1. Introduction
Neovascular age-related macular degeneration (nAMD) is the leading cause of permanent blindness among the elderly in industrialized nations [
1]. Vascular endothelial growth factor (VEGF) plays a crucial role in the regulation of macular neovascularization (MNV) and vascular permeability [
2].
The advent of anti-VEGF therapies has significantly reduced both the prevalence and severity of visual loss in patients with nAMD, and has greatly improved their visual outcomes [
3]. However, some patients are resistant to anti-VEGF treatment, and a number of them show resistance even after receiving monthly injections consecutively [
4,
5]. In the VIEW1/VIEW2 trials, active exudation persisted in approximately 19.7% and 36.6% of patients who received aflibercept (Eylea
®®, Regeneron Pharmaceuticals, Tarrytown, NY, USA) treatment every 4 and 8 weeks for one year, respectively [
6]. Hence, efforts have been made to improve efficacy and extend the duration of the intravitreal injections.
Brolucizumab (Beovu
®®, Novartis International, Basel, Switzerland), a humanized single-chain antibody fragment targeting VEGF is the smallest among commercially available anti-VEGF agents, enabling administration at higher molar doses [
7,
8]. In two prospective randomized phase 3 trials (HAWK and HARRIER) involving treatment-naïve nAMD patients, brolucizumab demonstrated noninferior visual outcomes and superior anatomical outcomes compared to aflibercept [
8,
9]. Faricimab (Vabysmo
®®, Roche/Genentech, Basel, Switzerland) acts through dual inhibition of angiopoietin-2 (Ang-2) and VEGF-A [
10]. According to the TENAYA and LUCERNE clinical trials, the improvement in best-corrected visual acuity (BCVA) in eyes treated with 6 mg of faricimab was comparable to that in eyes treated with 2 mg of aflibercept [
11].
Many prospective and retrospective, noncomparative studies have been conducted on patients who were switched to a different anti-VEGF agent after not responding to their current treatment regimen [
12,
13,
14,
15,
16,
17,
18,
19,
20]. However, there is currently no consensus on the most effective treatment for aflibercept-resistant nAMD. To our knowledge, the outcomes of brolucizumab versus faricimab for aflibercept-resistant nAMD have not been compared. Therefore, this study aimed to directly compare the short-term visual and structural outcomes of intravitreal brolucizumab versus faricimab treatment for aflibercept-resistant nAMD in a clinical setting.
2. Materials and Methods
2.1. Study Population
This study was approved by the Ethics Committee of Fuchu hospital (ID number: 2024009) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was not obtained because the study was retrospective. The need for informed consent is waived by the Ethics Committee of Fuchu eye center. The eye center website provided participants with an opportunity to opt out of the study. This was a retrospective, single-center, observational study. This study included 20 eyes of 20 patients switched to brolucizumab and 15 eyes of 14 patients switched to faricimab for aflibercept-resistant nAMD. All patients received the treatment from January 2022 until April 2024, and followed for three months after the switch.
2.2. Intravitreal Injection
Aflibercept 2.0 mg/0.05 mL, brolucizumab 6.0 mg/0.05 mL or faricimab 6.0 mg/0.05 mL was injected into the vitreous cavity of all patients. After instillation of the topical anesthetic (0.4% oxybuprocine hydrochloride; Benoxil™︎), all injections were conducted in a procedure room following the standard aseptic intravitreal technique. Aflibercept, brolucizumab or faricimab was injected into the vitreous cavity using the standard pars plana approach (3.5 mm posterior to the limbus) with a 30-gauge needle under sterile conditions. Following the treatment, patients were prescribed 0.5% MFLX ophthalmic solution (Vigamox™︎; Alcon Japan Ltd., Tokyo, Japan) for 3 days.
2.3. Inclusion Criteria
The inclusion criteria were as follows: (1) age over 40 years, (2) a diagnosis of MNV due to nAMD, (3) recurrence of any fluid within 16 weeks after IVA, (4) switched to brolucizumab or faricimab, and (5) three months of observation after switching.
2.4. Exclusion Criteria
The exclusion criteria were as follows: (1) history of treatments with photodynamic therapy, (2) any other retinal or optic nerve disease, and (3) presence of inflammatory or hereditary diseases that may induce MNV.
