1. Introduction
Chronic fatigue syndrome (CFS) is a debilitating biological medical condition (International Classification of Diseases ICD-11 8E49 [
1]) with no approved treatment. It is characterized by unexplained physical fatigue, cognitive problems (brain fog) and post-exertional malaise (PEM) [
2]. CFS´s etiology is controversial, and its underlying mechanisms not properly understood [
3]. Hypotheses of its pathophysiology include stress of the immune and the neurobehavioral systems [
4]. Dysregulation of the immune system and altered hypothalamic-pituitary-adrenal (HPA) axis are recurrent findings in CFS [
5]
. As a heterogeneous disorder, a close focus and monitoring of individual patient’s symptoms towards identification of subtypes and clustering is needed for personalized treatments.
The most referred cause of CFS is a viral infection, proposed to drive exhaustion of the immune system. Consistently, the literature supports a reduction of natural killer (NK) activity and anti-inflammatory cytokines, as well as increased levels of proinflammatory cytokines in CFS patients [
6,
7]. NK activity seems to indicate immune deficiency with potential stratification value [
8].
A subgroup of subjects diagnosed with CFS present stress induced CFS [
9,
10]. There is association of early life exposure to stress in the development of CFS [
11]. However, stressors during late adolescence or early adulthood (such as study/work related stress) could also elicit CFS. The prevalence of stress-related CFS is unknown, and the exact onset mechanism remains unclear. However, the literature recognizes that stress can disrupt physiological systems and behaviors, contributing to the onset and perpetuation of CFS symptoms. The impact of perceived stress management on CFS symptoms has been studied showing some benefits on physical symptoms such as PEM [
12,
13].
The HPA axis appears dysregulated in patients with CFS, thus explaining several symptoms of the disease, including PEM. A subset of CFS patients present hypocorticolism at awakening [
14], but proper stress management seems to regulate cortisol levels and PEM in CFS, to a certain extent [
13]. Functional alterations of the glucocorticoid receptor gene (NR3C1) have also been connected to CFS [
15].
Main catecholamines (dopamine (DA) and noradrenaline (NA) play an important role in HPA and the response to stress. Some CFS cases and individuals with postural orthostatic tachycardia syndrome (POTS), often present with an hyperadrenergic state [
16,
17]. This “sustained” arousal may derive from a chronic response against infections as well as an adaptation to psychological stressors that interact with predisposing genetic or epigenetic factors [
16]. Beta adrenergic blockers are recommended for those cases [
6,
18]. however, some CFS patients with specific genetic alterations show better response to beta blocker treatment than others [
6,
19]. Main catecholamine regulators, such as the catechol-O-methyltransferase (COMT), have shown to play an important role in CFS. A genetic variation in COMT has been recognized in a subset of CFS patients [
19,
20].
In addition to genetic factors, epigenetic modifications, such as differential DNA methylation to loci associated with differences in, for example, glucocorticoid sensitivity [
5], or the COMT gene [
21] may be important as biomarkers for future clinical testing of CFS.
The current case report follows the CARE guidelines for case reports [
22]. We introduce a male patient (body weight 76-82 kg; height 185cm) diagnosed with CFS in 2012 (Center for Disease Control (CDC/Fukuda) [
2]),and recognized as a stress-related CFS case. Patient´s diagnosis later included POTS, with overlapping symptoms to CFS. The genetics and biochemistry of the patient could indicate vulnerability and predisposition to stress-triggered disease, which added to personality traits of the patient may explain a major lack of response to a considerable list of medications
2. Case Description
2.1. Diagnostic Timeline
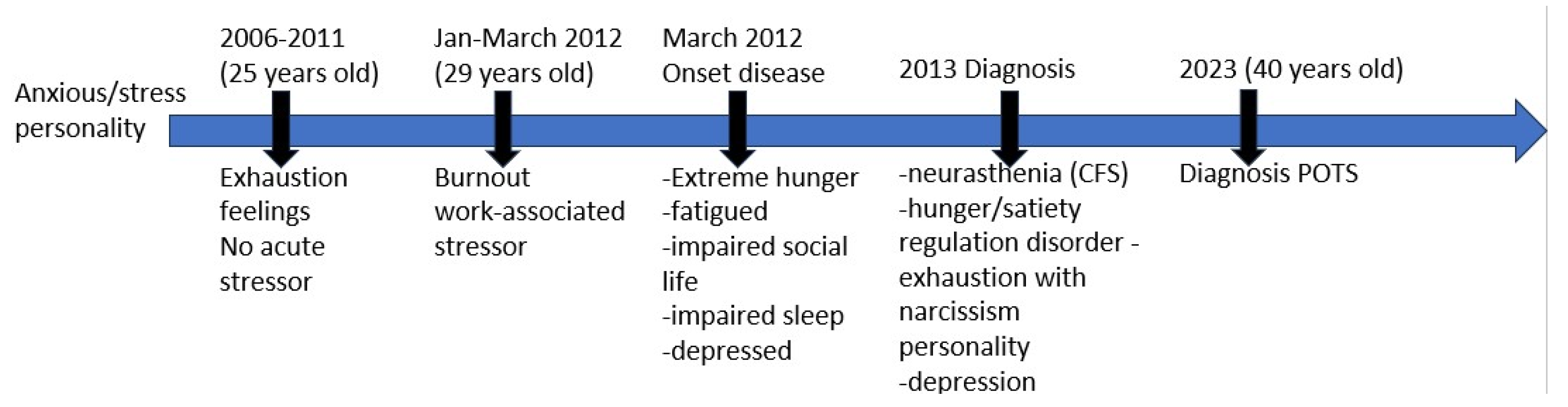
A 29-year-old man arrived at the hospital with extreme fatigue (2012), and a history of exhaustion (2008-2012), with the need of intermittent naps during the day. At hospitalization (2012) the patient described himself as anxious, stressed, obsessive and narcissistic, with a family history of psychiatric disorders (mother diagnosed with bipolar disorder at age of 40). Patient underwent stressors such as parents’ divorce, temporary separation from his mother and work-induced burnout. The patient described having few social contacts, being afraid of criticism and fearing disappointment. Symptoms persisted more than 6 months leading to strong physical and mental exhaustion (napping 5-6 times daily), reduced concentration capacity, loss of drive, with inability to work, depressed mood and stress.
