Submitted:
25 June 2024
Posted:
25 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Clearance
2.3. Chemiluminescent Immunoassay Anti-SARS-CoV-2 IgG Antibody Test
2.4. SARS-CoV-2 Neutralization Antibody Detection Kit
2.5. Statistical Analysis
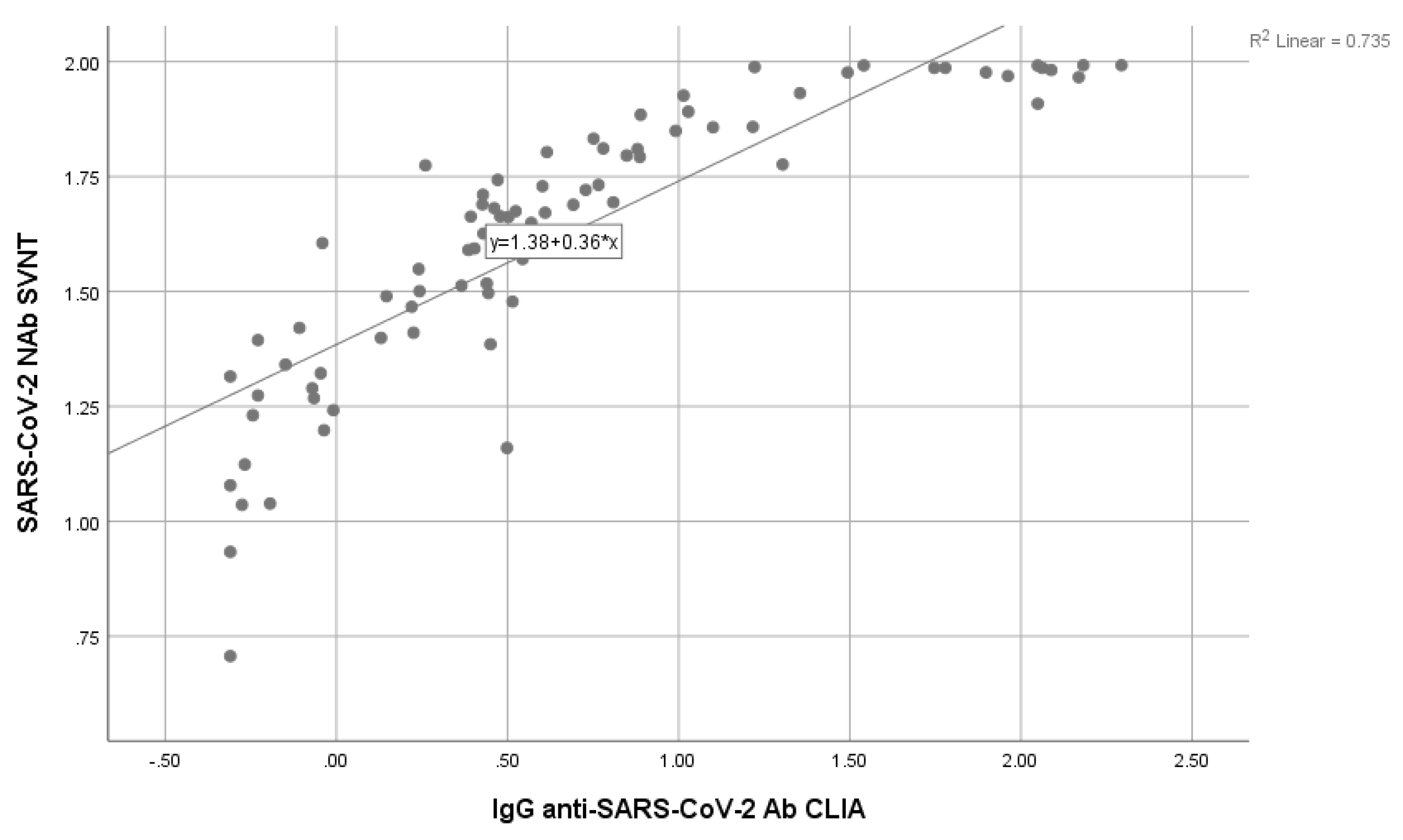
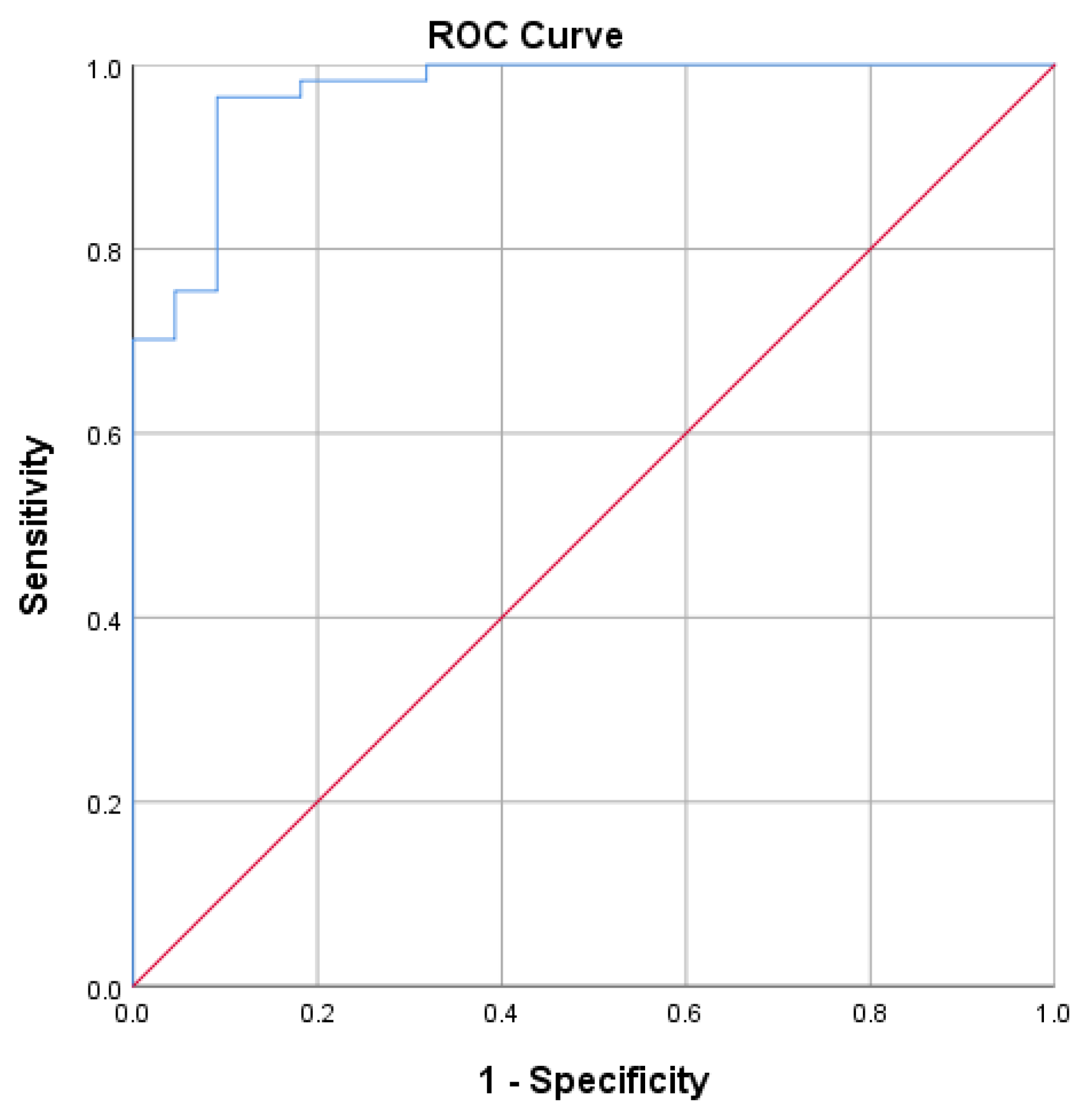
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021 Jul;27(7):1205–11.