2.5. Outcomes
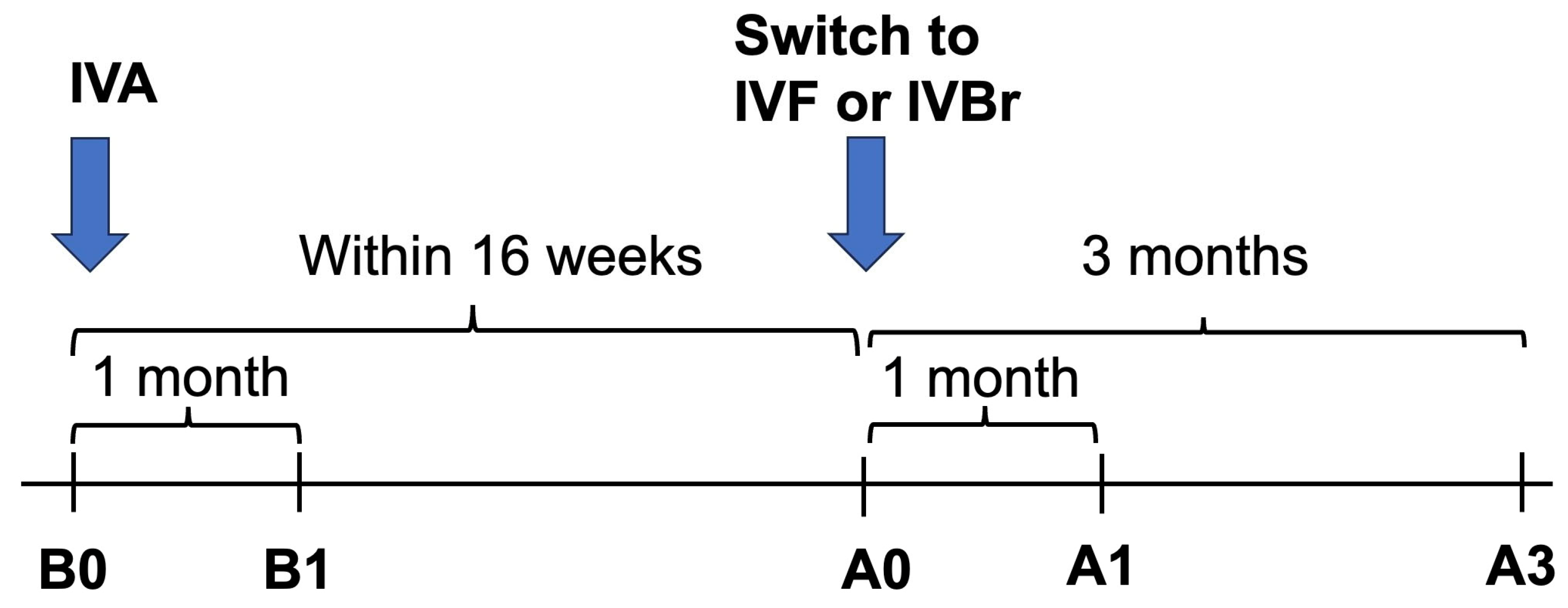
We measured the structural outcome (Central macular thickness (CMT)) and the visual outcome (BCVA) at five different time points: just before (B0), one month after the most recent IVA injection (B1), just before (A0), one month after (A1) and three months after (A3) the first IVBr or IVF injection as shown in
Figure 1.
Outcome measures included:
BCVA measured using the Landolt chart was converted into logarithm of the minimum angle of resolution (logMAR) values for statistical analyses;
CMT (μm) as measured with optical coherence tomography (OCT) (DRI OCT Triton, Topcon Inc., Tokyo, Japan) and defined as the distance from the internal limiting membrane to Bruch’s membrane at the fovea. For some cases who were not imaged by DRI OCT Triton, Cirrus high-definition-OCT (HD-OCT, Carl Zeiss Meditec Inc., Tokyo, Japan) was used.
2.6. Statistical Analyses
For statistical analyses, the baseline characteristics were compared using the unpaired t-test and Fisher’s exact tests. Wilcoxon signed-rank test was used to determine the significance of the difference between the values before and after switching. Unpaired t-test was used to compare the changes in BCVAs and CMTs at each timepoint between the two groups. Descriptive statistics were used to describe the sample in terms of mean and standard deviation (SD). p < 0.05 were considered statistically significant in all analyses. All analyses were performed using JMP Pro 17 software (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patients’ Characteristics
The baseline patient characteristics and clinical data are presented in
Table 1. In the IVBr group, the mean age was 77.00 ± 5.81, and 7 patients were women (35.00%). In the IVF group, the mean age was 74.87 ± 9.49, and 5 patients were women (33.33%). There were no statistically significant differences between the two groups for baseline data. The number of injections administered between the baseline switch (A0) and the three-month visit (A3) was 1.95 ± 0.78 in the IVBr group and 1.80 ± 0.77 in the IVF group (
p = 0.3539).
