1. Introduction
Obstetrical ultrasound diagnosis depends on highly skilled personnel to obtain images and interpret the findings. Fetal measurements are typically obtained by sonographers and reviewed and interpreted by sonologists (physicians). There is an inherent potential for diagnostic error because people are not perfect. Although there are well-defined standards for correct image planes and correct caliper placement for fetal biometry, sonographers have varying levels of skill in obtaining correct measurements. Some may systematically place the calipers too widely or may frequently measure in oblique planes, resulting in overmeasurement. Others may systematically place the calipers too narrowly, resulting in undermeasurement.
Measurement errors can have clinical consequences. The basic fetal biometry measurements, biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur length (FL), are used to calculate an estimated fetal weight (EFW). Although EFW is known to differ from birth weight by more than 10% in one quarter of exams [
1,
2], EFW and its percentile are often used to make clinical decisions. When EFW or AC are less than the 10
th percentile, a diagnosis of fetal growth restriction (FGR) is made and follow-up is recommended including fetal surveillance, repeat assessment of fetal growth, and sometimes preterm or early term delivery [
3]. When EFW is large, induction of labor is sometimes recommended and the risk of cesarean is increased, even if actual birth weight is not increased [2,4-11].
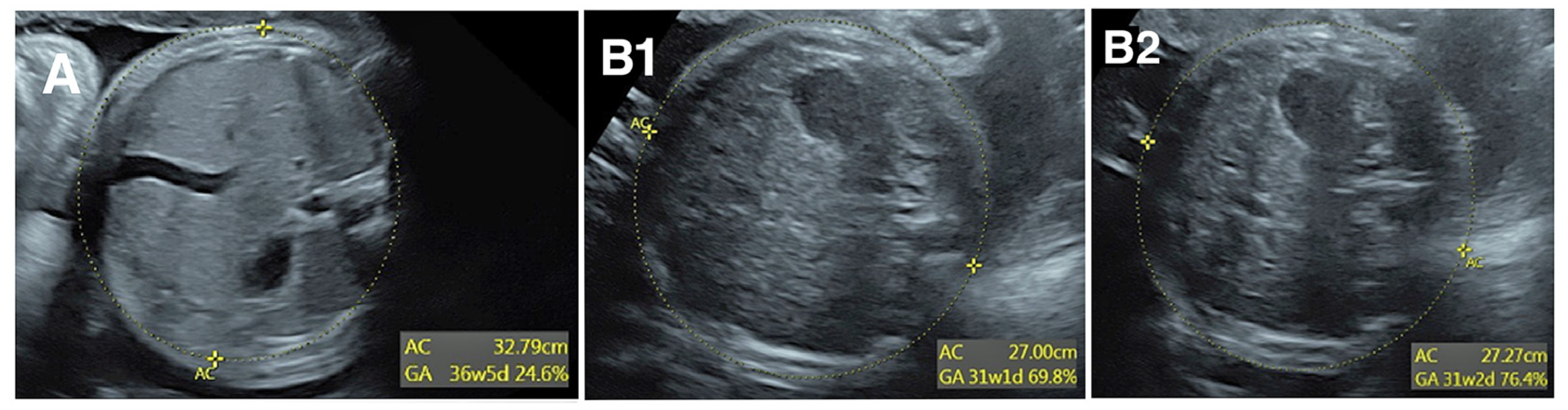
The interpreting physician is the first step in detecting and correcting measurement error, but this first level review does not prevent all errors. Consider the 3 images of AC in
Figure 1. In panel A, the calipers are placed far inside the fetal abdomen, so AC is clearly undermeasured. Though few providers would find this image acceptable, it was obtained in a very busy ultrasound practice and the measurement error was not detected by the sonographer or the reading physician. Panels B1 and B2 show two images from a different fetus taken a few seconds apart. An important limitation is how difficult it is to see the ellipses measuring AC. Notwithstanding that difficulty, the difference in AC between panels B1 and B2 is 2.7 mm or 1%, with AC slightly undermeasured in panel B1. Although this might seem inconsequential, the standard deviation (SD) of AC is 13.4 mm per Hadlock et al. [
12], so 2.7 mm is 0.2 SD. If a sonographer systematically undermeasures AC by 0.2 SD, they will find AC <10
th percentile in 14% of exams rather than the expected 10%. This would result in a 40% overdiagnosis of fetal growth restriction, which in turn would result in excess costs of fetal surveillance, repeat exams, and patient anxiety. Yet a systematic undermeasurement of this magnitude would probably go undetected.
