1. Introduction
To commence, it is crucial to define what is meant by "healthcare technology." The World Health Organization (WHO) offers a comprehensive definition: "devices, drugs, medical and surgical procedures – and the knowledge associated with these – used in the prevention, diagnosis, and treatment of disease as well as in rehabilitation, and the organizational and supportive systems within which care is provided" [
1]. In this paper, however, the term 'healthcare technology' specifically refers to the physical hardware and software components from the WHO definition, explicitly excluding drugs and pharmaceuticals, that require maintenance.
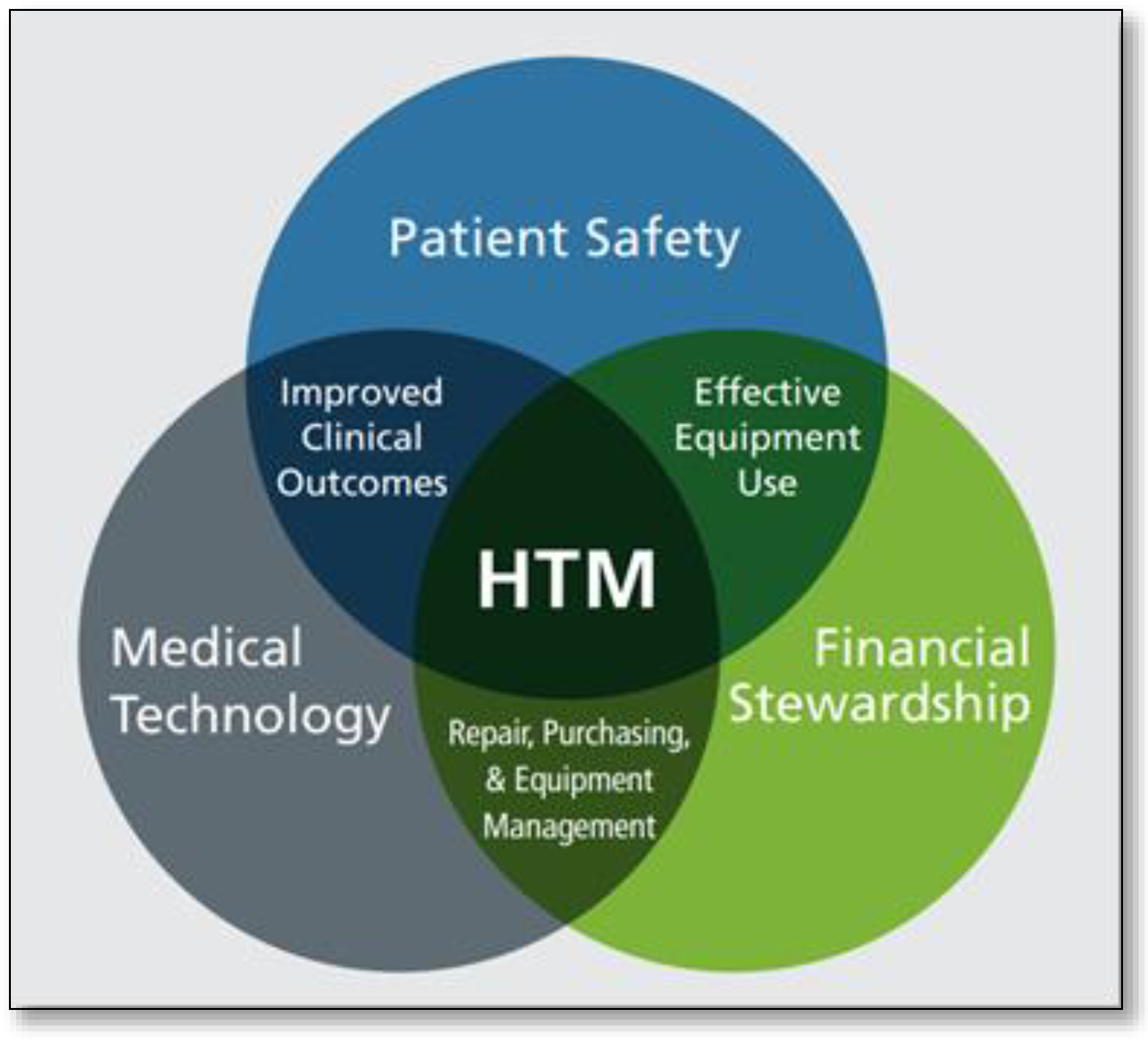
The Association for the Advancement of Medical Instrumentation (AAMI) defines Healthcare Technology Management (HTM) as the discipline dedicated to overseeing the selection, maintenance, and safe utilization of all health technology and medical equipment in healthcare settings [
2]. AAMI has also provided a diagram (
Figure 1) that illustrates how HTM roles and responsibilities intersect within the healthcare environment [
2].
Risk management in healthcare institutions began to take shape in the 1970s in the USA, following court rulings that held hospitals and clinical staff accountable for the quality of care provided. As a result, a formal risk management program became a critical requirement in healthcare facilities around the world and a prerequisite for hospital accreditation. In the Kingdom of Saudi Arabia, implementing a healthcare risk management program is a necessary condition for CBAHI and JCI accreditation [
3].
In their work cited as [
4], the authors pose the question, "What is enterprise risk management (ERM)?" They suggest that the definition of ERM should align with an organization's business model, strategy, culture, and climate, which means it can vary significantly across different organizations. Unlike traditional risk management, which typically focuses on specific risk categories and assesses the impact of isolated risks on a single aspect of organizational health, enterprise risk management provides a comprehensive overview of all risks an organization faces and their interrelationships. This comprehensive approach allows for more strategic planning.
Furthermore, traditional risk management often overlooks the interconnections between risks, which can lead to more efficient resource utilization—including people, money, time, and ideas—reduce redundant efforts, foster innovative thinking, and enhance the early identification and evaluation of risks.
