Submitted:
23 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
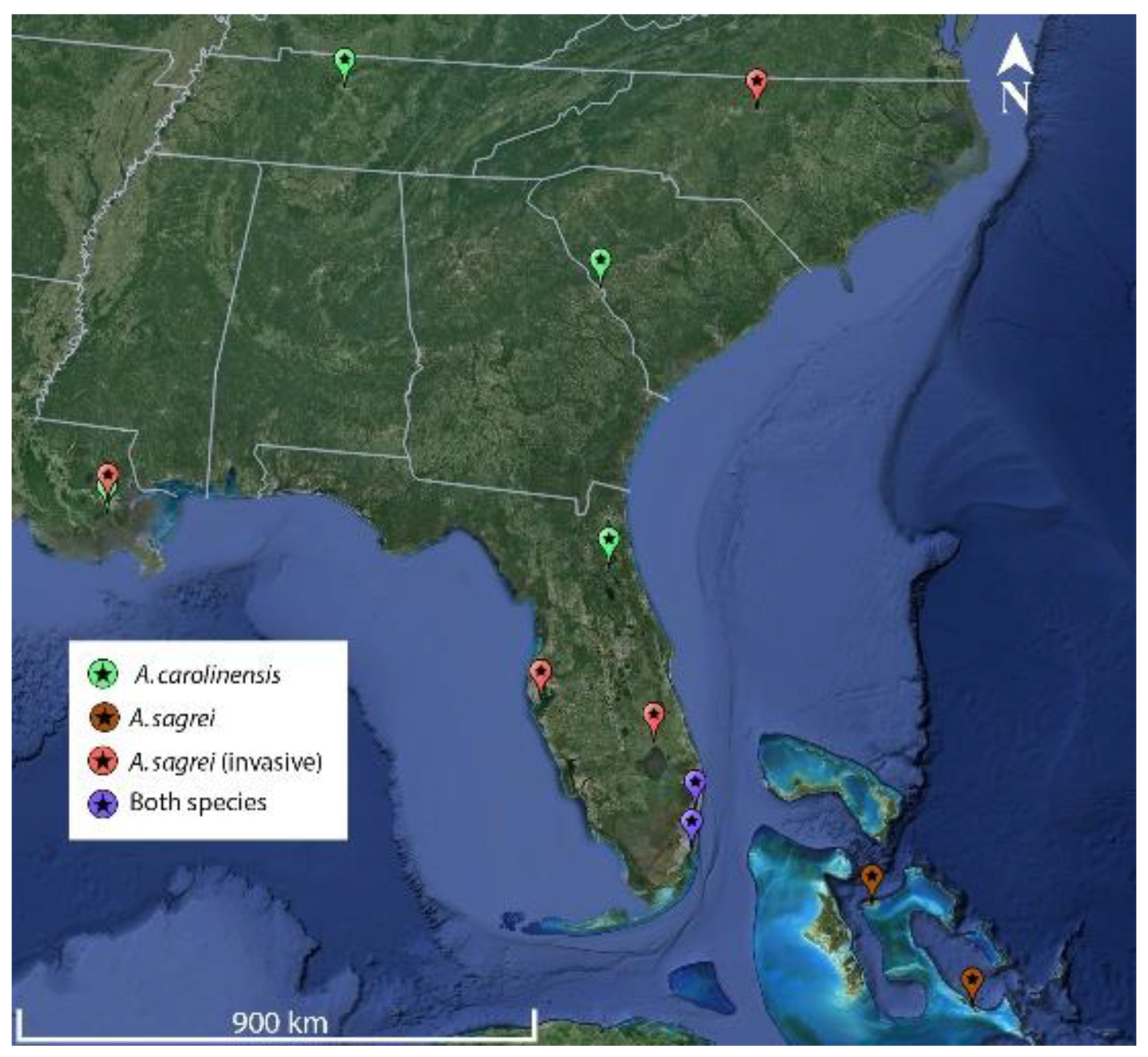
2.1. Study Organisms
2.2. Data Collection
2.3. Data Analyses
2.4. Meta Analyses
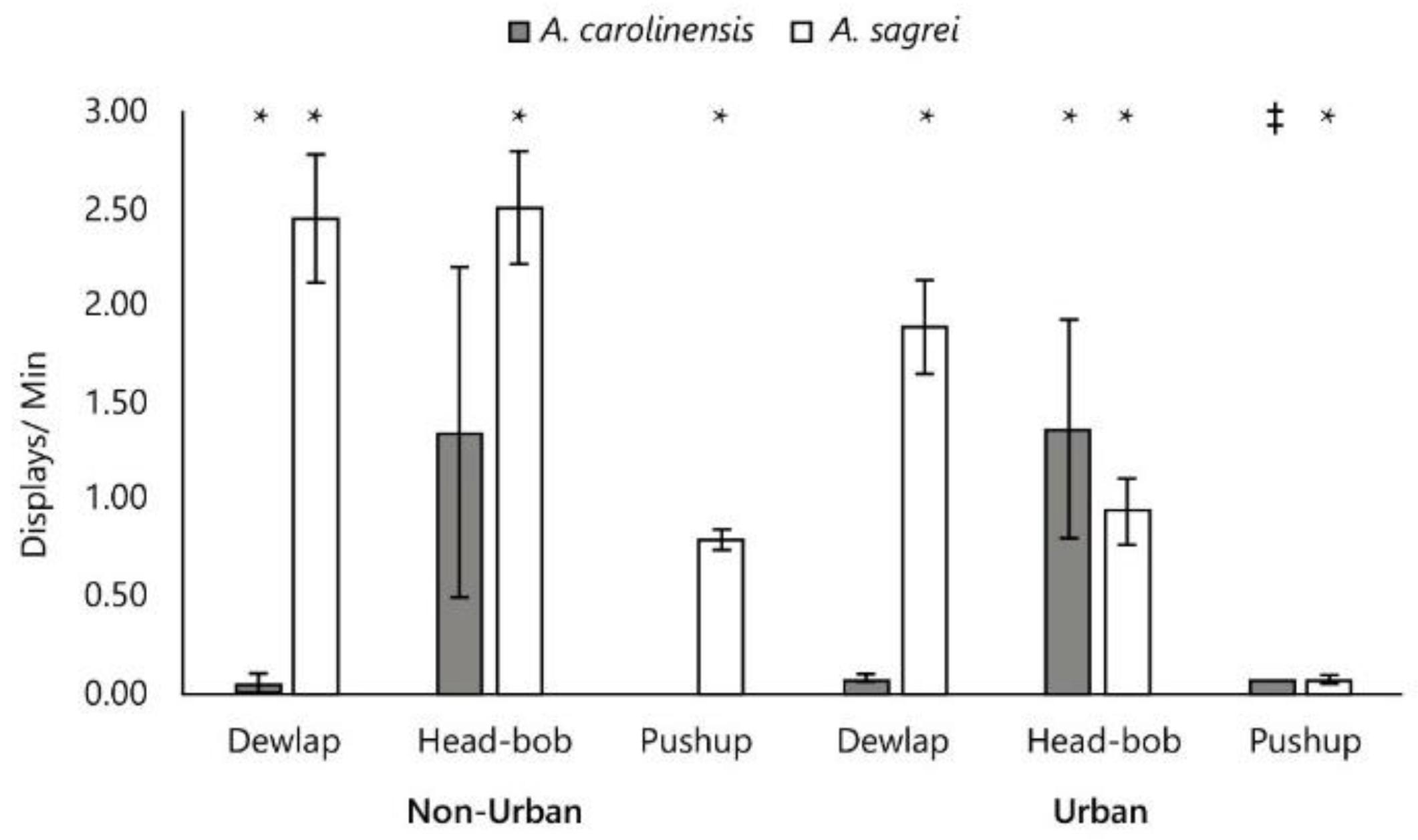
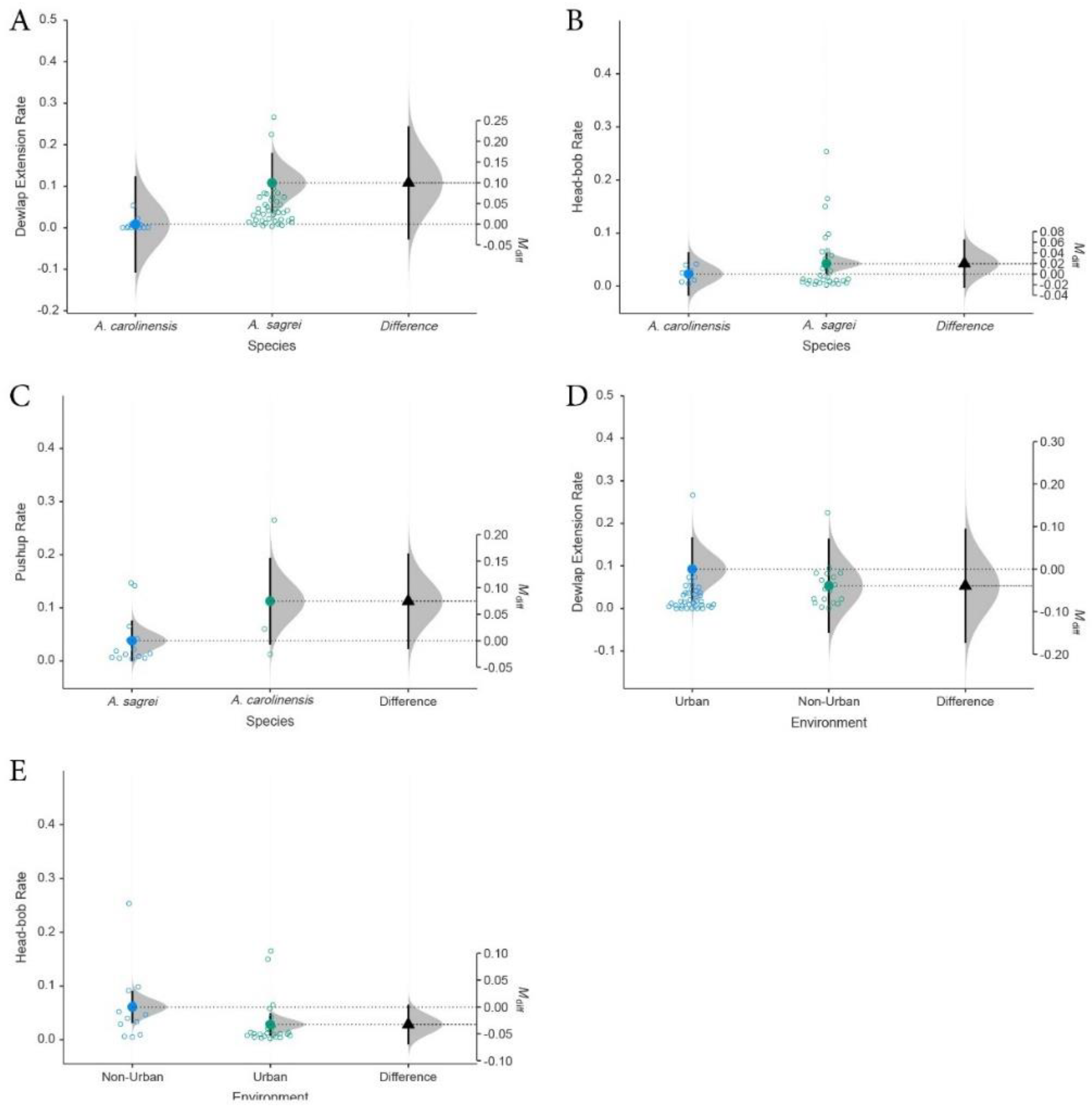
