Submitted:
24 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
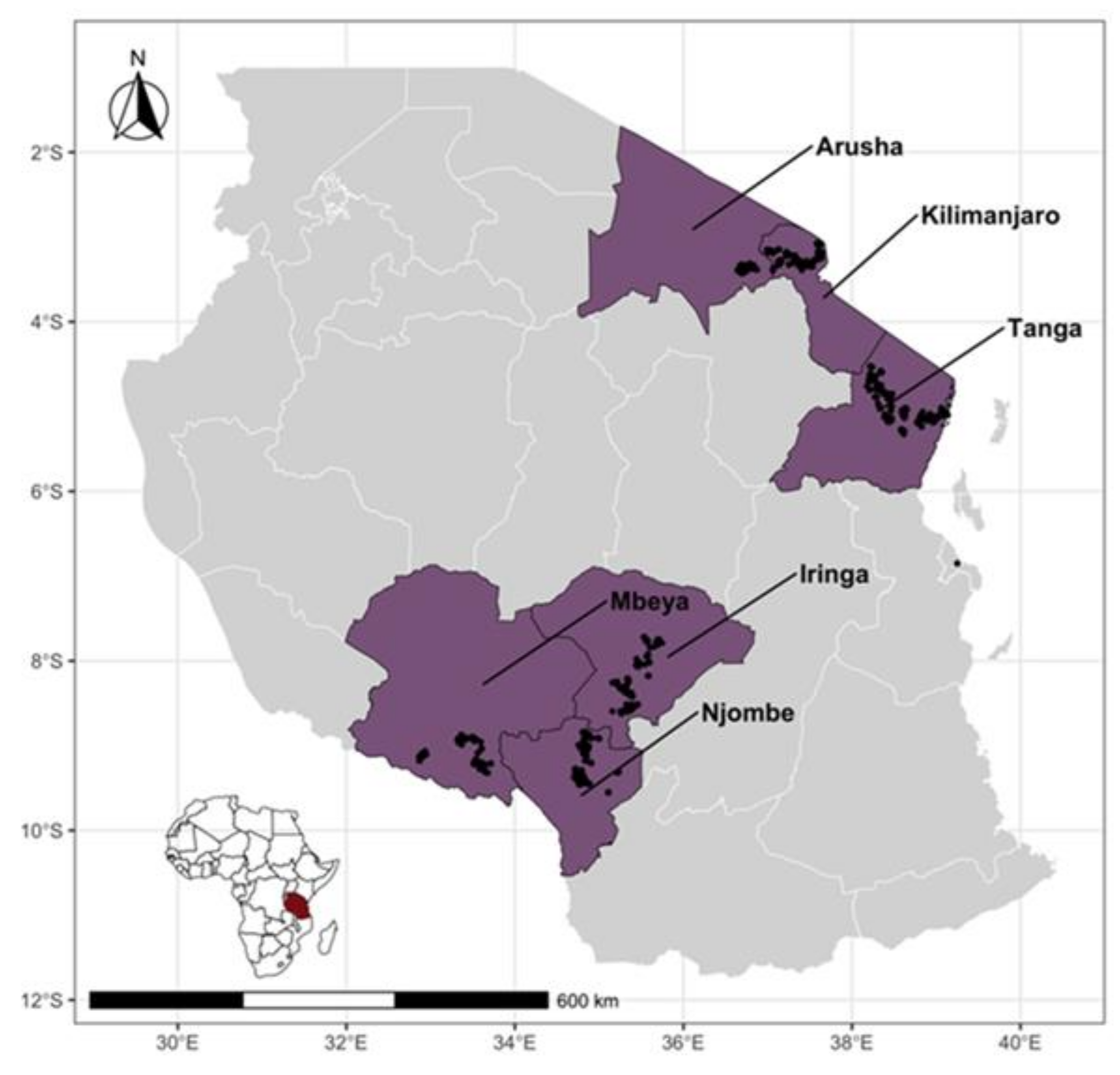
2.1. Study Area and Design
2.2. Study Population
2.3. Questionnaire Administration for Risk Factors Determination
2.4. Blood and Swab Sampling from Dairy Cattle and Samples Storage
2.5. DNA Extraction from EDTA Blood and PBS Swabs
2.6. Real Time PCR for Brucella Genus Detection and Species Characterization
2.7. Data Analysis for Calculation of Molecular Prevalence and Risk Factors
3. Results
3.1. Description of Sampled Dairy Cattle
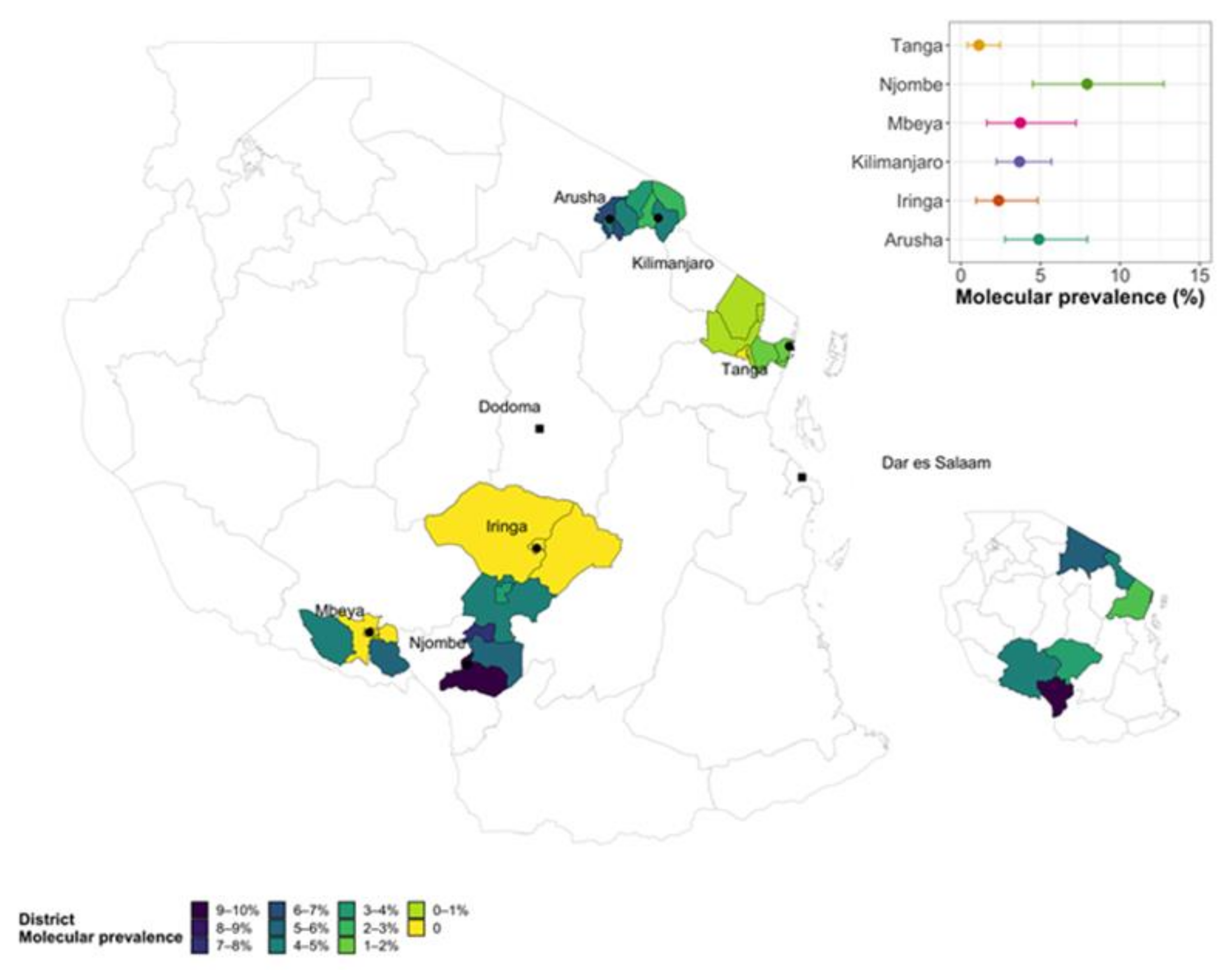
3.2. Brucellosis Molecular Prevalence of Dairy Cattle in Selected Regions of Tanzania
3.3. Brucella Species Circulating in Dairy Cattle Population Identified from Brucella Genus Positive Swab and Blood Samples
3.4. Brucellosis Hotspot Areas
3.5. Univariable Analysis Results to Combined PCR Positivity
3.6. Multivariable Logistic Regression Model for Association with Brucella PCR Positivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schelling, E., et al., Brucellosis and Q-fever seroprevalences of nomadic pastoralists and their livestock in Chad. Preventive Veterinary Medicine 2003, 61, 279–293. [CrossRef] [PubMed]
- World Organization for Animal Health (WOAH)., Brucellosis. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, twelfth edition 2023. Chapter 3.1.4, 2023: p. 1-35.
