1. Introduction
Hirschsprung´s disease (HSCR, incidence 1/5,000) or congenital megacolon is characterized by a local or general reduction or complete absence of the intrinsic gastrointestinal innervation, with individual variations from a local aganglionosis of the most distal colonic segments to a total aganglionosis [
1,
2]. Here the enteric nervous system (ENS) is completely absent or at least severely affected, resulting in varying grades of a- or hypoganglionosis. The compromised ENS leads to the impossibility of the intrinsic muscles to relax, while extrinsic innervation is still intact, thus resulting in a distal stenosis that impairs defecation and might lead to fatal co-morbidities such as toxic megacolon and enterocolitis.
HSCR is caused by a colonization failure of enteric precursor cells derived from neural crest (EPCs) to proliferate, migrate, survive or differentiate during ENS formation [
1,
3]. The regulation of this process is critical, and many different genes and proteins are involved in both migratory and colonization processes[
3]. Regarding genetics, HSCR shows a 4:1 male predominance, and a clear increased HSCR risk with Down syndrome [
1,
4]. The first HSCR-linked gene was RET kinase [
5], followed by endothelin receptor B (EDNRB) [
6], furthermore the combination of both mutations was reported to cause highly penetrant distal aganglionosis [
7]. Yet, in a prenatal diagnosis study [
8], fetuses carrying a RET variant did not develop any HSCR symptoms after years of follow-up. Thus, though RET gene is autosomal dominant; its mutation shows incomplete penetrance and does by itself not always lead to Hirschsprung diagnosis [
8]. In addition, a wide spectrum of mutations affecting many different genes (Plesin, ErbB, NTKR3, L-1CAM, etc.) has been associated to HSCR, confirming the multigenic inheritance and partial penetrance of the syndrome [
9,
10,
11,
12]. Nevertheless, the occurrence and severity of HSCR from many cases still remain unexplained by the genetics [
11,
12]. Thus, Hirschsprung is a multifactorial disease: although many genes influence HSCR occurrence, environmental factors could also impact the risk [
1,
9].
The ENS is closely linked to the local immune system, gastrointestinal macrophages, and dendritic cells within the intestinal wall. Neuroimmunological interactions and communications may be responsible for modulating physiological functions of the gastrointestinal tract (GIT), such as motility [
13]. The local immune system is in turn influenced by the microbiome [
14] and also influences the plasticity of the ENS [
15].
It is known that the ENS is not only affected by GIT disorders, it can be equally affected in systemic diseases such as diabetes, cancer or neurodegenerative diseases [
16]. For example, patients suffering from Parkinson's disease are known to be affected by motility disorders or gastric emptying disorders as the disease progresses [
17]. A hypothesis that PD has its first site of manifestation in the gastrointestinal tract is becoming increasingly established here [
18]. The brain and intestine are closely connected via the so-called brain-gut axis, and processes that take place in the intestine can also influence the brain [
18,
19,
20].
The NF-κB pathway consists in a family of transcription factors that can be found in most cells of the central and the peripheral nervous systems, mainly as NF-κB1/p50 homodimers and NF-κB1/RelA heterodimers [
21], which function as transcriptional activators in the canonical pro-inflammatory pathway [
22,
23,
24].
Indeed, the NF-κB pathway plays an important role in the structural and functional development of the nervous system [
21,
25]. Embryonic neurogenesis, neural progenitor migration and differentiation as well as synaptic signaling, neuroprotection, and neural plasticity are particularly regulated by the NF-κB system [
26,
27,
28].
Inflammatory and immune responses through NF-κB signaling are known to be implicated in many nervous system illnesses including neurodegenerative disorders such as Parkinson’s, Alzheimer’s, and Huntington’s diseases, multiple sclerosis, as well as neurodevelopmental diseases such as Hirschsprung disease [
16,
29]. Consequently, NF-κB signaling has been proposed as a therapeutic target for inflammatory neurodegeneration [
30,
31,
32].
In addition, the inflammatory environment specific immune cells (macrophages, dendritic cells) are a significant source of pro-inflammatory cytokines including IFNγ, IL-1, and TNFα, which induce inflammation through the NF-κB pathway [
33,
34]. Moreover, the inflammation itself plays a role in neurostimulation and enteric neuronal migration [
35,
36,
37,
38,
39], as well as in neuroregeneration through the NF-κB pathway [
40].
Altogether, the NF-κB pathway appears a relevant pathway for enteric neuronal migration and survival, and so, for a potential key role in HSCR disease development.
Therefore, in this study we have analyzed the expression of the main subunits of the NF-κB pathway, RelA/p65 and NFκB1/p50, together with other NF-κB-related and pro-inflammatory factors on HSCR samples, in an aim to analyze their potential role in Hirschsprung Disease.
2. Materials and Methods
2.1. Ethical Approval and Samples Collection
The collection and use of patient material have been performed according to informed consent signed by patients’ parents and approved by the “Medizinische Ethik-Kommission II” of the Medical Faculty Mannheim, University of Heidelberg (2011-237N-MA). Samples have only been identified by sequential code numbers with no other identifying details.
Colon tissue segments from Hirschsprung patients (27 samples) and from non-HSCR surgeries (8 samples from anastomosis) were obtained from the Pediatric Surgery Clinic at the University Hospital Clinic Mannheim, Germany.
Samples were divided into segments (A, B, C, D, etc.) indicating progressive HSCR pathology, from proximal end (closer to stomach, ganglionic healthy segment with normal innervation) to distal end (closer to rectum, aganglionic segment with pathological innervation). Each division was cut again in two parts, where one piece was immediately frozen in iso-pentane at (-80°C) and then used for RT-qPCR analysis; and the other piece shortly washed in PBS and fixed in 4% paraformaldehyde (PFA) for 24 hours, followed by paraffin embedment for immunohistochemical staining.
For the further experiments, from each patient the A segments (closer to the stomach) were considered as “proximal, ganglionic” and compared to their corresponding distal segment (closer to the rectum, usually D or afterwards), which were labeled as “distal, aganglionic with pathological innervation”.
2.2. RT-qPCR
27 HSCR patients (distal and proximal segments) and 7 non-HSCR samples (from anastomosis surgeries) were analyzed.
Tissue samples were diced in using a TissueLyser (Qiagen) and total RNA was extracted using TRIsure™ (BIO-38032, Bioline) and RNeasy Micro Kit (74004, Qiagen) following the manufacturer´s instructions. RNA concentration was measured in the Infinite M200 microplate reader (Tecan).
cDNA conversion was processed using a BioScript™ Reverse Transcriptase kit (BIO-27036, Bioline) and Random hexamer primers (BIO-38028, Bioline). The synthesis was done using a peqSTAR Thermocycler (PeqLab) as follows: 5 min. denaturation at 70°C, 10 min. annealing at 20°C, 60 min. elongation at 40°C and 10 min. inactivation at 70°C.