Systematic quality review is recommended by various professional organization to assure the accuracy of obstetrical ultrasound diagnoses [13-16]. Accreditation by the American Institute of Ultrasound in Medicine [
13] requires that a practice “must show ongoing monitoring of the clinical practice’s personnel performance, including all physicians and sonographers through regular, retrospective review. A record of quality assurance (QA) activities must be maintained and kept current.” The Society for Maternal Fetal Medicine (SMFM) [
16] states that “optimal QA monitoring includes large-scale audits and focused audits and should be used to provide constructive individual and group feedback.”
In a typical quality audit, an expert supervisor or peer will examine a sample of images obtained by a given provider, looking for proper image planes and caliper placement. However, image review has several limitations:
To address the limitations of image review, we have developed objective, quantitative methods to evaluate the findings of individual sonographers and physicians in our maternal-fetal medicine (MFM) practices. This article details our methods for quality review of fetal biometry measurements. Subsequent articles will address quantitative methods for quality review of fetal anatomy surveys and EFW.
2. Materials and Methods
Our approach to biometry review was modeled after the Nuchal Translucency Quality Review (NTQR) program of the Perinatal Quality Foundation [
20]. For the NTQR program, practices submitted limited data for review: exam date, crown-rump length (CRL), nuchal translucency (NT) measurement, and identifier codes for sonographer and physician. For each exam, the NTQR program calculated the difference between the observed NT and the expected value of NT for the given CRL. The program then summarized those differences and sent a quarterly report to each participating provider indicating whether the NTs they obtained were larger or smaller than expected, on average, and whether the variance was within the expected range. In this way, the NTQR program identified providers whose measurement technique needed scrutiny even though
providers did not send any images for review.
Our MFM practices use Viewpoint software (Version 6, GE Healthcare, Wauwatosa, WI) to store exam data and generate reports. The software includes a query tool to extract specific data from all exams meeting specified criteria. For each practice, we extract a full calendar year of data for all exams that have measurements of AC, biparietal diameter (BPD), head circumference (HC), and femur length (FL). In addition to the measurements, we extract the exam date, gestational age (GA), sonographer name, reading physician name, fetal cardiac activity (present or absent), plurality (singleton, twin, triplet, etc.), and exam status (final, revised, incomplete, etc). Viewpoint generates a data file in comma-separated value format (.csv file) with one row per exam and one column for each data field. We open the .csv file in Excel (Microsoft, Redmond, WA) and save it as an Excel Workbook (.xlsx file). We import the Excel file into Stata statistical software (Version 13, Statacorp, College Station, Texas). The Supplemental file shows an example of the Stata script used for the analysis.
Inclusion criteria for the quality audit are: finalized (signed) exam, fetal cardiac activity present, singleton pregnancy, and GA from 140/7 to 396/7 weeks. Exams not meeting these criteria are excluded from analysis.
To standardize the measurements across GAs, we calculate the z-score for BPD, HC, AC, and FL for each exam. The z-score is the number of SDs a measurement falls above or below the expected mean based on a standard or reference norm. We use the Hadlock references [
12] for BPD, HC, AC, and FL because those are the norms used for on-screen displays during the exams and in the reports from our practices. If a reference or standard is a perfect fit to a sample of measurements, the z-scores will be normally distributed with a mean of 0 and an SD of 1. Extreme outliers, defined as observations more than 6 SD from the mean (i.e. z-score <-6 or z-score >6), are excluded from the quantitative audit because of their potential to skew the mean but they are audited individually by comparing images to reported values. We summarize each provider’s measurements by calculating their mean and SD of z-score.
Differences between providers are tested with one-way analysis of variance (ANOVA) and Scheffe test. Two-tailed P-values <0.05 are considered significant. We present example scatterplots and histograms to illustrate certain points, but the figures are not a routine part of the quality reviews.
A focused image review is performed for providers whose z-scores differ significantly from their colleagues. The procedure for blinded image review of a randomly-selected subset of exams is detailed in a later section.
We have used this method for quality audits at 8 MFM practices in our national MFM group since 2019. The Results in the Figures and Tables below are actual data from a subset of sonographers and physicians at one of our AIUM-accredited practices. The sonographers were all certified by the Registry of Diagnostic Medical Sonographers and the physicians were all certified in MFM by the American Board of Obstetrics and Gynecology. The Results presented are typical examples of the findings in our complete audits, although the providers have been hand-selected to illustrate some of the issues that can be identified by a quantitative audit. To protect their confidentiality, we do not reveal individual names, practice location, or year of the exams. In the Tables and Figures that follow, we summarize analysis of fetal AC; analogous methods can be used for BPD, HC, and FL.
