Submitted:
21 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
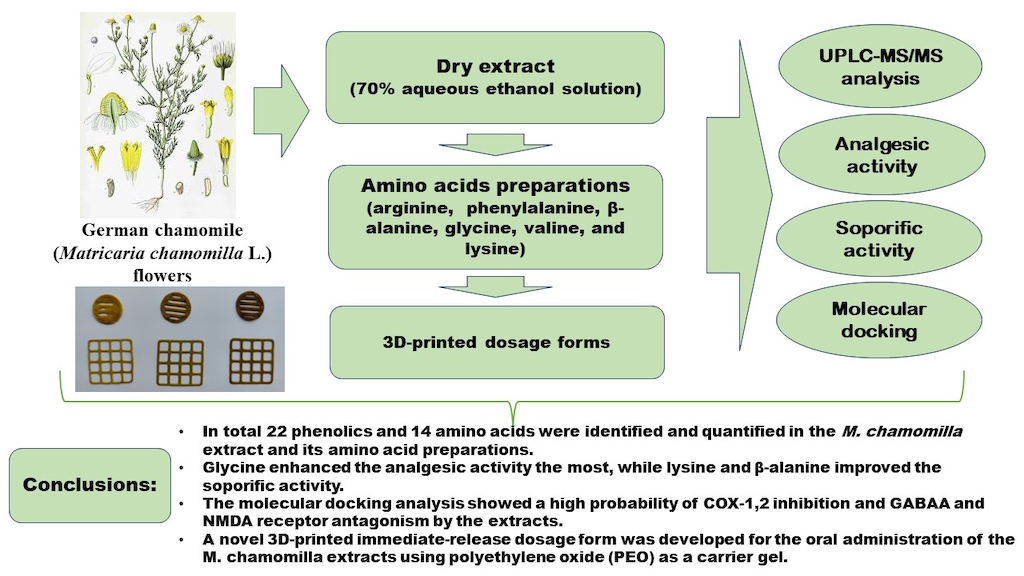
Keywords:
1. Introduction
2. Results
2.1. Phytochemical Study
2.2. Pharmacological Research in Analgesic and Soporific Activit, and Their Molecular Docking Study
2.2.1. Analgesic Activity
2.2.2. Soporific Activity
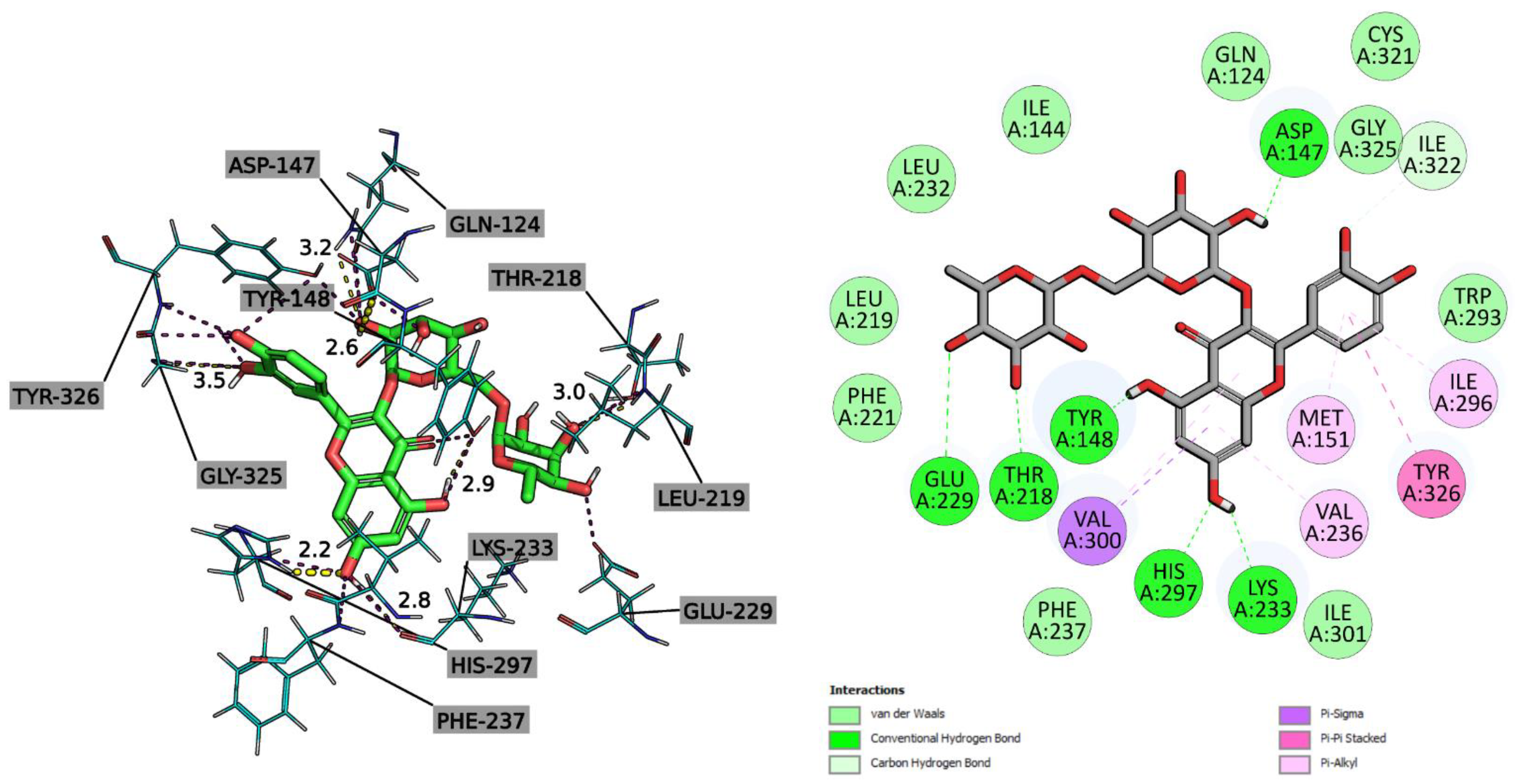
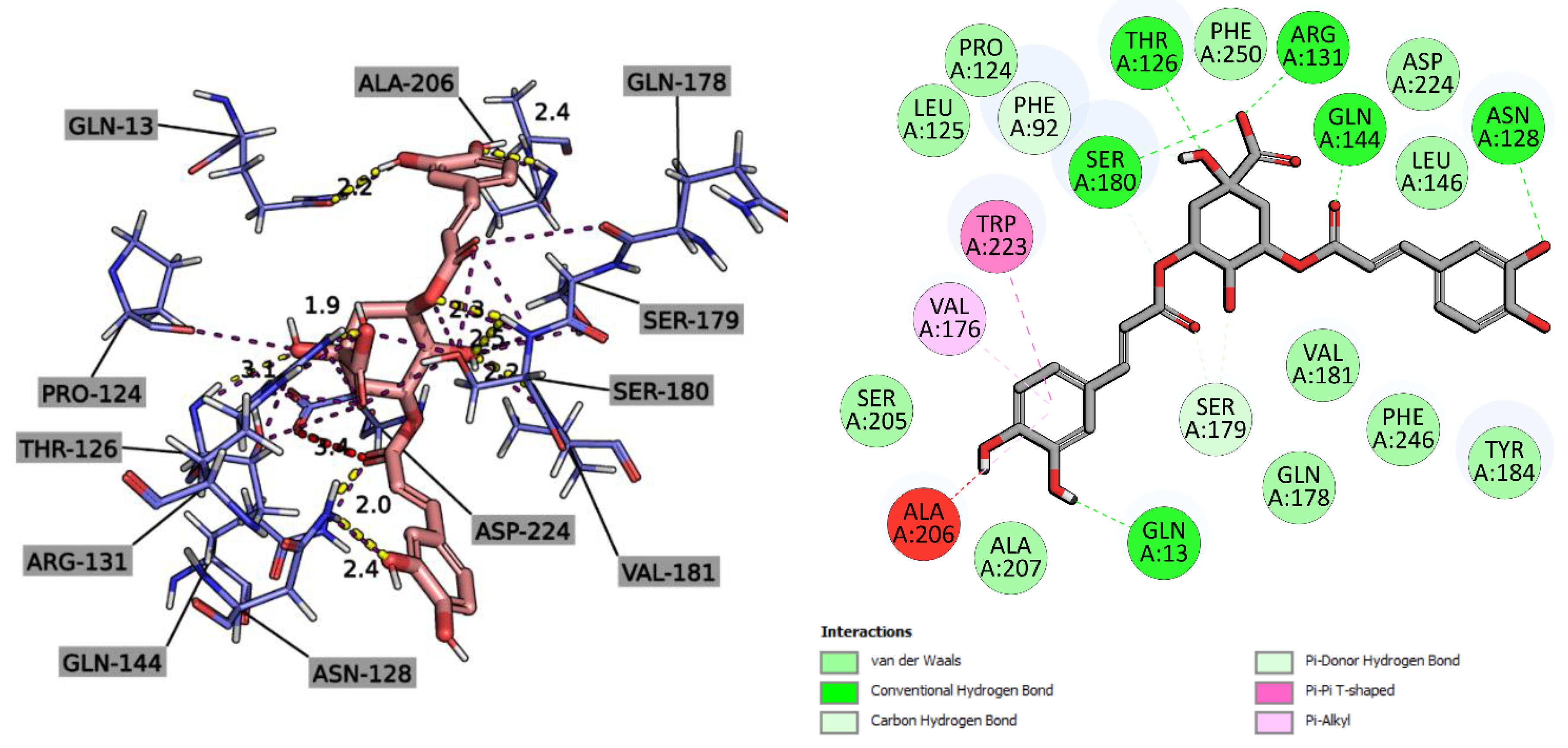
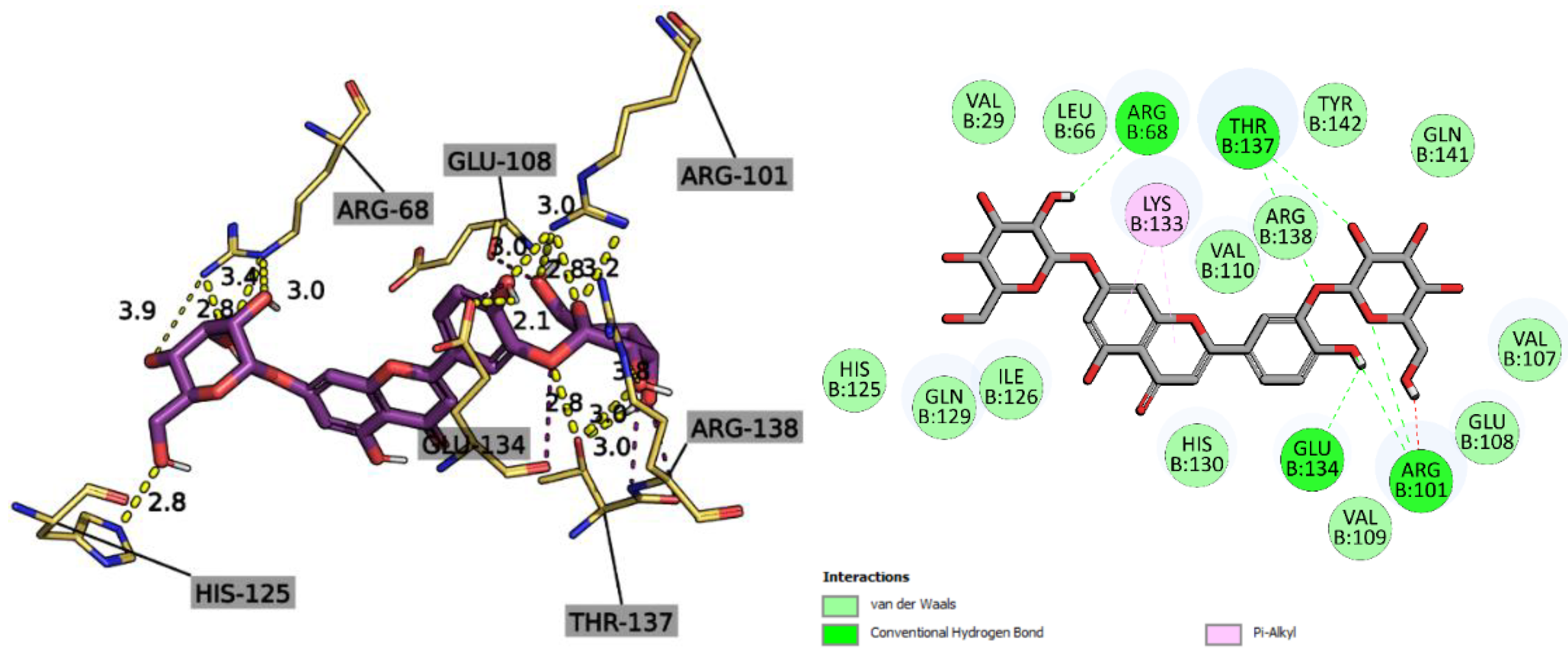
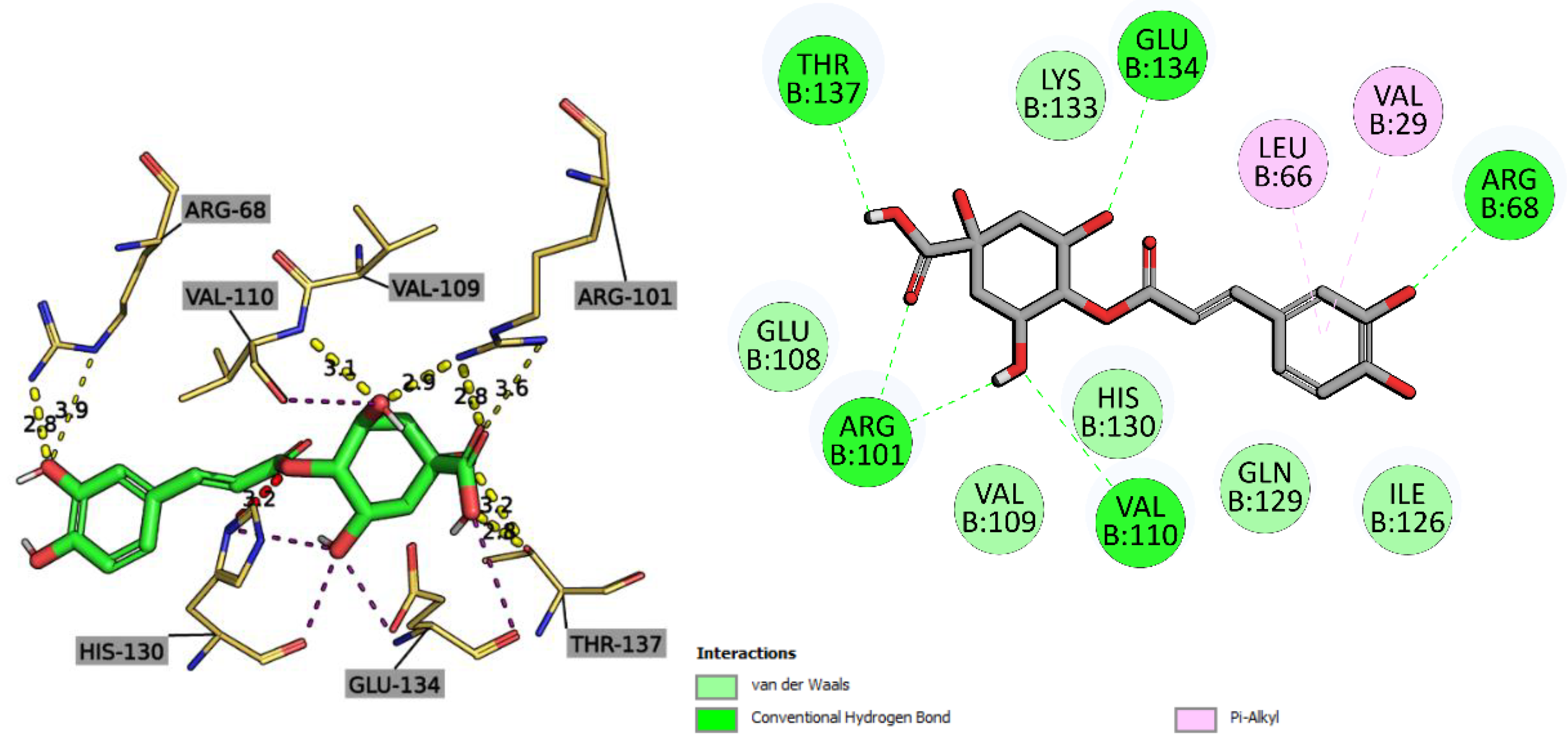
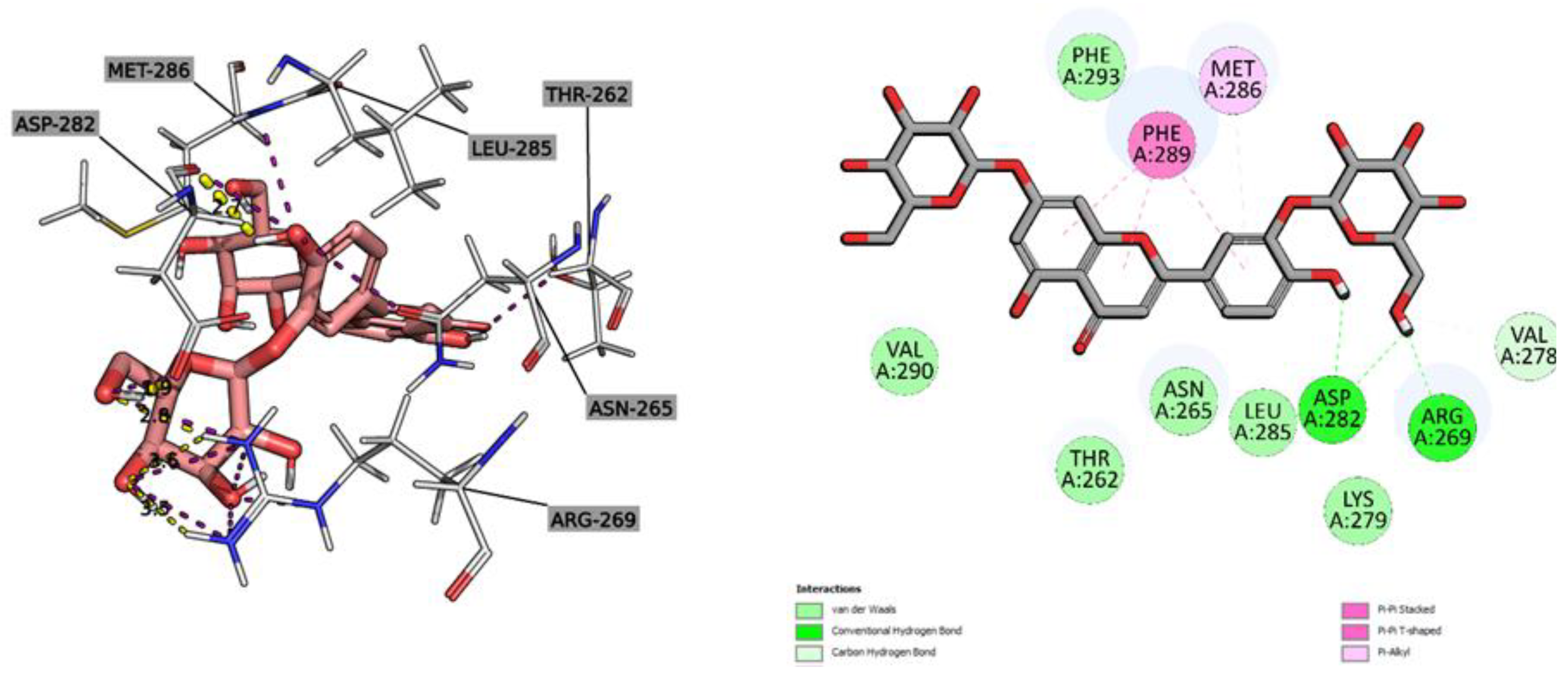
2.2.3. Molecular Docking Study
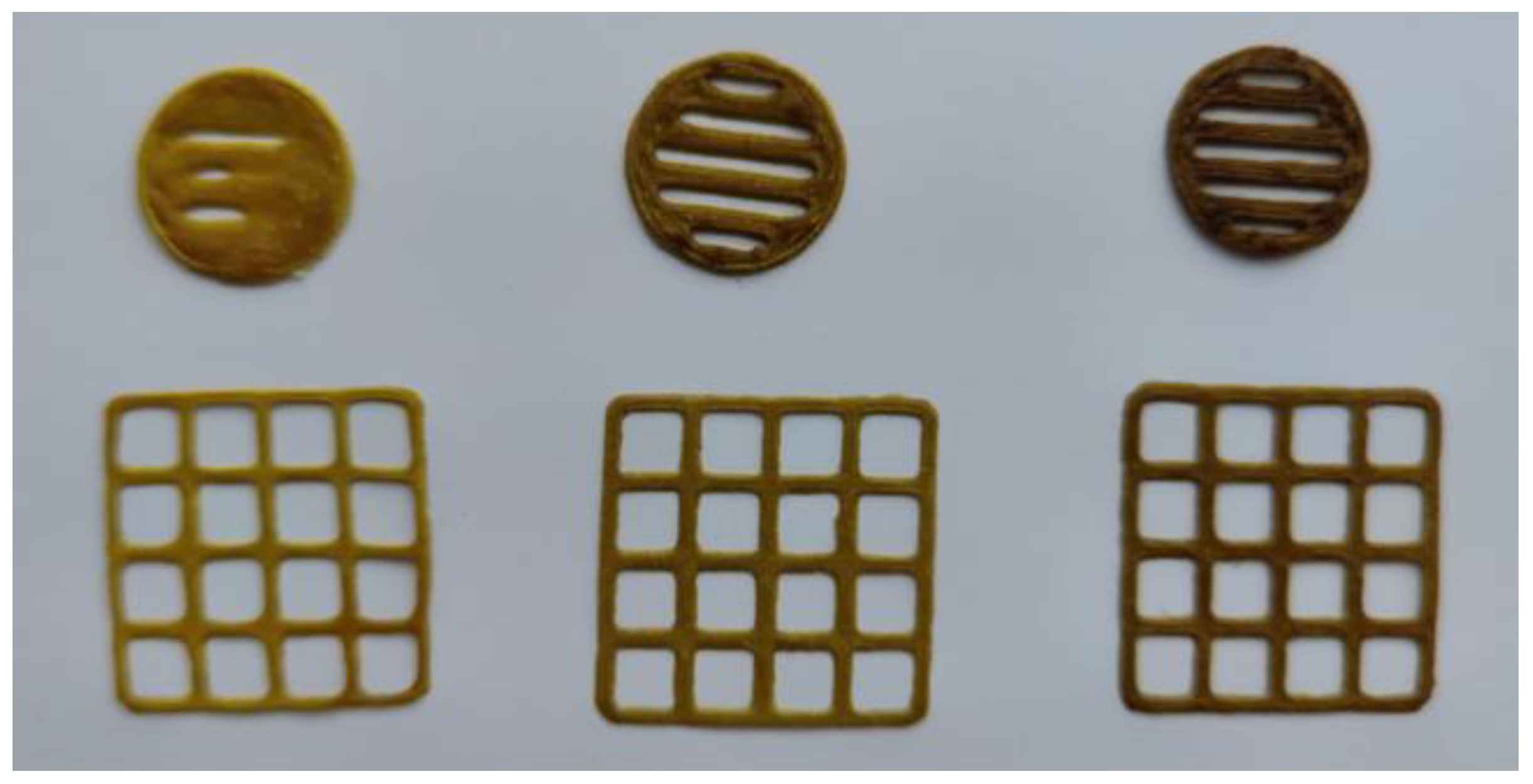
2.3. 3D-printed Oral Dosage forms with the M. chamomilla Dry Extract
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Preparation of Extracts
4.4. Spectrophotometric Assay of Main Groups of Phytochemicals
4.5. Assay of Phenolics by UPLC-MS/MS
4.6. Assay of Amino Acids by UPLC-MS/MS
4.7. Pharmacological Study
4.7.1. Analgesic Activity
4.7.2. Soporific Activity
4.7.3. In Silico Studies
4.8. 3D Printing of M. chamomilla Extract
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mittal, P.; Bansal, P.; Khokra, S.L.; Kaushik, D. Pharmacological potential of matricaria recutita—A review. International Journal of Pharmaceutical Sciences and Drug Research 2010, 2, 12–16. [Google Scholar]
- El Mihyaoui, A.; Esteves Da Silva, J.C.G.; Charfi, S.; Candela Castillo, M.E.; Lamarti, A.; Arnao, M.B. Chamomile (Matricaria chamomilla L.): A Review of Ethnomedicinal Use, Phytochemistry and Pharmacological Uses. Life 2022, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Menale, B.; De Castro, O.; Di Iorio, E.; Ranaldi, M.; Muoio, R. Discovering the Ethnobotanical Traditions of the Island of Procida (Campania, Southern Italy). Plant Biosystems—An International Journal Dealing with all Aspects of Plant Biology 2022, 156, 450–468. [Google Scholar] [CrossRef]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological Notes about Ancient Uses of Medicinal Plants in Trás-Os-Montes (Northern of Portugal). Journal of Ethnopharmacology 2009, 124, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Güzel, Y.; Güzelşemme, M.; Miski, M. Ethnobotany of Medicinal Plants Used in Antakya: A Multicultural District in Hatay Province of Turkey. Journal of Ethnopharmacology 2015, 174, 118–152. [Google Scholar] [CrossRef]
- Sepp, J.; Koshovyi, O.; Jakstas, V.; Žvikas, V.; Botsula, I.; Kireyev, I.; Tsemenko, K.; Kukhtenko, O.; Kogermann, K.; Heinämäki, J.; et al. Phytochemical, Technological, and Pharmacological Study on the Galenic Dry Extracts Prepared from German Chamomile (Matricaria chamomilla L.) Flowers. Plants 2024, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Orav, A.; Püssa, T.; Valner, C.; Malmiste, B.; Arak, E. Content of Essential Oil, Terpenoids and Polyphenols in Commercial Chamomile (Chamomilla Recutita L. Rauschert) Teas from Different Countries. Food Chemistry 2012, 131, 632–638. [Google Scholar] [CrossRef]