RT-qPCR reactions were performed with the SensiFASTTM SYBR Lo-ROX Kit from (BIO-94020, Bioline) using the QuantStudio 5 device (Applied Biosystems) as follows: 2 min. at 50°C, initial denaturation 10 min. at 95°C; 40 x cycles of (denaturation 15s. at 95°C, annealing 1 min. at 55°C), followed by a final Melting Curve of 15s. at 95°C, 1 min. at 55°C, 15s. at 95°C.
All experiments were performed in triplicates. Comparative 2-ΔΔCt method was used to calculate gene expression, where data was normalized to GAPDH as internal standard.
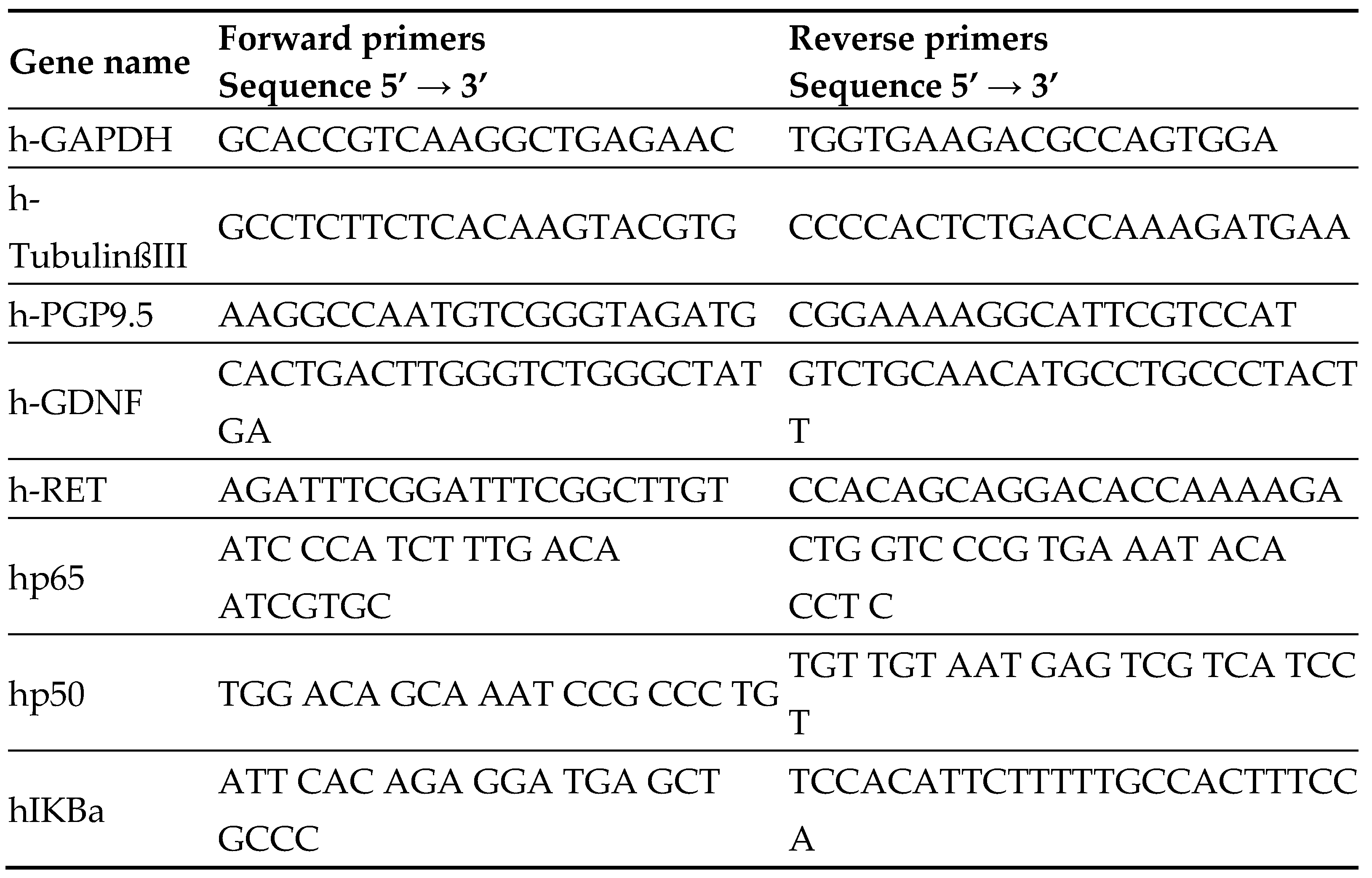
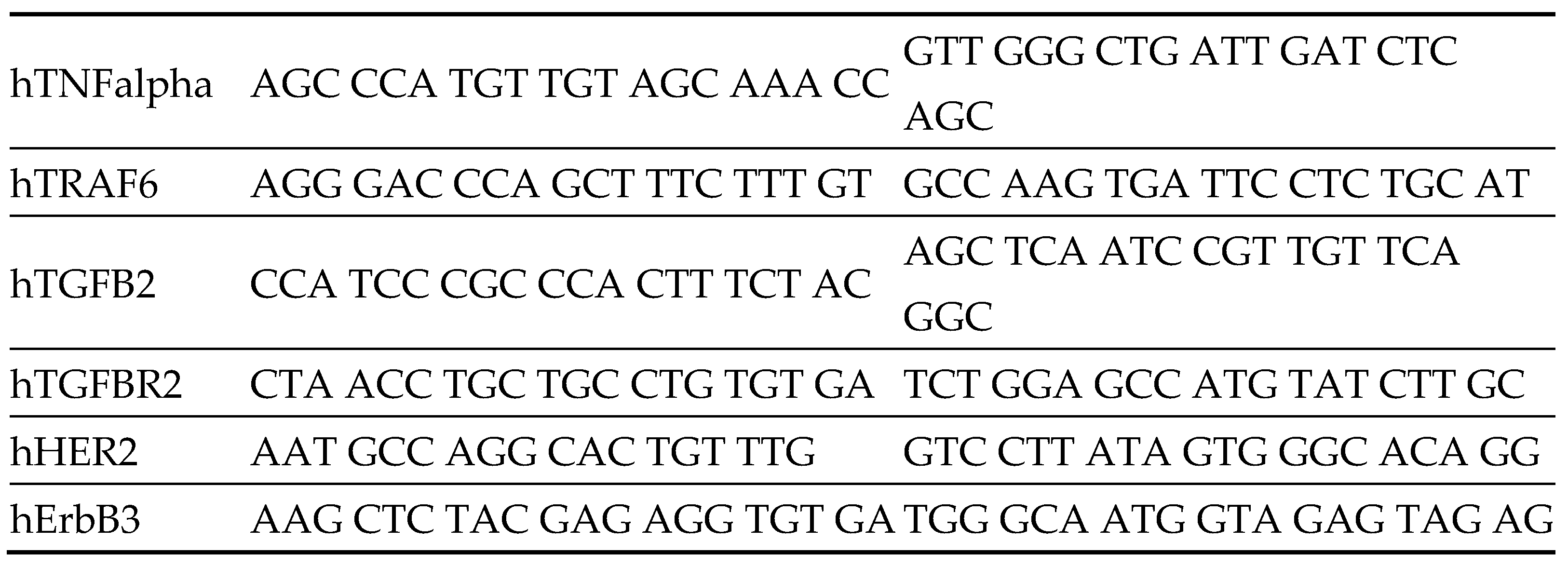
Table 1.