3. Results
3.1. Sonographer Mean z-score Values
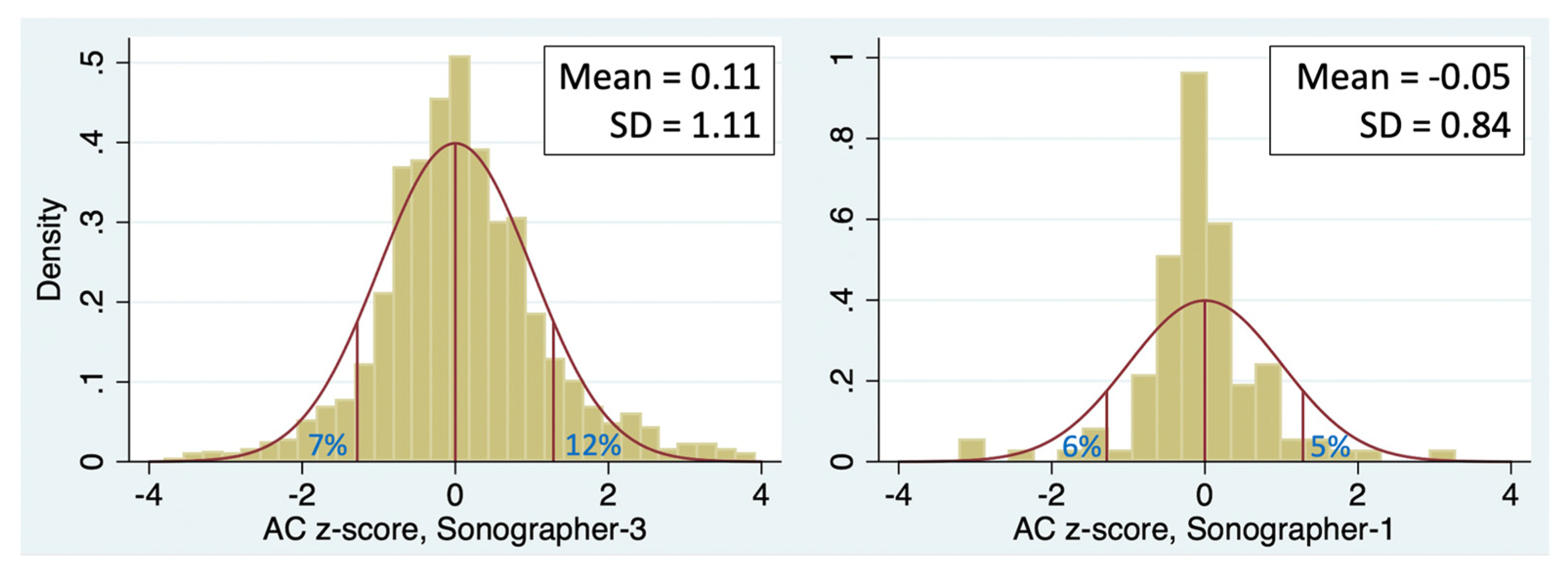
Figure 2 shows one year of AC measurements from 2 sonographers in the same practice. The upper panels are scatterplots of the AC measurements across GA. A z-score is calculated for each measurement to standardize the observations at different GAs. The lower panels show histograms of z-score for the two sonographers. Sonographer 2 (left panel) had a mean z-score of -0.03 (not significantly different than 0) and an SD of z-score very close to 1, indicating an excellent fit to the Hadlock reference [
12]. In contrast, the measurements of Sonographer 8 (right panel) are shifted far to the right, with a mean z-score of 0.63. This resulted in a paucity of exams with AC <10th percentile (2.4% compared to the expected 10%) and an excess of exams with AC >90th percentile (24% compared to the expected 10%).
Table 1 summarizes the mean and SD of the AC z-score for 8 sonographers from the same practice for one year. For the whole practice, the mean z-score was 0.33, meaning that the practice overall tends to find AC about 1/3 of an SD (4.4 mm) larger than expected based on the Hadlock reference [
12]. There are several possible explanations for this: the practice may have a high percentage of patients with obesity, diabetes, and other risk factors for large-for-gestational age (LGA) fetuses; the sonographers at this practice may have been trained to place their calipers slightly outside the fetal abdomen; a high percentage of exams may have been oblique cross-sections rather than perpendicular sections; or the Hadlock reference [
12] may have values that are too small for the modern US population. The data in hand do not permit a simple explanation for this deviation. If it is simply a reflection of a population enriched for LGA, it may not be an issue at all.
However,
variations between sonographers within the practice cannot be attributed to any of these explanations. If sonographers examine a random selection of patients, it is expected that they should all have approximately the same distribution of z-scores. Yet, Sonographers 1 and 2 had a mean z-scores very close to zero while Sonographers 6, 7, and 8 had a means >0.50, that is, over half an SD larger than expected. The impact of these differences is illustrated in the right two columns of
Table 1. Sonographers 6-8 all had a paucity of exams <10th percentile (3% of exams or fewer, compared to the expected 10%) and an excess of exams >90th percentile (20% of exams or more, compared to the expected 10%).