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; Van De Veen, W.; Brüggen, M.-C.; O'Mahony, L.; Gao, Y.; Nadeau, K.; A Akdis, C. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020 Jul;75(7):1564–81. [CrossRef]
- Kenny G, O’Reilly S, Wrigley Kelly N, Negi R, Gaillard C, Alalwan D, et al. Distinct receptor binding domain IgG thresholds predict protective host immunity across SARS-CoV-2 variants and time. Nat Commun. 2023 Nov 2;14(1):7015.
- Sil BK, Jahan N, Haq MdA, Oishee MJ, Ali T, Khandker SS, et al. Development and performance evaluation of a rapid in-house ELISA for retrospective serosurveillance of SARS-CoV-2. Ito E, editor. PLOS ONE. 2021 Feb 2;16(2):e0246346.
- Cao Y, Su B, Guo X, Sun W, Deng Y, Bao L, et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell. 2020 Jul;182(1):73-84.e16.
- Embregts CWE, Verstrepen B, Langermans JAM, Böszörményi KP, Sikkema RS, de Vries RD, et al. Evaluation of a multi-species SARS-CoV-2 surrogate virus neutralization test. One Health. 2021 Dec;13:100313.
- Tan CW, Chia WN, Qin X, Liu P, Chen MIC, Tiu C, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol. 2020 Sep;38(9):1073–8.
- Lynch KL, Zhou S, Kaul R, Walker R, Wu AH. Evaluation of Neutralizing Antibodies against SARS-CoV-2 Variants after Infection and Vaccination Using a Multiplexed Surrogate Virus Neutralization Test. Clin Chem. 2022 May 18;68(5):702–12.
- Kolesov DE, Sinegubova MV, Dayanova LK, Dolzhikova IV, Vorobiev II, Orlova NA. Fast and Accurate Surrogate Virus Neutralization Test Based on Antibody-Mediated Blocking of the Interaction of ACE2 and SARS-CoV-2 Spike Protein RBD. Diagnostics. 2022 Feb 3;12(2):393.
- Valcourt EJ, Manguiat K, Robinson A, Chen JCY, Dimitrova K, Philipson C, et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn Microbiol Infect Dis. 2021 Apr;99(4):115294.
- Siemens Healthcare Diagnostics Inc. SARS-CoV-2 IgG (sCOVG) Assay for the Detection of IgG Antibodies to SARS-CoV-2. 2021.
- GenScript. cPass. SARS-CoV-2 Neutralization Antibody Detection Kit Instruction for Use. GenScript; 2022.
- Qi, S.; Ngwa, C.; Scheihing, D.A.M.; Al Mamun, A.; Ahnstedt, H.W.; Finger, C.E.; Colpo, G.D.; Sharmeen, R.; Kim, Y.; Choi, H.A.; et al. Sex differences in the immune response to acute COVID-19 respiratory tract infection. Biol. Sex Differ. 2021, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between gender. Infectious Diseases (except HIV/AIDS); 2020 Mar.
- Bayram, A.; Demirbakan, H.; Karadeniz, P.G.; Erdoğan, M.; Koçer, I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in healthcare workers. J. Med Virol. 2021, 93, 5560–5567. [Google Scholar] [CrossRef] [PubMed]
- Li Z, Xiang T, Liang B, Deng H, Wang H, Feng X, et al. Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting. Front Immunol. 2021 Dec 22;12:802858.
- Mukherjee S, Pahan K. Is COVID-19 Gender-sensitive? J Neuroimmune Pharmacol. 2021 Mar;16(1):38–47.