List of primers.
Table 1.
List of primers.
2.3. Immunohistochemistry
Colon samples of 25 HSCR patients (subdivided in proximal and distal segments, total: 50 samples) and 5 samples from non-HSCR surgeries (anastomosis) were analyzed. Tissue sections from proximal (A) and distal (from D on) segments were cut at 3 μm thickness using a microtome (Leica RM2245).
Briefly, samples were de-paraffinized and re-hydrated through serial washes in xylene (5 min. x 2 times), ethanol (100% 2 min. x2, 90% 2 min., 80% 2 min., 70% 1 min.), and PBS (3 min.). After the HIER (heat antigen epitope retrieval) 30 min. in sodium citrate buffer (pH 6.0), samples were permeabylized using 0,5% Triton X100-PBS for 10 min., then washed in PBS for 5 min., and blocked in 10% normal goat serum (NGS) in PBS at RT for 1h. Next, sections were incubated for 1h at RT with the corresponding anti-NF-κB subunit antibody along with Tubulin Beta III.
After 3 x 5 min. washes in PBS 0.005%Tween 20, the secondary fluorescence antibody was added for 1h at RT. Nuclear staining was done using (DAPI (Sigma, Ref: 9542) in PBS, 1:1000 for 3 min.) followed by 3X washes in PBS for 5 min. each. Finally, samples were shortly rinsed in distilled water, and directly mounted on Dako Fluorescence Mounting Medium (S3023; Agilent Technologies). All samples were stored at 4°C in the darkness until image acquisition.
The following antibodies were used: NF-κB/p65 (sc-8008, Santa Cruz Biotechnology), NF-κB1 p105/50 (D4P4D) (#13586, Cell Signaling Technology), Anti-Beta III Tubulin Antibody Alexa Fluor® 488 Conjugate (AB15708A4, Millipore), Anti-Tubulin β3 isoform Antibody (MAB1637, Millipore), Alexa Fluor® 488 (Goat Anti-Mouse # A-10667, Molecular Probes, Invitrogen, Life Technologies Corporation), Alexa Fluor® 568 (Goat Anti-Rabbit # A-11011, Goat Anti-Mouse # A-11004, Molecular Probes, Invitrogen, Life Technologies Corporation).
In parallel, tissue sections of 5 HSCR patients were co- stained using NF-kB/p65 (sc-8008, Santa Cruz Biotechnology) and the Leukocyte Common Antigen/ CD45 (GA751, Dako Agilent) following the protocol described above. DCS double-staining reagent (LD591R015 AP polymer, anti-mouse) was used for 30 min. at RT. Then, instead of the secondary fluorescence antibody, samples were incubated with Fast Red Bright Red (HK182-5KE, Biogenex Laboratories) for 20 min. at 37°C and finally mounted on Aquerous Mounting Medium (EL017R 120, Dako).
2.4. Image Acquisition and Analysis
Pictures of the fluorescence-stained samples were taken using a confocal laser scanning microscope (Leica TCS SP8) at 40X magnification.
Samples stained with fast red reagent were pictured using an inverted phase-contrast microscope (KEYENCE BZ-9000) at objective magnifications X20 and X60.
The image quantification was performed using Image J. The integrated density was calculated for p65 (red) and Tubulin Beta III (green) in both the proximal and distal sections of each sample. At least 3-6 pictures (x40) per HSCR section were quantified in each sample. In total, 300 images were analyzed for each staining (p65 and Tubulin Beta III).
2.5. Statistical Analysis
Mann–Whitney U test (Wilcoxon rank-sum test) was used to determine whether there was a statistically significant difference in the gene and protein expression levels between HSCR proximal (ganglionic) and distal (aganglionic) tissue samples. Student’s T test was used to compare differences in gene expression between distal and proximal segments of HSCR patients. Differences were considered statistically significant at p-value ≤0.05.
3. Results
3.1. HSCR Proximal Ganglionic Segments Show Increased p65 and Pro-Inflammatory Genes Expression Profiles
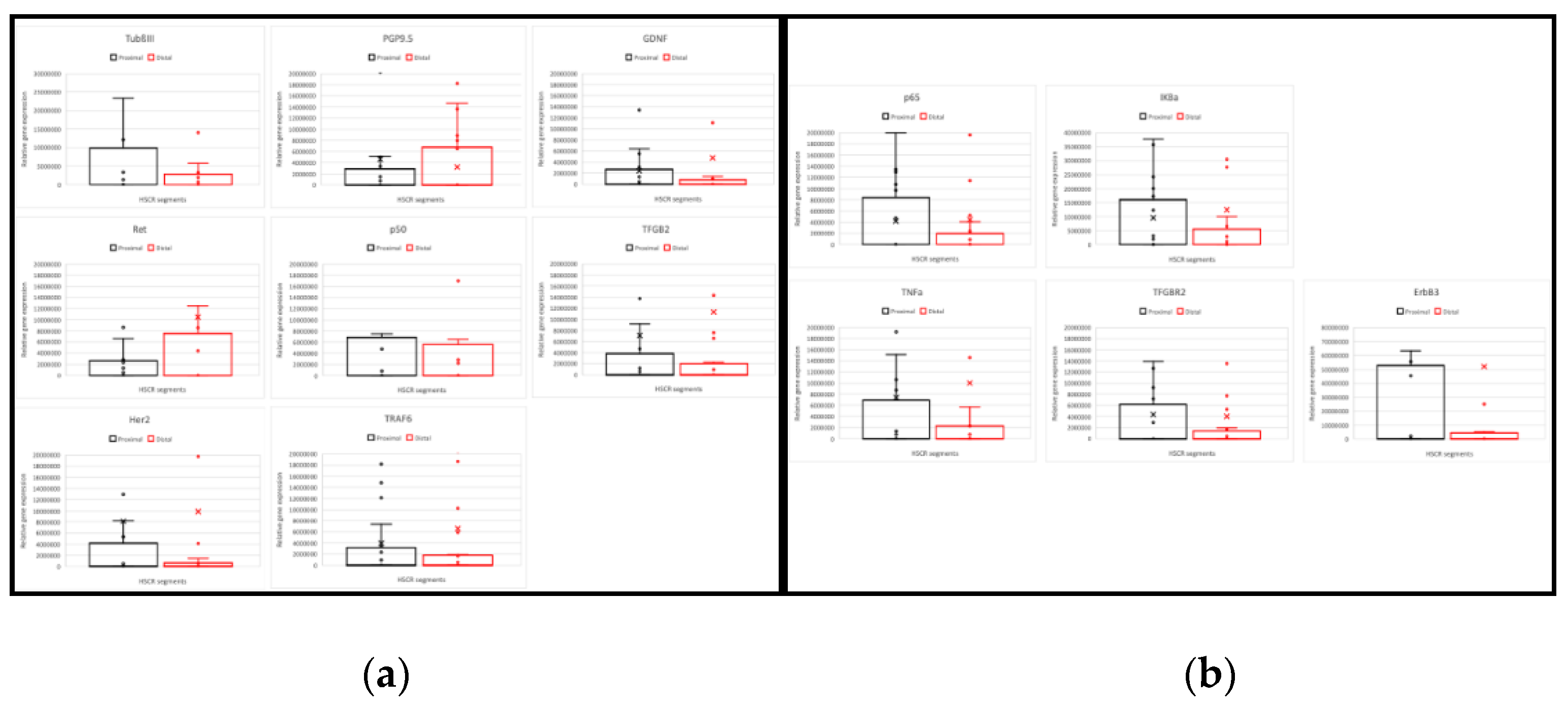
Firstly, we have examined the expression of genes that participate or are related to the NF-κB pathway, together with neuronal markers (
Figure 1a-b) in a cohort of 27 HSCR patients (in both proximal and distal colon sub-segments) and in further 7 non-HSCR colon surgeries samples (anastomosis) by RT-qPCR.
