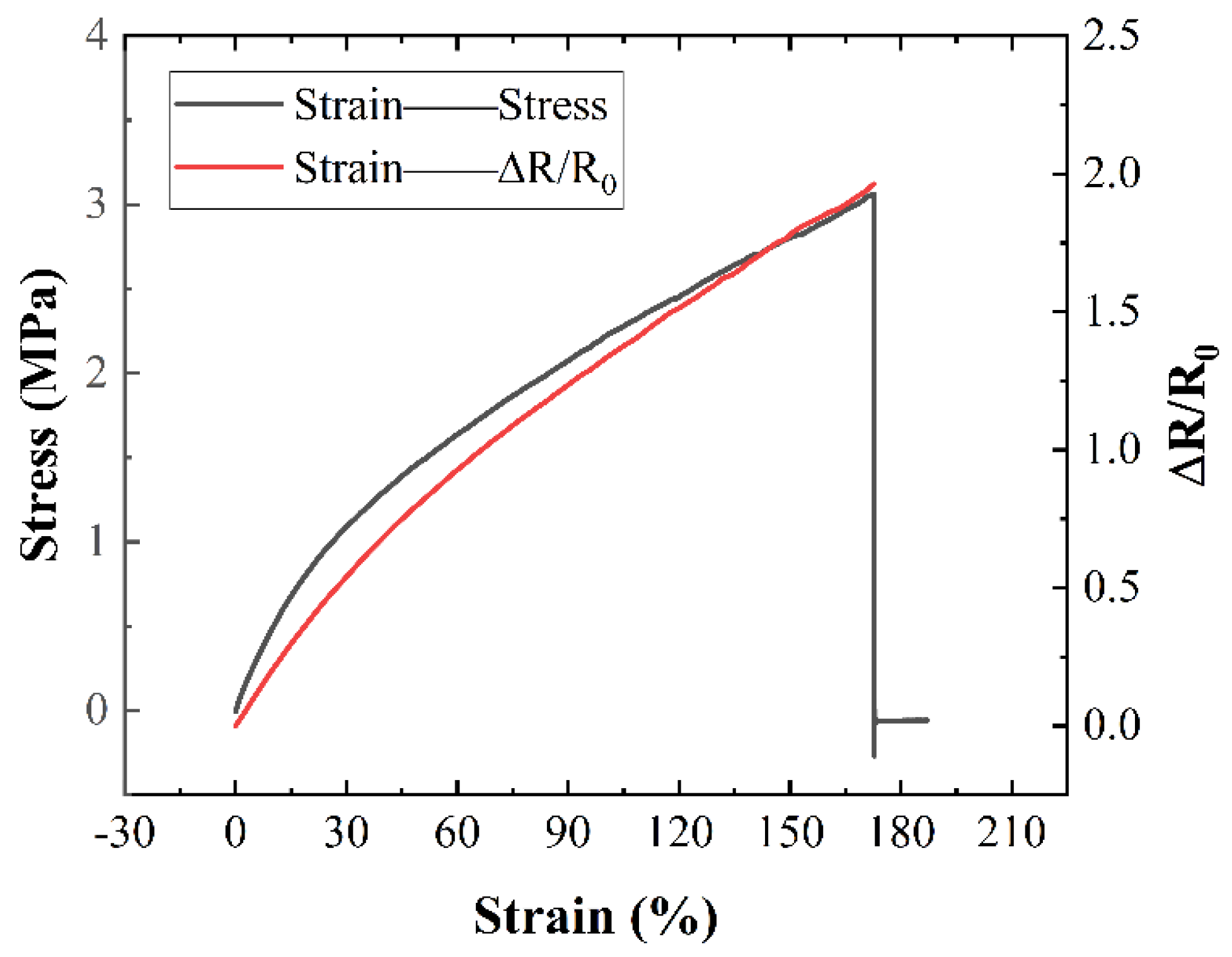
Following the conductivity test of the conductive line, bending radius and bending cycle tests were conducted to study the mechanical stability of the conductive network with different combinations of silver fillers. In
Figure 5, it can be observed that the mechanical properties of silver fillers with different characteristic parameters are superior to those of single micron silver fillers. Typically, with an increase in the degree of bending, the resistance of the conductive line increases, indicating that a smaller bending radius leads to greater resistance in the conductive line due to the formation of microcracks under bending stress. However, in
Figure 5, an opposite trend is noted where the resistance of the conductive line decreases with an increase in the degree of bending. This phenomenon occurs because during bending, the liquid metal in the conductive network breaks, resulting in the outflow of internal liquid metal, which mitigates the effects of microcracks caused by the bending of the conductive network. Simultaneously, there is a dynamic process of reconstruction and destruction of the conductive network. During bending, these processes compete: if the reconstruction rate exceeds the failure rate of the conductive network, the resistance of the conductive line decreases; otherwise, it increases.
Voids within the network can contribute to crack formation. Adding silver nanoparticles fills these voids, leading to lower resistance at smaller bending angles, although resistance might increase beyond a certain threshold. The inclusion of silver flakes further enhances bending stability, as shown by the lower resistance change rate compared to formulations lacking them (
Figure 5). This effect is likely due to the increased number of liquid metal particles, promoting faster network reconstruction relative to failure. Notably, conductive ink containing a combination of liquid metal, micron silver particles, and silver flakes exhibits a decreasing resistance change rate with increasing bending angle (
Figure 5), indicating exceptional mechanical stability. At maximum bending, resistance even decreases by 10.61%.