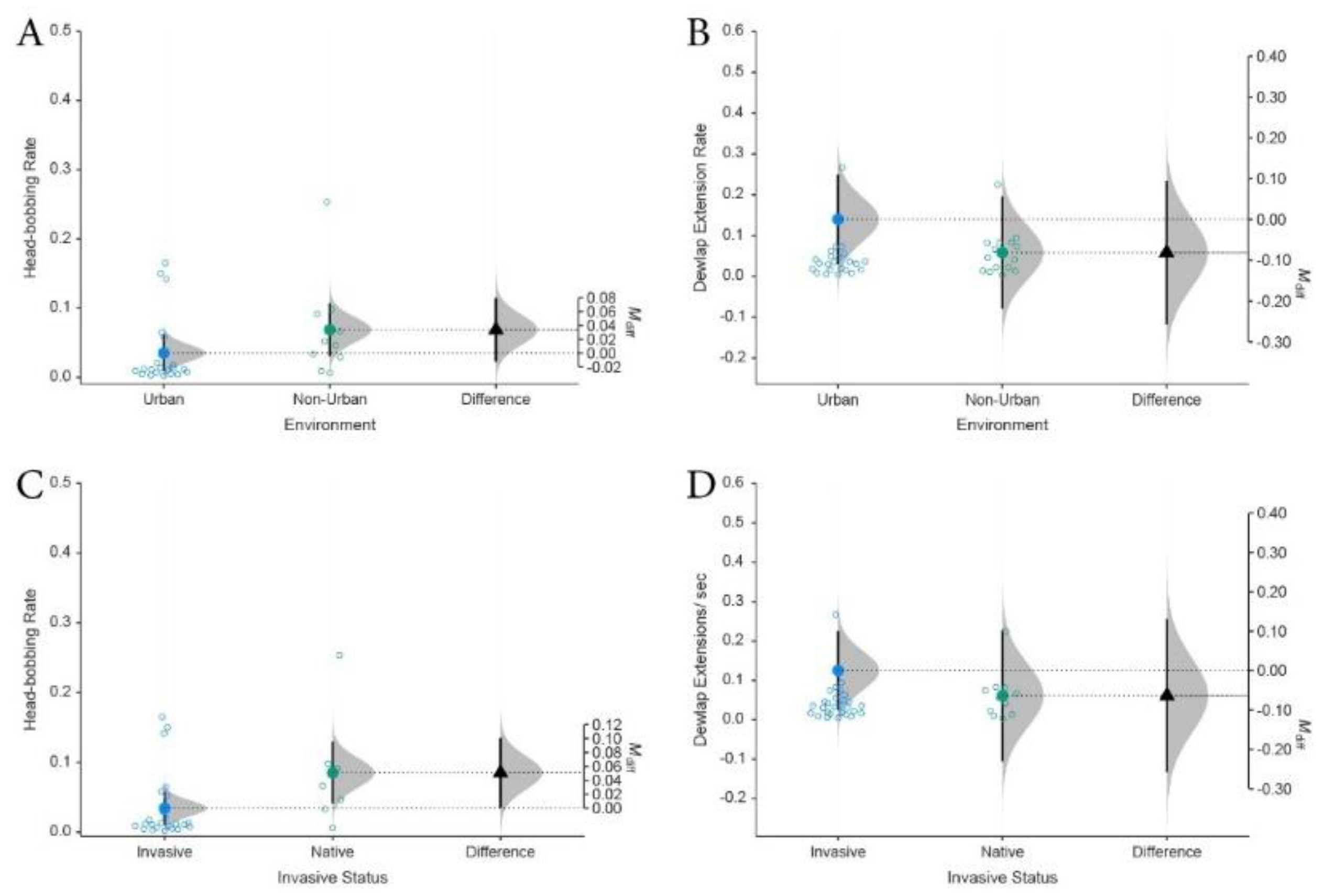
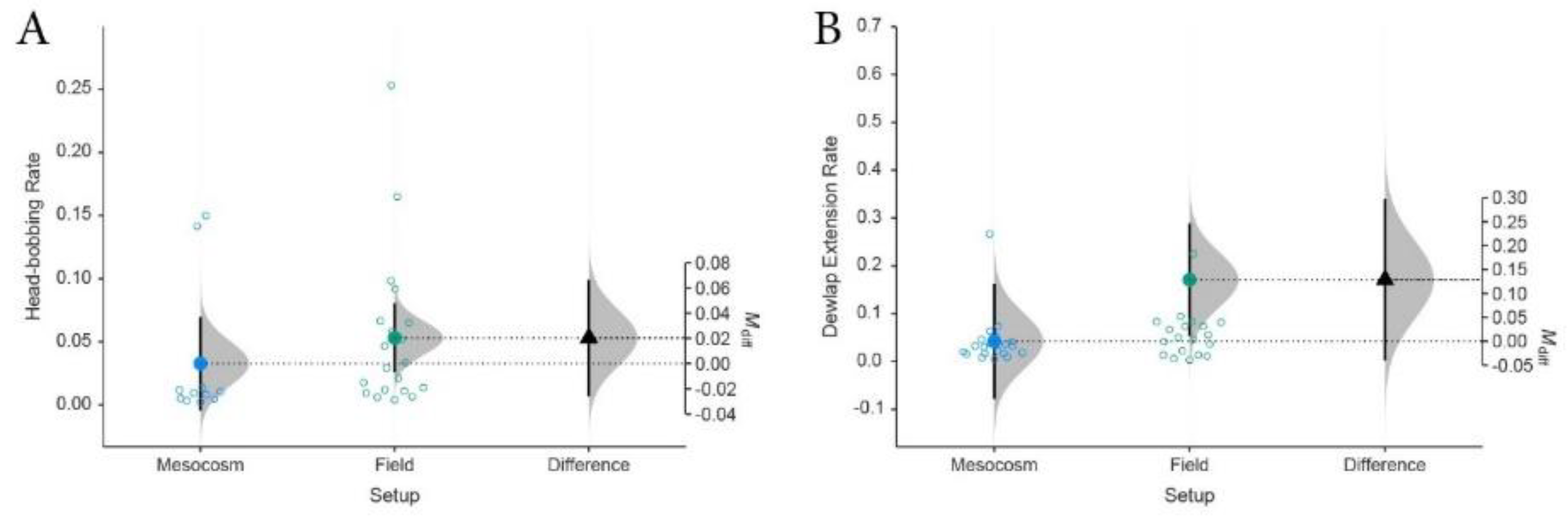
3. Results
4. Discussion
4.1. Frontline of Invasion Compared with Established Invasive Communities
4.1.1. Sex Over Aggression
4.1.2. Invasion Front and Habituated Populations
4.1.3. Marginal Costs of territorial Defense
4.1.4. Tolerance as an adaptive Trait to Higher Densities
4.2. Sexual Signals Counter Agonistic Signals
4.3. Publication Bias
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. Biodiversity; The National Academy Press, 1988.