The analyzed genes were NF-κB pathway genes (RelA/ p65, NFκB1/p50, NF-κB Inhibitor Alpha (IκBa), Tumor Necrosis Factor-Alpha (TNF⍺), and TNF Receptor-Associated Factor 6 (TRAF6)), and other NF-κB pathway-related genes (the transforming growth factor Beta 2 (TGFB2), the transforming growth factor Beta 2 receptor (TGFBR2), HER2 and ErbB3), and also neuronal and glia markers together with HSCR-associated genes (Tubulin Beta 3 Class III (TubβIII), PGP9.5, RET, GDNF) [
41].
Briefly, our results show a loss of RelA/p65 gene expression in the distal (aganglionic) segments of HSCR patients compared to the proximal (ganglionic) ones. In addition, the expression of the NF-κB inhibitor (IκBα), the pro-inflammatory cytokine (TNF⍺), and TFGBR2 was slightly decreased in the distal segment (Figure1b).
We also observed lower Erbb3 levels in the distal segments compared to the proximal ones (Figure1b).
Though about 50% of HSCR patients do not express RET gene [
5], we did not detect RET loss in the HSCR cohort analyzed in our study (Figure1a).
Furthermore, no significant alterations were obtained in the expression of the other analyzed genes (GDNF, TRAF6, NFκB1/p50, HER2 and TGFB2) between the proximal and distal HSCR segments (Figure1b).
Altogether, we observed that the proximal ganglionic HSCR segments present higher levels of RelA/p65 and other pro-inflammatory factors than the distal aganglionic sections, which, maybe as secondary effect, also present less or dysfunctional enteric neurons.
3.2. p65 Protein Levels Are Higher in HSCR Proximal Ganglionic Segments
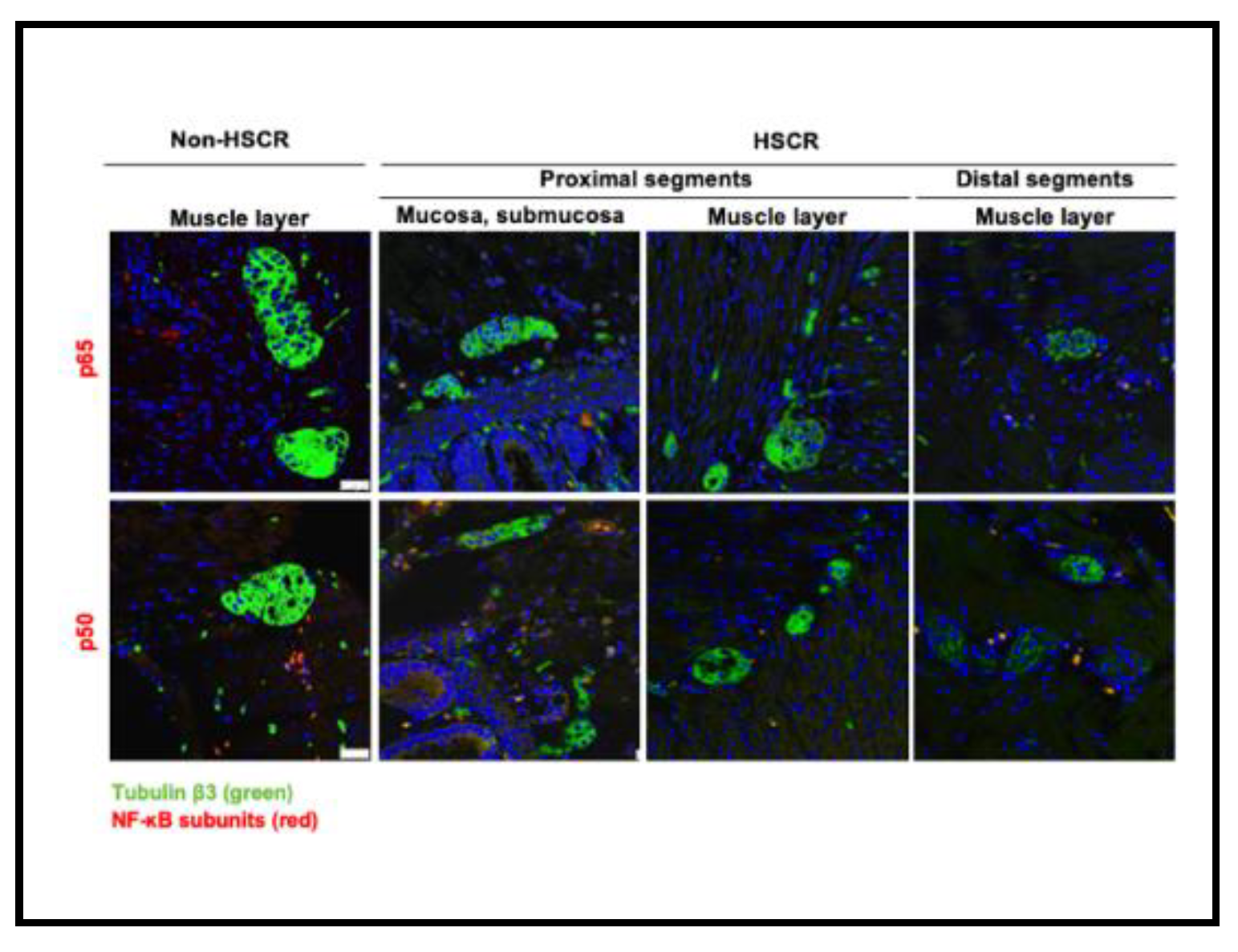
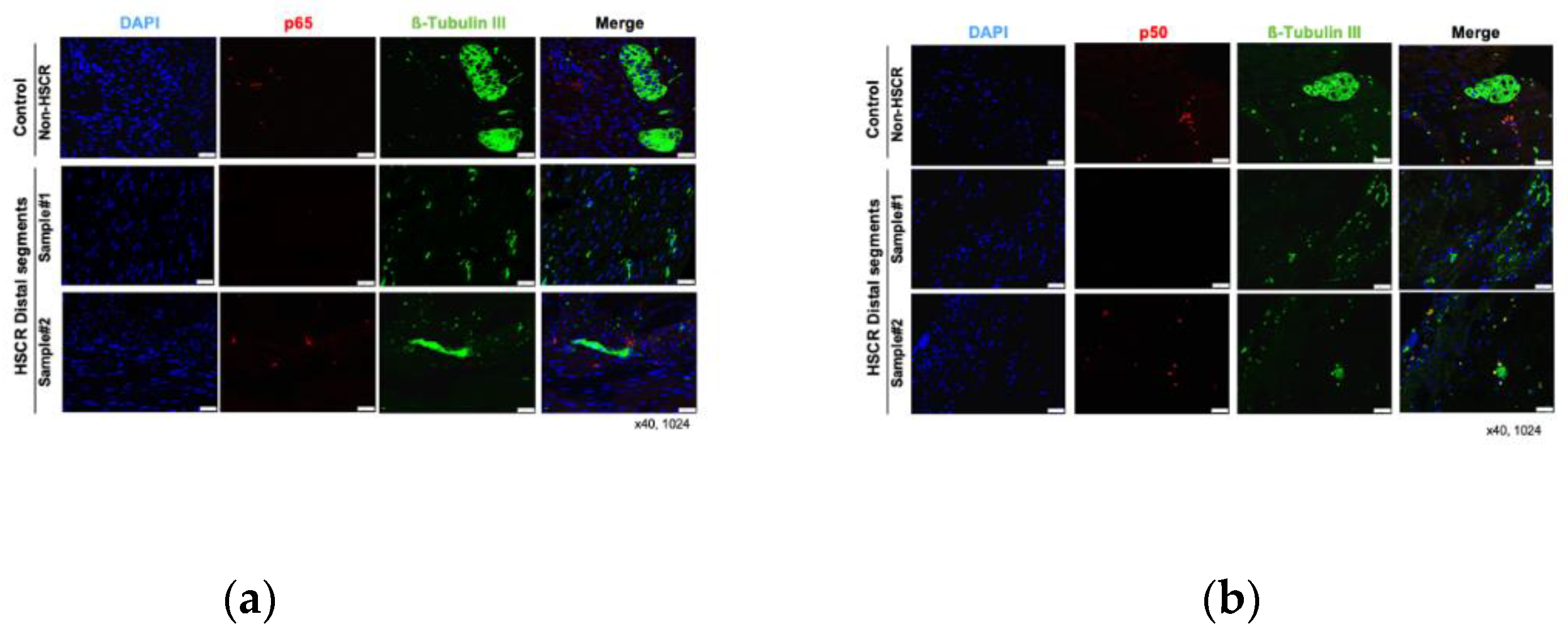
