1. Introduction
Infective endocarditis (IE) is a condition that has not experienced a significant decline in mortality rates despite advancements in current treatment, early diagnosis, and treatment efforts in recent decades. Prosthetic valve infective endocarditis was initially identified in the 1950s [
1] and has been the focus of numerous studies reporting extremely high mortality rates among these patients.
Research suggests that the mitral and aortic valves are most commonly affected, while the tricuspid valve is least commonly affected [
1,
2]
Bacteria are the primary cause of prosthetic valve endocarditis (PVE), although fungal species can also lead to IE. [
2,
3,
4]
S. aureus, E. faecalis, and
Coagulase-negative Staphylococci are the most commonly implicated bacteria in the pathogenesis of IE [
5].
One of the primary objectives of enhancing the survival rate of patients with PVE is to promptly identify the infectious agent and initiate antibiotic treatment as soon as possible.
The current research indicates that identifying the causative agent and initiating appropriate treatment at an early stage are crucial for improving outcomes among patients in this category.
Delays in both processes are associated with significantly high mortality rates [
4,
5].
Surgical management of patients diagnosed with PVE typically targets cases in which significant vegetation, prosthetic valve infections, valve dysfunction, or advanced cardiac insufficiency are identified through sonography. Frequently, when antibiotics fail to alleviate septic symptoms and persist for over a week, surgical intervention involving a prosthetic valve is usually warranted.
S. aureus is still associated with high mortality rates in patients with PVE, as well as early complications such as congestive heart failure (CHF) [
4,
5,
6].
Postoperative mortality rates in this category remained high, indicating virulence of the infectious pathogen. The prognosis for these individuals is challenging to evaluate, as it can fluctuate significantly depending on the specific infectious agent present.
2. Material and Method
The data for this retrospective cohort study were sourced from the electronic records of individuals admitted to the Infectious Diseases Department of the “Carol Davila” Central Military Emergency University Hospital in Bucharest between January 1, 2017, and December 31, 2021. The inclusion criterion for the present study was a definitive diagnosis of PVE. During this timeframe, our hospital treated 78 patients with PVE. Among these patients, 28 (35.8%) experienced symptoms of infective endocarditis within the first 12 months after surgery (early onset), while 50 (64.2%) developed symptoms more than 12 months after surgery (late onset).
Our study comprised 78 subjects, all of whom were included in the present investigation. The diagnosis of PVE was established according to the modified Duke criteria [
7]. Three sets of blood cultures were obtained from each patient, with a minimum interval of 30 min between each set and a maximum of 24 h.
Blood cultures were collected, and patients were administered empirical broad-spectrum antibiotic therapy.
Following the receipt of blood culture results at an average interval of 3-5 days, antibiotic therapy was directed by an antibiogram. The decision to administer combined therapy, comprising medical treatment and surgery, was made each time by a multidisciplinary team that included an infectious disease specialist, cardiologist, and cardiovascular surgeon.
The study was approved by the Ethics Committee of the Central Military Emergency Hospital “. Carol Davila” Bucharest (Decision no. 562/20.12.2022).
Data Management and Statistical Analysis
Frequency tables and descriptive statistics (mean and standard deviation) were used to analyze patient information, and two non-parametric statistical tests, the Mann-Whitney test and Kruskal-Wallis test, were applied to reveal gender and age differences in patient medical characteristics. These non-parametric tests have the main advantage of not making any assumptions about the shape of the distribution of the population from which the sample was drawn. The results of the Kruskal-Wallis test were expressed as an H-statistic and a p-value. The p-value represents the probability of obtaining differences as large or larger than those observed in our data, if the null hypothesis is true. If the p-value is less than the predefined significance level of 0.05, we can reject the null hypothesis and conclude that there are significant differences between at least two groups. SPSS software was used to conduct the statistical analyses.
3. Results
This study included 78 patients. The patient distribution per year was as follows: 23 patients in 2017, 20 in 2018, 10 in 2019, 13 in 2020, and 12 in 2021. With regard to age, the majority of patients fell within the range of 61 to 80 years (74.4%). Of the patients, 14.2% were aged between 41 and 60 years and 3.84% were aged between 21 and 40 years. Only 7.6% of the patients were > 80 years of age. Participants were aged between 25 and 95 years, with a mean age of 66 years.
