1. Introduction
In the context of global climate change, reducing CO
2 emissions and enhancing biological carbon stocks are critical measures to mitigate global warming [
1]. Tropical rainforests, despite covering only 7% of the global land area [
2], play a vital role in carbon absorption through photosynthesis, surpassing carbon emissions from human fossil fuel combustion by six times [
3]. These forests hold carbon stocks and net primary productivity exceeding 30% of the global land area [
4,
5]. Notably, tropical forest carbon sinks pose the most uncertain component of the global carbon budget, emphasizing the need for accurate carbon stock estimations in tropical forests to comprehend the global carbon balance and advance initiatives to reduce CO
2 emissions through forest management [
6].
Forest carbon stocks comprise three components: aboveground biomass, belowground biomass, and soil carbon stocks [
7]. Allometric regression models, typically utilizing parameters such as basal diameter (BD) or diameter at breast height (DBH), are commonly employed to estimate aboveground biomass, enabling the calculation of individual tree biomass [
8,
9]. However, in the case of surveys in tropical rainforests, the measurement position of tree stems with buttress roots is often determined based on the height of these roots. Trees with the highest point of buttress root attachment below 1.3 m are measured at 1.3m for DBH, while trees with the highest point of buttress root attachment above 1.3 m are measured at 0.5m above the highest point of the buttress roots [
10,
11]. Consequently, the biomass of buttress roots is often overlooked in the calculation of aboveground tree biomass. Similarly, limited empirical studies exist on belowground biomass due to the complex and time-consuming sampling process as well as the labor-intensive and costly nature of the research [
12]. Estimations frequently rely on relationships with aboveground biomass, disregarding the biomass of buttress roots. Accurately measuring aboveground, belowground, and total biomass is challenging due to the irregularities at the base of trees caused by buttress roots, and no reports have been published on constructing allometric growth equations for buttress-rooted trees based on diameter [
13].
Buttress roots are a common phenomenon in most tropical forests [
14]. Besides providing support and enhancing trunk mechanical stability, buttress roots fulfill other crucial ecological functions within the entire ecosystem [
15,
16]. For instance, they enhance heterogeneity and regulate understory diversity in tropical rainforests [
17]. Pandey discovered that soil organic carbon (SOC), total N, mineralized N, and soil particle size in buttress root zones of tropical rainforest in South Andaman Island, India, were 18%, 52%, 38%, and 13% higher, respectively, than in non-buttress root zones [
18]. However, there have been no studies that have adequately addressed the ecological roles of buttress roots in tropical forests in China; thus, this study is a novel undertaking to attempt to fill this gap. The influence of buttress roots on the surrounding topography results in higher accumulations of litter, surface soil nutrients, and water content near the upslope of buttress roots as compared to the downslope, establishing a persistent water gradient even during the dry season [
18,
19].
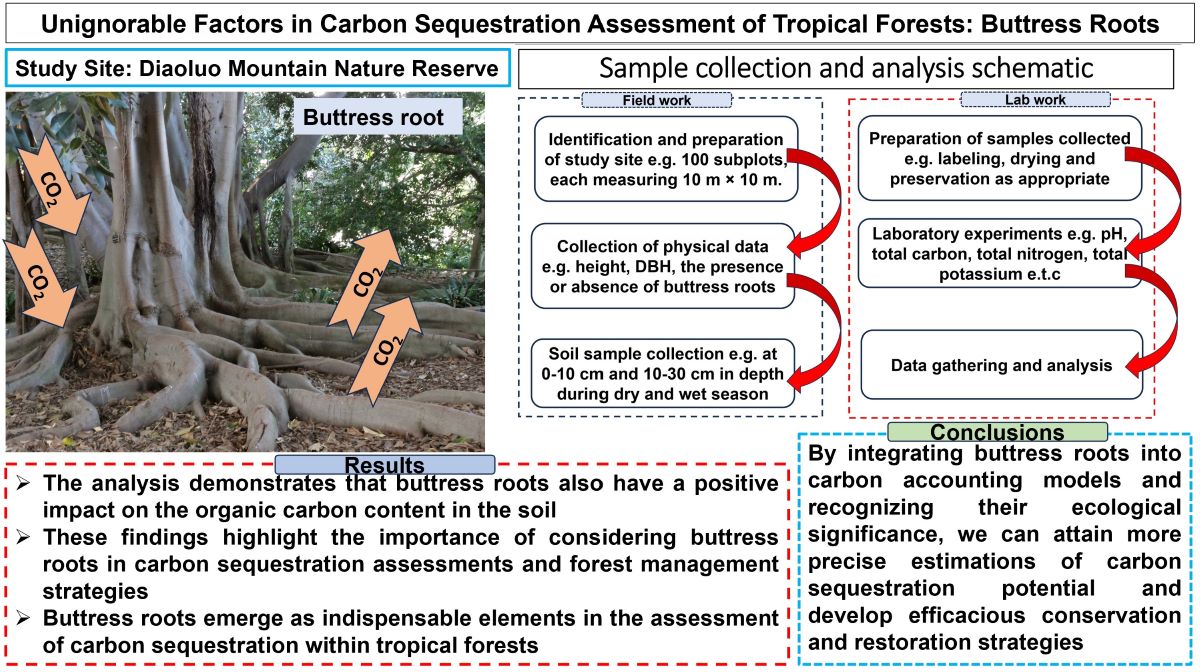
The role of buttress roots in environmental changes and increased spatial heterogeneity of soil has been overlooked in studies on the carbon stock capacity of tropical rainforests and therefore remains unclear. Thus, this study focuses on the tropical lowland rainforest of Diaoluo Mountain in Hainan Province, China. By analyzing the biomass of buttress roots, soil organic carbon in buttress root zones, and soil respiration, we aim to explore the contribution of buttress roots to carbon stocks in tropical forests. Additionally, this research provides theoretical support for understanding the carbon stock capacity of tropical rainforest ecosystems and global carbon accounting.
2. Materials and Methods
2.1. Study Site
The study site is situated within the pristine Diaoluo Mountain Nature Reserve located in Hainan Province, China (coordinates 18°43′-18°58′N, 109°45′-110°03′E) [
20]. This area is renowned for its unspoiled tropical rainforest, making it an important ecological site within China [
21].
The reserve has a tropical marine monsoon climate characterized by an average annual temperature of 24.6°C [
22]. The warmest month, July, sees an average temperature of 28.4°C, while the coolest month, January, has an average temperature of 15.3°C, with the relative humidity remaining consistently high at an average of 85.9% [
23]. The annual precipitation totals 2160 mm, with a distinct division between the wet season from the end of May to October and the dry season from November to early May of the following year, with April serving as a transitional period between these two seasons [
24].
Topographically, the reserve comprises predominantly middle mountains with elevations ranging from 100 m to 1499 m above sea level, with the terrain being higher in the northern part and gradually descending towards the southern region [
25]. The primary soil types are sandy red soil and mountain yellow soil derived from parent materials such as granite and diorite, characterized by depth, moisture, acidity, and richness in organic matter, although in some areas, exposed bedrock results in extremely shallow topsoil [
21].
The reserve's vegetation is exceptionally diverse, featuring a complex composition that includes extensive areas of pristine primary forests alongside extensive secondary forests. Prominent tree species found within the reserve include Vatica mangachapoi, Schima superba, Lithocarpus silvicolarum, Heritiera parvifolia, and Koilodepas hainanense [
21].