The ANOVA shows a significant overall difference between sonographer mean z-scores (footnote g, P<0.001). The Scheffe multiple-comparisons tests demonstrate significant pairwise differences between sonographers (footnotes b through e, all p<0.02).
3.2. Image Audit Focused on Outliers
Focused image audits are recommended for sonographers whose measurements are outliers. There is little value in auditing those whose measurements lie close to the practice mean because the majority of their images will be within accepted standards. For the practice illustrated in
Table 1, we recommended audits for sonographers 1, 2, and 8 because their mean z-scores had the largest deviations from the practice mean.
Image audits are performed anonymously, i.e., the auditor does not know the identity of the persons being audited and does not know whether their measurements are, on average, larger or smaller than expected. For this reason, it is ideal to simultaneously audit at least one sonographer whose mean is below the practice mean (possible systematic undermeasurement) and one whose mean is above the practice mean (possible systematic overmeasurement). At least two people are needed to perform a blinded review, one to select and prepare the images and another to evaluate them. If there are adequate personnel and time, an additional blinded auditor evaluates the same images. These tasks are performed by the lead sonographer, the physician ultrasound director, and other practice leaders as available.
A sample of 15-20 exams per sonographer is usually sufficient to identify systematic overmeasurement (oblique planes or calipers consistently placed too widely) or undermeasurement (calipers placed too narrowly). The example Stata script in the Appendix includes a section for generating a random subset of 20 exams for two sonographers selected for audit. If statistical software is not used, then an arbitrary set of recent exams by that sonographer can be selected manually.
Most ultrasound image storage systems allow the export of anonymized images. For each exam selected for audit, we anonymize and export all the images showing AC measurements. The person preparing the images keeps a key with exam identifiers and sonographer identifiers, but the key is not shared with the auditors. The selected images are compiled in a computer file folder and shared with the auditors. We review the image files on a computer monitor rather than printed on paper for two reasons: first, printed images are generally of lower quality; and second, the original exam and original interpretation are performed via monitors, not on paper.
Auditors keep a scoresheet, comparing the images for each exam to the reported value, judging whether the reported value represents overmeasurement, undermeasurement, or acceptable measurement, and recording any notes about improper image planes. Once the scoring is completed, the key is opened and each sonographer’s scores are compiled.
If the majority of images from a sonographer are rated as over- or undermeasurement, and especially if this matches the expected result based on the z-score, this is discussed privately with the sonographer. Sonographers often feel threatened by a quality audit and their privacy must be respected. The discussion is one-on-one, conducted by the lead sonographer or practice ultrasound director, depending on who the sonographer will likely find least threatening. The discussion emphasizes that the process is not intended to be punitive but rather to identify opportunities to improve measurement technique. The sonographer is told that the practice routinely monitors the measurement of all sonographers on an ongoing basis and that this sonographer was identified as an outlier. Any constructive suggestions about improving technique are discussed
If the majority of images from a sonographer are rated as acceptable, this is also shared with the sonographer. In a one-on-one session, the sonographer is told that they were identified as a possible outlier in routine monitoring of measurements but that review of their images did not find a systematic issue.
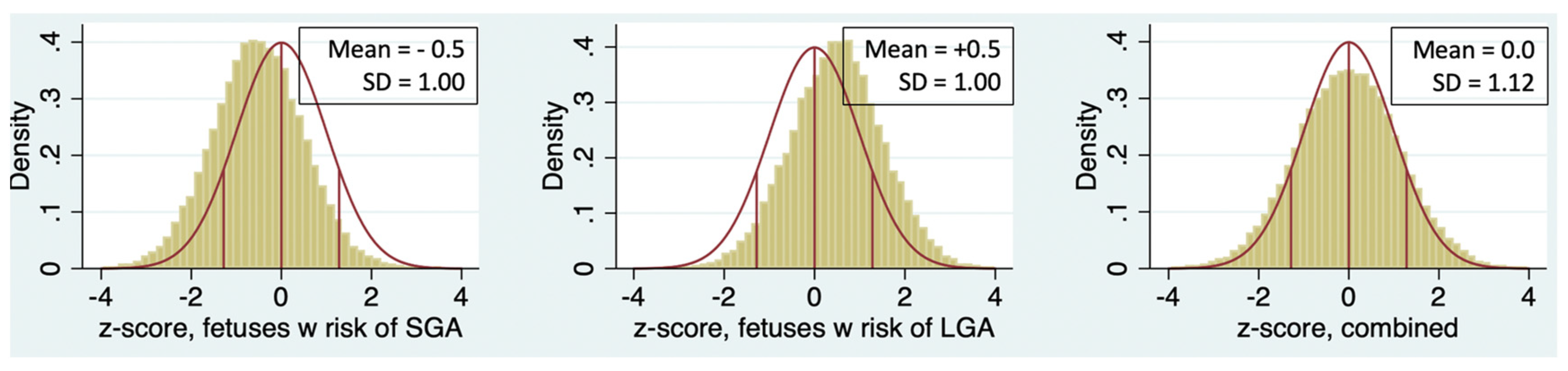
3.2. Evaluation of Standard Deviation (SD) of z-score
In
Table 1, the SD of z-score is greater than 1 for every sonographer except Sonographer 1. The population of patients undergoing ultrasound in a typical MFM practice is usually enriched with patients at risk for both LGA fetuses (e.g., diabetes, obesity) and small-for-gestational age (SGA) fetuses (e.g., hypertensive disorders, suspected growth restriction, advanced maternal age). The consequence is that the SD of z-score should be greater than 1, as illustrated in
Figure 3. It is unlikely that a sonographer examining a random selection of patients from a mixed-risk population will have an SD less than 1.