Upon admission, fever was a common symptom among all patients. In terms of hospitalization, the majority of the patients (66.4%) required hospitalization for 31–43 days, while 33.6% required more than 46 days of hospitalization.
The shortest hospital stay was 3 days, while the longest was 96 days, with an average hospital stay of 34.8 days.
Evolutionarily, 27 patients (34.7%) were admitted to the ICU, with an average duration of 11 days. The main characteristics of the male and female patients included in this study are presented in
Table 1 and
Table 2, respectively.
3.1. Analysis of the Distribution of Aetiological Agents in Patients with PVE
Etiological agents isolated from the blood cultures were identified in all 78 patients included in the study.
S. aureus was the most prevalent (38.5%). The remaining etiological agents were
E. faecalis (26.9%),
Streptococcus mitis (10.3%),
Escherichia Coli (7.7%),
Staphylococcus epidermidis (6.3%),
Streptococcus sanguinis (5.1%),
Streptococcus gallolyticus (1.3%),
Streptococcus pseudoporcinus (1.3%),
Enterobacter cloacae (1.3%), and
Klebsiella pneumoniae (1.3%). (
Table 3)
The analysis of the gender distribution of the most prevalent infectious agent demonstrated that 36.1% of male patients and 41.9% of female patients had positive S. aureus blood cultures, as shown in
Table 4.
Upon examining the dissemination of pathogenic organisms by age, it was found that the majority of patients in whom the primary causative factors were detected belonged to the 61-80 age cohort. (
Table 5)
3.2. Disease Onset
Examination of the onset of PVE indicated that 28 patients (35.8%) experienced early onset PVE (within less than 12 months after surgery). Among these patients, 18 (64.2%) were male and 10 (35.3%) were female.
Examination of the affected valves in patients with early onset disease revealed that the aortic valve was affected in 21 patients (67.9%) of which 13 (84.2%) were male, and 8 (15.8%) were female. The mitral valve was affected in 7 patients (32.1%), of which 5 (55.6%) were male, and 2 (44.4%) were female.
Late-onset disease, which developed more than 12 months after surgery, represented 50 of the patients included in the study (64.2%), with gender distribution of 29 males (58%) and 21 females (42%). Examination of the affected valves in individuals with late-onset disease revealed that 31 patients (62%) had an affected aortic valve, of which 17 (54.8%) were male and 14 (42.2%) were female. Additionally, 19 patients (38%) had an affected mitral valve, including 12 (63.2%) being male and 7 (36.8%) being female.
The frequency of etiological agents according to the disease onset was also analyzed. The data are summarized in
Table 6.
The Mann-Whitney U Test was employed to assess whether any noticeable distinctions existed between patient groups infected with specific pathogens. With regards to S. aureus, the p-value of 0.907 surpasses the conventional significance level of 0.05 (or 5%). On the other hand, for E. faecalis, the p-value of 0.054 falls below the standard significance level, thereby suggesting that there are notable discrepancies between patient groups affected by PVE caused by this pathogen.
3.3. Analysis of Secondary Diagnoses
Among the patients with PVE (n=78), the majority had secondary diagnoses, such as mitral valve insufficiency (36.3%), aortic valve insufficiency (31.2%), congestive heart failure (25.7%), and essential (primary) hypertension (29.4%).
The distribution of secondary diagnoses according to the age of the patients in the study revealed that the most affected age group was 61-80 years, followed by the age group 41-60 years.
Upon analyzing the distribution of the most prevalent secondary diagnoses for the age group of 61-80 years, it was found that mitral insufficiency occurred in 73.2% of cases, aortic insufficiency in 65.2%, and congestive heart failure in 70.2%. Additionally, essential hypertension was associated with this age group in 78.1% of cases (
Table 7).