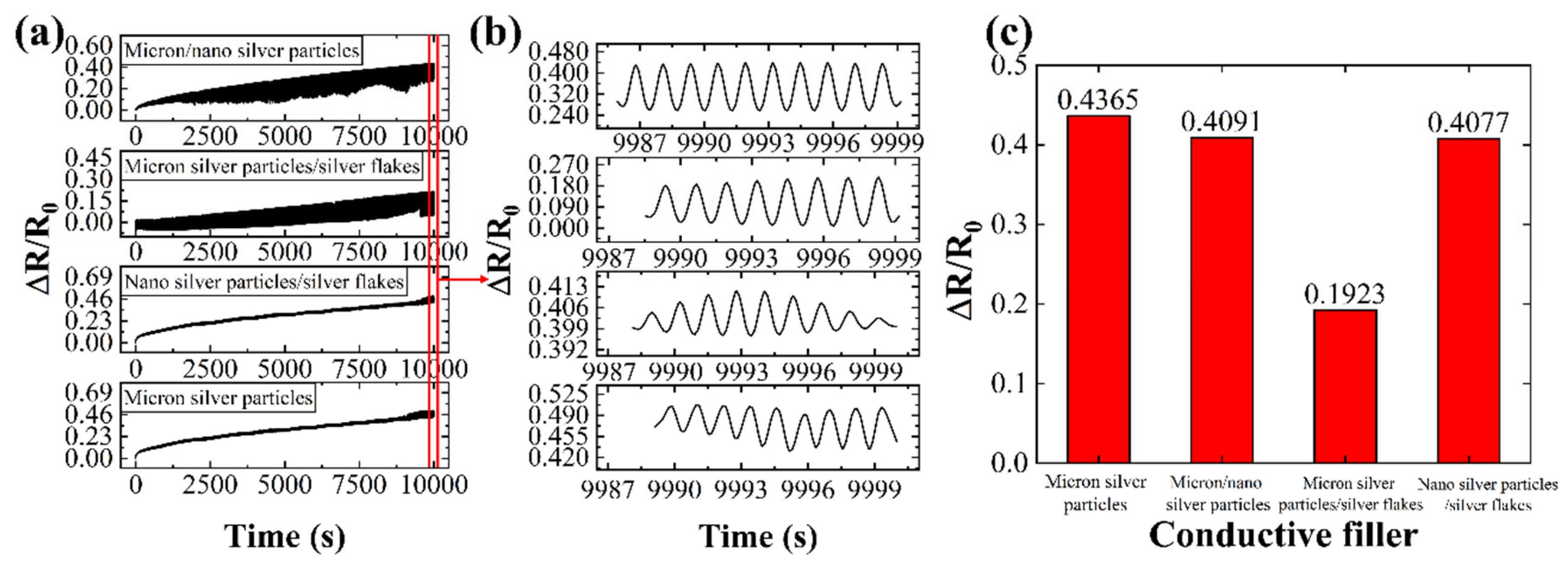
Conductive lines containing various conductive fillers were subjected to 5,000 bending cycles, with resistance changes monitored throughout and after the test. Compared to lines filled solely with micron silver particles, those incorporating silver fillers with different characteristics exhibited enhanced mechanical stability and bending durability.
Repetitive mechanical strain can induce microcracks within the conductive network [
16,
18,
25], compromising its continuity and potentially leading to network failure. However, this strain can also rupture the outer oxide film of the liquid metal, allowing the internal liquid metal to flow and repair the microcracks, ultimately facilitating network reconstruction. Additionally, incorporated silver nanoparticles (AgNPs) act as bridges between adjacent conductive fillers, increasing contact points and enhancing network stability. The larger surface area of silver flakes makes the liquid metal more susceptible to rupture under strain, resulting in more broken liquid metal connections and further improving network stability.
As shown in
Figure 6c, the resistance change rate of the conductive line containing a combination of liquid metal, micron silver particles, and silver flakes is significantly lower compared to lines with only micron silver particles. The optimal combination of conductive fillers (LM/micron silver particles/silver flakes) exhibited a resistance change rate of only 19.23% after 5,000 bending cycles, demonstrating the excellent mechanical stability and bending resistance of this conductive network.
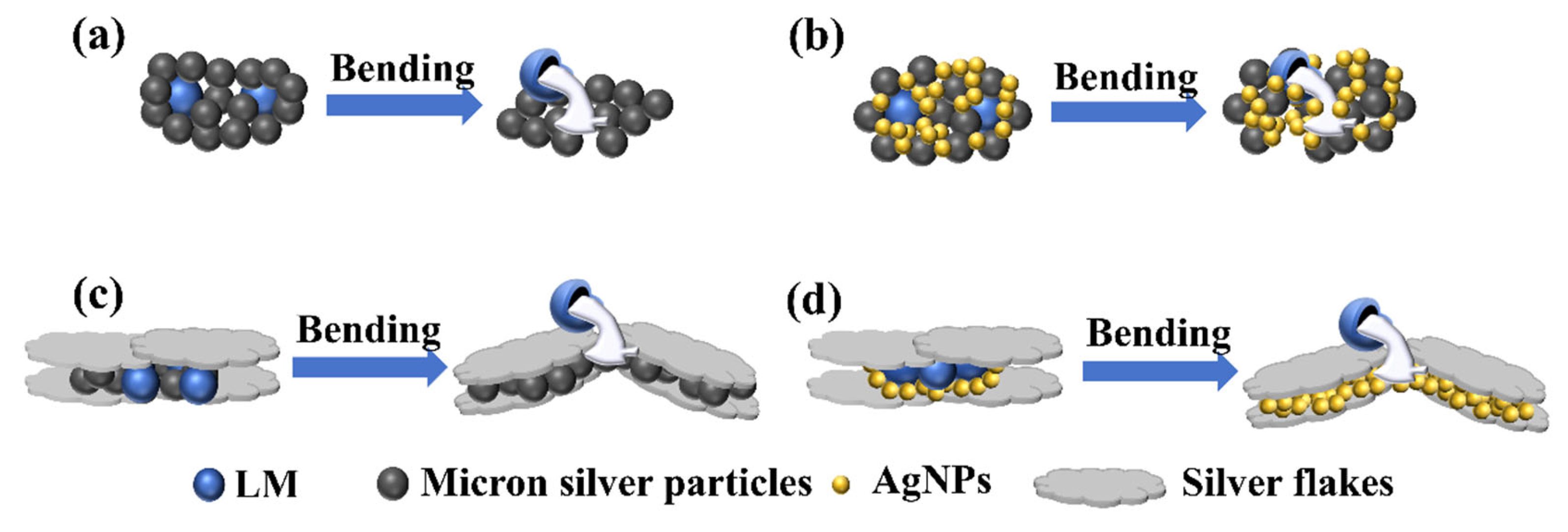
Figure 7 illustrates the mechanism underlying the difference in bending resistance of conductive lines. When the conductive network contains only micron silver particles, microcracks form within the network after numerous bends, leading to a sharp decline in electrical conductivity. With the addition of liquid metal, external mechanical force causes the liquid metal to rupture, and the overflow fills the gaps between the ruptured micron silver particles, thereby enhancing particle contact and improving bending resistance (as shown in
Figure 7(a)). Incorporating AgNPs into the network fills the voids and holes, increasing the number of contact points between adjacent conductive fillers when the liquid metal breaks. This enhances the bending resistance of the conductive lines (as depicted in
Figure 7(b) and (d)). After adding silver flakes, the reduction in electrical conductivity during bending primarily arises from the slip and separation of the flakes. When AgNPs or micron silver particles are present in the network, they fill the gaps between adjacent silver flakes, improving the contact between them and enhancing the flexibility of the conductive lines. Additionally, due to their large surface area after hot pressing, silver flakes facilitate the rupture of liquid metal under mechanical strain, increase the number of ruptured liquid metal connections, and thereby improve the mechanical stability of the conductive network (as shown in
Figure 7(c) and (d)).
