Submitted:
19 June 2024
Posted:
21 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Iron is the most abundant transition metal in the human body: Iron is the most important, and the most abundant transition metal in the human body. It is an essential element for DNA synthesis, transporting oxygen from the lungs to all other organs, and critical in many heme-containing enzymatic activities [2]. The human body contains approximately 4 grams of iron. In comparison, the second most abundant transition metal, copper, has an average body content of around 80 milligrams. Relatively speaking, the iron content in our bodies is 50 times that of copper content [3].
- Iron is the best-known transition metal that catalyzes oxidant formation through Fenton reaction: Transition metals are a group of metals in the middle of the periodic table of elements. They are called this because they form a “transition” between the metals on the left side of the periodic table and the nonmetals on the right. Transition metals are versatile and can change valences very easily. For example, they can transition from ferrous ions to ferric ions and vice versa enabling crucial functions in producing oxygen free radicals known as Fenton reaction [4]. Fe2+ + H2O2 -----→ Fe3+ + OH- + OH•
- Iron proteins are colorful, and they could contribute to skin tone and discoloration: For instance, the red color of hemoglobin, an oxygen-transporting protein in red blood cells, is due to iron. Similarly, ferritin, which stores excess iron, appears brown. Sleep deprivation and UV exposure can exacerbate dark circles under the eyes, often partly due to hemoglobin deposits from leaking micro blood vessels [5]. Additionally, hemosiderin—a complex of hemoglobin and ferritin—contributes to various skin pigmentations [6], such as age spots and sunspots. Bruises represent another form of skin discoloration, resulting from blood pooling under the skin due to vessel damage, which leads to hemoglobin accumulation and visible discoloration [5].
- Iron is excreted through the skin and makes the skin an important target for oxidative damage: In human physiology, the body typically loses approximately 1-2 mg of iron daily. Employing whole-body counting techniques to monitor radioactive Fe59 following intravenous injection, it was discovered that one-third of body iron is excreted via the intestines, while two-thirds are eliminated through the skin [7]. These findings underscore the skin's critical role not only in maintaining iron homeostasis but also as a principal site of oxidative damage due to iron deposition. Consequently, the implications of iron for skin appearance and health necessitate further investigation. This highlights the skin's dual function in iron regulation and its susceptibility to iron-induced oxidative stress, impacting overall skin condition and tone.
- Iron contributes to photoaging: Research has shown that ferritin, an iron storage protein that can bind up to 4,500 iron atoms per molecule, undergoes degradation when exposed to UVA radiation. This exposure releases significant amounts of 'free' iron, which then facilitates the formation of oxidants [8]. Concurrently, the interaction between ferritin and UVA radiation also increases the production of matrix metalloproteinase-1 (MMP-1) [9], an enzyme linked to skin aging. The oxidative damage and enzymatic activity resulting from these processes are crucial in accelerating the aging of the skin, leading to increased wrinkle formation and skin thinning. These findings highlight the significant impact of iron metabolism and UVA exposure on skin health and emphasize the urgent need for targeted research to develop preventive strategies against these detrimental effects.
2. Diets and Lifestyle on Skin Aging
3. Diets and Lifestyle on Iron
4. Limitations and Perspectives
5. Conclusions
- Abbreviations: AGEs: Advanced glycation end products; MMP: matrix metalloproteinase; TNF-a: tumor necrosis factor-alpha
References
- Tang X, Yang T, Yu D, Xiong H, Zhang S: Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ Int 2024, 185:108535. [CrossRef]
- Zeidan RS, Martenson M, Tamargo JA, McLaren C, Ezzati A, Lin Y, Yang JJ, Yoon HS, McElroy T, Collins JF et al: Iron homeostasis in older adults: balancing nutritional requirements and health risks. J Nutr Health Aging 2024, 28(5):100212. [CrossRef]
- Podgorska A, Kicman A, Naliwajko S, Wacewicz-Muczynska M, Niczyporuk M: Zinc, Copper, and Iron in Selected Skin Diseases. Int J Mol Sci 2024, 25(7). [CrossRef]
- Muranov KO: Fenton Reaction in vivo and in vitro. Possibilities and Limitations. Biochemistry (Mosc) 2024, 89(Suppl 1):S112-S126. [CrossRef]
- Urakov A, Urakova N, Nikolenko V, Belkharoeva R, Achkasov E, Kochurova E, Gavryushova L, Sinelnikov M: Current and emerging methods for treatment of hemoglobin related cutaneous discoloration: A literature review. Heliyon 2021, 7(1):e05954.