Analysis of the secondary diagnoses by agent type revealed that S. aureus and E. faecalis were the most prevalent infectious agents.(
Table 8)
The Kruskal-Wallis test results, which examine variations in the prevalence of secondary diagnoses across the primary age categories, indicate significant differences in cases of mitral insufficiency and congestive heart failure among the different age groups. Specifically, the probability associated with the test is below the maximum significance threshold of 10%. Consequently, patients aged between 61 and 80 are the most likely to have secondary diagnoses such as mitral insufficiency, aortic insufficiency and essential hypertension.
3.4. Evaluation of Prosthetic Valve Pathology
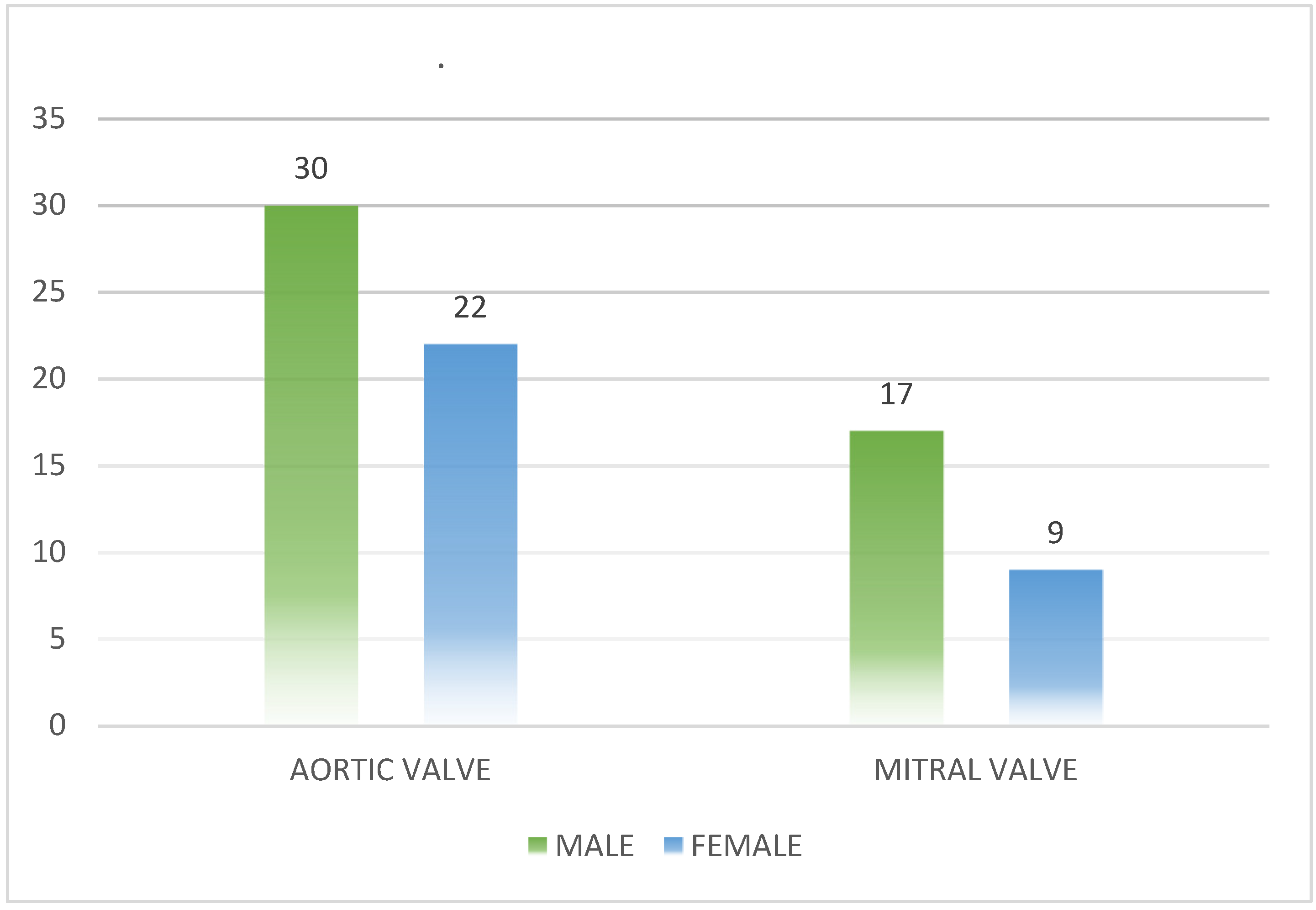
According to the evaluation of prosthetic valve pathology, all 78 patients in this study had metallic prosthetic valves. Upon analyzing the heart damage in these patients, it was found that the left heart was the most affected, with the aortic valve being affected in 52 patients (66.7%) and the mitral valve in 26 patients (33.3%). In terms of gender analysis of prosthetic valve damage, it was observed that in the case of aortic valve damage, 30 patients (57.7%) were male and 22 (42.3%) were female. For mitral valve damage, 17 patients (65,4%) were male and 9 patients (34.6%) were female.The data are summarized in
Figure 1.