2.2. Research Methods
2.2.1. Sample Collection
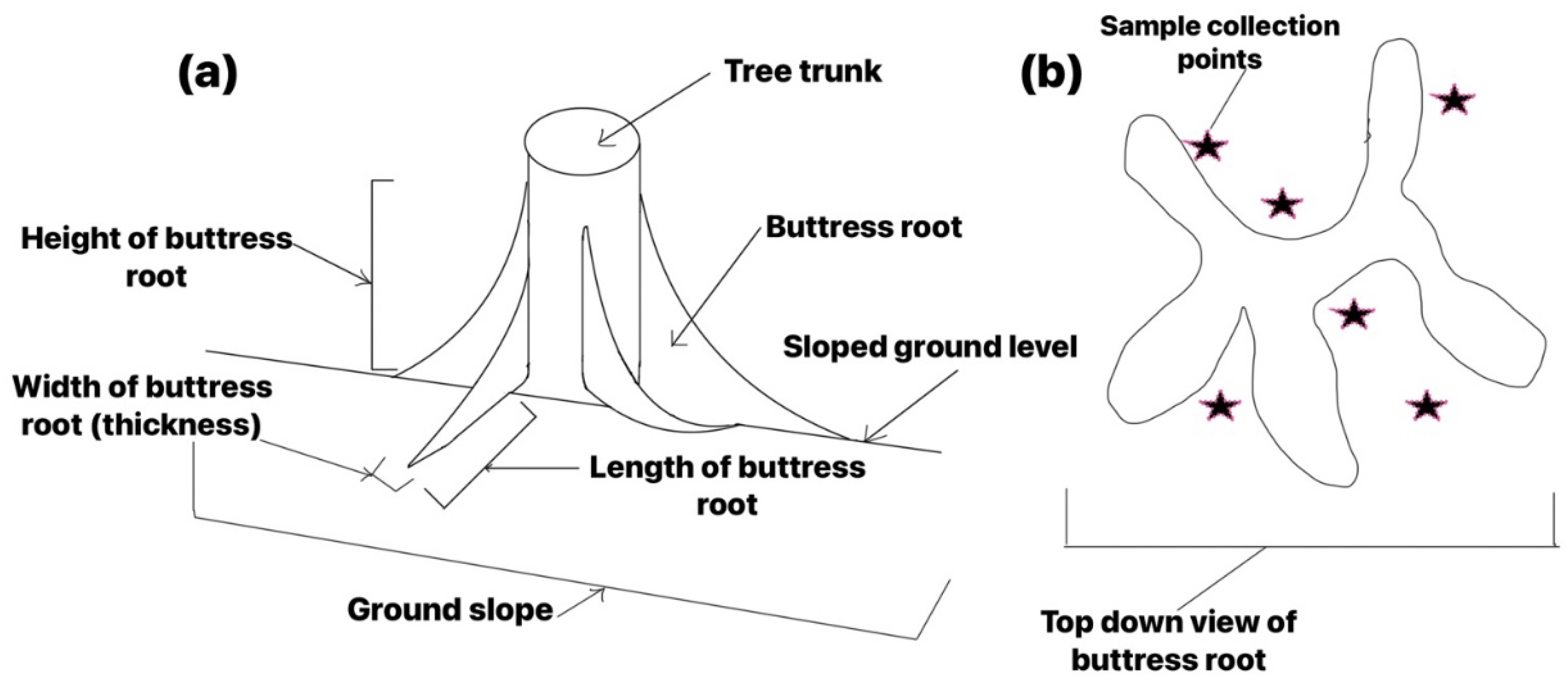
In the lowland rainforest region at an elevation of 300 m within the Diaoluo Mountain Nature Reserve, a 1-hectare (100 m × 100 m) vegetation plot was established in an undisturbed and representative forest area. This plot was further subdivided into 100 subplots, each measuring 10 m × 10 m. Within each subplot, comprehensive data were collected for all trees with a diameter at breast height (DBH) ≥10 cm. Spatial autocorrelation did not influence this study as we chose an area of varying vegetation density and varying elevation gradient; therefore, the difference recorded from one plot to the next was statistically significant. A buttress root was defined as a buttress-like projection of a tree root that extends from the trunk and is visible above the ground. This information included species identification, height, DBH, the presence of buttress roots, height at which buttress roots began, the number of buttress roots (each buttress protrusion from the stem), height and length of buttress roots, width of buttress roots at the proximal and distal ends of the tree trunk, and other relevant details (
Figure 1).
Within the 10 m x 10 m vegetation plot, five trees with distinct buttress root structures and an average DBH of 28.5±13.3 cm were selected as representatives of buttress-rooted trees totaling 840 trees. Additionally, five non-buttress-rooted trees with similar DBH and slope positions were chosen as control specimens, resulting in a total of 10 trees for analysis. This process was repeated five times to ensure statistical robustness.
To enhance sample representativeness, soil sampling points were designated at various positions around the base of each tree trunk. These positions included directly below the trunk, 50cm above it, as well as to the upper left, upper right, lower left, and lower right of the trunk (
Figure 1). For each buttress-rooted tree and non-buttress-rooted tree, three soil sampling points were established at equal distances of 50cm from the trunk in both the upslope and downslope directions.
Sampling was performed at two distinct soil layers: 0-10 cm and 10-30 cm in depth. Soil samples from each layer were carefully combined, and 100 g of soil was collected from each sampling point using the quadrant method. After eliminating extraneous materials like gravel and plant debris, soil samples from the same layer were thoroughly mixed in equal proportions. The mixed soil samples were subsequently divided into two portions.
One portion was placed in aluminum containers, meticulously labelled, and preserved with the original soil structure intact for subsequent determination of soil mechanical composition and density characteristics. The other portion was deposited in sealed bags, labelled accordingly, and securely stored for further analysis of various soil physicochemical properties. These properties encompassed parameters such as pH, total carbon, total nitrogen, total phosphorus, total potassium, hydrolyzed nitrogen, available potassium, available phosphorus, and more, all of which were examined in a laboratory setting.
Soil sample collection was conducted during two distinct periods: in August 2020, corresponding to the rainy season, and in January 2021, representative of the dry season. A total of 20 soil samples were collected for each soil layer during each sampling event. This resulted in a grand total of 40 bagged soil samples and 40 aluminium box samples being acquired during each sampling period, thus yielding a comprehensive dataset of 160 soil samples. Surface litter was removed before sample collection.
Upon collection, these soil samples were meticulously air-dried within a dedicated soil room. Subsequently, they were sieved through different mesh sizes according to specific analysis requirements. Finally, the prepared samples were dispatched to the laboratory for an examination of soil physicochemical properties. The determination of soil organic matter content was performed using the potassium dichromate oxidation-external heating method (LY/T1237-1999).
2.2.2. Biomass Calculation
Buttress Root Biomass Calculation [
26]:
H: Height of buttress root (m) [measured longitudinally from the ground level to the termination point on the stem]
L: Length of buttress root (m) [the distance between the buttress root’s furthest point from the stem to the closest point to the stem measured transversely at the ground level
W1: Width of buttress root near the tree trunk (m) [measured transversely at the highest point from the ground where the buttress root attaches to the stem]
W2: Width of buttress root away from the tree trunk (m) [measured transversely at the ground level]
Tree Biomass Calculation [
27]:
Where:
D: Diameter at breast height (m)
H: Tree height (m)
This research utilized the following volume-to-biomass conversion equation [
28]:
Where: Volumei is whole root volume m3 which is the volume for all the Biomass components (j), i.e., the predictor variable was the same but the dependent variable changed depending on the biomass component. bij= Least squares regression coefficient (slope) for biomass component j (j=1 - 0.4).
2.2.3. Determination of Soil Organic Carbon Components
The soil organic carbon was determined using the NaI heavy liquid fractionation method [
29]. Ten grams of air-dried soil samples, which had passed through a 2 mm sieve, were weighed into a 100 ml centrifuge tube. Subsequently, 40 ml of NaI solution with a density of 1.9g/cm
3 was added to the tube, and the mixture was oscillated for 60 minutes on a reciprocating shaker with a shaking speed of 250 times/min. The dispersed suspension was then centrifuged at 3000 rpm for 10 minutes. The suspended solids on the surface of the mixture were filtered through a 0.45μm microporous membrane to separate the light fraction organic matter. Following this, 20-30 ml of NaI solution was added to the centrifuge tube, and the same steps of separation, centrifugation, and collection of the reconstituted material were repeated (2-3 times). The collected reconstituted material was rinsed with a 0.01 mol/L CaCl
2 solution and further rinsed with distilled water until no Cl
- reaction occurred. The reconstituted material was transferred to a pre-weighed 25 ml beaker, dried at 60°C for 24 hours, and then weighed to determine the proportion of reconstituted material to the total sample mass.
The reconstituted organic carbon content was determined by grinding the material that passed through a 0.15mm sieve and using the potassium dichromate oxidation-external heating method.
2.2.4. Measurement of Soil Respiration
Within the selected tree disc area of each tree, at a distance of 80 cm from the trunk, PVC collars with an inner diameter of 20 cm and a height of 10 cm were installed for buttress-rooted trees on the upslope and downslope positions, and for non-buttress-rooted trees on the lateral position. The height of the PVC collar above the ground surface was approximately 2-3cm.
Soil respiration was measured using a Li-8100A Soil CO2 Flux System (Li-COR Inc., Lincoln, NE, USA). Measurements were conducted every 30 minutes from 9:00 a.m. to 5:00 p.m. on sunny days in August 2020, corresponding to the rainy season, and in January 2021, representative of the dry season. One month before soil respiration measurements, six PVC soil collars (with an inner diameter of 20 cm and a height of 12 cm) were installed in each 20 m × 20 m plot. The PVC soil collars were pressed into the soil at a depth of 8-10 cm, minimizing soil compaction caused by the PVC collars. All aboveground parts of plants within the collars were completely removed, and the soil around the outer ring of the PVC collar was compacted to ensure no gas leakage. The position of the PVC collars remained unchanged throughout the measurement period.
