Submitted:
21 June 2024
Posted:
24 June 2024
You are already at the latest version
Abstract
Keywords:
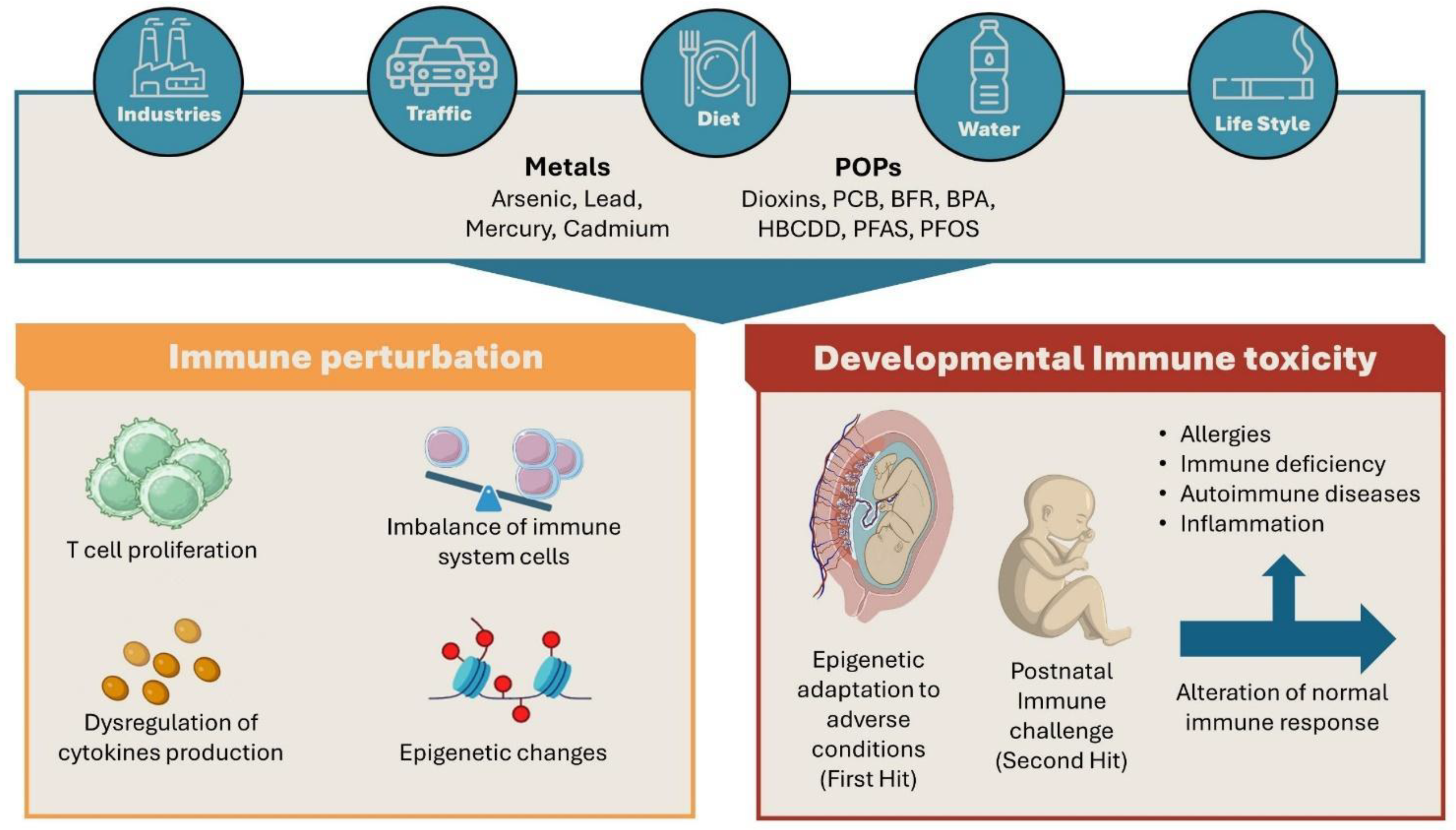
1. Introduction
2. Pollutants and Immune System Modulation: In Vitro, Ex Vivo and In Vivo Models
2.1. Metals
2.1.1. Arsenic
2.1.2. Lead
2.1.3. Mercury
2.1.4. Cadmium
2.2. Persistent Organic Pollutants (POPs)
2.2.1. Dioxins
2.2.2. Polychlorinated Biphenyls (PCBs)
2.2.3. Brominated Flame Retardants (BFRs)
2.2.3.1. Polybrominated Diphenyl Ethers (PBDEs)
2.2.3.2. Hexabromocyclododecane (HBCDD) and Tetrabromobisphenol A (TBBPA)
2.2.4. Bisphenol A
2.2.5. Perfluorooctanesulfonic Acid and Perfluoroctanoic Acid
3. Developmental Immunotoxicology
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| (o)Hg | Organic Mercury |
| A549 | Human lung adenocarcinoma cells |
| AhR | Aryl hydrocarbon receptor |
| AIM2 | Absent in Melanoma 2 receptor |
| AOP | Adverse Outcome Pathway |
| Arg1 | Arginase 1 |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| As | Arsenic |
| BD2 | Beta-defensin 2 |
| BDE-209 | Decabromodiphenyl ether |
| BDE-47 | Tetrabromodiphenyl ether |
| BFRs | Brominated flame retardants |
| BPA | Bisphenol A |
| BPF | Bisphenol F |
| BPS | Bisphenol S |
| Cd | Cadmium |
| CD11c | Cluster of Differentiation 11c |
| CD14 | Cluster of Differentiation 14 |
| CD19 | Cluster of Differentiation 19 |
| CD206 | Cluster of Differentiation 206 |
| CD4+ | Cluster of Differentiation 4 positive (T-helper cells) |
| CD70 | Cluster of Differentiation 70 |
| CD8+ | Cluster of Differentiation 8 positive (Cytotoxic T cells) |
| CpG | Cytosine-phosphate-guanine (DNA sequence) |
| deca-BDE | Decabromodiphenyl ether |
| DIT | Developmental Immunotoxicity |
| DNA | Deoxyribonucleic acid |
| DOHaD | Developmental Origins of Health and Disease |
| EEA | European Environment Agency |
| EFSA | European Food Safety Authority |
| EMT | Epithelial-mesenchymal transition |
| HBCDD | Hexabromocyclododecane |
| HBCDDs | Hexabromocyclododecanes |
| Hg | Mercury |
| Hg0 | Elemental Mercury |
| HgCl2 | Mercury (II) chloride |
| IFN-γ | Interferon-gamma |
| IgE | Immunoglobulin E |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-12p40 | Interleukin-12 subunit p40 |
| IL-17 | Interleukin-17 |
| IL-1β | Interleukin-1 beta |
| IL-2 | Interleukin-2 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| iNOS | Inducible nitric oxide synthase |
| IRF5 | Interferon regulatory factor 5 |
| JAK2 | Janus kinase 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| LPS | Lipopolysaccharide |
| M1 | Classically activated macrophages |
| M2 | Alternatively activated macrophages |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MD2 | Myeloid differentiation factor 2 |
| MeHg | Methylmercury |
| MyD88 | Myeloid differentiation primary response 88 |
| NDL-PCB | Non-dioxin-like polychlorinated biphenyls |
| NHANES | National Health and Nutrition Examination Survey |
| NK cells | Natural killer cells |
| NO | Nitric oxide |
| NOS2 | Nitric oxide synthase 2 |
| OCPs | Organochlorine pesticides |
| p65 | RelA (p65) transcription factor |
| Pb | Lead |
| PBBs | Polybrominated biphenyls |
| PBDEs | Polybrominated diphenyl ethers |
| PBMCs | Peripheral blood mononuclear cells |
| PCBs | Polychlorinated Biphenyls (PCBs) |
| PCFs | Perfluorinated chemicals |
| PFAS | Per- and polyfluoroalkyl substances |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctanesulfonic acid |
| PHAs | polycyclic aromatic hydrocarbons |
| POPs | Persistent organic pollutants |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PTEN | Phosphatase and tensin homolog |
| RAW264.7 | Murine macrophage cell line |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| sEVs | Small extracellular vesicles |
| TBBPA | Tetrabromobisphenol A |
| TCDD | 2,3,7,8-Tetrachlorodibenzo-p-dioxin |
| TGF-β1 | Transforming growth factor beta 1 |
| Th17 | T-helper 17 cells |
| Th2 | T-helper 2 cells |
| THP-1 | Human monocytic cell line |
| TLRs | Toll-like receptors |
| TNF-α | Tumor necrosis factor alpha |
| Treg | Regulatory T cells |
| WHO | World Health Organization |
| Wnt3a | Wingless-related integration site 3A |
| WQS | Weighted Quantile Sum regression |
References
- Kuppusamy, S.; Venkateswarlu, K.; Megharaj, M.; Mayilswami, S.; Lee, Y.B. Risk-based remediation of polluted sites: A critical perspective. Chemosphere 2017, 186, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. Preventing diseases through promotion of a healthier environment: World Health Organization. Ann Trop Med Public 2016, 9, 364–U115. [Google Scholar] [CrossRef]
- Wild, C.P. Complementing the genome with an "exposome": the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2005, 14, 1847–50. [Google Scholar]
- Rappaport, S.M. Genetic Factors Are Not the Major Causes of Chronic Diseases. PloS one 2016, 11, e0154387. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M. Redefining environmental exposure for disease etiology. NPJ Syst Biol Appl 2018, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Shah, P.P.; Nativio, R.; Berger, S.L. Epigenetic Mechanisms of Longevity and Aging. Cell 2016, 166, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. The exposome: from concept to utility. Int J Epidemiol 2012, 41, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, V.; Cardoso, R.C.; Puttabyatappa, M. Developmental Programming, a Pathway to Disease. Endocrinology 2016, 157, 1328–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Loganath, A.; Chong, Y.S.; Tan, J.; Obbard, J.P. Persistent organic pollutants and adverse health effects in humans. Journal of toxicology and environmental health 2006, 69. [Google Scholar]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; Serrrano, J.A.; Tietge, J.E.; Villeneuve, D.L. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 2010, 29, 730–41. [Google Scholar] [CrossRef]
- Bajard, L.; Adamovsky, O.; Audouze, K.; Baken, K.; Barouki, R.; Beltman, J.B.; Beronius, A.; Bonefeld-Jorgensen, E.C.; Cano-Sancho, G.; de Baat, M.L.; Di Tillio, F.; Fernandez, M.F.; FitzGerald, R.E.; Gundacker, C.; Hernandez, A.F.; Hilscherova, K.; Karakitsios, S.; Kuchovska, E.; Long, M.; Luijten, M.; Majid, S.; Marx-Stoelting, P.; Mustieles, V.; Negi, C.K.; Sarigiannis, D.; Scholz, S.; Sovadinova, I.; Stierum, R.; Tanabe, S.; Tollefsen, K.E.; van den Brand, A.D.; Vogs, C.; Wielsoe, M.; Wittwehr, C.; Blaha, L. Application of AOPs to assist regulatory assessment of chemical risks - Case studies, needs and recommendations. Environmental research 2023, 217, 114650. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; Miller, A.H.; Mantovani, A.; Weyand, C.M.; Barzilai, N.; Goronzy, J.J.; Rando, T.A.; Effros, R.B.; Lucia, A.; Kleinstreuer, N.; Slavich, G.M. Chronic inflammation in the etiology of disease across the life span. Nature medicine 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of Inflammation in Autoimmune Disease. J Immunol Res 2019, 2019, 7403796. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol 2014, 14, 463–77. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Henao-Mejia, J.; Flavell, R.A. Innate immune receptors: key regulators of metabolic disease progression. Cell Metab 2013, 17, 873–882. [Google Scholar] [CrossRef]
- Kazankov, K.; Jorgensen, S.M. D.; Thomsen, K.L.; Moller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Gronbaek, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Mangino, M.; Roederer, M.; Beddall, M.H.; Nestle, F.O.; Spector, T.D. Innate and adaptive immune traits are differentially affected by genetic and environmental factors. Nat Commun 2017, 8, 13850. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Rui, K.; Wang, X.; Peng, N.; Zhou, W.; Shi, X.; Lu, L.; Hu, D.; Tian, J. The aryl hydrocarbon receptor in immune regulation and autoimmune pathogenesis. J Autoimmun 2023, 138, 103049. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Vazquez, C.; Quintana, F.J. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 2018, 48, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Grosskopf, H.; Walter, K.; Karkossa, I.; von Bergen, M.; Schubert, K. Non-Genomic AhR-Signaling Modulates the Immune Response in Endotoxin-Activated Macrophages After Activation by the Environmental Stressor BaP. Frontiers in immunology 2021, 12, 620270. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Holfelder, P.; Prentzell, M.T.; Trump, S. The complex biology of aryl hydrocarbon receptor activation in cancer and beyond. Biochem Pharmacol 2023, 216, 115798. [Google Scholar] [CrossRef] [PubMed]