3.5. Treatment
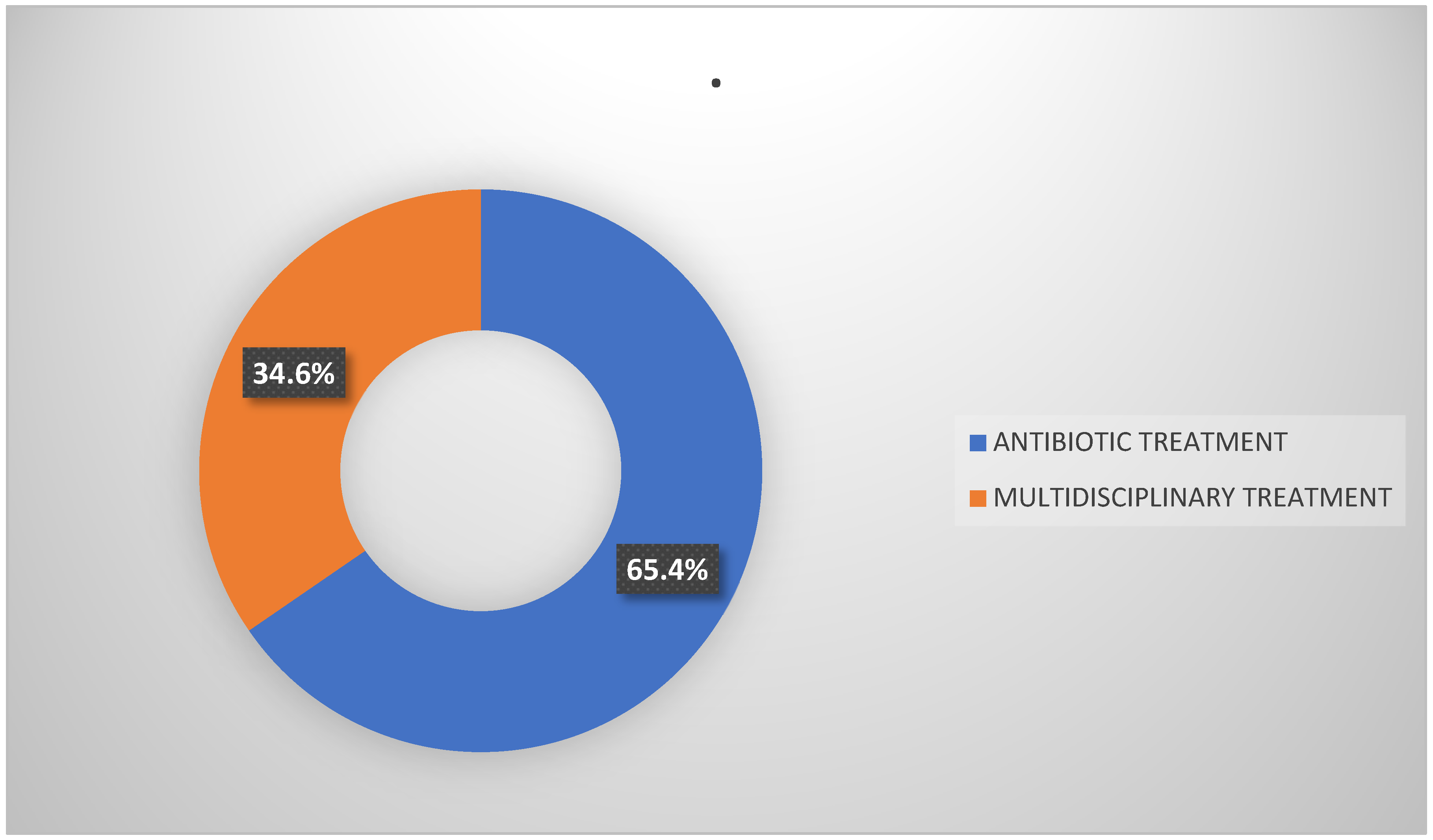
Among the individuals included in the study, 51 (65.4%) received antibiotic treatment alone. Among them, 27 (52.9%) were male and 24 (47.1%) were female. In addition, 27 patients (34.6%) received a combination of medical and surgical interventions. Of these, 20 patients (74.1%) were male and 7 patients (25.9%) were female (
Figure 2).
