Introduction
Pregnancy is a pivotal period in the life of both the mother and the developing foetus, characterized by a cascade of physiological changes and adaptations aimed at supporting growth and development of the foetus [
1]. From conception to birth, the pregnant woman navigates a complex interplay of hormonal, metabolic, and immunological shifts, all orchestrated to nurture and sustain the developing life within. During this time, the maternal body provides the necessary nutrients, oxygen, and protection for the growing fetus to thrive. However, the marvel of pregnancy comes with inherent risks and challenges, highlighting the critical nature of this period [
2]. Complications arising during pregnancy can have far-reaching implications, both impacting immediate health outcomes and predisposing individuals to a spectrum of chronic diseases later in life [
3]. Therefore, ensuring maternal health during pregnancy is essential for the successful progression of gestation as well as laying the foundation for the long-term well-being of both mother and child.
The placenta is a critical aspect of pregnancy, taking on the multifaceted role of supporting fetal growth and ensuring the well-being of both the developing fetus and the mother [
4]. Positioned at the interface between the maternal and fetal circulations, the placenta orchestrates a myriad of essential functions critical for the progression of gestation, and as an endocrine organ synthetizing and secreting various proteins, including PP13, a member of the galectin family [
5]. PP13 is secreted by placental syncytiotrophoblasts and enters maternal circulation as early as the 5th week of gestation [
6]. In healthy pregnancies PP13 plasma concentrations rise progressively throughout gestation, peaking at around 400pg/ml by end of pregnancy [
7]. PP13 content in the placenta is approximately 2.5 mg by the end of gestation, constituting about 7% of placental proteins [
6].
The significance of PP13 lies in its association with preeclampsia (PE) [
8], a hypertensive disorder of pregnancy, characterized by its onset in the first trimester and often leading to maternal and perinatal morbidity and mortality [
9]. Research indicates a stark decrease in PP13 levels during the first trimester of pregnancies complicated by PE, while normal pregnancies exhibit a steady increase [
10]. Altered levels of PP13 mRNA and protein, as well as polymorphic variants, found in women with PE suggest a potential role for PP13 in the pathogenesis of this disorder [
11,