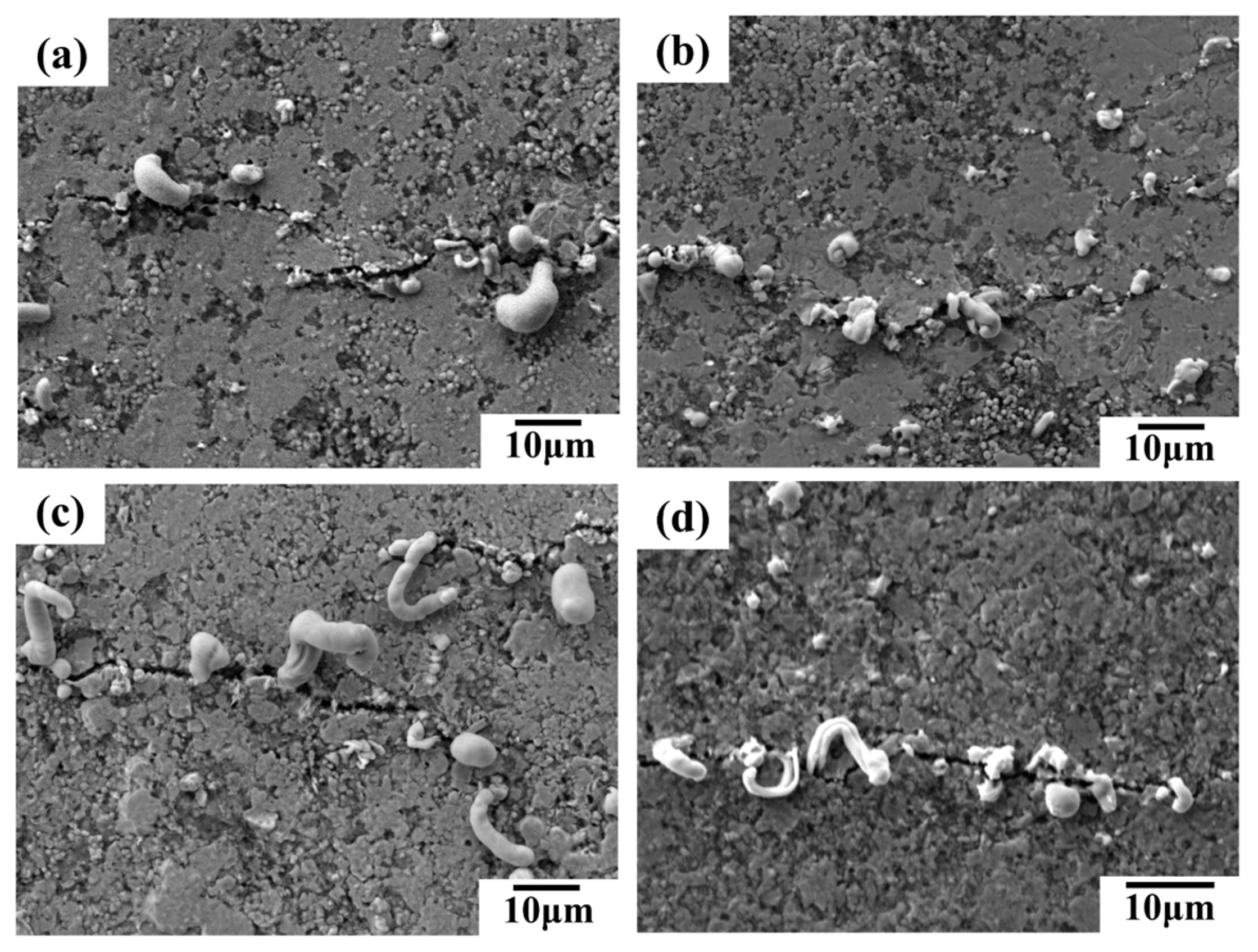
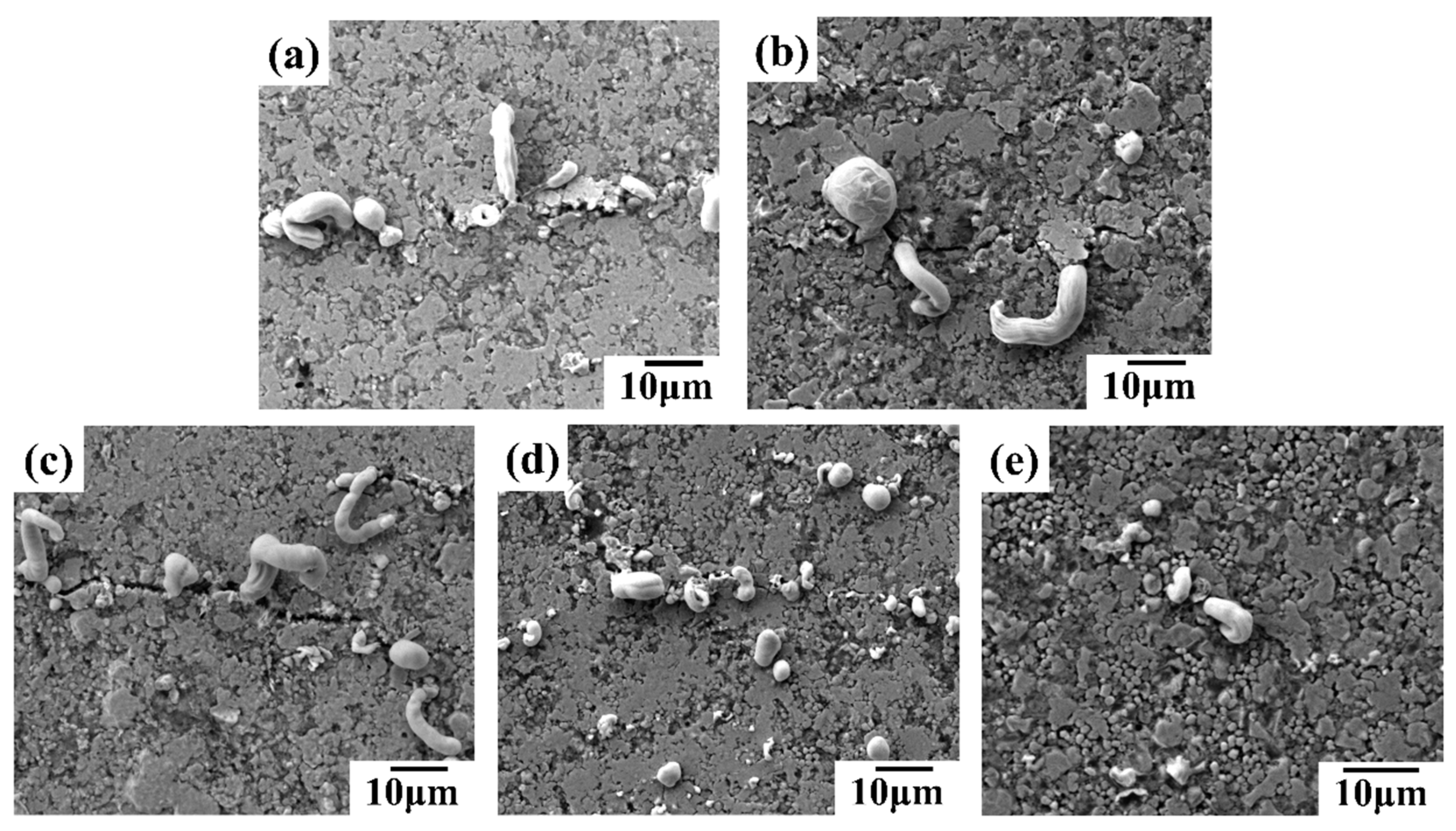
SEM images in
Figure 8 reveal the surface morphology of conductive lines prepared with different inks after 5,000 bending cycles. Microcracks, perpendicular to the bending direction, are observed on the surface of all lines (
Figure 8). These cracks contribute to the increased resistance shown in
Figure 6(a) and (b). The bending process likely induces liquid metal rupture within the conductive network, causing some metal to migrate towards the silver fillers and extrude at the crack sites. Notably, lines containing silver flakes exhibit more pronounced liquid metal extrusion compared to those without flakes, sometimes forming strips (
Figure 8(c) and (d)). Additionally, lines with silver nanoparticles (AgNPs) show some AgNPs extrusion at the cracks (
Figure 8(b) and (d)), potentially acting as bridges and enhancing the network's bending resistance. Consequently, conductive lines incorporating various silver fillers and liquid metal demonstrate superior mechanical stability and bending resistance compared to lines with only micron silver particles. Among these, the line formulated with liquid metal, micron silver particles, and silver flakes exhibits the best bending resistance.