When the SD of z-score is <1, a likely explanation is “expected-value bias” which occurs when a sonographer adjusts the caliper placement to make the measurement match the gestational age displayed on the screen [
21]. The observation that Sonographer 1 had both an SD <1 and a mean z-score very close to zero suggests that this is occurring (
Table 1). Sonographer 1 also had SD <1 for HC and FL z-scores (0.90 and 0.76, respectively), reinforcing the notion that expected-value bias may be operating. The problem with expected-value bias is that forcing the measurements to be “normal” will result in missed diagnoses of large or small measurements, that is, fewer than 10% of measurements will be <10
th percentile or >90
th percentile, as illustrated in
Figure 4.
Expected-value bias generally cannot be detected by image review. If it is suspected, as with Sonographer 1, our approach is to discuss the findings with the sonographer involved and ask whether their customary process is to “fine-tune” their measurements to match the gestational age. If so, it may be sufficient to give a brief educational intervention regarding why this should be avoided. A follow-up audit will determine whether the issue has been corrected.
Sonographer 7, on the other hand, had an SD much larger than the other sonographers (
Table 1). This suggests inconsistency in measurement, that is, the sonographer sporadically both overmeasures and undermeasures AC. This possibility is reinforced by the observation that Sonographer 7 also had a larger SD of z-scores for both HC and FL than all the other sonographers (data not shown). In such cases, we recommend mentoring on taking greater care in caliper placement. We also note that Sonographer 7 had a relatively small number of exams, so it is possible that the large SD might be spurious. A follow-up audit will reveal whether the issue is persistent.
3.3. Physician Mean Values
Table 2 shows the mean and SD of z-score for 5 physicians from the same practice as
Table 1. In our practices, the physicians generally read and interpret exams performed by sonographers and rarely perform the primary measurements themselves. Thus, differences between physicians likely reflect differences in the sonographers whose exams they interpret rather than different measurements by the physicians.
To adjust for the variance between sonographers, a formal multivariable regression can be used, but this is a complex task that will usually require professional statistical consultation. Instead, we perform a simpler adjustment: for each exam, we subtract the mean z-score of the sonographer who performed the exam. As shown in the right-hand section of
Table 2, this adjustment brings the mean z-score of most of the physicians very close to 0, meaning that most of the variance between physicians is attributable to the sonographers whose exams they are interpreting and that the physicians themselves are not generally driving the measurements higher or lower.
Table 2.
Abdominal circumference z-scores from selected physicians.
Table 2.
Abdominal circumference z-scores from selected physicians.
| |
|
Unadjusted |
|
Adjusted for |
Sonographer |
Physician
Number |
Number
of
Exams |
Mean
z-score |
Standard Deviation
of z-score |
Mean
z-score |
Standard Deviation
of z-score |
| 1 |
1348 |
0.17 a
|
1.06 |
-0.05 |
1.04 |
| 2 |
4739 |
0.26 a
|
1.06 |
0.00 |
1.04 |
| 3 |
2335 |
0.30 a
|
1.10 |
-0.02 |
1.09 |
| 4 |
2090 |
0.44 a
|
1.12 |
0.03 |
1.11 |
| 5 |
2044 |
0.57 a
|
1.06 |
0.09ab
|
1.07 |
| Total Practice |
--- c
|
0.33 a
|
1.08 |
0.00 |
1.07 |
Physician 5 is an outlier with an adjusted mean z-score of 0.09, significantly farther from 0 than the other physicians. A likely explanation is that this physician systematically changed the sonographers’ numbers, either by selecting a larger AC than the sonographer selected or by remeasuring the AC and entering a larger number. A deviation of 0.09 SD is unlikely to be detected by image audit. Our approach is to review the results with the physician, ask how often they are changing the measurements, and point out that this may bias their results. A follow-up audit will confirm whether the issue persists.
