1. Introduction
In the acute phase of Kawasaki disease, the normal vascular structure, including vascular support tissues such as internal and external elastic plates, is destroyed owing to severe vasculitis, and the coronary arteries that cannot withstand the intravascular pressure dilate, resulting in coronary artery aneurysms. After the acute phase of Kawasaki disease, the dilated coronary artery undergoes active vascular remodeling for a long period, with the proliferation of the intima, narrowing of the lumen, and sometimes re-dilation [
1]. As a result, coronary artery lesions (CALs) after Kawasaki disease are a mixture of dilated and stenotic lesions, which often appear in multiple branches and not infrequently have complex hemodynamics. Coronary angiographic morphology is insufficient to evaluate such complex CALs, and hemodynamic evaluation is necessary for subsequent treatment.
Coronary fractional flow reserve (FFR) and coronary flow reserve (CFR) are widely used in cardiology to evaluate coronary hemodynamics and can be used to diagnose myocardial ischemia and assess its severity. The FFR has been used as an indicator for coronary revascularization, as the DEFER and FAME studies have demonstrated its validity as a criterion for determining the indication for coronary revascularization therapy [
2,
3]. The CFR is an index that considers the morphological stenosis of coronary arteries and the microcirculation, which is a functional abnormality [
4]; it is considered an indicator of peripheral circulation and prognosis.
The agent
13N-ammonia PET, which has been covered by insurance in Japan since April 2012, is thought to be capable of accurately evaluating myocardial blood flow with lower radiation dose, higher sensitivity to γ-rays, and higher spatial resolution than single-photon emission computed tomography (PET) [
5,
6] . Therefore, it can evaluate myocardial blood flow (MBF) and myocardial flow reserve (MFR) in detail without cardiac catheterization and is considered useful in evaluating myocardial ischemia. The noninvasive evaluation of MFR with
13N-ammonia is generally considered to have the same clinical significance as the invasive evaluation of CFR [
7].
No report has evaluated the relationship between FFR and MFR in CAL after Kawasaki disease, nor has any report evaluated myocardial blood flow by MBF. Therefore, this study retrospectively evaluated FFR, MFR, and MBF in the complex hemodynamic situation of CAL after Kawasaki disease and examined the relationship between FFR, MFR, and MBF and their usefulness in CAL after Kawasaki disease.
2. Materials and Methods
2.1. Participants
Nineteen patients (18 male and 1 female) who underwent coronary angiography and FFR evaluation by cardiac catheterization and MFR evaluation by 13N-ammonia PET between April 2012 and December 2021 at Nippon Medical School Hospital, with 24 coronary branches with FFR and MFR evaluations, were included in the study. The 24 branches included 7 right coronary arteries (RCAs), 11 left anterior descending arteries (LADs), and 6 left circumflex arteries (LCXs). The average age at examination was 17.46 years (range: 12.67–28.58 years), and the average time from onset of Kawasaki disease to examination was 14.09 years (range: 3.42–26.92 years).
All participants provided written informed consent before the examinations. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the ethics committee of the Nippon Medical School (No. B-2021-360).
2.2. Methods
2.2.1. Cardiac Catheterization
2.2.1.1. Coronary Angiography
Coronary angiography was used to evaluate coronary aneurysms (location, shape, and size), stenotic lesions (location and extent), and the presence of collateral vessels. Coronary aneurysms were classified as small (4–6 mm), medium (6–8 mm), and giant (>8 mm) aneurysms [
1]. Regression was defined as the presence of a dilated lesion in the acute phase that disappeared during the disease course and was normalized on coronary angiography. In the present study, stenotic lesions were defined as lesions with ≥75% stenosis on angiography. Based on the above definitions, the morphology of CALs was classified into regression, small aneurysm, moderate aneurysm, giant aneurysm, and stenotic complications.
2.2.1.2. Coronary Fractional Flow Reserve (FFR)
A 5 F or 6 F guiding catheter was inserted into the coronary artery, and a pressure wire (PressureWire
TM by RADI Medical Systems, MA, USA) was inserted into the distal part of the CAL. The intracoronary pressure (Pd) at the distal part of the CAL and the pressure at the coronary artery inlet (Pa) were measured simultaneously. The right atrial pressure (Pv) was simultaneously measured with a 5 F or 6 F balloon catheter inserted into the right atrium. FFR was calculated by measuring Pd, Pa, and Pv during maximal coronary artery filling with intracoronary papaverine hydrochloride (0.1-0.2 mg/kg). FFR was calculated using the following formula [
8]:
A significant decrease in the value of FFR suggests ischemia in the perfused myocardial region of that coronary artery and is an indicator of the functional severity of regional coronary artery stenosis [
3]. The reference value for children is <0.80, as with adults [
9]. FFR ≥0.8 is considered normal, whereas FFR <0.8 is considered abnormal.