It is noteworthy that 74.1% of surgical patients were aged 61–80 years. The data are summarized in
Figure 2.
Patients who underwent valve reoperation were older than those who received only medical therapy (27 patients, 60 ± 20 years vs. 45 ± 23 years; p < 0.001) and had a higher proportion of male patients (74.1% vs. 25.9%; p < 0.005).
In contrast, patients who underwent surgery had a higher incidence of S. aureus PVE (41% vs. 35%; p = 0.002).
All patients included in the studies received antibiotic therapy regardless of whether they were treated with medical or surgical therapy. The mean time from diagnosis to valve reoperation in the surgical group was 13 days.
In comparison with medical therapy, valve reoperation outcomes were associated with a lower incidence of 60-day mortality. This observation was based on recent studies.
Specifically, in individuals with left-sided PVE, valve reoperation was also demonstrated to be associated with a lower risk of 60-day mortality than medical therapy alone.
3.6. Mortality Rate
Upon analyzing the mortality rate of the patient population included in the study, 28 deaths (35.9% of the total) were recorded. Of these, 17 patients (60.7%) were male and 11 (39.3%) were female.
In terms of the mortality rate based on treatment, the following findings were observed: among patients receiving medical therapy only, 20 deaths (39.2%) were recorded. Of these, 12 patients (60%) were male, and 8 patients (40%) were female.
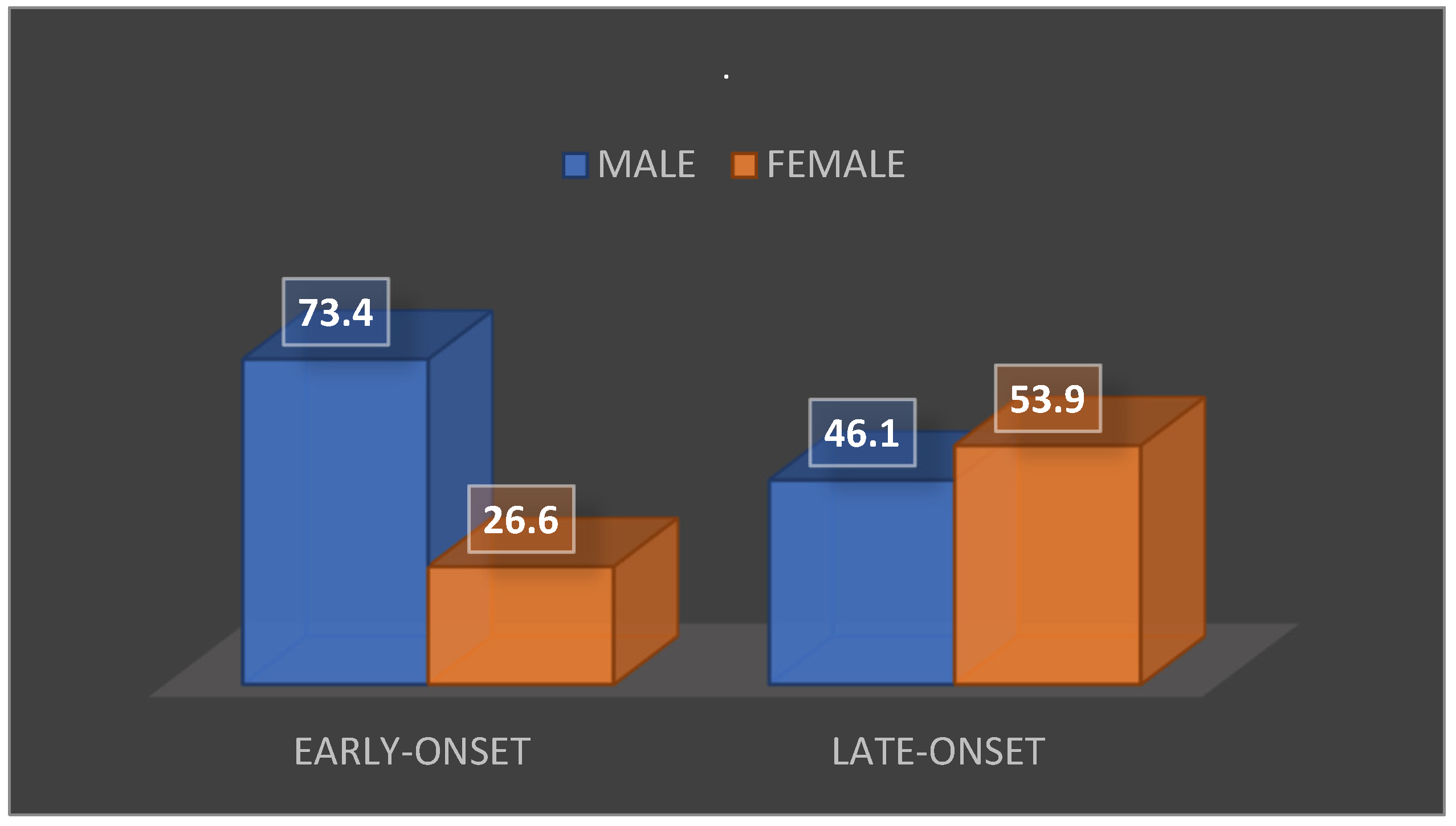