- Runge JS, Nakamura M, Sullivan AN, Harms PW, Chan MP: Pigmented Purpuric Dermatosis of the Hand: Clinicopathologic Analysis of Six Cases With Review of the Literature. Am J Dermatopathol 2022, 44(8):553-558. [CrossRef]
- Weintraub LR, Demis DJ, Conrad ME, Crosby WH: Iron Excretion by the Skin. Selective Localization of Iron-59 in Epithelial Cells. Am J Pathol 1965, 46(1):121-127. [CrossRef]
- Pourzand C, Watkin RD, Brown JE, Tyrrell RM: Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A 1999, 96(12):6751-6756. [CrossRef]
- Jian J, Pelle E, Yang Q, Pernodet N, Maes D, Huang X: Iron sensitizes keratinocytes and fibroblasts to UVA-mediated matrix metalloproteinase-1 through TNF-alpha and ERK activation. Exp Dermatol 2011, 20(3):249-254. [CrossRef]
- Purba MB, Kouris-Blazos A, Wattanapenpaiboon N, Lukito W, Rothenberg EM, Steen BC, Wahlqvist ML: Skin wrinkling: can food make a difference? J Am Coll Nutr 2001, 20(1):71-80. [CrossRef]
- Boelsma E, van de Vijver LP, Goldbohm RA, Klopping-Ketelaars IA, Hendriks HF, Roza L: Human skin condition and its associations with nutrient concentrations in serum and diet. Am J Clin Nutr 2003, 77(2):348-355.
- Cosgrove MC, Franco OH, Granger SP, Murray PG, Mayes AE: Dietary nutrient intakes and skin-aging appearance among middle-aged American women. Am J Clin Nutr 2007, 86(4):1225-1231. [CrossRef]
- Nagata C, Nakamura K, Wada K, Oba S, Hayashi M, Takeda N, Yasuda K: Association of dietary fat, vegetables and antioxidant micronutrients with skin ageing in Japanese women. Br J Nutr 2010, 103(10):1493-1498. [CrossRef]
- Daniell HW: Smoker's wrinkles. A study in the epidemiology of "crow's feet". Ann Intern Med 1971, 75(6):873-880.
- Mekic S, Jacobs LC, Hamer MA, Ikram MA, Schoufour JD, Gunn DA, Kiefte-de Jong JC, Nijsten T: A healthy diet in women is associated with less facial wrinkles in a large Dutch population-based cohort. J Am Acad Dermatol 2019, 80(5):1358-1363 e1352.
- Rosa DF, Sarandy MM, Novaes RD, da Matta SLP, Goncalves RV: Effect of a high-fat diet and alcohol on cutaneous repair: A systematic review of murine experimental models. PLoS One 2017, 12(5):e0176240. [CrossRef]
- Huang X, Xu Y, Partridge NC: Dancing with sex hormones, could iron contribute to the gender difference in osteoporosis? Bone 2013, 55(2):458-460. [CrossRef]