2.3. Statistical Analysis
The collected data underwent statistical analysis to determine significance and draw conclusions. Descriptive statistics, including means, standard deviations, and ranges, were calculated to summarize the distribution and variability of the data. Analysis of Variance (ANOVA) was conducted to assess the significance of differences among groups. Post-hoc LSD (Least Significant Difference) tests were performed to compare specific mean differences, with a significance level set at α = 0.05.
The statistical analysis and data visualization were conducted using R software 3.6.3, which facilitated data processing and analysis. Graphical representations, including bar charts and box plots, were employed to visually present the results and enhance their interpretability. By employing robust statistical techniques, this study ensured the reliability and validity of the conclusions drawn from the data.
3. Result
3.1. Buttress Root Biomass
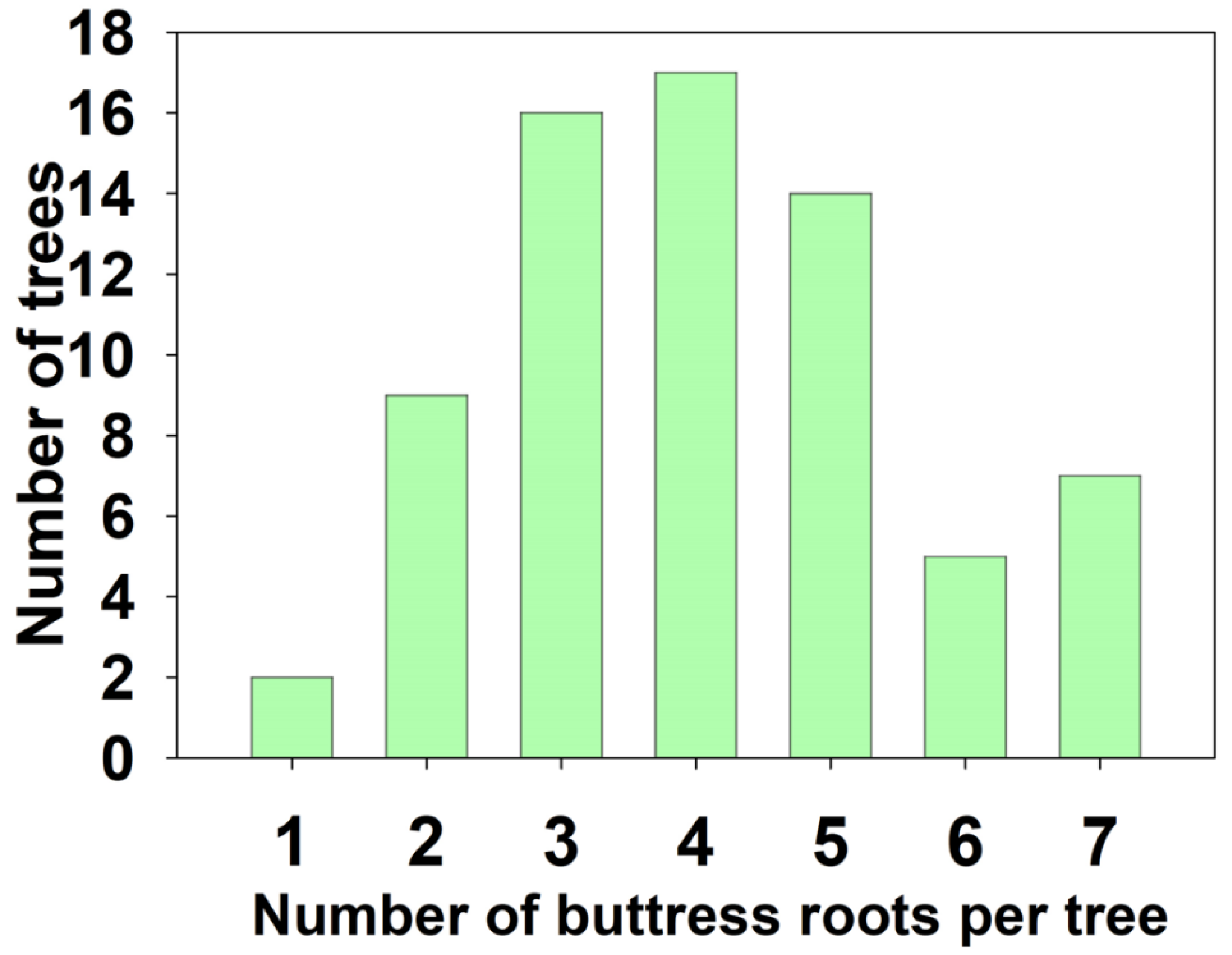
In this study, a total of 840 trees with a diameter at breast height (DBH) greater than 10 cm were observed in the sample plots. Among them, 69 trees were found to have buttress roots, with each tree having 1 to 7 buttress roots (
Figure 2). A statistically significant number of trees (69.57%) had 3 to 5 buttress roots per tree.
Using formulas (1) and (2), the total biomass of the buttress roots and the aboveground portion of the trees with buttress roots was calculated to be 8.5 tonnes/ha and 10.7 tonnes/ha, respectively. The buttress root biomass accounted for 44.27% of the total tree biomass. The minimum and maximum individual buttress root biomass were 2.3 tonnes/ha and 3.8 tonnes/ha, respectively. Compared to the total biomass of the tree, the minimum buttress root biomass was 1.07%, while the maximum was 88.72%.
3.2. Impact of Buttress Roots on Soil Organic Carbon
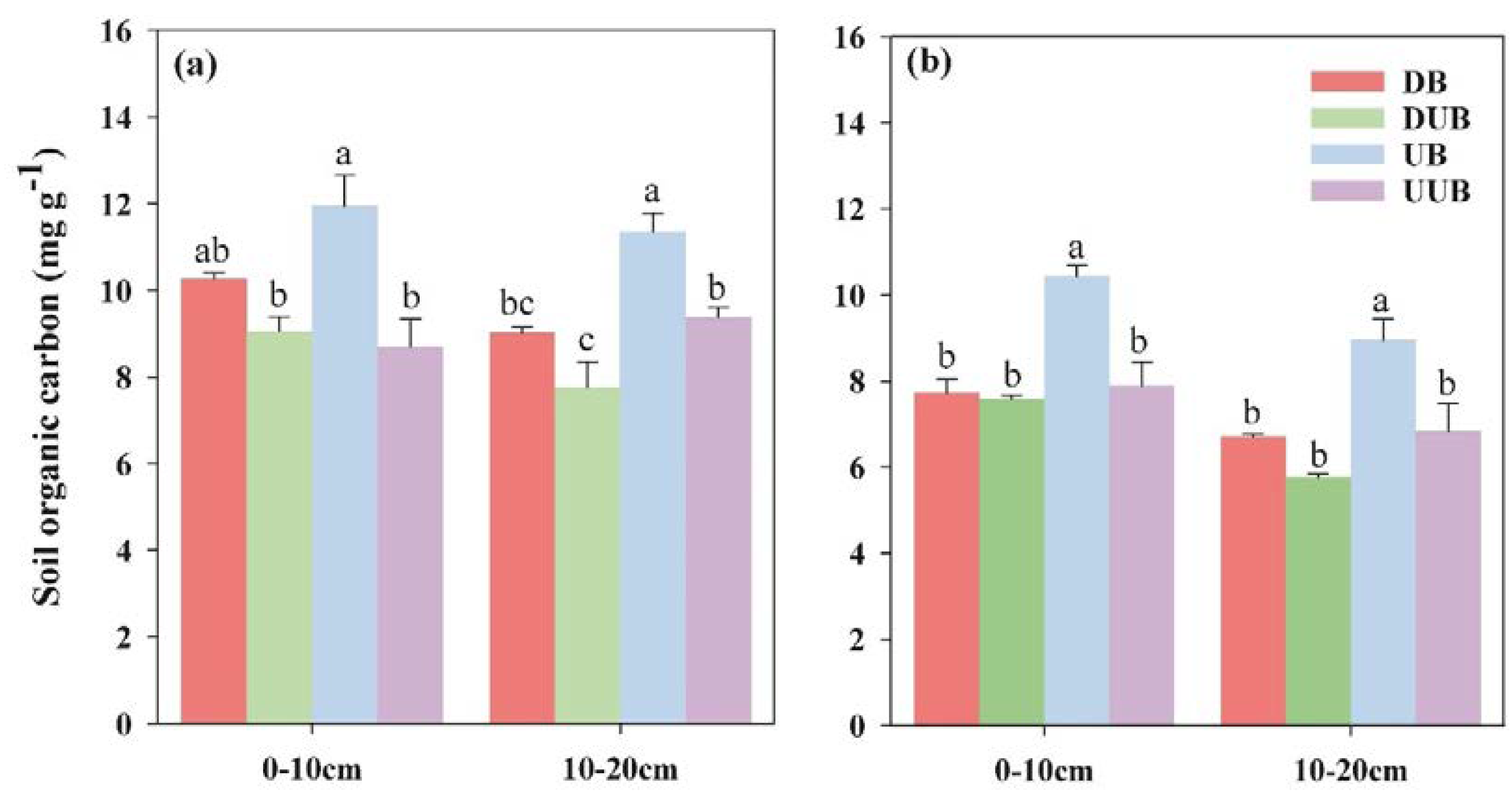
From
Figure 3, it was observed that the presence of buttress roots, during both the rainy and dry seasons, increased the soil organic carbon content in the up-slope area with buttress roots. During the rainy season, there were no statistically significant differences in the soil organic carbon content between the up-slope and down-slope areas with buttress roots or between the up-slope and down-slope areas without buttress roots in the 0-10cm soil layer. However, substantial differences were observed between the up-slope and down-slope areas with buttress roots and without buttress roots in the 10-30cm soil layer. In the 0-10cm soil layer, the average soil organic carbon content in the up-slope area with buttress roots was 11.948 mg/g, which was 16.34%, 31.95%, and 37.31% higher than the down-slope area with buttress roots, up-slope area without buttress roots, and down-slope area without buttress roots, respectively. In the 10-30 cm soil layer, the average soil organic carbon content in the up-slope area with buttress roots was 11.356 mg/g, which was 25.86%, 21.16%, and 46.21% higher than the down-slope area with buttress roots, up-slope area without buttress roots, and down-slope area without buttress roots, respectively.