- Hou, Y.; Chen, M.; Bian, Y.; Hu, Y.; Chuan, J.; Zhong, L.; Zhu, Y.; Tong, R. Insights into vaccines for elderly individuals: from the impacts of immunosenescence to delivery strategies. npj Vaccines 2024, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Ponticelli, D.; Agüero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, I.; Mantovani, L.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Heal. 2022, 203, 97–99. [Google Scholar] [CrossRef]
- Soegiarto G, Wulandari L, Purnomosari D, Dhia Fahmita K, Ikhwan Gautama H, Tri Hadmoko S, et al. Hypertension is associated with antibody response and breakthrough infection in health care workers following vaccination with inactivated SARS-CoV-2. Vaccine. 2022 Jun;40(30):4046–56.
- Soetedjo NNM, Iryaningrum MR, Lawrensia S, Permana H. Antibody response following SARS-CoV-2 vaccination among patients with type 2 diabetes mellitus: A systematic review. Diabetes Metab Syndr Clin Res Rev. 2022 Feb;16(2):102406.
- He, Y.-F.; Ouyang, J.; Hu, X.-D.; Wu, N.; Jiang, Z.-G.; Bian, N.; Wang, J. Correlation between COVID-19 vaccination and diabetes mellitus: A systematic review. World J. Diabetes 2023, 14, 892–918. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, C.; Retnakumar, S.V.; Bayry, J. Obesity negatively impacts maintenance of antibody response to COVID-19 vaccines. Cell Rep. Med. 2023, 4, 101117. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A.; et al. Previous COVID-19 Infection and Antibody Levels After Vaccination. Front. Public Heal. 2021, 9, 778243. [Google Scholar] [CrossRef] [PubMed]
- Zhang R, Khong KW, Leung KY, Liu D, Fan Y, Lu L, et al. Antibody Response of BNT162b2 and CoronaVac Platforms in Recovered Individuals Previously Infected by COVID-19 against SARS-CoV-2 Wild Type and Delta Variant. Vaccines. 2021 Dec 7;9(12):1442.
- Lau CS, Oh MLH, Phua SK, Liang YL, Li Y, Huo J, et al. Kinetics of the Neutralizing and Spike SARS-CoV-2 Antibodies following the Sinovac Inactivated Virus Vaccine Compared to the Pfizer mRNA Vaccine in Singapore. Antibodies. 2022 May 27;11(2):38.
- Liu KT, Han YJ, Wu GH, Huang KYA, Huang PN. Overview of Neutralization Assays and International Standard for Detecting SARS-CoV-2 Neutralizing Antibody. Viruses. 2022 Jul 18;14(7):1560.
- Tiwari AK, Negi G, Jaiswal RM, Aggarwal G, Yadav N, Kumar V, et al. Correlation of sample-to-cut-off ratio of anti-SARS-CoV-2 IgG antibody chemiluminescent assay with neutralization activity: a prospective multi-centric study in India. ISBT Sci Ser. 2021 Nov;16(4):269–75.
- Takahashi M, Saito K, Ai T, Nojiri S, Khasawneh A, Paran FJ, et al. Performance evaluation of the Ortho VITROS SARS-CoV-2 Spike-Specific Quantitative IgG test by comparison with the surrogate virus neutralizing antibody test and clinical assessment. Ito E, editor. PLOS ONE. 2023 Jan 24;18(1):e0279779.
- Kitagawa Y, Imai K, Matsuoka M, Fukada A, Kubota K, Sato M, et al. Evaluation of the correlation between the access SARS-CoV-2 IgM and IgG II antibody tests with the SARS-CoV-2 surrogate virus neutralization test. J Med Virol. 2022 Jan;94(1):335–41.
- Ye Q, Zhang T, Lu D. Potential false-positive reasons for SARS-CoV-2 antibody testing and its solution. J Med Virol. 2021 Jul;93(7):4242–6.