- Pappas, G. The changing Brucella ecology: novel reservoirs, new threats. International Journal of Antimicrobial Agents 2010, 36, S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Corbel, M.J. Brucellosis in Humans and Animals. 2006, Switzeland: World Health Organization (WHO), Food and Agriculture Organization (FAO) and World Organization for Animal Health (OIE).
- Akoko, J.M., et al., Molecular epidemiology of Brucella species in mixed livestock-human ecosystems in Kenya. Scientific Reports 2021, 11, 2045–2322. [CrossRef]
- Ntivuguruzwa, J.B., et al., Seroprevalence of brucellosis and molecular characterization of Brucella spp. from slaughtered cattle in Rwanda. PLoS ONE 2022, 17, e0261595. [CrossRef]
- ElTahir, Y., et al., Investigation on Brucella infection in farm animals in Saham, Sultanate of Oman with reference to human brucellosis outbreak. BMC Veterinary Research 2019, 15, 1–8. [CrossRef]
- Muendo, E.N., et al., Infection of cattle in Kenya with Brucella abortus biovar 3 and Brucella melitensis biovar 1 genotypes. Tropical Animal Health and Production 2012, 44, 17–20. [CrossRef] [PubMed]
- Moriyón, I., et al., Rough vaccines in animal brucellosis: structural and genetic basis and present status. Veterinary Research 2004, 35, 1–38. [CrossRef] [PubMed]
- Schurig, G.G., N. Sriranganathan, and M.J. Corbel, Brucellosis vaccines: past, present and future. Veterinary Microbiology 2002, 90, 479–496. [CrossRef] [PubMed]
- van Straten, M., et al., Brucella abortus S19 vaccine protects dairy cattle against natural infection with Brucella melitensis. Vaccine 2016, 34, 5837–5839. [CrossRef] [PubMed]
- Mahlau, E. Further brucellosis surveys in Tanzania. Bulletin of Epizootic Diseases of Africa. Bulletin des épizooties en Afrique 1967, 15, 373–378. [Google Scholar] [PubMed]
- Mathew, C., et al., First isolation, identification, phenotypic and genotypic characterization of Brucella abortus biovar 3 from dairy cattle in Tanzania. BMC Veterinary Research 2015, 11, 1–9. [CrossRef]
- Assenga, J.A., et al., Epidemiology of Brucella infection in the human, livestock and wildlife interface in the Katavi-Rukwa ecosystem, Tanzania. BMC Veterinary Research 2015, 11, 1–11. [CrossRef]
- Ducrotoy, M., et al., Brucellosis in Sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta Tropica 2017, 165, 179–193. [CrossRef] [PubMed]
- Mengele, I.J., et al., The status and risk factors of brucellosis in smallholder dairy cattle in selected regions of Tanzania. Veterinary Sciences 2023, 10, 155. [CrossRef]
- Makita, K., et al., Herd prevalence of bovine brucellosis and analysis of risk factors in cattle in urban and peri-urban areas of the Kampala economic zone, Uganda. BMC Veterinary Research 2011, 7, 1–8.