- Romeu M, Aranda N, Giralt M, Ribot B, Nogues MR, Arija V: Diet, iron biomarkers and oxidative stress in a representative sample of Mediterranean population. Nutr J 2013, 12:102. [CrossRef]
- Zhang Y, Li Q, Rao E, Sun Y, Grossmann ME, Morris RJ, Cleary MP, Li B: Epidermal Fatty Acid binding protein promotes skin inflammation induced by high-fat diet. Immunity 2015, 42(5):953-964. [CrossRef]
- Rosa DF, Sarandy MM, Novaes RD, Freitas MB, do Carmo Gouveia Peluzio M, Goncalves RV: High-Fat Diet and Alcohol Intake Promotes Inflammation and Impairs Skin Wound Healing in Wistar Rats. Mediators Inflamm 2018, 2018:4658583. [CrossRef]
- Higashi Y, Yamakuchi M, Fukushige T, Ibusuki A, Hashiguchi T, Kanekura T: High-fat diet exacerbates imiquimod-induced psoriasis-like dermatitis in mice. Exp Dermatol 2018, 27(2):178-184. [CrossRef]
- Marino MG, Carboni I, De Felice C, Maurici M, Maccari F, Franco E: Risk factors for psoriasis: a retrospective study on 501 outpatients clinical records. Ann Ig 2004, 16(6):753-758. [CrossRef]
- Herbert D, Franz S, Popkova Y, Anderegg U, Schiller J, Schwede K, Lorz A, Simon JC, Saalbach A: High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J Invest Dermatol 2018, 138(9):1999-2009. [CrossRef]
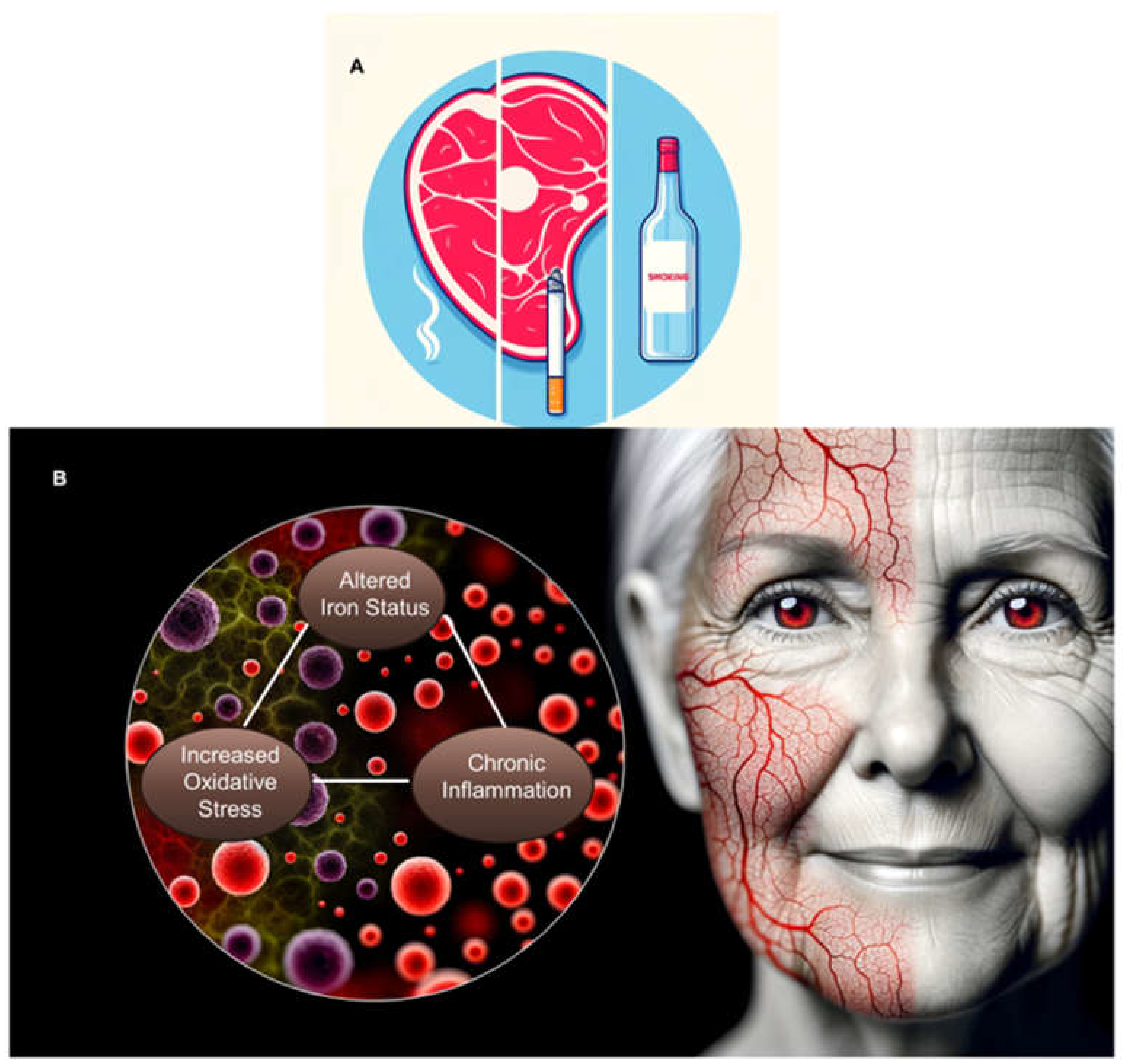
- Vaid M, Singh T, Prasad R, Katiyar SKFigure 1. Schematic representation of a holistic model of how extrinsic factors such as diet and lifestyle (A) interact with intrinsic factors (B) like altered iron status to influence skin appearance. Collectively, these elements may lead to increased oxidative stress and chronic inflammation, which in turn may impact skin health. The model suggests that the interplay between chronic inflammation and iron imbalance could sustain a cycle of oxidative stress, potentially accelerating skin aging. This holistic perspective underscores that merely targeting oxidative stress with antioxidant therapy may be insufficient. A comprehensive approach that also addresses iron levels and inflammation is likely essential for notably improving skin appearance: Intake of high-fat diet stimulates the risk of ultraviolet radiation-induced skin tumors and malignant progression of papillomas to carcinoma in SKH-1 hairless mice. Toxicol Appl Pharmacol 2014, 274(1):147-155.