- Kerkvliet, N.I.; Baecher-Steppan, L.; Smith, B.B.; Youngberg, J.A.; Henderson, M.C.; Buhler, D.R. Role of the Ah locus in suppression of cytotoxic T lymphocyte activity by halogenated aromatic hydrocarbons (PCBs and TCDD): structure-activity relationships and effects in C57Bl/6 mice congenic at the Ah locus. Fundam Appl Toxicol 1990, 14, 532–41. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.B.; Vorachek, W.R.; Steppan, L.B.; Mourich, D.V.; Kerkvliet, N.I. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Journal of immunology 2008, 181, 2382–91. [Google Scholar] [CrossRef] [PubMed]
- O'Driscoll, C.A.; Gallo, M.E.; Fechner, J.H.; Schauer, J.J.; Mezrich, J.D. Real-world PM extracts differentially enhance Th17 differentiation and activate the aryl hydrocarbon receptor (AHR). Toxicology 2019, 414, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.M.; Beitel, S.C.; Gutenkunst, S.L.; Billheimer, D.; Jahnke, S.A.; Littau, S.R.; White, M.; Hoppe-Jones, C.; Cherrington, N.; Burgess, J.L. Excretion of polybrominated diphenyl ethers and AhR activation in breastmilk among firefighters. Toxicol Sci 2023, 192, 223–32. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J Inflamm Res 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; Silva, C.P. E.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Ahmed, A.S. S.; Sultana, S.; Habib, A.; Ullah, H.; Musa, N.; Hossain, M.B.; Rahman, M.M.; Sarker, M.S. I. Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PloS one 2019, 14, e0219336. [Google Scholar] [CrossRef]
- Onakpa, M.M.; Njan, A.A.; Kalu, O.C. A Review of Heavy Metal Contamination of Food Crops in Nigeria. Ann Glob Health 2018, 84, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Orosun, M.M.; Nwabachili, S.; Alshehri, R.F.; Omeje, M.; Alshdoukhi, I.F.; Okoro, H.K.; Ogunkunle, C.O.; Louis, H.; Abdulhamid, F.A.; Osahon, S.E.; Mohammed, A.U.; Ehinlafa, E.O.; Yunus, S.O.; Ife-Adediran, O. Potentially toxic metals in irrigation water, soil, and vegetables and their health risks using Monte Carlo models. Scientific reports 2023, 13, 21220. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mahabbat-e Khoda, S.; Rekha, R.S.; Gardner, R.M.; Ameer, S.S.; Moore, S.; Ekstrom, E.C.; Vahter, M.; Raqib, R. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environmental health perspectives 2011, 119, 258–64. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Banerjee, S.; Sen, R.; Bandyopadhyay, A.; Sarma, N.; Majumder, P.; Das, J.K.; Chatterjee, M.; Kabir, S.N.; Giri, A.K. Chronic arsenic exposure impairs macrophage functions in the exposed individuals. J Clin Immunol 2009, 29, 582–94. [Google Scholar] [CrossRef]
- Cho, Y.; Ahn, K.H.; Back, M.J.; Choi, J.M.; Ji, J.E.; Won, J.H.; Fu, Z.; Jang, J.M.; Kim, D.K. Age-related effects of sodium arsenite on splenocyte proliferation and Th1/Th2 cytokine production. Arch Pharm Res 2012, 35, 375–82. [Google Scholar] [CrossRef] [PubMed]
- Conde, P.; Acosta-Saavedra, L.C.; Goytia-Acevedo, R.C.; Calderon-Aranda, E.S. Sodium arsenite-induced inhibition of cell proliferation is related to inhibition of IL-2 mRNA expression in mouse activated T cells. Arch Toxicol 2007, 81, 251–9. [Google Scholar] [CrossRef] [PubMed]
- Morzadec, C.; Bouezzedine, F.; Macoch, M.; Fardel, O.; Vernhet, L. Inorganic arsenic impairs proliferation and cytokine expression in human primary T lymphocytes. Toxicology 2012, 300, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Binet, F.; Antoine, F.; Girard, D. Interaction between arsenic trioxide and human primary cells: emphasis on human cells of myeloid origin. Inflamm Allergy Drug Targets 2009, 8, 21–7. [Google Scholar] [CrossRef] [PubMed]
- Binet, F.; Cavalli, H.; Moisan, E.; Girard, D. Arsenic trioxide (AT) is a novel human neutrophil pro-apoptotic agent: effects of catalase on AT-induced apoptosis, degradation of cytoskeletal proteins and de novo protein synthesis. Br J Haematol 2006, 132, 349–58. [Google Scholar] [CrossRef]
- Xiao, T.; Zou, Z.; Xue, J.; Syed, B.M.; Sun, J.; Dai, X.; Shi, M.; Li, J.; Wei, S.; Tang, H.; Zhang, A.; Liu, Q. LncRNA H19-mediated M2 polarization of macrophages promotes myofibroblast differentiation in pulmonary fibrosis induced by arsenic exposure. Environmental pollution 2021, 268, 115810. [Google Scholar] [CrossRef]
- Xue, J.; Xiao, T.; Wei, S.; Sun, J.; Zou, Z.; Shi, M.; Sun, Q.; Dai, X.; Wu, L.; Li, J.; Xia, H.; Tang, H.; Zhang, A.; Liu, Q. miR-21-regulated M2 polarization of macrophage is involved in arsenicosis-induced hepatic fibrosis through the activation of hepatic stellate cells. J Cell Physiol 2021, 236, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.M.; Chiou, H.Y.; Ho, I.C.; Chen, C.J.; Lee, T.C. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environmental health perspectives 2003, 111, 1429–38. [Google Scholar] [CrossRef] [PubMed]
- Pilsner, J.R.; Liu, X.; Ahsan, H.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Graziano, J.H.; Gamble, M.V. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environmental health perspectives 2009, 117, 254–60. [Google Scholar] [CrossRef] [PubMed]
- Dangleben, N.L.; Skibola, C.F.; Smith, M.T. Arsenic immunotoxicity: a review. Environmental health : a global access science source 2013, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Dashner-Titus, E.J.; Schilz, J.R.; Simmons, K.A.; Duncan, T.R.; Alvarez, S.C.; Hudson, L.G. Differential response of human T-lymphocytes to arsenic and uranium. Toxicol Lett 2020, 333, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.L.; Khan, M.I.; Wang, J.; Ali, R.; Ali, S.W.; Zahra, Q.U.; Kazmi, A.; Lolai, A.; Huang, Y.L.; Hussain, A.; Bilal, M.; Li, F.; Qiu, B. Role of receptor tyrosine kinases mediated signal transduction pathways in tumor growth and angiogenesis-New insight and futuristic vision. Int J Biol Macromol 2021, 180, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R.; Lee, J.E.; Hussain, I.; Piepenbrink, M. Developmental immunotoxicology of lead. Toxicol Appl Pharmacol 2004, 198, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.L.; Wu, K.H.; Wan, K.S. Effects of environmental lead exposure on T-helper cell-specific cytokines in children. J Immunotoxicol 2011, 8, 284–7. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.; Parsons, P.J.; Lawrence, D.A. Lead differentially modifies cytokine production in vitro and in vivo. Toxicol Appl Pharmacol 1996, 138, 149–57. [Google Scholar] [CrossRef] [PubMed]
- Krocova, Z.; Macela, A.; Kroca, M.; Hernychova, L. The immunomodulatory effect(s) of lead and cadmium on the cells of immune system in vitro. Toxicol In Vitro 2000, 14, 33–40. [Google Scholar] [CrossRef]
- Mishra, K.P.; Singh, V.K.; Rani, R.; Yadav, V.S.; Chandran, V.; Srivastava, S.P.; Seth, P.K. Effect of lead exposure on the immune response of some occupationally exposed individuals. Toxicology 2003, 188, 251–9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Xu, H.; Li, Q.; Zhang, Y.; Zhai, Y.; Tang, M.; Liu, Y.; Liu, T.; Ye, Y.; He, M.; He, R.; Xu, Y.; Zhou, Z.; Kan, H.; Zhang, Y. Lead exposure suppresses the Wnt3a/beta-catenin signaling to increase the quiescence of hematopoietic stem cells via reducing the expression of CD70 on bone marrow-resident macrophages. Toxicol Sci 2023, 195, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Dobrakowski, M.; Boron, M.; Czuba, Z.P.; Kasperczyk, A.; Machon-Grecka, A.; Kasperczyk, S. Cytokines related to three major types of cell-mediated immunity in short- and long-term exposures to lead compounds. J Immunotoxicol 2016, 13, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Metryka, E.; Kupnicka, P.; Kapczuk, P.; Aszakiewicz, B.; Piotrowska, K.; Tkacz, M.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Lead (Pb) Accumulation in Human THP-1 Monocytes/Macrophages In Vitro and the Influence on Cell Apoptosis. Biol Trace Elem Res 2021, 199, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Osman, K.; Akesson, A.; Berglund, M.; Bremme, K.; Schutz, A.; Ask, K.; Vahter, M. Toxic and essential elements in placentas of Swedish women. Clin Biochem 2000, 33, 131–8. [Google Scholar] [CrossRef] [PubMed]