- Orav, A.; Raal, A.; Arak, E. Content and Composition of the Essential Oil of Chamomilla Recutita (L.) Rauschert from Some European Countries. Natural Product Research 2010, 24, 48–55. [Google Scholar] [CrossRef]
- Rawat, A.; Gupta, A.; Kholiya, S.; Chauhan, A.; Kumar, D.; Venkatesha, K.T.; Upadhyay, R.K.; Padalia, R.C. Comparative Study of Chemical Composition of Two Cultivars of German Chamomile, Matricaria chamomilla L. Syn Chamomilla recutita L. Rauschert. Journal of Biologically Active Products from Nature 2022, 12, 488–506. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J. Chemical Composition, Antioxidant and Antimicrobial Activity of Chamomile Flowers Essential Oil (Matricaria chamomilla L.). Journal of Essential Oil Bearing Plants 2016, 19, 2017–2028. [Google Scholar] [CrossRef]
- Kravchenko, G.; Krasilnikova, O.; Raal, A.; Mazen, M.; Chaika, N.; Kireyev, I.; Grytsyk, A.; Koshovyi, O. Arctostaphylos uva-ursi L. Leaves Extract and Its Modified Cysteine Preparation for the Management of Insulin Resistance: Chemical Analysis and Bioactivity. Nat. Prod. Bioprospect. 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Koshovyi, O.; Granica, S.; Piwowarski, J.P.; Stremoukhov, O.; Kostenko, Y.; Kravchenko, G.; Krasilnikova, O.; Zagayko, A. Highbush Blueberry (Vaccinium corymbosum L.) Leaves Extract and Its Modified Arginine Preparation for the Management of Metabolic Syndrome—Chemical Analysis and Bioactivity in Rat Model. Nutrients 2021, 13, 2870. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, V.N. Compendium 2020. Medicines; MORION: Kyiv, Ukraine, 2020. [Google Scholar]
- Parfenov, V.A. Use of L-Lysine Aescinate in Central Nervous System Diseases. Neurology, Neuropsychiatry, Psychosomatics 2011, 0, 99. [Google Scholar] [CrossRef]
- Koshovyi, O.; Raal, A.; Kireyev, I.; Tryshchuk, N.; Ilina, T.; Romanenko, Y.; Kovalenko, S.M.; Bunyatyan, N. Phytochemical and Psychotropic Research of Motherwort (Leonurus cardiaca L.) Modified Dry Extracts. Plants 2021, 10, 230. [Google Scholar] [CrossRef]
- Koshovyi, O.M.; Zagayko, A.L.; Kolychev, I.O.; Akhmedov, E.Yu.; Komissarenko, A.N. Phytochemical Study of the Dry Extract from Bilberry Leaves. Azerbaijan Pharmaceutical and Pharmacotherapy Journal 2016, 16, 18–23. [Google Scholar]
- Chaika, N.; Koshovyi, O.; Ain, R.; Kireyev, I.; Zupanets, A.; Odyntsova, V. Phytochemical Profile and Pharmacological Activity of the Dry Extract from Arctostaphylos Uva-Ursi Leaves Modified with Phenylalanine. ScienceRise: Pharmaceutical Science 2020, 0, 74–84. [Google Scholar] [CrossRef]