To corroborate the RT-qPCR results, we examined the protein expression of the main subunits involved in the NF-κB canonical activation pathway, RelA/p65 and NF-κB1 p105/p50. Here, 25 of the previous HSCR patients (again subdivided in proximal and distal segments) were analyzed by immunohistochemistry staining, where TubulinßIII was used as internal control for neuronal marker (
Figure 2). Further 5 non-Hischprung sections were included as staining controls.
While we did not detect any remarkable difference on the levels of NF-κB1 p105/p50 between the distal and proximal segments, the expression of the main NF-κB subunit, p65, was lower in the distal segments compared to the proximal HSCR colon tissue samples (
Figure 3 and
Figure 4), which confirms our previous RT-PCR results.
Despite the expression of Tubulin ßIII (
Figure 2 and
Figure 3), a neuronal marker, was not completely lost in the distal segments of all the HSCR patients, we could histologically observe that the innervations stained by Tubulin ßIII were either not complete or are not forming healthy ganglia, indicating a non-complete enteric innervation in those patients.
Regarding the muscle layer, we observed variable immuno-reactivities of RelA/p65 (
Figure 3a) and NF-κB1/p50 (
Figure 3b), which indicates a highly heterogeneous expression of NF-κB proteins among HSCR patients. However, the NF-κB expression within the muscle layer of most of the HSCR samples was low or undetectable. In general, both RelA/p65 and NFκB1/p50 were mainly detected in the mucosa and submucosa layers of the colon wall. (
Figure 3).
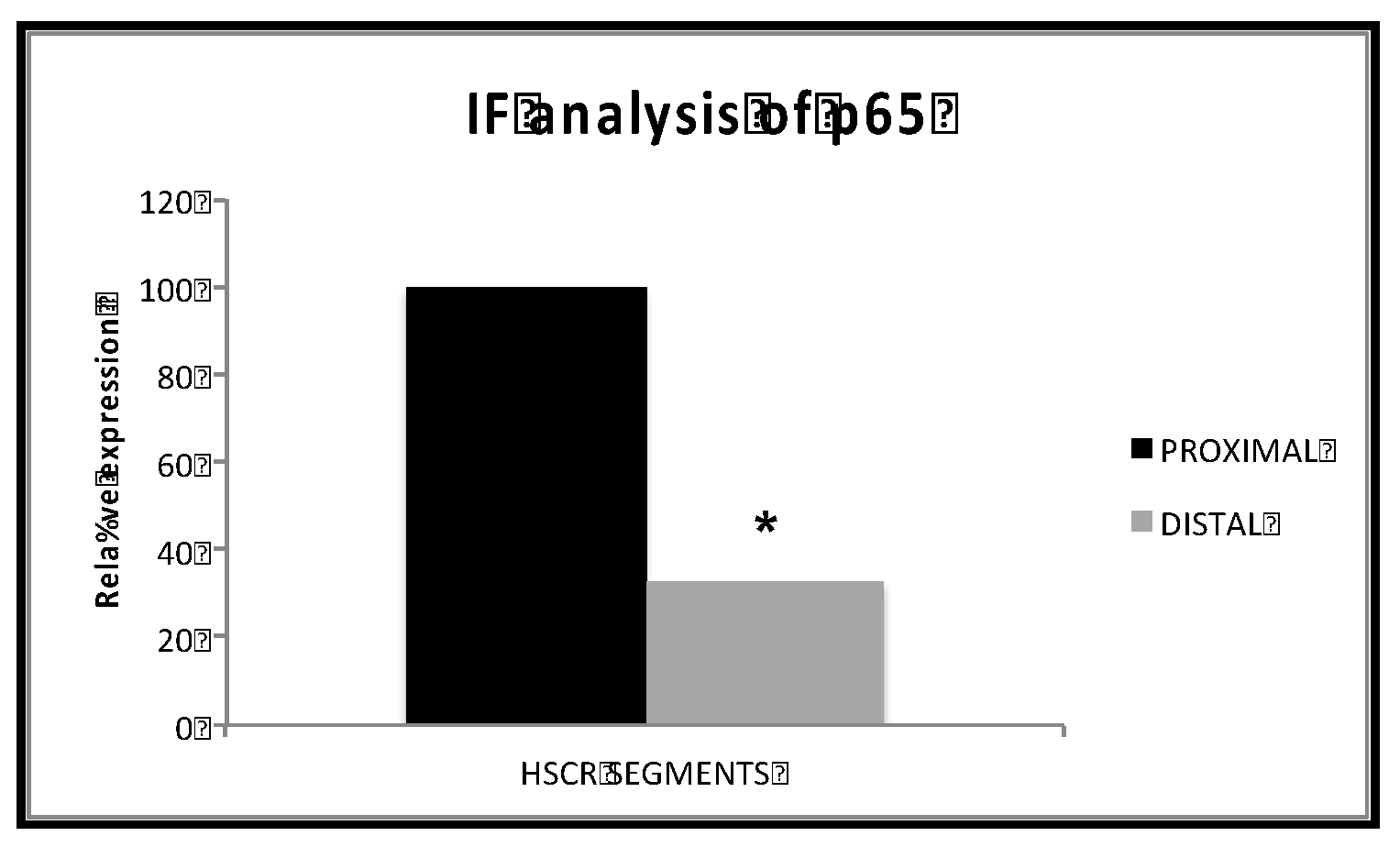
Searching for accurate results, we quantified the immunofluorescence intensity from the taken pictures of the distal and proximal colon segments of HSCR patients. Again, we could prove that proximal HSCR samples present higher amounts of RelA/p65 protein than distal HSCR sections (p< 0.05) (
Figure 4).