During the dry season, statistically significant differences in soil organic carbon content were observed between the up-slope and down-slope areas with buttress roots and between the up-slope areas with buttress roots and without buttress roots in both the 0-10cm and 10-30cm soil layers. However, no substantial differences were found between the down-slope area with buttress roots and the up-slope areas without buttress roots. In the 0-10cm soil layer, the average soil organic carbon content in the up-slope area with buttress roots was 10.442 mg/g, which was 34.91%, 32.14%, and 37.72% higher than the down-slope area with buttress roots, up-slope area without buttress roots, and down-slope area without buttress roots, respectively. In the 10-30cm soil layer, the average soil organic carbon content in the up-slope area with buttress roots was 8.948 mg/g, which was 33.55%, 31.09%, and 54.99% higher than the down-slope area with buttress roots, up-slope area without buttress roots, and down-slope area without buttress roots, respectively.
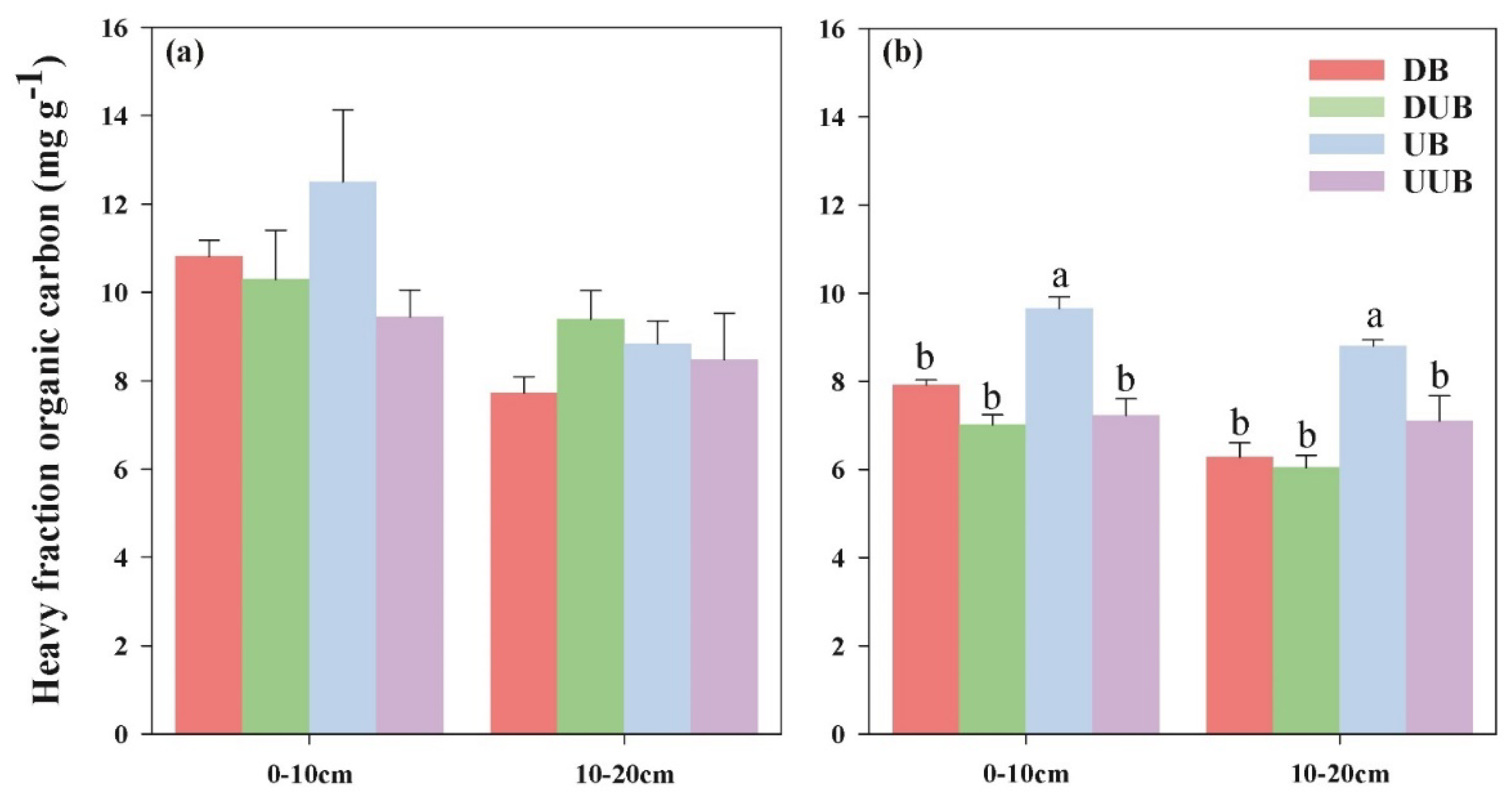
3.3. Impact of Buttress Roots on Soil Heavy Fraction Organic Carbon Content
Figure 4 displays the differences in soil heavy fraction organic carbon content between areas with buttress roots and areas without buttress roots during different seasons. The heavy fraction organic carbon in soil refers to the organic-inorganic composite carbon bound to soil mineral colloids. It represents the primary form of soil organic carbon and is an important indicator of soil carbon stocks capacity. From
Figure 4, it was observed that during the rainy season, the presence of buttress roots did not affect the heavy fraction organic carbon in the soil layers in a statistically significant way, as there were no substantial differences observed between the areas with and without buttress roots. However, during the dry season, in both the 0-10 cm and 10-30 cm soil layers, the up-slope areas with buttress roots exhibited higher levels of heavy fraction organic carbon compared to the down-slope areas with buttress roots and the up-slope and down-slope areas without buttress roots. In the 0-10 cm soil layer, the average content of heavy fraction organic carbon in the up-slope area with buttress roots (10.962 mg/g) was higher by 32.01%, 30.25%, and 47.54% compared to the down-slope area with buttress roots, up-slope area without buttress roots, and down-slope area without buttress roots, respectively. Similarly, in the 10-30 cm soil layer, the average content of heavy fraction organic carbon in the up-slope area with buttress roots (9.518 mg/g) was higher by 28.22%, 25.12%, and 41.93% compared to the down-slope area with buttress roots, up-slope area without buttress roots, and down-slope area without buttress roots, respectively.