12]. PE poses significant risks to maternal and fetal health, including increased cardiovascular disease risk and improper vascular remodeling, which can lead to intrauterine growth restriction. Correct vascular remodeling during pregnancy is essential for adequate utero-placental blood flow, with the uterine circulation undergoing expansive remodeling to accommodate the increased demand for oxygen and nutrients required for fetal growth [
13].
PP13 modulates several key processes critical for ensuring a successful pregnancy, including maternal immune responses to prevent maternal-fetal rejection [
14], modulating oxidative stress and inflammation to mitigate oxidative damage and inflammatory responses [
15,
16], promoting cytotrophoblast invasion into the myometrium and ensuring spiral artery remodelling [
17,
18]. Animal studies have also shown promising results, demonstrating PP13’s effect on modulating endothelial cell function [
19,
20], promoting angiogenesis and facilitating vascular remodeling necessary for proper placental and fetal development [
21].
Despite these findings, the specific effects of PP13 on human vasculature remain unclear. Therefore, the objective of this study is to investigate the effects of PP13 on the vasculature of pregnant and non-pregnant women, aiming to elucidate its mechanisms of action and potential implications in pregnancy-related vascular physiology. A comprehensive understanding of PP13 blood vessels effects is essential for elucidating the pathophysiology of pregnancy-related vascular disorders. This research may provide valuable insights into the diagnostic and therapeutic applications of PP13 in pregnancy-related disorders.
Results
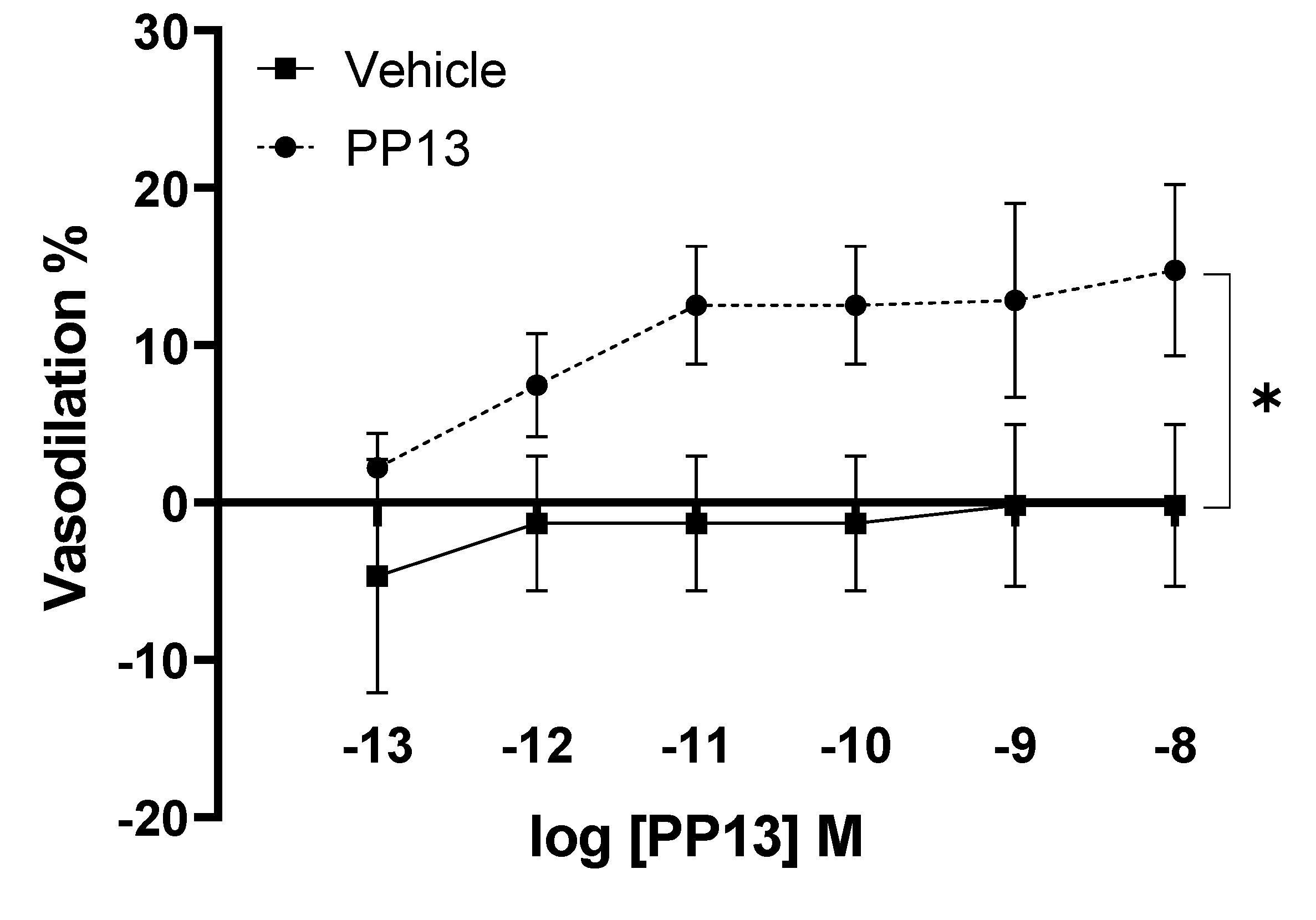
PP13 and its vehicle were assessed for their effects on uterine arteries isolated from pregnant women. The results demonstrate a dose-dependent vasodilation effect elicited by PP13, with a maximal dilation of 14.7 ± 5.4% observed at a concentration of 10
-8 M. The EC
50 value for PP13 was calculated to be 1.7 x 10
−12 M. In contrast, administration of the vehicle did not induce any significant effect on arterial diameter (
Figure 1). These findings indicate a specific vasodilatory action of PP13, highlighting the biological activity of PP13 in regulating uterine vascular tone.
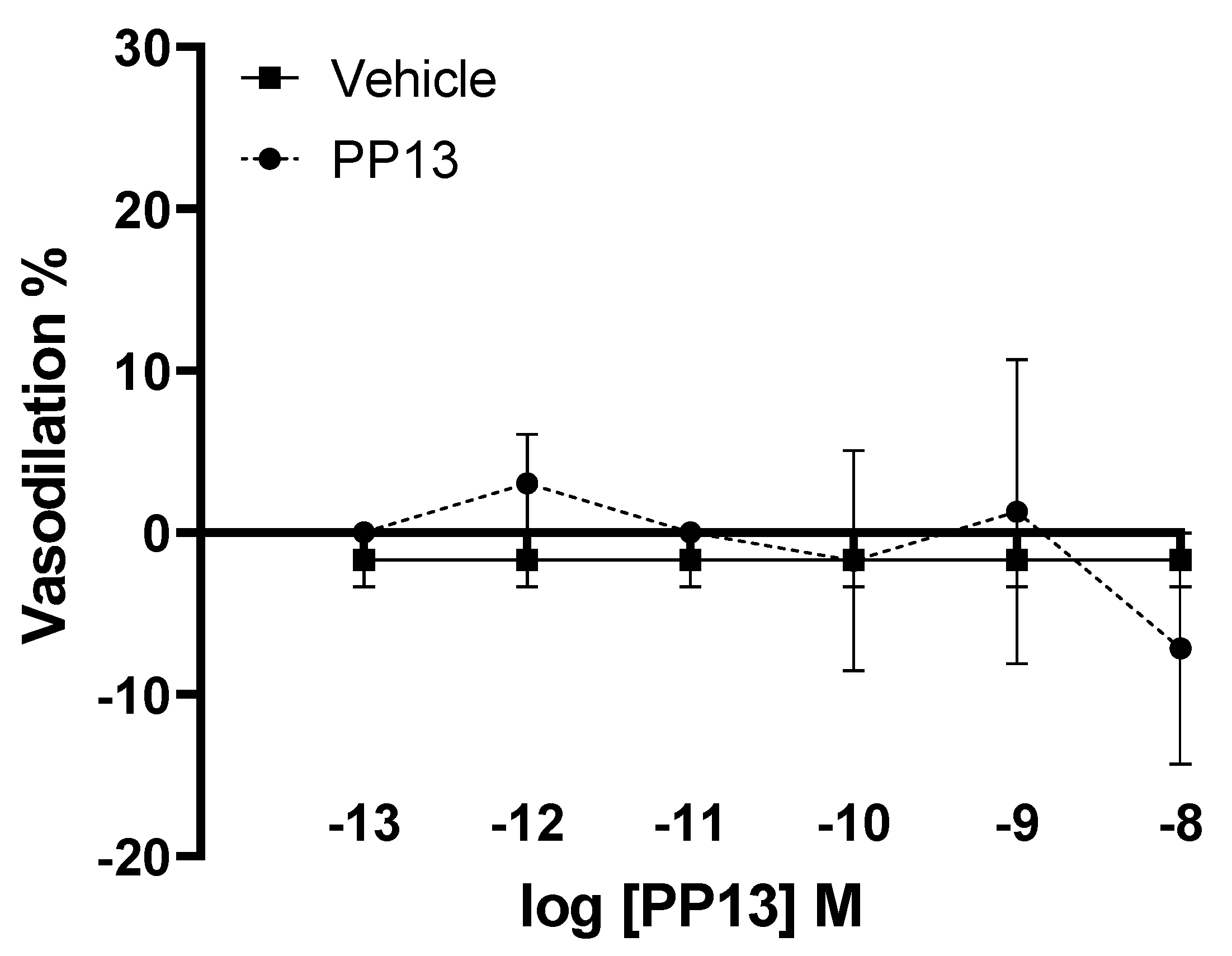
PP13 and its vehicle were also evaluated on subcutaneous arteries obtained from pregnant women. However, unlike uterine arteries, neither PP13 nor its vehicle significantly effected subcutaneous arterial function (
Figure 2). These results suggest a specificity of PP13 action to uterine arteries in this experimental context.