2.3. 13N-Ammonia PET
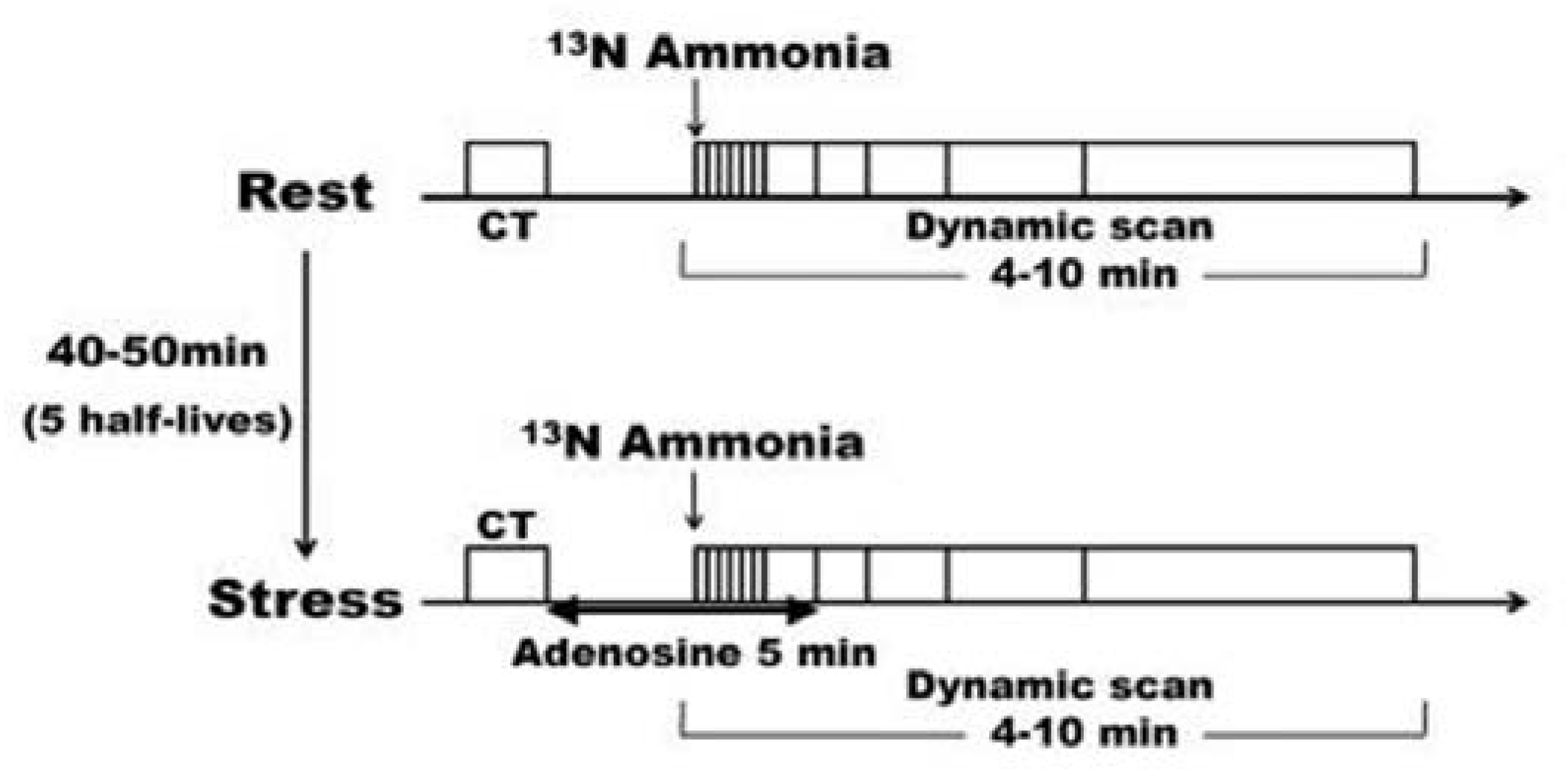
The protocol for the adenosine-stressed
13N-ammonia PET scan is shown in
Figure 1. After attenuation correction with a chest CT scan, a bolus of 7.4 MBq/kg of 13N-ammonia tracer with 30 ml of saline was administered via the right antecubital vein within 20 s, and data collection for 10 min began simultaneously with administration. Absolute MBF and MFR values were calculated by a 1-issue (intravascular – intramyocardial) 2-compartment model analysis using a list mode data from 4 min (6 s/frame × 20, 30 s/frame × 2, 60 s/frame × 1) after administration. An electrocardiogram-gated left ventricular function analysis was also performed. Pharmacological stress imaging using adenosine was initiated 40–50 min after resting imaging (4–5 half-lives). Adenosine (144 μg/kg/min) was administered through the left antecubital vein for over 5 min while monitoring the electrocardiogram, blood pressure, and oxygen saturation. Three minutes after the start of adenosine administration, a
13N-ammonia tracer was administered using the same tracer dose and procedure as at rest. MBF at rest and during adenosine stress, and MFR, the MBF ratio between stress and resting states, were calculated.
In the normal myocardium, vasodilators decrease vascular resistance and increase MFR. However, when coronary artery damage occurs, the increase in blood flow in the area is restricted, and the MFR is low. Myocardial ischemia occurs when the MFR is <2.0 [
10]; hence, a value of 2.0 or higher is considered normal. MFR ≥2.0 is considered normal, and MFR <2.0 is considered abnormal.
2.4. Evaluation Items
Based on the above data, we compared MBF (at rest and during stress) between normal and abnormal FFR groups, compared MBF (at rest and during stress) between normal and abnormal MFR groups, compared FFR and MFR with and without collateral vessels, and examined the relationship between collateral vessels and MBF (at rest and during stress). The hemodynamics of cases with inconsistent FFR and MFR were also discussed.
2.5. Statistics
For continuous variables of FFR, MFR, and MBF, the data are expressed as mean ± standard deviation, and analysis of variance was used for comparisons. Fisher’s two-tailed test was used for comparison of definition scales. Statistical analysis was performed using JMP statistical software version 16 (SAS Institute Inc, Cary, NC, USA). P<0.05 was considered statistically significant.
3. Results
The contrast findings of the 24 coronary branches showed two small aneurysms, seven moderate aneurysms, and nine giant aneurysms, including six cases of regression (
Table 1). Six cases were complicated by stenosis (four giant aneurysms and two regressions). Six cases had an abnormal FFR <0.8, and five had an abnormal MFR <2.0. Sixteen patients (Cases A-P) had normal FFR and MFR, three (Cases Q-S) had abnormal values for both, three (Cases V-X) had abnormal FFR, and two (Cases T and U) had abnormal MFR. All six patients with stenosis had abnormal FFR, and three of them had collaterals. In addition, the collateral blood circulation in case T was supplying the LAD and RCA regions from the LCX and not the receiving side of the LCX region. Six cases underwent coronary artery bypass grafting (CABG) after the examination.