3.3. RelA/p65 Expression in HSCR Tissue Correlates with Lymphocyte Infiltration
Since RelA/p65 was mostly detected in the mucosal and submucosal layers, we wanted to confirm if this expression was related to tissue-infiltrating lymphocytes. Thus, we compared RelA/p65 protein expression with the expression of the leukocyte common antigen (LCA) in the distal segments of selected HSCR patients.
Results show a co-expression of both proteins in the tissue, indicating a high expression of RelA/p65 in the tissue-infiltrated lymphocytes in the submucosa and mucosa layer. Again, no RelA/p65 expression was observed in the muscle layer (
Figure 5).
Hence, proximal ganglionic HSCR segments present an inflammatory status, as suggested by RelA/p65 and LCA levels.
4. Discussion
In this study, we have found that HSCR proximal (ganglionic) segments present higher levels of of RelA/p65 gene and protein expression compared to the distal (aganglionic) segments by RT-qPCR and IHC. In addition, the expression of the pro-inflammatory cytokine (TNF⍺), as well as the TFGBR2, were increased in the proximal segments respect to the distal sections (
Figure 1a).
Additionally, we observed lower Erbb3 levels in the distal segments compared to the proximal ones (
Figure 1b). Supporting this, Erbb3 was previously reported to be deregulated in enteric neuropathies [
42].
Also, we detected a co-expression of RelA/p65 and LCA, particularly in the mucosa and not in the muscle layer of HSCR proximal samples, indicating lymphocyte infiltration (
Figure 5).
These observations indicate a higher expression of RelA/p65 and other pro- inflammatory factors in the HSCR proximal sections, but a lower inflammation in the HSCR distal segments, which contain either less quantity or dysfunctional enteric neurons. Here it could also be, that the infiltration rate is correlated to dilatation and thus a result of a defect mucosal barrier.
Thus, our observed p65 levels and pro-inflammatory status of the HSCR proximal segments may contribute to the neurodegeneration that leads to the neuronal loss and impairment observed in the HSCR distal segments.
Consistently with our results, previous studies have already related pointed out the relation of the NF-κB pathway to the enteric neuronal survival. For instance, in a mouse model of HSCR; (a model with a mutation in c-Ret, the major susceptibility gene in Hirschsprung), the impaired phosphorylation of NF-κB was pointed to as the possible cause of neurodegeneration of the spiral ganglion neurons (SGNs) in the inner ears and subsequent syndromic deafness [
43]. In another mouse model of Parkinson Disease, a debilitating neurodegenerative disorder, NF-κB inhibition prevented the loss of enteric neurons induced by inflammation [
44].
Concerning the infiltration of pro-inflammatory macrophages, it has been associated with myenteric neurons injury, while their depletion helped to rescue the enteric neurons [
45,
46]. Moreover, an impaired lymphocyte function has been associated with Hirschsprung-related enterocolitis [
47,
48]. In addition, post-surgical dysfunction of intestinal smooth muscle and enteric neurons, have been attributed to inflammation and increased expression of TNF-α, IL-6 and IL-1α [
49]. Importantly, NF-κB has been implicated in enteric neuronal loss by mediating 5-Fluorouracil intestinal inflammation and activating enteric glial cells [
50], which also supports our hypothesis of a pro-inflammatory neurodegenerative role of NF-κB in HSCR development.
Altogether, the NF-κB pathway and inflammation seem to play an important role in the fate of the enteric nervous system, and so, in the development of Hirschprung disease. Considering the high availability of specific NF-κB inhibitors [
24,
51] and pro- and anti- inflammatory drugs in the market, new treatments based on the NF-κB pathway seem promising in a short future for prevention and therapy of Hirschsprung disease.
Our observations set the stage for further studies on the role of NF-κB in neuro-enteric development. Modulation of NF-κB can be integrated with neural stem cell and regenerative research to potentiate neural progenitor migration and differentiation and to optimize outcomes of stem cell transplantation. Furthermore, NF-κB manipulation in animal models of Hirschsprung as well as healthy animals, to confirm the in-vivo its effect on the enteric nervous system, would allow better disease characterization.
Given the intricate aetiology of HSCR disease, the complex interaction between genetic and environmental factors, the varying severity, and the lack of treatment; currently, the only available solution is surgery which, indeed, comes with its complications, more extensive investigation of the NF-κB pathway is warranted to elucidate molecular mechanisms underlying the pathogenesis of the disease.
Finally, screening programs for the use of anti-inflammatory drugs in pregnant women would provide useful data that can be utilized in correlative studies with incidence of Hirschsprung and other neurodevelopmental disorders. They also help in directing the discovery of potential disease mediators and biomarkers, not only for diagnosis, and treatment, but also strategies for disease prevention.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: An example of amplification plot of completed RT-qPCR reactions.
Author Contributions
Conceptualization, M.A.T.L. and K.H.S.; validation, M.A.T.L. and K.H.S; formal analysis, E.Z.E. and R.K. and M.A.T.L.; investigation, E.Z.E., A.A.A and S.M.O.; resources, L.M.W., M.B., K.H.S.; writing—original draft preparation, E.Z.E. and M.A.T.L.; writing—review and editing, M.A.T.L. and K.H.S.; supervision, M.A.T.L., L.M.W. and K.H.S.; project administration, M.A.T.L.; funding acquisition, L.M.W., M.B. and K.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Translational Medical Research” Master Program of the Medical Faculty of Mannheim, University of Heidelberg.