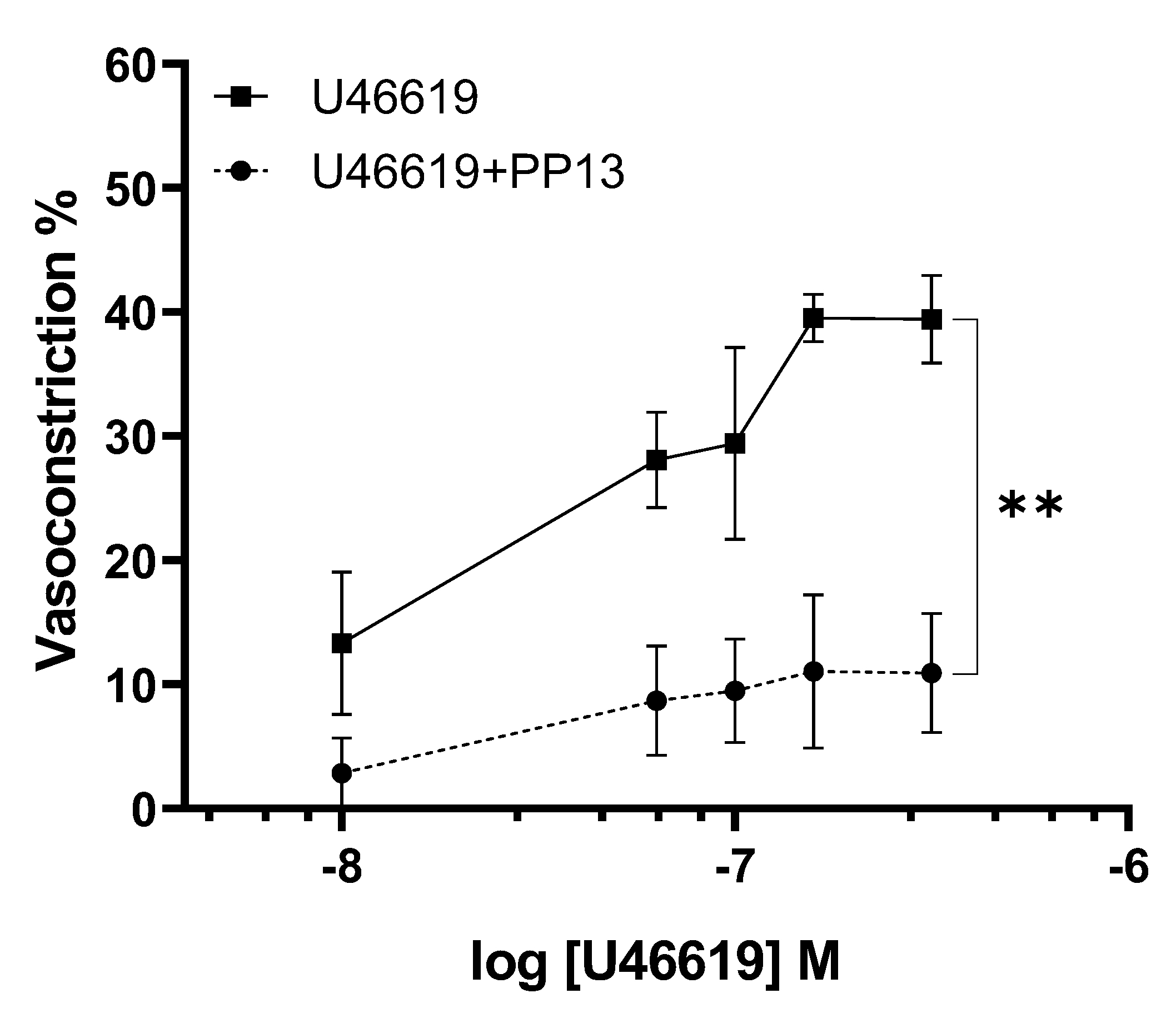
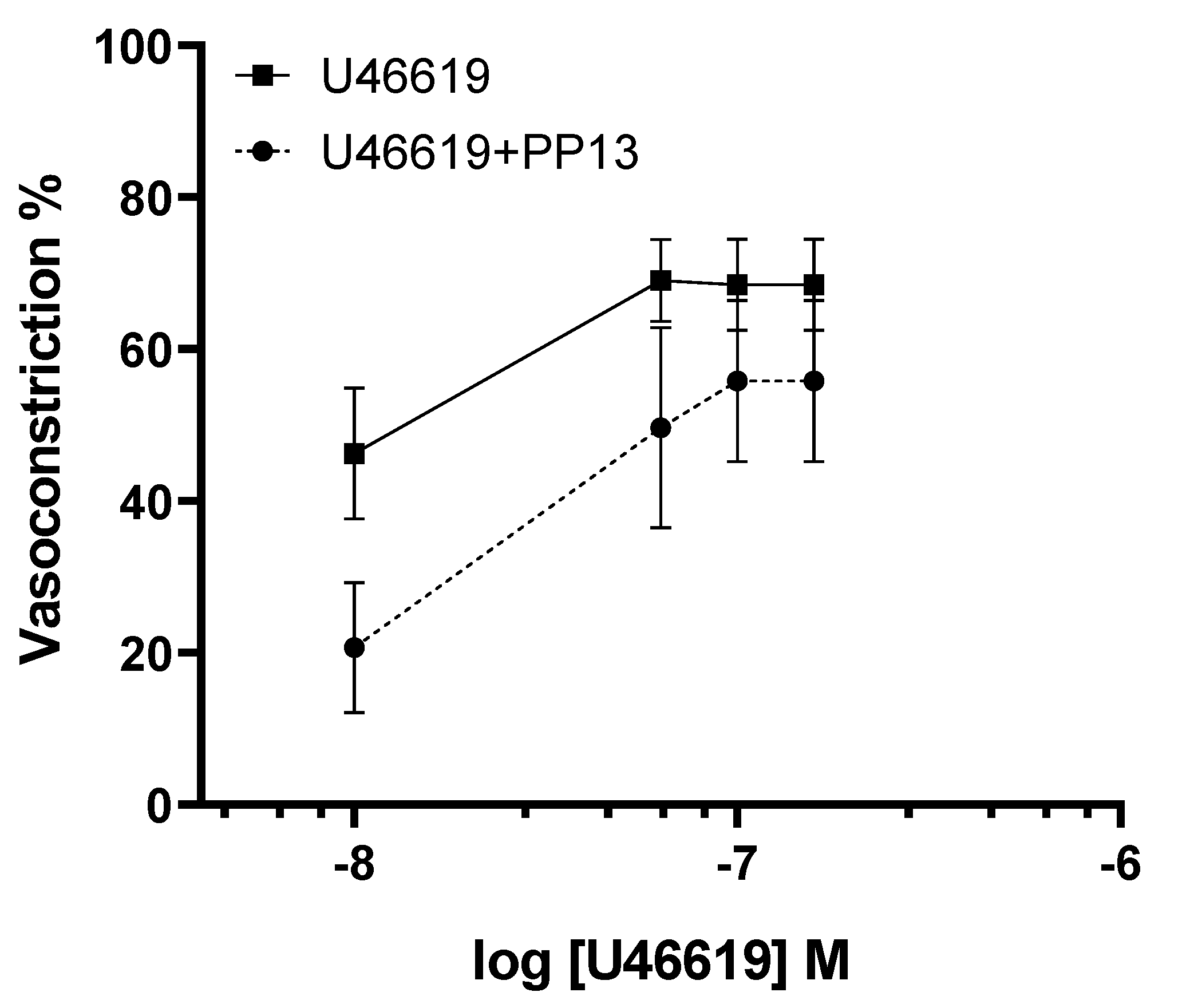
Given the evidence suggesting a vasodilatory role for PP13 in uterine arteries, we sought to investigate its potential counteractive effect against the potent vasoconstrictor U46619, a thromboxane receptor agonist. Uterine arteries isolated from pregnant women were exposed to U46619 in the presence and absence of PP13. As anticipated, U46619 induced a dose-dependent contraction of uterine arteries. However, intriguingly, the contraction curve was significantly attenuated in the presence of PP13 (
Figure 3). The maximum U46619-contraction was reduced 71 %, from 40.4 ± 6.3% to 11.6 ± 4.8% by PP13. These findings suggest a possible antagonistic interaction between PP13 and U46619, highlighting the vasomodulatory potential of PP13 in regulating uterine vascular tone.
To determine whether PP13's effect on vascular tone is dependent on pregnancy we conducted experiments using uterine arteries isolated from non-pregnant women. Interestingly, PP13 reduced U46619-induced contraction in these arteries as well, but it was not significant as depicted in
Figure 4. PP13 was less effective in attenuating U46619 contraction in non-pregnant women than in pregnant women. In uterine arteries from non-pregnant women, PP13 reduced the maximum U46619-contraction only 32%, from 72.2 ± 9.2% to 48.9± 14.6%. These results suggest PP13 acts differently in pregnant and non-pregnant states, indicating a potential modulation of PP13's vasomodulatory effects by pregnancy status.