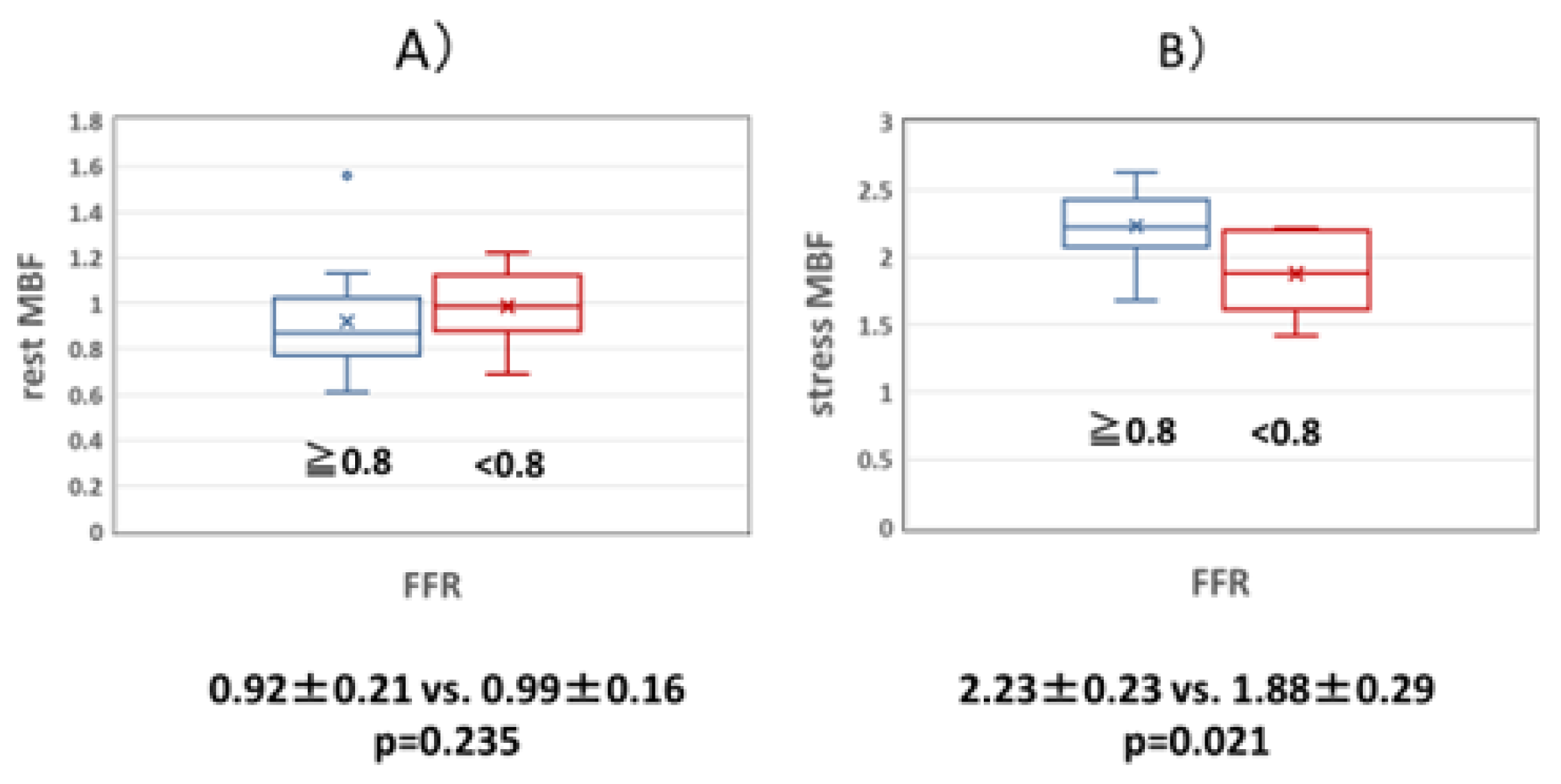
The relationship between MBF, FFR, and MFR was examined. Although there was no significant difference in resting MBF between the normal and abnormal FFR groups (0.92±0.21 vs. 0.99±0.16, p=0.235), the MBF during adenosine loading was significantly lower in the abnormal FFR group than in the normal FFR group (2.23±0.23 vs. 1.88±0.29, p=0.021) (
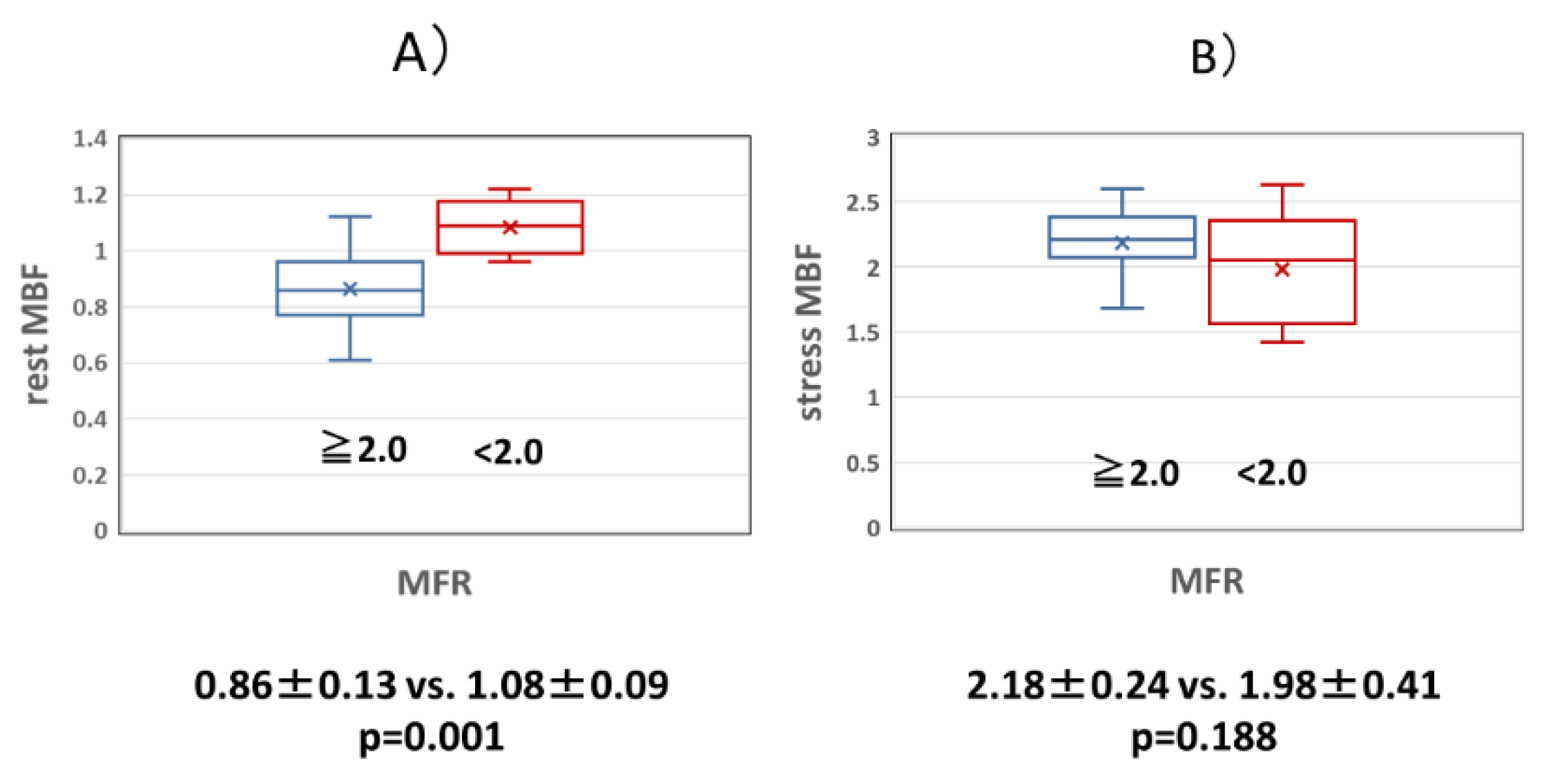
Figure 2). MBF at rest in the abnormal MFR group was significantly higher than that in the normal MFR group (0.86±0.13 vs. 1.08±0.09, p=0.001), but MBF during adenosine loading was not significantly different between the normal and abnormal MFR groups (2.18±0.24 vs. 1.98±0.41, p=0. 188) (
Figure 3).