3.2. BCVA outcomes
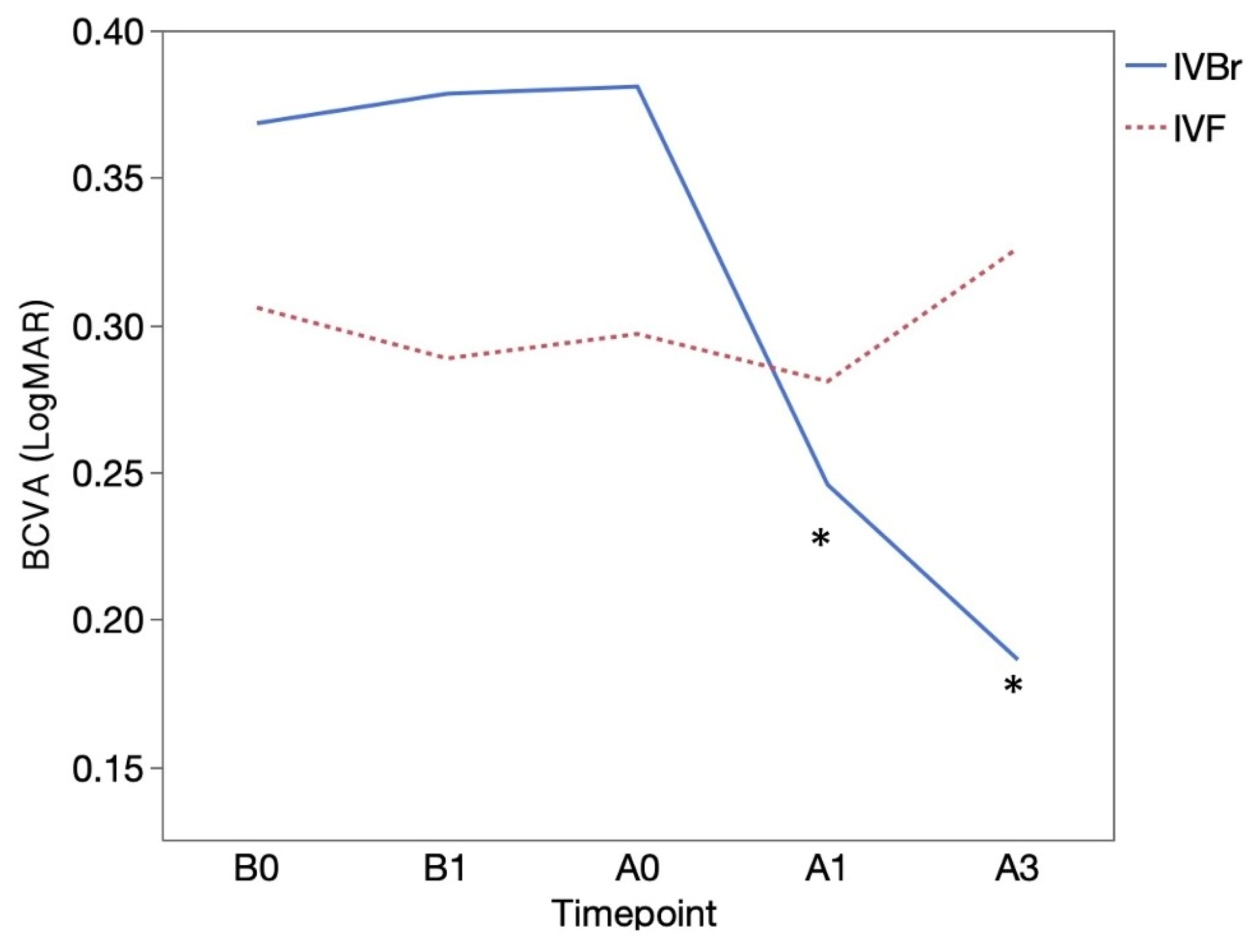
BCVA (logMAR) was 0.37 ± 0.35 at B0 and 0.38 ± 0.35 at B1 in the IVBr group, 0.31 ± 0.36 at B0 and 0.29 ± 0.37 at B1 in the IVF group and the BCVA before switching treatments (between B0 and B1) showed no significant difference in both groups (
p > 0.05 for all). BCVA showed significant improvement at A1 (0.25 ± 0.34) and at A3 (0.19 ± 0.24) than at A0 (0.38 ± 0.35) (
p = 0.0156,