The most appropriate definition of enterprise risk, found in [
4] titled "Enterprise Risk Management—An Analytic Approach" describes it as "the possibility that something, directly or indirectly, will obstruct the achievement of business objectives." Based on [
4], the categories of Enterprise Risk Management (ERM) include:
− Financial
− Operational/Clinical
− Human Capital
− Strategic
− Legal/Regulatory
− Technological
− Natural Disaster/Hazard [
4]
S Taghipour et al. examined 16 factors in their study titled "Prioritization of medical equipment for maintenance decisions," which included: function, mission criticality, utilization, availability of alternative devices, age, total risk, failure frequency, detectability, failure consequence, operational downtime, non-operational, downtime, cost of repair, safety and environment, recalls and hazard alerts, maintenance requirement [
5]. Conversely, Malek Masmoudi et al. examined 13 criteria in their research "Quantitative techniques for medical equipment maintenance management," which included: Degree of complexity of the maintenance, detectability, frequency, safety, downtime, degree of importance of the mission, utilization rate, availability of alternative devices, age, recalls and user errors, classes of equipment, function, risk [
6]. Both studies derived their selection of criteria from the risk assessment method proposed by Fennigkoh and Smith in 1989, which introduced Equipment Management (EM) numbers [
7]. They also considered the subsequent adaptations found in Wang and Levenson's algorithms, which include the Equipment Management Rating (EMR) and the adjusted EMR [
8].
Ron Kaye and Jay Crowley [
9] emphasize that addressing medical device hazards during the development phase can improve the risk management process. They advocate for the inclusion of human factors engineering (HFE) to identify potential hazards effectively. The authors outline the key elements of HFE as follows:
End users (such as patients, family members, physicians, nurses, and professional caregivers),
Typical and atypical uses of the device,
Device specifications,
Characteristics of the environments where the device will be used,
The interactions among users, devices, and their environments. [
9]
John Collins suggests that a more precise assessment of potential physical risks to patients can be achieved by analyzing data from the Food and Drug Administration (FDA) [
10]. By reviewing adverse event reports for each medical device, a risk score can be assigned, according to Collins. The author presents a data-driven method that aims to lower the total risk score and preventive maintenance costs of devices that pose physical risks to patients. Collins also recommends an annual reevaluation of maintenance data along with FDA records for individual hospitals, although he does not specify the analysis tools to be used. His method assigns risk scores annually using historical data from these two sources, but it does not predict future trends. Additionally, Collins' approach includes a modification of the Fennigkoh and Smith scoring protocol based on insights from the FDA data analysis [
10].
Amran et al. introduced a multi-criteria decision-making (MCDM) model that employs quality function deployment (QFD) and fuzzy logic to rank medical devices according to their criticality [
11]. This model was applied to assess customer requirements and the technical characteristics within the House of Quality (HOQ). However, it did not consider factors related to the facility, internal environmental conditions, or external influences.
Abd Rahman et al. developed a predictive model to categorize critical medical devices in healthcare facilities across Malaysia [
12]. The model divides devices into three classes based on their projected failure times: (i) class 1 for devices unlikely to fail within the first three years after purchase, (ii) class 2 for devices likely to fail within three years of purchase, and (iii) class 3 for devices expected to fail more than three years post-purchase, utilizing both machine learning and deep learning techniques [
12]. The analysis incorporated 17 input parameters, including Service support, Asset condition, Service intention of function, Frequency of maintenance requirements, Maintenance complexity, Total downtime, Alternatives and backups, Operations, Maintenance costs, and details specific to the device's manufacturing country, model, manufacturer, and brand . Notably, the study focused solely on factors inherent to the medical devices or their maintenance operations, excluding external or facility-related factors.
Khalaf et al. [
13] proposed a preliminary model, which posits that the operational probability of equipment can be modeled as an exponential function of its age and the time since last maintenance, presents a simplified view that may not hold up under the complex realities of equipment management in practical settings. This model's primary limitation is its reductionist approach, assuming that all factors influencing equipment reliability can be adequately captured merely through age and maintenance intervals. This hypothesis fails to consider the inherent differences between types of equipment, the other critical factors such as the quality of maintenance, the environment in which the equipment is used, operator errors, and manufacturing defects. These factors can significantly impact the actual operational lifetime and reliability of equipment. Furthermore, the model does not account for the strategic and economic implications of maintenance decisions. In many cases, maintenance strategies are influenced by cost considerations, availability of parts, and technological obsolescence, factors that an exponential model based solely on age and maintenance history cannot predict or accommodate.
In their 2021 study, Zamzam et al. examined smart prioritization strategies, focusing on preventive, corrective, and replacement programs [
14]. They developed three robust models that categorize healthcare facilities into low, medium, and high priority groups for better management. The importance of such prioritization is further emphasized in references [
15] and [
16], which discuss the complexity and the numerous interconnected components of most medical equipment. These complexities have direct implications on patient care, underscoring the need for strategically planned maintenance to prevent equipment failures, as detailed in [
16].
In their 2015 study, Jamshidi et al. employed a fuzzy multi-criteria decision-making approach to prioritize medical devices, incorporating a variety of expert opinions and addressing uncertainties by proposing a risk-based framework for device replacement [
17]. This approach utilized a risk-based methodology that assessed both the probability and severity of failures. The framework considered four main factors and seven additional dimensions, including use-related hazards, age, and utilization, to cover all relevant aspects of hazards and risks. These factors were primarily technology-related, in addition to considering the economic impact of monetary losses and safety concerns for patients and workers. However, the study did not encompass strategic, managerial, and facility-related aspects, nor did it consider internal or external environmental factors, as its primary aim was to improve decisions related to device replacement.