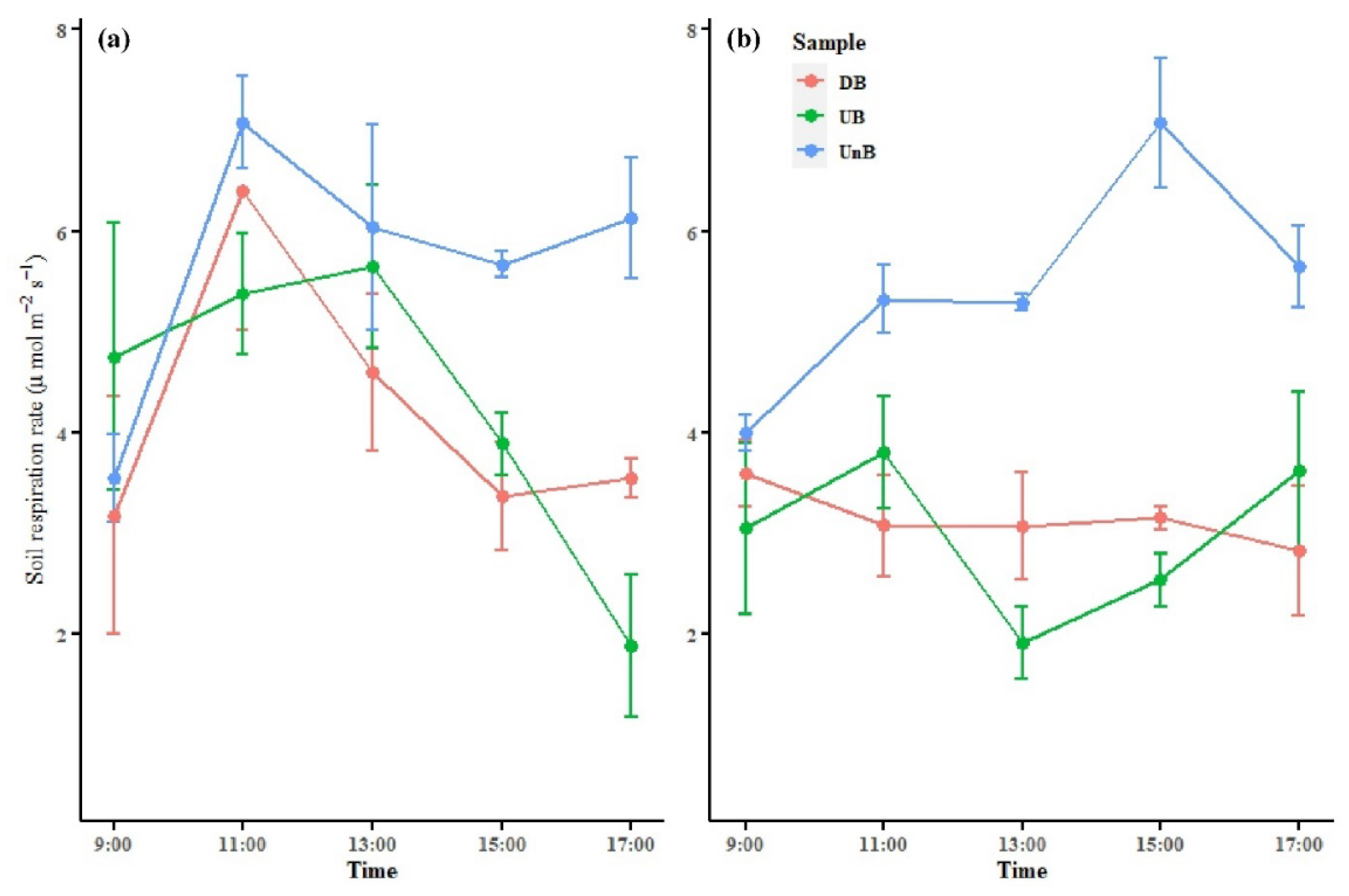
3.4. Impact of Buttress Roots on Soil Respiration
Figure 5 illustrates the diurnal variation of soil respiration between areas with buttress roots and areas without buttress roots during different seasons. Soil respiration is the main pathway through which CO
2 is released from the soil to the atmosphere. From
Figure 5, it can be observed that the diurnal variation of soil respiration differs between the up-slope and down-slope areas of the buttress roots, as well as the areas without buttress roots. Overall, regardless of the season, the soil respiration rate in the areas without buttress roots was higher than that in the areas with buttress roots.
During the rainy season, both the areas with and without buttress roots exhibit a similar pattern of increasing and then decreasing soil respiration, with the peak occurring around 11:00. However, the peak of soil respiration in the areas with buttress roots is slightly delayed, occurring around 13:00.
During the dry season, the variation patterns of soil respiration differ among the up-slope and down-slope areas of the buttress roots and the areas without buttress roots. In the up-slope area with buttress roots, soil respiration shows an increasing trend followed by a decrease and then another increase, with peaks occurring around 11:00 and 17:00. In the down-slope area with buttress roots, soil respiration generally exhibits a decreasing trend. In the areas without buttress roots, soil respiration shows an initial increase followed by a decrease, with the peak occurring around 15:00.
3.5. Impact of Buttress Roots on Various Soil Nutrient Components
Table 1 reveals a noteworthy link (P<0.05) between the primary physical and chemical indicators of the soil in the root region, excluding phosphorus, throughout the dry season. The correlation analysis reveals that the highest correlation coefficient (r=0.728) was observed between soil organic carbon and nitrogen. This is followed by the correlation between organic carbon and potassium (r=0.059), nitrogen and potassium (r=0.298), nitrogen and available phosphorus (r=0.260), and phosphorus and potassium (r=0.236). All of these correlations demonstrate a positive relationship. A tenuous positive association exists between the overall potassium content in soil and other physicochemical indices. However, there is no correlation (P>0.05) between soil-available potassium and phosphorus or nitrogen. A statistically significant weak negative association (P<0.05) exists between soil pH and the levels of organic carbon and nitrogen. There was no statistically significant relationship between phosphorus and other soil physicochemical indices (P>0.05). Overall, the presence of buttress roots had a positive effect on soil nutrient concentration in all seasons creating a healthier environment for trees.
Table 2 reveals a minor alteration in the association among several indicators during the wet season. In addition to soil pH, there is a strong positive association (P<0.05) between the physical and chemical indicators of the soil in the root area during the wet season. The correlation coefficient between nitrogen and accessible phosphorus is the highest (r=0.919), followed by the correlation between organic carbon and nitrogen (r=0.897), and the correlation between organic carbon and phosphorus (r=0.786). The relationship between soil pH and other physicochemical properties is not strong. There is a significant positive correlation between soil pH and nitrogen (P<0.05). On the other hand, there is a significant negative correlation between soil pH and potassium (P<0.05). Overall, soil organic carbon was observed to be influenced by soil chemical properties with soil nitrogen having the highest statistical influence followed by phosphorus and potassium both during the dry and wet seasons.
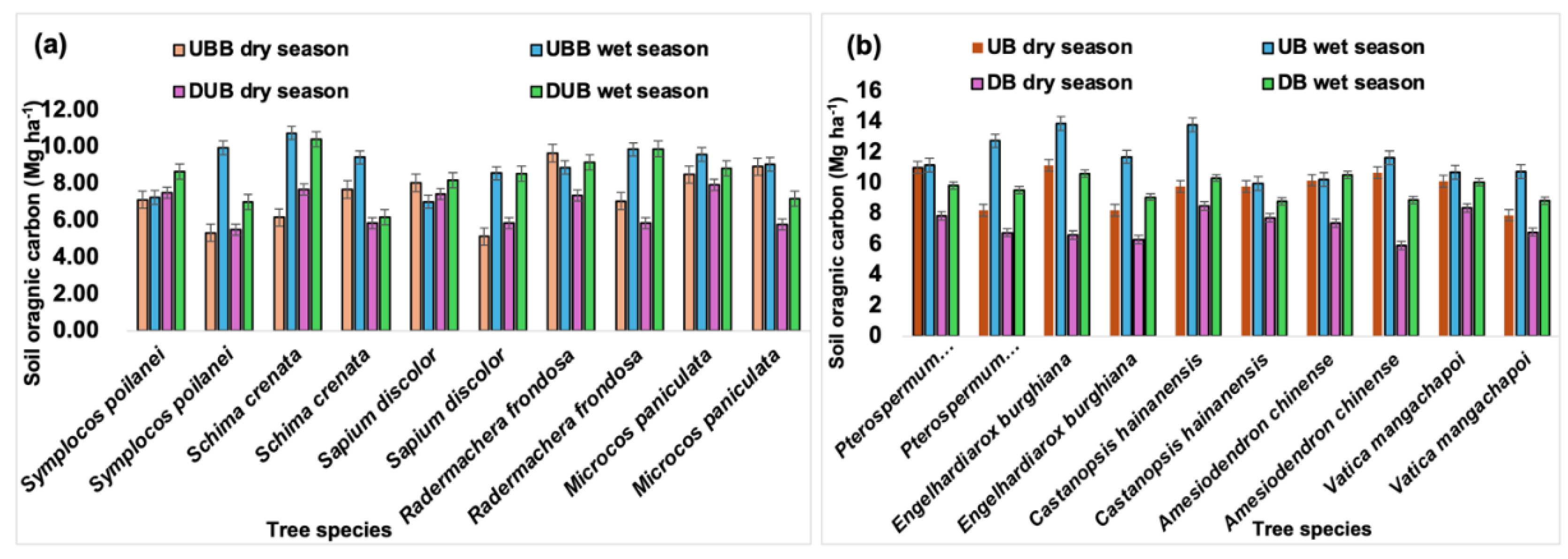
3.6. The Impact of Buttress Roots on Soil Organic Carbon in Different Tree Species
From the analysis in
Figure 6, tree species with buttress roots had on average 20% higher organic carbon content from the samples collected both during the wet and dry seasons. Among the trees without buttress roots, the tree species Schima crenata had the highest soil organic carbon (11.2 Mg ha
-1) during the wet season compared to the lowest which was Symplocos poilanei (7.2 Mg ha
-1) during the wet season. Both buttressed and unbuttressed trees had significantly lower soil organic carbon content (25% lower) during the dry season compared to the wet season. Among the trees with buttress roots, Engelhardiarox burghiana had the highest organic carbon content (14.1 Mg ha
-1) compared to Castanopsis hainanensis which has the lowest (9.2 Mg ha
-1) during the wet season.
