Submitted:
22 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
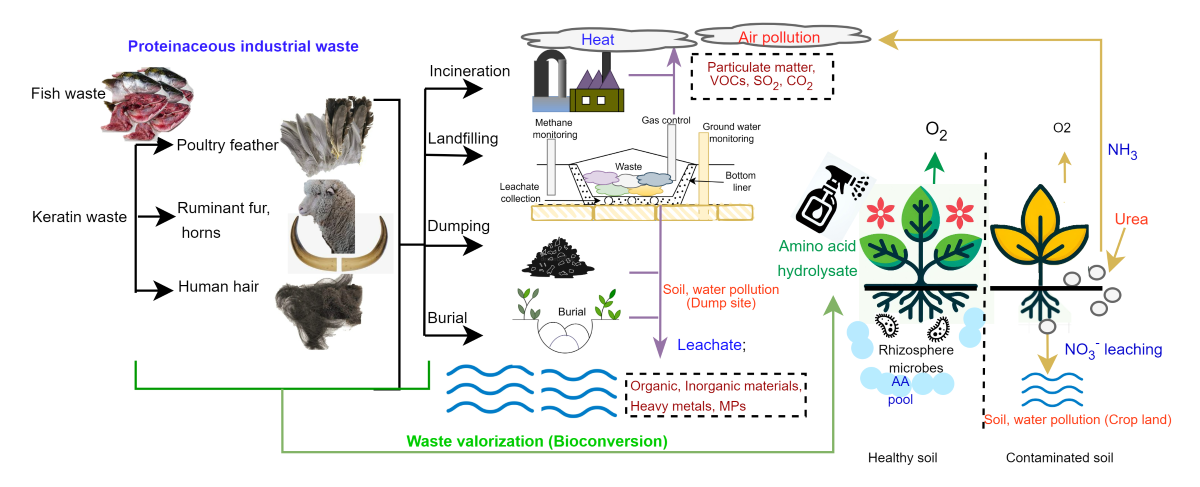
Keywords:
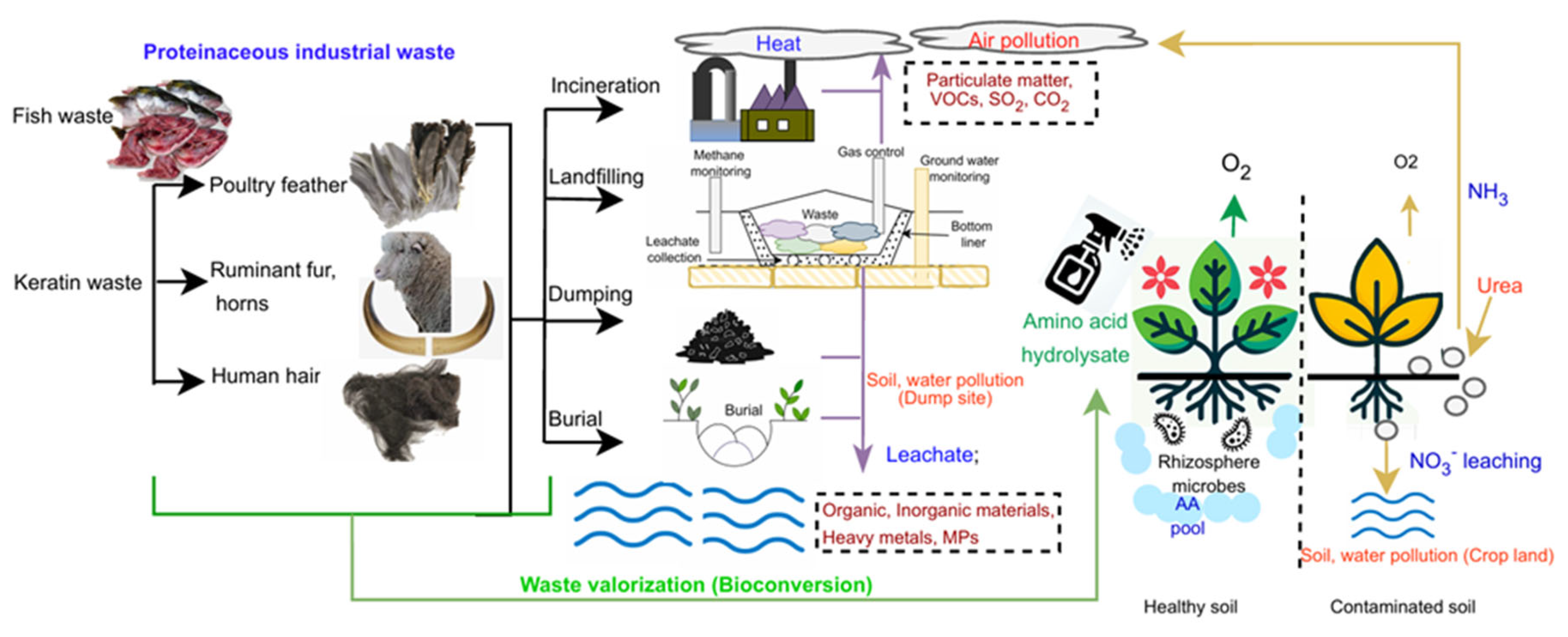
Graphical Abstract
1. Introduction
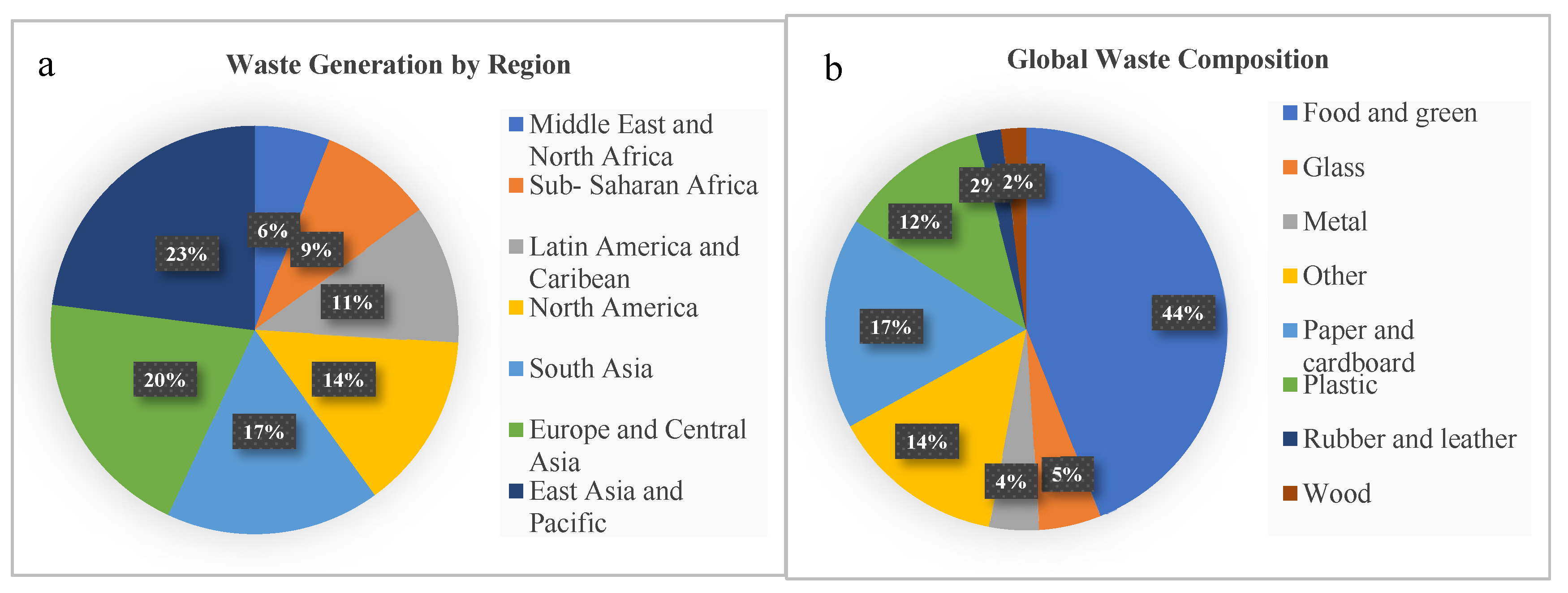
2. Global waste generation
3. Food and Agricultural wastes as a major portion of waste generation
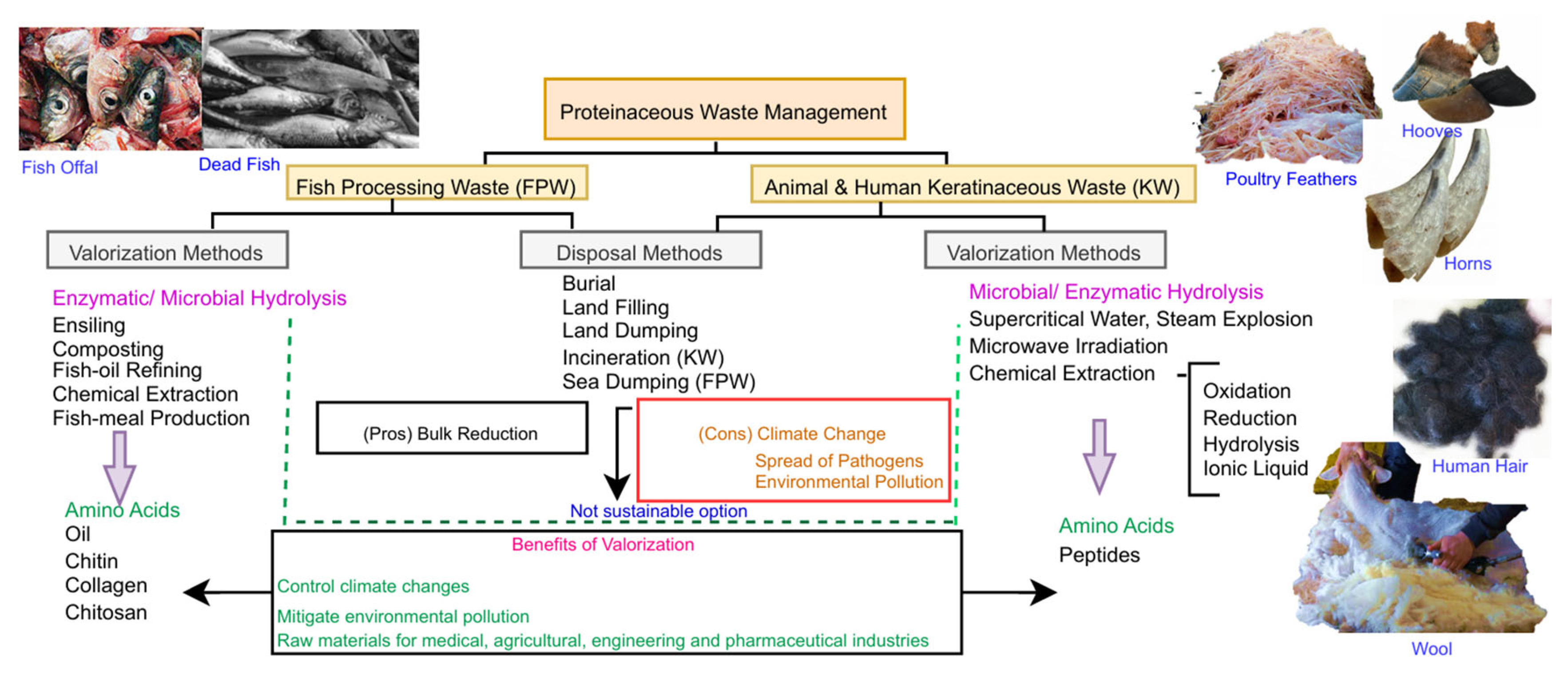
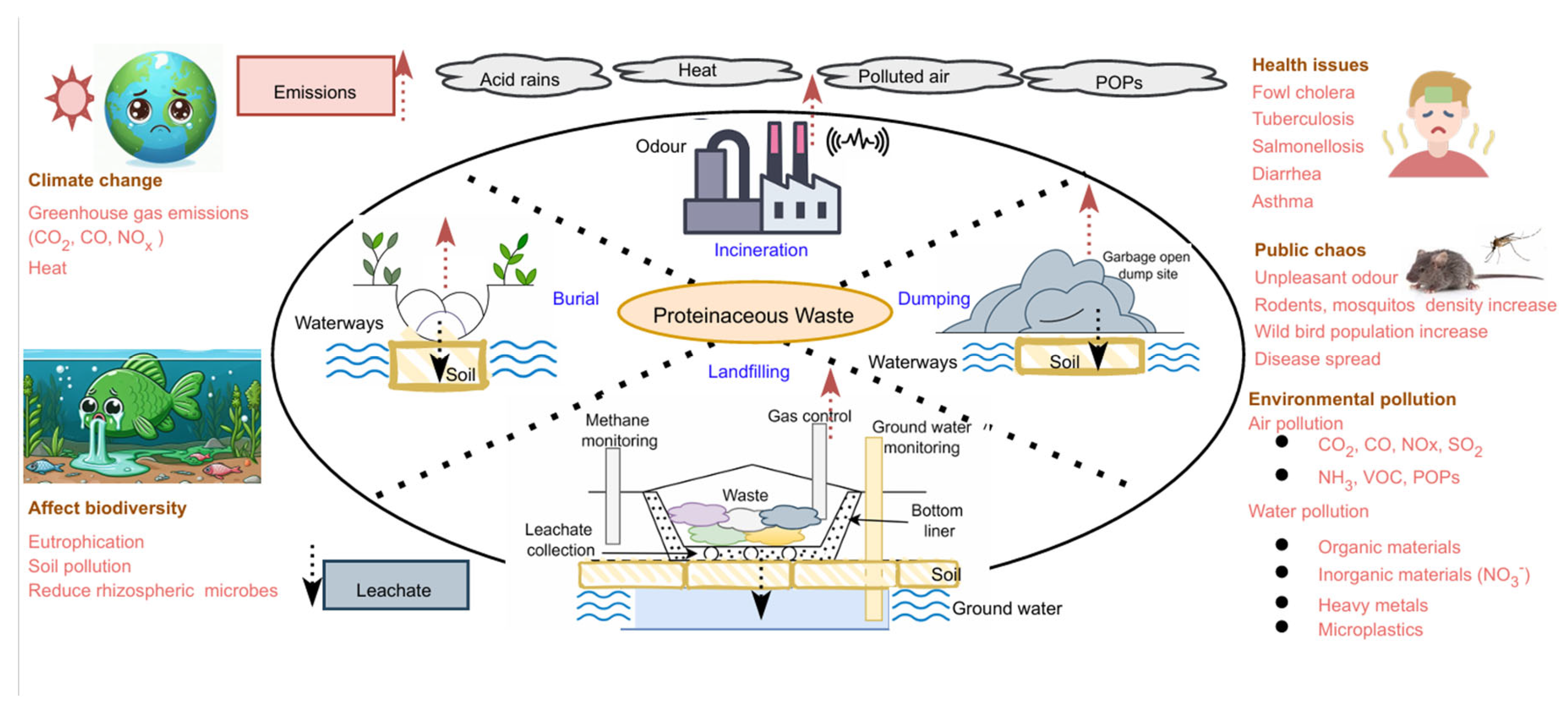
4. Challenges in proteinaceous waste management
5. Current management of proteinaceous waste