3.2. Optimizing Conductive Ink Performance through Mass Ratio of Fillers
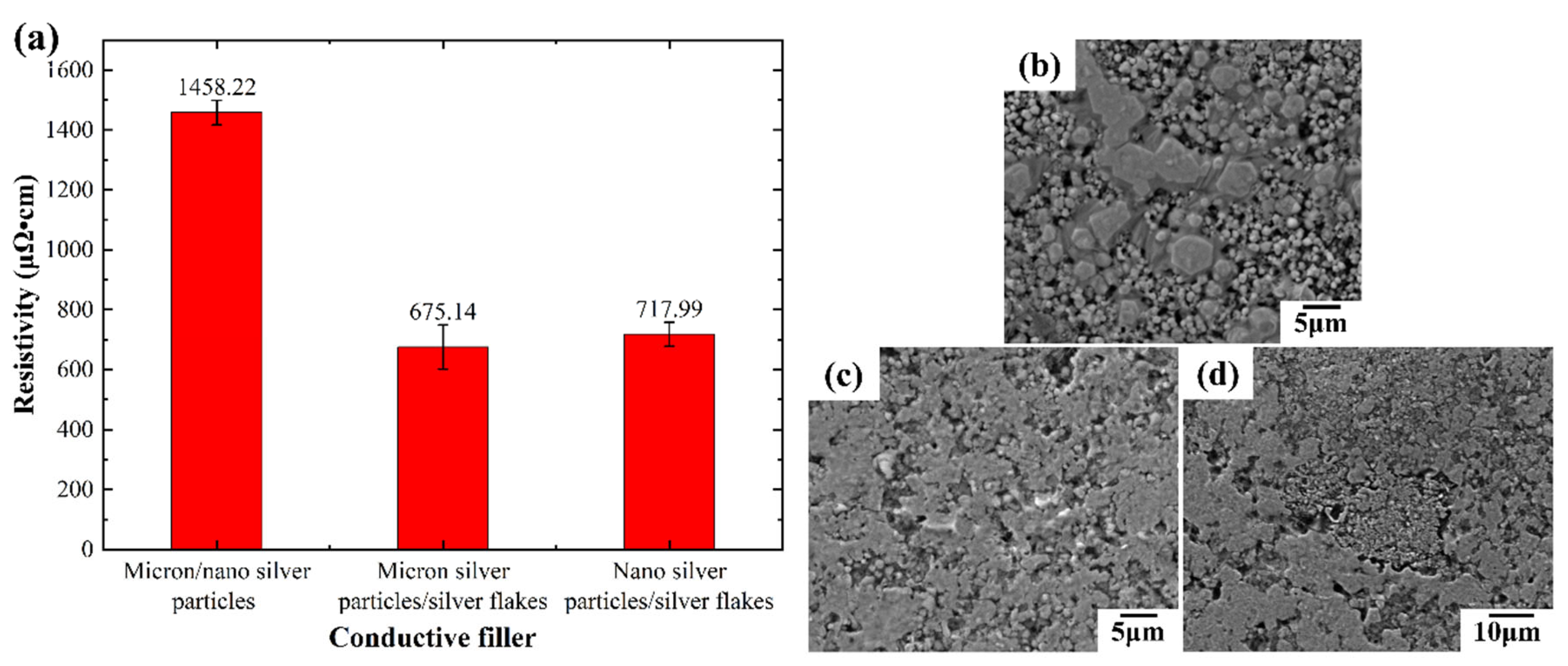
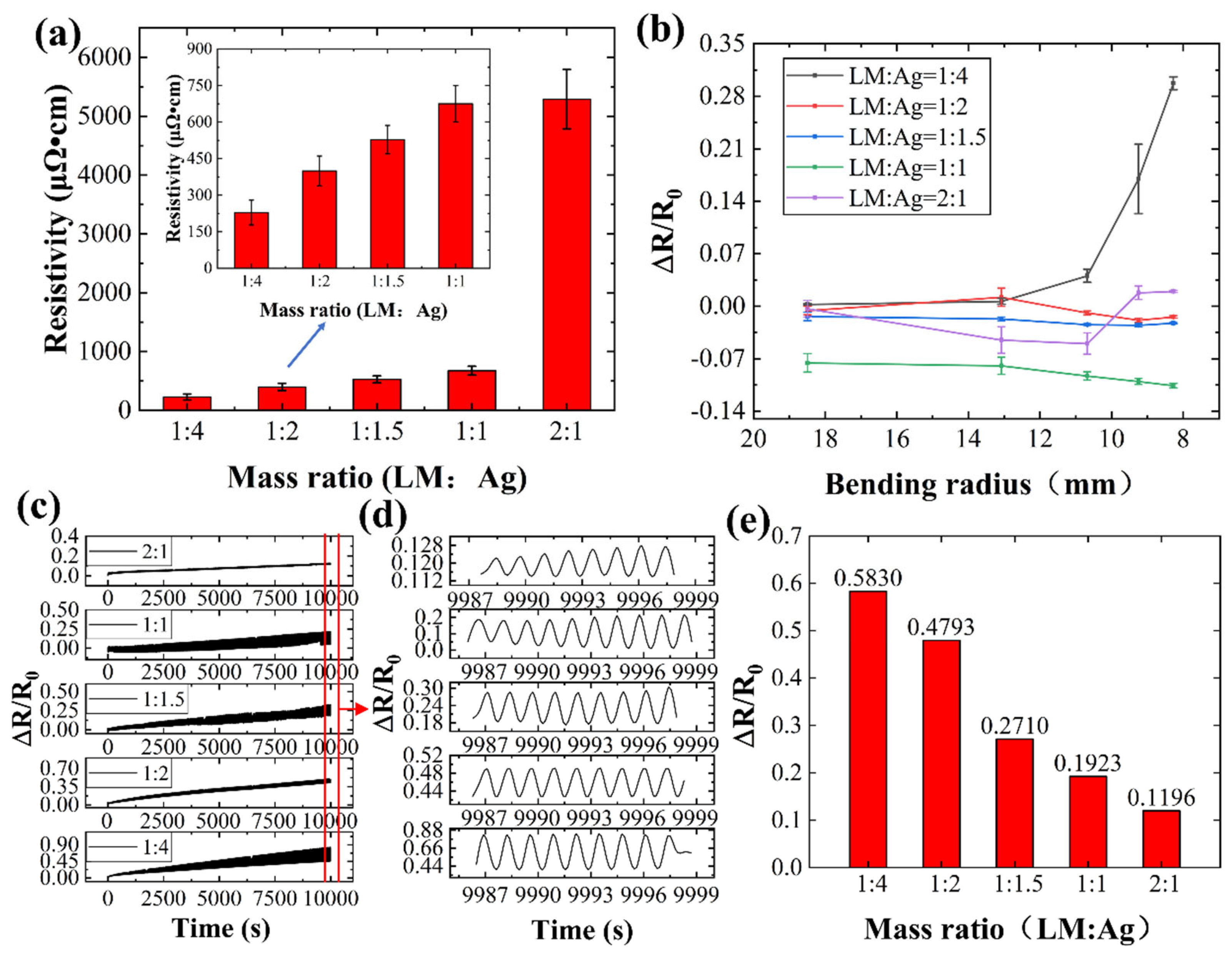
Five conductive inks with varying liquid metal (LM) to silver mass ratios were prepared and their corresponding conductive lines were tested (
Figure 9). As shown in
Figure 9(a), conductivity in LM/silver inks depends primarily on the silver network and fractured LM particles. Lower LM content translates to a higher silver fraction, leading to lower resistivity and improved electrical performance. For example, the conductive line exhibits a resistivity of 675.14 μΩ·cm at a 1:1 LM:silver ratio, which decreases to 228.77 μΩ·cm with a 1:4 ratio.
Bending tests were performed on conductive lines with varying liquid metal to silver mass ratios to investigate their resistance changes at different bending radii (
Figure 9(b)). As shown in
Figure 9(b), only the conductive line with a 1:1 mass ratio exhibits a continuous decrease in resistance change rate with increasing bending radius. Other lines show an initial decrease at small bending angles, followed by an increase beyond a certain point. At maximum bending, the 1:4 ratio line exhibits the highest resistance change rate, with a 29.74% increase.