Doctors advised to focus his recovery on stress-release techniques. Psychotherapy, yoga and meditation helped control psychological variables and stress factors (2012). Patient´s cortisol levels were elevated at the time of diagnosis (2012-2013), in support of an hyperadrenergic state of the patient. In 2013, the patient was diagnosed with neurasthenia, exhaustion with narcissistic personality, depressive disorder, and finally CFS.
The patient reports gradual increase of fatigue and stress over the years. Looking for psychological support due to his anxiety/stress symptoms (2021) the patient arrived at Dr. Marta Pardo (later referred as MP), PhD in Psychology, corresponding author of this report. MP was the support psychologist for the patient (2021-2023). The patient provided MP all personal and medical files and written informed consent for publication. Across medical files, it could be recognized a variety of symptoms related to multiple system dysfunctions. At this moment, and due to patient´s symptomatology related to the increased arousal, the patient looked for new doctors with emphasis on the stress symptoms. In 2021, the patient reports assisting to the clinic of a specialist on CFS and stress, who developed a genetic profile study focused on the HPA axis and other genes relevant for the stress response in CFS patients. From 2021-2023 the patient showed PEM after medium level of exercise, insomnia, reduced libido, high startle response, altered response to sweet taste, foggy brain and mental fatigue. During the time frame of 2021-2022, the patient tried nootropics and RIMAs (reversible inhibitors of monooxidase A, or MAO-A), L-tryptophan, and MAO-A inhibitors (Syrian rue), but also, following doctor’s indication, he started an intensive treatment based on supplements. The patient reported this period as “not comfortable to be taking supplements several times a day”. Some medication “helped” him manage some of the symptoms for a period of maximum 2 weeks, presenting with strong buildup tolerance shortly after.
In 2023, the patient tried “Parasym”, a neuromodulation device of the vagal nerve shown to have therapeutic benefits [
23]. Patient reported “worked a bit”. In 2023 the patient was additionally diagnosed with POTS (
Figure 1).
The patient completed a structured clinical interview to provide clinical, socio-demographic treatmentdata (June 2022-July 2023) while reported to continue attending regular medical doctor’s visits. Most biochemical analyses based on the catecholamine system were performed during this time frame. Biomolecule measurement at the end of 2021 (see details in
Table 1) could be influenced by patient´s treatment with supplements that did not cease until beginning 2022. However, patient’s health status continued to decline. Physical fatigue continued. Sleep was controlled with zopiclone (January-June 2023) which helped stabilize an average of 7 hours of sleep and reduced insomnia. The perceived patient’s quality of life worsened, with a high impact in social relationships.
2.2. Biochemical and Genetic Analyses
Biochemical parameters included measuring of neurotransmitter circulating levels. The potential involvement of main catecholamines (DA and NA), as well as the role of glutamate and gamma-aminobutyric acid (GABA) neurotransmitters and related molecules was studied in blood/urine at different time points (see
Table 1 for details and
Supplementary Table S1 for complete data).
In addition, a comprehensive panel of single nucleotide polymorphisms (SNP) (November 2021) (McMind company:
https://shop.mcmind.de/) showed the presence of some SNPs with potential relevance for patient’s risk to develop CFS (
Table 2, for complete data set please see
Supplementary Table S2). Some implied a direct impact on the regulation of the monoamines and neurotransmitters (COMT and MAO) and other indicated a direct involvement in the response to stress (e.g. NR3C1, 24) and methylenetetrahydrofolate reductase (MTHFR) [
25] (
Table 2). The change of wt “G” by an “A” in the SNP rs46080 of the COMT gene (Val158Met) is associated with lower COMT activity which may lead to higher DA levels in the brain cortex, reduced pain threshold and enhanced vulnerability to stress, what could align with the upper limit blood DA levels found in the patient (
Table 1). Additional missense variants in heterozygosis: Ala222Val of the MTHFR leads to reduced processing of folic acid and Val16Ala of the superoxide dismutase (SOD) gene translates into oxidative stress alterations. Importantly, some of these genetic conditions have previously been associated with CFS patients [
26].
In addition, HPA axis and immune system composition and function were studied by measuring glucocorticoid and cytokine levels as well as the presence of autoantibodies.
The patient presented with elevated proinflammatory cytokine levels (interleukin-6 (IL6), IL10 and interferon gamma (IFN
ϒ)) (2021-2022), indicative of neuroinflammation and reduction of NK cells suggesting a compromised immune system, (
Supplementary Table S1). In addition, the presence of Epstein-Barr virus (EBV) antibodies (
Supplementary Table S1, and several receptor autoantibodies (
Table 1) further supported immune problems. Patient´s immune system alterations go in agreement with previously described CFS classical phenotype [
6,
7].
Itemized results for additional blood parameters including blood counts, hormones, vitamins, etc. are broadly documented on
Supplementary Table S1.
In summary the patient presented altered parameters in neurotransmitter pathways and an hyperadrenergic state with increased levels of main metabolites of serotonin and kynurenine giving support to increased tryptophan catabolism. Increased levels of glutamate and GABA were confirmed indicating aberrant system activity with altered autoregulation (
Table 1).