- Llanos, M.N.; Ronco, A.M. Fetal growth restriction is related to placental levels of cadmium, lead and arsenic but not with antioxidant activities. Reproductive toxicology 2009, 27, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zahir, F.; Rizwi, S.J.; Haq, S.K.; Khan, R.H. Low dose mercury toxicity and human health. Environ Toxicol Pharmacol 2005, 20, 351–60. [Google Scholar] [CrossRef] [PubMed]
- Jansson, G.; Harms-Ringdahl, M. Stimulating effects of mercuric- and silver ions on the superoxide anion production in human polymorphonuclear leukocytes. Free Radic Res Commun 1993, 18, 87–98. [Google Scholar] [CrossRef]
- Christensen, M.M.; Ellermann-Eriksen, S.; Rungby, J.; Mogensen, S.C. Comparison of the interaction of methyl mercury and mercuric chloride with murine macrophages. Arch Toxicol 1993, 67, 205–11. [Google Scholar] [CrossRef]
- InSug, O.; Datar, S.; Koch, C.J.; Shapiro, I.M.; Shenker, B.J. Mercuric compounds inhibit human monocyte function by inducing apoptosis: evidence for formation of reactive oxygen species, development of mitochondrial membrane permeability transition and loss of reductive reserve. Toxicology 1997, 124, 211–24. [Google Scholar] [CrossRef]
- Rodriguez-Viso, P.; Domene, A.; Velez, D.; Devesa, V.; Monedero, V.; Zuniga, M. Mercury toxic effects on the intestinal mucosa assayed on a bicameral in vitro model: Possible role of inflammatory response and oxidative stress. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2022, 166, 113224. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alarcon, J.; Milic, M.; Bustamante-Montes, L.P.; Isaac-Olive, K.; Valencia-Quintana, R.; Ramirez-Duran, N. Genotoxicity of Mercury and Its Derivatives Demonstrated In Vitro and In Vivo in Human Populations Studies. Systematic Review. Toxics 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Ilback, N.G. Effects of methyl mercury exposure on spleen and blood natural killer (NK) cell activity in the mouse. Toxicology 1991, 67, 117–24. [Google Scholar] [CrossRef] [PubMed]
- Ilback, N.G.; Sundberg, J.; Oskarsson, A. Methyl mercury exposure via placenta and milk impairs natural killer (NK) cell function in newborn rats. Toxicol Lett 1991, 58, 149–58. [Google Scholar] [CrossRef] [PubMed]
- Migdal, C.; Foggia, L.; Tailhardat, M.; Courtellemont, P.; Haftek, M.; Serres, M. Sensitization effect of thimerosal is mediated in vitro via reactive oxygen species and calcium signaling. Toxicology 2010, 274, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Migdal, C.; Tailhardat, M.; Courtellemont, P.; Haftek, M.; Serres, M. Responsiveness of human monocyte-derived dendritic cells to thimerosal and mercury derivatives. Toxicol Appl Pharmacol 2010, 246, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Shukla, G.S.; Chandra, S.V. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: in vivo and in vitro studies. Pharmacol Toxicol 1987, 60, 355–8. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Roles of dioxins and heavy metals in cancer and neurological diseases using ROS-mediated mechanisms. Free Radic Biol Med 2010, 49, 1328–41. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mendoza, D.; Han, B.; van den Berg, H.; van den Brink, N.W. Cell-specific immune-modulation of cadmium on murine macrophages and mast cell lines in vitro. J Appl Toxicol 2019, 39, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Mo, L.; Huang, H.; Mo, L.; Zhu, W.; Li, W.; Yang, G.; Chen, L.; Wu, Y.; Song, J.; Yang, X. Cadmium contributes to atherosclerosis by affecting macrophage polarization. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2023, 173, 113603. [Google Scholar] [CrossRef]
- Hanson, M.L.; Holaskova, I.; Elliott, M.; Brundage, K.M.; Schafer, R.; Barnett, J.B. Prenatal cadmium exposure alters postnatal immune cell development and function. Toxicol Appl Pharmacol 2012, 261, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Holaskova, I.; Elliott, M.; Hanson, M.L.; Schafer, R.; Barnett, J.B. Prenatal cadmium exposure produces persistent changes to thymus and spleen cell phenotypic repertoire as well as the acquired immune response. Toxicol Appl Pharmacol 2012, 265, 181–9. [Google Scholar] [CrossRef] [PubMed]
- Goyal, T.; Mitra, P.; Singh, P.; Ghosh, R.; Sharma, S.; Sharma, P. Association of microRNA expression with changes in immune markers in workers with cadmium exposure. Chemosphere 2021, 274, 129615. [Google Scholar] [CrossRef] [PubMed]
- El-Shahawi, M.S.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W.T. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–97. [Google Scholar] [CrossRef] [PubMed]
- Haddad, S.; Ayotte, P.; Verner, M.A. Derivation of exposure factors for infant lactational exposure to persistent organic pollutants (POPs). Regul Toxicol Pharmacol 2015, 71, 135–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Tao, F.M.; Zeng, E.Y. Theoretical study on the chemical properties of polybrominated diphenyl ethers. Chemosphere 2008, 70, 901–7. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.P.; Warren, T.K.; Luong, H. Fewer T lymphocytes and decreased pulmonary influenza virus burden in mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). J Toxicol Environ Health A 2000, 61, 39–53. [Google Scholar] [PubMed]
- Marshall, N.B.; Kerkvliet, N.I. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann N Y Acad Sci 2010, 1183, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Mitchell, K.A.; Lawrence, B.P. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) suppresses the humoral and cell-mediated immune responses to influenza A virus without affecting cytolytic activity in the lung. Toxicol Sci 2000, 56, 114–23. [Google Scholar] [CrossRef]
- Kerkvliet, N.I. Recent advances in understanding the mechanisms of TCDD immunotoxicity. Int Immunopharmacol 2002, 2, 277–91. [Google Scholar] [CrossRef]
- Torti, M.F.; Giovannoni, F.; Quintana, F.J.; Garcia, C.C. The Aryl Hydrocarbon Receptor as a Modulator of Anti-viral Immunity. Frontiers in immunology 2021, 12, 624293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, N.; Han, Y.; Rao, K.; Ji, X.; Ma, M. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of immunity in THP-1-derived macrophages and the possible mechanisms. Environmental pollution 2021, 287, 117302. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Gnecco, J.; Ding, T.; Glore, D.R.; Pensabene, V.; Osteen, K.G. Exposure to the environmental endocrine disruptor TCDD and human reproductive dysfunction: Translating lessons from murine models. Reproductive toxicology 2017, 68, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Petriello, M.C.; Zhu, B.; Hennig, B. PCB 126 induces monocyte/macrophage polarization and inflammation through AhR and NF-kappaB pathways. Toxicol Appl Pharmacol 2019, 367, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Ferrante, M.C.; Di Guida, F.; Pirozzi, C.; Lama, A.; Simeoli, R.; Clausi, M.T.; Monnolo, A.; Mollica, M.P.; Mattace Raso, G.; Meli, R. Polychlorinated Biphenyls (PCB 101, 153, and 180) Impair Murine Macrophage Responsiveness to Lipopolysaccharide: Involvement of NF-kappaB Pathway. Toxicol Sci 2015, 147, 255–69. [Google Scholar] [CrossRef] [PubMed]
