1. Introduction
The European Biostimulants Industry Council (EBIC) defines biostimulants as materials containing substance(s) and/or microorganisms whose function, when applied to plants or the rhizosphere, is to stimulate natural processes to improve/benefit nutrient absorption, nutrient efficiency, abiotic stress tolerance and crop quality ("European Biostimulants Industry Council," 2013). HPs contain mixtures of amino acids, oligopeptides, and peptides obtained through chemical or enzymatic hydrolysis of proteins from agro-industrial residues, which can originate from either plant or animal sources [
1]. When provided to plants as biostimulants, HPs act on nitrogen absorption and assimilation, regulate enzymes involved in nitrogen assimilation and their structural genes, enhance response efficiency to biotic and abiotic stresses, increasing the production of secondary compounds and the activity of antioxidant enzymes [
2,
3,
4].
Several studies have demonstrated the potential use of biostimulant products in soybean (
Glycine max L. Merril), with most focusing on inducing tolerance to water stress. These studies have shown that with the application of biostimulants, plants adjust their turgor in relation to leaf temperature [
5], increase nutrient concentration in grains, and exhibit enhanced growth parameters [
6]. Additionally, they alter the expression of stress-responsive genes [
7] and increase antioxidant activity, photosynthetic activity, and assimilate production [
8].
Soybean cultivation has the most varied production purposes, including feed and meal production, oil consumption, processing into products such as paints and cosmetics, and pharmaceutical manufacturing, among others [
9,
10,
11]. Hence, soybean is considered one of the primary agricultural commodities globally. In Brazil, soybean cultivation covers 45 million hectares, yielding 162 million tons of grains [
12]. Due to the importance of soybean cultivation and the increasing demand for food worldwide, there is a need to increase the productivity of soybeans and other crops within the same cultivated area. However, it is essential to consider reducing environmental impacts caused by agricultural activities by employing more sustainable resources [
13,
14].
N is the main nutrient for soybean crops (Tamagno et al., 2017). Increasing the rate of N assimilation by the crop tends to increase its productivity, as the number of grains is directly related to the number of retained pods, which depend on nitrogen availability during the flowering period (Thibodeau and Jaworski, 1975). In this case, biostimulants can be used as a resource to achieve higher yields by enhancing nitrogen metabolism efficiency. This study aimed to evaluate the effect of a protein hydrolysate-based biostimulant on nitrogen metabolism in nodulated soybean plants and their productivity.
2. Material and Methods
The experiment was conducted using soybean seeds cv. Intact RR2 Pro 57HO123 TP IPRO (HO-Genetics), inoculated with Bradyrhizobium elkanii and B. japonicum. These were sown in 10L pots filled with a mixture of soil, organic gardening substrate and vermiculite (2:1:1, v/v/v). HP 0.20% was tested in the seed and foliar application. Such concentration was defined in previous results where the growth of seedlings was measured. The HP was obtained by acid treatment of commercial collagen.
The seed application occurred at the time of sowing when 1ml of 0.20% HP was applied to each pot. Foliar application was performed at vegetative stages V3 and V5 [
15], using a pressurised CO2 sprayer with a pressure of 2 bars. Six plants were used for each treatment. Control plants remained untreated with HP. The experiment was conducted in a greenhouse, irrigated with a drip system, and once a week, the plants received a nutrient solution without N. The experiment was repeated twice; in the first, biochemical and gene expression parameters were evaluated at stage R5.1 of the plants, and in the second experiment, productivity was assessed at full plant maturity [
15].
Enzymatic and biochemical analyses: Leaves were collected for RN activity determination. The newest fully expanded leaf from each plant was collected, placed in plastic bags, and transported to the laboratory in a cooler with ice, where
in vivo analysis was carried out [
16]. Leaves collected and kept in a freezer at -20°C were used for the determination of the enzyme glutamine synthetase (GS) [
17] and glutamate synthetase (GOGAT) [
18], with the protein concentration in the enzymatic extracts obtained by the Bradford reagent method (Bio-Rad). Nitrogen compounds such as nitrate (Cataldo et al. 1975), free amino acids (W. Yemm et al., 1955), and ureides (Vogels and Van Der Drift, 1970) were analysed in lyophilised and ground leaves, as well as in xylem sap collected and kept in a freezer at -20°C. The concentration of chlorophyll and carotenoids was determined after extraction in DMSO [
20]. The foliar contents of nutrients N, phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) were determined in the leaves by plasma emission spectrometry (ICP-OES; JobinYvon, JY50P Longjumeau, France).