- Rypel, A.L. Do Invasive Freshwater Fish Species Grow Better When They Are Invasive? Oikos 2014, 123, 279–289. [Google Scholar] [CrossRef]
- Leishman, M.R.; Cooke, J.; Richardson, D.M. Evidence for Shifts to Faster Growth Strategies in the New Ranges of Invasive Alien Plants. J. Ecol. 2014, 102, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Graebner, R.C.; Callaway, R.M.; Montesinos, D. Invasive Species Grows Faster, Competes Better, and Shows Greater Evolution toward Increased Seed Size and Growth than Exotic Non-Invasive Congeners. Plant Ecol. 2012, 213, 545–553. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Zheng, Y.L.; Lei, Y.B.; Feng, Y.L. Evolutionary Increases in Defense during a Biological Invasion. Oecologia 2014, 174, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Stigall, A.L. Invasive Species and Evolution. Evol. Educ. Outreach 2012, 5, 526–533. [Google Scholar] [CrossRef]
- Snyder, W.E.; Evans, E.W. Ecological Effects of Invasive Arthropod Generalist Predators. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 95–122. [Google Scholar] [CrossRef]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.F.; Chapuis, E.; Loeuille, N. Impacts of Invasive Species on Food Webs: A Review of Empirical Data. Adv. Ecol. Res. 2017, 56, 1–60. [Google Scholar] [CrossRef]
- Bleicher, S.S.; Rosenzweig, M.L. Too Much of a Good Thing? A Landscape-of-Fear Analysis for Collared Peccaries ( Pecari Tajacu ) Reveals Hikers Act as a Greater Deterrent than Thorny or Bitter Food. Can. J. Zool. 2018, 96, 317–324. [Google Scholar] [CrossRef]
- Bleicher, S.S.; Dickman, C.R. On the Landscape of Fear: Shelters Affect Foraging by Dunnarts (Marsupialia, Sminthopsis Spp.) in a Sandridge Desert Environment. J. Mammal. 2020, 101, 281–290. [Google Scholar] [CrossRef]
- Lailvaux, S.P.; Edwards, J.R.; Lailvaux, S.P.; Winchell, K.M.; Reynolds, R.G.; Prado-Irwin, S.R.; Puente-Rolón, A.R.; Revell, L.J.; Stroud, J.T.; Colom, M.; et al. Hurricane Irma Induces Divergent Behavioral and Hormonal Impacts on an Urban and Forest Population of Invasive Anolis Lizards: Evidence for an Urban Resilience Hypothesis. Front. Ecol. Evol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Gruber, J.; Brown, G.; Whiting, M.J.; Shine, R. Geographic Divergence in Dispersal-Related Behaviour in Cane Toads from Range-Front versus Range-Core Populations in Australia. Behav. Ecol. Sociobiol. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Tsutsui, N.D.; Suarez, A. V; Grosberg, R.K. Genetic Diversity, Asymmetrical Aggression, and Recognition in a Widespread Invasive Species. PNAS 2002, 100, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Magellan, K.; Garcıa-Berthou, E. Influences of Size and Sex on Invasive Species Aggression and Native Species Vulnerability : A Case for Modern Regression Techniques. Rev. Fish Biol. Fish. 2015, 25, 537–549. [Google Scholar] [CrossRef]
- Hudina, S.; Hock, K.; Žganec, K. The Role of Aggression in Range Expansion and Biological Invasions. Curr. Zool. 2014, 60, 401–409. [Google Scholar] [CrossRef]
- Pintor, L.M.; Brown, J.S.; Vincent, T.L. Evolutionary Game Theory as a Framework for Studying Biological Invasions. Am. Nat. 2011, 177, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Mckinney, M.L. Urbanization as a Major Cause of Biotic Homogenization. Biol. Conserv. 2006, 127, 246–260. [Google Scholar] [CrossRef]
- Magle, S.B.; Simoni, L.S.; Lehrer, E.W.; Brown, J.S. Urban Predator-Prey Association: Coyote and Deer Distributions in the Chicago Metropolitan Area. Urban Ecosyst. 2014, 17, 875–891. [Google Scholar] [CrossRef]
- Bradley, C. a.; Altizer, S. Urbanization and the Ecology of Wildlife Diseases. Trends Ecol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Meek, P.; Ballard, G.; Fleming, P.; Falzon, G. Are We Getting the Full Picture? Animal Responses to Camera Traps and Implications for Predator Studies. Ecol. Evol. 2016, 1, 1–10. [Google Scholar] [CrossRef]
- Avilés-Rodríguez, K.J.; Kolbe, J.J. Escape in the City: Urbanization Alters the Escape Behavior of Anolis Lizards. Urban Ecosyst. 2019, 22, 733–742. [Google Scholar] [CrossRef]
- St. Juliana, J.; Bleicher, S.S.; Mukherjee, S.; Sundararaj, V.; Brown, J.S.; Kotler, B.P. Human-Commensal Threats; Combinations of Predators Alter Gerbil Risk-Perception in Additive, Substitutable, or Antagonistic Manners. Journal: Conserv. Biol. 2023, 1–20. (In review).
- Pham, A.; Riley, J.; Bleicher, S.S. Human-Wildlife Conflicts and Sensory Disinformation, a Review of Evolutionary Trajectories Caused by Human Land- Management and Rapidly Expanding Urban Landscapes. (In Review). J. Urban Ecol. 2024, 1–33.