In the abnormal FFR group with stenosis in all cases, rest and stress MBF were examined in the presence and absence of collateral vessels, respectively. Rest MBF was significantly lower with collateral vessels (0.86±0.12 vs. 1.11±0.08, p=0.041). Moreover, stress MBF did not differ significantly between patients with and without collateral vessels (2.03±0.25 vs. 1.73±0.26, p=0.149) but tended to be higher when collateral vessels were present. Therefore, MFR was significantly higher in the presence of collateral blood vessels (
Table 2). Regarding classification by aneurysm size, no stenosis, abnormal FFR, abnormal MFR, or collateral vessels were observed in small and medium aneurysms.
Among the cases with inconsistent FFR and MFR results, 13N-ammonia PET images and coronary angiography images of Cases T, U, and V are shown.
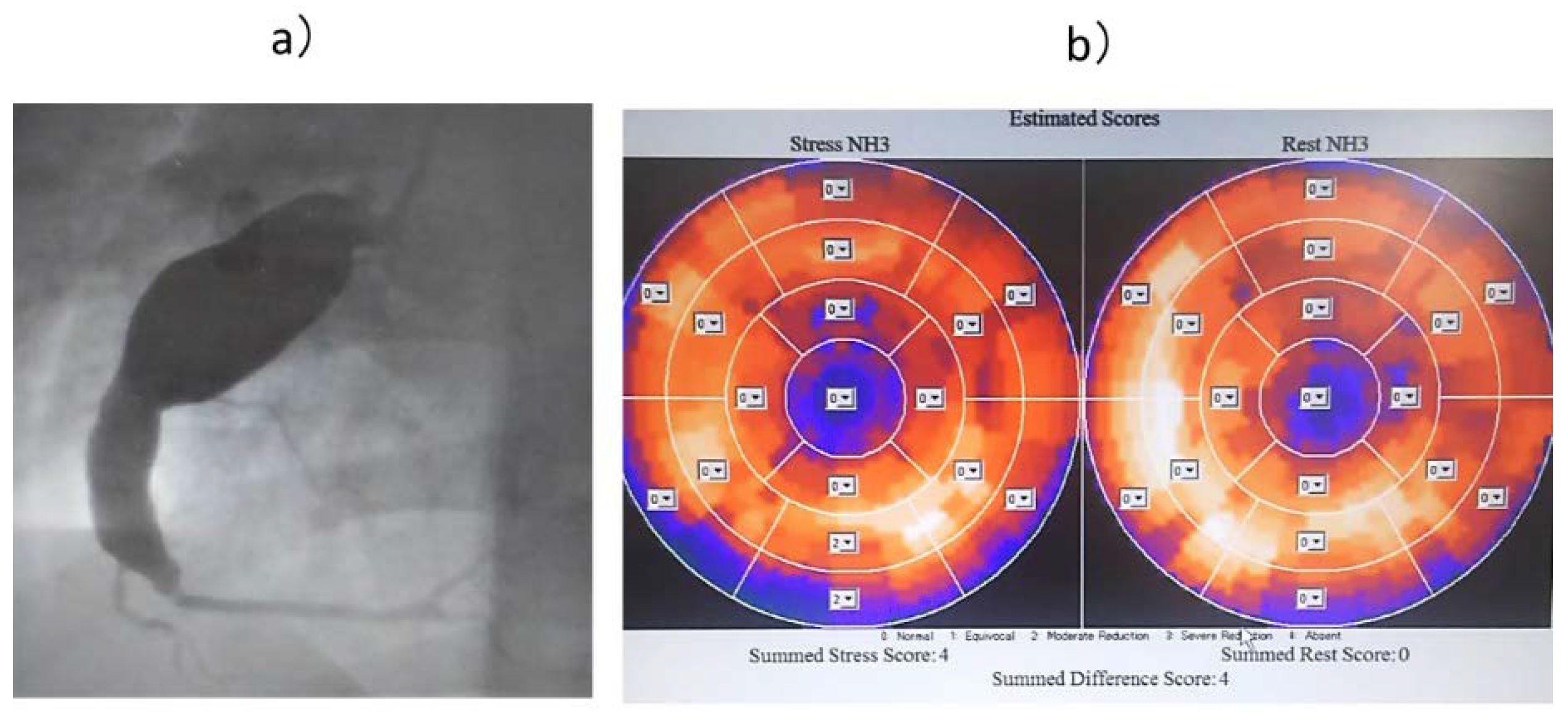
In Case T (
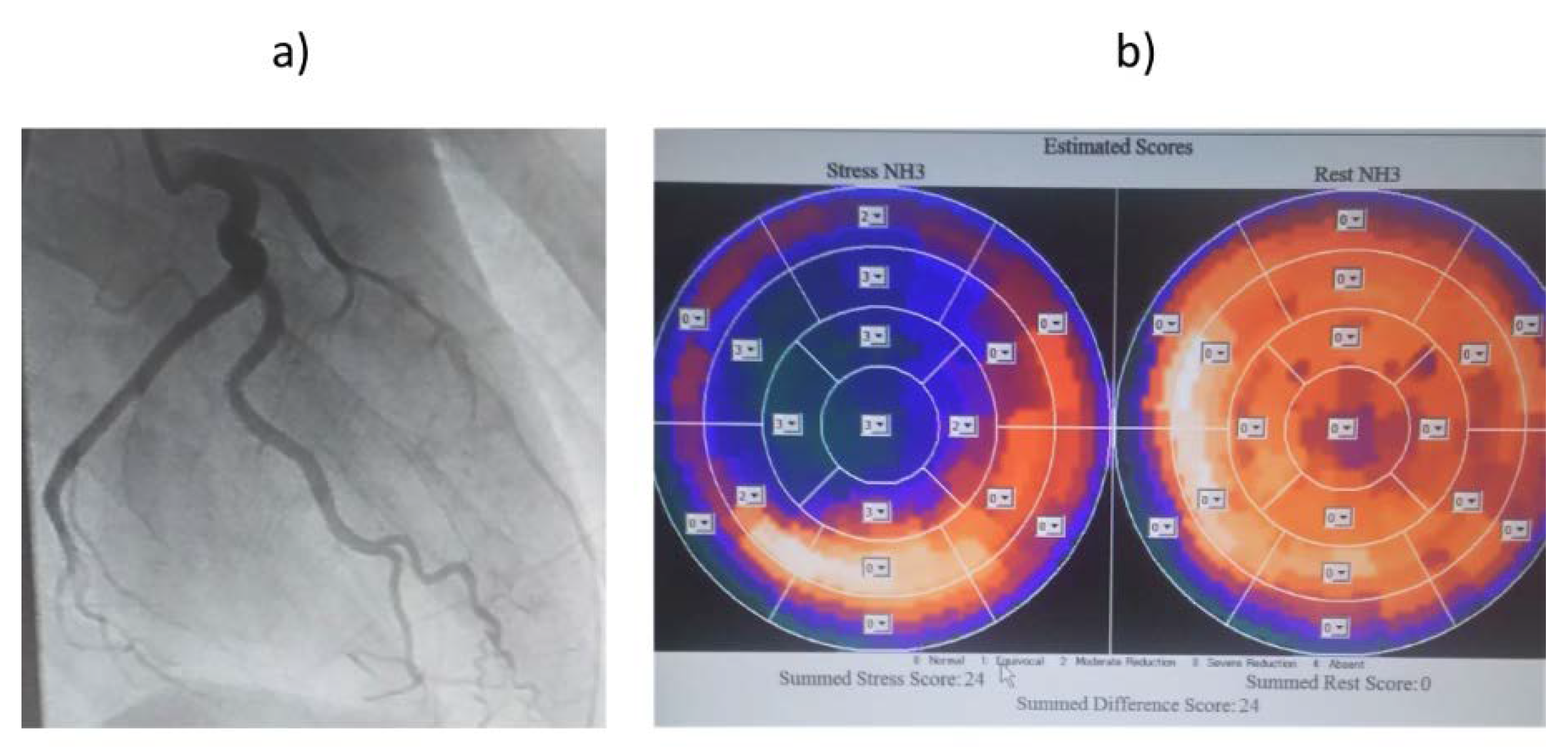