4. Discussion
4.1. Buttress Roots' Contribution to Soil Carbon Content
The results of this study reaffirm the important role of buttress roots in influencing soil carbon content within tropical forests, aligning with previous research findings [
30]. Biomass, a crucial indicator of forest carbon stocks capacity, has often overlooked the contribution of buttress roots in previous studies due to the unique and irregular nature of these structures. However, our findings indicate that the biomass of buttress roots can account for a substantial portion of the total biomass in plate-rooted trees, representing 44.27% in this study. The individual variation in buttress root biomass ranged from 2.3 tonnes/ha and 3.8 tonnes/ha contributing between 1.07% and 88.72% to the total tree biomass. It is important to recognize the biomass of buttress roots when assessing the carbon stocks potential of tropical forests, as plate-rooted trees are commonly found in these ecosystems, typically representing 12% to 35% of the forest composition [
31,
32]. Additionally, plate-rooted trees tend to have tall trunks and canopies, with the length and height of their plate roots increasing as the trees age and grow in diameter at breast height [
33,
34].
The advent of three-dimensional laser scanning technology offers a promising avenue for further studying buttress roots [
35]. Given the complexities of measuring underground biomass and the resource-intensive nature of these measurements, estimates of below-ground biomass often rely on above-ground biomass data [
36]. However, due to the distinctive characteristics of plate-rooted trees, future research should prioritize improving plate-root biomass models and investigating the relationship between above-ground and below-ground biomass for these trees.
4.2. Influence of Buttress Roots on Soil Organic Carbon
Buttress roots play a multifaceted role in shaping soil organic carbon dynamics within tropical forests [
29]. Our findings underscore the pivotal role of buttress roots in affecting soil organic carbon levels and distribution within the ecosystem. The presence of buttress roots gives rise to "root walls" [
37], which act as barriers, impeding down-slope material flow. This reduction in surface runoff and erosion caused by rainfall results in the creation of unique ground biogeochemical zones [
18]. The observed increase in soil organic carbon content in areas with buttress roots can be attributed to several factors. First, buttress roots contribute to the accumulation of organic matter derived from their own structure, and their presence fosters enhanced nutrient cycling [
18]. The large surface area of buttress roots, along with their stabilizing influence on tree trunks, creates microenvironments conducive to organic matter accumulation and the development of nutrient-rich soil conditions [
38].
Leaf litter represents a primary source of soil organic carbon. An increase in leaf litter quantity in plate-rooted areas, as indicated by Pandey may contribute to the elevated soil organic carbon content in these regions [
18]. However, it is noteworthy that some studies have shown that even a doubling of leaf litter quantity over 15 consecutive years did not result in increased soil carbon storage in tropical forests [
39].
Soil organisms and microorganisms, the primary decomposers of leaf litter, play a crucial role in soil organic carbon dynamics [
40]. Buttress-rooted areas exhibit higher leaf litter quantities compared to non-buttress-rooted areas, leading to increased species diversity and abundance of soil animals. These conditions enhance biogeochemical cycling, potentially contributing to higher soil organic carbon levels, particularly in upper slope positions.
The influence of buttress roots extends beyond soil organic carbon content to encompass other soil properties, including moisture retention, nutrient availability, and soil structure [
41]. The elevated soil moisture near buttress roots supports plant growth and enhances overall ecosystem productivity. Alterations in soil structure and nutrient distribution, induced by the presence of buttress roots, contribute to the ecological functioning of the forest ecosystem as our results show.
4.3. Impact of Buttress Roots on Soil Respiration
Soil respiration, a critical pathway for releasing CO
2 from the soil to the atmosphere, exhibits varying diurnal patterns in areas with and without buttress roots [
42]. Our findings indicate that, overall, soil respiration rates in areas without buttress roots surpass those in areas with buttress roots during both the rainy and dry seasons. These observed differences in soil respiration patterns can be attributed to several factors.
During the rainy season, both areas with and without buttress roots display similar patterns of increasing and decreasing soil respiration, with peak rates occurring around 11:00. However, areas with buttress roots exhibit a slight delay in the timing of the peak, which occurs around 13:00. In contrast, during the dry season, the variation in soil respiration differs among the up-slope and down-slope areas of the buttress roots and the areas without buttress roots. In the up-slope area with buttress roots, soil respiration shows an increasing trend, followed by a decrease and another increase, with peaks around 11:00 and 17:00. In the down-slope area with buttress roots, soil respiration generally exhibits a decreasing trend. In areas without buttress roots, soil respiration follows an initial increase, followed by a decrease, with the peak occurring around 15:00. This are similar patterns as what has been found out by other researchers [
43].
The lower soil respiration rates observed in areas with buttress roots can be attributed to multiple factors. Soil respiration is primarily driven by tree roots and microbial activity; the Plate Root Nutrient Hypothesis posits that plate roots are adapted for efficient nutrient and water uptake to overcome nutrient-poor soil conditions [
44]. The enhanced nutrient uptake capacity of plate roots may result in a reduced overall quantity of roots in the soil, including root respiration.
Moreover, higher soil phosphorus content in buttress-rooted areas could potentially lead to reduced soil respiration rates. However, it is essential to investigate whether fine root quantity in buttress-rooted trees substantially differs from that in non-buttress-rooted trees and whether microbial biomass in buttress-rooted areas is lower than in non-buttress-rooted areas. These factors warrant further research.
4.4. Impact of Buttress Roots on Various Soil Nutrient Components
The chemical characteristics of the soil have a direct impact on the development of plants. An examination of the chemical properties of soil in the upper and lower slopes of the buttress root zone revealed significant differences in the indicators of soil chemical properties. Furthermore, the nutrient gradient between the slopes of the buttress root zone was found to be higher compared to the non-buttress root zone. This suggests that a zone of increased soil nutrient enrichment had developed along the slope gradient of the buttress root zone, leading to an increase in the amount of soil organic carbon and consequently enhancing the variability of the soil in the root zone. This finding further validates the concept that the existence of buttress roots can enhance soil heterogeneity [
18]. This study detected variations in pH levels between the higher and lower slopes of the buttress root zone, as well as across distinct soil layers, throughout both the dry and rainy seasons. During the dry season, the pH value of the soil in the 0-10 cm layer where the buttress roots are located was notably higher compared to the non-buttress root zone. This finding contradicts the research results of Mack [
45]. While Mack's data indicated a rise in soil pH, there was no notable distinction between the buttress root zone and the non-buttress root zone [
45]. During the wet season, the impact of buttress roots on the soil pH value in the 0-10 cm soil layer aligns well with Mack's findings. Through a differential comparison of nitrogen, it was discovered that the basal portion of the trees with buttress roots contained a higher nitrogen content compared to the non-buttress root portion. This suggests that buttress roots enhance the process of nitrogen cycling at a micro-environmental level, leading to the formation of nitrogen reserves and increased availability of nitrogen for plants in tropical rainforests. This finding aligns with the findings of He in the Xishuangbanna region [
46], and further corroborates Pandey et al.'s claim that buttress roots enhance the efficiency of nitrogen element consumption in tropical rainforests [
18].