- Ciuti, S.; Muhly, T.B.; Paton, D.G.; McDevitt, A.D.; Musiani, M.; Boyce, M.S. Human Selection of Elk Behavioural Traits in a Landscape of Fear. Proc. Biol. Sci. 2012, 279, 4407–4416. [Google Scholar] [CrossRef] [PubMed]
- Ament, R.; Clevenger, A.P.; Yu, O.; Hardy, A. An Assessment of Road Impacts on Wildlife Populations in U. S. National Parks. Environ. Manage. 2008, 42, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.G.; Long, R.A.; Berger, J.; Bergen, S.; Beckmann, J.P. Identifying Impediments to Long-Distance Mammal Migrations. Conserv. Biol. 2015, 29, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga-Palacios, J.; Zuria, I.; Castellanos, I.; Lara, C.; Sánchez-Rojas, G. What Do We Know (and Need to Know) about the Role of Urban Habitats as Ecological Traps? Systematic Review and Meta-Analysis. Sci. Total Environ. 2021, 780, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Magle, S.B.; Reyes, P.; Zhu, J.; Crooks, K.R. Extirpation, Colonization, and Habitat Dynamics of a Keystone Species along an Urban Gradient. Biol. Conserv. 2010, 143, 2146–2155. [Google Scholar] [CrossRef]
- Barrett, M.A.; Telesco, D.J.; Barrett, S.E.; Katelyn, M.; Leone, E.H. Testing Bear-Resistant Trash Cans in Residential Areas of Florida. Southeast. Nat. 2014, 13, 26–39. [Google Scholar] [CrossRef]
- Kruszyk, R.; Ciach, M. White Storks, Ciconia Ciconia, Forage on Rubbish Dumps in Poland-a Novel Behaviour in Population. Eur. J. Wildl. Res. 2010, 56, 83–87. [Google Scholar] [CrossRef]
- Theimer, T.C.; Clayton, A.C.; Martinez, A.; Peterson, D.L.; Bergman, D.L. Visitation Rate and Behavior of Urban Mesocarnivores Differs in the Presence of Two Common Anthropogenic Food Sources. Urban Ecosyst. 2015, 18, 895–906. [Google Scholar] [CrossRef]
- Deatherage, N.A.; Cypher, B.; Westall, T.; Kelly, E. Spatial Relationships between Mesocarnivores and Domestic Species in an Urban Environment and Implications for Endangered San Joaquin Kit Foxes. Urban Ecosyst. 2024, 27, 321–334. [Google Scholar] [CrossRef]
- Losos, J.B. Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles; Unviersity of California Press: Berkley, CA, USA, 2009. [Google Scholar]
- Roughgarden, J. Resource Partitioning among Competing Spcecies-- A Coevolutionary Approach. Theor. Popul. Biol. 1976, 9, 388–424. [Google Scholar] [CrossRef]
- Roughgarden, J. Competition and Theory in Community Ecology. Am. Nat. 1983, 122, 583–601. [Google Scholar] [CrossRef]
- Les, A.M.; Gifford, M.E.; Parmerlee, J.S.; Powell, R. Do Polymorphic Female Brown Anoles (Anolis Sagrei ) Differ in Sprint Speed or Escape Behavior? Herpetologica 2014, 70, 47–55. [Google Scholar] [CrossRef]
- Cooper, W.E. Risk Factors Affecting Escape Behaviour by Puerto Rican Anolis Lizards. Can. J. Zool. 2006, 84, 495–504. [Google Scholar] [CrossRef]
- Bloch, N.; Irschick, D.J. An Analysis of Inter-Population Divergence in Visual Display Behavior of the Green Anole Lizard (Anolis carolinensis). Ethology 2006, 112, 370–378. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Irschick, D.J.; Lailvaux, S.P. Trait Compensation between Boldness and the Propensity for Tail Autotomy under Different Food Availabilities in Similarly Aged Brown Anole Lizards. Funct. Ecol. 2015, 29, 385–392. [Google Scholar] [CrossRef]
- Losos, J.B. Integrative Approaches to Evolutionary Ecology: Anolis Lizards as Model Systems. Annu. Rev. Ecol. Systamatics 1994, 25, 467–469. [Google Scholar] [CrossRef]
- Hall, J.M.; Warner, D.A. Thermal Spikes from the Urban Heat Island Increase Mortality and Alter Physiology of Lizard Embryos. J. Exp. Biol. 2018, 221, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Losos, J.B. Adaptive Radiation, Ecological Opportunity, and Evolutionary Determinism. American Society of Naturalists E. O. Wilson Award Address. Am. Nat. 2010, 175, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, J.J.; Leal, M.; Schoener, T.W.; Spiller, D.A.; Losos, J.B. Founder Effects Persist Despite Adaptive Differentiation : A Field Experiment with Lizards. Science (80-. ). 2012, 335, 1086–1089. [Google Scholar] [CrossRef]
- Lovern, M.B.; Jenssen, T.A.; Orrell, K.S.; Tuchak, T.; Herpetologica, S.; Jun, N. Herpetologists’ League Comparisons of Temporal Display Structure across Contexts and Populations in Male Anolis carolinensis: Signal Stability or Lability? Herpetologica 1999, 55, 222–234. [Google Scholar]
- Eals, J.; Thorpe, R.S.; Malhotra, A. Weak Founder Effect Signal in a Recent Introduction of Caribbean Anolis. 2Molecular Ecol. 2008, 17, 1416–1426. [Google Scholar] [CrossRef]
- Manríquez-morán, Norma, L.; Villagrán-santa Cuz, M.; Méndez-de La Cruz, Fausto, R. Reproductive Biology of the Parthenogenetic Lizard, Aspidoscelis Cozumela. Herpetologica 2005, 61, 435–439. [Google Scholar] [CrossRef]
- Fujita, M.K.; Singhal, S.; Brunes, T.O.; Maldonado, J.A.; Queen, R. Evolutionary Dynamics and Consequences of Parthenogenesis in Vertebrates. Annu. Rev. Ecol. Systamatics 2020, 51, 191–216. [Google Scholar] [CrossRef]
- Jancuchova-Lanskova, J.; Landova, E.; Frynta, D. Are Genetically Distinct Lizard Species Able to Hybridize ? A Review. Curr. Zool. 2015, 61, 155–180. [Google Scholar] [CrossRef]
- Fujita, M.K.; Moritz, C. Origin and Evolution of Parthenogenetic Genomes in Lizards: Current State and Future Directions. Cytogenet. Genome Res. 2010, 127, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.; Losos, J. Reconsidering Territoriality Is Necessary for Understanding Anolis Mating Systems. Behav. Ecol. Sociobiol. 2018, 72, 106. [Google Scholar] [CrossRef]
- Kamath, A.; Losos, J. The Erratic and Contingent Progression of Research on Territoriality : A Case. Behav. Ecol. Sociobiol. 2017, 71, 1–13. [Google Scholar] [CrossRef]
- Jenssen, T.A.; Orrell, K.S.; Lovern, M.B. Sexual Dimorphisms in Aggressive Signal Structure and Use by a Polygynous Lizard, Anolis carolinensis. Copeia 2000, 2000, 140–149. [Google Scholar] [CrossRef]
- Jenssen, T.A.; Garrett, S.; Sydor, W.J. Complex Signal Usage by Advertising Male Green Anoles (Anolis carolinensis): A Test of Assumptions. Herpetologica 2012, 68, 345–357. [Google Scholar] [CrossRef]
- Bush, J.M.; Simberloff, D. A Case for Anole Territoriality. Behav. Ecol. Sociobiol. 2018, 72, 111. [Google Scholar] [CrossRef]
- Losos, J.B.; Schneider, C.J. Anolis Lizards. Curr. Biol. 2009, 19, R316–R318. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, T.A.; Nunez, S.C. Spatial and Breeding Relationships of the Lizard, Anolis carolinensis: Evidence of Intrasexual Selection. Behaviour 1998, 135, 981–1003. [Google Scholar] [CrossRef]
- Orrell, K.S.; Jenssen, T.A. Heterosexual Signalling by the Lizard Anolis carolinensis, with Intersexual Comparisons across Contexts; 2003; Vol. 140; ISBN 1568539033221.