- Koshovyi, O.; Vlasova, I.; Laur, H.; Kravchenko, G.; Krasilnikova, O.; Granica, S.; Piwowarski, J.P.; Heinämäki, J.; Raal, A. Chemical Composition and Insulin-Resistance Activity of Arginine-Loaded American Cranberry (Vaccinium Macrocarpon Aiton, Ericaceae) Leaf Extracts. Pharmaceutics 2023, 15, 2528. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 11th ed.; Council of Europe: Strasbourg, 2022.
- Koshovyi, O.; Heinämäki, J.; Raal, A.; Laidmäe, I.; Topelius, N.S.; Komisarenko, M.; Komissarenko, A. Pharmaceutical 3D-Printing of Nanoemulsified Eucalypt Extracts and Their Antimicrobial Activity. European Journal of Pharmaceutical Sciences 2023, 187, 106487. [Google Scholar] [CrossRef]
- Viidik, L.; Seera, D.; Antikainen, O.; Kogermann, K.; Heinämäki, J.; Laidmäe, I. 3D-Printability of Aqueous Poly(Ethylene Oxide) Gels. European Polymer Journal 2019, 120, 109206. [Google Scholar] [CrossRef]
- Catani, M.V.; Rinaldi, F.; Tullio, V.; Gasperi, V.; Savini, I. Comparative Analysis of Phenolic Composition of Six Commercially Available Chamomile (Matricaria chamomilla L.) Extracts: Potential Biological Implications. IJMS 2021, 22, 10601. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Romani, A.; Pinelli, P.; Vincieri, F.F.; Prucher, D. Characterization of Matricaria recutita L. Flower Extracts by HPLC-MS and HPLC-DAD Analysis. Chromatographia 2000, 51, 301–307. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, Z.; Chang, X.; Xue, H.; Yahefu, W.; Zhang, X. 4-Hydroxyphenylacetic Acid Prevents Acute APAP-Induced Liver Injury by Increasing Phase II and Antioxidant Enzymes in Mice. Front. Pharmacol. 2018, 9, 653. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, B.F.; Zlotnik, A.; Oleshko, A.; Matalon, F.; Shiyntum, H.N.; Frenkel, A.; Boyko, M. The Relationship between Post-Traumatic Stress Disorder Due to Brain Injury and Glutamate Intake: A Systematic Review. Nutrients 2024, 16, 901. [Google Scholar] [CrossRef]
- Owens, S.L.; Ahmed, S.R.; Lang Harman, R.M.; Stewart, L.E.; Mori, S. Natural Products That Contain Higher Homologated Amino Acids. ChemBioChem 2024, e202300822. [Google Scholar] [CrossRef] [PubMed]
- Henzi, V.; Reichling, D.B.; Helm, S.W.; MacDermott, A.B. L-Proline Activates Glutamate and Glycine Receptors in Cultured Rat Dorsal Horn Neurons. Mol Pharmacol 1992, 41, 793–801. [Google Scholar]