Among the patients who received both surgical and medical therapy, eight deaths (representing 29.6% of the total) were recorded. Of these, five patients (62.5%) were male and three (37.5%) were female. When analyzing the mortality rate according to the time of onset of IE episodes, the following was found: among patients with early onset of the disease (28 patients), 15 deaths (53.6%) were recorded. Of these, 11 patients (73.4%) were male and 4 patients (26.6%) were female. Among the patients with late-onset disease (50 patients), there were 13 deaths (26%). Of these, 6 patients (46.1%) were male and 7 patients (53.9%) were female. The data are summarized in
Figure 3.
4. Discussions
This study aimed to analyze and evaluate the etiology, clinical outcomes, and mortality rates associated with various bacterial pathogens that cause PVE in individuals who received surgical, antibiotic, and combined therapies.
Despite advancements in IE diagnosis and treatment of infective endocarditis, PVE remains a severe and potentially fatal complication. Consequently, a combination of medical theraphy and surgery is typically regarded as the preferred course of action in managing this condition.[
7]
Numerous studies have demonstrated the superiority of surgical treatment compared to a strategy solely reliant on antibiotics.[
8,
9,
10]
According to a more current investigation, the medical group experienced a 46% mortality rate, while the surgical group reported a 26% mortality rate.[
11]
In our study, we discovered that 35.8% of the patients had early onset PVE, while 64.2% had late-onset PVE. The prevalence of PVE in the aortic position was greater than that in the mitral position, at 67.9% compared with 32.1% in the early-onset group and 62% compared to 38% in the late-onset group, respectively. Our findings align with those of other studies that have reported similar rates of PVE [
12,
13,
14].