- Danby FW: Nutrition and aging skin: sugar and glycation. Clin Dermatol 2010, 28(4):409-411.
- Draelos ZD: Aging skin: the role of diet: facts and controversies. Clin Dermatol 2013, 31(6):701-706. [CrossRef]
- Nguyen HP, Katta R: Sugar Sag: Glycation and the Role of Diet in Aging Skin. Skin Therapy Lett 2015, 20(6):1-5.
- Ortiz A, Grando SA: Smoking and the skin. Int J Dermatol 2012, 51(3):250-262.
- Ernster VL, Grady D, Miike R, Black D, Selby J, Kerlikowske K: Facial wrinkling in men and women, by smoking status. Am J Public Health 1995, 85(1):78-82. [CrossRef]
- Sandby-Moller J, Poulsen T, Wulf HC: Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol 2003, 83(6):410-413. [CrossRef]
- Doshi DN, Hanneman KK, Cooper KD: Smoking and skin aging in identical twins. Arch Dermatol 2007, 143(12):1543-1546. [CrossRef]
- Martires KJ, Fu P, Polster AM, Cooper KD, Baron ED: Factors that affect skin aging: a cohort-based survey on twins. Arch Dermatol 2009, 145(12):1375-1379. [CrossRef]
- Ichibori R, Fujiwara T, Tanigawa T, Kanazawa S, Shingaki K, Torii K, Tomita K, Yano K, Osaka Twin Research G, Sakai Y et al: Objective assessment of facial skin aging and the associated environmental factors in Japanese monozygotic twins. J Cosmet Dermatol 2014, 13(2):158-163.
- Chung JH, Lee SH, Youn CS, Park BJ, Kim KH, Park KC, Cho KH, Eun HC: Cutaneous photodamage in Koreans: influence of sex, sun exposure, smoking, and skin color. Arch Dermatol 2001, 137(8):1043-1051.
- Kadunce DP, Burr R, Gress R, Kanner R, Lyon JL, Zone JJ: Cigarette smoking: risk factor for premature facial wrinkling. Ann Intern Med 1991, 114(10):840-844. [CrossRef]
- Leung WC, Harvey I: Is skin ageing in the elderly caused by sun exposure or smoking? Br J Dermatol 2002, 147(6):1187-1191. [CrossRef]
- Rexbye H, Petersen I, Johansens M, Klitkou L, Jeune B, Christensen K: Influence of environmental factors on facial ageing. Age Ageing 2006, 35(2):110-115. [CrossRef]
- Just M, Ribera M, Monso E, Lorenzo JC, Ferrandiz C: Effect of smoking on skin elastic fibres: morphometric and immunohistochemical analysis. Br J Dermatol 2007, 156(1):85-91.
- Lahmann C, Bergemann J, Harrison G, Young AR: Matrix metalloproteinase-1 and skin ageing in smokers. Lancet 2001, 357(9260):935-936. [CrossRef]
- Morita A: Tobacco smoke causes premature skin aging. J Dermatol Sci 2007, 48(3):169-175. [CrossRef]
- Morita A, Torii K, Maeda A, Yamaguchi Y: Molecular basis of tobacco smoke-induced premature skin aging. J Investig Dermatol Symp Proc 2009, 14(1):53-55. [CrossRef]
- Yin L, Morita A, Tsuji T: Tobacco smoke extract induces age-related changes due to modulation of TGF-beta. Exp Dermatol 2003, 12 Suppl 2:51-56. [CrossRef]
- Smith JB, Fenske NA: Cutaneous manifestations and consequences of smoking. J Am Acad Dermatol 1996, 34(5 Pt 1):717-732; quiz 733-714. [CrossRef]
- Goodman GD, Kaufman J, Day D, Weiss R, Kawata AK, Garcia JK, Santangelo S, Gallagher CJ: Impact of Smoking and Alcohol Use on Facial Aging in Women: Results of a Large Multinational, Multiracial, Cross-sectional Survey. J Clin Aesthet Dermatol 2019, 12(8):28-39.