As previously discussed, bending induces both network reconstruction and failure within the conductive lines. When reconstruction dominates (resistance decrease), the network maintains conductivity. Conversely, failure (resistance increase) weakens the network. High liquid metal or silver content weakens the reconstruction relative to failure. For high liquid metal content, the network becomes more rigid, hindering reconstruction. High silver content increases the probability of particle fracture and network disruption. These factors lead to increased resistance and decreased electrical conductivity at high bending degrees (
Figure 9(b)).
Figure 9(c-e) illustrate the resistance change rate of conductive lines with different mass ratios over 5000 bending cycles. As evident in
Figure 9(c,d), higher liquid metal content leads to smaller resistance changes during bending, indicating enhanced network stability. Additionally, higher liquid metal content results in lower post-bending resistance (
Figure 9(d)). Notably, the line with a 2:1 liquid metal to silver ratio exhibits only an 11.96% increase in resistance after 5000 bending cycles (
Figure 9(e)).
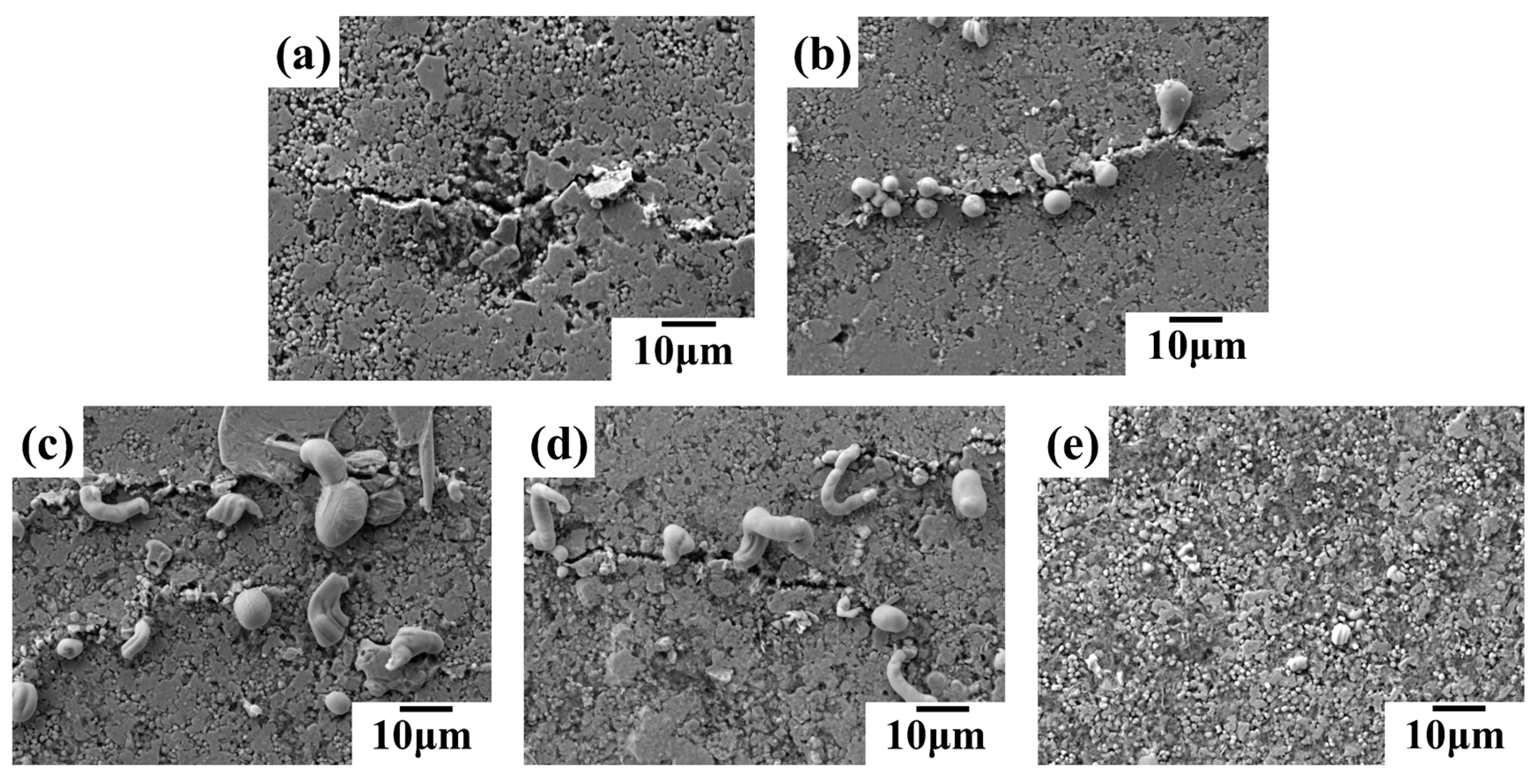
SEM images in
Figure 10 reveal the influence of the liquid metal (LM) to silver mass ratio on the morphology of conductive lines after 5,000 bending cycles. The line with a 1:4 LM:silver ratio (
Figure 10(a)) exhibits microcracks without any LM extrusion at these cracks. As the LM content increases from 1:2 (
Figure 10(b)) to 1:1 (