Figure 4), the LAD and RCA were occluded, and the LCX alone was responsible for the coronary circulation, with collateral blood flow from the LCX to the LAD and RCA regions. Therefore, MFR was thought to be low because of the high resting blood flow of 1.56 ml/min/g in the LCX. We judged that there was no myocardial ischemia in the perfused area of the LCX, and no therapeutic intervention was performed on the LCX.
In Case U (
Figure 5), the FFR was normal because the aneurysm was so large that a pressure gradient was unlikely to occur, and the MFR was low because the MBF at rest was as high as 1.13 ml/min/g because the peripheral coronary arteries were already dilated at rest.
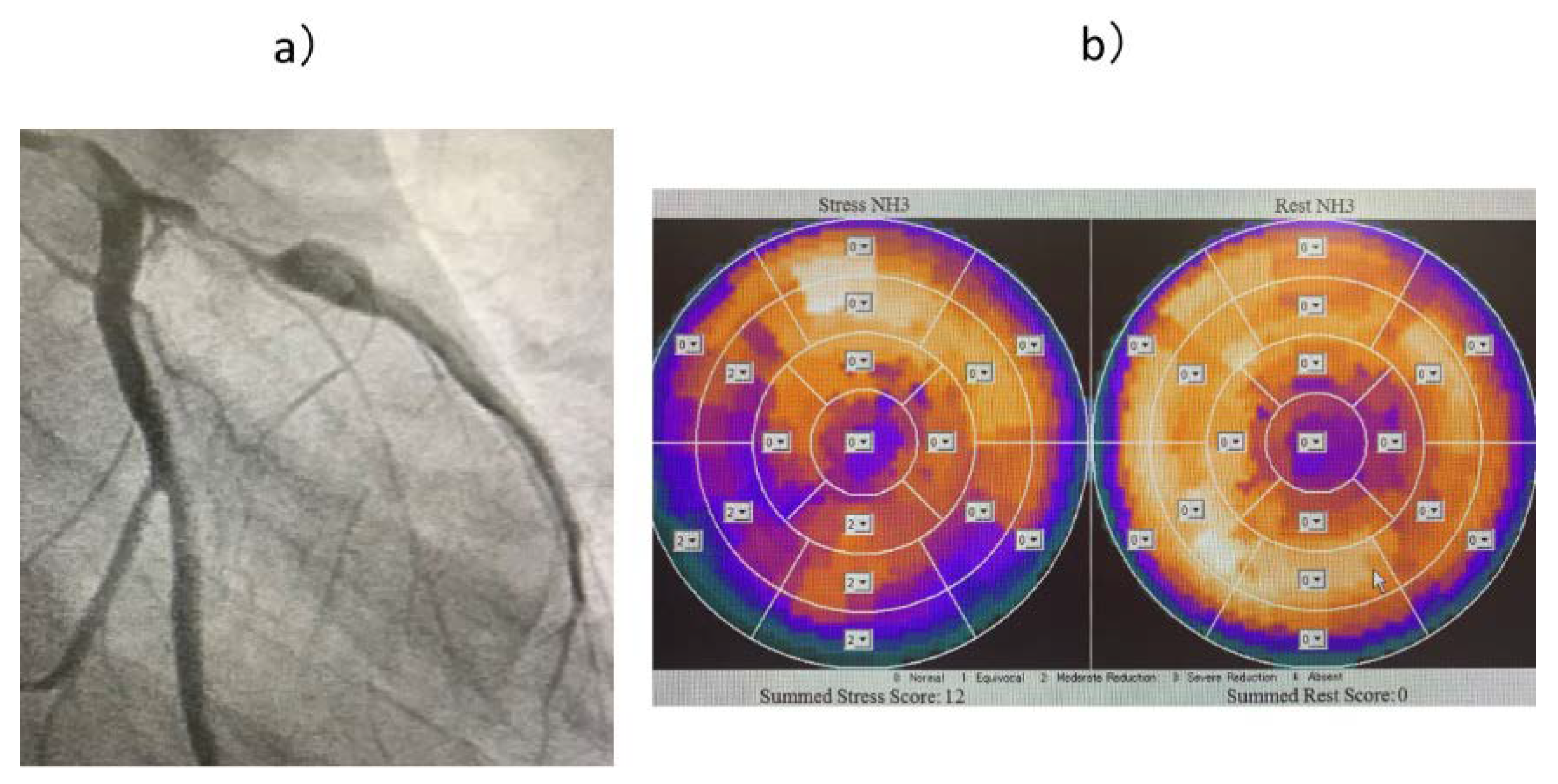
In Cases V (
Figure 6), W, and X, only the FFR was abnormal, and although there was no evidence of myocardial peripheral circulatory disturbance, the FFR was thought to be low owing to a stenotic lesion. All three patients were considered to have significant stenotic lesions and underwent CABG.