3. Treatment and Course of Symptoms
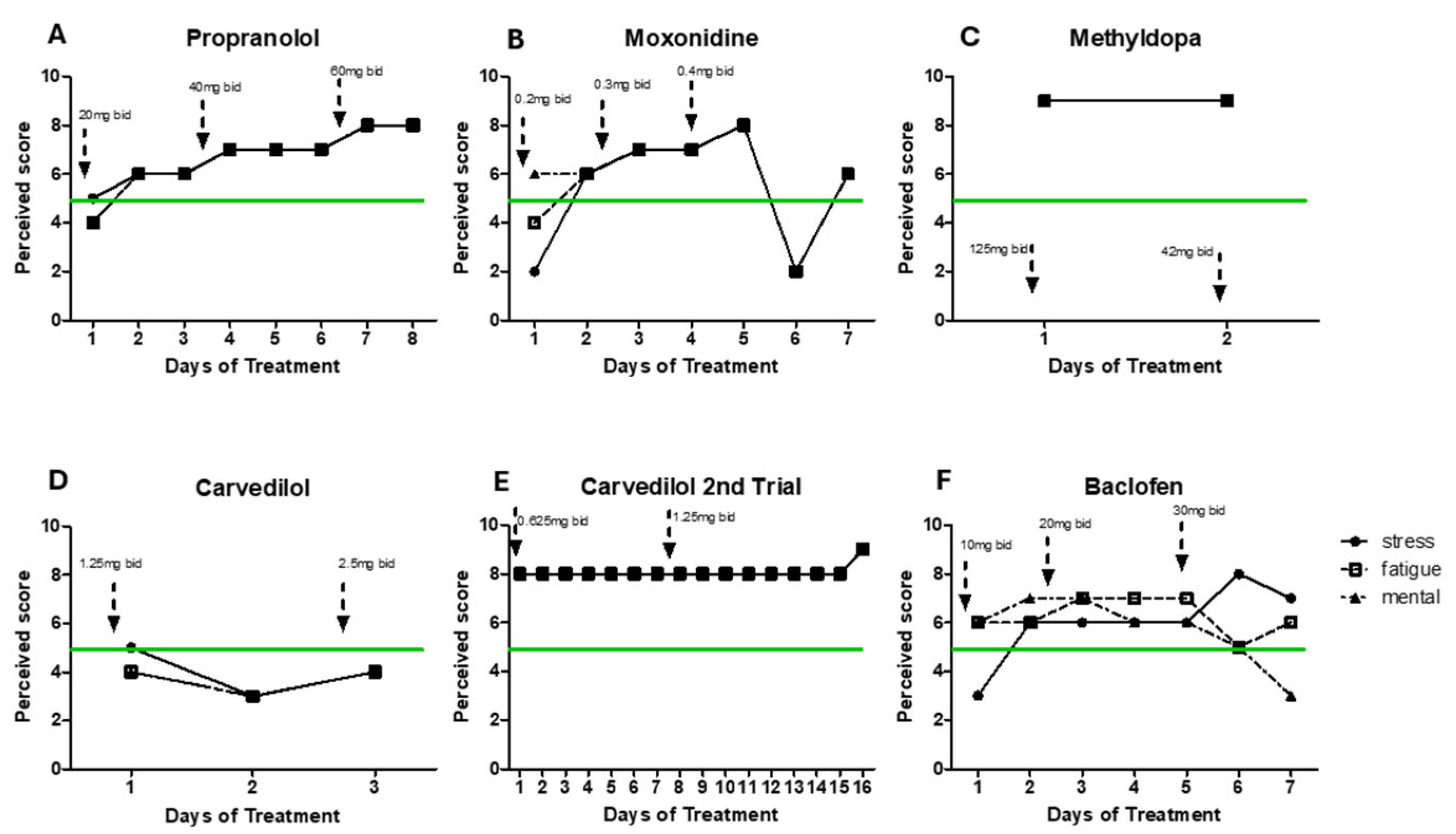
After a decade of treatments with supplements and other approaches offering little or no help at all, drug treatments reported by the patient to MP (summer 2022- 2023) seemed to have a basis to alleviate patient’s hyperadrenergic state. Propranolol (non-selective beta-adrenergic blocker) is currently approved treatment for CFS [
6] and has shown beneficial effects on CFS patients. Patient´s response to treatment was self-scored based on patient’s daily notes (see below for more details). However, following increasing doses (20 mg bid/40 mg bid/60 mg bid), the patient reported increased rates of stress and fatigue across all doses (data not shown).
When beta-blockers are not appropriate or irresponsive, moxonidine is recommended (reduces the activity of the sympathetic nervous system via activation of I1-imidazoline receptors resulting in a decrease of adrenaline and NA in humans [
27]. With moxonidine all symptoms worsened after trial of increasing doses (0.2 mg bid/ 0.3 mg bid/ 0.4 mg bid). Additionally, a GABAb agonist (Baclofen, 10mg bid/20mg bid/30mg bid) did not improve mental and physical fatigue. Carvedilol (1.25 mg bid and 2.50 mg bid), an alpha 1 adrenergic receptor blocker led to same negative results. Methyldopa treatment (a centrally alpha 2 adrenergic agonist, inhibits the adrenergic neuronal outflow and depletes NA) led to extreme levels of stress and physical fatigue after only 2 days making the patient to also stop this treatment (1.25 mg bid). Detailed treatment information is provided in
Figure 2.