Institutional Review Board Statement
The collection and use of patient material have been performed according to in-formed consent signed by patients’ parents and approved by the “Medizinische Ethik-Kommission II” of the Medical Faculty Mannheim, University of Heidelberg (2011-237N-MA). Samples have only been identified by sequential code numbers with no other identifying details.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We want to acknowledge the support of the LIMa: Live Cell Imaging at Microscopy Core Facility Platform Mannheim (CFPM), Medical Faculty of Mannheim, University of Heidelberg, Germany.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Heuckeroth, R.O. Hirschsprung Disease — Integrating Basic Science and Clinical Medicine to Improve Outcomes. Nat Rev Gastroenterol Hepatol 2018, 15, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Calkins, C. Hirschsprung Disease beyond Infancy. Clin Colon Rectal Surg 2018, 31, 051–060. [Google Scholar] [CrossRef] [PubMed]
- Lake, J.I.; Heuckeroth, R.O. Enteric Nervous System Development: Migration, Differentiation, and Disease. American Journal of Physiology-Gastrointestinal and Liver Physiology 2013, 305, G1–G24. [Google Scholar] [CrossRef] [PubMed]
- Heuckeroth, R.O. Hirschsprung’s Disease, Down Syndrome, and Missing Heritability: Too Much Collagen Slows Migration. Journal of Clinical Investigation 2015, 125, 4323–4326. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ceccherini, I.; Pasini, B.; Matera, I.; Bicocchi, M.P.; Barone, V.; Bocclardi, R.; Kääriänen, H.; Weber, D.; Devoto, M.; et al. Close Linkage with the RET Protooncogene and Boundaries of Deletion Mutations in Autosomal Dominant Hirschsprung Disease. Hum Mol Genet 1993, 2, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Puffenberger, E.G.; Hosoda, K.; Washington, S.S.; Nakao, K.; deWit, D.; Yanagisawa, M.; Chakravarti, A. A Missense Mutation of the Endothelin-B Receptor Gene in Multigenic Hirschsprung’s Disease. Cell 1994, 79, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- McCallion, A.S.; Stames, E.; Conlon, R.A.; Chakravarti, A. Phenotype Variation in Two-Locus Mouse Models of Hirschsprung Disease: Tissue-Specific Interaction between Ret and Ednrb. Proceedings of the National Academy of Sciences 2003, 100, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Camilleri, M. Hirschsprung Disease: Insights on Genes, Penetrance, and Prenatal Diagnosis. Neurogastroenterology & Motility 2019, 31. [Google Scholar] [CrossRef]
- Heuckeroth, R.O. Even When You Know Everything, There Is Still More to Learn About Hirschsprung Disease. Gastroenterology 2018, 155, 1681–1684. [Google Scholar] [CrossRef]
- Heuckeroth, R.O.; Schäfer, K.-H. Gene-Environment Interactions and the Enteric Nervous System: Neural Plasticity and Hirschsprung Disease Prevention. Dev Biol 2016, 417, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Luzón-Toro, B.; Villalba-Benito, L.; Torroglosa, A.; Fernández, R.M.; Antiñolo, G.; Borrego, S. What Is New about the Genetic Background of Hirschsprung Disease? Clin Genet 2020, 97, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.S.; Li, P.; Lai, F.P.-L.; Fu, A.X.; Lau, S.-T.; So, M.T.; Lui, K.N.-C.; Li, Z.; Zhuang, X.; Yu, M.; et al. Identification of Genes Associated with Hirschsprung Disease, Based on Whole-Genome Sequence Analysis, and Potential Effects on Enteric Nervous System Development. Gastroenterology 2018, 155, 1908–1922. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.A.; Koscsó, B.; Rajani, G.M.; Stevanovic, K.; Berres, M.-L.; Hashimoto, D.; Mortha, A.; Leboeuf, M.; Li, X.-M.; Mucida, D.; et al. Crosstalk between Muscularis Macrophages and Enteric Neurons Regulates Gastrointestinal Motility. Cell 2014, 158, 1210. [Google Scholar] [CrossRef] [PubMed]
- Geuking, M.B.; Köller, Y.; Rupp, S.; McCoy, K.D. The Interplay between the Gut Microbiota and the Immune System. Gut Microbes 2014, 5, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Schäfer, K.-H.; Tieftrunk, E.; Friess, H.; Ceyhan, G.O. Neural Plasticity in the Gastrointestinal Tract: Chronic Inflammation, Neurotrophic Signals, and Hypersensitivity. Acta Neuropathol 2013, 125, 491–509. [Google Scholar] [CrossRef]
- Niesler, B.; Kuerten, S.; Demir, I.E.; Schäfer, K.-H. Disorders of the Enteric Nervous System — a Holistic View. Nat Rev Gastroenterol Hepatol 2021, 18, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Marrinan, S.; Emmanuel, A.V.; Burn, D.J. Delayed Gastric Emptying in Parkinson’s Disease. Movement Disorders 2014, 29, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s Disease: A Dual-hit Hypothesis. Neuropathol Appl Neurobiol 2007, 33, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Gries, M.; Christmann, A.; Schulte, S.; Weyland, M.; Rommel, S.; Martin, M.; Baller, M.; Röth, R.; Schmitteckert, S.; Unger, M.; et al. Parkinson Mice Show Functional and Molecular Changes in the Gut Long before Motoric Disease Onset. Mol Neurodegener 2021, 16, 34. [Google Scholar] [CrossRef]
- Schäfer, K.; Christmann, A.; Gries, M. Can We Trust the Gut? The Role of the Intestine in Neurodegeneration. J Physiol 2020, 598, 4141–4142. [Google Scholar] [CrossRef]
- Meffert, M.K.; Chang, J.M.; Wiltgen, B.J.; Fanselow, M.S.; Baltimore, D. NF-ΚB Functions in Synaptic Signaling and Behavior. Nat Neurosci 2003, 6, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear Factor-ΚB in Cancer Development and Progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D. Integrating Cell-Signalling Pathways with NF-ΚB and IKK Function. Nat Rev Mol Cell Biol 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.A.; González-Navarrete, I.; Dalmases, A.; Bosch, M.; Rodriguez-Fanjul, V.; Rolfe, M.; Ross, J.S.; Mezquita, J.; Mezquita, C.; Bachs, O.; et al. Inhibition of the Canonical IKK/NFKappaB Pathway Sensitizes Human Cancer Cells to Doxorubicin. Cell Cycle 2007, 6, 2284–2292. [Google Scholar] [CrossRef]