Gene expression analysis RT-qPCR: We analysed the expression of NR, nitrite reductase (NiR), GS, GOGAT, nitrate transporter NTR1, asparagine synthetase, arginase, and urease (
Table S1). The genes
CYP2 and
ACTII were used for gene expression normalisation [
21]. Total RNA was extracted from three replicates of leaves and roots using Trizol reagent (Sigma, Kawasaki, Japan), following the manufacturer's instructions. The RNA was treated with "Turbo DNA-free" DNase (Ambion, Inc., Austin, TX, USA) and quantified using a spectrophotometer at 260nm. RNA integrity was checked by 1.5% agarose gel electrophoresis with ethidium bromide and UV light observation. Three µg of total RNA was used to synthesise the first strand with the SuperScript III First-Strand kit (Invitrogen, Waltham, MA, USA), according to the manufacturer's instructions. The primers were designed using the Primer 3 program (//bioinfo.ut.ee/primer3-0.4.0/, accessed on April 1, 2019) (
Table S1) using gene sequences obtained from the Phytozome database (
https://phytozome.jgi.doe.gov/pz/portal.html, accessed on April 1, 2019). Real-time reverse transcription quantitative PCR (RT-qPCR) reactions were prepared with a final volume of 10 µL, containing 3 µL of diluted cDNA, 1.6 µL of milliQ water, 0.2 µL of sense primer, 0.2 µL of antisense primer, and 5 µL of Sybr Green Master Mix (BioRad, Hercules, CA, USA). A StepOnePlus Real-Time PCR System (Thermo Fisher, Waltham, MA, USA) was used for gene amplification (95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 60°C for 30 s as melting curve). Seven biological replicates were performed for each treatment. Relative expression quantification was determined by comparing transcriptional expression between target genes and reference genes using the 2
-ΔCt method [
22].
After collecting the material for laboratory analysis, the dry weights of leaves, stems, and roots were determined. Nodules were counted, and their fresh and dry weights (72h, 70°C) were determined. The assessment of productivity was conducted in the second repetition of the experiment. Plant height, number of productive nodes, number of pods per plant, dry weight of pods per plant, number of seeds per pod and plant, dry weight of seeds, seed weight, and productivity were analysed.
The experiment was conducted in a completely randomised design, with mode of application (seed and foliar) x treatment (HP 0.20% and control) x six replicates, except for gene expression (seven replicates) and nutrient analysis (three replicates). The data obtained were subjected to the Shapiro-Wilk normality test of residuals, and the Bartlett test tested the homogeneity of variances. The data were subjected to logarithmic transformation for variables that did not meet the test assumptions. Subsequently, the data were subjected to Analysis of Variance (ANOVA), and the means were compared using the Tukey test with a 5% probability of error. All analyses were performed using R software v.4.0.0 (
http://www.r-project.org/).
3. Results
The results of the analyses of photosynthetic pigments, nitrogenous compounds and enzyme activity are presented in
Table 1. The mode of application is shown only for those parameters with significant differences. Treatment with HP increased the nitrate content in soybean leaves in relation to control plants, although no interference was found in the mode of application. The ureide content in the xylem sap showed a difference in the modes of application, being higher when the treatment with HP occurred in the seed; this difference also occurred in comparison to the control treatment, however, when applied to the leaves, the plants control showed higher ureide content in the xylem sap.
The amino acid content in the leaves and xylem sap were affected by the HP treatment. Although there was no effect on the mode of application, plants treated with HP had a higher content of total free amino acids. GOGAT activity did not change with HP foliar application, but its was reduced when HP was applied via seed. The modes of application did not differ for this enzyme.
No significant changes were observed in most of the foliar nutrients analysed (
Table 2). Only for P and K, plants treated with HP 0.20% showed a reduction in content. The effect of the mode of application, either seed or foliar, was not observed.