- Waugh, C.A.; Arukwe, A.; Jaspers, V.L. B. Deregulation of microRNA-155 and its transcription factor NF-kB by polychlorinated biphenyls during viral infections. APMIS 2018, 126, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C.L.; Spiegelhoff, A.; Wang, K.; Lavery, T.; Nunez, A.; Manuel, R.; Hillers-Ziemer, L.; Arendt, L.M.; Stietz, K.P. K. The Bladder Is a Novel Target of Developmental Polychlorinated Biphenyl Exposure Linked to Increased Inflammatory Cells in the Bladder of Young Mice. Toxics 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Curtis, S.W.; Cobb, D.O.; Kilaru, V.; Terrell, M.L.; Marder, M.E.; Barr, D.B.; Marsit, C.J.; Marcus, M.; Conneely, K.N.; Smith, A.K. Genome-wide DNA methylation differences and polychlorinated biphenyl (PCB) exposure in a US population. Epigenetics 2021, 16, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Sjodin, A.; Patterson, D.G. Jr.; Bergman, A. A review on human exposure to brominated flame retardants--particularly polybrominated diphenyl ethers. Environment international 2003, 29, 829–39. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.J.; Ball, A.S.; Clarke, B.O. Critical review of soil contamination by polybrominated diphenyl ethers (PBDEs) and novel brominated flame retardants (NBFRs); concentrations, sources and congener profiles. Environmental pollution 2017, 230, 741–757. [Google Scholar] [CrossRef]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S. Halogenated flame retardants: do the fire safety benefits justify the risks? Rev Environ Health 2010, 25, 261–305. [Google Scholar] [CrossRef] [PubMed]
- Chain, E.P. o. C. i. t. F.; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Ron Hoogenboom, L.; Leblanc, J.-C.; Nebbia, C.S.; Nielsen, E.; Ntzani, E.; Petersen, A.; Sand, S.; Schwerdtle, T.; Wallace, H.; Benford, D.; Furst, P.; Hart, A.; Rose, M.; Schroeder, H.; Vrijheid, M.; Ioannidou, S.; Nikolic, M.; Bordajandi, L.R.; Vleminckx, C. Update of the risk assessment of polybrominated diphenyl ethers (PBDEs) in food. EFSA journal European Food Safety Authority 2024, 22, e8497. [Google Scholar]
- Xue, J.; Xiao, Q.; Zhang, M.; Li, D.; Wang, X. Toxic Effects and Mechanisms of Polybrominated Diphenyl Ethers. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Alzualde, A.; Behl, M.; Sipes, N.S.; Hsieh, J.H.; Alday, A.; Tice, R.R.; Paules, R.S.; Muriana, A.; Quevedo, C. Toxicity profiling of flame retardants in zebrafish embryos using a battery of assays for developmental toxicity, neurotoxicity, cardiotoxicity and hepatotoxicity toward human relevance. Neurotoxicology and teratology 2018, 70, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Lema, S.C.; Schultz, I.R.; Scholz, N.L.; Incardona, J.P.; Swanson, P. Neural defects and cardiac arrhythmia in fish larvae following embryonic exposure to 2,2',4,4'-tetrabromodiphenyl ether (PBDE 47). Aquat Toxicol 2007, 82, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A.; Lange, A.; Hutchinson, T.H.; Miyagawa, S.; Iguchi, T.; Kudoh, T.; Tyler, C.R. Molecular mechanisms and tissue targets of brominated flame retardants, BDE-47 and TBBPA, in embryo-larval life stages of zebrafish (Danio rerio). Aquat Toxicol 2019, 209, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Longo, A.; Di Sano, C.; Cigna, D.; Cibella, F.; Di Felice, G.; Colombo, P. In vitro exposure to 2,2',4,4'-tetrabromodiphenyl ether (PBDE-47) impairs innate inflammatory response. Chemosphere 2019, 219, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Longo, A.; Adamo, G.; Fiannaca, A.; Picciotto, S.; La Paglia, L.; Romancino, D.; La Rosa, M.; Urso, A.; Cibella, F.; Bongiovanni, A.; Colombo, P. 2,2'4,4'-Tetrabromodiphenyl Ether (PBDE-47) Modulates the Intracellular miRNA Profile, sEV Biogenesis and Their miRNA Cargo Exacerbating the LPS-Induced Pro-Inflammatory Response in THP-1 Macrophages. Frontiers in immunology 2021, 12, 664534. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Aloi, N.; Lo Presti, E.; Fiannaca, A.; Longo, A.; Adamo, G.; Urso, A.; Meraviglia, S.; Bongiovanni, A.; Cibella, F.; Colombo, P. Impact of the flame retardant 2,2'4,4'-tetrabromodiphenyl ether (PBDE-47) in THP-1 macrophage-like cell function via small extracellular vesicles. Frontiers in immunology 2022, 13, 1069207. [Google Scholar] [CrossRef]
- Albano, G.D.; Longo, V.; Montalbano, A.M.; Aloi, N.; Barone, R.; Cibella, F.; Profita, M.; Colombo, P. Extracellular vesicles from PBDE-47 treated M(LPS) THP-1 macrophages modulate the expression of markers of epithelial integrity, EMT, inflammation and muco-secretion in ALI culture of airway epithelium. Life Sci 2023, 322, 121616. [Google Scholar] [CrossRef]
- Ren, Q.; Xie, X.; Zhao, C.; Wen, Q.; Pan, R.; Du, Y. 2,2',4,4'-Tetrabromodiphenyl Ether (PBDE 47) Selectively Stimulates Proatherogenic PPARgamma Signatures in Human THP-1 Macrophages to Contribute to Foam Cell Formation. Chem Res Toxicol 2022, 35, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Babior, B.M. Phagocytes and oxidative stress. Am J Med 2000, 109, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, F.; Hui, Y.; Xu, Y.; Lu, Y.; Liu, J. Cytotoxicity and apoptosis induction on RTG-2 cells of 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) and decabrominated diphenyl ether (BDE-209). Toxicol In Vitro 2010, 24, 1190–6. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Y.; Wan, B.; Guo, L.H.; Yang, Y.; Ren, X.M.; Zhang, H. In vivo immunotoxicity of perfluorooctane sulfonate in BALB/c mice: Identification of T-cell receptor and calcium-mediated signaling pathway disruption through gene expression profiling of the spleen. Chem Biol Interact 2015, 240, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Barletta, B.; Corinti, S.; Maranghi, F.; Tait, S.; Tassinari, R.; Martinelli, A.; Longo, A.; Longo, V.; Colombo, P.; Di Felice, G.; Butteroni, C. The environmental pollutant BDE-47 modulates immune responses in invitro and in vivo murine models. Chemosphere 2024, 349, 140739. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: cause for concern? Environmental health perspectives 2004, 112, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Szabo, D.T.; Miller, J.; Gent, T.L.; Malik-Bass, N.; Petersen, M.; Paepke, O.; Colacino, J.A.; Hynan, L.S.; Harris, T.R.; Malla, S.; Birnbaum, L.S. Hexabromocyclododecane (HBCD) stereoisomers in U.S. food from Dallas, Texas. Environmental health perspectives 2012, 120, 1260–4. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, N.; Sueoka, M.; Ohiwa, T.; Takigami, H. Determination of flame-retardant hexabromocyclododecane diastereomers in textiles. Chemosphere 2009, 74, 1485–9. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.A.; Harrad, S.; Covaci, A. Hexabromocyclododecanes and tetrabromobisphenol-A in indoor air and dust in Birmingham, U.K: implications for human exposure. Environmental science & technology 2008, 42, 6855–61. [Google Scholar]
- Remberger, M.; Sternbeck, J.; Palm, A.; Kaj, L.; Stromberg, K.; Brorstrom-Lunden, E. The environmental occurrence of hexabromocyclododecane in Sweden. Chemosphere 2004, 54, 9–21. [Google Scholar] [CrossRef]
- Kakimoto, K.; Akutsu, K.; Konishi, Y.; Tanaka, Y. Time trend of hexabromocyclododecane in the breast milk of Japanese women. Chemosphere 2008, 71, 1110–4. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Kvalem, H.E.; Thomsen, C.; Froshaug, M.; Haugen, M.; Becher, G.; Alexander, J.; Meltzer, H.M. Dietary exposure to brominated flame retardants correlates with male blood levels in a selected group of Norwegians with a wide range of seafood consumption. Mol Nutr Food Res 2008, 52, 217–27. [Google Scholar] [CrossRef] [PubMed]
- Pulkrabova, J.; Hradkova, P.; Hajslova, J.; Poustka, J.; Napravnikova, M.; Polacek, V. Brominated flame retardants and other organochlorine pollutants in human adipose tissue samples from the Czech Republic. Environment international 2009, 35, 63–8. [Google Scholar] [CrossRef] [PubMed]
- Chain, E.P. C. i. t. F.; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; Nebbia, C.S.; Nielsen, E.; Ntzani, E.; Petersen, A.; Sand, S.; Schwerdtle, T.; Wallace, H.; Benford, D.; Furst, P.; Rose, M.; Ioannidou, S.; Nikolic, M.; Bordajandi, L.R.; Vleminckx, C. Update of the risk assessment of hexabromocyclododecanes (HBCDDs) in food. EFSA journal European Food Safety Authority 2021, 19, e06421. [Google Scholar]