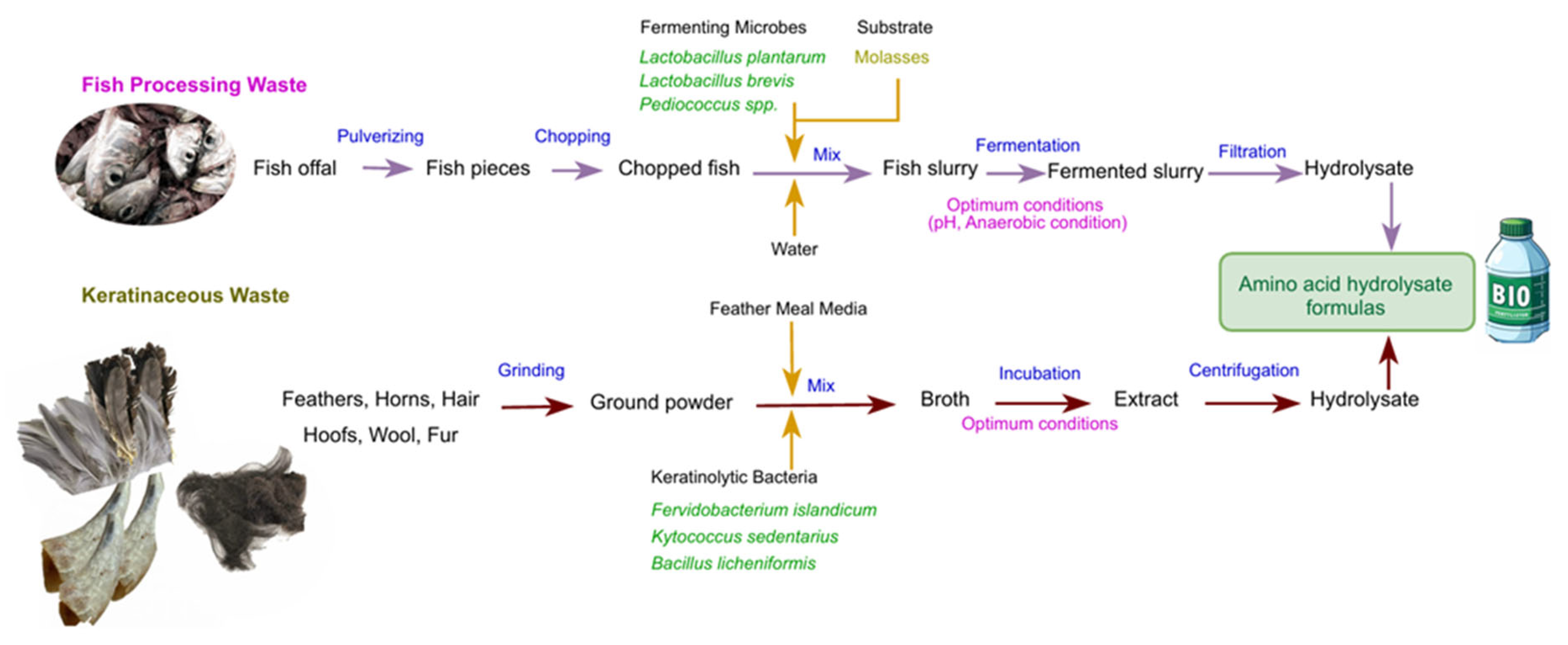
6. Potential for valorization and fertilizer formulation
6.1. Composition of FPW fertilizer
6.2. Composition of keratin waste
6.3. Introduce amino acid formulations from proteinaceous wastes
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reznikova, N., et al., Global circular e-chain in overcoming the global waste. Procedia Environmental Science, Engineering and Management, 2019. 6(4): p. 641-647.
- Quested, T.E., et al., Spaghetti soup: The complex world of food waste behaviours. Resources, Conservation and Recycling, 2013. 79: p. 43-51. [CrossRef]
- Zhang, J., Ç. Akyol, and E. Meers, Nutrient recovery and recycling from fishery waste and by-products. Journal of Environmental Management, 2023. 348: p. 119266. [CrossRef]
- FAO, Agriculture Organization of the United Nations The state of world fisheries and aquaculture 2020: Sustainability in action. Rome: Food and Agriculture Organization of the United Nations, , 2020: p. pp.1-244.
- Martínez-Alvarez, O., S. Chamorro, and A. Brenes, Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Research International, 2015. 73: p. 204-212. [CrossRef]
- Timorshina, S., E. Popova, and A. Osmolovskiy, Sustainable applications of animal waste proteins. Polymers, 2022. 14(8): p. 1601. [CrossRef]
- Zheljazkov, V.D., et al., Human hair as a nutrient source for horticultural crops. HortTechnology, 2008. 18(4): p. 592-596. [CrossRef]
- Gupta, A., Human hair “waste” and its utilization: gaps and possibilities. Journal of waste management, 2014. 2014(1): p. 498018.