In the gene expression analyses in soybean leaves, urease and RN showed significant differences (
Table 3). The mode of application is shown only for those parameters with significant differences. There was variation in the application of HP on soybean seeds for urease, increasing expression compared to untreated plants or when treated via foliar. RN expressions also increased in the leaves, but there was no difference regarding mode of application. In root expression results, treatment with HP increased the asparagine synthetase expression when applied via foliar; however, when applied via seed treatment, the expression was lower than in control plants. The same occurred for RN, although foliar treatment did not show a difference.
The growth parameters of the plant analysed are shown in
Table 4. The root dry weight was different between control and HP plants, and a difference was found regarding the application mode. In the foliar application of HP, a greater root dry weight was observed compared to the control treatment, and this mode of application was also greater in relation to seed application. Leaves, stem and nodules dry weight showed no treatment effect.
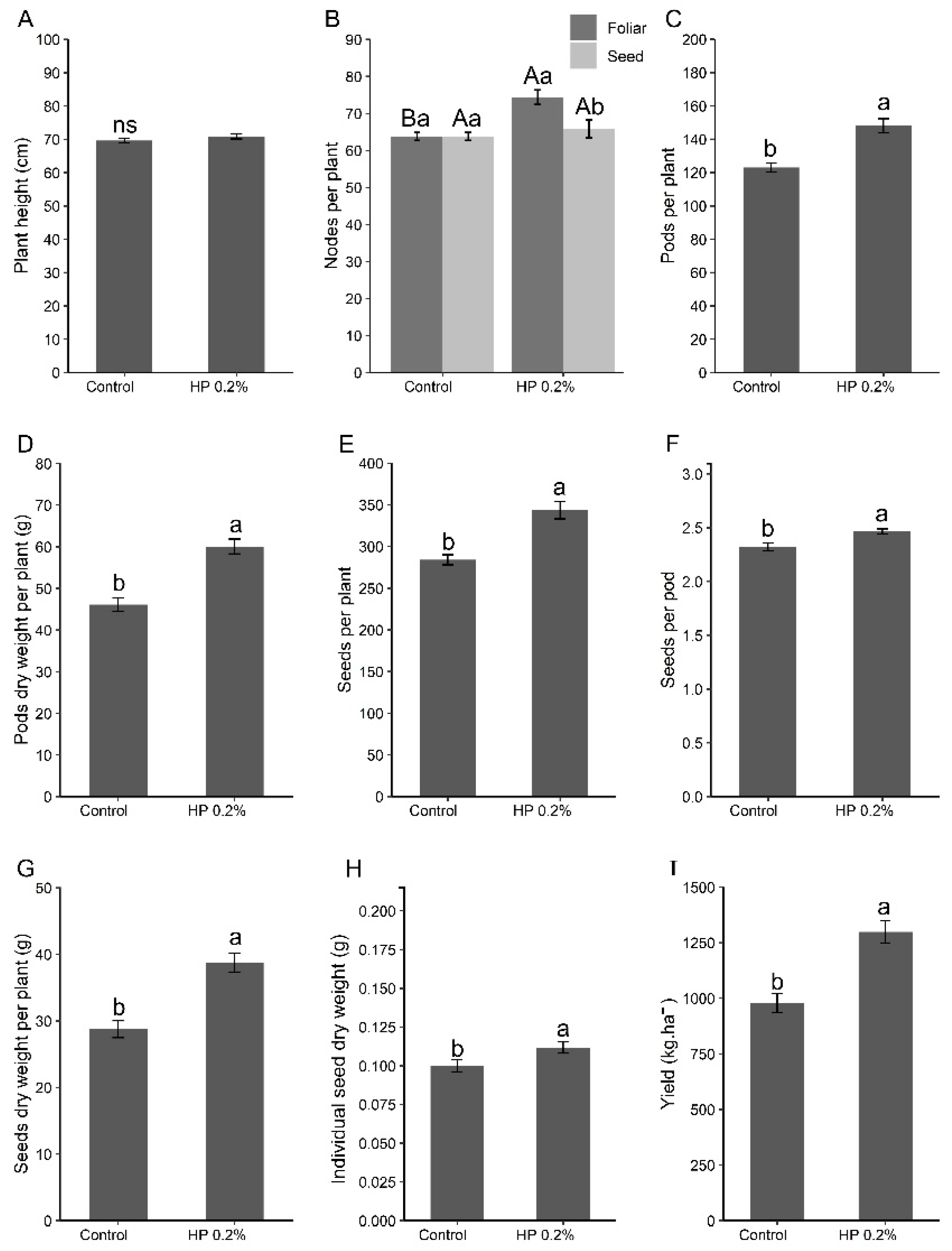
In the experiment carried out until plant maturity, for the evaluation of productivity parameters, it was visually observed that the plants treated with the biostimulant maintained green leaves for a longer period (
Figure 1). Additionally, the treatment with HP practically altered all analysed productivity parameters (
Figure 2). Plants treated with HP were not taller than the control plants; however, they showed a higher number of productive nodes, influenced by the mode of application, being greater when applied via foliar. They also exhibited a higher number and dry weight of pods, seeds per plant and per pod, seed dry weight, and greater productivity per area.