- Abdelnour, S.A.; Khalil, W.A.; Khalifa, N.E.; Khalil, F.M.A.; Hassan, M.A.E. L-Proline: A Promising Tool for Boosting Cryotolerance and Fertilizing Ability of Cryopreserved Sperm in Animals. Animal Reproduction Science 2024, 263, 107429. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Donapetry-García, C.; Domínguez-Montero, A. Insulin Resistance and Glycine Metabolism in Humans. Amino Acids 2018, 50, 11–27. [Google Scholar] [CrossRef]
- Memmott, R.J.; Young, L.A. An Encounter with Homeless Mothers and Children: Gaining an Awareness. Issues Ment Health Nurs 1993, 14, 357–365. [Google Scholar] [CrossRef]
- Kozlov, V.A. Proteinogenic Acids; Cheboksary, 2012. [Google Scholar]
- Chaves, P.F.P.; Hocayen, P.D.A.S.; Dallazen, J.L.; De Paula Werner, M.F.; Iacomini, M.; Andreatini, R.; Cordeiro, L.M.C. Chamomile Tea: Source of a Glucuronoxylan with Antinociceptive, Sedative and Anxiolytic-like Effects. International Journal of Biological Macromolecules 2020, 164, 1675–1682. [Google Scholar] [CrossRef]
- Kvist, T.; Steffensen, T.B.; Greenwood, J.R.; Mehrzad Tabrizi, F.; Hansen, K.B.; Gajhede, M.; Pickering, D.S.; Traynelis, S.F.; Kastrup, J.S.; Bräuner-Osborne, H. Crystal Structure and Pharmacological Characterization of a Novel N-Methyl-d-Aspartate (NMDA) Receptor Antagonist at the GluN1 Glycine Binding Site. Journal of Biological Chemistry 2013, 288, 33124–33135. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and Mechanistic Insights into 5-Lipoxygenase Inhibition by Natural Products. Nat Chem Biol 2020, 16, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Dobrochaeva, D.N.; Kotov, M.I.; Prokudin, Y.N.; Barbarich, A.I. Key to Higher Plants of Ukraine; Naukova Dumka: Kyiv, Ukraine, 1999. [Google Scholar]
- Vlasova, I.; Gontova, T.; Grytsyk, L.; Zhumashova, G.; Sayakova, G.; Boshkayeva, A.; Shanaida, M.; Koshovyi, O. Determination of Standardization Parameters of Oxycoccus Macrocarpus (Ait.) Pursh and Oxycoccus Palustris Pers. Leaves. SR: PS 2022, 48–57. [Google Scholar] [CrossRef]
- Huzio, N.; Grytsyk, A.; Raal, A.; Grytsyk, L.; Koshovyi, O. Phytochemical and Pharmacological Research in Agrimonia Eupatoria L. Herb Extract with Anti-Inflammatory and Hepatoprotective Properties. Plants 2022, 11, 2371. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Raudone, L.; Petrikaite, V. Phenolic Fractions from Vaccinium vitis-idaea L. and Their Antioxidant and Anticancer Activities Assessment. Antioxidants 2020, 9, 1261. [Google Scholar] [CrossRef]
- Marzullo, L.; Ochkur, O.; Orlandini, S.; Renai, L.; Gotti, R.; Koshovyi, O.; Furlanetto, S.; Del Bubba, M. Quality by Design in Optimizing the Extraction of (Poly)Phenolic Compounds from Vaccinium Myrtillus Berries. Journal of Chromatography A 2022, 1677, 463329. [Google Scholar] [CrossRef] [PubMed]
- European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes; 1986; Vol. Official Journal L 222, p. P 0031-0037.