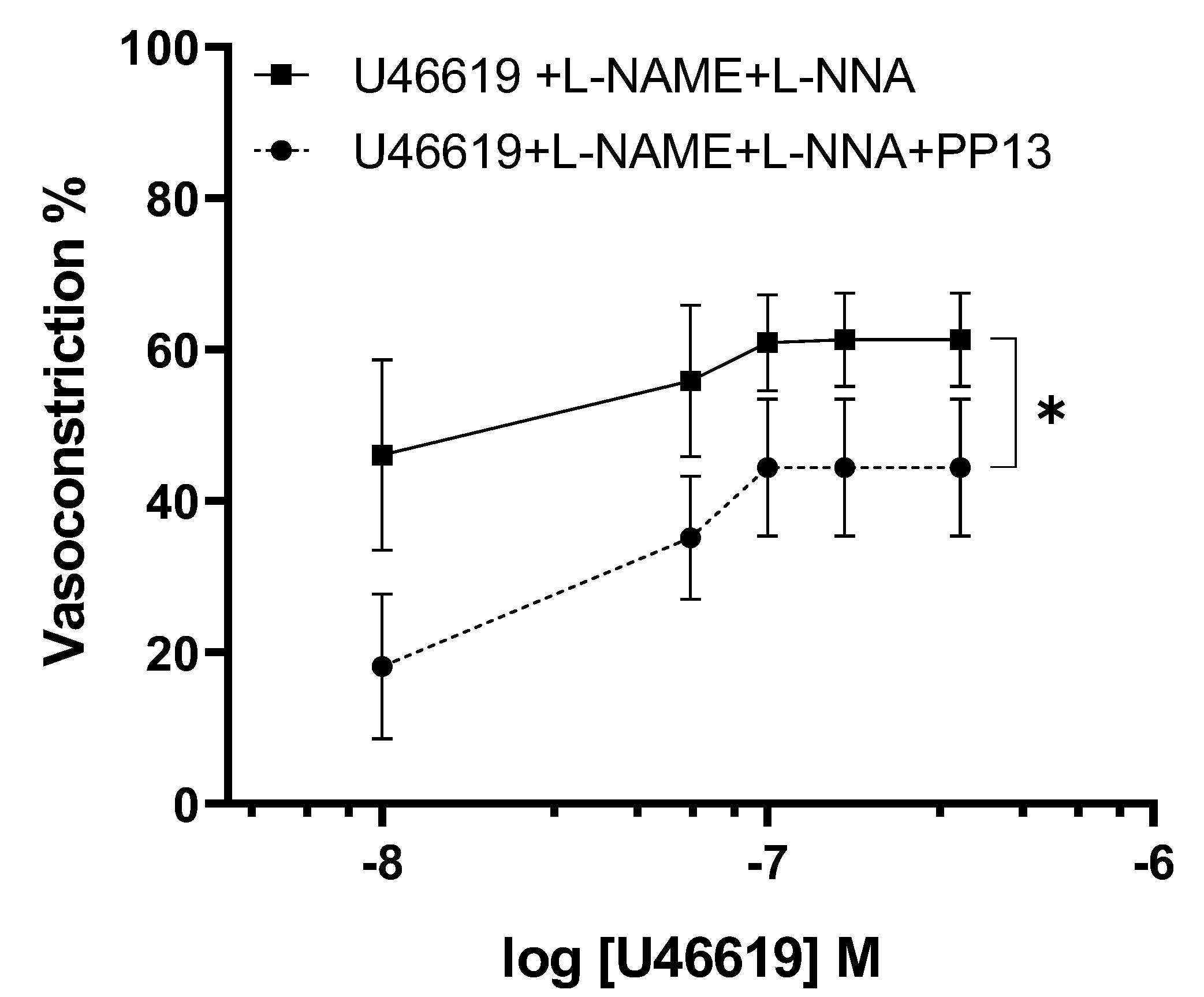
To elucidate the mechanism by which PP13 counteracts U46619-induced contraction in uterine arteries isolated from pregnant women, we investigated whether PP13 acts through the nitric oxide pathway. To this end, uterine arteries were preincubated with specific inhibitors of the enzyme NOS, L-NAME and L-NNA, then exposed to U46619 in either the presence or absence of PP13 (
Figure 5). Although, the dose-response curve for U46619 contraction remained significantly (p<0.05) reduced, the counteracting action of PP13 was much lower than reported in fig.3, (p<0.01). This indicates that the inhibition of NO production prevents in part the observed antagonistic action of PP13 on U46619-induced contraction. These findings suggest that PP13 may exert its vasomodulatory effects by modulating the production or availability of nitric oxide.