- Reedy, A.M.; Pope, B.D.; Kiriazis, N.M.; Giordano, C.L.; Sams, C.L.; Warner, D.A.; Cox, R.M. Female Anoles Display Less but Attack More Quickly than Males in Response to Territorial Intrusions. Behav. Ecol. 2017, 28, 1323–1328. [Google Scholar] [CrossRef]
- Evans, L.T. Courtship Behavior and Sexual Selection of Anolis. J. Comp. Psychol. 1938, 26, 475–494. [Google Scholar] [CrossRef]
- Noble, G.K.; Bradly, H.T. The Mating Behavior of Lizards; Its Bearing on the Theory of Sexual Selection. Ann. N. Y. Acad. Sci. 1993, 35, 25–100. [Google Scholar] [CrossRef]
- Wilczynski, W.; Black, M.P.; Salem, S.J.; Ezeoke, C.B. Behavioural Persistence during an Agonistic Encounter Differentiates Winners from Losers in Green Anole Lizards. Behaviour 2015, 152, 563–591. [Google Scholar] [CrossRef]
- Calsbeek, R.; Cox, R.M. Experimentally Assessing the Relative Importance of Predation and Competition as Agents of Selection. Nature 2010, 465, 613–616. [Google Scholar] [CrossRef]
- Scott, M.P. Agonistic and Courtship Displays of Male Anolis sagrei. Brevoria 1984, 479, 1–22. [Google Scholar]
- Jenssen, T.A.A. Evolution of Anoline Lizard Display Behavior. Am. Zool. 1977, 17, 203–215. [Google Scholar] [CrossRef]
- Macedonia, J.M.; Stamps, J.A. Species Recognition in Anolis grahami (Sauria, Iguanidae): Evidence from Responses to Video Playbacks of Conspecific and Heterospecific Displays. Ethology 1994, 98, 246–264. [Google Scholar] [CrossRef]
- Leal, M.; Rodriguez-Robles, J.A.; Rodri, J.A. Signalling Displays during Predator – Prey Interactions in a Puerto Rican Anole, Anolis cristatellus. Anim. Behav. 1997, 54, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.B. Not All Signals Are Equal: Male Brown Anole Lizards (Anolis sagrei) Selectively Decrease Pushup Frequency Following a Simulated Predatory Attack. Ethology 2007, 113, 793–801. [Google Scholar] [CrossRef]
- Tokarz, R.R.; Mcmann, S.; Smith, L.C.; John-alder, H. Effects of Testosterone Treatment and Season on the Frequency of Dewlap Extensions during Male – Male Interactions in the Lizard Anolis sagrei. Horm. Behav. 2002, 41, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, R.R.; Patterson, A. V.; McMann, S. Laboratory and Field Test of the Functional Significance of the Male’s Dewlap in the Lizard Anolis sagrei. Copeia 2003, 3, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.E.; Harmon, L.J.; Losos, J.B. Evolution of Anolis Lizard Dewlap Diversity. PLoS One 2007, 2, e274. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, R.R. An Experimental Test of the Importance of the Dewlap Inm Male Mating Success in the Lizard Anolis aagrei. Herpetologica 2002, 58, 87–94. [Google Scholar] [CrossRef]
- Battles, A.C.; Kolbe, J.J. Miami Heat : Urban Heat Islands Influence the Thermal Suitability of Habitats for Ectotherms. Glob. Chang. Biol. 2019, 25, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.M.; Stenquist, D.S.; Henningsen, J.P.; Calsbeek, R. Manipulating Testosterone to Assess Links between Behavior, Morphology, and Performance in the Brown Anole Anolis sagrei. Physiol. Biochem. Zool. 2009, 82, 686–698. [Google Scholar] [CrossRef]
- Trivers, R.L. Sexual Selection and Resource-Accruing Abilities in Anolis garmani. Evolution (N. Y). 1976, 30, 253–269. [Google Scholar]
- Crawford, N.G.; McGreevy, T.J.; Mullen, S.P.; Schneider, C.J. The Genetic Basis of Conspicuous Coloration in the Guadeloupean Anole: Evolution by Sexual and Ecological Selection. Ecol. Evol. 2023, 13, 1–13. [Google Scholar] [CrossRef]
- Lailvaux, S.P.; Gilbert, R.L.; Edwards, J.R. A Performance-Based Cost to Honest Signalling in Male Green Anole Lizards (Anolis carolinensis). Proc. R. Soc. B Biol. Sci. 2012, 279, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.A. Sexual Selection, Honest Advertisment and the Handicape Principle: Reviewing the Evidence. Biol. Rev. 1995, 70, 1–65. [Google Scholar] [CrossRef] [PubMed]
- McMann, S.; Patterson, A.V. Display Behavior of Resident Brown Anoles (Anolis sagrei) during Close Encounters with Neighbors and Non-Neighbors. Herpetol. Conserv. Biol. 2012, 7, 27–37. [Google Scholar]
- Lovern, M.B.; Jenssen, T.A. Form Emergence and Fixation of Head Bobbing Displays in the Green Anole Lizard (Anolis carolinensis): A Reptilian Model of Signal Ontogeny. J. Comp. Psychol. 2003, 117, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bernal, E.; Brown, G.P.; Shine, R. Invasive Cane Toads : Social Facilitation Depends upon an Individual ’ s Personality. PLoS One 2014, 9, e102880. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.S.; Losos, J.B.; Schoener, T.W.; Spiller, D.A.; Kolbe, J.J.; Leal, M. Predation-Associated Modulation of Movement-Based Signals by a Bahamian Lizard. Proc. Natl. Acad. Sci. 2014, 111, 9187–9192. [Google Scholar] [CrossRef] [PubMed]
- Côté, I.M.; Darling, E.S.; Malpica-Cruz, L.; Smith, N.S.; Green, S.J.; Curtis-Quick, J.; Layman, C. What Doesn’t Kill You Makes You Wary? Effect of Repeated Culling on the Behaviour of an Invasive Predator. PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R.; Bursey, C.R. Transport of Helminths To Hawaii Via the Brown Anole, Anolis sagrei (Polychrotidae). J. Parasitol. 2000, 86, 750. [Google Scholar] [CrossRef]
- Schwartz, A.; Henderson, R.W. Amphibians and Reptiles of the West Indies: Descriptions, Distributions, and Natural History; University of Florida Press: Gainesville, FL, USA, 1991. [Google Scholar]
- Fisher, S.R.R.; Del Pinto, L.A.A.; Fisher, R.N.N. Establishment of Brown Anoles (Anolis sagrei) across a Southern California County and Potential Interactions with a Native Lizard Species. PeerJ 2019, 8, e8937. [Google Scholar] [CrossRef]
- Horr, D.M. The Ecology of Dynamic Body Color in the Green Anole, Anolis Carolinensis (Honors Thesis), 2019.