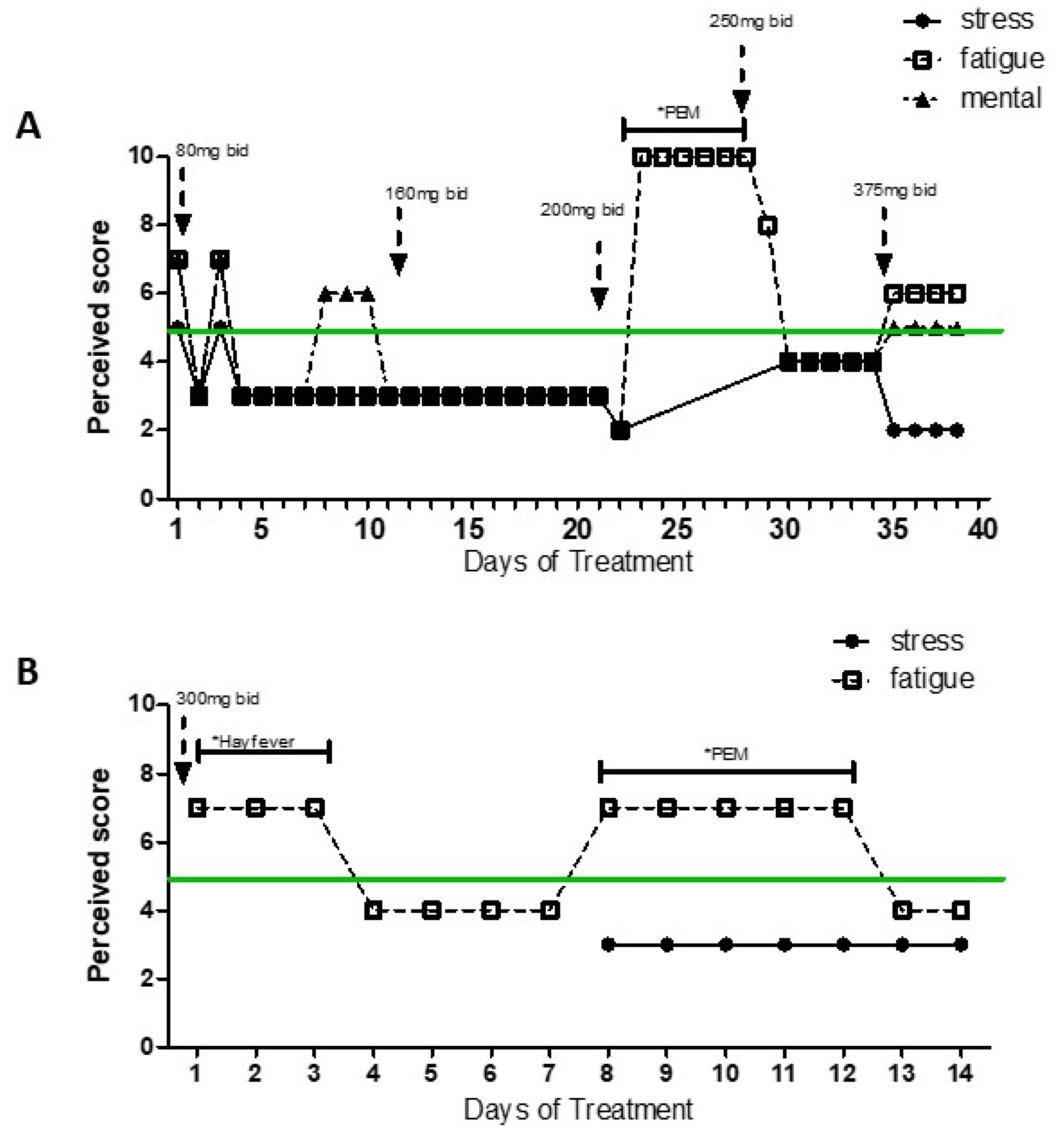
Due to prior patient’s inability to report daily response to treatment in a more standardized and quantifiable way, MP created a simplified instrument to evaluate patient´s response to the pharmaceutical treatments. What the patient described as his daily baseline levels of stress, physical fatigue and mental fatigue were equal to 5. The data presented on
Figure 2 and
Figure 3 was standardized based on the brief daily notes of the patient and his comparison to what he considered his basal level (represented as 5) (light/low/a bit/more=±1, very/high=±2, extreme=±3, very extreme=±4) to represent improvement or worsened of his symptoms (differentiated between physical fatigue, mental fatigue and stress, when provided).
However, following treatment failure to target his potential hyperadrenergic state, the patient reports to MP having experienced improvement with AMPT (alpha-methyl-p-tyrosine or methyrosine) at a dose of 80 to 300 mg bid, a compound that reduces synthesis of NA and DA (
Figure 3 and 4).
Figure 3 shows daily levels of stress, physical and mental fatigue (compared to baseline patients’ level) across time in two separate periods of treatment with AMPT. As the patient felt better, he engaged in more physical activity that led to few periods of PEM (as indicated on the graphs), what may explain increased levels of physical fatigue independently of treatment dose. Difficulty for compliance with treatment for personal reasons led to interruption of AMPT treatment. However, symptom worsening drove the patient to restart medication a few weeks later. Although AMPT doses 80-200 mg bid seemed efficient at reducing fatigue (trial 1), the patient reported to self-increase the dose in trial 2 in search of more drastic positive results. However, higher dose (300 mg bid) did not improve the symptoms. Doses of AMPT up to 1500 mg showed absence of secondary effects [
28], what supports the safety of the dose used by the patients (300 mg bid).
Figure 4.