- Council Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes 2010.
- On the Protection of Animals from Cruel Treatment; 2009.
- On Approval of the Procedure for Preclinical Study of Medicinal Products and Examination of Materials of Preclinical Study of Medicinal Products; 2009.
- Regulating the Application of Principles of Good Laboratory Practice and the Verification of Their Applications for Tests on Chemical Substances; 1986; Vol. 1, pp. 145–146.
- Masocha, W.; Kombian, S.B.; Edafiogho, I.O. Evaluation of the Antinociceptive Activities of Enaminone Compounds on the Formalin and Hot Plate Tests in Mice. Sci Rep 2016, 6, 21582. [Google Scholar] [CrossRef]
- Inaltekin, A.; Kivrak, Y. Evaluation of the Effect of Vortioxetine on Pain Threshold by Hot-Plate Test in Mice. Archives of Neuropsychiatry 2021. [Google Scholar] [CrossRef]
- Sepp, J.; Koshovyi, O.; Jakštas, V.; Žvikas, V.; Botsula, I.; Kireyev, I.; Severina, H.; Kukhtenko, O.; Põhako-Palu, K.; Kogermann, K.; et al. Phytochemical, Pharmacological, and Molecular Docking Study of Dry Extracts of Matricaria Discoidea DC. with Analgesic and Soporific Activities. Biomolecules 2024, 14, 361. [Google Scholar] [CrossRef]
- Hossain, Md.F.; Talukder, B.; Rana, M.N.; Tasnim, R.; Nipun, T.S.; Uddin, S.M.N.; Hossen, S.M.M. In Vivo Sedative Activity of Methanolic Extract of Stericulia Villosa Roxb. Leaves. BMC Complement Altern Med 2016, 16, 398. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Ghorbani, A.; Hosseini, M.; Rakhshandeh, H. Hydroalcoholic Extract of Needles of Pinus Eldarica Enhances Pentobarbital-Induced Sleep: Possible Involvement of GABAergic System. Avicenna J Phytomed 2016, 6, 449–457. [Google Scholar] [PubMed]
- Huang, W.; Manglik, A.; Venkatakrishnan, A.J.; Laeremans, T.; Feinberg, E.N.; Sanborn, A.L.; Kato, H.E.; Livingston, K.E.; Thorsen, T.S.; Kling, R.C.; et al. Structural Insights into Μ-Opioid Receptor Activation. Nature 2015, 524, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural Basis for Selective Inhibition of Cyclooxygenase-2 by Anti-Inflammatory Agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Selinsky, B.S.; Gupta, K.; Sharkey, C.T.; Loll, P.J. Structural Analysis of NSAID Binding by Prostaglandin H2 Synthase: Time-Dependent and Time-Independent Inhibitors Elicit Identical Enzyme Conformations. Biochemistry 2001, 40, 5172–5180. [Google Scholar] [CrossRef]
- Che, T.; Majumdar, S.; Zaidi, S.A.; Ondachi, P.; McCorvy, J.D.; Wang, S.; Mosier, P.D.; Uprety, R.; Vardy, E.; Krumm, B.E.; et al. Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell 2018, 172, 55–67.e15. [Google Scholar] [CrossRef]
- Aritake, K.; Kado, Y.; Inoue, T.; Miyano, M.; Urade, Y. Structural and Functional Characterization of HQL-79, an Orally Selective Inhibitor of Human Hematopoietic Prostaglandin D Synthase. Journal of Biological Chemistry 2006, 281, 15277–15286. [Google Scholar] [CrossRef]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M.; Lindahl, E.; Hibbs, R.E. Shared Structural Mechanisms of General Anaesthetics and Benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef]
- McNutt, A.T.; Francoeur, P.; Aggarwal, R.; Masuda, T.; Meli, R.; Ragoza, M.; Sunseri, J.; Koes, D.R. GNINA 1.0: Molecular Docking with Deep Learning. J Cheminform 2021, 13, 43. [Google Scholar] [CrossRef]
- Moazzem Hossen, S.M.; Akramul Hoque Tanim, M.; Shahadat Hossain, M.; Ahmed Sami, S.; Uddin Emon, N. Deciphering the CNS Anti-Depressant, Antioxidant and Cytotoxic Profiling of Methanol and Aqueous Extracts of Trametes Versicolor and Molecular Interactions of Its Phenolic Compounds. Saudi Journal of Biological Sciences 2021, 28, 6375–6383. [Google Scholar] [CrossRef]
- Riegel, J.; Mayer, W.; Havre, Y.V. FreeCAD 2001.
- Lapach, S.N.; Chubenko, A.V.; Babich, P.N. Statistical Methods in Biomedical Research Using Excel; MORION: Kyiv, 2000. [Google Scholar]
| Substance | Content in the extract | ||||||
|---|---|---|---|---|---|---|---|
| Gch [7] | Gch-Arg | Gch-Phe | Gch-β-Ala | Gch-Gly | Gch-Val | Gch-Lys | |
| UPLC-MS/MS, µg/g of a dry extract | |||||||
| Neochlorogenic acid | 444.94 ± 20.16 | 329.21 ± 4.47 | 322.26 ± 8.51 | 373.81 ± 7.33 | 385.18 ± 13.11 | 382.70 ± 27.35 | 359.65 ± 12.79 |
| Luteolin | 310.93 ± 22.73 | 270.78 ± 15.81 | 320.87 ± 9.72 | 283.67 ± 8.20 | 309.48 ± 12.86 | 320.93 ± 27.22 | 301.31 ± 6.67 |
| Isoquercitrin | 921.16 ± 85.20 | 774.55 ± 41.11 | 850.92 ± 85.24 | 872.54 ± 34.08 | 880.89 ± 79.41 | 894.15 ± 43.80 | 838.97 ± 25.75 |
| Cryptochlorogenic acid | 80.74 ± 13.48 | 62.26 ± 9.76 | 70.97 ± 10.16 | 58.20 ± 5.03 | 62.47 ± 5.47 | 75.10 ± 20.95 | 55.98 ± 12.71 |
| Luteolin-4-O-glucoside | 45.11 ± 3.67 | 37.98 ± 1.88 | 49.59 ± 4.86 | 44.06 ± 3.86 | 41.68 ± 3.82 | 49.25 ± 6.75 | 43.68 ± 6.82 |
| Chlorogenic acid | 11742.31 ± 376.34 | 8984.21 ± 397.30 | 9532.56 ± 179.37 | 10341.94 ± 211.50 | 10567.30 ± 220.17 | 10014.55 ± 167.55 | 9342.78 ± 355.66 |
| Quercetin | 172.15 ± 12.01 | 138.19 ± 1.54 | 162.89 ± 8.50 | 149.96 ± 6.55 | 156.29 ± 6.19 | 149.27 ± 17.77 | 141.29 ± 6.20 |