- Lovern, M.B.; Holmes, M.M.; Wade, J. The Green Anole (Anolis carolinensis): A Reptilian Model for Laboratory Studies of Reproductive Morphology and Behavior. ILAR J. 2004, 45, 54–64. [Google Scholar] [CrossRef]
- Cox, C.L.; Peaden, R.T.; Cox, R.M. The Metabolic Cost of Mounting an Immune Response in Male Brown Anoles (Anolis sagrei). J. Exp. Zool. 2015, 323, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.; Adkins, K. Hormones and Social Behavior in the Lizard, Anolis carolinensis. Horm. Behav. 1976, 86, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Vigil, S. Brown Anole (Anolis sagrei). In Natural History Publication Series; Mengak, M.T., Ed.; Tampa, FL, FL, 2006; pp. 1–6.
- Yasumiba, K.; Okada, A.; Okochi, I.; Iwai, N. Minimum Longevity and Growth of the Invasive Green Anole, Anolis carolinensis, in Chichi-Jima of the Ogasawara Islands, Japan. Curr. Herpetol. 2016, 35, 101–105. [Google Scholar] [CrossRef]
- Battles, A.C.; Whittle, T.K.; Stehle, C.M.; Johnson, M.A.; Attles, A.N.C.B.; Hittle, T.A.R.A.K.W.; Tehle, C.H.M.S.; Ohnson, M.I.A.J. Effects of Human Land Use on Prey Availability and Body Condition in the Green Anole Lizard, Anolis carolinensis. Herpetol. Conserv. Biol. 2013, 8, 16–26. [Google Scholar]
- Norval, G.; Chen, C.K.; Hsiao, W.-F.; Huang, S.-C.; Chen, C.-K. The Diet of an Introduced Lizard Species, the Brown Anole (Anolis sagrei), in Chiayi County, Taiwan. Russ. J. Herpetol. 2010, 17, 131–138. [Google Scholar]
- Schaefer, R.R.; Fleet, R.R.; Rudolph, D.C.; Koerth, N.E. Habitat Selection by Anolis carolinensis (Green Anole) in Open Pine Forests in Eastern Texas. In Proceedings of the Proceedings of the 4th Big Thicket Science Conference Southeastern Naturalist; 2009; pp. 63–76.
- Horr, D.M.; Payne, A.A.; Mcentire, K.D.; Johnson, M.A. Sexual Dimorphism in Dynamic Body Color in the Green Anole Lizard. Behav. Ecol. Sociobiol. 2023. [Google Scholar] [CrossRef]
- Cumming, G. Understanding the New Statistics; Effect Sizes, Confidence Intervals, and Meta Analyses; Routledge: New York, New York, 2012.
- Bonnet, D.G.; Calin-Jageman, R.J. ESCI (Effect Sizes and Confidence Intervals); Statpsych: Statistical Methods for Psychologists 2023, 1.5.0.
- Jamovi The Jamovi Project 2023, Version 2.4.
- R Core Team R: A Langauage and Environment for Statistical Computing 2022, Version 4.1.
- Bonnet, D.G. Confidence Intervals for Standardized Linear Contrast Means. Psychol. Methods 2008, 13, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Kay, M. Ggdist: Visualization of Distributions and Uncertinty in the Grammar of Graphics. IEEE Trans. Visulaization Comput. Graph. 2002, 30, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Heavener, S.J.; Carthey, A.J.R.; Banks, P.B. Competitive Naïveté between a Highly Successful Invader and a Functionally Similar Native Species. Oecologia 2014, 175, 73–84. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral Syndromes: An Ecological and Evolutionary Overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef]
- Carpintero, S.; Reyes, J. Capter 7: The Role of Agression in the Success of the Invasive Argentine Ant. In Handbook of Aggressive Behavior Research Ed:; Tawse, C.Q. and S., Ed.; Nova Science Publishers, Inc, 2009; pp. 241–268 ISBN 9781607415831.
- Molina-Montenegro, M. a.; Cleland, E.E.; Watts, S.M.; Broitman, B.R. Can a Breakdown in Competition-Colonization Tradeoffs Help Explain the Success of Exotic Species in the California Flora? Oikos 2012, 121, 389–395. [Google Scholar] [CrossRef]
- Mason, R.A.B.; Cooke, J.; Moles, A.T.; Leishman, M.R. Reproductive Output of Invasive versus Native Plants. Glob. Ecol. Biogeogr. 2008, 17, 633–640. [Google Scholar] [CrossRef]
- Kamath, A.; Losos, J.B. Estimating Encounter Rates as the First Step of Sexual Selection in the Lizard Anolis sagrei. Proc. R. Soc. B Biol. Sci. 2018, 285. [Google Scholar] [CrossRef] [PubMed]
- Hudina, S.; Žganec, K.; Hock, K. Differences in Aggressive Behaviour along the Expanding Range of an Invasive Crayfish: An Important Component of Invasion Dynamics. Biol. Invasions 2015, 17, 3101–3112. [Google Scholar] [CrossRef]
- Michelangeli, M.; Smith, C.R.; Wong, B.B.M.; Chapple, D.G. Aggression Mediates Dispersal Tendency in an Invasive Lizard. Anim. Behav. 2017, 133, 29–34. [Google Scholar] [CrossRef]
- Putman, B.J.; Pauly, G.B.; Blumstein, D.T. Urban Invaders Are Not Bold Risk-Takers: A Study of 3 Invasive Lizards in Southern California. Curr. Zool. 2020, 66, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.C.; Ruiz-Navarro, A.; Britton, J.R. Population Density Modifies the Ecological Impacts of Invasive Species. Oikos 2015, 124, 880–887. [Google Scholar] [CrossRef]