- Simmons, L.J.; Surles-Zeigler, M.C.; Li, Y.; Ford, G.D.; Newman, G.D.; Ford, B.D. Regulation of Inflammatory Responses by Neuregulin-1 in Brain Ischemia and Microglial Cells in Vitro Involves the NF-Kappa B Pathway. J Neuroinflammation 2016, 13, 237. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct Target Ther 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Mémet, S. NF-ΚB Functions in the Nervous System: From Development to Disease. Biochem Pharmacol 2006, 72, 1180–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, W. NFκB Signaling Regulates Embryonic and Adult Neurogenesis. Front Biol (Beijing) 2012, 7, 277–291. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-ΚB Function in the Nervous System. Front Immunol 2019, 10. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M.P. NF-ΚB as a Therapeutic Target in Neurodegenerative Diseases. Expert Opin Ther Targets 2007, 11, 123–132. [Google Scholar] [CrossRef]
- Flood, P.M.; Qian, L.; Peterson, L.J.; Zhang, F.; Shi, J.-S.; Gao, H.-M.; Hong, J.-S. Transcriptional Factor NF-KB as a Target for Therapy in Parkinson’s Disease. Parkinsons Dis 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.K.; Jha, S.K.; Kar, R.; Nand, P.; Swati, K.; Goswami, V.K. Nuclear Factor-kappa β as a Therapeutic Target for Alzheimer’s Disease. J Neurochem 2019, 150, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-C. The Non-Canonical NF-ΚB Pathway in Immunity and Inflammation. Nat Rev Immunol 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Burgueño, J.F.; Barba, A.; Eyre, E.; Romero, C.; Neunlist, M.; Fernández, E. TLR2 and TLR9 Modulate Enteric Nervous System Inflammatory Responses to Lipopolysaccharide. J Neuroinflammation 2016, 13, 187. [Google Scholar] [CrossRef]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory Response in the CNS: Friend or Foe? Mol Neurobiol 2017, 54, 8071–8089. [Google Scholar] [CrossRef] [PubMed]
- Di Liddo, R.; Bertalot, T.; Schuster, A.; Schrenk, S.; Tasso, A.; Zanusso, I.; Conconi, M.; Schäfer, K. Anti-Inflammatory Activity of Wnt Signaling in Enteric Nervous System: In Vitro Preliminary Evidences in Rat Primary Cultures. J Neuroinflammation 2015, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Di Liddo, R.; Bertalot, T.; Schuster, A.; Schrenk, S.; Müller, O.; Apfel, J.; Reischmann, P.; Rajendran, S.; Sfriso, R.; Gasparella, M.; et al. Fluorescence-Based Gene Reporter Plasmid to Track Canonical Wnt Signaling in ENS Inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology 2016, 310, G337–G346. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Klotz, M.; Schwab, T.; Di Liddo, R.; Bertalot, T.; Schrenk, S.; Martin, M.; Nguyen, T.D.; Nguyen, T.N.Q.; Gries, M.; et al. Maintenance of the Enteric Stem Cell Niche by Bacterial Lipopolysaccharides? Evidence and Perspectives. J Cell Mol Med 2014, 18, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- von Boyen, G.B.T. Proinflammatory Cytokines Increase Glial Fibrillary Acidic Protein Expression in Enteric Glia. Gut 2004, 53, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Reed, R.R.; Lane, A.P. Acute Inflammation Regulates Neuroregeneration through the NF-ΚB Pathway in Olfactory Epithelium. Proceedings of the National Academy of Sciences 2017, 114, 8089–8094. [Google Scholar] [CrossRef] [PubMed]
- Hagl, C.I.; Wink, E.; Weiss, C.; Wessel, L.; Gretz, N.; Schäfer, K.H. Enteric Neurons from Postnatal Fgf2 Knockout Mice Differ in Neurite Outgrowth Responses. Autonomic Neuroscience 2012, 170, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-L.; Galmiche, L.; Levy, J.; Suwannarat, P.; Hellebrekers, D.M.E.I.; Morarach, K.; Boismoreau, F.; Theunissen, T.E.J.; Lefebvre, M.; Pelet, A.; et al. Dysregulation of the NRG1/ERBB Pathway Causes a Developmental Disorder with Gastrointestinal Dysmotility in Humans. Journal of Clinical Investigation 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, N.; Ida-Eto, M.; Shimotake, T.; Sakashita, N.; Sone, M.; Nakashima, T.; Tabuchi, K.; Hoshino, T.; Shimada, A.; Tsuzuki, T.; et al. C-Ret–Mediated Hearing Loss in Mice with Hirschsprung Disease. Proceedings of the National Academy of Sciences 2010, 107, 13051–13056. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.-A.; Côté, M.; Bourque, M.; Morissette, M.; Di Paolo, T.; Soulet, D. Neuroprotective and Immunomodulatory Effects of Raloxifene in the Myenteric Plexus of a Mouse Model of Parkinson’s Disease. Neurobiol Aging 2016, 48, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, X.; Zhang, H.; Feng, C.; Wang, B.; Li, N.; Abdullahi, K.M.; Wu, X.; Yang, J.; Li, Z.; et al. Intestinal Proinflammatory Macrophages Induce a Phenotypic Switch in Interstitial Cells of Cajal. Journal of Clinical Investigation 2020, 130, 6443–6456. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tang, Y.; Zhang, H.; Chu, X.; Yan, B.; Li, J.; Liu, C. Vincristine Leads to Colonic Myenteric Neurons Injury via Pro-Inflammatory Macrophages Activation. Biochem Pharmacol 2021, 186, 114479. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; Brinkman, A.S. Hirschsprung’s Associated Enterocolitis. Curr Opin Pediatr 2015, 27, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, D.; Klotz, M.; Laures, K.; Clasohm, J.; Bischof, M.; Schäfer, K.-H. The Mesenterially Perfused Rat Small Intestine: A Versatile Approach for Pharmacological Testings. Annals of Anatomy - Anatomischer Anzeiger 2014, 196, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Farro, G.; Gomez-Pinilla, P.J.; Di Giovangiulio, M.; Stakenborg, N.; Auteri, M.; Thijs, T.; Depoortere, I.; Matteoli, G.; Boeckxstaens, G.E. Smooth Muscle and Neural Dysfunction Contribute to Different Phases of Murine Postoperative Ileus. Neurogastroenterology & Motility 2016, 28, 934–947. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Bon-Frauches, A.C.; Silva, A.M.H.P.; Lima-Júnior, R.C.P.; Martins, C.S.; Leitão, R.F.C.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L.; et al. 5-Fluorouracil Induces Enteric Neuron Death and Glial Activation During Intestinal Mucositis via a S100B-RAGE-NFκB-Dependent Pathway. Sci Rep 2019, 9, 665. [Google Scholar] [CrossRef]
- Domingo-Domènech, J.; Pippa, R.; Tápia, M.; Gascón, P.; Bachs, O.; Bosch, M. Inactivation of NF-ΚB by Proteasome Inhibition Contributes to Increased Apoptosis Induced by Histone Deacetylase Inhibitors in Human Breast Cancer Cells. Breast Cancer Res Treat 2008, 112, 53–62. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).