In [
18], the authors introduced a mathematical model that issues alerts in an informatics system for medical equipment needing maintenance or replacement. Derived using an event tree, the model evaluates several factors: safety risks to patients or operators, equipment reaching or exceeding its useful life, high maintenance costs, low maintenance levels, unavailability due to catastrophic events, and performance issues caused by user or technical errors.
In [
19], the author analyzed 15 criteria to aid in the maintenance management of medical equipment. However, the assessment did not account for several important aspects. These omitted aspects include facility-specific and internal environmental factors, external influences, training for users, and contractual considerations. Additionally, the management of alerts and recalls was not covered in the criteria.
Previous research in the literature primarily focused on prioritizing risk to improve maintenance management or replacement decisions. This study expands on that approach by considering risk as an integral part of the entire lifecycle of healthcare technology. This broader perspective aims to enhance management decisions across all stages of healthcare technology management. These stages include building the appropriate infrastructure, ensuring the proper maintenance of the healthcare technology infrastructure, collecting reliable information, planning and assessing technology needs, allocating adequate funding, procuring technology, providing necessary resources for usage, operating technology effectively and safely, maintaining and repairing technology, and finally, decommissioning, disposing of, and replacing unsafe or obsolete items. Furthermore, this study also explores additional factors such as contractual aspects, staff training, workforce development, and user experience.
Using only the fuzzy AHP model to assess risk might not capture the unique attributes of each healthcare technology or the differences among healthcare organizations. Additionally, this method could overlook the probability of risk events occurring, which may result in less accurate risk assessments. Thus, there is a vital need to amalgamate the findings from the fuzzy AHP model with the foundational risk equation to enhance the accuracy and comprehensiveness of the risk assessment process.
In this paper, we propose a novel risk equation specifically designed for healthcare technology management (HTM), advancing the classic risk calculation by integrating a more comprehensive view of equipment utilization, as initially suggested by Wang and Levenson (2000). We redefine this utilization concept to include both extremes of equipment use, from overuse to underuse, terming it 'optimal utilization.' The equation leverages global weights derived from the fuzzy Analytic Hierarchy