The primary etiological agent identified in our investigation was S. aureus, with
E. faecalis following closely behind. In both the early and late onset groups, S. aureus was the most prevalent infectious agent, with E. faecalis being the second most common in the early onset group and S. mitis in the late onset group.
It is worth noting that infection with
S. aureus is frequently cited as the primary cause of PVE in numerous epidemiological studies and is considered a high-risk subgroup within this population.[
15,
16]
In our study, the mortality rate was higher in the antibiotic group (39.3% vs. 29.6%), which is consistent with the majority of the studies. Despite the high rates observed in the antibiotic group (39.2%), our findings on in-hospital mortality (35.9% mortality rate) were comparable to data published in major studies. [
17,
18]
The relationship between the infectious agent
S. aureus and deterioration of clinical outcomes in infective endocarditis (IE) has been established. Over the course of a five-year study at our institution,
S. aureus was the most frequently identified causative microorganism in patients with prosthetic valve endocarditis (PVE). As a pathogen known for its aggressive nature,
S. aureus is often associated with severe clinical presentation. These findings are consistent with those of previous studies. [
19,
20,
21].
The key findings of the present study, which examined the efficacy of surgical intervention versus medical management in individuals with PVE, revealed that the surgical method was associated with a lower 60-day mortality rate than the medical therapy approach. Our results align with the outcomes reported in other studies.[
22,
23,
24]
5. Conclusions
The present investigation indicates a lower 60-day mortality rate and greater survival at follow-up for individuals with PVE who underwent valve reoperations than for those who received medical therapy. Moreover, secondary diagnosis, infectious agents, and age also affect survival rates.
Limitations
The current study has certain limitations that should be acknowledged. Firstly, the study was limited to only one hospital, which may have influenced the results. Future investigations should be conducted in a larger population to gain a more comprehensive understanding of patient outcomes in PVE.
Author Contributions
Conceptualization: C.-I. A., I. Ș.; methodology A. D.; software: A.-T. Ș., S. B.; validation: A. S.-C., Ș. I.; formal analysis: C.-I. A.; investigation: C.-I. A.; resources: I. Ș.; data curation: C.-I. A., writing, original draft preparation, C.-I. A., C.-A. B.; writing, review and editing: C.-I. A., visualization: S.-M. S.; supervision: C.-I. A.; project administration: I. Ș.. All authors have read and agreed to the published version of the manuscript.
Funding
This study received no external funding.
Informed Consent Statement
All patients who have been admitted and examined within our institution are invited to read and sign a document authorizing the department to anonymously analyze their data for medical research purposes, preserving the confidentiality of their identity.
Data Availability Statement
The data presented in this study are available upon request. The data are not publicly available because of the confidentiality of health data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Glaser, N.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Prosthetic Valve Endocarditis After Surgical Aortic Valve Replacement. Circulation. 2017, 136, 329–331 [PubMed]. [Google Scholar] [CrossRef] [PubMed]
- Glaser, N.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50-69 years.Eur Heart J. 2016, 37, 2658–2667. [Google Scholar] [CrossRef]
- Rajani, R.; Klein, J.L. Clin Med (Lond), Infective endocarditis: a contemporary update. 2020, 20, 31–35.
- Baddour LM, Wilson WR, Bayer AS, et al. , Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. 2015, 132, 1435–1486.
- Calderón-Parra J, Kestler M, Ramos-Martínez A, et al. Clinical factors associated with reinfection versus relapse in infective endocarditis: prospective cohort study. J Clin Med. 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Alicia Galar,corresponding authora, Ana A. Weil, David M. Dudzinski, Patricia Muñoz, Mark J. Siednerc, Methicillin-Resistant Staphylococcus aureus Prosthetic Valve Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management, Clin Microbiol Rev. 2019 Apr; 32(2): e00041-18.2019 Feb 13. [CrossRef]
- Brennan, J.M.; Edwards, F.H.; Zhao, Y.; O’Brien, S.; Booth, M.E.; Dokholyan, R.S.; Douglas, P.S.; Peterson, E.D. Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database.2013, 127, 1647–1655.
- Hassan Khalil, Shadi Soufi, Prosthetic Valve Endocarditis, StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. 2022 Dec 11.