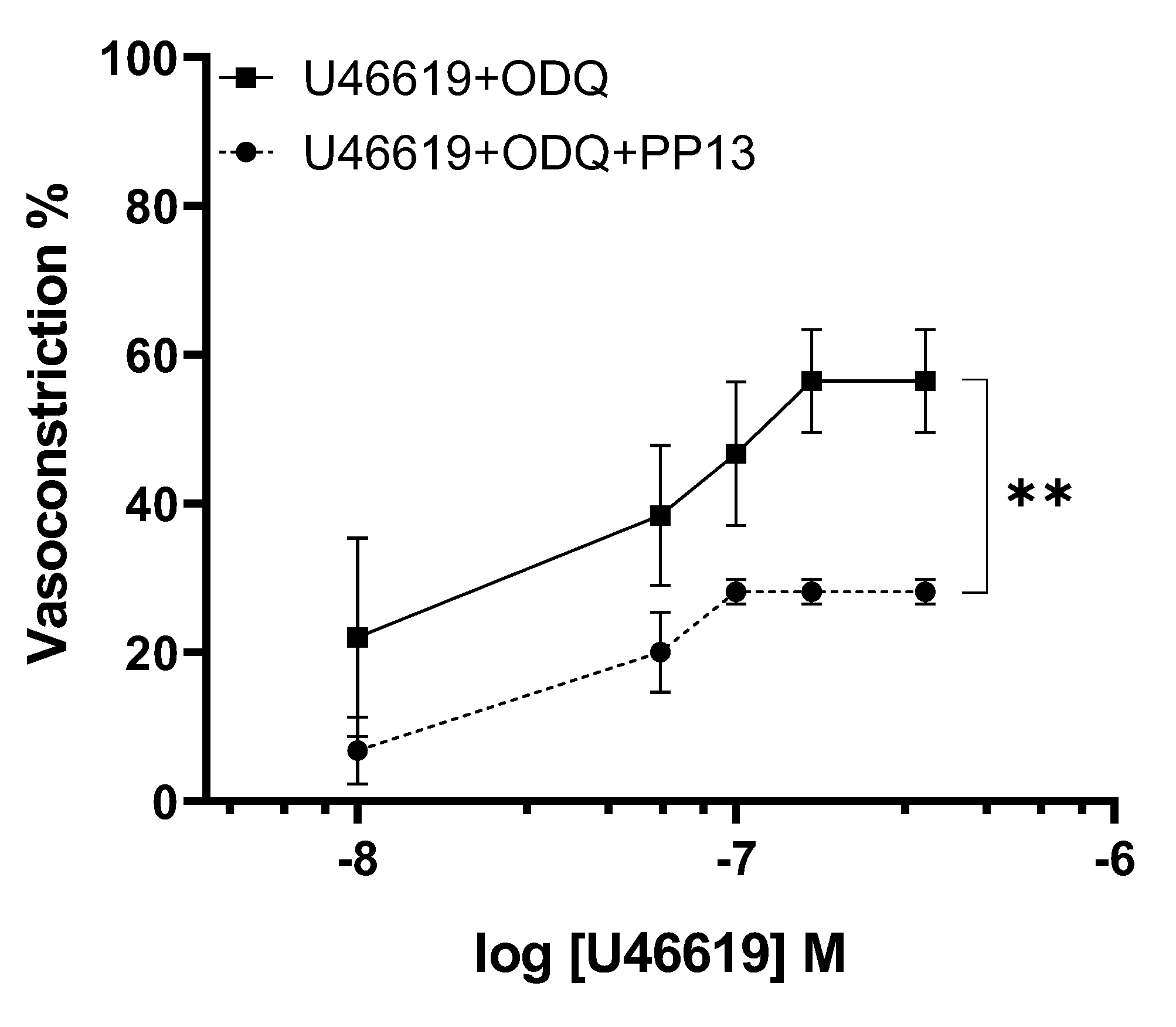
To further elucidate the transduction pathway through which PP13 exerts its effects, we investigated its action in the presence of a specific inhibitor of guanylate cyclase, ODQ. Uterine arteries were preincubated with ODQ to inhibit the production of the nucleotide cGMP. In the presence of ODQ, the dose-response curve for U46619 contraction in PP13 treated vessels remained significantly reduced (p<0.01),
Figure 6. These results suggest that PP13’s mechanism of action is cGMP independent.
Discussions
This study investigated the impact of PP13 on human vasculature, specifically focusing on both uterine (reproductive) and subcutaneous (systemic) arteries isolated from pregnant and non-pregnant women. The results revealed several key findings: 1) PP13 exhibited a pregnancy specific vasodilatory action in uterine arteries, while no such effect was observed in subcutaneous arteries, indicating a selectivity of PP13 action on reproductive vasculature; 2) PP13 mitigated the vasoconstriction induced by U46619, which is particularly pronounced during pregnancy, 3) PP13 partially exerted its effects through the NO pathway.
This study was performed on small subcutaneous and uterine arteries with a lumen diameter of less than 250 μm. These vessels are recognized as pivotal regulators of vascular resistance, thereby controlling blood flow to downstream organs and tissues. Specifically, uterine arteries are responsible for supplying blood to the uterus, role which becomes even more critical during pregnancy as they orchestrate the circulation to the feto-placental unit. This process ensures optimal delivery of the oxygen and nutrients required for the growing fetus. Profound adaptations in the structural and functional uterine vasculature occur to meet the escalating metabolic demands of fetal development and optimize utero-placental blood flow [
13]. These multifaceted adaptations include angiogenesis, vasodilation, and alterations in vascular tone and compliance, and are meticulously regulated by a complex interplay of hormonal, mechanical, and paracrine factors [
22]. Ultimately, this remodeling leads to a substantial enhancement in utero-placental blood flow, increasing approximately 10-20 times non-pregnant levels. Studies utilizing Doppler ultrasound and MRI have reported utero-placental blood flow values hovering around 50 ml/min in non-pregnant women, escalating to levels of 500-600 ml/min during late pregnancy [
23,
24,
25]. This significant increase underscores the dynamic nature of the tissue and the vascular adaptations necessary to sustain fetal growth and development.