- Polmann, G., et al., Non-conventional nuts: An overview of reported composition and bioactivity and new approaches for its consumption and valorization of co-products. Future Foods, 2021. 4: p. 100099. [CrossRef]
- Zarkadas, I., et al., Exploring the potential of fur farming wastes and byproducts as substrates to anaerobic digestion process. Renewable Energy, 2016. 96: p. 1063-1070. [CrossRef]
- Xia, Y., et al., Anaerobic digestibility of beef hooves with swine manure or slaughterhouse sludge. Waste Management, 2015. 38: p. 443-448. [CrossRef]
- Coppola, D., et al., Fish waste: From problem to valuable resource. Marine drugs, 2021. 19(2): p. 116. [CrossRef]
- Tesfaye, T., B. Sithole, and D. Ramjugernath, Valorisation of chicken feathers: a review on recycling and recovery route—current status and future prospects. Clean Technologies and Environmental Policy, 2017. 19: p. 2363-2378. [CrossRef]
- Olgunoğlu, İ.A., Salmonella in fish and fishery products. Salmonella: a dangerous foodborne pathogen, 2012: p. 91-105.
- Said, M. Potential development of poultry feather waste resources as raw material in industry: A review. in IOP Conference Series: Earth and Environmental Science. 2020. IOP Publishing. [CrossRef]
- Kaza, S., et al., What a waste 2.0: a global snapshot of solid waste management to 2050. 2018: World Bank Publications.
- FAO, Food and Agriculture Organization of the UN FAO, Case study, . 2023.
- Hasan, Z. and M. Lateef, Transforming food waste into animal feeds: an in-depth overview of conversion technologies and environmental benefits. Environmental Science and Pollution Research, 2024. 31(12): p. 17951-17963. [CrossRef]
- Ahmed, A.E. and F. Alzahrani, Food Loss and Waste in Saudi Arabia: Analysis, Causes, and Interventions, in Food and Nutrition Security in the Kingdom of Saudi Arabia, Vol. 2: Macroeconomic Policy and Its Implication on Food and Nutrition Security. 2024, Springer. p. 241-274.
- Ebuete, A.W., P.-E.D. Wodu, and E. Ebuete, Dumping on Waters: The Lacunae in Waste Management in the Niger Delta, Nigeria. American Journal of Environment and Climate, 2022. 1(2): p. 100-109. [CrossRef]
- Ahuja, I., et al., Fish and fish waste-based fertilizers in organic farming–With status in Norway: A review. Waste Management, 2020. 115: p. 95-112. [CrossRef]
- Love, D.C., et al., Aquatic food loss and waste rate in the United States is half of earlier estimates. Nature Food, 2023. 4(12): p. 1058-1069. [CrossRef]
- Mi, X., et al., Transferring feather wastes to ductile keratin filaments towards a sustainable poultry industry. Waste Management, 2020. 115: p. 65-73. [CrossRef]
- Shavandi, A., et al., Keratin: dissolution, extraction and biomedical application. Biomaterials science, 2017. 5(9): p. 1699-1735. [CrossRef]
- Cottle, D., World sheep and wool production. 2010: Nottingham University Press, Nottingham, UK.
- Doyle, E.K., et al., The science behind the wool industry. The importance and value of wool production from sheep. Animal Frontiers, 2021. 11(2): p. 15-23.
- Russell, S., et al. Review of wool recycling and reuse. in Natural Fibres: advances in science and technology towards industrial applications: from science to market. 2016. Springer.
- Tian, H., X. Wang, and Y.W. Tong, Sustainability assessment: focusing on different technologies recovering energy from waste. Waste-to-Energy, 2020: p. 235-264.
- Mrajji, O., et al., Feather waste as a thermal insulation solution: Treatment, elaboration and characterization. Journal of Industrial Textiles, 2021. 50(10): p. 1674-1697.
- Islam, M.S., S. Khan, and M. Tanaka, Waste loading in shrimp and fish processing effluents: potential source of hazards to the coastal and nearshore environments. Marine pollution bulletin, 2004. 49(1-2): p. 103-110.
- Read, P. and T. Fernandes, Management of environmental impacts of marine aquaculture in Europe. Aquaculture, 2003. 226(1-4): p. 139-163. [CrossRef]
- Naylor, R.L., et al., Effect of aquaculture on world fish supplies. Nature, 2000. 405(6790): p. 1017-1024.
- Arvanitoyannis, I.S. and A. Kassaveti, Fish industry waste: treatments, environmental impacts, current and potential uses. International journal of food science & technology, 2008. 43(4): p. 726-745.
- Joardar, J. and M. Rahman, Poultry feather waste management and effects on plant growth. International Journal of Recycling of Organic Waste in Agriculture, 2018. 7: p. 183-188.
- Vijayalakshmi, E., Hair pollution hits Karnataka. Down to Earth. 2003.
- Grundwaldt, J.J., T. Tilsworth, and S.E. Clark, Solid waste disposal in Alaska. F. Carlson, 1974: p. 331.