4. Discussion
4.1. Quantitative Analysis and Focused Image Audits
The methods outlined fulfill the SMFM recommendation that QA for prenatal ultrasound should include both large-scale and focused audits [16, 25]. For large-scale audit, we use the quantitative analysis of an entire practice for an entire year using standard parametric statistical techniques. For focused audit, we perform image review for sonographers with outlier mean values. This approach avoids the time, complexity, and expense of performing image reviews for the majority of sonographers whose measurements fall within the expected range.
Beyond mean values, we provide examples of three other issues that can be identified in the practice-wide quantitative analysis: (1) standard deviation of z-score (SD) less than 1 suggests “expected-value bias” [
21]; (2) large SD suggests inconsistency of technique; and (3) physicians whose adjusted mean z-scores differ significantly from 0 may be systematically overriding sonographer measurements. These issues cannot easily be evaluated by image audit but discussion with the involved providers may yield insights into areas for improvement.
4.2. Alternative Approaches
An entirely different approach to quality review is the RADPEER program of the American College of Radiology [
22] in which a percentage of examinations are randomly selected for retrospective review by a second physician. The program was primarily designed for radiologic exams, but it can also be applied to ultrasound exams [
18]. The RADPEER “double-reading” approach is time-consuming; for example, performing a review of 5% of just 5 types of radiological exams would require over 60 hours per year for a skilled radiologist [
23]. We are not aware of studies that assess the time requirements to double-read ultrasound exams. In RADPEER studies, the rates of discrepancy between the initial reading and the second reading are typically less than 10%, and most of these are judged not to be clinically significant [17, 19, 23]. In one study, the rate of significant discrepancy for ultrasound exams was less than 1% [
18]. An important limitation of the RADPEER program is that the reviews are not blinded, so there is a potential for bias if, for example, the auditor is a subordinate of the person being reviewed [
19]. Finally, the program evaluates only the reading physician, not the sonographer or radiology technician who obtains the images.
Another approach to quality control (QC) monitoring of fetal biometry measurements was adopted by the INTERGROWTH-21st project for its international prospective study of fetal growth [
24]. For QC, each sonographer self-rated the quality of each biometry image using a standardized rating scale. Then, a random 10% sample of exams was selected for reevaluation by a central Quality Unit. There was a high level of agreement between sonographers and the central unit on both the qualitative assessment (kappa statistics 0.99 for HC, 0.98 for AC, and 0.96 for FL) and the measurements (interobserver limits of agreement ±4.4%, ±6.0%, and ±5.5%, respectively). The authors concluded that qualitative and quantitative QC are feasible and highly reproducible. They recommended these methods for both future research studies and clinical practice. We agree that comparable methods should be applied to well-funded research, but they may be too time-consuming and labor-intensive (and therefore expensive) to incorporate into routine clinical practice.
4.3. Biometry Quality Review in Context
The review of biometric measurements is only one component of a comprehensive quality program for prenatal ultrasound. Other components include review of fetal anatomy imaging and diagnostic accuracy. In forthcoming articles, we will describe our quantitative approach to evaluation of performance on the fetal anatomy survey and accuracy of fetal weight and sex determination.
Beyond review of examination results, there are several structural components generally recommended for a high-quality ultrasound practice [15, 16]. These include accreditation of the practice by an organization such as AIUM [
13] or ACR [
14]. Accreditation, in turn, requires that all personnel have adequate formal education and training in the theory and practice of the types of ultrasound exams performed by the practice, have certification by the appropriate body, and have several hours of continuing education annually. Accreditation also requires the practice to have written protocols to ensure uniformity of exams, timely interpretation and communication of findings, disinfection and cleaning of transducers, maintenance of equipment, and patient safety and confidentiality. Practices should also have a protocol for onboarding new sonographers and physicians that includes a formal orientation to practice protocols and formal assessment of competency in performance of various types of examinations [15, 25].
4.4. Strengths and Limitations
A strength of the quantitative summary (
Table 1 and
Table 2) is that it provides a large-scale overview of an entire practice for a full year. Once the Stata script is written, the entire process requires only a few minutes each year to export the data and run the analysis. The method readily detects outlier sonographers and physicians for focused review. Analyzing a large number of exams for each provider, the method is highly sensitive to small variations.