The placenta assumes a central role in orchestrating physiological changes within the maternal uterine vasculature during pregnancy through the secretion of hormones, growth factors, and cytokines that exert paracrine effects [
26,
27]. Given its marked decrease in plasma concentration in PE, a leading pregnancy complication associated with increased maternal and fetal mortality [
8], evaluating the effects of the placental protein PP13 on vasculature is paramount. Notably, preeclampsia is linked to impaired uterine vascular remodeling, resulting in elevated vascular resistance and reduced utero-placental blood flow, akin to a "vascular pathology" [
14]. Hence, the primary objective of this study was to assess PP13's impact on uterine arteries, vital for fetal-placental blood supply during pregnancy.
The effects of PP13 on subcutaneous and uterine arteries was assessed using pressure myography, a well-established
ex vivo technique renowned for its ability to closely replicate
in vivo physiological conditions. This method allows for the examination of vascular responses under pressure, more reflective of
in vivo biology than traditional wire myography. Our findings underscored PP13's specificity action on uterine arteries, indicating a dependence on the type of vascular bed. This aligns with PP13's role as a placental protein primarily involved in reproductive processes. Although prior studies demonstrated PP13 induced vasodilation in rat uterine arteries as well as in mesenteric arteries [
19,
20,
28], this study is the first to examine PP13's effects on human uterine arteries, critical given that preeclampsia occurs exclusively in humans.
Moreover, PP13's ability to counteract U46619-induced vasoconstriction, which is particularly enhanced during pregnancy, may be facilitated by gestation-associated vascular conditions, such as increased endothelial NO levels in uterine arteries [
29]. Endothelial cells line the lumen of all blood vessels, including uterine arteries, are surrounded by vascular smooth muscle cells (VSMCs). These endothelial cells serve as sensors and effectors of multiple inputs, such as pressure, neural signals and substances present in the bloodstream. They release various mediators including NO, prostaglandins, and other hyperpolarizing factors, which induce relaxation of VSMCs (endothelium-dependent dilation).
Our results elucidated PP13's vasodilatory action mediated through NO, consistent with previous findings in rats, indicating an endothelium-dependent action via NO. The NO-signaling pathway induces vasodilation [
30] and counteracts increased contraction in various vascular beds, including the rat aorta [
31].
During pregnancy the expression of endothelial nitric oxide synthase (eNOS) undergoes significant upregulation, primarily influenced by estrogens [
32]and shear stress [
33]. This upregulation of eNOS is pivotal for the endothelium-dependent dilation mechanism, which plays a crucial role in relaxing VSMCs. As a result, uterine vascular resistance is reduced, leading to enhanced blood flow to the uterus and the feto-placental unit. This augmented blood flow is essential for optimal oxygen and nutrient transport, ensuring normal fetal growth throughout pregnancy.
In our conventional understanding, the effects of NO are predominantly attributed to the activation of soluble guanylyl cyclase, leading to the formation of cyclic guanosine-3,5-monophosphate (cGMP). However, our findings indicate that PP13-NO-induced vasodilation operates through a pathway independent of cGMP. This suggests the existence of alternative mechanisms that contribute to the vasodilatory effects of nitric oxide beyond the traditional NO-cGMP pathway. Emerging evidence has shed light on these alternative signaling pathways. For instance, nitric oxide has been shown to directly stimulate potassium channels [
34] and calcium channels [
35]. These findings underscore the complexity of nitric oxide signaling and its diverse physiological implications, paving the way for further exploration into the intricate mechanisms underlying vascular regulation. Therefore, although the study provided insights into PP13's vasodilatory effects mediated by NO, it did not delve into the precise molecular mechanisms underlying this action. Further investigations are warranted to elucidate the specific pathways involved. Another limitation to consider is that our studies focused on isolated arteries, which may not fully replicate the complex in vivo vascular environment.
In conclusion, the insights gained from this study offer valuable groundwork for future investigations into a potential therapeutic role for PP13 in treating preeclampsia, a condition affecting approximately 8% of women worldwide without a definitive cure. However, in clinical studies are warranted to ascertain the efficacy and safety of PP13 administration in preeclamptic women, ensuring positive outcomes for both maternal and fetal health.
Material and Methods
Patient Recruitment and Selection
The study involved recruiting patients undergoing cesarean delivery or hysterectomy, all within reproductive age (ranging from 20 to 45 years). A total of 17 patients participated, comprising 5 non-pregnant individuals and 12 pregnant patients with normal pregnancies. Patients were informed about the project upon hospitalization and provided with detailed explanations. Those who agreed to participate underwent a health assessment and provided informed consent. Ethical approval was obtained from the local Research Ethics Committee [approval number: Prot. 8177, January 01,2022)], adhering to the principles outlined in the Declaration of Helsinki. Exclusion criteria included twin pregnancies and pre-existing conditions such as chronic arterial hypertension, autoimmune, renal, or hepatic diseases, ensuring the enrolled patients were free from debilitating pathologies.
Samples Collection
Samples of subcutaneous fat and myometrium (~1 cm3) were obtained from consenting healthy women at the Department of Gynecology and Obstetrics, Annunziata Hospital of Cosenza, Italy. Tissues were immediately immersed in cold HEPES-physiological salt solution (HEPES-PSS), kept on ice, and transported within 30 minutes to the Vascular Physiology laboratory for arteriole isolation.