- Baranska, A.; Bukowska, B.; Michalowicz, J. Determination of Apoptotic Mechanism of Action of Tetrabromobisphenol A and Tetrabromobisphenol S in Human Peripheral Blood Mononuclear Cells: A Comparative Study. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Baranska, A.; Wozniak, A.; Mokra, K.; Michalowicz, J. Genotoxic Mechanism of Action of TBBPA, TBBPS and Selected Bromophenols in Human Peripheral Blood Mononuclear Cells. Frontiers in immunology 2022, 13, 869741. [Google Scholar] [CrossRef] [PubMed]
- Canbaz, D.; Lebre, M.C.; Logiantara, A.; van Ree, R.; van Rijt, L.S. Indoor pollutant hexabromocyclododecane enhances house dust mite-induced activation of human monocyte-derived dendritic cells. J Immunotoxicol 2016, 13, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, Z.; Chen, H.; Han, Y.; Xiang, M.; Chen, X.; Ma, R.; Wang, Z. Tetrabromobisphenol A: Disposition, kinetics and toxicity in animals and humans. Environmental pollution 2019, 253, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Feiteiro, J.; Mariana, M.; Cairrao, E. Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environmental pollution 2021, 285, 117475. [Google Scholar] [CrossRef]
- Miao, B.; Yakubu, S.; Zhu, Q.; Issaka, E.; Zhang, Y.; Adams, M. A Review on Tetrabromobisphenol A: Human Biomonitoring, Toxicity, Detection and Treatment in the Environment. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Balistrieri, A.; Hobohm, L.; Srivastava, T.; Meier, A.; Corriden, R. Alterations in human neutrophil function caused by bisphenol A. Am J Physiol Cell Physiol 2018, 315, C636–C642. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, M.; Wu, C.; Zhou, C.; Zhang, J.; Zhu, Q.; Shen, T. Bisphenol A promotes macrophage proinflammatory subtype polarization via upregulation of IRF5 expression in vitro. Toxicol In Vitro 2019, 60, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kodila, A.; Franko, N.; Sollner Dolenc, M. A review on immunomodulatory effects of BPA analogues. Arch Toxicol 2023, 97, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, Z.; Ye, L.; Chen, X.; Zhang, W.; Zhang, Z.; Luo, F.; Liu, Y.; Shi, M. Estrogen receptor-regulated SOCS3 modulation via JAK2/STAT3 pathway is involved in BPF-induced M1 polarization of macrophages. Toxicology 2020, 433-434, 152404. [Google Scholar] [CrossRef]
- Hernandez Avila, R.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Morales-Montor, J.; Ostoa-Saloma, P. Neonatal Bisphenol A Exposure Affects the IgM Humoral Immune Response to 4T1 Breast Carcinoma Cells in Mice. International journal of environmental research and public health 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Liu, T.; Yang, S.; Sun, L.; Zhao, Z.Y.; Li, L.Y.; She, Y.C.; Zheng, Y.Y.; Ye, X.Y.; Bao, Q.; Dong, G.H.; Li, C.W.; Cui, J. Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat Commun 2021, 12, 2915. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.M.; Chen, D.; Han, F.J.; Guo, Y.; Zeng, L.; Lu, X.; Wang, F. A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). The Science of the total environment 2018, 636, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Nadal, M.; Navarro-Ortega, A.; Fabrega, F.; Domingo, J.L.; Barcelo, D.; Farre, M. Accumulation of perfluoroalkyl substances in human tissues. Environment international 2013, 59, 354–62. [Google Scholar] [CrossRef] [PubMed]
- Midgett, K.; Peden-Adams, M.M.; Gilkeson, G.S.; Kamen, D.L. In vitro evaluation of the effects of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on IL-2 production in human T-cells. J Appl Toxicol 2015, 35, 459–65. [Google Scholar] [CrossRef]
- Torres, L.; Redko, A.; Limper, C.; Imbiakha, B.; Chang, S.; August, A. Effect of Perfluorooctanesulfonic acid (PFOS) on immune cell development and function in mice. Immunol Lett 2021, 233, 31–41. [Google Scholar] [CrossRef]
- Taylor, K.D.; Woodlief, T.L.; Ahmed, A.; Hu, Q.; Duncker, P.C.; DeWitt, J.C. Quantifying the impact of PFOA exposure on B-cell development and antibody production. Toxicol Sci 2023, 194, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Dietert, R.R. Developmental Immunotoxicity, Perinatal Programming, and Noncommunicable Diseases: Focus on Human Studies. Adv Med 2014, 2014, 867805. [Google Scholar] [CrossRef] [PubMed]
- Holsapple, M.P.; West, L.J.; Landreth, K.S. Species comparison of anatomical and functional immune system development. Birth Defects Res B Dev Reprod Toxicol 2003, 68, 321–34. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; Hanson, M.A.; Forrester, T.; Gluckman, P.D.; Godfrey, K.M. Origins of lifetime health around the time of conception: causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; Rychen, G.; Schlatter, J.R.; Silano, V.; Solecki, R.; Turck, D.; Bresson, J.L.; Dusemund, B.; Gundert-Remy, U.; Kersting, M.; Lambré, C.; Penninks, A.; Tritscher, A.; Waalkens-Berendsen, I.; Woutersen, R.; Arcella, D.; Marques, D.C.; Dorne, J.L.; Kass, G.E. N.; Mortensen, A.; Comm, E.S. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. Efsa Journal 2017, 15. [Google Scholar]
- Dietert, R.R.; DeWitt, J.C.; Germolec, D.R.; Zelikoff, J.T. Breaking patterns of environmentally influenced disease for health risk reduction: immune perspectives. Environmental health perspectives 2010, 118, 1091–9. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; Feldman, T.B.; Chitnis, T. Interplay Between Endocrine Disruptors and Immunity: Implications for Diseases of Autoreactive Etiology. Front Pharmacol 2021, 12, 626107. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Chavan, A.R.; Protopapas, S.; Maziarz, J.; Romero, R.; Wagner, G.P. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci U S A 2017, 114, E6566–E6575. [Google Scholar] [CrossRef] [PubMed]
- Jarmund, A.H.; Giskeodegard, G.F.; Ryssdal, M.; Steinkjer, B.; Stokkeland, L.M. T.; Madssen, T.S.; Stafne, S.N.; Stridsklev, S.; Moholdt, T.; Heimstad, R.; Vanky, E.; Iversen, A.C. Cytokine Patterns in Maternal Serum From First Trimester to Term and Beyond. Frontiers in immunology 2021, 12, 752660. [Google Scholar] [CrossRef]
- Kaislasuo, J.; Simpson, S.; Petersen, J.F.; Peng, G.; Aldo, P.; Lokkegaard, E.; Paidas, M.; Pal, L.; Guller, S.; Mor, G. IL-10 to TNFalpha ratios throughout early first trimester can discriminate healthy pregnancies from pregnancy losses. Am J Reprod Immunol 2020, 83, e13195. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.C.; Cornelius, D.C.; Amaral, L.M.; Faulkner, J.L.; Cunningham, M.W. Jr.; Wallace, K.; LaMarca, B. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 2016, 130, 409–19. [Google Scholar] [CrossRef] [PubMed]
- Prairie, E.; Cote, F.; Tsakpinoglou, M.; Mina, M.; Quiniou, C.; Leimert, K.; Olson, D.; Chemtob, S. The determinant role of IL-6 in the establishment of inflammation leading to spontaneous preterm birth. Cytokine Growth Factor Rev 2021, 59, 118–130. [Google Scholar] [CrossRef]
- Atladottir, H.O.; Thorsen, P.; Ostergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 2010, 40, 1423–30. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.J.; Hallenbeck, J.M. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res 2002, 70, 570–9. [Google Scholar] [CrossRef]
- Ponzio, N.M.; Servatius, R.; Beck, K.; Marzouk, A.; Kreider, T. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann N Y Acad Sci 2007, 1107, 118–28. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 2007, 27, 10695–702. [Google Scholar] [CrossRef] [PubMed]
- Lu-Culligan, A.; Iwasaki, A. The Role of Immune Factors in Shaping Fetal Neurodevelopment. Annu Rev Cell Dev Biol 2020, 36, 441–468. [Google Scholar] [CrossRef]
- Tomlinson, M.S.; Bommarito, P.A.; Martin, E.M.; Smeester, L.; Fichorova, R.N.; Onderdonk, A.B.; Kuban, K.C. K.; O'Shea, T.M.; Fry, R.C. Microorganisms in the human placenta are associated with altered CpG methylation of immune and inflammation-related genes. PloS one 2017, 12, e0188664. [Google Scholar] [CrossRef]
- Schaub, B.; Liu, J.; Hoppler, S.; Schleich, I.; Huehn, J.; Olek, S.; Wieczorek, G.; Illi, S.; von Mutius, E. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 2009, 123, 774–82. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Green, E.S.; Overduin, T.S.; Mah, C.Y.; Russell, D.L.; Robertson, S.A. Endocrine Disruptor Compounds-A Cause of Impaired Immune Tolerance Driving Inflammatory Disorders of Pregnancy? Front Endocrinol (Lausanne) 2021, 12, 607539. [Google Scholar] [CrossRef] [PubMed]