- Bodenham, R.F., et al., Prevalence and speciation of brucellosis in febrile patients from a pastoralist community of Tanzania. Scientific Reports 2020, 10, 7081. [CrossRef]
- Njombe, A., et al. Dairy Industry Status in Tanzania. Ministry of Livestock Development and Fisheries. Available online: https://dairyafrica.com/africadairyportal/wp-content/uploads/2020/06/Dairy_Industry_Status_in_Tanzania_2011.pdf (accessed on 10 May 2021). in 7th African Dairy Conference and Exhibition. 2011. Dar Es Salaam, Tanzania, 25-27 May..
- National Bureau of Statistics, National Sample Census of Agriculture 2019-2020. National Report. Ministry of Finance and Planning, United Republic of Tanzania, 2021: p. 1-317.
- Ministry of Finance and Planning (MoFP), Tanzania Mainland Household Budget Survey 2017-2018, Key indicators Report: Poverty Eradication Division. National Bureau of Statistics, United Republic of Tanzania. Poverty Eradication Division. National Bureau of Statistics, United Republic of Tanzania, 2019.
- Mrode, R., et al., Genomic prediction of crossbred dairy cattle in Tanzania: A route to productivity gains in smallholder dairy systems. Journal of Dairy Science 2021, 104, 11779–11789. [CrossRef] [PubMed]
- Shirima, G.M. The epidemiology of brucellosis in animals and humans in Arusha and Manyara regions in Tanzania. PhD Thesis, University of Glasgow, UK, 2005. [Google Scholar]
- R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.R-project.org/.
- Lumley, T. Analysis of complex survey samples. Journal of Statistical Software 2004, 9, 1–19. [Google Scholar] [CrossRef]
- Shirima, G., B. Lyimo, and N. Kanuya, Re-emergence of Bovine Brucellosis in Smallholder Dairy Farms in Urban Settings of Tanzania. Journal of Applied Life Sciences International 2018, 17, 1–7. [Google Scholar] [CrossRef]
- Mengele, I.J., et al., Diagnostic challenges of brucellosis in humans and livestock in Tanzania: A thematic review. CABI One Health 2023, ohcs20230001. [CrossRef]
- Aliyev, J., et al., Identification and molecular characterization of Brucella abortus and Brucella melitensis isolated from milk in cattle in Azerbaijan. BMC Veterinary Research 2022, 18, 71. [CrossRef]
- Oliveira, M.S., et al., Molecular epidemiology of Brucella abortus isolated from cattle in Brazil, 2009–2013. Acta Tropica 2017, 166, 106–113. [CrossRef] [PubMed]
- Akoko, J.M., et al., Mapping brucellosis risk in Kenya and its implications for control strategies in sub-Saharan Africa. Scientific Reports 2023, 13, 20192. [CrossRef] [PubMed]
- Mitterran, K.N.R., et al., Detection of Brucella abortus and Brucellla melitensis in cattle and sheep from southern Cameroon. Research Square 2020, 2, 1–13. [CrossRef]
- Abnaroodheleh, F., et al., Shedding rate of Brucella spp. in the milk of seropositive and seronegative dairy cattle. Heliyon 2023, 9, 1–8.