p = 0.0166, respectively) in the IVBr group. In the IVF group, BCVA at A1 (0.28 ± 0.42) and at A3 (0.33 ± 0.34) showed no significant improvement compared to at A0 (0.30 ± 0.36) (
p > 0.05 for all) (
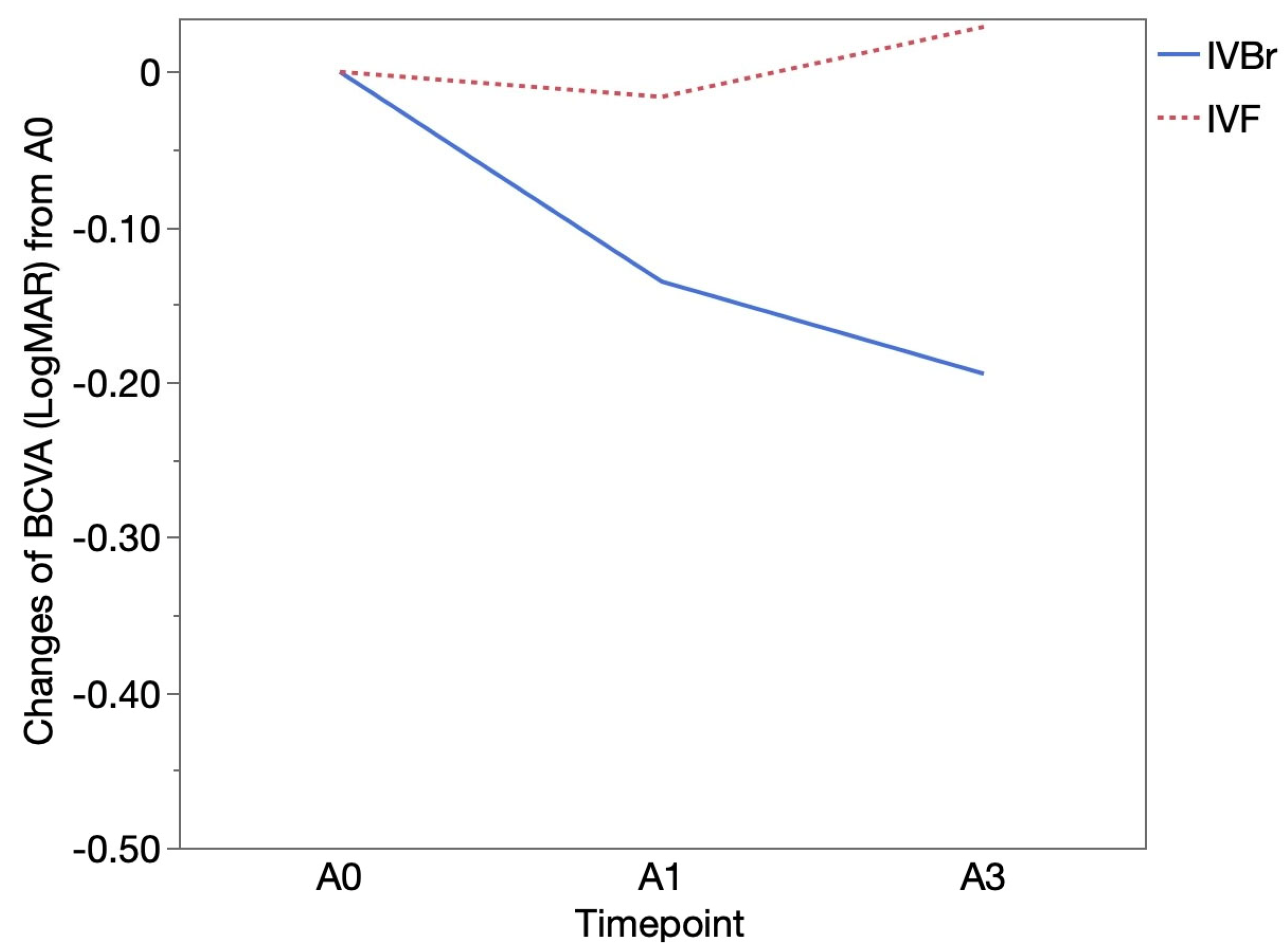
Figure 2). There was no significant difference in BCVA improvement observed at either A1 or A3 between two groups (
p > 0.05 for all) (
Figure 3).
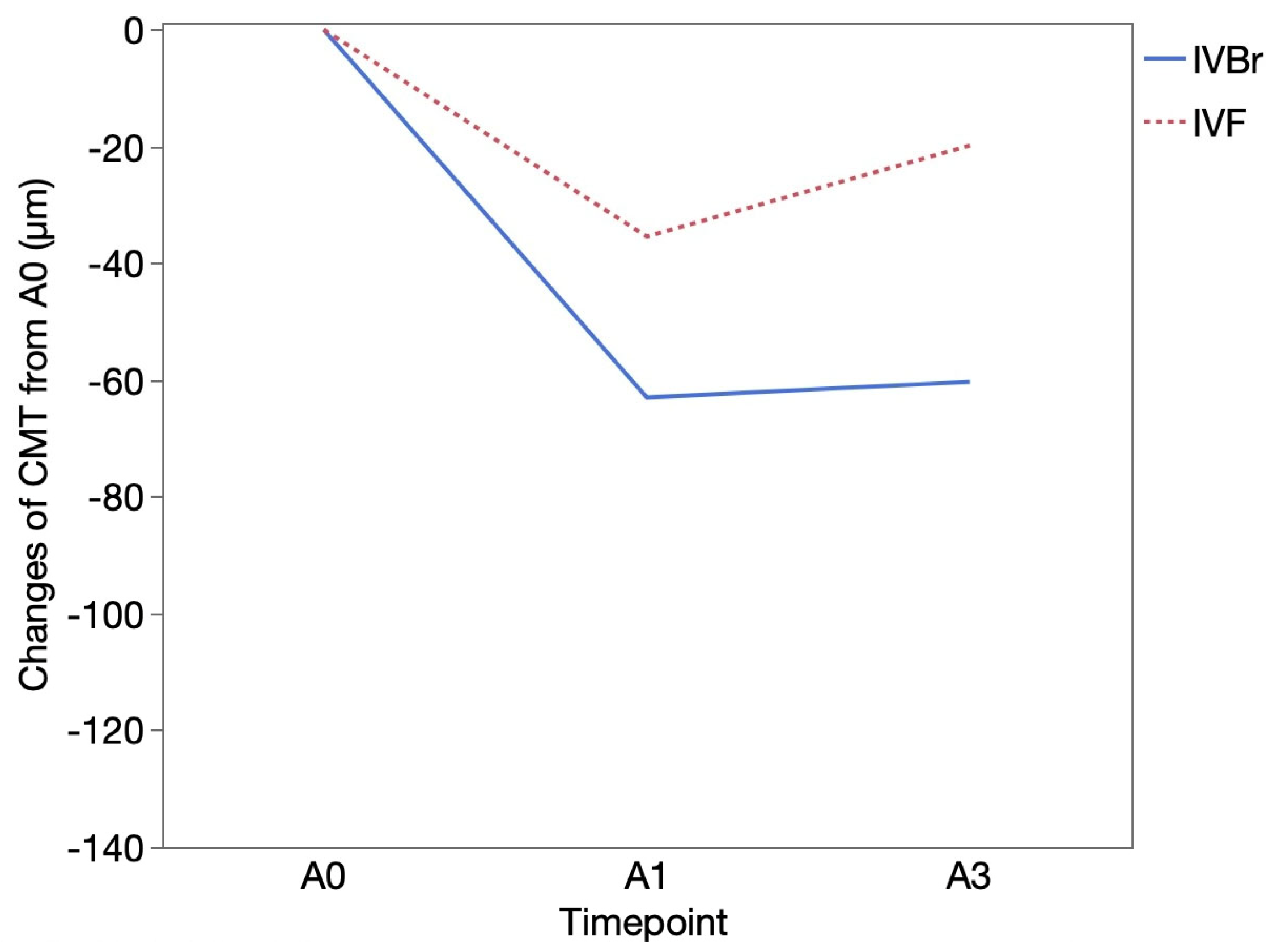
3.3. CMT outcomes
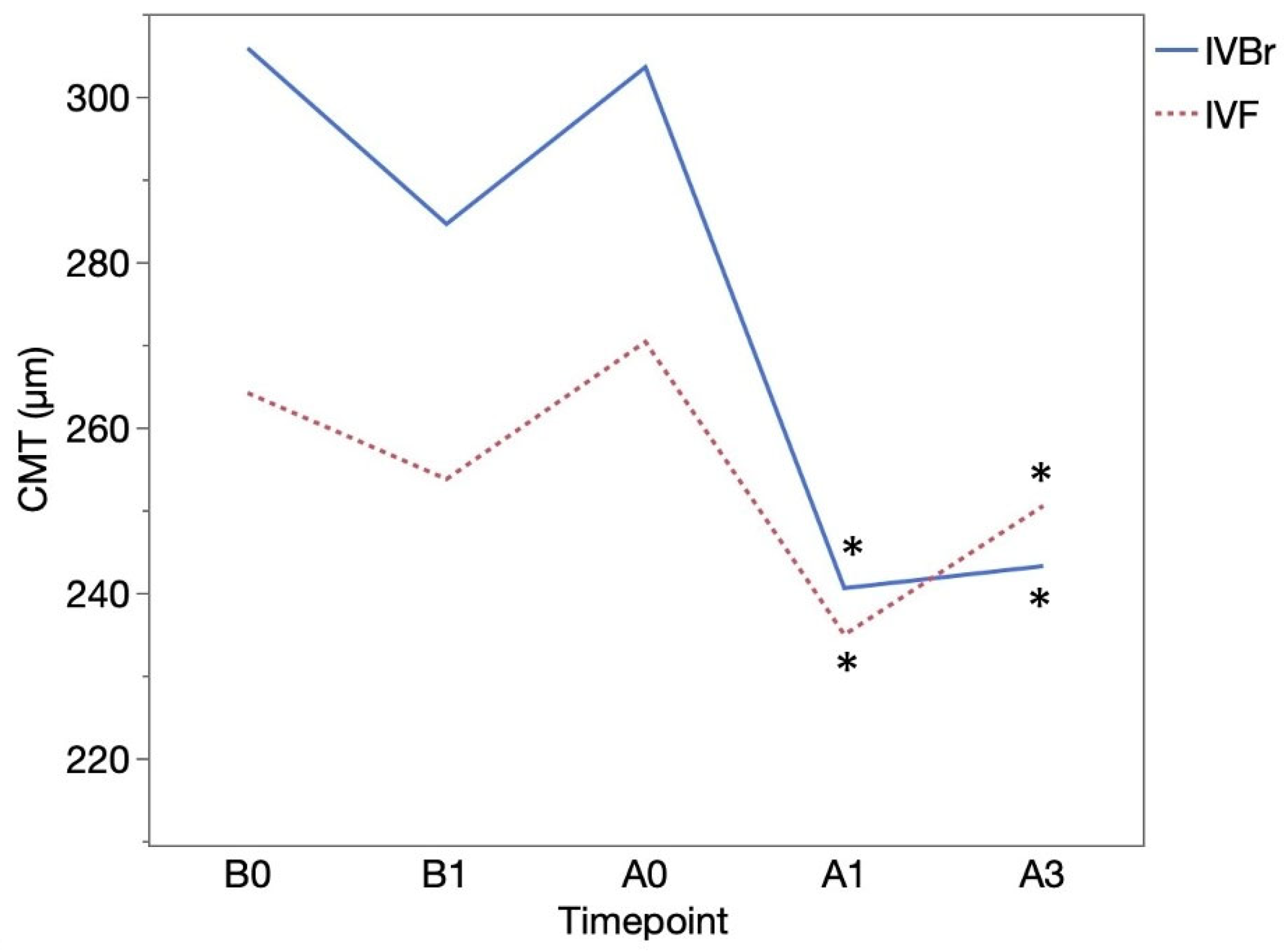