- Attreed, S.E.; Navas-Acien, A.; Heaney, C.D. Arsenic and Immune Response to Infection During Pregnancy and Early Life. Curr Environ Health Rep 2017, 4, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, X.; Burchiel, S.W. Toxicity of environmentally-relevant concentrations of arsenic on developing T lymphocyte. Environ Toxicol Pharmacol 2018, 62, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Kile, M.L.; Houseman, E.A.; Baccarelli, A.A.; Quamruzzaman, Q.; Rahman, M.; Mostofa, G.; Cardenas, A.; Wright, R.O.; Christiani, D.C. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics 2014, 9, 774–82. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.C.; Li, Z.; Palys, T.; Jackson, B.; Subbiah, M.; Malipatlolla, M.; Sampath, V.; Maecker, H.; Karagas, M.R.; Nadeau, K.C. Cord blood T cell subpopulations and associations with maternal cadmium and arsenic exposures. PloS one 2017, 12, e0179606. [Google Scholar] [CrossRef] [PubMed]
- Farzan, S.F.; Li, Z.; Korrick, S.A.; Spiegelman, D.; Enelow, R.; Nadeau, K.; Baker, E.; Karagas, M.R. Infant Infections and Respiratory Symptoms in Relation to in Utero Arsenic Exposure in a U.S. Cohort. Environmental health perspectives 2016, 124, 840–7. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Vahter, M.; Ekstrom, E.C.; Persson, L.A. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environmental health perspectives 2011, 119, 719–24. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.; Varela-Nallar, L.; Coddou, C.; Nelson, P.; Maisey, K.; Valdes, D.; Aspee, A.; Espinosa, V.; Rozas, C.; Montoya, M.; Mandiola, C.; Rodriguez, F.E.; Acuna-Castillo, C.; Escobar, A.; Fernandez, R.; Diaz, H.; Sandoval, M.; Imarai, M.; Rios, M. Oxidative damage in lymphocytes of copper smelter workers correlated to higher levels of excreted arsenic. Mediators Inflamm 2010, 2010, 403830. [Google Scholar] [CrossRef] [PubMed]
- Luna, A.L.; Acosta-Saavedra, L.C.; Lopez-Carrillo, L.; Conde, P.; Vera, E.; De Vizcaya-Ruiz, A.; Bastida, M.; Cebrian, M.E.; Calderon-Aranda, E.S. Arsenic alters monocyte superoxide anion and nitric oxide production in environmentally exposed children. Toxicol Appl Pharmacol 2010, 245, 244–51. [Google Scholar] [CrossRef] [PubMed]
- Fry, R.C.; Navasumrit, P.; Valiathan, C.; Svensson, J.P.; Hogan, B.J.; Luo, M.; Bhattacharya, S.; Kandjanapa, K.; Soontararuks, S.; Nookabkaew, S.; Mahidol, C.; Ruchirawat, M.; Samson, L.D. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genet 2007, 3, e207. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Fu, G.; Feng, Y.; Wu, W.; Yang, H.; Zhang, Y.; Wang, S. DNA methylation analysis reveals the effect of arsenic on gestational diabetes mellitus. Genomics 2023, 115, 110674. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.D.; Lam, W.L. Health Effects Associated With Pre- and Perinatal Exposure to Arsenic. Front Genet 2021, 12, 664717. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, J.; Benbrahim-Tallaa, L.; Ward, J.M.; Logsdon, D.; Diwan, B.A.; Waalkes, M.P. Aberrant DNA methylation and gene expression in livers of newborn mice transplacentally exposed to a hepatocarcinogenic dose of inorganic arsenic. Toxicology 2007, 236, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol 2014, 13, 330–8. [Google Scholar] [CrossRef] [PubMed]
- Emeny, R.T.; Korrick, S.A.; Li, Z.; Nadeau, K.; Madan, J.; Jackson, B.; Baker, E.; Karagas, M.R. Prenatal exposure to mercury in relation to infant infections and respiratory symptoms in the New Hampshire Birth Cohort Study. Environmental research 2019, 171, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Movassagh, H.; Halchenko, Y.; Sampath, V.; Nygaard, U.C.; Jackson, B.; Robbins, D.; Li, Z.; Nadeau, K.C.; Karagas, M.R. Maternal gestational mercury exposure in relation to cord blood T cell alterations and placental gene expression signatures. Environmental research 2021, 201, 111385. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; Shamim, Z.; Kielsen, K.; Weihe, P.; Grandjean, P.; Ryder, L.P.; Heilmann, C. Children's white blood cell counts in relation to developmental exposures to methylmercury and persistent organic pollutants. Reproductive toxicology 2017, 68, 207–214. [Google Scholar] [CrossRef]
- Stratakis, N.; Conti, D.V.; Borras, E.; Sabido, E.; Roumeliotaki, T.; Papadopoulou, E.; Agier, L.; Basagana, X.; Bustamante, M.; Casas, M.; Farzan, S.F.; Fossati, S.; Gonzalez, J.R.; Grazuleviciene, R.; Heude, B.; Maitre, L.; McEachan, R.R. C.; Theologidis, I.; Urquiza, J.; Vafeiadi, M.; West, J.; Wright, J.; McConnell, R.; Brantsaeter, A.L.; Meltzer, H.M.; Vrijheid, M.; Chatzi, L. Association of Fish Consumption and Mercury Exposure During Pregnancy With Metabolic Health and Inflammatory Biomarkers in Children. JAMA Netw Open 2020, 3, e201007. [Google Scholar] [CrossRef] [PubMed]
- Nyland, J.F.; Fillion, M.; Barbosa, F. Jr.; Shirley, D.L.; Chine, C.; Lemire, M.; Mergler, D.; Silbergeld, E.K. Biomarkers of methylmercury exposure immunotoxicity among fish consumers in Amazonian Brazil. Environmental health perspectives 2011, 119, 1733–8. [Google Scholar] [CrossRef]
- Pinheiro, M.C. N.; Macchi, B.M.; Vieira, J.L. F.; Oikawa, T.; Amoras, W.W.; Guimaraes, G.A.; Costa, C.A.; Crespo-Lopez, M.E.; Herculano, A.M.; Silveira, L.C. L.; do Nascimento, J.L. M. Mercury exposure and antioxidant defenses in women: a comparative study in the Amazon. Environmental research 2008, 107, 53–9. [Google Scholar] [CrossRef]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environment international 2018, 120, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.A.; McCabe, M.J. Jr. Immunomodulation by metals. Int Immunopharmacol 2002, 2, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Dietert, R.R. Developmental immunotoxicity of lead: impact on thymic function. Birth Defects Res A Clin Mol Teratol 2003, 67, 861–7. [Google Scholar] [CrossRef] [PubMed]
- Annesi-Maesano, I.; Pollitt, R.; King, G.; Bousquet, J.; Hellier, G.; Sahuquillo, J.; Huel, G. In utero exposure to lead and cord blood total IgE. Is there a connection? Allergy 2003, 58, 589–94. [Google Scholar] [CrossRef] [PubMed]
- Wells, E.M.; Bonfield, T.L.; Dearborn, D.G.; Jackson, L.W. The relationship of blood lead with immunoglobulin E, eosinophils, and asthma among children: NHANES 2005-2006. International journal of hygiene and environmental health 2014, 217, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Sekovanic, A.; Dorotic, A.; Pasalic, D.; Orct, T.; Kljakovic-Gaspic, Z.; Grgec, A.S.; Stasenko, S.; Mioc, T.; Piasek, M.; Jurasovic, J. The effects of maternal cigarette smoking on cadmium and lead levels, miRNA expression and biochemical parameters across the feto-placental unit. Heliyon 2022, 8, e12568. [Google Scholar] [CrossRef] [PubMed]
- Canepari, S.; Astolfi, M.L.; Drago, G.; Ruggieri, S.; Tavormina, E.E.; Cibella, F.; Perrino, C. PM(2.5) elemental composition in indoor residential environments and co-exposure effects on respiratory health in an industrial area. Environmental research 2023, 216, 114630. [Google Scholar] [CrossRef] [PubMed]
- Drago, G.; Perrino, C.; Canepari, S.; Ruggieri, S.; L'Abbate, L.; Longo, V.; Colombo, P.; Frasca, D.; Balzan, M.; Cuttitta, G.; Scaccianoce, G.; Piva, G.; Bucchieri, S.; Melis, M.; Viegi, G.; Cibella, F.; Group, R.C.P.; Indoor, *!!! REPLACE !!!*; Outdoor Air, Q.; Respiratory Health in, M.; Sicily, R.S. G.; Balzan, M.; Bilocca, D.; Borg, C.; Montefort, S.; Zammit, C.; Bucchieri, S.; Cibella, F.; Colombo, P.; Cuttitta, G.; Drago, G.; Ferrante, G.; L'Abbate, L.; Grutta, S.; Longo, V.; Melis, M.R.; Ruggieri, S.; Viegi, G.; Minardi, R.; Piva, G.; Ristagno, R.; Rizzo, G.; Scaccianoce, G. Relationship between domestic smoking and metals and rare earth elements concentration in indoor PM(2.5). Environmental research 2018, 165, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Breen, J.G.; Eisenmann, C.; Horowitz, S.; Miller, R.K. Cell-specific increases in metallothionein expression in the human placenta perfused with cadmium. Reproductive toxicology 1994, 8, 297–306. [Google Scholar] [CrossRef]