| Isorhamnetin-3-O-rutinoside | 15.40 ± 1.60 | 12.03 ± 1.51 | 11.58 ± 1.28 | 12.78 ± 1.05 | 15.18 ± 1.55 | 14.68 ± 0.98 | 12.94 ± 1.50 |
| Isorhamnetin-3-glucoside | 410.75 ± 52.07 | 355.44 ± 24.71 | 402.53 ± 13.93 | 385.13 ± 32.20 | 448.75 ± 38.82 | 429.60 ± 16.85 | 406.41 ± 15.58 |
| Luteolin-3,7-diglucoside | 20.72 ± 1.88 | 15.60 ± 0.98 | 24.16 ± 0.70 | 19.03 ± 4.42 | 21.86 ± 1.36 | 20.04 ± 2.27 | 22.53 ± 6.19 |
| Vanilic acid | 86.58 ± 5.54 | 64.91 ± 5.23 | 69.80 ± 7.89 | 90.63 ± 2.98 | 88.66 ± 3.46 | 79.04 ± 3.04 | 85.28 ± 12.40 |
| Caffeic acid | 43.04 ± 3.22 | 40.58 ± 3.01 | 45.80 ± 6.485 | 47.91 ± 7.11 | 37.44 ± 4.50 | 36.52 ± 6.60 | 45.18 ± 4.35 |
| 3,4-Dihydroxyphenylacetic acid | 184.05 ± 13.38 | 149.40 ± 12.77 | 145.05 ± 5.55 | 180.78 ± 11.94 | 154.06 ± 3.07 | 170.92 ± 4.40 | 151.33 ± 8.57 |
| Isorhamnetin | 125.32 ± 12.71 | 92.98 ± 5.25 | 116.50 ± 7.41 | 106.09 ± 5.58 | 101.81 ± 1.42 | 112.95 ± 3.76 | 102.56 ± 5.78 |
| Apigenin | 578.65 ± 63.91 | 462.21 ± 23.88 | 537.43 ± 47.49 | 506.71 ± 11.63 | 502.22 ± 3.14 | 549.22 ± 26.09 | 493.34 ± 29.54 |
| Kaempherol-3-O-glucoside | 50.76 ± 2.10 | 40.41 ± 3.67 | 49.94 ± 3.18 | 50.78 ± 5.32 | 55.22 ± 3.52 | 58.84 ± 2.27 | 48.15 ± 2.58 |
| Rutin | 126.49 ± 5.73 | 104.66 ± 3.94 | 102.88 ± 7.79 | 113.02 ± 14.86 | 109.13 ± 10.54 | 107.17 ± 10.24 | 99.26 ± 7.71 |
| Hyperoside | 366.82 ± 21.21 | 308.22 ± 21.78 | 315.79 ± 16.66 | 357.39 ± 12.20 | 387.16 ± 16.43 | 345.88 ± 21.19 | 347.21 ± 19.20 |
| Luteolin-7-O-glucoside | 1061.82 ± 83.68 | 874.36 ± 62.18 | 975.10 ± 57.44 | 1031.03 ± 73.93 | 1094.50 ± 72.63 | 1021.56 ± 59.44 | 1070.74 ± 37.66 |
| 4.5-Dicaffeoylquinic acid | 4912.17 ± 416.85 | 3844.32 ± 149.55 | 4375.54 ± 208.35 | 4320.08 ± 175.34 | 4671.16 ± 242.58 | 4336.20 ± 352.82 | 4227.72 ± 365.27 |
| 3.5-Dicaffeoylquinic acid | 2512.69 ± 213.23 | 1966.47 ± 76.50 | 2238.19 ± 106.57 | 2209.83 ± 89.69 | 2389.41 ± 124.08 | 2218.07 ± 180.47 | 2162.58 ± 186.85 |
| 3.4-Dicaffeoylquinic acid | 5152.17 ± 437.22 | 4032.151 ± 156.86 | 4589.32 ± 218.53 | 4531.15 ± 183.91 | 4899.39 ± 254.43 | 4548.06 ± 370.05 | 4434.28 ± 383.12 |
| Spectrophotometry, % | |||||||
| Phenolic compounds | 9.70 ± 0.52 | 7.87 ± 0.41 | 8.50 ± 0.34 | 9.40 ± 0.62 | 7.43 ± 0.59 | 8.29 ± 0.53 | 7.49 ± 0.09 |
| Hydrocinnamic acids | 3.47 ± 0.15 | 2.75 ± 0.29 | 3.28 ± 0.33 | 3.20 ± 0.14 | 2.64 ± 0.21 | 2.94 ± 0.31 | 2.50 ± 0.32 |
| Flavonoids | 9.92 ± 0.32 | 7.16 ± 0.38 | 8.50 ± 0.37 | 7.95 ± 0.09 | 7.55 ± 0.32 | 7.71 ± 0.77 | 7.26 ± 0.57 |
| Substance | Content in the extract, mg/g | ||||||
|---|---|---|---|---|---|---|---|
| Gch | Gch-Arg | Gch-Phe | Gch-β-Ala | Gch-Gly | Gch-Val | Gch-Lys | |
| Alanine (Ala) | 2.90 ± 0.10 | 2.27 ± 0.10 | 2.10 ± 0.07 | 2.59 ± 0.14 | 2.61 ± 0.23 | 2.59 ± 0.15 | 2.31 ± 0.11 |
| Arginine (Arg) | 1.34 ± 0.27 | 105.99 ± 2.03 | 3.09 ± 0.39 | 1.33 ± 0.16 | 1.03 ± 0.49 | 0.83 ± 0.07 | 7.48 ± 0.58 |
| Aspartic acid (Asn) | 1.44 ± 0.13 | 1.14 ± 0.04 | 1.10 ± 0.9 | 1.31 ± 0.14 | 0.99 ± 0.05 | 1.08 ± 0.05 | 1.20 ± 0.28 |
| Glutamic acid (Glu) | 2.13 ± 0.14 | 1.61 ± 0.06 | 1.73 ± 0.16 | 1.94 ± 0.30 | 2.04 ± 0.15 | 1.87 ± 0.16 | 2.02 ± 0.36 |
| Glycine (Gly) | 0.38 ± 0.01 | 0.37 ± 0.01 | 0.36 ± 0.01 | 0.32 ± 0.02 | 107.30 ± 8.80 | 0.92 ± 0.09 | 0.46 ± 0.07 |
| Isoleucine (Ile) | 1.76 ± 0.08 | 1.35 ± 0.04 | 1.43 ± 0.06 | 1.51 ± 0.07 | 1.43 ± 0.15 | 1.51 ± 0.09 | 1.32 ± 0.22 |
| Leucine (Leu) | 1.77 ± 0.11 | 1.37 ± 0.19 | 1.35 ± 0.14 | 1.53 ± 0.37 | 1.35 ± 0.43 | 3.07 ± 0.31 | 1.22 ± 0.09 |
| Lysine (Lys) | 1.10 ± 0.02 | 0.58 ± 0.09 | 0.69 ± 0.04 | 0.90 ± 0.01 | 0.90 ± 0.04 | 0.81 ± 0.02 | 298.56 ± 11.13 |
| Phenylalanine (Phe) | 1.07 ± 0.07 | 0.84 ± 0.08 | 181.68 ± 17.38 | 1.98 ± 0.69 | 0.96 ± 0.06 | 1.05 ± 0.15 | 0.74 ± 0,08 |
| Proline (Pro) | 2.70 ± 0.20 | 2.21 ± 0.02 | 2.25 ± 0.18 | 2.48 ± 0.25 | 2.56 ± 0.09 | 3.52 ± 0.19 | 2.63 ± 0.17 |
| Serine (Ser) | 1.96 ± 0.27 | 1.60 ± 0.04 | 1.45 ± 0.19 | 1.66 ± 0.06 | 1.77 ± 0.09 | 1.51 ± 0.09 | 1.61 ± 0.09 |
| Threonine (Thr) | 2.19 ± 0.22 | 1.81 ± 0.30 | 1.75 ± 0.13 | 1.94 ± 0.132 | 2.84 ± 0.09 | 2.06 ± 0.48 | 1.96 ± 0.19 |
| Tyrosine (Tyr) | 1.13 ± 0.13 | 0.85 ± 0.06 | 0.98 ± 0.01 | 1.02 ± 0.09 | 1.05 ± 0.05 | 1.00 ± 0.09 | 1.06 ± 0.11 |
| Valine (Val) | 1.52 ± 0,20 | 1.29 ± 0.11 | 1.34 ± 0.20 | 1.53 ± 0.15 | 1.47 ± 0.14 | 140.05 ± 7.33 | 1.86 ± 0.17 |
| β-Alanine (β-Ala) | 0 | 0 | 0 | 138.14 ± 9.77 | 0 | 0 | 0 |
| Sum of amino acids | 23.40 | 123.26 | 201.29 | 160.18 | 128.30 | 161.86 | 324.43 |
| Phytosubstance | Group | Dose (mg/kg) | The time of response (s)/Analgesic effect (%) in comparison to (reference drug) and [control] | |||||
|---|---|---|---|---|---|---|---|---|
| Before | after administration in | |||||||
| before | 30 min | 60 min | 120 min | 180 min | 240 min | |||
| Control | 1 | 6.84 ± 0.47 | 7.20 ± 0.29 | 7.10 ± 0.61 | 7.08 ± 0.27 | 7.15 ± 0.65 | 6.73 ± 0.94 | |
| Gch | 2 | 25 | 7.93 ± 0.29 | 9.63 ± 0.54 [34%] (-8%) * |
9.97 ± 0.60 [40%] (-4%) * |
8.65 ± 0.48 [22%] # (-18%) * |
7.98 ± 0.12 [12%] (-16%) |
8.43 ± 0.21 [25%] (1%) |