CMT (μm) was 305.85 ± 110.29 at B0 and 284.58± 75.11 at B1 in the IVBr group (
p = 0.1034), 264.15 ± 103.72 at B0 and 253.73 ± 64.00 at B1 in the IVF group (
p = 0.3370). CMT showed significant improvement at A1 (240.55 ± 51.82) and at A3 (243.21 ± 76.15) than at A0 (303.55 ± 79.18) (
p = 0.0093,
p = 0.0026, respectively) in the IVBr group. In the IVF group, CMT also showed significant improvement at A1 (234.91 ± 47.29) and at A3 (250.50 ± 72.61) than at A0 (270.33 ± 77.62) (
p = 0.0161,
p = 0.0093, respectively) (
Figure 4). There was no significant difference in CMT improvement observed at either A1 or A3 between two groups (
p > 0.05 for all). (
Figure 5).
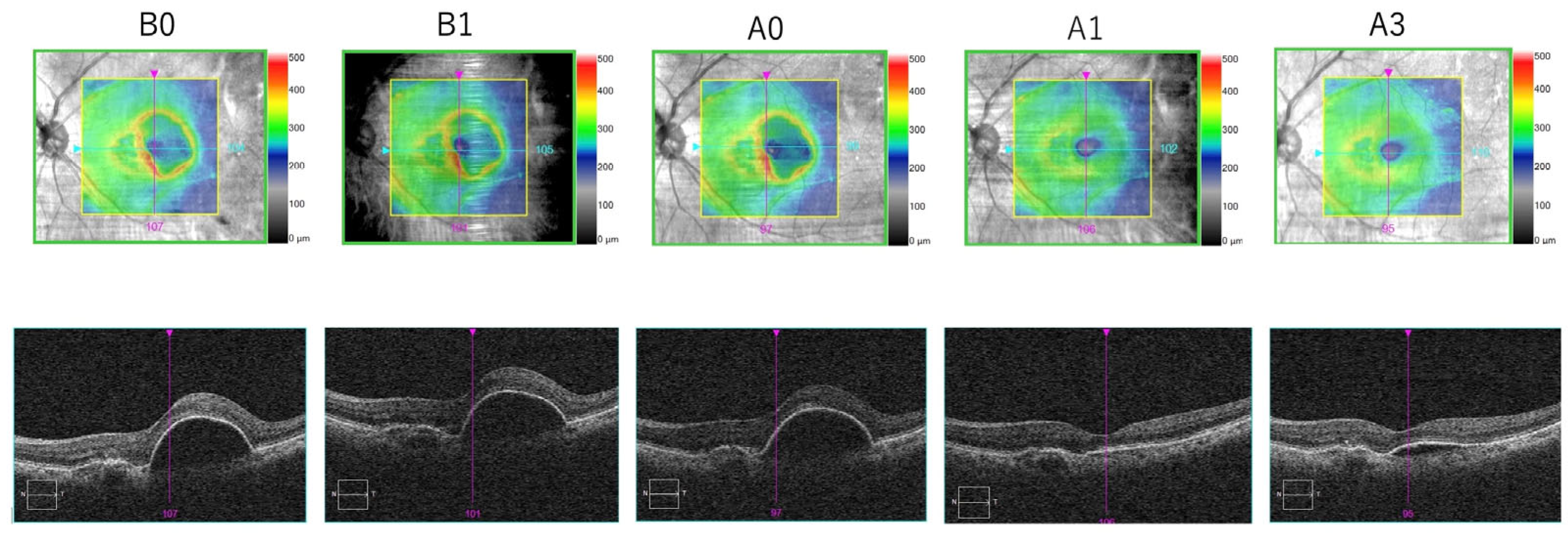
3.4. Representative Case
Figure 6 shows the follow-up of one of the patients included in our study.
3.5. Safety
No endophthalmitis, occlusive vasculitis, intraocular inflammation (IOI) or other ocular adverse event were seen, and no systemic adverse events were noted following the treatment switch.