Process (AHP) and readiness assessments from healthcare organizations to establish a refined risk metric. The quantification of the effect of optimal utilization on risk, represented by f(Uopt), will be the focus of a subsequent paper, which aims to empirically validate the model using data from specific healthcare technologies. This advancement holds significant implications for the precision and applicability of risk assessment in HTM. This approach also enhances healthcare technology management effectiveness, reducing resource depletion and contributing to more sustainable healthcare practices.
2. Materials and Methods
Methodologically, the study employed a mixed-methods approach. Initially, a systematic literature review was conducted to identify existing risk factors in healthcare technology management. The reliability and validity of the identified risk factors were further tested through a questionnaire survey, which also explored additional risks not previously documented in the literature. To gain deeper insights, face-to-face interviews were conducted, evaluating the practical applicability of the proposed hierarchical model. Following this, a Fuzzy Analytic Hierarchy Process (Fuzzy AHP) survey was undertaken, and subsequently, a Fuzzy AHP model was developed to prioritize the identified risk factors, thereby enhancing the effectiveness of the risk assessment process. A novel risk equation for healthcare technology management is introduced, to be optimized and empirically validated in our subsequent paper using data from specific healthcare technologies.
2.1. Risk Evaluation Using Fuzzy Analytic Hierarchy Process (Fuzzy AHP)
The structure of this process is divided into five main stages:
Stage 1: Identification of Risk Factors
The process of establishing criteria and sub-criteria for healthcare technology management risks (HTMR) commenced with an extensive review of existing literature. This was complemented by a field survey and interviews with professionals to identify the most pertinent risk factors.
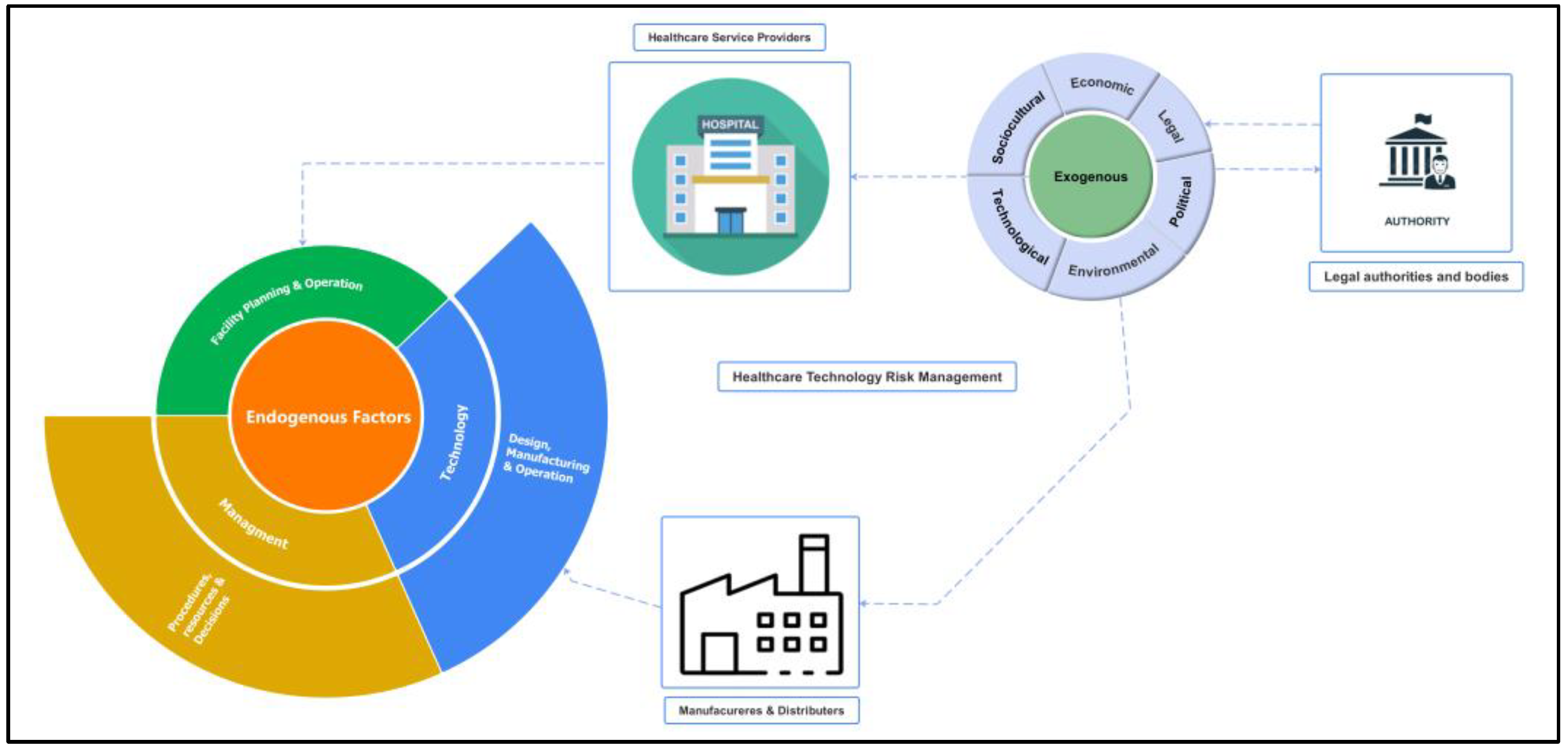
The PESTLE (Political, Economic, Social, Technological, Legal, and Environmental) analysis technique was employed to identify external risk factors affecting healthcare technology management. This approach ensures a comprehensive understanding of the broader macro-environmental influences that can impact HTM performance. By examining each element of the PESTLE framework, this study captures a wide array of external risks, ranging from regulatory changes and economic fluctuations to technological advancements and societal trends. The use of PESTLE analysis in identifying external risks is a well-established practice in the healthcare industry, providing a structured method to anticipate challenges and adapt strategies accordingly. This methodological inclusion enriches the depth of the risk assessment and aligns with industry best practices as referenced in recent healthcare management literature [