- Lobo AR, Gaievski EHS, de Mesquita CH, De Carli E, Teixeira PDS, Pereira RMR, Borelli P, de Sa LRM, Colli C: Increased adiposity by feeding growing rats a high-fat diet results in iron decompartmentalisation. Br J Nutr 2020, 123(10):1094-1108. [CrossRef]
- Yamano N, Ikeda Y, Sakama M, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, Miyamoto L, Tomita S, Tsuchiya K, Tamaki T: A long-term high-fat diet changes iron distribution in the body, increasing iron accumulation specifically in the mouse spleen. J Nutr Sci Vitaminol (Tokyo) 2015, 61(1):20-27. [CrossRef]
- Basak T, Kanwar RK: Iron imbalance in cancer: Intersection of deficiency and overload. Cancer Med 2022, 11(20):3837-3853. [CrossRef]
- Petzer V, Theurl I, Weiss G: Established and Emerging Concepts to Treat Imbalances of Iron Homeostasis in Inflammatory Diseases. Pharmaceuticals (Basel) 2018, 11(4). [CrossRef]
- Chen CY, Zhang JQ, Li L, Guo MM, He YF, Dong YM, Meng H, Yi F: Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front Med (Lausanne) 2022, 9:837222. [CrossRef]
- Ghio AJ, Hilborn ED, Stonehuerner JG, Dailey LA, Carter JD, Richards JH, Crissman KM, Foronjy RF, Uyeminami DL, Pinkerton KE: Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am J Respir Crit Care Med 2008, 178(11):1130-1138. [CrossRef]
- Lee CH, Goag EK, Lee SH, Chung KS, Jung JY, Park MS, Kim YS, Kim SK, Chang J, Song JH: Association of serum ferritin levels with smoking and lung function in the Korean adult population: analysis of the fourth and fifth Korean National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis 2016, 11:3001-3006. [CrossRef]
- Whitfield JB, Zhu G, Heath AC, Powell LW, Martin NG: Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res 2001, 25(7):1037-1045. [CrossRef]
- Harrison-Findik DD: Role of alcohol in the regulation of iron metabolism. World J Gastroenterol 2007, 13(37):4925-4930. [CrossRef]
- Paulke A, Sohling N, Held H, Wurglics M, Skopp G, Toennes SW: Chronic alcohol abuse may lead to high skin iron content, but not to hepatic siderosis. Forensic Sci Int 2019, 304:109851. [CrossRef]
- Kuprys PV, Tsukamoto H, Gao B, Jia L, McGowan J, Coopersmith CM, Moreno MC, Hulsebus H, Meena AS, Souza-Smith FM et al: Summary of the 2018 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol 2019, 77:11-18. [CrossRef]
- Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT: Dietary Advanced Glycation End Products and Risk Factors for Chronic Disease: A Systematic Review of Randomised Controlled Trials. Nutrients 2016, 8(3):125. [CrossRef]
- Baylis D, Bartlett DB, Patel HP, Roberts HC: Understanding how we age: insights into inflammaging. Longev Healthspan 2013, 2(1):8. [CrossRef]
- Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K: Oxidative stress in aging human skin. Biomolecules 2015, 5(2):545-589.
- Rahman K: Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2007, 2(2):219-236.
- Schagen SK, Zampeli VA, Makrantonaki E, Zouboulis CC: Discovering the link between nutrition and skin aging. Dermatoendocrinol 2012, 4(3):298-307. [CrossRef]
- Liu J, Chen T, Zhao Y, Ding Z, Ge W, Zhang J: Blood donation improves skin aging through the reduction of iron deposits and the increase of TGF-beta1 in elderly skin. Mech Ageing Dev 2022, 205:111687. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