- Kolo, F.B., et al., Seroprevalence and characterization of Brucella species in cattle slaughtered at Gauteng abattoirs, South Africa. Veterinary Medicine and Science 2019, 5, 545–555. [CrossRef]
- Morales-Estrada, A.I., et al., Characterization of Brucella species in Mexico by Bruce-Ladder polymerase chain reaction (PCR). African Journal of Microbiology Research 2012, 6, 2793–2796. [CrossRef]
- Ewalt, D.R., et al., Brucella suis biovar 1 in naturally infected cattle: a bacteriological, serological, and histological study. Journal of Veterinary Diagnostic Investigation 1997, 9, 417–420. [CrossRef]
- Fretin, D., et al., Unexpected Brucella suis biovar 2 infection in a dairy cow, Belgium. Emerging Infectious Diseases 2013, 19, 2053. [CrossRef] [PubMed]
- Baek, B., et al., The first detection of Brucella canis in cattle in the Republic of Korea. Zoonoses and Public Health 2012, 59, 77–82. [CrossRef] [PubMed]
- Segwagwe, B.E., et al., Prevalence and risk factors for brucellosis seropositivity in cattle in Nyagatare District, Eastern Province, Rwanda. Journal of the South African Veterinary Association 2018, 89, 1–8. [CrossRef]
- Alamian, S. and M. Dadar, Brucella melitensis infection in dog: a critical issue in the control of brucellosis in ruminant farms. Comparative Immunology, Microbiology and Infectious Diseases 2020, 73, 101554. [CrossRef]
- Mol, J.P., et al., Diagnosis of canine brucellosis: comparison of various serologic tests and PCR. Journal of Veterinary Diagnostic Investigation 2020, 32, 77–86. [CrossRef]
- Shome, R., et al., Management of bovine brucellosis in organized dairy herds through the identification of risk factors: A cross-sectional study from Karnataka, India. Veterinary World 2023, 16.
- Aguiar, D.M.d., et al., Risk factors and seroprevalence of Brucella spp. in cattle from western Amazon, Brazil. J Arquivos do Instituto Biológico, 2022, 74, 301–305. [CrossRef]
- Bagenda, I., et al., Case Study of Impact and Risk Factors of Brucellosis (Brucella abortus) in Beef Cattle. Hasanuddin Journal of Animal Science 2023, 5, 66–83. [CrossRef]
- Chagunda, M.G., et al., Risk, knowledge and preventive measures of smallholder dairy farmers in northern Malawi with regard to zoonotic brucellosis and bovine tuberculosis. Onderstepoort Journal of Veterinary Research 2014, 81, 1–6.
- Getahun, T.K., B. Urge, and G. Mamo, Seroprevalence of bovine brucellosis and associated factors among dairy cows with recent cases of abortion in Ethiopia. Public Health Challenges 2023, 2, 1–8. [CrossRef]
- Cárdenas, L., et al., Risk factors for new bovine brucellosis infections in Colombian herds. BMC Veterinary Research 2019, 15, 81. [CrossRef] [PubMed]
- Shome, R., et al., Management of bovine brucellosis in organized dairy herds through the identification of risk factors: A cross-sectional study from Karnataka, India. Veterinary World 2023, 16, 1122–1130. [CrossRef]