- Radziemska, M., et al., Valorization of fish waste compost as a fertilizer for agricultural use. Waste and Biomass Valorization, 2019. 10: p. 2537-2545. [CrossRef]
- Pagarkar, A., et al., Preparation of bio-fermented and acid silage from fish waste and its biochemical characteristic. Asian Journal of Microbiology Biotechnology and Environmental Sciences, 2006. 8(2): p. 381.
- Salih, A.W., S.M. Najim, and J.M. Al-Noor, Some physical, chemical and sensory properties of fish oil extracted from fish wastes by physical and chemical methods. Biological and applied environmental research, 2021. 5(1): p. 152-162.
- Ivanovs, K. and D. Blumberga, Extraction of fish oil using green extraction methods: A short review. Energy Procedia, 2017. 128: p. 477-483. [CrossRef]
- Ghaly, A., et al., Fish processing wastes as a potential source of proteins. Amino acids and oils: A critical review. J. Microb. Biochem. Technol, 2013. 5(4): p. 107-129.
- Thiele, C.J., et al., Microplastics in fish and fishmeal: an emerging environmental challenge? Scientific reports, 2021. 11(1): p. 2045.
- Hall, G.M., Fishmeal production and sustainability. Fish Processing: Sustainability and New Opportunities, 2010: p. 207-235.
- al., B.e., U.S. Patents, Editor. 2010: United States.
- Farhad ALI, M., et al., BIODEGRADABLE RETANNING MATERIAL FROM TANNERY TRIMMING WASTE: EXTRACTION, PREPARATION AND APPLICATION. Leather & Footwear Journal/Revista de Pielarie Incaltaminte, 2023. 23(4).
- Choudhary, B.L., et al., Replacement of synthetic nitrogenous fertilizer by human hair hydrolysates in cultivation of mung bean (Vigna radiata L.). Waste and Biomass Valorization, 2022. 13(7): p. 3147-3159. [CrossRef]
- Bindal, S., et al., In-Situ and Cell-Free Goat Hair Hydrolysis by a Consortium of Proteases from Bacillus licheniformis Strain ER-15: Hair Hydrolysate Valorization by Melanin Extraction. Waste and Biomass Valorization, 2022. 13(7): p. 3265-3282. [CrossRef]
- Anbesaw, M.S., Bioconversion of Keratin Wastes Using Keratinolytic Microorganisms to Generate Value-Added Products. International Journal of Biomaterials, 2022. 2022(1): p. 2048031.
- Maity, T.K., et al., Efficient isolation of keratin from protein-rich waste biomass: a practical approach to minimize environmental impact and valorize waste biomass. Sustainable Environment Research, 2022. 32(1): p. 42. [CrossRef]
- Qin, X., et al., A sustainable and efficient recycling strategy of feather waste into keratin peptides with antimicrobial activity. Waste Management, 2022. 144: p. 421-430.
- Yan, R.-R., et al., Preparation and applications of keratin biomaterials from natural keratin wastes. Applied Microbiology and Biotechnology, 2022. 106(7): p. 2349-2366.
- Lebedytė, M. and D. Sun, A review: can waste wool keratin be regenerated as a novel textile fibre via the reduction method? The Journal of The Textile Institute, 2022. 113(8): p. 1750-1766.
- Araujo, J., et al., Enzymatic hydrolysis of fish waste as an alternative to produce high value-added products. Waste and Biomass Valorization, 2021. 12: p. 847-855.
- Lv, S., et al., Recycling fish scale powder in improving the performance of asphalt: A sustainable utilization of fish scale waste in asphalt. Journal of Cleaner Production, 2021. 288: p. 125682.
- Petek, B. and R. Marinšek Logar, Management of waste sheep wool as valuable organic substrate in European Union countries. Journal of Material Cycles and Waste Management, 2021. 23: p. 44-54. [CrossRef]
- Choe, U., et al., Anaerobic co-digestion of fish processing waste with a liquid fraction of hydrothermal carbonization of bamboo residue. Bioresource technology, 2020. 297: p. 122542.
- Li, Z.-W., et al., The feather degradation mechanisms of a new Streptomyces sp. isolate SCUT-3. Communications biology, 2020. 3(1): p. 191.
- Rajabinejad, H., I.-I. Bucişcanu, and S.S. Maier, Current approaches for raw wool waste management and unconventional valorization: A review. Environmental Engineering & Management Journal (EEMJ), 2019. 18(7).
- Peng, Z., et al., Keratin waste recycling based on microbial degradation: mechanisms and prospects. ACS sustainable chemistry & engineering, 2019. 7(11): p. 9727-9736.
- Łaba, W., et al., New keratinolytic bacteria in valorization of chicken feather waste. AMB Express, 2018. 8: p. 1-14.
- Holkar, C.R., et al., Valorization of keratin based waste. Process Safety and Environmental Protection, 2018. 115: p. 85-98.
- Lakhal, D., et al., Mixture experimental design in the development of a bio fertilizer from fish waste, molasses and scum. International Journal of Engineering Research & Technology, 2017. 6(6): p. 588-594.