Vessel Preparation
In the laboratory setting, tissues were initially rinsed with fresh cold HEPES-PSS to ensure removal of any blood residue from the sample surface. Subsequently, the tissues were delicately transferred into small glass dissecting dishes (90 mm) filled with cold HEPES-PSS solution. Once in the dish, they were carefully positioned at the bottom and secured in place using insect pins. The bottom of the dishes is covered with a smooth layer of silicone elastomer (Sylgard 184) to provide a stable working surface.
Arterioles were meticulously dissected under a stereomicroscope (Leica MZ 12.5 Ergo) utilizing fine forceps (Dumont #55) and sharp Vannas spring scissors (Fine Science Tools, Foster City, CA, USA). This precise instrumentation was indispensable for the meticulous dissection of microvessels, ensuring the preservation of their structural integrity and minimizing any potential damage.
Pressure Myography
Artery segments dissected free of surrounding adipose and connective tissue, were isolated and transferred to a pressure myograph chamber (Instrumentation and Model Facility, University of Vermont, Burlington, VT, USA). Cannulation at both ends of the vessel was conducted between two opposing glass cannulas filled with HEPES-PSS, requiring the use of forceps with fine tips to ensure precision. Arterial segments of a sufficient length, approximately 3 mm, were mounted to ensure an undamaged segment between the two cannulas.
To elaborate on the cannulation process, one end of the vessel was mounted and tied onto glass pipettes using thin nylon suture thread, and any residual blood within the lumen was gently flushed out with solution. Subsequently, the other end of the vessel was tied onto the downstream cannula, which was closed off to establish a “blind sack” preparation. Meanwhile, the inflow cannula, connected to a pressure-servo system (Living Systems Instrumentation, Burlington, VT, USA) was adjusted to a physiological pressure level of 50 mmHg to mimic in vivo conditions. This pressure-servo system was coupled with a pressure transducer to meticulously monitor the pressure applied to the vessels throughout the experiment. Vessel was evaluated for leaks and discharges as these can influence vasodilation and vasoconstriction. The preparation was continuously superfused with HEPES-PSS warmed at 37 oC and a pH of 7.The arterial lumen diameter was continuously monitored using video microscopy, coupled with specialized data-acquisition software (Ionoptix, Westwood, MA, USA) which accurately tracks the relative positions of the inner and/or outer vascular walls.
Experimental Protocol
Arteries were tested for viability using vasoactive agents U46619 (constrictor) and acetylcholine (dilator). Non-responsive vessels were excluded. Functioning arteries were equilibrated for 30-45 minutes and subjected to PP13 testing using two protocols: 1) Vessels pre-contracted with U46619 were exposed to increasing PP13 concentrations (10
-13 - 10
-8 M), and 2) A dose response curve for U46619 was generated in the absence or presence of PP13 (10
-8 M, pre-incubated for 1 hour). At the end vessels were superfused with HEPES-PSS without calcium plus the phosphodiesterase inhibitor, papaverine (10
-4 M) to induce maximal vasodilation and to measure maximal vessel diameter. The underlying molecular mechanism of PP13 action was investigated using the following pharmacological inhibitors: 1) Nω-nitro-L-arginine methylester (L-NAME 10
-4 M) and Nω-nitro-l-arginin (LNNA 10
-4 M) for the eNOS enzyme, and 2) 1H-[
1,
2,
4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) for the guanylate cyclase enzyme. In detail, uterine arteries were preincubated with the inhibitors for 20 minutes, then contracted with U46619 and tested with PP13.
Chemicals and Solutions
The HEPES-PSS solution consists of the following components in mM: 141.8 NaCl; 4.7 KCl; 1.7 MgSO4; 0.5 EDTA; 2.8 CaCl2; 10.0 HEPES; 1.2 KH2PO4; 5.0 Glucose. The solutions were prepared in deionized water and titrated with sodium hydroxide to a physiologic pH of 7.Chemicals were purchased from Sigma-Aldrich (Milan, Italy), Fisher Scientific (Milan, Italy), and Cayman Chemical Co. (Hamburg, Germany), unless otherwise specified. All drugs tested were administered from stock solutions prepared daily, while PP13, U46619 and ODQ stock solutions, were stored in aliquots and were thawed as needed.
Recombinant PP13 was obtained from Protein Laboratories Rehovot (PLR) Ltd. in collaboration with HyLaboratories Ltd., (Rehovot, Israel). The protein was dissolved in sterile water (vehicle).
Statistical Analyses
The vasodilatory effect of PP13 was expressed as percentage of maximal diameter, which was determined in the presence of the relaxing solution. EC50 calculated as the concentration inducing 50% of the maximum response. Area under the dose-response curve (AUC) was compared across experimental conditions. Data were expressed as Means ± Standard Error Mean (SEM), with statistical significance set at p < 0.05 using unpaired Student’s t-test.
Author Contributions
Investigation, Mc.G. and M.E.; Data Curation, Mc.G., M.E. and Maurizio M.; Writing – Original Draft Preparation, Mc.G. and M.E.; Conceptualization, Maurizio M., S.G., Michele M., H.M. and DR.S.; Methodology, Maurizio M., Mc.G. and M.E; Validation, Maurizio M.; Formal Analysis, Maurizio M., S.G., Michele M., H.M. and DR.S.; Writing – Review & Editing, Maurizio M., S.G., H.M., Michele M., and DR.S.;; Resources, DR.S. and Michele M.; Funding Acquisition, S.G. and Maurizio M. . All authors have read and agreed to the published version of the manuscript.