- Meriem Drissa, Sana Helali, Marwa Chebbi, Khaled Ezzaouia, Fadwa Omri, Habiba Drissa, Prosthetic valve endocarditis: clinical, bacteriological and therapeutic aspects. Tunis Med. 2017, 95, 461–465.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis. Eur Heart J. 2015;36: 3075–123. 10. 1093.
- del Val, D. , Panagides V., Mestres C.A., Miró J.M., Rodés-Cabau J. Infective endocarditis after transcatheter aortic valve replacement: JACC state-of-the-art review. J Am Coll Cardiol. [CrossRef]
- Habib, G. , Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.-P., Del Zotti F., Dulgheru R., el Khoury G., Erba P.A., Iung B., et al. Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–312. [Google Scholar] [PubMed]
- Kreitmann, L.; Montaigne, D.; Launay, D.; Morell-Dubois, S.; Maillard, H.; Lambert, M.; Hachulla, E.; Sobanski, V. Clinical Characteristics and Outcome of Patients with Infective Endocarditis Diagnosed in a Department of Internal Medicine. J. Clin. Med. 2020;9:864. [CrossRef]
- Fernandez-Felix, B.M. , Barca L.V., Garcia-Esquinas E., Correa-Pérez A., Fernández-Hidalgo N., Muriel A., Lopez-Alcalde J., Álvarez-Diaz N., Pijoan J.I., Ribera A., et al. Prognostic models for mortality after cardiac surgery in patients with infective endocarditis: A systematic review and aggregation of prediction models. Clin. Microbiol. Infect. 2021;27:1422–1430. [CrossRef]
- Pettersson, G.B.; Hussain, S.T. Current AATS guidelines on surgical treatment of infective endocarditis. Ann. Cardiothorac. Surg. 2019;8:630–644. [CrossRef]
- Otto, C.M. , Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., Gentile F., Jneid H., Krieger E.V., Mack M., McLeod C., et al. Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines2020 ACC/AHA. [CrossRef]
- Williams JB, Shah AA, Zhang S, et al. Impact of Microbiological Organism Type on Surgically Managed Endocarditis. Ann Thorac Surg, 1325. [CrossRef]
- Cabezón, G.; López, J.; Vilacosta, I.; Sáez, C.; García-Granja, P.E.; Olmos, C.; Jerónimo, A.; Gutiérrez, A.; Pulido, P.; de Miguel, M.; et al. Reassessment of Vegetation Size as a Sole Indication for Surgery in Left-Sided Infective Endocarditis, J. Am. Soc. Echocardiogr. 2021. [CrossRef] [PubMed]
- Blerand Berisha, Sigurdur Ragnarsson, Lars Olaison, Magnus Rasmussen, Microbiological etiology in prosthetic valve endocarditis: A nationwide registry study, J Intern Med. 2022 Sep; 292(3): 428–437.2022 Apr 19. [CrossRef]
- Luciani I, Mossuto E, Ricci D, Luciani M, Russo M, Salsano A, et al. Prosthetic valve endocarditis: predictors of early outcome of surgical therapy. A multicentric study. Eur J Cardiothorac Surg. 2017;52:768–74.
- Han, S.M.; Sorabella, R.A.; Vasan, S.; Grbic, M.; Lambert, D.; Prasad, R.; Wang, C.; Kurlansky, P.; Borger, M.A.; Gordon, R.; et al. Influence of Staphylococcus aureus on Outcomes after Valvular Surgery for Infective Endocarditis, J. Cardiothorac. Surg. 2017;12:57. [CrossRef]
- Østergaard L, Valeur N, Ihlemann N, Smerup M, Bundgaard H, Gislason G, et al. Incidence and factors associated with infective endocarditis in patients undergoing left-sided heart valve replacement. Eur Heart J. 2018, 39, 2668. [Google Scholar] [CrossRef] [PubMed]
- Slipczuk L, Codolosa JN, Davila CD, et al. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One 2013;8:e82665.24.
- Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisci- plinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol 2013, 112, 1171–6. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).