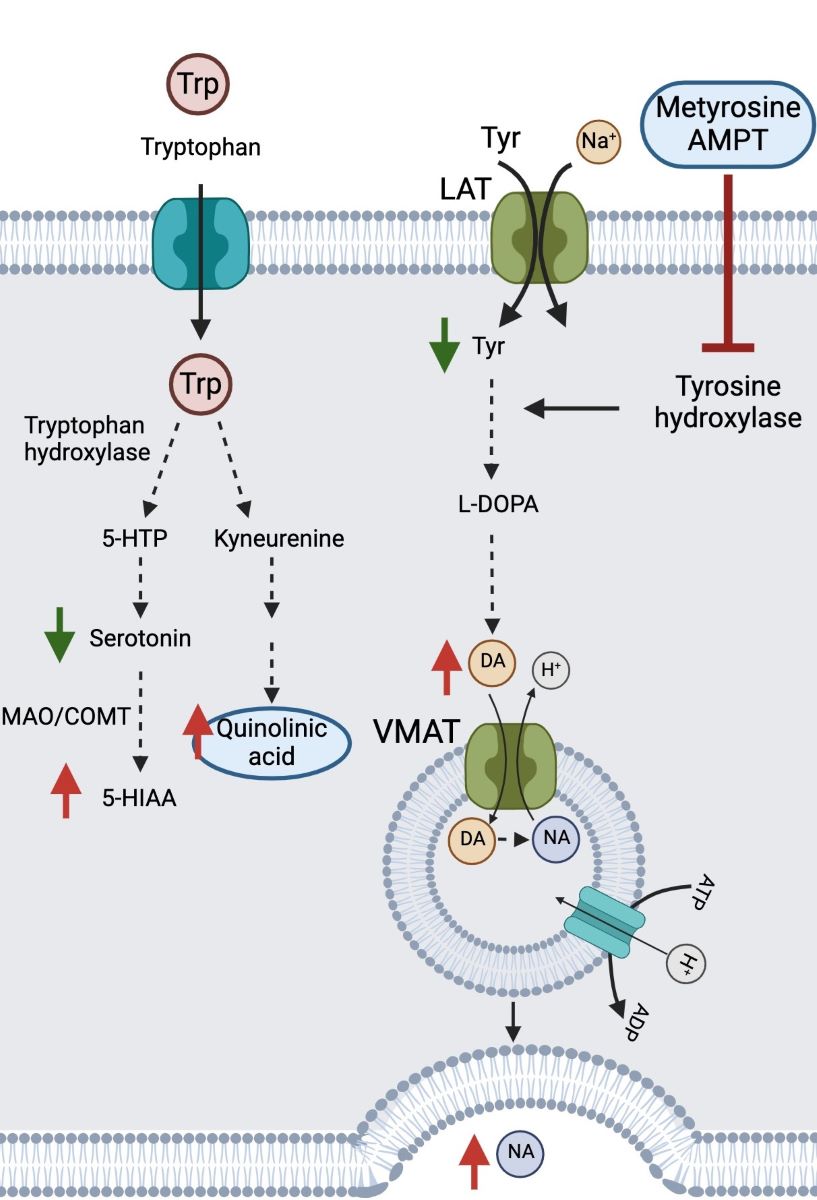
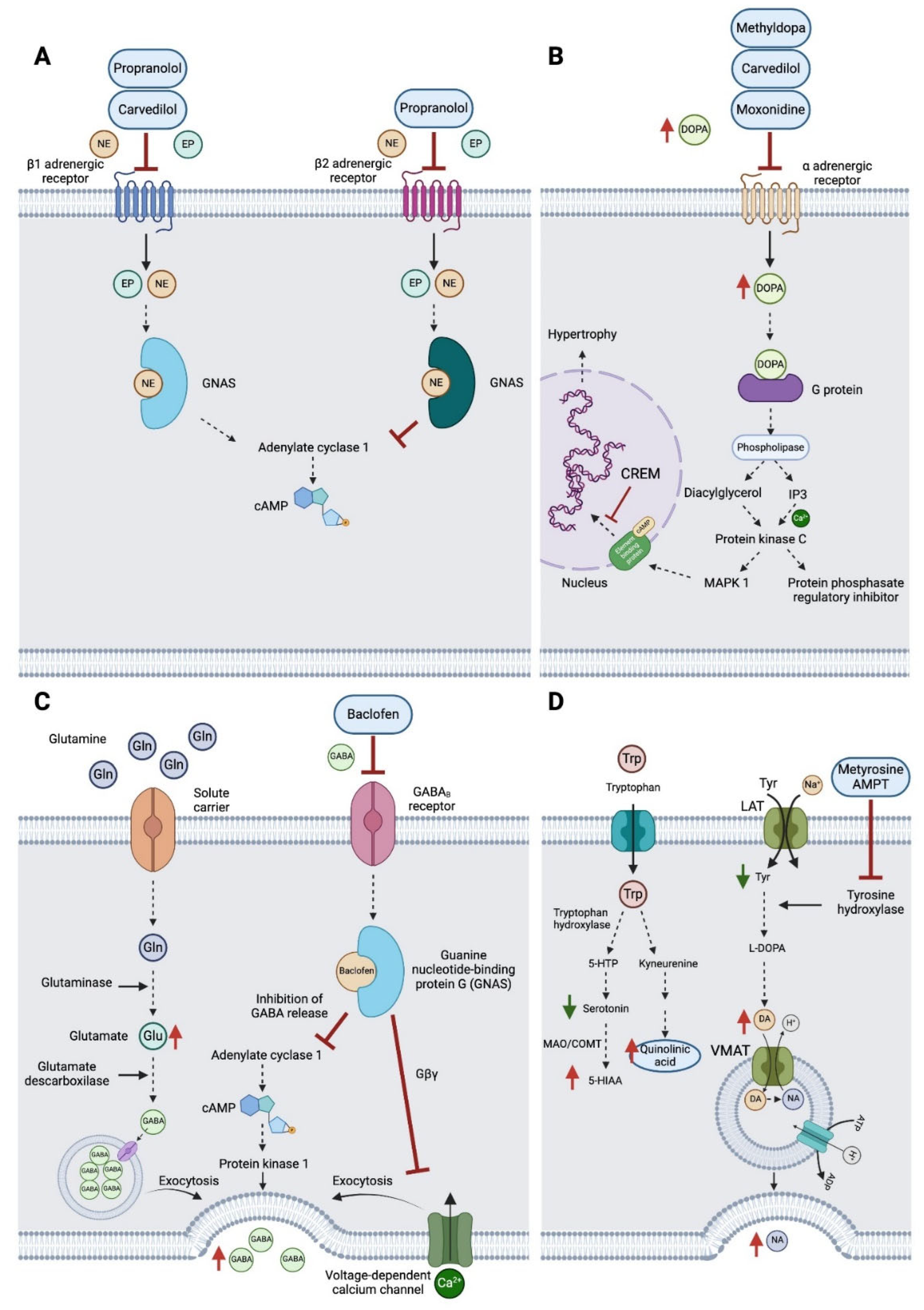
Molecular pathways affected and drug treatments. (A) Propranolol and carvedilol mechanism of action targeting β1 and β2 adrenergic receptors; (B) Methyldopa, carvedilol and moxonidine mechanism of action targeting α adrenergic receptors; (C) Baclofen mechanism of action targeting GABAB receptors; and (D) AMPT or metyrosine inhibition of tyrosine hydroxylase, affecting tyrosine metabolism and the related tryptophan pathway (Created with Biorender.com).