- Sharma, S. and A. Gupta, Sustainable management of keratin waste biomass: applications and future perspectives. Brazilian Archives of Biology and Technology, 2016. 59: p. e16150684. [CrossRef]
- Jin, X., et al., Extraction, characterization, and NO release potential of keratin from human hair. Materials Letters, 2016. 175: p. 188-190. [CrossRef]
- Lee, H., et al., Human hair keratin and its-based biomaterials for biomedical applications. Tissue Engineering and Regenerative Medicine, 2014. 11: p. 255-265.
- Kakkar, P., B. Madhan, and G. Shanmugam, Extraction and characterization of keratin from bovine hoof: A potential material for biomedical applications. SpringerPlus, 2014. 3: p. 1-9.
- Fakhfakh, N., et al., Wool-waste valorization: production of protein hydrolysate with high antioxidative potential by fermentation with a new keratinolytic bacterium, Bacillus pumilus A1. Journal of applied microbiology, 2013. 115(2): p. 424-433. [CrossRef]
- Chalamaiah, M., R. Hemalatha, and T. Jyothirmayi, Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food chemistry, 2012. 135(4): p. 3020-3038.
- Gupta, A., et al., Extraction of keratin protein from chicken feather. Journal of Chemistry and Chemical Engineering, 2012. 6(8): p. 732.
- Stingone, J.A. and S. Wing, Poultry litter incineration as a source of energy: reviewing the potential for impacts on environmental health and justice. New Solutions: A Journal of Environmental and Occupational Health Policy, 2011. 21(1): p. 27-42.
- Beckley, L., S. Fennessy, and B. Everett, Few fish but many fishers: a case study of shore-based recreational angling in a major South African estuarine port. African Journal of Marine Science, 2008. 30(1): p. 11-24.
- El-Tarabily, K.A., et al., Fish emulsion as a food base for rhizobacteria promoting growth of radish (Raphanus sativus L. var. sativus) in a sandy soil. Plant and soil, 2003. 252: p. 397-411.
- Abbasi, P.A., Establishing suppressive conditions against soilborne potato diseases with low rates of fish emulsion applied serially as a pre-plant soil amendment. Canadian journal of plant pathology, 2013. 35(1): p. 10-19. [CrossRef]
- Santos, V.P., et al., Seafood waste as attractive source of chitin and chitosan production and their applications. International journal of molecular sciences, 2020. 21(12): p. 4290.
- Chilakamarry, C.R., et al., Extraction and application of keratin from natural resources: a review. 3 Biotech, 2021. 11: p. 1-12. [CrossRef]
- Srivastava, B., et al., Microbial keratinases: an overview of biochemical characterization and its eco-friendly approach for industrial applications. Journal of Cleaner Production, 2020. 252: p. 119847.
- Feroz, S., et al., Keratin-Based materials for biomedical applications. Bioactive materials, 2020. 5(3): p. 496-509.
- Bhari, R., et al., Bioconversion of chicken feathers by Bacillus aerius NSMk2: a potential approach in poultry waste management. Bioresource Technology Reports, 2018. 3: p. 224-230. [CrossRef]
- de Menezes, C.L.A., et al., Industrial sustainability of microbial keratinases: production and potential applications. World Journal of Microbiology and Biotechnology, 2021. 37(5): p. 86.
- Nnolim, N.E. and U.U. Nwodo, Microbial keratinase and the bio-economy: a three-decade meta-analysis of research exploit. AMB Express, 2021. 11: p. 1-16.
- Qiu, J., et al., Bioinformatics based discovery of new keratinases in protease family M36. New Biotechnology, 2022. 68: p. 19-27. [CrossRef]
- Bohacz, J., Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus Chrysosporium and statistical optimization of feather mass loss. World Journal of Microbiology and Biotechnology, 2017. 33: p. 1-16.
- Gurung, S.K., et al., Discovery of two chrysosporium species with keratinolytic activity from field soil in Korea. Mycobiology, 2018. 46(3): p. 260-268.
- Dari, B., C.W. Rogers, and O.S. Walsh, Understanding factors controlling ammonia volatilization from fertilizer nitrogen applications. Univ. Ida. Ext. Bul, 2019. 926: p. 1-4.
- Liyanage, L., A. Jayakody, and G. Gunaratne, Ammonia volatilization from frequently applied fertilizers for the low-country tea growing soils of Sri Lanka. 2014. [CrossRef]
- Elliott, M., et al., “And DPSIR begat DAPSI (W) R (M)!”-a unifying framework for marine environmental management. Marine Pollution Bulletin, 2017. 118(1-2): p. 27-40.
- Coskun, D., et al., Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nature Plants, 2017. 3(6): p. 1-10.
- Gonzalez Perez, P., et al., Characterization of the amino acid composition of soils under organic and conventional management after addition of different fertilizers. Journal of Soils and Sediments, 2015. 15: p. 890-901. [CrossRef]
- Wang, X., et al., The fate of 15 N-labelled urea in an alkaline calcareous soil under different N application rates and N splits. Nutrient Cycling in Agroecosystems, 2016. 106: p. 311-324.