The findings of an increased quantity of organic carbon in the soil of tropical forests through buttress roots align with the study conducted by Dean on eucalyptus trees in Australia [
30]. The correlation analysis of the primary chemical characteristics of soil indicated a strong link between the total phosphorus element and several other soil indicators in the wet season in the buttress root zone. During the rainy season, there is a strong association between the phosphorus content and organic carbon, nitrogen, and available potassium in the root zone. However, there is a weak correlation with potassium, nitrogen, and phosphorus. The results suggest that the microenvironment created by buttress roots has a significant impact on the phosphorus content in the soil. Phosphorus exhibits a significant leaching rate in tropical rainforests [
47] and it frequently becomes a restricting factor for the growth of trees [
48]. Buttress roots contribute to the increase of both the overall amount and efficient use of phosphorus in the soil. In tropical settings with phosphorus-deficient soil, trees with buttress roots have a growth advantage, which may explain their large stature in tropical forests. In general, buttress roots exert a substantial positive influence on the three primary elements in soil, with the exception of potassium. This may be attributed to several factors. Firstly, buttress roots have a consolidating effect on litter [
18]. Secondly, the presence of buttress roots enhances soil moisture, promoting litter decomposition [
37]. Thirdly, buttress roots contribute to an increase in the population of vertebrates, reptiles, and invertebrates in the buttress root zone [
49]. This rise in soil animal population accelerates litter decomposition, facilitating rapid nutrient replenishment [
50]. Lastly, the heterogeneous habitat created in the buttress root zone can alter the structure and composition of soil microbial communities, influencing the pathways of soil nitrogen and phosphorus decomposition and reduction, consequently impacting soil chemical properties.
4.5. Implications for Carbon Stocks and Ecosystem Conservation
Recognizing the role of buttress roots in carbon stocks assessments and forest management strategies is essential. Neglecting the contribution of buttress roots may lead to underestimated carbon stocks potential in tropical forests. Integrating buttress roots into carbon accounting models will yield more accurate estimations, guiding effective conservation and restoration strategies aimed at enhancing the carbon stocks capacity of tropical forests.
The findings of this study underscore the ecological significance of buttress roots in tropical forests. Beyond their carbon stocks role, buttress roots enhance ecosystem heterogeneity and regulate understory diversity, contributing to broader biodiversity conservation efforts. The presence of buttress roots ripples through the ecosystem, influencing soil properties and supporting the growth and functioning of various plant and microbial communities.
5. Conclusions
In conclusion, buttress roots emerge as indispensable elements in assessing carbon stocks within tropical forests. Their substantial contribution to soil carbon content and multifaceted ecological functions underscore their pivotal role in carbon assessment frameworks and forest management strategies. Acknowledging and incorporating the influence of buttress roots in our methodologies not only enhance our comprehension of carbon dynamics in tropical forests but also amplify their capacity as formidable carbon sinks, while promoting sustainable forest management practices that preserve biodiversity and mitigate climate change.
The findings of this study emphasize the imperative need to acknowledge and appreciate the diverse contributions of buttress roots to tropical forest ecosystems. By integrating buttress roots into carbon accounting models and recognizing their ecological significance, we can achieve more precise estimations of carbon stock potential and develop effective conservation and restoration strategies. This comprehensive understanding of buttress roots will catalyse the formulation of sustainable forest management practices that bolster the vital role of tropical forests as crucial carbon sinks and biodiversity reservoirs.
Future research should delve deeper into unraveling the mechanisms through which buttress roots influence soil carbon dynamics. Moreover, exploring the long-term impacts of buttress roots on ecosystem stability and resilience will be instrumental in advancing our knowledge of the intricate carbon dynamics in tropical forests. Addressing these knowledge gaps will further refine our strategies for conserving and managing these critical ecosystems.
Overall, acknowledging and valuing the role of buttress roots in carbon storage and ecosystem functioning will serve as a cornerstone for developing effective conservation and management strategies for tropical forest ecosystems. These collective efforts are of paramount importance in the broader context of climate change mitigation, global biodiversity preservation, and the long-term sustainability of our planet.
Author Contributions
Xu Wang: data processing and writing; Brian Njoroge Mwangi: data processing and writing; Houben Zhao: site maintenance; Guangyi Zhou: experimental design; Zhiiun Qiu: data collection; Mengmeng Yang: writing; Zhongmin Wu: experimental design; Yuelin Li: data processing and writing.
Funding
This work was supported by the Central Non-profit Research Institution of Chinese Academy of Forestry [CAFYBB2021ZE001; CAFYBB2017SY017], and the National Natural Science Foundation of China [31961143023].
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to express our gratitude to Tianyu Han and Haiwei Liu for their diligent manual labor during the fieldwork.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ma, X.; Wu, L.; Zhu, Y.; Wu, J.; Qin, Y. Simulation of Vegetation Carbon Sink of Arbor Forest and Carbon Mitigation of Forestry Bioenergy in China. International Journal of Environmental Research and Public Health 2022, 19, 13507. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Meir, P.; Brown, S. Forests, carbon and global climate. Philosophical Transactions of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences 2002, 360, 1567–1591. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, C. Tropical ecology: an overview. International Society for Tropical Ecology 2009.
- Townsend, A.R.; Cleveland, C.C.; Houlton, B.Z.; Alden, C.B.; White, J.W. Multi-element regulation of the tropical forest carbon cycle. Frontiers in Ecology and the Environment 2011, 9, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hubau, W.; Lewis, S.L.; Phillips, O.L.; Affum-Baffoe, K.; Beeckman, H.; Cuní-Sanchez, A.; et al. Asynchronous carbon sink saturation in African and Amazonian tropical forests. Nature 2020, 579, 80–87. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; et al. Global Carbon Budget 2022. Earth System Science Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Yadav, V.S.; Yadav, S.S.; Gupta, S.R.; Meena, R.S.; Lal, R.; Sheoran, N.S.; et al. Carbon sequestration potential and CO2 fluxes in a tropical forest ecosystem. Ecological Engineering 2022, 176, 106541. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Coe, R.; van Noordwijk, M.; Ambagau’, Y.; Palm, C.A. Reducing uncertainty in the use of allometric biomass equations for predicting above-ground tree biomass in mixed secondary forests. Forest Ecology and Management 2001, 146, 199–209. [Google Scholar] [CrossRef]
- Piotto, D. A meta-analysis comparing tree growth in monocultures and mixed plantations. Forest Ecology and Management 2008, 255, 781–786. [Google Scholar] [CrossRef]
- Mequanint, F.; Wassie, A.; Aynalem, S.; Adgo, E.; Nyssen, J.; Frankl, A.; et al. Biodiversity conservation in the sacred groves of north-west Ethiopia: diversity and community structure of woody species. Global Ecology and Conservation 2020, 24, e01377. [Google Scholar] [CrossRef]
- Yoshifuji, N.; Kumagai, T.; Ichie, T.; Kume, T.; Tateishi, M.; Inoue, Y.; et al. Limited stomatal regulation of the largest-size class of Dryobalanops aromatica in a Bornean tropical rainforest in response to artificial soil moisture reduction. Journal of Plant Research 2019, 133, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kraenzel, M.; Castillo, A.; Moore, T.; Potvin, C. Carbon storage of harvest-age teak (Tectona grandis) plantations, Panama. Forest Ecology and Management 2003, 173, 213–225. [Google Scholar] [CrossRef]