An important limitation is the assumption that each sonographer performs exams on a random subset of patients. Each practice needs to carefully evaluate this assumption in order to properly interpret results. An example of a non-random selection might be a sonographer who only works on Tuesdays and Thursdays, which are, coincidentally, the 2 days when the practice sees and scans all the patients with diabetes, patients with a high rate of LGA fetuses; this sonographer would be expected to have a high z-score for AC even with perfect measurement. Another example might be a new-hire sonographer who has not yet been approved to scan patients with body mass index over 30 kg/m2, another risk factor for LGA fetuses; this sonographer would be expected to have a lower than average z-score for AC.
Another consideration is that high numbers of exams can result in very low P-values even with very small differences in z-score, that is differences may be statistically significant even though they are too small to be clinically relevant or indeed, too small to be detected by image audit.
Figure 1, Panels B & C shows an example of how difficult it is to see differences in AC of 0.2 SD, corresponding to differences in z-score of 0.2. As a “rule-of-thumb”, we find that image audits are generally not useful unless a sonographer’s z-score differs from the practice mean by at least 0.3. This criterion was met for sonographers 1, 2, and 8 in
Table 1.
4.5. Future directions
A major barrier to regular quality review is that personnel time must be dedicated to it, time that is not compensated by payers. The techniques we describe for the quantitative audit can be adopted with a few hours of development time by a person skilled with statistical software to adapt the analysis script. Once implemented, it takes less than an hour annually to export the data and run and interpret the analyses. Blinded image audits and provision of feedback to providers take considerably more time.
A preferred alternative would be for developers and vendors of commercial ultrasound reporting software to include a suite of tools that would allow practices to readily summarize and compare z-scores for all the personnel in the practice for a variety of measurements including basic biometry, special biometry, EFW, and other measurements. The tools should also include methods to generate a random sample of exams for focused audit as well as tools to readily identify and review extreme outlier observations.
Some modern ultrasound systems are capable of using artificial intelligence (AI) to detect image planes and perform fetal biometry measurements. We hypothesize that use of AI should reduce or eliminate between-sonographer variance in measurements. This hypothesis warrants testing as AI becomes increasingly adopted
5. Conclusions
The large-scale quantitative analysis provides an overview of the biometry measurements of all the sonographers and physicians in a practice so that image audits can be focused on those whose measurements are outliers. The method also identifies several distinct types of systematic errors that image review alone would be unlikely to identify. The analysis takes little time to perform after initial development and avoids the time, complexity, and expense of auditing providers whose measurements fall within the expected range. We encourage commercial software developers, including those using AI for fetal biometry measurements, to include tools in their ultrasound reporting software to facilitate such quantitative review.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org: Sample Stata script for analysis (PDF) and sample Excel file (XLSX) with pseudodata for 885 exams.
Author Contributions
Conceptualization, CAC, SA, CK.; methodology, CAC; software, CAC; validation, SA, CK, OAB, ZSB; formal analysis, CAC; investigation, all authors; data curation, CAC; writing—original draft preparation, CAC; writing—review and editing, all authors; visualization, supervision, and project administration, CAC; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The protocol was reviewed by the Institutional Review Board (IRB) of Good Samaritan Hospital, San Jose. Because the study involved retrospective review of existing data and posed negligible risk to persons, it was determined to be Exempt.
Informed Consent Statement
Patient consent was waived due to the Exempt status of this retrospective analysis of existing data.
Data Availability Statement
For investigators wishing to develop their own analysis script, we provide a Supplemental Excel .XLSX file containing pseudodata with 885 observations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Milner J, Arezina J. The accuracy of ultrasound estimation of fetal weight in comparison to birthweight: a systematic review. Ultrasound 2018; 24:32-41. [CrossRef]
- Stubert J, Peschel A, Bolz M, Glass A, Gerber B. Accuracy of immediate antepartum ultrasound estimated fetal weight and its impact on mode of delivery and outcome – a cohort analysis. BMC Pregnancy Childbirth 2018; 18:118. [CrossRef]
- Society for Maternal-Fetal Medicine (SMFM); Martins JG, Biggio JR, Abuhamad A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction. Am J Obstet Gynecol 2020; 223(4): B2-B19. [CrossRef]
- Parry S, Severs CP, Sehdev HM, Macones GA, White LM, Morgan MA. Ultrasonic prediction of fetal macrosomia. Association with cesarean delivery. J Reprod Med 2000; 45:17-22.