Figure 10(d)), LM starts to appear at the microcracks, transitioning from droplets to strips, suggesting more pronounced LM extrusion. Interestingly, at a 2:1 LM:silver ratio (
Figure 10(e)), the extruded LM reverts to a droplet shape, while the number of microcracks decreases. This behavior can be attributed to the higher LM content. Upon rupture of the LM oxide film, the numerous LM droplets merge, exhibiting self-healing properties and suppressing microcrack formation.
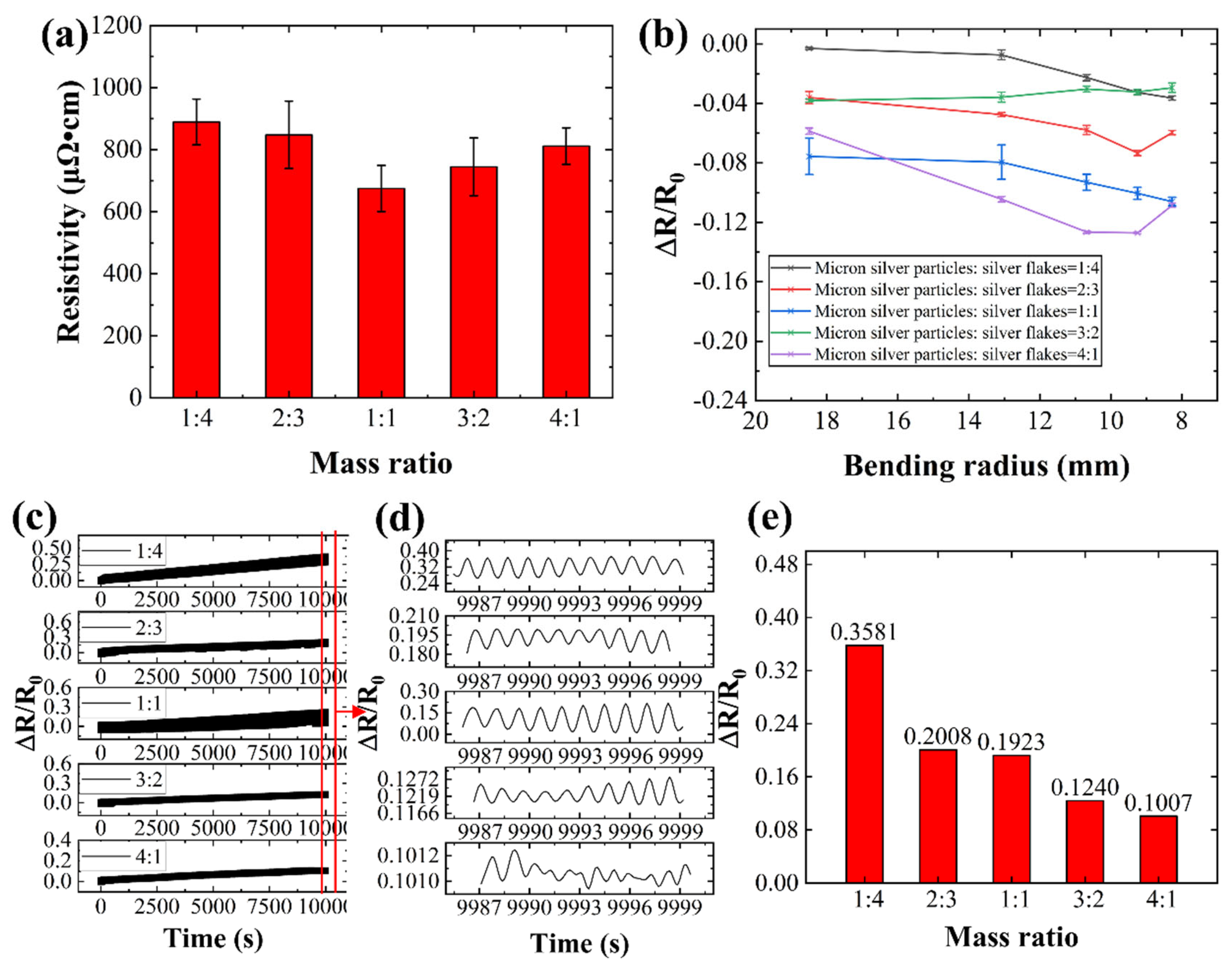
We investigated the impact of the mass ratio between micron silver particles and silver flakes on conductive ink performance. Five conductive inks were formulated with varying mass ratios, and corresponding conductive lines were fabricated. The performance of these lines was evaluated after hot pressing treatment (
Figure 11).
Figure 11(a) reveals an initial decrease in the conductive line's resistivity followed by an increase with increasing silver flake mass fraction. The lowest resistivity of 675.14 μΩ·cm is achieved with a 1:1 mass ratio of micron silver particles to silver flakes. This initial improvement can be attributed to the increased number of contact points within the conductive network formed by the additional silver flakes. However, exceeding a 12.5% mass fraction of silver flakes leads to a decline in conductivity. This is likely due to the accumulation of flakes and the formation of voids between them, hindering electrical pathways.