4. Discussion
CALs after Kawasaki disease differ from adult coronary circulation disorders in that collateral vessels often develop into stenotic lesions. CALs in Kawasaki disease begin acutely as dilated lesions but may develop into stenotic lesions due to ongoing vascular remodeling [
11]. Progression of the stenotic lesion is slow, during which collateral blood vessels begin to develop in the perfusion area of the stenotic lesion [
12]. As a result, it can be difficult to assess myocardial ischemia and determine therapeutic intervention, as the stenotic lesions are often asymptomatic even when advanced. In our study, FFR and MFR are indicators of myocardial ischemia, but even in CALs after Kawasaki disease, FFR is abnormal in all cases of stenosis, and stress MBF was also significantly lower in the group with abnormal FFR, which may be a useful indicator of stenotic lesions. On the other hand, 13N-ammonia PET can calculate MFR, an index that takes peripheral circulatory disturbance into account, as well as the absolute value of myocardial blood flow at rest and under load. Although the presence of collateral vessels and high resting coronary blood flow can result in low MFR values even in the absence of peripheral circulatory impairment, more detailed assessment of coronary circulatory dynamics is possible by considering the absolute value of MBF.
FFR is a measure of the percentage contribution of vascular regional lesions to overall blood flow impairment, and the DEFER and FAME studies have shown the validity of FFR as a criterion (FFR < 0.8) to determine the indication for revascularization therapy [
2,
3]. Conversely, CFR is reduced owing to increased resting coronary blood flow and decreased blood flow during maximal coronary dilatation. Resting coronary blood flow increases with increasing heart rate, systolic blood pressure, and myocardial contractility. Resting coronary blood flow also increases when epicardial stenosis is present because the microvasculature dilates from rest, to increase the vascular bed and decrease peripheral vascular resistance, to prevent the myocardium from becoming ischemic. Maximal coronary blood flow at the diastole is reduced by local vascular lesions and myocardial microcirculatory disturbances. In other words, CFR is an index that considers the morphological stenosis of the coronary arteries and the functional abnormality of the microcirculation [
4]. Cases with CFR ≥2.0 have a cardiovascular accident rate of <1.0%, and CFR is considered an indicator of peripheral circulation and prognosis [
13].
Inconsistency between anatomic stenosis and CFR has been reported in the adult cardiovascular field [
14]. A relationship exists between FFR and CFR (stress MBF); cases with higher CFR (stress MBF) tend to have lower FFR, indicating higher myocardial blood flow at maximal hyperemia. In addition, patients with low stress MBF (CFR), such as older adults and patients with diabetes mellitus and chronic kidney disease, tend to have low blood flow at maximal hyperemia, making it difficult for a pressure gradient to occur before and after stenosis, resulting in a high FFR value. Considering the relationship between both indicators, it is inferred that cases with an FFR of ≥0.8 and a CFR of <2.0 are those with a strong microcirculatory disturbance, whereas cases with an FFR of ≤0.8 and a CFR of ≥2.0 are local lesions with adequate microcirculation [
15]. The prognosis of patients with CFR <2.0 and FFR >0.8 was reported to be the worst, whereas that of patients with CFR >2 and FFR <0.8 was almost equal to that of the group with both indices at normal values [
4].
In this study, five branches had inconsistent FFR and MFR results. Two branches (Cases T and U) with abnormal MFR only had no stenotic lesions and normal FFR. In Case T, the LCX alone was responsible for the coronary circulation, and the MFR was thought to be low because of the high rest MBF. Case U showed no obvious stenotic lesion, but because of the giant aneurysm, the blood flow velocity was reduced, and it was assumed that the small coronary artery was already highly dilated at rest. In general, peripheral vascular resistance decreases with the progression of stenosis in coronary artery lesions; the more advanced the stenosis, the more dilated the small coronary arteries become to reduce resistance and maintain constant myocardial blood flow at rest. This decreases vasodilatory reserve and reduces stress MBF and CFR [
16]. However, since Cases U and T were children or young adults, even if rest MBF was elevated, they still had sufficient remaining coronary vasodilatory reserve; both stress MBF values were >2.0, suggesting that these patients did not have myocardial ischemia. It has been reported that even in cases with coronary aneurysms, low MFR is associated with a depressed coronary vascular resistance response [
17]; the present study showed that rest MBF was significantly higher in the group with abnormal MFR, suggesting that the peripheral coronary arteries were dilated at rest. The fact that the stress MBF did not differ significantly between the normal and abnormal MFR groups suggests that children and young adults maintain a high vasodilatory reserve, unlike middle-aged and older patients with epicardial stenosis who have clinical risk factors and advanced atherosclerosis, including microangiopathy.
The six branches with abnormal FFR all had more than 75% stenotic lesions on contrast. As stenotic lesions in coronary arteries progress, the CFR decreases, and its value correlates with the stenosis rate [
16]. For the six branches with abnormal FFR only, the reason for the normal MFR values besides the negative presence of peripheral circulatory disturbance and despite stenosis of >90% in three branches, was speculated to be the presence of collateral vessels. We also examined collateral vessels in relation to FFR, MFR, and MBF and found that rest MBF was significantly lower and stress MBF tended to be higher when collateral vessels were present. Therefore, MFR was normal in cases with collateral vessels. This is thought to be due to the low myocardial blood flow, which led to the presence of collateral vessels, while the presence of collateral vessels contributed to the increased myocardial blood flow during adenosine loading. In other words, all three branches with collateral vessels received blood supply from collateral vessels in the dominant region. Thus, MBF was maintained under load, which may have resulted in normal MFR values.
As mentioned above, a high blood flow (stress MBF) at maximal coronary dilatation increases the pressure gradient before and after a stenotic lesion, decreasing FFR. The LAD has a large perfusion area, and the increase in blood flow during maximal coronary dilatation is considered significant. Therefore, a large increase in blood flow through the stenotic lesion increases the pressure gradient and decreases FFR [
4]. In this case, FFR would be treated as a false positive, but since the three branches in this study have stenotic lesions, the abnormal FFR value was considered significant.