Figure 4.
Molecular pathways affected and drug treatments. (A) Propranolol and carvedilol mechanism of action targeting β1 and β2 adrenergic receptors; (B) Methyldopa, carvedilol and moxonidine mechanism of action targeting α adrenergic receptors; (C) Baclofen mechanism of action targeting GABAB receptors; and (D) AMPT or metyrosine inhibition of tyrosine hydroxylase, affecting tyrosine metabolism and the related tryptophan pathway (Created with Biorender.com).
4. Materials and Methods
All data was provided by the patient.
Clinical evaluation and diagnosis were performed by medical doctors
Blood and urine samples were analyzed by several medical doctors across disease progression.
Genetic analyses of SNP were performed by the following companies: McMind, Ancestry, 23andMe.
Pharmacological treatments reported by the patient included: Atenolol (Aliud Pharma), alpha methyldopa (Stada), carvedilol (1A Pharma), moxonidine (Ratiopharm), propranolol (Aliud Pharma), mirtazapine (1A Pharma), zopiclone (Ratiopharm), Alpha-methyl-p-tyrosine (AMPT) (Angene International Limited).
Evaluation of pharmacological treatments: Due to the patient´s inability to report daily response to treatment in a more standardized and quantifiable way, MP created a simplified instrument to evaluate patient´s response to the pharmaceutical treatments. What the patient described as his daily baseline levels of stress, physical fatigue and mental fatigue were equal to 5. The data presented in
Figure 2 and
Figure 3 was standardized based on the brief daily notes of the patient and his comparison to what he considered his basal level (light/low/ a bit/more =±1, very/high=±2; extreme=±3, very extreme=±4) to represent improvement or worsened of his symptoms (differentiated between physical fatigue, mental fatigue and stress)
5. Summary and Conclusions
The current case report presents the case of a patient diagnosed with CFS over 10 years ago longitudinally followed for response to treatment. We highlight the lack of positive response to classical approaches to treat CFS, reflecting the limitations of CFS diagnosis and available treatments to alleviate patients´ symptoms. Additionally, we show the lack of adherence of this CFS patient to medication (common trend among CFS patients possibly a consequence of improvement failure). The current case supports the implication of stress factors on the etiology of CFS as well as a role of catecholamines on the symptoms described.
Data obtained revealed biochemical indicators of altered HPA axis as well as genetic predisposition or vulnerability to stress, what, in other cases could be of epigenetic nature. The current pathomechanism hypothesis highlights monoamine alterations (hyperadrenergic state) on the DA/adrenergic system and a dysfunctional autonomic nervous system resulting from sympathetic overactivity.
Along years of trial-and-error series of treatments, the patient highlights his unexpected positive response to AMPT. AMPT inhibits TH (converts tyrosine to DA) (
Figure 4D). The patient had presented decreased levels of tyrosine and increased levels of DA and NA as well as other downstream metabolites such as vanillylmandelic acid (VMA) and B1/B2 adrenergic autoantibody receptors (
Table 1). Additionally, the tryptophan pathway had shown elevated levels of quinolinic acid, toxic product of activated microglia in the brain (
Figure 4D). Due to previous hyper adrenergic alteration history of the patient, it does not seem surprising that the patient showed reduced stress and fatigue levels after AMPT treatment (low-intermediate doses 80-300 mg bid). A limitation being not counting with post- AMPT treatment analytics.
AMPT treatment also attenuates oxidative stress and inflammation [
29], aspects possibly altered in the patient according to his genotype. AMPT has been tested in clinical trials of 22q11.2 deletion syndrome (what is characteristic of COMT polymorphism) [
28,
30]. Other disorders have given AMPT a chance (obsessive compulsive disorder [
31], schizophrenia [
32,
33] and mood disorders [
34] with some positive results. Currently a metyrosine derivate is being tested for Autism Spectrum Disorder (ClinicalTrials.gov Identifier: NCT05067582). We are not aware of previous studies having reported the use of AMPT for the treatment of CFS patients. Our data supports the relevance of personalized treatment, focused on the patient’s specific symptoms, biochemical findings (including circulating levels of relevant neurotransmitters), and genotype towards tailored improvement of patient´s quality of life. CFS is a devastating heterogenic disorder with lack of effective treatments.