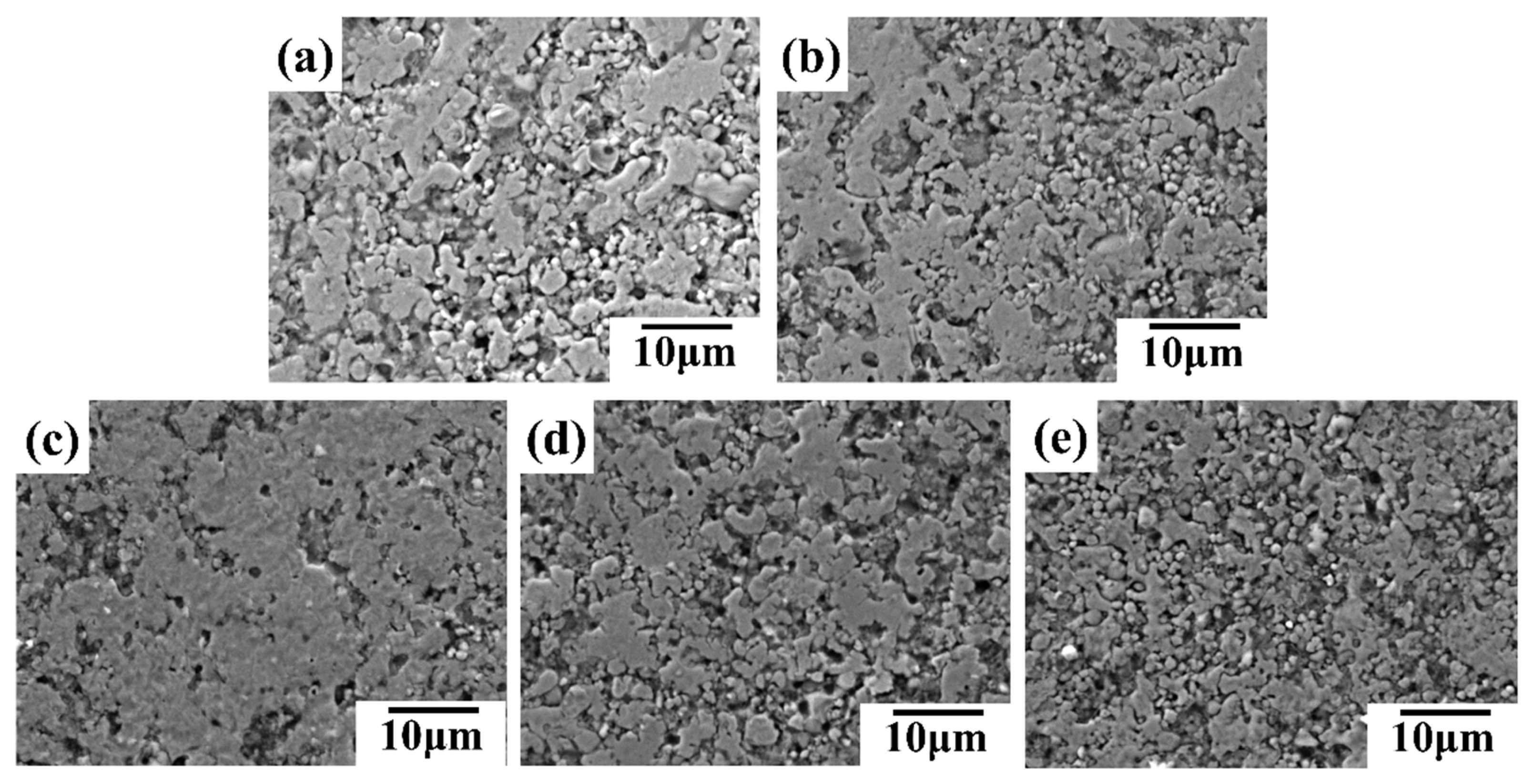
SEM images in
Figure 12 support this observation.
Figure 12(a,b) depict excessive silver flake content leading to accumulation and void formation, resulting in a lower surface density of the conductive line and decreased electrical conductivity. Conversely, insufficient silver content (
Figure 12(d,e)) also leads to a less dense surface. Therefore, the silver flake content significantly affects the electrical conductivity of the conductive coating. An optimal balance is achieved at a 12.5% silver flake mass fraction, leading to a denser conductive line surface and maximized performance.
Figure 11(b) depicts the resistance changes of conductive lines with varying mass fractions of silver flakes (5%, 10%, 12.5%, 15%, and 20%) subjected to different bending radii. Lines with lower silver flake content (5%) exhibit a sharp increase in resistance at higher bending angles, indicating a greater susceptibility to mechanical stress. Conversely, lines with a higher silver flake content (20%) show a more stable resistance profile under larger bending radii.
Figure 11(c-e) illustrate the results of bending fatigue tests (5000 cycles) on conductive lines with different silver flake contents. The resistance of the lines generally decreases with increasing silver content. This trend might be attributed to the vulnerability of silver flakes to mechanical stress, as evidenced by microcrack formation and disruption of the outer oxide film on the liquid metal particles (
Figure 13). At lower silver flake content (5%,
Figure 13(e)), fewer microcracks are observed after bending cycles, suggesting better structural integrity. In contrast, samples with a high silver flake content (20%,
Figure 13(a)) exhibit extensive microcracks and breakage of silver flakes, leading to a significant decrease in bending resistance.
3.3 Influence of Sintering Parameters on the Properties of Conductive Inks
A conductive ink was prepared using a filler composition of 25 wt% liquid metal (LM), 12.5 wt% micron silver particles, and 12.5 wt% silver flakes to study the influence of hot pressing parameters on the resistivity of conductive lines. Five samples were measured under each parameter to minimize errors.