- Blackwell SC, Refuerzo J, Chadha R, Carreno CA. Overestimation of fetal weight by ultrasound: does it influence the likelihood of cesarean delivery for labor arrest? Am J Obstet Gynecol 2009; 200:340.e1-e3. [CrossRef]
- Melamed N, Yogev Y, Meizner I, Mashiach R, Ben-Haroush A. Sonographic prediction of fetal macrosomia. The consequences of false diagnosis. J Ultrasound Med 2010; 29:225-230. [CrossRef]
- Little SE, Edlow AG, Thomas AM, Smith NA. Estimated fetal weight by ultrasound: a modifiable risk factor for cesarean delivery? Am J JObstet Gynecol 2012; 207:309.e1-e6. [CrossRef]
- Yee LM, Grobman WA. Relationship between third-trimester sonographic estimation of fetal weight and mode of delivery. J Ultrasound Med 2016; 35:701-706. [CrossRef]
- Froehlich RJ, Gandoval G, Bailit JL, et al. Association of recorded estimated fetal weight and cesarean delivery in attempted vaginal delivery at term. Obstet Gynecol 2016; 128:487-494. [CrossRef]
- Matthews KC, Williamson J, Supta S, et al. The effect of a sonographic estimated fetal weight on the risk of cesarean delivery in macrosomic and small for gestational-age infants. J Matern Fetal Neonatal Med 2017; 30:1172-1176. [CrossRef]
- Dude AM, Davis B, Delaney K, Yee LM. Sonographic estimated fetal weight and cesarean delivery among nulliparous women with obesity. Am J Perinatol Rep 2019; 9:e127-e132. [CrossRef]
- Hadlock FP, Deter RL, Harrist RB, Park SK. Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiol 1984; 152:497-501. [CrossRef]
- American Institute of Ultrasound in Medicine. Standards and guidelines for the accreditation of ultrasound practices. Available online: https://www.aium.org/resources/official-statements/view/standards-and-guidelines-for-the-accreditation-of-ultrasound-practices (accessed 28 May 2024).
- American College of Radiology. Physician QA requirements: CT, MRI, nuclear medicine/PET, ultrasound (revised 1-3-2024). Available online: https://accreditationsupport.acr.org/support/solutions/articles/11000068451-physician-qa-requirements-ct-mri-nuclear-medicine-pet-ultrasound-revised-9-7-2021- (accessed 28 May 2024).
- Benacerraf BF, Minton KK, Benson CB, et al. Proceedings: Beyond Ultrasound First Forum on improving the quality of ultrasound imaging in obstetrics and gynecology. Am J Obstet Gynecol 2018; 218:19-28. [CrossRef]
- Society for Maternal-Fetal Medicine. Executive summary: Workshop on developing an optimal maternal-fetal medicine ultrasound practice, February 7-8, 2023, cosponsored by the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, American Institue of Ultrasound in Medicine, American Registry for Diagnostic Medical Sonography, Internation Society of Ultrasound in Obstetrics and Gynecology, Gottesfeld-Hohler Memorial Foundation, and Perinatal Quality Foundation. Am J Obstet Gynecol 2023; 229:B20-4. [CrossRef]
- Geijer H, Geijer M. Added value of double reading in diagnostic radiology, a systematic review. Insights Imaging 2018; 9:287-301. [CrossRef]
- Dinh ML, Yazdani R, Godiyal N, Pfeifer CM. Overnight radiology resident discrepancies at a large pediatric hospital: categorization by year of training, program, imaging modality, and report type. Acta Radiol 2022; 63:122-6. [CrossRef]
- Moriarity AK, Hawkins CM, Geis JR, et al. Meaningful peer review in radiology: a review of current practices and future directions. J Am Coll Radiol 2016; 13:1519-24. [CrossRef]
- Cuckle H, Platt LD, Thornburg LL, et al. Nuchal Translucency Quality Review (NTQR) program: first one and half million results. Ultrasound Obstet Gynecol 2015; 45:199-204. [CrossRef]
- Drukker L, Droste R, Chatelain P, Noble JA, Papageorghiou AT. Expected-value bias in routine third-trimester growth scans. Ultrasound Obstet Gynecol 2020; 55:375-82. [CrossRef]
- Chaudhry H, Del Gaizo AJ, Frigini LA, et al. Forty-one million RADPEER reviews later: what we have learned and are still learning. J Am Coll Radiol 2020; 17:779-785. [CrossRef]
- Maurer MH, Bronnimann M, Schroeder C, et al. Time requirement and feasibility of a systematic quality peer review of reporting in radiology. Fortschr Rontgenstr 2021; 193:160-167. [CrossRef]
- Cavallaro A, Ash ST, Napolitano R, et al. Quality control of ultrasound for fetal biometry: results from the INTERGROWTH-21st project. Ultrasound Obstet Gynecol 2018; 52:332-339. [CrossRef]
- Society for Maternal-Fetal Medicine, Stone J, Bromley B, et al. Developing an optimal maternal-fetal medicine ultrasound practice: a report and recommendations of the workshop of the Society for Maternal-Fetal Medicine. Am J Obstet Gynecol 2024; in press.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).