- de Alencar Mota, A.L.A., et al., Large-scale study of herd-level risk factors for bovine brucellosis in Brazil. Acta Tropica 2016, 164, 226–232. [CrossRef] [PubMed]
- Sanz, C., et al., Mass vaccination as a complementary tool in the control of a severe outbreak of bovine brucellosis due to Brucella abortus in Extremadura, Spain. Preventive Veterinary Medicine 2010, 97, 119–125. [CrossRef] [PubMed]
- Lord, V.R., et al., Field study of vaccination of cattle with Brucella abortus strains RB51 and 19 under high and low disease prevalence. American Journal of Veterinary Research 1998, 59, 1016–1020. [CrossRef]
- Keid, L.B., et al., A polymerase chain reaction for detection of Brucella canis in vaginal swabs of naturally infected bitches. Theriogenology 2007, 68, 1260–1270. [CrossRef] [PubMed]
| Target | Targeted gene | Sequences of primers and probes (5’ -3’) | Fluorophore/ quencher | Reference |
|---|---|---|---|---|
| Genus Brucella | IS711 | Probe: AAG CCA ACA CCC GGC Forward: GGC CTA CCG CTG CGA AT Reverse: TTG CGG ACA GTC ACC ATA ATG |
FAM/-MGBNFQ | Matero, 2011 |
| B. melitensis | IS711 downstream of BMEI1162 | Probe CAGGAGTGTTTCGGCTCAGAATAATCCACA Forward AACAAGCGGCACCCCTAAAA Reverse CATGCGCTATGATCTGGTTACG |
Texas Red/BHQ2 | Probert. 2004 |
| B. abortus | IS711 downstream of alkB | Probe: CGCTCATGCTCGCCAGACTTCAATG Forward: GCGGCTTTTCTATCACGGTATTC Reverse: CATGCGCTATGATCTGGTTACG |
JOE/BHQ1 |
| Region | Total Animal Sampled | Number of positive_blood samples (%) | Number of positive_swab samples (%) |
|---|---|---|---|
| Arusha | 318 | 5/318 (1.6%) | 10/294 (3.4%) |
| Tanga | 524 | 6/524 (1.0%) | 1/412 (0.2%) |
| Kilimanjaro | 521 | 11/519 (2.1%) | 8/513 (1.6%) |
| Iringa | 281 | 7/281 (2.5%) | 1/273 (0.4%) |
| Njombe | 187 | 1/186 (1.1%) | 14/186 (7.5%) |
| Mbeya | 218 | 5/218 (2.3%) | 3/215 (1.4%) |
| Total | 2049 | 35/2046 (1.7%) | 37/1893 (2.0%) |
| Region | Negative | Positive | Total | PCR Prevalence % | 95% CI | Dairy cattle Population |
|---|---|---|---|---|---|---|
| Arusha | 303 | 15 | 318 | 4.7 | 2.7-7.7 | 78,637 |
| Tanga | 517 | 7 | 524 | 1.3 | 0.5-2.7 | 41,639 |
| Kilimanjaro | 500 | 19 | 519 | 3.7 | 2.2-5.7 | 161,984 |
| Iringa | 273 | 8 | 281 | 2.8 | 1.2-5.5 | 7,081 |
| Njombe | 171 | 15 | 186 | 8.1 | 4.6-13.0 | 7,177 |
| Mbeya | 210 | 8 | 218 | 3.8 | 1.7-7.4 | 72,724 |
| Total | 1974 | 72 | 2046 | 3.5 | 2.8-4.4 | 369,242 |
| Region | Sample | B. abortus | B. melitensis | Mixed | Unidentified |
|---|---|---|---|---|---|
| Arusha | Blood n=5 | 0 | 3 | 2 | 0 |
| Swabs n=10 | 0 | 9 | 1 | 0 | |
| Kilimanjaro | Blood n=11 | 1 | 9 | 0 | 1 |
| Swabs n=8 | 0 | 6 | 2 | 0 | |
| Tanga | Blood n=6 | 0 | 2 | 3 | 1 |
| Swabs n=1 | 0 | 1 | 0 | 0 | |
| Njombe | Blood n=1 | 0 | 1 | 0 | 0 |
| Swabs n=14 | 0 | 11 | 2 | 1 | |
| Iringa | Blood n=7 | 0 | 3 | 2 | 2 |
| Swabs n=1 | 0 | 1 | 0 | 0 | |
| Mbeya | Blood n=5 | 1 | 1 | 3 | 0 |
| Swabs n=3 | 0 | 1 | 2 | 0 | |
| Total | Blood n=35 | 2 | 19 | 10 | 4 |
| Swabs n=37 | 0 | 29 | 7 | 1 |
| Variable | Level | Negative | Positive | OR | 95% Confid. Interval | P_value | |