4. Discussion
The use of biostimulants in agriculture aims to stimulate the physiological processes of crops, primarily under conditions of abiotic stress, thereby improving nutrient use efficiency and product quality [
7,
23]. Due to the complexity of formulating HP, it is challenging to elucidate their mode of action and how they interfere with plant metabolism [
24]. In the results presented in this study, an increase in productivity parameters was observed with the application of the biostimulant HP, such as the number and weight of pods and seeds per plant. Despite that, few biochemical and genetic analysed parameters related to N metabolism changed significantly. The mode of HP application was not a determining factor in the examined results.
We have decided to evaluate the N-related biochemical parameters and gene expression at R5.1 phenological stage when seeds are starting to accumulate biomass [
15]. We chose this stage because the NR and NiR activities and NO3 concentration in the xylem sap at the filling pod are still high [
25] and N content due to biological fixation is near its maximum [
26]. Thus, further evaluations at more frequent intervals during plant development are necessary to clarify at which point the HP application is acting on its metabolism, justifying the observed increase in productivity.
There were changes of the activity of the enzymes NR, urease, and asparagine synthetase. NR catalyses the initial step of reducing absorbed nitrate to ammonia, while urease hydrolyses urea into ammonia and carbon dioxide, and asparagine synthetase synthesises asparagine, an amino acid involved in N transport, mainly in seeds and roots [
27]. The increase in the expression of genes, such as RN and urease in leaves, was not followed by an increase in activity. There was also an accumulation of nitrogenous compounds influenced by the application of HP. Considering that ureides are compounds originating from biological N fixation (symbiosis with
Bradyrhizobium), an increase in ureides, amino acids and nitrate in the leaves was observed. In 14-day-old maize seedlings grown hydroponically, the application of alfalfa-based HP also increased the dry root mass of the plants but led to a lower concentration of nitrate in the leaves [
3]. These results contrast with our findings. The authors explained the reduction by the higher activity of the enzymes NR and GS [
3].
Mamatha et al. [
28] studied the application of different doses of plant-based HP on soybean, pepper, and chickpea grown under thermal and water stress conditions. Foliar application of the biostimulant at a dose of 4 ml.l
-1 increased the photosynthetic efficiency of treated plants. The treatment also increased the productivity of soybean, chickpea, and pepper crops by 17%, 30%, and 25%, respectively, as also observed in our study with soybean plants. The effect on the productivity of plants treated with biostimulants has been attributed to the action of phytohormones, increased defence response to biotic and abiotic stresses, maintenance of water balance in plants and soil, and improvement in nutrient uptake [
1,
29,
30]. Here, we did not observe significant changes in nutrient levels because HP application.
Maintaining leaf area during grain filling can be decisive for greater plant productivity [
31,
32]. Francesca et al. [
33] attributed the greater biomass production of tomato plants treated with HP and subjected to thermal and water stress, to the presence of amino acids in the biostimulant that promoted the action of phytohormones that stimulated their development. The presence of leaves at the end of maturation observed in this study can be attributed to the action of phytohormones such as cytokinin, which acts on cell division, leaf senescence, nutritional signalling and stress tolerance triggered by the biostimulant [
34]. Aremu et al. [
35] applied a biostimulant based on algae extract for five weeks in
Eucomis autumnalis plants grown in hydroponics and observed a higher concentration of cytokinin in the leaves of the treated plants, in addition to greater root mass and bulb size of the plants.