| 3 | 50 | 7.68 ± 0.20 | 11.43 ± 0.85 [59%] # (9%) |
11.83 ± 0.77 [67%] # (14%) |
11.72 ± 0.73 [65%] # (11%) |
11.13 ± 0.73 [56%] # (18%) |
8.67 ± 0.31 [29%] # (4%) |
|
| 4 | 100 | 8.46 ± 0.42 | 12.50 ± 0.36 [74%] # (20%) |
12.52 ± 0.31 [76%] # (21%) * |
12.47 ± 0.30 [76%] # (18%) * |
9.63 ± 0.50 [35%] # (2%) |
9.02 ± 0.39 [34%] # (8%) |
|
| Gch-Arg | 5 | 25 | 7.39 ± 0.31 | 8.36 ± 0.45 [16%] # (-20%) * |
8.12 ± 0.60 [14%] (-22%) * |
7.86 ± 0.41 [11%] (-25%) * |
8.93 ± 0.33 [25%] # (-6%) |
8.24 ± 0.52 [21%] (-1%) |
| 6 | 50 | 7.52 ± 0.16 | 8.40 ± 0.11 [17%] (-20%) |
8.52 ± 1.11 [20%] (-18%) |
8.85 ± 0.93 [25%] (-16%) |
9.00 ± 0.99 [26%] (-5%) |
7.82 ± 0.51 [16%] (-6%) |
|
| 7 | 100 | 7.63 ± 0.41 | 8.43 ± 0.63 [17%] (-19%) |
8.48 ± 0.77 [19%] (-18%) |
9.20 ± 0.53 [30%] # (-13%) |
9.05 ± 0.80 [27%] (-4%) |
7.45 ± 0.47 [11%] (-11%) |
|
| Gch-Phe | 8 | 25 | 7.54 ± 0.31 | 8.04 ± 0.42 [12%] (-23%) * |
8.51 ± 0.49 [20%] (-18%) * |
9.04 ± 0.23 [28%] # (-14%) |
9.10 ± 0.16 [27%] # (-4%) |
8.81 ± 0.60 [31%] (6%) |
| 9 | 50 | 7.35 ± 0.26 | 9.58 ± 0.86 [33%] # (-8%) |
10.67 ± 0.95 [50%] # (-3%) |
10.37 ± 0.86 [46%] # (-2%) |
9.70 ± 0.68 [36%] # (-3%) |
8.12 ± 0.51 [21%] (-3%) |
|
| 10 | 100 | 7.43 ± 0.57 | 8.65 ± 0.68 [20%] # (-17%) |
9.13 ± 0.65 [29%] # (-12%) |
10.00 ± 0.50 [41%] # (-5%) |
9.88 ± 0.51 [38%] # (5%) |
8.03 ± 0.68 [19%] (-4%) |
|
| Gch-β-Ala | 11 | 25 | 7.47 ± 0.27 | 8.79 ± 0.53 [22%] # (-16%) * |
9.79 ± 0.72 [38%] # (-6%) |
8.71 ± 0.44 [23%] # (-17%) * |
8.73 ± 0.68 [22%] (-8%) |
7.83 ± 0.41 [16%] (-6%) |
| 12 | 50 | 7.60 ± 0.16 | 8.33 ± 1.00 [16%] (-20%) |
8.92 ± 1.01 [26%] (-14%) |
8.93 ± 0.87 [26%] # (-15%) |
9.17 ± 0.75 [28%] # (-3%) |
7.98 ± 0.25 [19%] (-4%) |
|
| 13 | 100 | 7.59 ± 0.39 | 8.55 ± 0.58 [19%] (-18%) |
8.53 ± 0.68 [20%] (-18%) |
8.55 ± 0.60 [21%] (-19%) |
8.47 ± 0.37 [18%] (-10%) |
8.33 ± 0.29 [24%] (0%) |
|
| Gch-Gly | 14 | 25 | 7.47 ± 0.27 | 9.86 ± 0.60 [37%] # (-6%) |
10.46 ± 0.84 [47%] # (1%) |
9.86 ± 0.49 [39%] # (-6%) |
10.11 ± 0.58 [41%] # (7%) |
9.89 ± 0.72 [47%] # (18%) * |
| 15 | 50 | 7.60 ± 0.16 | 10.02 ± 0.67 [39%] # (-4%) |
10.35 ± 0.67 [46%] # (-0.3%) |
10.38 ± 0.59 [47%] # (-1%) |
10.00 ± 0.59 [40%] (6%) |
9.46 ± 0.16 [40%] (13%) |
|
| 16 | 100 | 7.59 ± 0.39 | 11.25 ± 0.59 [56%] # (8%) |
11.40 ± 0.66 [61%] # (10%) |
11.68 ± 0.65 [65%] # (11%) |
11.23 ± 0.64 [57%] # (19%) * |
10.75 ± 0.45 [60%] # (29%) * |
|
| Gch-Val | 17 | 25 | 7.50 ± 0.28 | 8.20 ± 0.49 [14%] (-22%) * |
8.45 ± 0.48 [19%] (-19%) * |
8.70 ± 0.53 [23%] # (-17%) |
8.35 ± 0.33 [17%] (-12%) |
7.52 ± 0.41 [12%] (-10%) |
| 18 | 50 | 7.53 ± 0.16 | 9.23 ± 0.83 [28%] # (-12%) |
9.67 ± 0.83 [36%] # (-7%) |
10.00 ± 0.81 [43%] # (-4%) |
10.23 ± 0.73 [43%] # (8%) |
9.60 ± 0.60 [43%] # (15%) |
|
| 19 | 100 | 7.56 ± 0.38 | 8.87 ± 0.57 [23%] # (-15%) |
9.50 ± 0.55 [34%] # (-9%) |
9.93 ± 0.54 [40%] # (-6%) |
10.15 ± 0.40 [42%] # (7%) |
9.33 ± 0.51 [39%] # (12%) |
|
| Gch-Lys | 20 | 25 | 7.48 ± 0.29 | 9.83 ± 0.50 [37%] # (-6%) |
10.12 ± 0.47 [42%] # (-3%) |
9.73 ± 0.51 [37%] # (-8%) |
9.52 ± 0.42 [33%] # (1%) |
9.13 ± 0.40 [36%] # (9%) |
| 21 | 50 | 7.57 ± 0.15 | 9.55 ± 0.79 [33%] # (-9%) |
9.57 ± 0.86 [35%] # (-8%) |
9.48 ± 0.82 [34%] # (-10%) |
9.30 ± 0.79 [30%] # (-2%) |
8.34 ± 0.25 [24%] (-0.1%) |
|
| 22 | 100 | 7.58 ± 0.38 | 9.03 ± 0.50 [25%] # (-14%) |
9.45 ± 0.42 [33%] # (-9%) |
9.40 ± 0.43 [33%] # (-11%) |
9.42 ± 0.47 [32%] # (-0.4%) |
9.03 ± 0.48 [34%] # (-8%) |
|
| Acetaminophen | 23 | 50 | 7.23 ± 0.72 | 10.54 ± 0.73 | 10.38 ± 0.62 | 10.53 ± 0.74 | 9.45 ± 0.60 | 8.35 ± 0.36 |
| Active ingredient/group (n = 6) | Dose (mg/kg) | Average duration of sleep (min). | Soporific effect (%) in comparison to the group given thiopental | Soporific effect (%) in comparison to the group given the chamomile extract | |
|---|---|---|---|---|---|
| Control group (1) | 0 | - | |||
| Gch | 2 | 25 | 79.67 ± 7.18 | 75.99 | 100 |
| 3 | 50 | 115.33 ± 12.60 | 110.02 | 100 | |
| 4 | 100 | 76.67 ± 9.91 * | 73.13 | 100 | |
| Gch-Arg | 5 | 25 | 74.99 ± 6.51 *# | 71.54 | -5.87 |
| 6 | 50 | 40.02 ± 5.93 *# | 40.09 | -65.30 | |
| 7 | 100 | 50.85 ± 7.55 *# | 48.50 | -33.68 | |
| Gch-Phe | 8 | 25 | 43.32 ± 8.33 *# | 41.32 | -45.63 |
| 9 | 50 | 54.46 ± 11.12 *# | 51.95 | -52.78 | |
| 10 | 100 | 57.77 ± 8.17 *# | 55.10 | -24.65 | |
| Gch-β-Ala | 11 | 25 | 132.88 ± 7.91 *# | 126.76 | 66.79 |
| 12 | 50 | 137.14 ± 3.19 *# | 130.82 | 18.91 | |
| 13 | 100 | 196.61 ± 11.69 *# | 187.55 | 156.44 | |
| Gch-Gly | 14 | 25 | 73.22 ± 6.10 *# | 71.54 | -8.10 |
| 15 | 50 | 105.76 ± 15.15 | 100.89 | -8.30 | |
| 16 | 100 | 93.21 ± 12.46 | 88.91 | 21.57 | |
| Gch-Val | 17 | 25 | 53.86 ± 5.52 *# | 51.37 | -32.40 |
| 18 | 50 | 61.13 ± 5.99 *# | 51.95 | -47.00 | |
| 19 | 100 | 95.54 ± 4.62 | 91.13 | 24.61 | |
| Gch-Lys | 20 | 25 | 206.27 ± 8.52 *# | 196.76 | 158.91 |
| 21 | 50 | 201.65 ± 6.88 *# | 192.35 | 74.85 | |
| 22 | 100 | 193.90 ± 14.71 *# | 184.96 | 152.90 | |
| Valerian extract (23) | 2,15 | 96.83 ± 8.46 | 92.37 | ||
| Thiopental (24) | 40 | 104.83 ± 8.76 | 100 | ||