- Kippler, M.; Hoque, A.M.; Raqib, R.; Ohrvik, H.; Ekstrom, E.C.; Vahter, M. Accumulation of cadmium in human placenta interacts with the transport of micronutrients to the fetus. Toxicol Lett 2010, 192, 162–8. [Google Scholar] [CrossRef]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental toxicity of cadmium in infants and children: a review. Environ Anal Health Toxicol 2021, 36, e2021003–0. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.P.; Smeester, L.; Rojas, D.; DeBussycher, T.; Wu, M.C.; Wright, F.A.; Zhou, Y.H.; Laine, J.E.; Rager, J.E.; Swamy, G.K.; Ashley-Koch, A.; Lynn Miranda, M.; Fry, R.C. Cadmium exposure and the epigenome: Exposure-associated patterns of DNA methylation in leukocytes from mother-baby pairs. Epigenetics 2014, 9, 212–21. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhang, W.X.; Zheng, T.Z.; Zhou, B.; Li, J.X.; Zhang, B.; Xia, W.; Li, Y.Y.; Xu, S.Q. Prenatal and postnatal cadmium exposure and cellular immune responses among pre-school children. Environment international 2020, 134, 105282. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Rooney, A.A.; Bouquegneau, J.-M.; Cyr, D.G.; Fournier, M. Sex-specific effects of neonatal exposures to low levels of cadmium through maternal milk on development and immune functions of juvenile and adult rats. Toxicology 2005, 209, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Budtz-Jorgensen, E. Serum Vaccine Antibody Concentrations in Adolescents Exposed to Perfluorinated Compounds. Environmental health perspectives 2017, 125, 077018. [Google Scholar] [CrossRef]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.B.; Timmermann, A.; Budtz-Jorgensen, E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J Immunotoxicol 2017, 14, 188–195. [Google Scholar] [CrossRef]
- Granum, B.; Haug, L.S.; Namork, E.; Stolevik, S.B.; Thomsen, C.; Aaberge, I.S.; van Loveren, H.; Lovik, M.; Nygaard, U.C. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 2013, 10, 373–9. [Google Scholar] [CrossRef] [PubMed]
- Osuna, C.E.; Grandjean, P.; Weihe, P.; El-Fawal, H.A. Autoantibodies associated with prenatal and childhood exposure to environmental chemicals in Faroese children. Toxicol Sci 2014, 142, 158–66. [Google Scholar] [CrossRef] [PubMed]
- Ait Bamai, Y.; Goudarzi, H.; Araki, A.; Okada, E.; Kashino, I.; Miyashita, C.; Kishi, R. Effect of prenatal exposure to per- and polyfluoroalkyl substances on childhood allergies and common infectious diseases in children up to age 7years: The Hokkaido study on environment and children's health. Environment international 2020, 143, 105979. [Google Scholar] [CrossRef]
- Impinen, A.; Longnecker, M.P.; Nygaard, U.C.; London, S.J.; Ferguson, K.K.; Haug, L.S.; Granum, B. Maternal levels of perfluoroalkyl substances (PFASs) during pregnancy and childhood allergy and asthma related outcomes and infections in the Norwegian Mother and Child (MoBa) cohort. Environment international 2019, 124, 462–472. [Google Scholar] [CrossRef]
- Stein, C.R.; McGovern, K.J.; Pajak, A.M.; Maglione, P.J.; Wolff, M.S. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12-19 y: National Health and Nutrition Examination Survey. Pediatr Res 2016, 79, 348–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Hsieh, W.-S.; Chen, C.-Y.; Fletcher, T.; Lien, G.-W.; Chiang, H.-L.; Chiang, C.-F.; Wu, T.-N.; Chen, P.-C. The effect of prenatal perfluorinated chemicals exposures on pediatric atopy. Environmental research 2011, 111, 785–91. [Google Scholar] [CrossRef] [PubMed]
- von Holst, H.; Nayak, P.; Dembek, Z.; Buehler, S.; Echeverria, D.; Fallacara, D.; John, L. Perfluoroalkyl substances exposure and immunity, allergic response, infection, and asthma in children: review of epidemiologic studies. Heliyon 2021, 7, e08160. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Grandjean, P.; Weihe, P.; Nielsen, F.; Budtz-Jorgensen, E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med 2006, 3, e311. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Hertz-Picciotto, I.; Petrik, J.; Palkovicova, L.; Kocan, A.; Trnovec, T. Prenatal PCB exposure and thymus size at birth in neonates in Eastern Slovakia. Environmental health perspectives 2008, 116, 104–9. [Google Scholar] [CrossRef] [PubMed]
- Horvathova, M.; Jahnova, E.; Palkovicova, L.; Trnovec, T.; Hertz-Picciotto, I. The kinetics of cell surface receptor expression in children perinatally exposed to polychlorinated biphenyls. J Immunotoxicol 2011, 8, 367–80. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Singh, U.P.; Guan, H.; Nagarkatti, P.; Nagarkatti, M. Prenatal exposure to TCDD triggers significant modulation of microRNA expression profile in the thymus that affects consequent gene expression. PloS one 2012, 7, e45054. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Paris, F.; Kalfa, N.; Soyer-Gobillard, M.O.; Sultan, C.; Hamamah, S. Experimental Evidence of 2,3,7,8-Tetrachlordibenzo-p-Dioxin (TCDD) Transgenerational Effects on Reproductive Health. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- O'Boyle, N.M.; Delaine, T.; Luthman, K.; Natsch, A.; Karlberg, A.T. Analogues of the epoxy resin monomer diglycidyl ether of bisphenol F: effects on contact allergenic potency and cytotoxicity. Chem Res Toxicol 2012, 25, 2469–78. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.M.; Miller, R.L.; Perzanowski, M.S.; Just, A.C.; Hoepner, L.A.; Arunajadai, S.; Canfield, S.; Resnick, D.; Calafat, A.M.; Perera, F.P.; Whyatt, R.M. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol 2013, 131, 736–42. [Google Scholar] [CrossRef]
- Gascon, M.; Casas, M.; Morales, E.; Valvi, D.; Ballesteros-Gomez, A.; Luque, N.; Rubio, S.; Monfort, N.; Ventura, R.; Martinez, D.; Sunyer, J.; Vrijheid, M. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J Allergy Clin Immunol 2015, 135, 370–8. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A.; Salo, P.M.; Wilkerson, J.; Feinstein, L.; Ferguson, K.K.; Fessler, M.B.; Thorne, P.S.; Zeldin, D.C. Association of urinary levels of bisphenols F and S used as bisphenol A substitutes with asthma and hay fever outcomes. Environmental research 2020, 183, 108944. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Hong, X.; Wang, X.; Tang, W.Y. Aberrant 5'-CpG Methylation of Cord Blood TNFalpha Associated with Maternal Exposure to Polybrominated Diphenyl Ethers. PloS one 2015, 10, e0138815. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Drago, G.; Longo, A.; Ruggieri, S.; Sprovieri, M.; Cibella, F.; Colombo, P. A multipollutant low-grade exposure regulates the expression of miR-30b, Let-7a and miR-223 in maternal sera: Evidence from the NEHO cohort. The Science of the total environment 2022, 844, 157051. [Google Scholar] [CrossRef] [PubMed]
- Shaddick, G.; Thomas, M.L.; Amini, H.; Broday, D.; Cohen, A.; Frostad, J.; Green, A.; Gumy, S.; Liu, Y.; Martin, R.V.; Pruss-Ustun, A.; Simpson, D.; van Donkelaar, A.; Brauer, M. Data Integration for the Assessment of Population Exposure to Ambient Air Pollution for Global Burden of Disease Assessment. Environmental science & technology 2018, 52, 9069–9078. [Google Scholar]
- Chen, Y.; Liu, S.; Xu, H.; Zheng, H.; Bai, C.; Pan, W.; Zhou, H.; Liao, M.; Huang, C.; Dong, Q. Maternal exposure to low dose BDE209 and Pb mixture induced neurobehavioral anomalies in C57BL/6 male offspring. Toxicology 2019, 418, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Mesa, M.; Narduzzi, L.; Ouzia, S.; Soetart, N.; Jaillardon, L.; Guitton, Y.; Le Bizec, B.; Dervilly, G. Metabolomics and lipidomics to identify biomarkers of effect related to exposure to non-dioxin-like polychlorinated biphenyls in pigs. Chemosphere 2022, 296, 133957. [Google Scholar] [CrossRef] [PubMed]