The nutrient absorption in soybeans increases significantly from the V3 vegetative stage, reaching a maximum proportion close to the R5.3 stage [
25,
26,
36]. At reproductive stage R5, soybean plants have fully developed pods and seeds at the beginning of development [
15]. Sampling plants at this developmental stage did not show significant differences in many of the evaluated nitrogen metabolism analyses, such as chlorophyll and carotenoid content, NR, GS, and GOGAT enzyme activity, as well as gene expression. However, there was significant expression in productivity parameters, indicating an effect of the application on soybean plants.
Biostimulants, such as HP, have great potential in increasing the productivity of agricultural crops, improving sustainability, even if plants are not subjected to stress conditions [
4,
37]. In this sense, more research is still necessary to clarify which mechanisms are related to the responses to increased productivity in soybeans since different results can occur due to the variation in biostimulants such as manufacturing, components, number, time and method of application, in addition to the plant genotype [
38,
39].
5. Conclusions
In recent years, there has been a shift towards more sustainable strategies in agriculture, as the intensive use of pesticides and fertilisers raises concerns. Including biostimulant products in plant management represents a more sustainable approach to mitigate productivity losses due to stress conditions, enhance crop quality and yield, and improve efficiency of nutrient and resource use. Biostimulants such as HPs demonstrate the potential for increasing soybean crop productivity by increasing the production of pod and grain in plants. However, it's not definitive to attribute this exclusively to the stimulation of nitrogen metabolism. Therefore, further studies with periodic evaluation of their effects throughout plant development are necessary for a better understanding.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
DCHE, DF, MR and JLCB executed the experiment and conducted data analyses; DCHE wrote the first manuscript draft; PM planned the experiment, helped with data analyses and reviewed the manuscript. All authors read and approved the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
For additional information contact the corresponding author.
Acknowledgments
DCHE thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES and Fundação de Amparo à Pesquisa do Estado de São Paulo - Fapesp for a MSc (2019/20211-2) fellowships. DF thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES for postdoctoral fellowships. MR and JLCB thanks Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq for doctoral fellowship. PM thanks The National Council for Scientific and Technological Development (CNPq-Brazil) for a research fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maise seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. in response to an alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal imaging of soybean response to drought stress : The effect of Ascophyllum nodosum seaweed extract. Springerplus 2016, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. South African J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 2018, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- do Rosário Rosa, V.; Farias dos Santos, A.L.; Alves da Silva, A.; Peduti Vicentini Sab, M.; Germino, G.H.; Barcellos Cardoso, F.; de Almeida Silva, M. Increased soybean tolerance to water deficiency through biostimulant based on fulvic acids and Ascophyllum nodosum (L.) seaweed extract. Plant Physiol. Biochem. 2021, 158, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-I.; Erh, M.-H.; Su, N.-W.; Liu, W.-H.; Chou, C.-C.; Cheng, K.-C. Soyfoods and soybean products: From traditional use to modern applications. Appl. Microbiol. Biotechnol. 2012, 96, 9–22. [Google Scholar] [CrossRef]
- Goldsmith, P.D. Economics of soybean production, marketing, and utilisation. In Soybeans Chemistry, Production, Processing, and Utilisation; Johnson, L.A., White, P.J., Galloway, R.B.T.-S., Eds.; AOCS Press, 2008; pp. 117–150, ISBN 978-1-893997-64-6.
- Kim, I.-S.; Yang, W.-S.; Kim, C.-H. Beneficial effects of soybean-derived bioactive peptides. Int. J. Mol. Sci. 2021, 22, 8570. [Google Scholar] [CrossRef]
- CONAB Acompanhamento da safra brasileira: Grãos - Safra 2023/2024 2o levantamento; 2023; Vol. 11. Available online: https://www.conab.gov.br/info-agro/safras/graos/boletim-da-safra-de-graos.
- Hirel, B.; Bertin, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maise. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.; Correia, S.; Guinan, K.J.; Sujeeth, N.; Bragança, R.; Gonçalves, B. Recent advances in the molecular effects of biostimulants in plants: An overview. Biomolecules 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Fehr, W.R.; Caviness, C.E. Stages of soybean development. 1977, 80.