|---|---|---|---|---|---|---|---|
| Farmer’s gender | female | 541 | 26 | ref | |||
| male | 643 | 31 | 1.003 | 0.59 | 1.71 | 1 | |
| Herd size | 1-2animals | 589 | 31 | ref | |||
| 3-4animals >4animals |
429 163 |
22 4 |
0.97 0.47 |
0.53 0.12 |
1.77 1.35 |
0.341 0.353 |
|
| Farmer’s experience | ≤5years | 105 | 6 | ref | |||
| in dairy farming | >5years | 1079 | 51 | 0.83 | 0.35 | 1.97 | 0.63 |
| Farmer’s education | basic | 969 | 49 | ref | |||
| secondary_plus | 215 | 8 | 0.74 | 0.34 | 1.57 | 0.59 | |
| Farm or neighbor | have_goat | 795 | 38 | ref | |||
| keep goat | no_goat | 387 | 19 | 1.03 | 0.58 | 1.80 | 1 |
| Farm or neighbor | no_dog | 773 | 36 | ref | |||
| keep dog | have_dog | 411 | 21 | 1.09 | 0.63 | 1.90 | 0.78 |
| Farm or neighbor | have_sheep | 271 | 12 | ref | |||
| keep sheep Farm or neighbor keep pig |
no_sheep no_pig have_pig |
911 891 293 |
45 47 10 |
1.11 ref 0.65 |
0.58 0.32 |
2.14 1.29 |
0.87 0.26 |
| Keeping bull | no_bull | 909 | 50 | ref | |||
| have_bull | 275 | 7 | 0.46 | 0.21 | 1.03 | 0.05 | |
| Body condition score | good | 630 | 29 | ref | |||
| poor | 554 | 28 | 1.09 | 0.64 | 1.86 | 0.78 | |
| Introduction of new | no | 1070 | 49 | ref | |||
| animal | yes | 114 | 8 | 1.53 | 0.71 | 3.31 | 0.26 |
| Placenta management | correct_dispose (bury/burn) |
956 | 44 | ref | |||
| Incorrect_dispose | 228 | 13 | 1.24 | 0.65 | 2.34 | 0.49 | |
| Farm’s distance | <100 | 946 | 41 | ref | |||
| ≥100 | 238 | 16 | 1.55 | 0.85 | 2.81 | 0.17 | |
| Cattle breed | SHZxAyrshire | 264 | 10 | ref | |||
| SHZxFriesian | 850 | 40 | 1.24 | 0.6 | 2.8 | 0.550 | |
| SHZxJersey+SHZ | 70 | 7 | 2.62 | 0.82 | 7.95 | 0.058 | |
| Animal sex | female | 1147 | 57 | ref | |||
| male | 37 | 0 | 0 | 0 | NaN | 0.41 | |
| Animal age | 1-4years | 501 | 21 | ref | |||
| 5-7years >7years |
488 192 |
27 9 |
1.32 1.12 |
0.71 0.44 |
2.49 2.60 |
0.351 0.784 |
|
| Region | Arusha Kilimanjaro Mbeya Njombe |
303 500 210 172 |
15 19 8 15 |
ref 0.77 1.77 1.80 |
0.38 0.32 0.85 |
1.53 1.85 3.71 |
0.47 0.66 0.17 |
| History of abortion (Animal) |
no yes |
1049 32 |
53 4 |
ref 2.51 |
0.86 |
7.38 |
0.056 |
| History of abortion | no | 1110 | 52 | ref | |||
| (Farm) Feeding management |
yes pasture intensive |
71 166 1915 |
5 6 51 |
1.51 ref 1.39 |
0.58 0.59 |
3.89 3.29 |
0.39 0.452 |
| Variable | Level | OR | Lower | Upper | P-value |
|---|---|---|---|---|---|
| History of abortion (Animal) | no | ref | |||
| yes | 3.17 | 1.05 | 9.62 | 0.04 | |
| Farm or neighbour | have dog | ref | |||
| keep Dog | no dog | 2.68 | 1.30 | 5.55 | 0.008 |
| Introduction of | no | ref | |||
| new animal | yes | 1.87 | 0.82 | 4.24 | 0.14 |
| Farm or neighbor | have_pig | ref | |||
| keep pig | no_pig | 2.19 | 1.21 | 3.98 | 0.01 |
| Keeping bull | no | ref | |||
| yes | 0.39 | 0.16 | 0.96 | 0.04 | |
| Region | Arusha | ref | |||
| Kilimanjaro | 1.20 | 0.56 | 2.56 | 0.6 | |
| Mbeya Njombe |
1.39 2.72 |
0.50 1.21 |
3.86 6.10 |
0.5 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