- Ajmal Siddique S, I., N., Reshma, J., and Harish, N., , Fish Amino Acid – A Review. International Journal of Advanced Research in Science, Communication and Technology (IJARSCT), 2023. 3 (1): p. pp 235-240.
- Balraj, T.H., S. Palani, and G. Arumugam, Influence of Gunapaselam, a liquid fermented fish waste on the growth characteristics of Solanum melongena. Journal of Chemical and Pharmaceutical Research, 2014. 6(12): p. 58-66.
- Hepsibha, B.T. and A. Geetha, Physicochemical characterization of traditionally fermented liquid manure from fish waste (Gunapaselam). 2019.
- Ihemanma, A. and C. Ebutex, A contrast between fish offal's fertilizer, chemical fertilizer and manure applied to tomato and onion. Advances in Aquaculture and Fisheries Management, 2013. 1(9): p. i+ 90-93.
- Xu, H.L., et al. Yield and quality of leafy vegetables grown with organic fertilizations. in XXVI International Horticultural Congress: Toward Ecologically Sound Fertilization Strategies for Field Vegetable Production 627. 2002.
- Barone, J.R., W.F. Schmidt, and C.F. Liebner, Thermally processed keratin films. Journal of applied polymer science, 2005. 97(4): p. 1644-1651.
- Strnad, P., et al., Unique amino acid signatures that are evolutionarily conserved distinguish simple-type, epidermal and hair keratins. Journal of Cell Science, 2011. 124(24): p. 4221-4232.
- Baqir, H., N. Zeboon, and A. Al-Behadili, The role and importance of amino acids within plants: A review. Plant Archives, 2019. 19(2): p. 1402-1410.
- Gauthankar, M., et al., Comparative assessment of amino acids composition in two types of marine fish silage. Scientific Reports, 2021. 11(1): p. 15235. [CrossRef]
| Proteinaceous Waste | Valorization method | References |
|---|---|---|
| Keratin Waste | Keratin from human and animal sources; Chemical hydrolysis | [45] |
| Human Hair | Chemical hydrolysis using alkalis | [46] |
| Goat Hair | Biological hydrolysis (Bacillus licheniformis Strain ER-15) | [47] |
| Keratin Waste | Bioconversion (Vibrio sp.), hydrothermal and chemical hydrolysis | [48] |
| Keratin Waste | Chemical hydrolysis (tetramethyl ammonium hydroxide (TMAOH) | [49] |
| Feather Waste | Physical method-catapult steam explosion (ICSE), ICSE-keratinolysis process | [50] |
| Keratin Waste | Summarizes physical, chemical, enzymatic methods | [51] |
| Wool | Physical method (reduction method) | [52] |
| FPW | Enzymatic hydrolysis | [53] |
| FPW | Physical, chemical, and biological extraction methods | [12] |
| FPW | Specific methodology to use fish scale powder in improving the performance of asphalt | [54] |
| Wool | Biotechnological approaches, such as microbial or enzymatic pretreatment, and composting | [55] |
| FPW | Composting, hydrolysis, anaerobic digestion | [21] |
| FPW | Anaerobic co-digestion with a liquid fraction of hydrothermal carbonization | [56] |
| Feather Waste | Biological hydrolysis (Streptomyces sp. isolate SCUT-3) | [57] |
| Wool | Current approaches for raw wool waste management and unconventional valorization | [58] |
| Keratin Waste | Biological hydrolysis | [59] |
| Feather Waste | Biological hydrolysis | [60] |
| Keratin Waste | Summarizes valorization of keratin-based (wools, feathers, hair) waste | [61] |
| FPW | Biological hydrolysis (early 68% of fish waste, 13% of molasses and 19% of scum) to produce biofertilizer | [62] |
| Keratin Waste | Different extraction methods to produce value-added products | [24] |
| Feather Waste | Different technologies to obtain high-value products | [13] |
| Keratin Waste | Different strategies for extraction and use in pharmaceutical and cosmetics industries | [63] |
| Human Hair | Specific method for extraction and use in biomedical and biotechnological applications | [64] |
| Human Hair | Extraction for biomedical applications | [65] |
| Horns, Hooves | Extraction for biomedical applications | [66] |
| Wool | Biological hydrolysis (Bacillus pumilus A1) | [67] |
| FPW | Fish protein hydrolysates | [68] |
| Feather Waste | Specific valorization methods to extract keratin for cosmetics | [69] |
| Feather Waste | Incineration to produce energy | [70] |
| Composition | Percentage (%) |
|---|---|
| 1. Macro Nutrients | |
| Total organic carbon | 25% |
| Nitrogen (N) | 6.5% |
| Phosphorous (P) | 1% |
| Potassium (K) | 1.5% |
| Sulphur(S) | 0.8% |
| Calcium (Ca) | 15ppm |
| Magnesium (Mg) | 15ppm |
| 2. Micronutrients | |
| Sodium (Na) | 1% |
| Manganese (Mn) | 5ppm |
| Zinc (Zn) | 17ppm |
| Copper | 5ppm |
| Boron | 7ppm |
| Molybdenum | 0.5ppm |
| 3. Parameters | |
| pH | 6.5% |
| C:N ratio | 4:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