4. Discussion
In the present study, we retrospectively evaluated the effects before and after switching to either brolucizumab or faricimab in aflibercept-resistant nAMD in a clinical setting. The current findings demonstrated that both IVBr and IVF improved structural outcome in aflibercept-resistant nAMD patients. No significant differences in visual and structural outcomes were observed between both groups throughout the follow-up period. To our knowledge, this report is the first to directly compare the outcomes of brolucizumab versus faricimab for aflibercept-resistant nAMD in routine clinical practice.
In this study, although there were no significant differences, the IVBr group tended to show a greater reduction in CMT and greater improvement in BCVA compared to the IVF group. Similar to our study, Maruyama-Inoue et al. compared functional and morphologic changes in the loading phase between patients with treatment-naïve nAMD treated with either brolucizumab or faricimab [
21]. They showed brolucizumab had greater reductions of the central foveal thickness than the faricimab and also brolucizumab caused a trend toward faster visual improvements in the BCVA. Brolucizumab appears to be advantageous when compared to faricimab in improving structural and visual outcomes both in treatment-naïve and aflibercept-resistant nAMD. Despite of structural improvement, no significant improvement in visual outcomes was reported when switched to faricimab in short-term follow-up, which was consistent with our study [
16,
18,
22,
23]. In these studies, photoreceptor damage at the time of switch was thought to be the reason for suboptimal improvement in BCVA. However, we found significant BCVA improvement when switched to brolucizumab. The rapid improvement in BCVA observed in the IVBr group may be attributed to the differences in mechanisms of action, molecular weight and VEGF affinity between the two agents as mentioned in previous report [
21]. Brolucizumab is a humanized single-chain antibody fragment that binds all the isoforms of VEGF-A while faricimab is a bispecific molecule bound to an optimized Fc fragment that binds both VEGF-A and Ang-2 [
24]. Brolucizumab, with a molecular weight of 26 kDa, is smaller than faricimab, which has a molecular weight of 146 kDa, potentially allowing for a higher drug concentration per injection [
7,
10]. This potentially enhances tissue penetration and increases its effectiveness compared to other agents. Additionally, the single-chain anitibody fragment of brolucizumab enables its full binding capacity to VEGF. As a result, a higher number of molecules can be administered per injection within the same volume, leading to increased bioavailability. This may account for the greater reduction in CMT observed in the IVBr group compared to the IVF group. Faricimab helps restore vascular stability and promote vessel maturation by inhibiting Ang-2 [
10,
11]. Although a three-month period may have been insufficient for faricimab to fully demonstrate its potential, both treatments achieved significant reduction in CMT compared to baseline at 1 and 3months.
Previous studies suggest that some patients require three monthly intravitreal injections to control retinal exudate, even after switching to a different anti-VEGF drug [
16,
20]. However, in our studies, the IVBr group received 1.95 ± 0.78 injections and the IVF group received 1.80 ± 0.77 injections within 3 months after the switch, which may not be sufficient. Further studies are needed to determine whether the loading dose regimen leads to additional improvements in structural and visual outcomes in aflibercept-resistant nAMD patients.
IOI has become a clinical concern following the approval of brolucizumab for nAMD by the U.S. Food and Drug Administration in October 2019. The HAWK/HARRIER study reported that the incidence of brolucizumab-associated definite/probable IOI was 4.6% [
25]. In this study, there was no complications such as IOI and RPE tears in both groups.
This study had several limitations. First, this study only evaluated a short period (3 months after the switch). Second, although no substantial differences were observed between the two groups in the number of injections within 3 months after the switch, the number of injections was determined by each physician and was not consistent. Third, since this study was retrospective, further research and a large prospective study are needed to validate the present conclusions.
5. Conclusions
Switching from aflibercept to either brolucizumab or faricimab has a significant anatomical effect on eyes with aflibercept-resistant nAMD and both treatments appear to be effective short-term treatment options. There is a trend towards greater visual improvements and reductions in CMT with brolucizumab.
Author Contributions
Conceptualization, A.K. and T.M.; Methodology, A.K., T.M., S.U. and S.M.; Formal Analysis, A.K. and T.M.; Investigation, A.K. and T.M.; Data Curation, A.K., T.M. and S.U.; Writing – Original Draft Preparation, A.K. and T.M.; Writing – Review & Editing, S.U. and S.M.; Supervision, S.M. and Y.S.; Project Administration, Y.S. All the authors have read and approved the final version of the manuscript. K.A. and T.M. contributed equally to this work.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Fuchu Hospital Ethical Committee (Izumi, Osaka, Japan) (Approval number: 2024009. Date of approval: June 20th, 2024).
Informed Consent Statement
Given the retrospective nature of this study, the requirement for informed consent was waived and this was approved by ethic committee. The eye center website provided participants with an opportunity to opt out of the study.