20,
21].
From these insights, an Initial Hierarchical Framework of Healthcare Technology Management Risks was constructed (
Figure 2).
Stage 2: Validation of Risk Factors and Development of the Final HTMR Hierarchy
The initially proposed criteria were refined and validated through a survey conducted with HTM professionals from the Kingdom of Saudi Arabia and the Gulf region. An expert panel specializing in HTM was formed to oversee this phase. The impact of different experts on ultimate decisions and results differs, leading to the development of evaluation weighting criteria assigned to each expert based on their job role, qualifications, and professional experience. According to authors in [
22], expert weighting criteria serve as a method to assess the relevance of data considering an expert's organizational position and years of work experience [
22]. The specific details and descriptions of the weighting assigned to each expert evaluation criterion used in identifying respondents are presented in
Table 1. The sample size selected for this phase was deemed suitable based on recommendations from prior studies, which indicate that fewer than ten expert responses are typically adequate for Analytic Hierarchy Process (AHP) studies. This is due to the expected uniformity in expert opinions, which diminishes the necessity for a larger sample [
23,
24].
In
Figure 3, the two lines represent the Mean and the Weighted Average for each risk factor, demonstrating a close alignment between the two metrics. This proximity corroborates the reliability of the expert weighting criteria utilized [
24]. Risk factors with Weighted Average (W avg.) values below 3 were excluded from further analysis due to their lesser significance, and these excluded factors are denoted in Yellow in
Table 2.
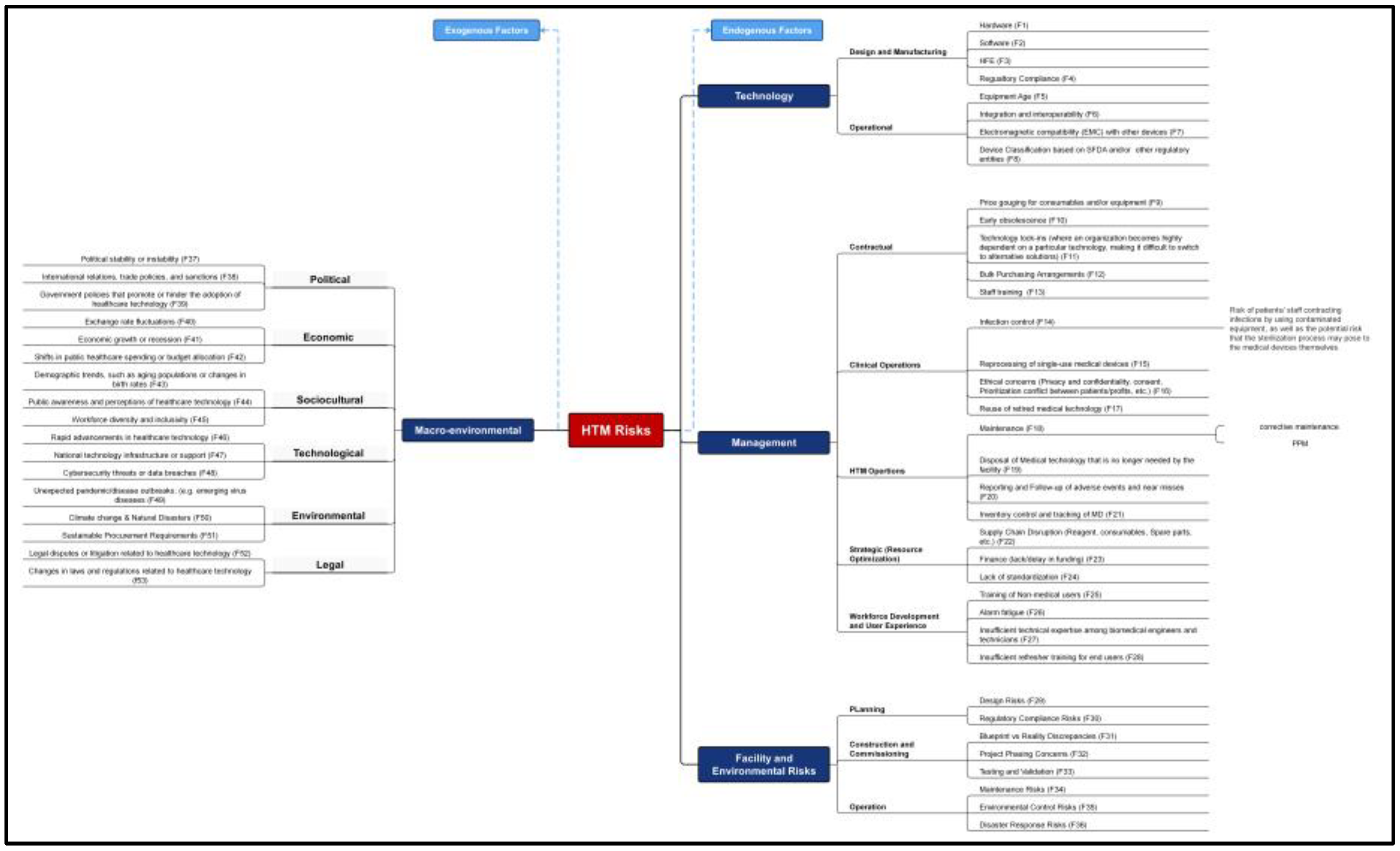
A comprehensive conceptual diagram was also created to offer a detailed view of the various risk sources, including technology, management, facility and environmental, and macroenvironmental factors (
Figure 4). This diagram aims to pinpoint all potential threats that might adversely affect HTM’s performance.
To ensure the thoroughness and accuracy of the identified risks, a qualitative survey was conducted. This survey analyzed the risks to confirm the comprehensiveness and appropriateness of the risk classification method. An initial assessment was also performed to determine the significance of the identified risks, focusing on those experts identified as the most critical.
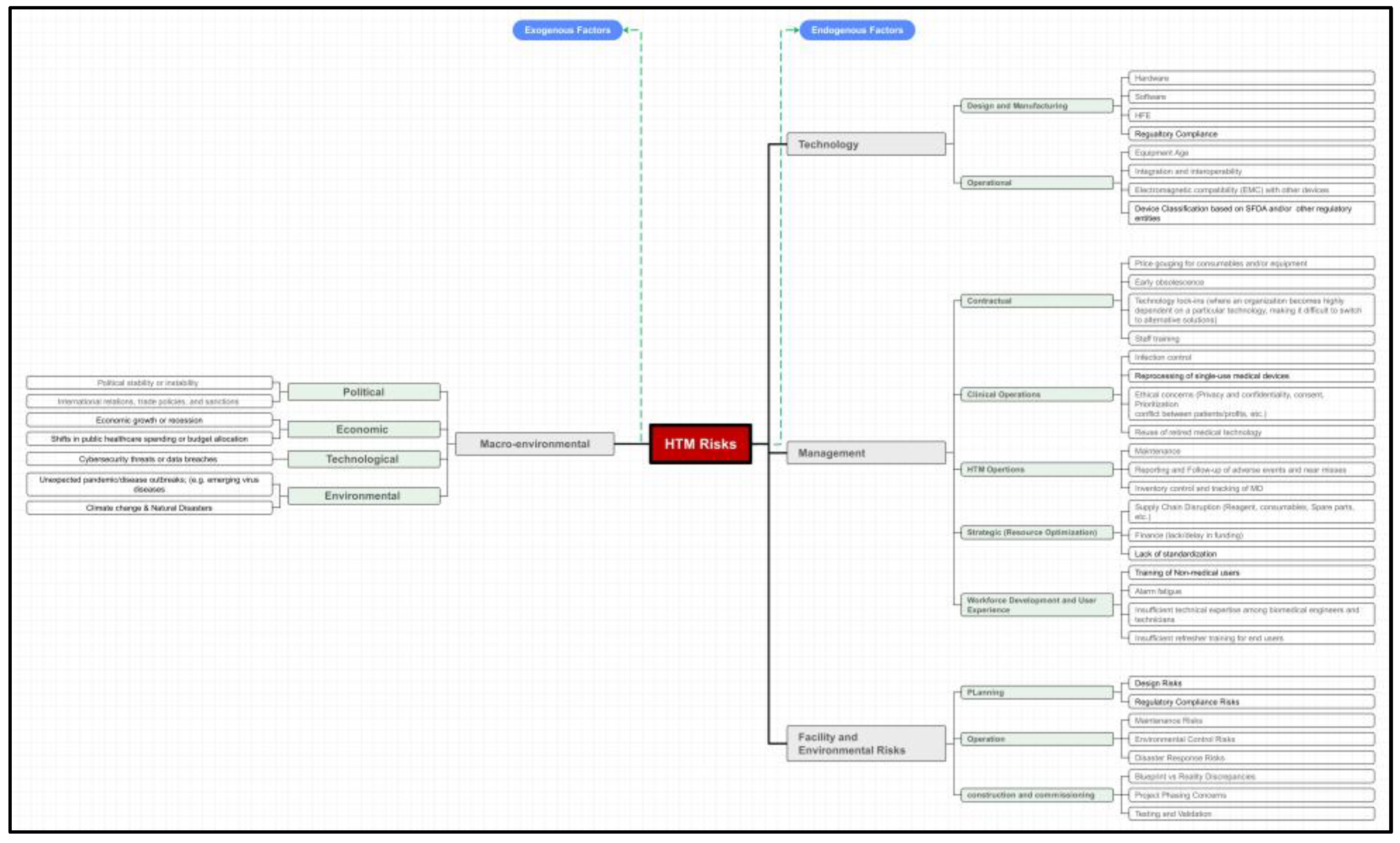
Following this comprehensive review and consultation with experts, a refined HTM risk hierarchy was established (
Figure 5).