Since stress MBF can be high even when FFR and CFR are abnormal, it seemed important to consider MBF along with FFR and MFR in addition to morphological diagnosis including collateral vessels. As a result, more detailed assessment of peripheral circulation and stenotic lesions in the coronary arteries may be useful in determining treatment strategy and timing of intervention. In this study, stress MBF tended to be high, and if the stress MBF is high enough, ischemia during exercise is unlikely to occur. Even if FFR and MFR are low, surgical intervention may not be required if sufficient stress MBF is maintained, so further findings are needed.
The study had some limitations. The number of cases is limited, and the absolute number is small. Although cardiac function may be impaired in cases of suspected myocardial peripheral circulatory disturbance, we could not assess cardiac function in cases with low MFR. Furthermore, only the presence or absence of collateral vessels was evaluated in this study. The blood flow in the collateral vessels was not evaluated, necessitating a more detailed evaluation of collateral blood flow involvement in the future.
5. Conclusions
Because of the complex hemodynamics of CAL, it is often challenging to assess myocardial ischemia in clinical settings. The combination of FFR from cardiac catheterization and MFR and MBF from 13N-ammonia PET, in addition to morphological diagnostics such as collateral vessels, will enable a more accurate assessment of peripheral coronary circulation and stenotic lesions in CAL and may be useful in determining treatment strategy and timing of intervention.
Author Contributions
Conceptualization, M.W. and R.F.; methodology, M.W.; software, R.F.; validation, M.W., R.F., T.K., R.M.; formal analysis, R.F., T.K., S.I.; investigation, M.W., K.H., Y.H., and K.S.; data curation, R.F.; writing—original draft preparation, M.W.; writing—review and editing, R.F., T.K.; visualization, Y.I.; supervision, M.A. and M.K.; project administration, R.F. and Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the ethics committee of the Nippon Medical School (No. B-2021-360).
Informed Consent Statement
All participants provided written informed consent before the examinations.
Data Availability Statement
The original contributions presented in the study are included in the article material; further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank Editage (
www.editage.com) for English language editing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fukazawa, R.; Kobayashi, J.; Ayusawa, M.; et al. JCS/JSCS 2020 Guideline on Diagnosis and Management of Cardiovascular Sequelae in Kawasaki Disease. Circ J 2020, 84, 1348–1407. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.J.; van Schaardenburgh, P.; Manoharan, G.; et al. Percutaneous coronary intervention of functional nonsignificant stenosis: 5-year follow up of the DEFER study. J Am Coll Cardiol 2007, 49, 2105–2111. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, B.; Piljs, N.H.J.; Kalesan, B.; et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012, 367, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- van de Hoef, T.P.; van Lavieren, M.A.; Damman, P.; et al. Physiological Basis and Long-Term Clinical Outcome of Discordance Between Fractional Flow Reserve and Coronary Flow Velocity Reserve in Coronary Stenoses of Intermediate Severity. Circ Cardiovasc Interv 2014, 7, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Machac, J.; Bacharach, S.L.; Bateman, T.M.; et al. Positron emission tomography myocardial perfusion and glucose metabolism imaging. J Nucl Cardiol 2006, 13, e121–151. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Gropler, R.J.; Jones, T.; et al. The impact of myocardial blood flow quantitation with PET on the understanding of cardiac diseases. Eur Heart J 1996, 17, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Bateman, T.M.; Beanlands, R.S.; et al. Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Med 2018, 59, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Pijls, N.H.; van Son, J.A.; Kirkeeide, R.L.; De Bruyne, B.; Gould, KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation 1993, 87, 1354–1367. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Ohkubo, T.; Fukazawa, R.; et al. Estimation of myocardial hemodynamics before and after intervention in children with Kawasaki disease. J Am Coll Cardiol 2004, 43, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.J.; de Bruyne, B.; Pijls, N.H. From research to clinical practice: current role of intracoronary physiologically based decision making in the cardiac catheterization laboratory. J Am Coll Cardiol 1997, 30, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, J.M.; Shulman, S.T.; Fox, L.M.; et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS One 2012, 7, e38998. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Miyagawa-Tomita, S.; Nakazawa, M.; et al. Remodeling of coronary artery lesions due to Kawasaki disease: comparison of arteriographic and immunohistochemical findings. Jpn Heart J 2000, 41, 245–256. [Google Scholar] [PubMed]
- Gould, K.L. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging 2009, 2, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Manabe, O.; Naya, M.; Tamaki, N. Feasibility of PET for the management of coronary artery disease: Comparison between CFR and FFR. J Cardiol 2017, 70, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Kirkeeide, R.L.; Gould, K.L. Is discordance of coronary flow reserve and fractional flow reserve due to methodology or clinically relevant coronary pathophysiology? JACC Cardiovasc Imaging 2012, 5, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Tsuno, K.; Fukazawa, R.; Kiriyama, T.; et al. Peripheral Coronary Artery Circulatory Dysfunction in Remote Stage Kawasaki Disease Patients Detected by Adenosine Stress 13N-Ammonia Myocardial Perfusion Positron Emission Tomography. J Clin Med 2022, 11, 1134. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Lipscomb, K.; Hamilton, G.W. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol 1974, 33, 87–94. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Adenosine-loaded 13N-ammonia PET examination protocol. The same protocol was used for both the resting and loading examinations except for the adenosine administration, and the interval between the resting and loading examinations was 4–5 half-lives. CT: computed tomography, PET: positron emission tomography.
Figure 1.
Adenosine-loaded 13N-ammonia PET examination protocol. The same protocol was used for both the resting and loading examinations except for the adenosine administration, and the interval between the resting and loading examinations was 4–5 half-lives. CT: computed tomography, PET: positron emission tomography.
Figure 2.
(a) Comparison of resting MBF in the normal and abnormal FFR groups. There was no significant difference in resting MBF between the normal and abnormal FFR groups. (b) Comparison of stress MBF in the normal and abnormal FFR groups. The abnormal FFR group had significantly lower stress MBF than the normal FFR group. FFR: coronary fractional flow reserve, MBF: myocardial blood flow volume fraction.
Figure 2.
(a) Comparison of resting MBF in the normal and abnormal FFR groups. There was no significant difference in resting MBF between the normal and abnormal FFR groups. (b) Comparison of stress MBF in the normal and abnormal FFR groups. The abnormal FFR group had significantly lower stress MBF than the normal FFR group. FFR: coronary fractional flow reserve, MBF: myocardial blood flow volume fraction.
Figure 3.