Data Availability Statement
The data used and analyzed for this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Andreoli CM, Miller JW. Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol. Nov 2007;18(6):502-8. [CrossRef]
- Chappelow AV, Kaiser PK. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68(8):1029-36. [CrossRef]
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. Oct 5 2006;355(14):1419-31. [CrossRef]
- Fu Y, Zhang Z, Webster KA, Paulus YM. Treatment Strategies for Anti-VEGF Resistance in Neovascular Age-Related Macular Degeneration by Targeting Arteriolar Choroidal Neovascularization. Biomolecules. 2024. [CrossRef]
- Mettu PS, Allingham MJ, Cousins SW. Incomplete response to Anti-VEGF therapy in neovascular AMD: Exploring disease mechanisms and therapeutic opportunities. Prog Retin Eye Res. 21;82:100906. 20 May. [CrossRef]
- Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. Dec 2012;119(12):2537-48. [CrossRef]
- Tadayoni R, Sararols L, Weissgerber G, Verma R, Clemens A, Holz FG. Brolucizumab: A Newly Developed Anti-VEGF Molecule for the Treatment of Neovascular Age-Related Macular Degeneration. Ophthalmologica. 2021;244(2):93-101. [CrossRef]
- Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. Jan 2020;127(1):72-84. [CrossRef]
- Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: Ninety-Six-Week Outcomes from the Phase 3 Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. Jan 2021;128(1):89-99. [CrossRef]
- Shirley, M. Faricimab: First Approval. Drugs. 22;82(7):825-830. 20 May. [CrossRef]
- Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. Feb 19 2022;399(10326):729-740. [CrossRef]
- Kawashima Y, Oishi A, Tsujikawa A, et al. Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. Sep 2015;253(9):1471-7. [CrossRef]
- Waizel M, Todorova MG, Masyk M, et al. Switch to aflibercept or ranibizumab after initial treatment with bevacizumab in eyes with neovascular AMD. BMC Ophthalmol. 2017;17(1):79. 23 May. [CrossRef]
- Rush, RB. One-Year Outcomes of Faricimab Treatment for Aflibercept-Resistant Neovascular Age-Related Macular Degeneration. Clin Ophthalmol. 2023;17:2201-2208. [CrossRef]
- Takahashi H, Inoda S, Takahashi R, et al. One-year visual and anatomical outcomes of intravitreal faricimab injection for neovascular age-related macular degeneration after prior brolucizumab treatment. Sci Rep. Apr 20 2024;14(1):9087. [CrossRef]
- Raimondi R, Falfeli T, Bogdanova-Bennet A, et al. Outcomes of Treatment-Resistant Neovascular Age-Related Macular Degeneration Switched from Aflibercept to Faricimab. Ophthalmol Retina. Jun 2024;8(6):537-544. [CrossRef]
- Archambault SD, Nichols MM, McCullum JC, Zhang Y, Steinberger EE, Ramsey DJ. Patient adherence to therapy after switch to aflibercept from bevacizumab or ranibizumab for treatment-refractory neovascular age-related macular degeneration. Indian J Ophthalmol. Jan 1 2024;72(Suppl 1):S101-s105. [CrossRef]
- Szigiato A, Mohan N, Talcott KE, et al. Short-Term Outcomes of Faricimab in Patients with Neovascular Age-Related Macular Degeneration on Prior Anti-VEGF Therapy. Ophthalmol Retina. Jan 2024;8(1):10-17. [CrossRef]
- Kishi M, Miki A, Kamimura A, et al. Short-Term Outcomes of Faricimab Treatment in Aflibercept-Refractory Eyes with Neovascular Age-Related Macular Degeneration. J Clin Med. 2023. [CrossRef]
- Kamao H, Mitsui E, Date Y, Goto K, Mizukawa K, Miki A. The Effect of a Loading Dose Regimen in the Switch to Brolucizumab for Patients with Aflibercept-Resistant nAMD. J Ophthalmol. 2024;2024:3673930. [CrossRef]
- Maruyama-Inoue M, Yanagi Y, Inoue T, Kadonosono K. Comparison of functional and morphologic changes between brolucizumab and faricimab in neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. Feb 2024;262(2):589-599. [CrossRef]
- Schneider M, Bjerager J, Hodzic-Hadzibegovic D, Klefter ON, Subhi Y, Hajari J. Short-term outcomes of treatment switch to faricimab in patients with aflibercept-resistant neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2024. [CrossRef]
- Inoda S, Takahashi H, Takahashi R, et al. Visual and Anatomical Outcomes After Initial Intravitreal Faricimab Injection for Neovascular Age-Related Macular Degeneration in Patients with Prior Treatment History. Ophthalmol Ther. Oct 2023;12(5):2703-2712. [CrossRef]
- Fragiotta S, Bassis L, Abdolrahimzadeh B, Marino A, Sepe M, Abdolrahimzadeh S. Exploring Current Molecular Targets in the Treatment of Neovascular Age-Related Macular Degeneration toward the Perspective of Long-Term Agents. Int J Mol Sci. 2024. [CrossRef]
- Monés J, Srivastava SK, Jaffe GJ, et al. Risk of Inflammation, Retinal Vasculitis, and Retinal Occlusion-Related Events with Brolucizumab: Post Hoc Review of HAWK and HARRIER. Ophthalmology. Jul 2021;128(7):1050-1059. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).