- Čuda, J.; Skálová, H.; Janovský, Z.; Pyšek, P. Competition among Native and Invasive Impatiens Species: The Roles of Environmental Factors, Population Density and Life Stage. AoB Plants 2015, 7, 1–12. [Google Scholar] [CrossRef]
- Akin-Fajiye, M.; Gurevitch, J. The Influence of Environmental Factors on the Distribution and Density of Invasive Centaurea Stoebe across Northeastern USA. Biol. Invasions 2018, 20, 3009–3023. [Google Scholar] [CrossRef]
- Angetter, L.S.; Lötters, S.; Rödder, D. Climate Niche Shift in Invasive Species: The Case of the Brown Anole. Biol. J. Linn. Soc. 2011, 104, 943–954. [Google Scholar] [CrossRef]
- Campbell, T.S.; Echternacht, A.C. Introduced Species as Moving Targets: Changes in Body Sizes of Introduced Lizards Following Experimental Introductions and Historical Invasions. Biol. Invasions 2003, 5, 193–212. [Google Scholar] [CrossRef]
- Chejanovski, Z.A.; Giery, S.T.; Kolbe, J.J. Effects of Urbanization on the Trophic Niche of the Brown Anole, a Widespread Invasive Lizard. Food Webs 2022, 33, e00257. [Google Scholar] [CrossRef]
- Morris, D.W. Density-Dependent Habitat Selection : Testing the Theory with Fitness Data. Evol. Ecol. 1989, 3, 80–94. [Google Scholar] [CrossRef]
- Grand, T.C.; Dill, L.M. Predation Risk, Unequal Competitors and the Ideal Free Distribution. Evol. Ecol. Res. 1999, 1, 389–409. [Google Scholar]
- Morris, D.W. How Can We Apply Theories of Habitat Selection to Wildlife Conservation and Management? Wildl. Res. 2003, 30, 303–319. [Google Scholar] [CrossRef]
- Brown, J.S.; Kotler, B.P. Hazardous Duty Pay and the Foraging Cost of Predation. Ecol. Lett. 2004, 7, 999–1014. [Google Scholar] [CrossRef]
- Schoener, T.W.; Schoener, A. Ecological and Demographic Correlates of Injury Rates in Some Bahamian Anolis Lizards. Copeia 1980, 1980, 839–850. [Google Scholar] [CrossRef]
- Losos, J.B. Detective Work in the West Indies : Integrating Historical and Experimental Approaches to Study Island Lizard Evolution. Teach. Biol. 2007, 57, 585–597. [Google Scholar] [CrossRef]
- Kolbe, J.J.; VanMiddlesworth, P.; Battles, A.C.; Stroud, J.T.; Buffum, B.; Forman, R.T.T.; Losos, J.B. Determinants of Spread in an Urban Landscape by an Introduced Lizard. Landsc. Ecol. 2016, 31, 1795–1813. [Google Scholar] [CrossRef]
- Bleicher, S.S. Anole Behavior Meta-Analysis. Mendeley Data 2024, V1, 1. [Google Scholar] [CrossRef]
| Category | Factor | Total | A. carolinensis | A. sagrei | |||||||||
| (N) | DE | HB | PU | N | DE | HB | PU | N | DE | HB | PU | ||
| Species | AC | 26 | 16 | 7 | 3 | ||||||||
| AS | 88 | 42 | 31 | 15 | |||||||||
| Environment | Non-Urban | 36 | 18 | 12 | 16 | 4 | 2 | 2 | ND | 32 | 16 | 10 | 6 |
| Urban | 78 | 40 | 26 | 12 | 22 | 14 | 5 | 3 | 56 | 26 | 21 | 9 | |
| Sex | Female | 15 | 7 | 5 | 3 | 7 | 5 | 2 | ND | 8 | 2 | 3 | 3 |
| Male | 99 | 51 | 33 | 15 | 19 | 11 | 5 | 3 | 80 | 40 | 28 | 12 | |
| Invasive status | Invasive | 65 | 31 | 24 | 10 | ND | ND | ND | ND | 65 | 31 | 24 | 10 |
| Native | 49 | 27 | 14 | 8 | 26 | 16 | 7 | 3 | 23 | 11 | 7 | 5 | |
| Experimental Setup |
Field | 49 | 22 | 20 | 7 | 1 | 1 | ND | ND | 48 | 21 | 20 | 7 |
| Mesocosm | 65 | 36 | 18 | 11 | 25 | 15 | 7 | 3 | 40 | 21 | 11 | 8 | |
| Abbreviations: N- Data collected, DE- Dewlap Extensions, HB – Head-Bobs, PU- Pushups, ND – No Data, AC- Anolis carolinensis, AS – Anolis sagrei | |||||||||||||
| Publication | Species |
Sex |
Display | |||
|---|---|---|---|---|---|---|
| DE | HB | PU | # of data | |||
| Calsbeek & Marnocha, 2006* | AS | M | √ | √ | 4 | |
| Cox et al., 2009* | AS | M | √ | 2 | ||
| Decourcy & Jenssen, 1994 | AC | M | √ | 2 | ||
| Driessens et al., 2014* | AS | Both | √ | √ | 10 | |
| Edwards & Lailvaux, 2012 | AS | M | √ | √ | 4 | |
| Farrell et al., 2016 | AC | M | √ | √ | 4 | |
| Johnson & Wade, 2010* | AC | M | √ | 1 | ||
| Magaña, 2017*‡ | Both | Both | √ | √ | 19 | |
| McMann & Paterson, 2003 | AS | M | √ | 1 | ||
| McMann & Patterson, 2012 | AS | M | √ | √ | 4 | |
| Orrell, 2002‡ | AC | Both | √ | 6 | ||
| Partan et al., 2011* | AS | Both | √ | √ | √ | 6 |
| Paterson, 1999‡ | AS | M | √ | √ | 10 | |
| Paterson, 2002 | AS | M | √ | √ | 6 | |
| Simon, 2002*‡ | AS | M | √ | √ | √ | 10 |
| Simon, 2007* | AS | M | √ | √ | √ | 3 |
| Simon, 2011* | AS | M | √ | √ | 8 | |
| Stroud et al., 2019 | AS | M | √ | √ | 4 | |
| Tokarz & Beck, 1987 | Both | M | √ | 3 | ||
| Tokarz et al., 2002* | AS | M | √ | 1 | ||
| Tokarz et al., 2003 | AS | M | √ | √ | 3 | |
| Tokarz et al., 2005 | AS | M | √ | 1 | ||
| Yang & Wilczynski, 2002* | AS | M | √ | √ | 2 | |
| Total | 111 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