The reliability of the conducted survey was assessed using Cronbach's Alpha method. The questionnaire evaluated 289 questions in total, yielding a Cronbach's Alpha value of 0.990. In practical terms, a Cronbach's Alpha value of 0.8 or above is considered indicative of reliable data [
25].
Stage 3: Implementation of Fuzzy AHP Survey
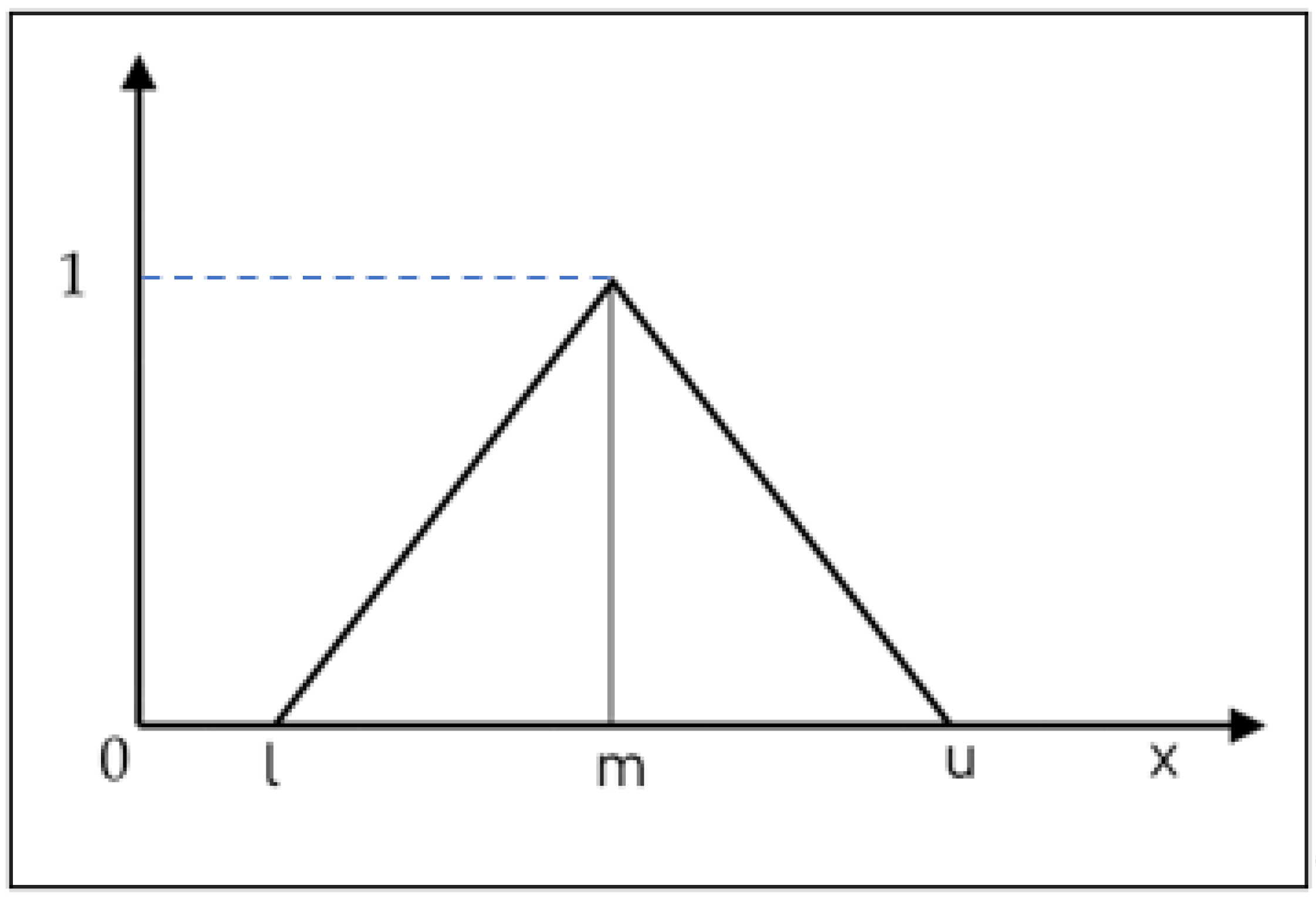
A questionnaire survey utilizing the Fuzzy Analytic Hierarchy Process (AHP) was carried out to capture the expert judgments on the importance of structured risk factors. This assessment was based on predefined linguistic variables. These linguistic variables were subsequently transformed into Triangular Fuzzy Numbers (TFNs) to support pairwise comparisons.
Stage 4: Evaluation and Prioritization of Risk Factors
Using the data gathered, the weights of the risk factors were determined through the experts' judgments and pairwise comparison matrices. The risk factors were then ranked based on their numerical values to underscore their relative importance.
Stage 5: Verifying the reliability of the Proposed Method
To ensure the reliability of the data, a consistency check was conducted on the comparison matrix to confirm the rationality of the experts' judgments and pairwise comparisons. Additionally, a Sensitivity analysis was performed.