The response to AMPT treatment highlights the relevance of pacing regarding to stressful situations and increased activity. We acknowledge some limitations of the study as a case report reflects individual features and responses. We focus the current report on the discussion of data with direct relationship with the main mechanisms related with AMPT treatment. It would have been informative to monitor biochemical levels of the main catecholamines after AMPT treatment, what could have allowed establish mechanistic causality of patient improvement. Current data is based on biochemical and genetic analyses and patient´s reports. Importantly, the results do not indicate causality between AMPT and its action on the monoamine system and future studies should evaluate the implication of other targets. Additionally, we acknowledge that neurotransmitter levels in blood are not the best indicative of autonomic dysfunction itself.
This longitudinal study provides support for the hyper adrenergic hypothesis possibly for a subset of patients and provides, for the first time, positive results in response to AMPT. Our results open the possibility of AMPT repurposing through clinical trials suited for a specific subgroup of CFS patients with altered DA/NA system and HPA axis function.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1: Biochemical Laboratory analyses; Table S2: Genetics.
Author Contributions
MP designed, acquired, analyzed and interpreted the data; MP and EO drafted the manuscript and critically revised the content: ML drafted figures and tables; all coauthors have given final approval of the version to be published.
Funding
This research was funded by several economic support. ML is supported by UCV predoctoral fellowship. EO funding support UCV2020-270-001. MP funding support by Ministerio de Ciencia e Innovacion (RYC2022-035438-I)
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written Informed consent was obtained from the patient to publish this paper.
Data Availability Statement
All data is available in the article and additional supplementary tables.
Acknowledgments
We acknowledge the patient on whom the case report is based.
Conflicts of Interest
The authors declare no conflicts of interest. The research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest
References
- Harrison, J.E.; Weber, S.; Jakob, R.; Chute, C.G. ICD-11: an international classification of diseases for the twenty-first century. BMC Med Informatics Decis. Mak. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Van De Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.; Speight, N.; Vallings, R.; et al. Myalgic encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef]
- Sapra, A., & Bhandari, P. (2023). Chronic fatigue syndrome. In StatPearls [Internet]. StatPearls Publishing. Available from https://www.ncbi.nlm.nih.gov/books/NBK557676/.
- Abdulla, J. , & Torpy, B. D. J. (2000). Chronic fatigue syndrome. In Feingold, K. R., Anawalt, B., Blackman, M. R., Boyce, A., Chrousos, G., Corpas, E.,... & Wilson, D. P. (Eds.), *Endotext*. MDText.com, Inc.
- de Vega, W.C.; Herrera, S.; Vernon, S.D.; McGowan, P.O. Epigenetic modifications and glucocorticoid sensitivity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). BMC Med Genom. 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Allen, C. D. (2014). Use of low-dose beta-blockers to treat symptoms of chronic fatigue syndrome (Master's thesis, The University of Utah).
- Montoya, J.G.; Holmes, T.H.; Anderson, J.N.; Maecker, H.T.; Rosenberg-Hasson, Y.; Valencia, I.J.; Chu, L.; Younger, J.W.; Tato, C.M.; Davis, M.M. Cytokine signature associated with disease severity in chronic fatigue syndrome patients. Proc. Natl. Acad. Sci. 2017, 114, E7150–E7158. [Google Scholar] [CrossRef]
- Brenu, E.W.; Hardcastle, S.L.; Atkinson, G.M.; van Driel, M.L.; Kreijkamp-Kaspers, S.; Ashton, K.J.; Staines, D.R.; Marshall-Gradisnik, S.M. Natural killer cells in patients with severe chronic fatigue syndrome. Autoimmun. Highlights 2013, 4, 69–80. [Google Scholar] [CrossRef]
- Janal, M.N.; Ciccone, D.S.; Natelson, B.H. Sub-typing CFS patients on the basis of ‘minor’ symptoms. Biol. Psychol. 2006, 73, 124–131. [Google Scholar] [CrossRef]
- Pi, I.G.; Sein-Echaluce, M.L.G.; Aubach, L.R.; Puig-Gros, J.T.; Solà, J.F. Stressful Events in the Onset of Chronic Fatigue Syndrome. . 2016, 90, e1–7. [Google Scholar]
- Borsini, A.; Hepgul, N.; Mondelli, V.; Chalder, T.; Pariante, C.M. Childhood stressors in the development of fatigue syndromes: a review of the past 20 years of research. Psychol. Med. 2013, 44, 1809–1823. [Google Scholar] [CrossRef]
- Hall, D.L.; Lattie, E.G.; Milrad, S.F.; Czaja, S.; Fletcher, M.A.; Klimas, N.; Perdomo, D.; Antoni, M.H. Telephone-administered versus live group cognitive behavioral stress management for adults with CFS. J. Psychosom. Res. 2016, 93, 41–47. [Google Scholar] [CrossRef]
- Hall, D.L.; Lattie, E.G.; Antoni, M.H.; Fletcher, M.A.; Czaja, S.; Perdomo, D.; Klimas, N.G. Stress management skills, cortisol awakening response, and post-exertional malaise in Chronic Fatigue Syndrome. Psychoneuroendocrinology 2014, 49, 26–31. [Google Scholar] [CrossRef]
- Powell, D.J.; Liossi, C.; Moss-Morris, R.; Schlotz, W. Unstimulated cortisol secretory activity in everyday life and its relationship with fatigue and chronic fatigue syndrome: A systematic review and subset meta-analysis. Psychoneuroendocrinology 2013, 38, 2405–2422. [Google Scholar] [CrossRef]
- Rajeevan, M.S.; Smith, A.K.; Dimulescu, I.; Unger, E.R.; Vernon, S.D.; Heim, C.; Reeves, W.C. Glucocorticoid receptor polymorphisms and haplotypes associated with chronic fatigue syndrome. Genes, Brain Behav. 2006, 6, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wyller, V.B.; Eriksen, H.R.; Malterud, K. Can sustained arousal explain the Chronic Fatigue Syndrome? Behav. Brain Funct. 2009, 5, 10–10. [Google Scholar] [CrossRef]
- Mar, P.L.; Raj, S.R. Postural Orthostatic Tachycardia Syndrome: Mechanisms and New Therapies. Annu. Rev. Med. 2020, 71, 235–248. [Google Scholar] [CrossRef]
- Clinician Coalition ME/CFS Treatment Recommendations US ME/CFS Version 1. (2021). *Bateman Horne Center*. Retrieved from https://batemanhornecenter.org/wp-content/uploads/filebase/Treatment-Recs-MECFS-Clinician-Coalition-V1-Feb.-2021.pdf.