| Ligand | 5C1M/mu-opioid | 1CX2/COX-2 | 1EQG/COX-1 | 4KFQ/NMDA-receptor | 6B73/kappa-opioid | 2CVD/PTGES | 6NCF/5-LOX | 6X3X/GABAa receptor | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Affinity (kcal/mol) | CNN pose score | CNN affinity | Affinity (kcal/mol) | CNN pose score | CNN affinity | Affinity (kcal/mol) | CNN pose score | CNN affinity | Affinity (kcal/mol) | CNN pose score | CNN affinity | Affinity (kcal/mol) | CNN pose score | CNN affinity | Affinity (kcal/mol) | CNN pose score | CNN affinity | Affinity (kcal/mol) | CNN pose score | CNN affinity | Affinity (kcal/mol) | CNN pose score | CNN affinity | |
| 4,5-Dicaffeoylquinic acid | -10.19 | 0.4587 | 5.896 | -1.78 | 0.5513 | 6.424 | -1.38 | 0.4501 | 6.242 | -9.45 | 0.4413 | 6.042 | -6.84 | 0.7377 | 4.908 | -8.20 | 0.3372 | 5.217 | -8.06 | 0.7459 | 5.636 | -6.4 | 0.3781 | 5.109 |
| 3,4-Dicaffeoylquinic acid | -10.19 | 0.4587 | 5.896 | -1.78 | 0.5513 | 6.424 | -1.38 | 0.4501 | 6.242 | -9.45 | 0.4413 | 6.042 | -6.84 | 0.7377 | 4.908 | -8.20 | 0.3372 | 5.217 | -8.06 | 0.7459 | 5.636 | -5.6 | 0.4765 | 5.05 |
| Luteolin 7,3′-diglucoside | -9.29 | 0.4652 | 5.633 | 0 | 0.5548 | 6.591 | 0 | 0.4685 | 6.659 | -8.05 | 0.3440 | 5.598 | -6.53 | 0.548 | 4.789 | -9.46 | 0.3781 | 5.388 | -10.85 | 0.3891 | 5.219 | -7.17 | 0.5276 | 4.759 |
| 3,5-Dicaffeoyl-quinic acid | -9.80 | 0.5466 | 5.714 | -2.37 | 0.5267 | 5.852 | -6.4 | 0.5493 | 6.401 | -10.70 | 0.7496 | 6.361 | -5.85 | 0.6750 | 4.807 | -9.54 | 0.4659 | 5.364 | -10.05 | 0.4505 | 5.099 | -7.06 | 0.5644 | 4.538 |
| Rutin | -11.12 | 0.3228 | 5.738 | 0 | 0.5586 | 6.675 | 0 | 0.5043 | 6.284 | -5.80 | 0.4154 | 5.613 | -6.83 | 0.7248 | 4.935 | -7.82 | 0.4735 | 4.92 | -8.76 | 0.4502 | 4.943 | -6.56 | 0.7816 | 4.815 |
| Luteolin-7-O-glucoside | -9.56 | 0.6739 | 5.884 | 0 | 0.4945 | 5.261 | -0.38 | 0.5469 | 6.672 | -9.11 | 0.2525 | 5.192 | -2.26 | 0.6834 | 4.345 | -8.45 | 0.3774 | 5.211 | -8.24 | 0.5702 | 4.935 | -5.87 | 0.6119 | 4.747 |
| Isorhamnetin | -7.99 | 0.7924 | 5.645 | -8.15 | 0.6803 | 6.101 | 0 | 0.6922 | 6.566 | -8.53 | 0.6732 | 5.100 | -5.18 | 0.7515 | 4.517 | -9.4 | 0.646 | 5.517 | -8.52 | 0.7166 | 4.927 | -6.3 | 0.8264 | 4.903 |
| Luteolin | -8.21 | 0.8413 | 5.526 | 0 | 0.5640 | 5.002 | -6.95 | 0.6118 | 6.131 | -4.88 | 0.4978 | 5.136 | -5.57 | 0.7010 | 4.32 | -8.94 | 0.5414 | 5.098 | -8.58 | 0.7573 | 4.886 | -6.47 | 0.761 | 4.699 |
| Cryptochlorogenic acid | -8.41 | 0.8107 | 5.056 | -3.88 | 0.5025 | 5.612 | -1.41 | 0.4915 | 5.184 | -8.07 | 0.3818 | 4.514 | -5.46 | 0.6593 | 4.018 | -8.07 | 0.3780 | 4.151 | -8.65 | 0.8774 | 4.860 | -6.14 | 0.3561 | 3.749 |
| Chlorogenic acid | -8.27 | 0.7236 | 5.005 | -4.97 | 0.4685 | 5.577 | -5.99 | 0.5017 | 5.635 | -8.81 | 0.4930 | 4.985 | -5.39 | 0.7198 | 4.143 | -7.72 | 0.4304 | 5.017 | -7.68 | 0.5863 | 4.803 | -5.91 | 0.6869 | 4.286 |
| Hyperoside | -9.78 | 0.3398 | 5.640 | 0 | 0.4976 | 5.883 | 0 | 0.4257 | 5.864 | -4.89 | 0.4491 | 5.527 | -5.47 | 0.7574 | 4.694 | -8.1 | 0.3867 | 4.833 | -8.27 | 0.4721 | 4.612 | -6.09 | 0.7415 | 4.627 |
| Isorhamnetin-3-glucoside | -10.28 | 0.2467 | 5.633 | 0 | 0.501 | 6.609 | 0 | 0.5186 | 6.516 | -10.11 | 0.5157 | 5.600 | -5.28 | 0.674 | 4.764 | -7.56 | 0.3456 | 4.278 | -8.08 | 0.762 | 4.563 | -6.16 | 0.7908 | 4.703 |
| Luteolin-4-O-glucoside | -9.50 | 0.6026 | 5.424 | -4.49 | 0.5279 | 6.47 | 0 | 0.619 | 6.331 | -9.59 | 0.6868 | 5.422 | -5.54 | 0.7177 | 4.601 | -8.33 | 0.3488 | 4.772 | -7.89 | 0.619 | 4.53 | -4.98 | 0.5845 | 4.984 |
| Neochlorogenic acid | -6.94 | 0.4411 | 4.648 | -5.45 | 0.4810 | 5.717 | -0.66 | 0.5154 | 6.087 | -9.00 | 0.6977 | 5.053 | -5.45 | 0.6040 | 4.031 | -7.01 | 0.3980 | 4.306 | -7.39 | 0.8060 | 4.485 | -5.71 | 0.5854 | 4.054 |
| Caffeic acid | -5.40 | 0.8155 | 4.474 | -6.89 | 0.6681 | 4.986 | -6.64 | 0.6885 | 4.797 | -7.04 | 0.7202 | 4.359 | -3.76 | 0.6726 | 3.384 | -5.61 | 0.8203 | 4.69 | -5.51 | 0.7808 | 3.871 | -5.61 | 0.7254 | 3.823 |
| 3,4-Dihydroxy phenylacetic acid | -5.53 | 0.7237 | 3.999 | -6.04 | 0.5821 | 4.396 | -6.15 | 0.7965 | 4.58 | -5.84 | 0.7776 | 4.128 | -3.48 | 0.6418 | 3.225 | -5.11 | 0.7007 | 3.331 | -5.41 | 0.7654 | 3.771 | -5.36 | 0.7053 | 3.632 |
| Vanilic acid | -5.67 | 0.9025 | 4.038 | -5.75 | 0.6248 | 4.096 | -5.64 | 0.7333 | 4.369 | -6.34 | 0.9128 | 4.316 | -3.18 | 0.6980 | 2.988 | -6.46 | 0.7923 | 4.085 | -5.09 | 0.6891 | 3.456 | -4.86 | 0.77 | 3.388 |
| Reference ligand | -11.10 | 0.9215 | 7.847 | -11.51 | 0.9735 | 7.432 | -7.78 | 0.9496 | 6.941 | -8.33 | 0.983 | 6.599 | -6.51 | 0.8109 | 6.063 | -10.28 | 0.9167 | 6.396 | -10.34 | 0.8341 | 6.362 | -7.08 | 0.8793 | 6.578 |
| Gels: Extract (g)/10 g |
Viscosity, cP (22 ± 2 °C) |
Surface area of the 3D lattices, mm2 | S practical/S theoretical ratio | Mass of lattices, mg | Mass of round-shaped discs, mg |
|---|---|---|---|---|---|
| 0.5 | 137100 ± 9908 | 362.7 ± 59.9 | 1.19 | 129.9 ± 9.1 | 117.4 ± 1.2 |
| 1.0 | 179633 ± 9785 | 456.0 ± 67.4 | 1.41 | 149.4 ± 3.4 | 146.5 ± 7.9 |
| 1.5 | 181767 ± 9887 | 453.2 ± 68.1 | 1.40 | 187.4 ± 7.9 | 180.4 ± 9.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