- Naville, D.; Gaillard, G.; Julien, B.; Vega, N.; Pinteur, C.; Chanon, S.; Vidal, H.; Le Magueresse-Battistoni, B. Chronic exposure to a pollutant mixture at low doses led to tissue-specific metabolic alterations in male mice fed standard andhigh-fat high-sucrose diet. Chemosphere 2019, 220, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, J.P.; Parent, A.S.; Kleinjans, J.C. S.; Nawrot, T.S.; Schoeters, G.; Van Larebeke, N. Rationale for Environmental Hygiene towards global protection of fetuses and young children from adverse lifestyle factors. Environmental health : a global access science source 2018, 17, 42. [Google Scholar] [CrossRef]
- Cori, L.; Bianchi, F.; Sprovieri, M.; Cuttitta, A.; Ruggieri, S.; Alessi, A.L.; Biondo, G.; Gorini, F. Communication and Community Involvement to Support Risk Governance. International journal of environmental research and public health 2019, 16. [Google Scholar] [CrossRef]
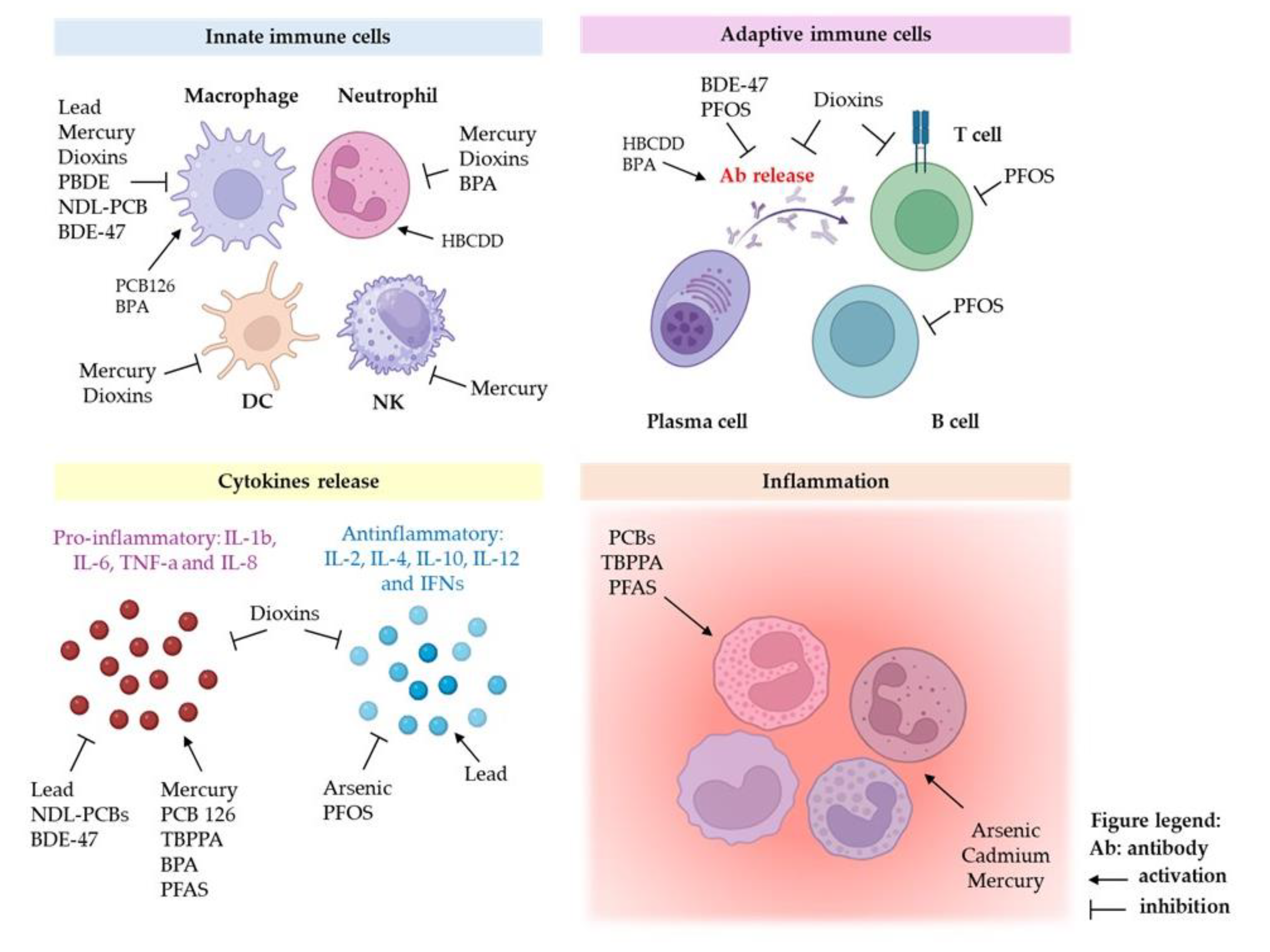
| Pollutant | Effects | Model systems (in vitro/ex vivo/in vivo) | Reference |
|---|---|---|---|
| Arsenic | Reduction of (IFN-γ, IL-4 and IL-10) | Murine splenocytes, mouse activated T cell (ex vivo) |
[34,35] |
| Macrophages, neutrophil, B and T cell apoptosis induction |
Human primary cultures (ex vivo) |
[36] |
|
| Modulation of T cell Receptor, cell cycle and apoptosis |
Human PBMCs (ex vivo) |
[37,38] |
|
| Involvement in fibrinogenic processes and chronic hepatic fibrosis | Co-cultures of THP-1 macrophages or BMDM and primary lung fibroblasts from C57BL/6 mice (in vivo/in vitro/ex vivo); co-cultures of THP-1 monocytes and LX2 cell lines (in vitro) |
[39,40] |
|
| Hypomethylation of leukocyte DNA |
Human blood samples (ex vivo) | [42] |
|
| Oxidative stress signalling, DNA damage and cytotoxicity in T cells and in human polymorphonuclear neutrophils | Human primary cell cultures and human cell lines (ex vivo, in vitro) | [44,45] | |
| Lead | Induction of allergies, infectious and autoimmune diseases in human |
Human ex vivo studies | [46,47] |
| Dysregulation of proinflammatory cytokine and impairment of THP-1 monocyte/macrophage cell viability |
Human THP-1 Monocyte/Macrophage cultures (in vitro) |
[48,49,50] | |
| Quiescence of haematopoietic stem cells |
C57BL/6 murine model (in vivo and ex vivo); | [51] | |
| Induction of higher levels of IFN-γ, IL-2, IL-12 and IL-17 in exposed workers | Human THP-1 Monocyte/Macrophage (in vitro); Human serum samples and primary T cell cultures (ex vivo) |
[52,53] |
|
| Mercury | Genetic damage, neurological, kidney, cardiac and immunological diseases in human | In vitro, ex vivo and in vivo model systems | [56] |
| Superoxide ion production and cytotoxic effect in neutrophils |
Human neutrophils (ex vivo) |
[57] |
|
| Impairment of macrophage migratory and phagocytic activity; NO and pro-inflammatory cytokines production |
BALB/cABOM peritoneal macrophages (ex vivo); Monocytes from Human PBMCs; co-cultures of Caco-2, HT29-MTX intestinal epithelial cells and THP-1 macrophages (in vitro). |
[58,59,60] |
|
| Genotoxic effects. Suppression both tumoricidal activity of blood and splenic NK cells and T and B cells proliferation |
Human cell lines (in vitro) and blood samples (ex vivo); Balb/c mouse and rat primary cell cultures (in vivo and ex vivo) | [61,62,63] | |
| Cadmium | Inflammation and oxidative damage induction in neutrophils and macrophages | Rat liver and kidney primary cell cultures (in vivo and ex vivo); various cells and tissues in vitro and ex vivo models. | [66,67] |
|
Down regulation of TNF-α, IL-12p40, TLRs, CD14, MD2, BD2, MyD88, p65 and NOS2 |
Wild boars’ macrophages (ex vivo) |
[69] |
|
|
Impairment of adaptive immunity cell populations in offsprings |
C57Bl/6 mice (in vivo and ex vivo) |
[70,71] |
|
| Modulation of miRNAs associated with inflammation and carcinogenesis and alteration oh Th17 and Treg lymphocytes subpopulation in exposed workers | Human blood samples (ex vivo) | [72] |
| Pollutant | Effects | Model systems (in vitro/ex vivo/in vivo) | Reference | |||
|---|---|---|---|---|---|---|
| Dioxins | Impairment of macrophages, NK, neutrophils and dendritic cells |
in vivo murine models; in vitro and ex vivo cell cultures |
[76,77,78] |
|||
| Reduction both of antibody’s production by B cell and cytotoxic activity of T lymphocytes |
In vitro and ex vivo cell cultures; in vivo murine models |
[79] | ||||
| Attenuation of IgE mediated hypersensitivity response |
In vivo and ex vivo studies |
[80] |
||||
| Alterations of THP-1 macrophages adherence, adhesion molecule expression, morphology, multiple cytokine/chemokine production and total mRNA expression |
THP-1 monocyte/macrophage cell line (in vitro) | [81] |
||||
| Effects on human reproductive health | Murine models; Human ex vivo studies |
[82] | ||||
| PCBs | Proinflammatory activity in macrophages |
THP-1 monocyte/macrophage cell line (in vitro) |
[83] |
|||
| In vitro immunosuppressive effects and expression of reactive species | J774A.1 cell line and primary murine macrophages (in vitro and ex vivo) | [84] |
||||
|
In vivo inflammatory effects and impairment of immune system functions in mouse model |
Wild-type mice C57BL/6J and SVJ129 (in vivo and ex vivo) |
[86] |
||||
| DNA methylation differences in PCB-exposed populations | Human PBMCs (ex vivo) |
[87] | ||||
| PBDEs | Liver, kidney, gut, and thyroid toxicity |
In vitro, ex vivo and in vivo studies |
[91] |
|||
| Neurotoxic, cardiotoxic, hepatotoxic and teratogenic effects on zebrafish and fish |
Zebrafish embryos (in vivo and ex vivo); | [92,93,94] | ||||
| Impairment of proinflammatory response modulation of small extracellular vesicle biogenesis and miRNA cargo and exacerbation of LPS-induced pro-inflammatory response in macrophage cell line. |
THP-1 monocyte/macrophage cell line (in vitro) |
[95,96,97] |
||||
| Alteration of tight junctions, adhesion molecules, cytokines and EMT (epithelial-mesenchymal transition) markers expression in epithelial lung cells |
ALI cultures of human A549 cell line (in vitro) | [98] |
||||
| Cardiovascular toxicity |
THP-1 monocyte/macrophage cell line (in vitro) |
[99] | ||||
| Destruction of macrophage functional activity in animal model systems |
RTG-2 cell line (in vitro) |
[100,101,102] | ||||
| Reduction of antibody response, histopathological effects on liver, spleen, small intestine, and thyroid |
BALB/c murine model (in vivo and ex vivo) | [103] | ||||
| HBCDD | Bioaccumulation in human blood, adipose tissue and breast milk |
Human blood, adipose tissue and maternal milk (ex vivo) |
[109,110,111] | |||
| TBBPA | Genotoxic effects | Human PBMCs (ex vivo) |
[112,113,114] |
|||
| Induction of inflammatory phenotype in human dendritic cells from healthy subjects | Human monocyte-derived dendritic cells (ex vivo) | [115] |
||||
|
Neurotoxic, nephrotoxic, hepatotoxic and immunotoxic effects |
[116] |
|||||
| Correlation with human thyroid and neurological disorders, reproductive health, immunological, oncological, and cardiovascular diseases |
Laboratory animals and human samples (in vivo and ex vivo) | [117,118] | ||||
| BPA | Impairment of neutrophils chemotactic function |
Neutrophils isolated from human blood | [119] |
|||
| Induction of M1 macrophages polarization | Peritoneal macrophages from C57BL/6 J mice (ex vivo) |
[120] |
||||
| Upregulation of proinflammatory cytokines | RAW264.7 murine macrophage cell line (in vitro) |
[121,122] |
||||
| Immunotoxic effects on adaptive immune response and effect on IgM reactivity against tumour antigens | Guinea pig model system (in vivo and ex vivo) |
[123] | ||||
| Inhibition of T cell proliferation | BALB/c murine model (in vivo and ex vivo) |
[102] |
||||
| PFAS (PFOS and PFOA) |
PFOS bioaccumulation in human liver, kidneys, lungs, hair, breast milk urine and blood |
Human blood, urine, milk, hair, nails and tissue samples |
[125,126] | |||
| In vitro reduction of IL-2 expression in human T cells | Jurkat cell line and primary T cell (in vitro and ex vivo) | [127] |
||||
| De novo localization of immune cell populations in organs like the spleen and liver | C57BL/6 murine model (in vivo and ex vivo) |
[128] | ||||
| Reduction of B-cell subtypes and IgM antibody primary response in female mice induced by PFOA | C57BL/6 murine model (in vivo and ex vivo) |
[129] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