2.2. Integrating Fuzzy AHP Outcomes with Foundational Risk Equation and Optimal Utilization Metrics
Relying solely on the outcomes of the fuzzy AHP model to measure risk may overlook the unique characteristics of each healthcare technology and the variations among healthcare organizations. Additionally, this approach might omit the crucial aspect of the probability of a risk event occurring, which could result in inaccuracies in risk assessment. Therefore, there is a critical need to integrate the results from the fuzzy AHP model with the foundational risk equation. This integration should include the concept of optimal utilization as outlined by Wang and Levenson (2000). By incorporating a function f(Uopt) that reflects optimal utilization, we can more accurately assess the likelihood that current levels of equipment usage might lead to risk events. This approach ensures a more comprehensive and precise quantification of risk, tailored to the specific conditions and usage patterns of healthcare technology within different organizational contexts.
The derivation of the proposed equation, which modifies the foundational risk equation for specific applications, is outlined in the results section of this paper
4. Discussion and Conclusion
This research represents the first comprehensive study of HTM risk factors in the Kingdom of Saudi Arabia and the Gulf region, and it is the inaugural research to employ the PESTEL framework for a thorough examination of all external risk factors and their influence on HTM performance. The approach taken in this study is entirely innovative, as previous research primarily relied on the Fennigkoh and Smith (1989) mathematical model or its modified version by Wang and Levenson (2000). Some variations of the same classifications have been explored in [
5,
6,
11]. Previously, studies focused on assessing medical device risk have predominantly concentrated on internal maintenance factors, overlooking the importance of incorporating broader external elements into risk analysis. Furthermore, the scope of these studies was often restricted to improving medical device maintenance strategies, which is seen as a limitation since it does not cover the wider spectrum of healthcare technology management.
In this study, the evaluation of risk factors in healthcare technology management (HTM) reveals a broad spectrum of concerns that span from highly technical issues to macro-environmental influences.
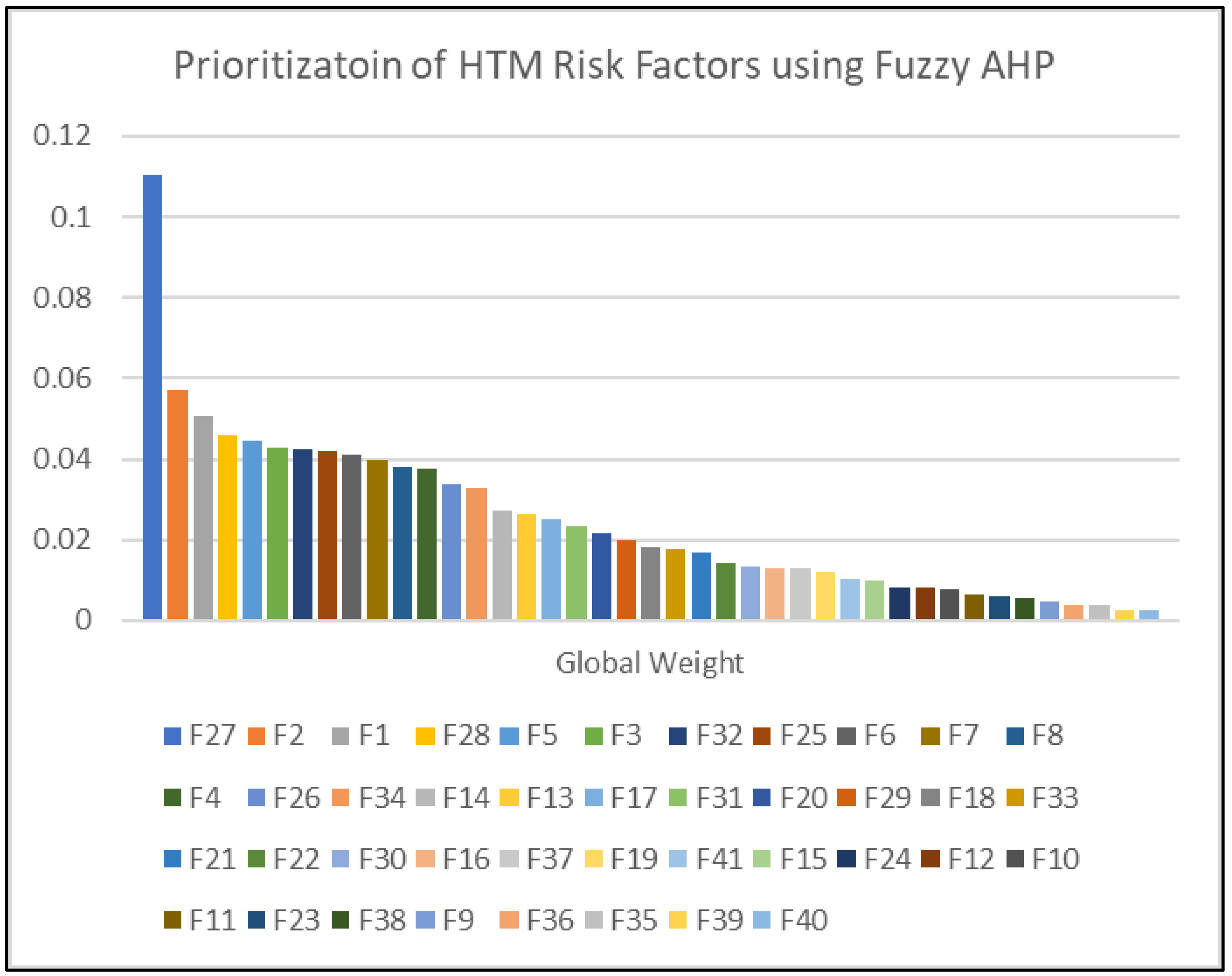
Using the Fuzzy AHP model, the top 20 risk factors identified based on their global weights are:
Design Risks (Global Weight: 0.110548984) - Under Facility and Internal Environmental Risks in planning.
Software Risks (Global Weight: 0.057322275) - Under Technology Risks in design and manufacturing.
Hardware Risks (Global Weight: 0.050745647) - Under Technology Risks in design and manufacturing.