- Foy, C.D.; Fleming, A.L. Aluminum tolerances of two wheat genotypes related to nitrate reductase activities. J. Plant Nutr. 1982, 5, 1313–1333. [Google Scholar] [CrossRef]
- Rhodes, D.; Rendon, G.A.; Stewart, G.R. The control of glutamine synthetase level in Lemna minor L. Planta 1975, 125, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Dougall, D.K. Evidence for the presence of glutamate synthase in extracts of carrot cells cultures. Biochem. Biophys. Res. Commun. 1974, 58, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- de Souza, S.C.R.; Sodek, L.; Polacco, J.C.; Mazzafera, P. Urease deficiency alters nitrogen metabolism and gene expression in urease-null soybean without affecting growth or productivity under nitrate supply. Acta Physiol. Plant. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Agliassa, C.; Mannino, G.; Molino, D.; Cavalletto, S.; Contartese, V.; Bertea, C.M.; Secchi, F. A new protein hydrolysate-based biostimulant applied by fertigation promotes relief from drought stress in Capsicum annuum L. Plant Physiol. Biochem. PPB 2021, 166, 1076–1086. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, P.S.; Jaworskki, E.G. Patterns of nitrogen utilization in the soybean. Planta 1975, 127, 133–147. [Google Scholar] [CrossRef]
- Balboa, G.R.; Ciampitti, I.A. Estimating biological nitrogen fixation in field-grown soybeans: Impact of B value. Plant Soil 2020, 446, 195–210. [Google Scholar] [CrossRef]
- Taiz, L.; Møller, I.A.; Murphy, A.; Zeiger, E. Plant Physiology and Development, 7th ed.; 2022; ISBN: 9780197577240.
- Mamatha, B.C.; Rudresh, K.; Karthikeyan, N.; Kumar, M.; Das, R.; Taware, P.B.; Khapte, P.S.; Soren, K.R.; Rane, J.; Gurumurthy, S. Vegetal protein hydrolysates reduce the yield losses in off-season crops under combined heat and drought stress. Physiol. Mol. Biol. Plants 2023, 29, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Reichert, J.L.; Costa, E.C. Desfolhamentos contínuos e seqüenciais simulando danos de pragas sobre a cultivar de soja BRS 137. Ciência Rural 2003, 33, 1–6. [Google Scholar] [CrossRef]
- Tagliapietra, E.L.; Streck, N.A.; da Rocha, T.S.M.; Richter, G.L.; da Silva, M.R.; Cera, J.C.; Guedes, J.V.C.; Zanon, A.J. Optimum leaf area index to reach soybean yield potential in subtropical environment. Agron. J. 2018, 110, 932–938. [Google Scholar] [CrossRef]
- Francesca, S.; Najai, S.; Zhou, R.; Decros, G.; Cassan, C.; Delmas, F.; Ottosen, C.-O.; Barone, A.; Rigano, M.M. Phenotyping to dissect the biostimulant action of a protein hydrolysate in tomato plants under combined abiotic stress. Plant Physiol. Biochem. 2022, 179, 32–43. [Google Scholar] [CrossRef]
- Matsuo, S.; Kikuchi, K.; Fukuda, M.; Honda, I.; Imanishi, S. Roles and regulation of cytokinins in tomato fruit development. J. Exp. Bot. 2012, 63, 5569–5579. [Google Scholar] [CrossRef]
- Aremu, A.O.; Plačková, L.; Gruz, J.; Bíba, O.; Novák, O.; Stirk, W.A.; Doležal, K.; Van Staden, J. Seaweed-derived biostimulant (Kelpak®) influences endogenous cytokinins and bioactive compounds in hydroponically grown Eucomis autumnalis. J. Plant Growth Regul. 2016, 35, 151–162. [Google Scholar] [CrossRef]
- Zobiole, L.H.S.; Oliveira, R.S., Jr.; Constantin, J.; Oliveira JR, A.; Castro, C.; Oliveira, F.A.; Kremer, R.J.; Moreira, A.; Romagnoli, L.M. Acúmulo de nutrientes em soja convencional e soja RR em diferentes tipos de controle de planta daninha. Planta Daninha 2012, 30, 75–85. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolysed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Paradiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of growth, yield, and the nutraceutical and antioxidative potential of soybean through the use of synthetic biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).