- Sinacore, K.; Hall, J.S.; Potvin, C.; Royo, A.A.; Ducey, M.J.; Ashton, M.S. Unearthing the hidden world of roots: Root biomass and architecture differ among species within the same guild. PLOS ONE 2017, 12, e0185934. [Google Scholar] [CrossRef] [PubMed]
- Alencar, G.M.; de Castilho, C.V.; Costa FR, C. When are buttresses and stilt roots necessary for a tree in terra-firme Amazonian forests? Biotropica 2023, 55, 665–673. [Google Scholar] [CrossRef]
- Newbery, D.M.; Schwan, S.; Chuyong, G.B.; van der Burgt, X.M. Buttress form of the central African rain forest tree Microberlinia bisulcata, and its possible role in nutrient acquisition. Trees 2008, 23, 219–234. [Google Scholar] [CrossRef]
- Zhiyuan, H.; Yong, T.; Xiaobao, D.; Min, C. Buttress trees in a 20-hectare tropical dipterocarp rainforest in Xishuangbanna, SW China. Journal of Plant Ecology 2012, 6, 187–192. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, X.; Cao, M.; Baskin, C.C.; Baskin, J.M. Buttress trees elevate soil heterogeneity and regulate seedling diversity in a tropical rainforest. Plant and soil 2011, 338, 301–309. [Google Scholar] [CrossRef]
- Pandey, C.B.; Singh, L.; Singh, S.K. Buttresses induced habitat heterogeneity increases nitrogen availability in tropical rainforests. Forest Ecology and Management 2011, 262, 1679–1685. [Google Scholar] [CrossRef]
- Facelli, J.M.; Pickett ST, A. Plant litter: Its dynamics and effects on plant community structure. The Botanical Review 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Wu, X.; Qiao, S.; Tan, Q. Destination Management for Ecotourism Activity Using Analytical Hierarchy Process. Scientific Programming 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Zhu, Z.-X.; Nizamani, M.M.; Sahu, S.K.; Kunasingam, A.; Wang, H.-F. Tree abundance, richness, and phylogenetic diversity along an elevation gradient in the tropical forest of Diaoluo Mountain in Hainan, China. Acta Oecologica 2019, 101, 103481. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Zhang, X.; Ma, L. Carbon Sink Calculation and Time Variation in Hainan Tropical Rainforest National Park—A Case Study of Diaoluo Mountain Forest Area. Journal of Physics: Conference Series 2020, 1637, 012002. [Google Scholar] [CrossRef]
- Lin, Z.; Zhao, C.; Li, P. The Application of RS and GIS in Forest Park Planning: A Case Study of Diaoluoshan National Forest Park. Advances in Intelligent Systems and Computing 2014, 353–365. [Google Scholar] [CrossRef]
- Wang, X.; Han, T.; Ruan, L.P. Effects of buttressed roots on soil physical and chemical properties of soil in lowland rainforests of Diaoluoshan, Hainan Province. acta ecologica sinica 2022. [Google Scholar]
- Yin, J.; Tang, X.; Zhang, W.; Liang, X.; Zhu, J. Where to Preserve? Evaluating the Integrity Principle for Delineating Protection Scopes of Kaiping Diaolou and Villages. Sustainability 2019, 11, 2196. [Google Scholar] [CrossRef]
- Warren, S.D.; Black, H.L.; Eastmond, D.A.; Whaley, W.H. Structural Function of Buttresses of Tachigalia Versicolor. Ecology 1988, 69, 532–536. [Google Scholar] [CrossRef]
- Li, Y.D. Comparative analysis of biomass estimation methods of tropical high-altitude stands in Hainan Island. Acta ecological sinica 1993, 13, 313–320. [Google Scholar]
- Zhou, X.; Brandle, J.R.; Awada, T.N.; Schoeneberger, M.M.; Martin, D.L.; Xin, Y.; Tang, Z. The use of forest-derived specific gravity for the conversion of volume to biomass for open-grown trees on agricultural land. biomass and bioenergy 2011, 35, 1721–1731. [Google Scholar] [CrossRef]
- Ola, A.; Gauthier AR, G.; Xiong, Y.; Lovelock, C.E. The roots of blue carbon: responses of mangrove stilt roots to variation in soil bulk density. Biology Letters 2019, 15, 20180866. [Google Scholar] [CrossRef]
- Dean, C.; Kirkpatrick, J.B.; Doyle, R.B.; Osborn, J.; Fitzgerald, N.B.; Roxburgh, S.H. The overlooked soil carbon under large, old trees. Geoderma 2020, 376, 114541. [Google Scholar] [CrossRef]
- Chapman, C.A.; Kaufman, L.; Chapman, L.J. Buttress formation and directional stress experienced during critical phases of tree development. Journal of Tropical Ecology 1998, 14, 341–349. [Google Scholar] [CrossRef]
- Milliken2, W. Structure and Composition of One Hectare of Central Amazonian Terra Firme Forest1. Biotropica 1998, 30, 530–537. [Google Scholar] [CrossRef]
- Woodcock, D. The Buttressed Blue Marble Tree: Wood and Growth Characteristics of Elaeocarpus angustifolius (Elaeocarpaceae). Annals of Botany 2000, 85, 1–6. [Google Scholar] [CrossRef]
- Warner, A.J.; Jamroenprucksa, M.; Puangchit, L. Buttressing impact on diameter estimation in plantation teak (Tectona grandis Lf.) sample trees in northern Thailand. Agriculture and Natural Resources 2017, 51, 520–525. [Google Scholar] [CrossRef]
- Næsset, E.; Gobakken, T. Estimation of above- and below-ground biomass across regions of the boreal forest zone using airborne laser. Remote Sensing of Environment 2008, 112, 3079–3090. [Google Scholar] [CrossRef]
- Kenzo, T.; Himmapan, W.; Yoneda, R.; Tedsorn, N.; Vacharangkura, T.; Hitsuma, G.; et al. General estimation models for above- and below-ground biomass of teak (Tectona grandis) plantations in Thailand. Forest Ecology and Management 2020, 457, 117701. [Google Scholar] [CrossRef]
- Herwitz, S.R. Buttresses of tropical rainforest trees influence hillslope processes. Earth Surf Processes Landforms. 1988, 13, 563–567. [Google Scholar] [CrossRef]
- Epron, D.; Mochidome, T.; Bassar, A.T.M.Z.; Suwa, R. Variability in methane emissions from stems and buttress roots of Bruguiera gymnorrhiza trees in a subtropical mangrove forest. Ecological Research 2023. [Google Scholar] [CrossRef]
- Sayer, E.J.; Lopez-Sangil, L.; Crawford, J.A.; Bréchet, L.M.; Birkett, A.J.; Baxendale, C.; et al. Tropical forest soil carbon stocks do not increase despite 15 years of doubled litter inputs. Scientific Reports 2019, 9. [Google Scholar] [CrossRef]
- Strecker, T.; Jesch, A.; Bachmann, D.; Jüds, M.; Karbstein, K.; Ravenek, J.; et al. Incorporation of mineral nitrogen into the soil food web as affected by plant community composition. Ecology and Evolution 2021, 11, 4295–4309. [Google Scholar] [CrossRef]
- Zürcher, N. In consideration of the tree: the importance of structure and function in the realization of ecological Design. In Urban Services to Ecosystems: Green Infrastructure Benefits from the Landscape to the Urban Scale; 2021; pp. 509–527. [Google Scholar]
- Ishikura, K.; Hirata, R.; Hirano, T.; Okimoto, Y.; Wong, G.X.; Melling, L.; et al. Carbon Dioxide and Methane Emissions from Peat Soil in an Undrained Tropical Peat Swamp Forest. Ecosystems 2019, 22, 1852–1868. [Google Scholar] [CrossRef]
- Nottingham, A.T.; Cheesman, A.W.; Riutta, T.; Doughty, C.E.; Telford, E.; Huaraca Huasco, W.; et al. Large contribution of recent photosynthate to soil respiration in tropical dipterocarp forest revealed by girdling. Journal of Ecology 2021, 110, 387–403. [Google Scholar] [CrossRef]
- Black, H.L.; Harper, K.T. The Adaptive Value of Buttresses to Tropical Trees: Additional Hypotheses. Biotropica 1979, 11, 240. [Google Scholar] [CrossRef]
- Mack, A. Effects of tree buttresses on nutrient availability and macroinvertebrate species richness. CIEE Fall Monteverde Costa Rica. 2003, 21–27. [Google Scholar]
- He, Z.Y. The buttress root and its effect on litter decomposition in tropical monsoon rainforest in Xishuangbanna, China [D]. Graduate University of the Chinese Academy of Sciences, 2012. [Google Scholar]
- Shi, L.L. Study on the spatial heterogeneity of soil physical and chemical properties of primary tropical montane rainforest in Jianfengling, Hainan Island [D]. China Academic of Forestry, 2012. [Google Scholar]
- Turner, B.L.; Brenes-Arguedas, T.; Condit, R. Pervasive phosphorus limitation of tree species but not communities in tropical forests [J]. Nature 2018, 555, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, S.M.; Pierce, M.S. Tree buttress microhabitat use by a Neotropical leaf-litter herpetofauna. J Herpetol 2005, 39, 192–198. [Google Scholar] [CrossRef]
- Marian, F.; Brown, L.; Sandmann, D.; Maraun, M.; Scheu, S. Roots, mycorrhizal fungi and altitude as determinants of litter decomposition and soil animal communities in tropical montane rainforests[J]. Plant Soil 2019, 438, 1–18. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).