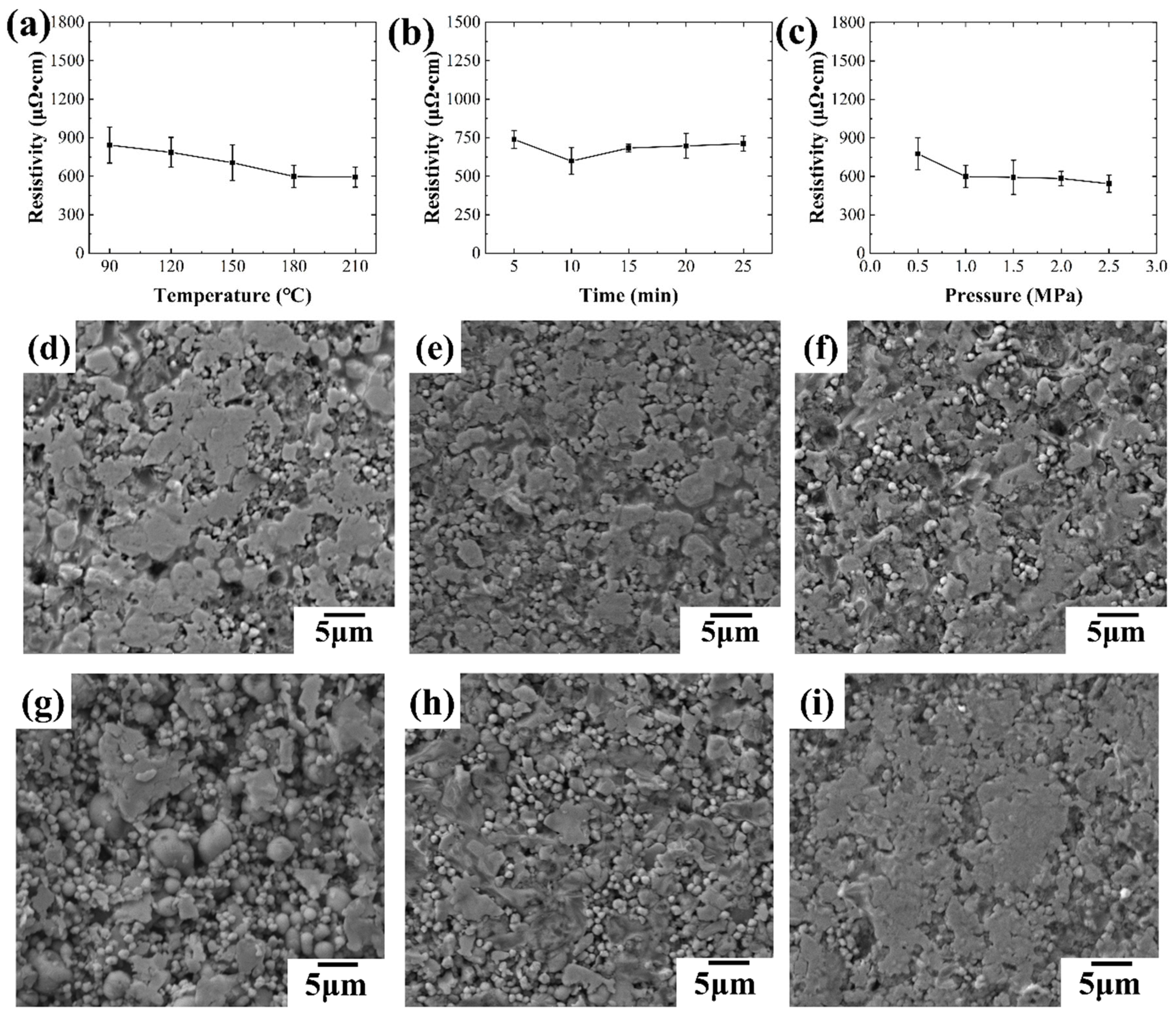
The effects of sintering temperature (10 minutes, 1.0 MPa) and time (180 ℃, 1.0 MPa) were investigated first, as shown in
Figure 14.
Figure 14(a) depicts the impact of varying sintering temperatures on the resistivity of conductive lines. The results demonstrate a decrease in resistivity with increasing temperature, likely due to the gradual decomposition of solvents and organic compounds within the ink, leading to improv ed conductivity. Notably, the decreasing trend plateaus at 180 ℃, suggesting a diminishing effect of temperature on resistivity at higher values.
Figure 14(b) illustrates the effect of different sintering times on the resistivity of conductive lines during hot pressing sintering. As shown in the figure, the resistivity of the conductive line decreases with increasing sintering time up to 10 minutes. However, after 10 minutes, the resistivity begins to increase with further increases in sintering time, leading to a decrease in electrical conductivity. To investigate this behavior, conductive lines treated with various sintering times were characterized microscopically, and SEM images are presented in
Figure 14(d-f). After hot pressing, the oxide layer of the liquid metal on the surface of the conductive line breaks under pressure, causing internal liquid metal to flow out and fill holes in the surface layer. Additionally, silver flakes adhere to the surface of the conductive coating, resulting in a smoother surface for the conductive line. A smoother surface typically indicates better conductivity. From
Figure 14(d-f), it can be observed that with a sintering time of 5 minutes, the surface of the conductive line is smooth, resulting in improved conductive performance. However, as the sintering time increases to 15 minutes, the surface of the conductive line becomes rougher, leading to a decline in electrical conductivity. Further increasing the sintering time to 25 minutes results in an even rougher surface of the conductive line, further increasing the resistivity.
Additionally, to explore the effect of different sintering pressures on the electrical conductivity of the conductive line, the line was sintered under varying pressures of 0.5 MPa, 1.0 MPa, and 2.5 MPa, at a sintering temperature of 180 ℃ and for a duration of 10 minutes.
Figure 14 illustrates the impact of sintering pressure on the resistivity of the conductive lines. As shown in
Figure 14(c), the resistivity of the conductive line decreases with increasing sintering pressure. Higher sintering pressures result in better conductivity of the conductive line.
Figure 14(g-i) displays the micro-morphology of conductive lines under different sintering pressures. At a low sintering pressure (0.5 MPa), the surface of the conductive line is uneven with numerous holes visible. Additionally, many liquid metal particles remain unbroken, with silver flakes adhering to the outer surface of these particles, significantly affecting the electrical conductivity of the line. With an increase in pressure, more liquid metal ruptures inside the conductive line, leading to a smoother surface and fewer holes in the conductive line. This reduction in surface defects contributes to the decreased resistivity of the conductive line.
To further investigate the effect of sintering process parameters on the conductive line of liquid metal-micron silver conductive ink, a set of orthogonal experiments was designed using liquid metal, micron silver particles, and silver flakes at a mass ratio of 1:1:1, as detailed in
Table 1. The orthogonal experiment included three factors with four levels each. The factors were temperature, time, and pressure. Four levels for each was used in the experiment, with a total of 16 groups of experiments. According to the designed sintering process parameters, the conductive lines were hot-pressed and sintered, and then the resistance was measured. Each measurement was averaged over 10 trials to reduce experimental error. The resistivity of the conductive lines under different sintering process parameters is summarized in
Table 1.
After obtaining the test results in
Table 1, the range analysis method was employed to analyze the experimental data, and the results are summarized in
Table 2. In
Table 2,
represents the sum of the experiments of this factor in the case of horizontal I;
represents the mean of
, which is used to calculate R value; R is the range value, which is obtained by the maximum mean of
minus the minimum mean of
, and the primary and secondary of factors can be sorted according to the size of R value; The number of repetitions per level of factors represents the average number of experiments conducted horizontally.
Table 2 shows that the sintering temperature has a greater effect on the resistivity of conductive lines compared to sintering pressure and sintering time.