(a) Comparison of resting MBF in the normal and abnormal MFR groups. The rest MBF was significantly higher in the abnormal MFR group than in the normal MFR group. (b) Comparison of stress MBF in the normal and abnormal MFR groups. There was no significant difference in stress MBF between the normal and abnormal MFR groups. MBF: myocardial blood flow volume fraction, MFR: myocardial blood flow reserve.
Figure 3.
(a) Comparison of resting MBF in the normal and abnormal MFR groups. The rest MBF was significantly higher in the abnormal MFR group than in the normal MFR group. (b) Comparison of stress MBF in the normal and abnormal MFR groups. There was no significant difference in stress MBF between the normal and abnormal MFR groups. MBF: myocardial blood flow volume fraction, MFR: myocardial blood flow reserve.
Figure 4.
(a) Coronary angiography image of Case T. (b) 13N-ammonia PET image of Case T. Owing to occlusion of the LAD and RCA, only LCX is responsible for coronary circulation. MFR may be low due to high blood flow at rest. LAD: left anterior descending artery, LCX: left circumflex artery, MFR: myocardial blood flow reserve, RCA: right coronary artery, PET: positron emission tomography.
Figure 4.
(a) Coronary angiography image of Case T. (b) 13N-ammonia PET image of Case T. Owing to occlusion of the LAD and RCA, only LCX is responsible for coronary circulation. MFR may be low due to high blood flow at rest. LAD: left anterior descending artery, LCX: left circumflex artery, MFR: myocardial blood flow reserve, RCA: right coronary artery, PET: positron emission tomography.
Figure 5.
(a) Coronary angiography image of Case U. (b) 13N-ammonia PET image of Case U. The FFR was normal because of the giant aneurysm, and the MFR was low because the peripheral coronary arteries were already dilated at rest. FFR: coronary fractional flow reserve, MFR: myocardial blood flow reserve, PET: positron emission tomography.
Figure 5.
(a) Coronary angiography image of Case U. (b) 13N-ammonia PET image of Case U. The FFR was normal because of the giant aneurysm, and the MFR was low because the peripheral coronary arteries were already dilated at rest. FFR: coronary fractional flow reserve, MFR: myocardial blood flow reserve, PET: positron emission tomography.
Figure 6.
(a) Coronary angiography image of Case V. (b) 13N-ammonia PET image of Case V. The FFR was low due to the presence of a stenotic lesion, but it was thought the peripheral circulation was not impaired due to the collateral blood vessels. FFR: coronary fractional flow reserve; PET: positron emission tomography.
Figure 6.
(a) Coronary angiography image of Case V. (b) 13N-ammonia PET image of Case V. The FFR was low due to the presence of a stenotic lesion, but it was thought the peripheral circulation was not impaired due to the collateral blood vessels. FFR: coronary fractional flow reserve; PET: positron emission tomography.
Table 1.
Case background.
Table 1.
Case background.
| Case |
Site |
AN |
Stenosis |
FFR |
MFR |
Rest MBF |
Stress MBF |
Collateral |
Intervention |
Stenosis ratio(%) |
| A |
#8 |
reg |
- |
0.93 |
2.61 |
0.96 |
2.6 |
- |
- |
|
| B |
#2 |
giant |
- |
0.82 |
2.3 |
0.9 |
2.07 |
- |
- |
|
| C |
#13 |
mod |
- |
0.91 |
2.28 |
1.06 |
2.42 |
- |
- |
|
| D |
#7 |
mod |
- |
0.96 |
2.89 |
0.77 |
2.24 |
- |
- |
|
| E |
#7 |
giant |
- |
0.8 |
2.76 |
0.61 |
1.68 |
- |
- |
|
| F |
#12 |
mod |
- |
0.93 |
2.22 |
1.10 |
2.26 |
- |
- |
|
| G |
#2 |
giant |
- |
0.83 |
3.26 |
0.77 |
2.5 |
- |
- |
|
| H |
#2 |
small |
- |
0.96 |
2.19 |
1.12 |
2.45 |
- |
- |
|
| I |
#7 |
mod |
- |
0.95 |
2.75 |
0.8 |
2.21 |
- |
- |
|
| J |
#1 |
small |
- |
0.88 |
3.18 |
0.75 |
2.38 |
- |
- |
|
| K |
#7 |
giant |
- |
0.83 |
2.48 |
0.86 |
2.13 |
- |
- |
|
| L |
#2 |
mod |
- |
0.95 |
2.34 |
0.85 |
1.99 |
- |
- |
|
| M |
#9 |
mod |
- |
0.95 |
2.14 |
0.99 |
2.11 |
- |
- |
|
| N |
#13 |
mod |
- |
0.85 |
2.43 |
0.88 |
2.14 |
- |
- |
|
| O |
#4 |
reg |
- |
0.94 |
2.43 |
0.77 |
2.24 |
- |
- |
|
| P |
#13 |
reg |
- |
0.93 |
2.29 |
0.75 |
1.99 |
- |
- |
|
| Q |
#7 |
giant |
+ |
0.6 |
1.68 |
1.02 |
1.71 |
- |
CABG |
90 |
| R |
#8 |
reg |
+ |
0.5 |
1.3 |
1.09 |
1.42 |
- |
CABG |
99 |
| S |
#7 |
giant |
+ |
0.75 |
1.67 |
1.22 |
2.05 |
- |
CABG |
75 |
| T |
#13 |
reg |
- |
1.04 |
1.69 |
1.56 |
2.63 |
(+)* |
- |
|
| U |
#2 |
giant |
- |
0.96 |
1.83 |
1.13 |
2.08 |
- |
- |
|
| V |
#8 |
reg |
+ |
0.44 |
2.33 |
0.94 |
2.19 |
+ |
CABG |
99 |
| W |
#7 |
Giant |
+ |
0.73 |
2.33 |
0.95 |
2.21 |
+ |
CABG |
90 |
| X |
#11 |
Giant |
+ |
0.55 |
2.42 |
0.69 |
1.68 |
+ |
CABG |
99 |
Table 2.
Comparison of FFR, MFR, rest MBF, and stress MBF with and without collateral blood flow.
Table 2.
Comparison of FFR, MFR, rest MBF, and stress MBF with and without collateral blood flow.
| |
FFR |
MFR |
rest MBF |
stress MBF |
| Collateral (+) |
0.57±0.12 |
2.36±0.04 |
0.86±0.12 |
2.03±0.25 |
| Collateral (-) |
0.62±0.10 |
1.55±0.18 |
1.11±0.08 |
1.73±0.26 |
| P-value |
0.359 |
0.009 |
0.041 |
0.149 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).