Regulatory Compliance related to Facility Design (Global Weight: 0.045956915) - Under Facilities and Internal Environmental Risks in planning.
Equipment Age (Global Weight: 0.044375066) - Under Technology Risks in the operational category.
Human Factor Engineering (Global Weight: 0.042752409) - Under Technology Risks in design and manufacturing.
Maintenance Risks (Global Weight: 0.042342317) - Under Facility and Internal Environmental Risks.
Insufficient Technical Expertise (Global Weight: 0.041855872) - Among biomedical engineers and technicians under Management in workforce development.
Integration and Interoperability (Global Weight: 0.040933724) - Under Technology Risks in the operational category.
Electromagnetic Compatibility (Global Weight: 0.039631732) - With other devices under Technology Risks in operational category.
Device Classification (Global Weight: 0.038091717) - Based on regulatory entities Under Technology Risks in operational category.
Regulatory Compliance (Global Weight: 0.03760016) - Under Technology Risks in design and manufacturing.
Insufficient Refresher Training (Global Weight: 0.03359518) - For end users under Management in workforce development.
Disaster Response Risks (Global Weight: 0.032762298) - Under Facility and Internal Environmental Risks in operation category.
Reprocessing of Single-Use Devices (Global Weight: 0.027418526) - Under Management risks in Clinical operations.
Infection Control (Global Weight: 0.026351732) - Under Management risks in Clinical operations.
Maintenance (Global Weight: 0.025177756) - Under management Risks in HTM Operations.
Testing and Validation (Global Weight: 0.023179116) - Under Facility and Internal Environmental Risks in construction and commissioning.
Supply Chain Disruption (Global Weight: 0.021573761) - Under Management risks in strategic risks category.
Blueprint vs Reality Discrepancies (Global Weight: 0.020009202) - Under Facility and Internal Environmental Risks in construction and commissioning.
These top 20 risks reflect a mix of technological, operational, managerial, and Facility and Internal Environmental challenges that require comprehensive and proactive management strategies to mitigate their impact.
The most critical risk identified is Design Risks under the Facilities and Internal Environmental Risks sub-criteria of planning, holding the highest global weight of 0.110548984. This suggests that design flaws or inadequacies in facility planning can significantly jeopardize healthcare operations, emphasizing the need for meticulous design processes that consider both current needs and future adaptability.
Software risks and hardware risks under Technology Risks in the design and manufacturing category follow, highlighting the pivotal role of technological reliability and integration in modern healthcare. These elements are crucial for ensuring that medical devices and software systems function seamlessly and support rather than hinder healthcare delivery.
Interestingly, Regulatory Compliance related to facility design also ranks highly, underscoring the importance of adhering to established standards and regulations to mitigate legal and operational risks. The focus on regulatory compliance reflects an acute awareness of the potential repercussions of non-compliance, including penalties and compromised patient safety.
Another significant area of concern is the aging of equipment under Technology Risks in the operational category. As medical equipment ages, it often requires more frequent maintenance, may become incompatible with newer technologies, or fail to meet the latest clinical standards, thus posing risks to patient safety and healthcare efficiency. Human Factor Engineering (HFE) also emerges as a critical consideration, indicating a growing recognition of the need to design medical technology and environments that are intuitive and minimize the potential for human error. This factor is essential in enhancing overall safety and effectiveness in healthcare settings.
Among the various categories evaluated for risk in healthcare technology management, macro-environmental risks capture external factors that can significantly influence healthcare operations. These are often beyond the direct control of healthcare facilities but are crucial for strategic planning. Macro-environmental factors such as cybersecurity threats, shifts in public healthcare spending, political stability, and unexpected pandemics have broader implications that transcend individual healthcare facilities. For example, cybersecurity threats, while ranked 27th in terms of global weight, pose significant risks to the integrity of healthcare data and the privacy of patient information. Similarly, shifts in public healthcare spending or budget allocations directly influence the financial resources available for upgrading technology, conducting training, and maintaining equipment.
Political instability can lead to changes in healthcare policy and funding, which may disrupt service provision and delay the implementation of crucial healthcare projects. The recent global experience with the COVID-19 pandemic underscores the dramatic impact that sudden health crises can have on the demand for medical services, supply chains, and the overall capacity of healthcare systems.
The relatively lower ranking of these macro-environmental factors in our study does not diminish their importance. Instead, it highlights the need for healthcare administrators to maintain a dual focus: managing immediate and direct risks associated with healthcare operations and technology, while also developing strategies to mitigate the effects of broader environmental and systemic challenges. Effective risk management in healthcare must include preparedness and resilience planning to address these macro-environmental risks, which, though they may seem less immediate, can cause significant disruptions.
To effectively mitigate the top risk factors identified in healthcare technology management (HTM), a focused approach is necessary, one that prioritizes both immediate risk reduction and the long-term sustainability of healthcare systems. It starts with robust design and planning to mitigate design risks and ensure compliance from the outset. A crucial aspect is updating and upgrading technology, especially software and hardware, to handle risks associated with aging equipment, which can often be resolved through a well-considered replacement strategy.
Equally important is a well-structured preventive maintenance strategy that includes regular checks and proactive measures to maintain operational efficiency and ensure system integration. Continuous training and professional development are essential to improve the use of healthcare technology and address gaps in technical expertise.
Moreover, developing comprehensive disaster response plans and strengthening supply chain resilience are key for managing unforeseen disruptions and sustaining operations. By embedding these strategies into a dynamic risk management framework that evolves with new challenges, healthcare facilities can greatly improve the safety and efficiency of their HTM systems.
In the early stages of this study, some risks were assessed as less significant and therefore removed from the pairwise comparison matrix. These excluded risks encompassed Bulk Purchasing Arrangements, the Disposal of Medical Technology no longer needed by the facility, and certain macro-environmental factors, particularly those related to sociocultural and legal dimensions Figure (2).