- Sommerfeldt, L.; Portilla, H.; Jacobsen, L.; Gjerstad, J.; Wyller, V.B. Polymorphisms of adrenergic cardiovascular control genes are associated with adolescent chronic fatigue syndrome. Acta Paediatr. 2011, 100, 293–298. [Google Scholar] [CrossRef]
- Hall, K.T.; Kossowsky, J.; Oberlander, T.F.; Kaptchuk, T.J.; Saul, J.P.; Wyller, V.B.; Fagermoen, E.; Sulheim, D.; Gjerstad, J.; Winger, A.; et al. Genetic variation in catechol-O-methyltransferase modifies effects of clonidine treatment in chronic fatigue syndrome. Pharmacogenomics J. 2016, 16, 454–60. [Google Scholar] [CrossRef]
- Polli, A.; Hendrix, J.; Ickmans, K.; Bakusic, J.; Ghosh, M.; Monteyne, D.; Velkeniers, B.; Bekaert, B.; Nijs, J.; Godderis, L. Genetic and epigenetic regulation of Catechol-O-methyltransferase in relation to inflammation in chronic fatigue syndrome and Fibromyalgia. J. Transl. Med. 2022, 20, 1–11. [Google Scholar] [CrossRef]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Pan, Y.-W.; Zhang, Y.-N.; Wang, Y.-C.; Lu, Y.-B.; Huang, X.-L.; Lao, Y.-F.; Zhang, L.; Yang, J.; Shi, M.; Ma, H.-L. Myeloperoxidase: a new target for the treatment of stroke? Neural Regen. Res. 2022, 17, 1711–1716. [Google Scholar] [CrossRef]
- Palma-Gudiel, H.; Córdova-Palomera, A.; Leza, J.C.; Fañanás, L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: A critical review. Neurosci. Biobehav. Rev. 2015, 55, 520–535. [Google Scholar] [CrossRef]
- Jiménez, K.M.; Pereira-Morales, A.J.; Forero, D.A. MTHFR gene methylation is associated with perceived stress in healthy young adults. Psychiatr. Genet. 2018, 28, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yin, J.; Miller, A.H.; Xiao, C. A systematic review of the association between fatigue and genetic polymorphisms. Brain, Behav. Immun. 2017, 62, 230–244. [Google Scholar] [CrossRef]
- Prichard, B.N.; Owens, C.W.; Graham, B.R. Pharmacology and clinical use of moxonidine, a new centrally acting sympatholytic antihypertensive agent. 1997, S29–45. [Google Scholar]
- Boot, E.; Booij, J.; Hasler, G.; Zinkstok, J.R.; de Haan, L.; Linszen, D.H.; van Amelsvoort, T.A. AMPT-induced monoamine depletion in humans: evaluation of two alternative [123I]IBZM SPECT procedures. Eur. J. Nucl. Med. 2008, 35, 1350–1356. [Google Scholar] [CrossRef]
- Ballester-Servera, C.; Cañes, L.; Alonso, J.; Puertas, L.; Taurón, M.; Rodríguez, C.; Martínez-González, J. El receptor nuclear NOR-1 (Neuron-derived Orphan Receptor-1) en el remodelado vascular patológico. Clin. E Investig. En Arter. 2022, 34, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Graf, W.D.; Unis, A.S.; Yates, C.M.; Sulzbacher, S.; Dinulos, M.B.; Jack, R.M.; Dugaw, K.A.; Paddock, M.N.; Parson, W.W. Catecholamines in patients with 22q11.2 deletion syndrome and the low-activity COMT polymorphism. Neurology 2001, 57, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Longhurst, J.G.; Carpenter, L.L.; Epperson, C.; Price, L.H.; McDougle, C.J. Effects of catecholamine depletion with AMPT (alpha-methyl-para-tyrosine) in obsessive-compulsive disorder. Biol. Psychiatry 1999, 46, 573–576. [Google Scholar] [CrossRef]
- Voruganti, L.; Msc; Slomka, P.; Msc, P.Z.; Bsc, G.C.; Bsc, A.S.; Mattar, A.; Awad, A.G. Subjective Effects of AMPT-induced Dopamine Depletion in Schizophrenia Correlation between Dysphoric Responses and Striatal D2 Binding Ratios on SPECT Imaging. Neuropsychopharmacology 2001, 25, 642–650. [Google Scholar] [CrossRef]
- Alves, F.d.S.; Bakker, G.; Schmitz, N.; Abeling, N.; Hasler, G.; van der Meer, J.; Nederveen, A.; de Haan, L.; Linszen, D.; van Amelsvoort, T. Dopaminergic modulation of the reward system in schizophrenia: A placebo-controlled dopamine depletion fMRI study. Eur. Neuropsychopharmacol. 2013, 23, 1577–1586. [Google Scholar] [CrossRef]
- Miller, H.L.; Delgado, P.L.; Salomon, R.M.; Heninger, G.R.; Charney, D.S. Effects of α-methyl-para-tyrosine (AMPT) in drug-free depressed patients. Neuropsychopharmacology 1996, 14, 151–157. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).