- Hicks J, Klumpp-Thomas C, Kalish H, Shunmugavel A, Mehalko J, Denson JP, et al. Serologic Cross-Reactivity of SARS-CoV-2 with Endemic and Seasonal Betacoronaviruses. J Clin Immunol. 2021 Jul;41(5):906–13.
- Lokida D, Karyana M, Kosasih H, Mardian Y, Sugiyono RI, Arlinda D, et al. Performance and correlation of ten commercial immunoassays for the detection of SARS-CoV-2 antibodies. Heliyon. 2022 Dec;8(12):e12614.
- Vilibic-Cavlek T, Bogdanic M, Borko E, Hruskar Z, Zilic D, Ferenc T, et al. Detection of SARS-CoV-2 Antibodies: Comparison of Enzyme Immunoassay, Surrogate Neutralization and Virus Neutralization Test. Antibodies. 2023 May 10;12(2):35.
- Pieri, M.; Infantino, M.; Manfredi, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Sarubbi, S.; Russo, E.; Amedei, A.; et al. Performance evaluation of four surrogate Virus Neutralization Tests (sVNTs) in comparison to the in vivo gold standard test. Front. Biosci. 2022, 27, 74. [Google Scholar] [CrossRef] [PubMed]
- Shibata S, Ishiguro T, Kobayashi Y, Koike M, Numano T, Shimizu Y, et al. High incidence of false-positive results of IgG antibody against SARS-CoV-2 with rapid immunochromatographic antibody test due to human common cold coronavirus infection. Respir Med Case Rep. 2020;31:101180.
- Liu, G.; Rusling, J.F. COVID-19 Antibody Tests and Their Limitations. ACS Sensors 2021, 6, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang H, Jia Y, Ji Y, Cong X, Liu Y, Yang R, et al. Inactivated Vaccines Against SARS-CoV-2: Neutralizing Antibody Titers in Vaccine Recipients. Front Microbiol. 2022 Mar 10;13:816778.
- Gillot C, Favresse J, David C, Maloteau V, Dogne JM, Douxfils J. An Evaluation of a SARS-CoV-2 Pseudovirus Neutralization Test and A Comparison to a SARS-CoV-2 Surrogate Virus Neutralization Test in a COVID-19 Long-Term Follow-Up Cohort. Microbiol Res. 2024 Mar 21;15(1):422–30.
| Charateristics | Frequency (n=79) |
Percentage (%) |
|
|---|---|---|---|
| Age, (years) | |||
| Median | 48 | ||
| Min-Max | 21-76 | ||
| Sex | |||
| Male | 34 | 43 | |
| Female | 45 | 57 | |
| History of Smoking | |||
| No | 74 | 93.7 | |
| Yes | 5 | 6.3 | |
| Comorbidity | |||
| History of Chronic Illness | 19 | 24.1 | |
| Hypertension | 12 | 15.2 | |
| Type 2 Diabetes Mellitus | 5 | 6.3 | |
| Asthma Bronchiale | 3 | 3.8 | |
| Chronic Obstructive Pulmonary Disease | 1 | 1.3 | |
| Body Mass Index (kg/m2) | |||
| Underweight (<18.5) | 1 | 1.3 | |
| Normal weight (18.5-24.9) | 33 | 41.8 | |
| Overweight (25-29.9) | 34 | 43 | |
| Obese (≥30) | 11 | 10.1 | |
| Complete COVID-19 Vaccination* | 58 | 73.4 | |
| Previous COVID-19 Infection | 2 | 2.5 | |
| IgG anti-SARS-CoV-2 Ab CLIA (U/L) | |||
| Median | 3.18 | ||
| Min-Max | 0.49-196.66 | ||
| SARS-CoV-2 SVNT (%) | |||
| Median | 46.91 | ||
| Min-Max | 5.09-98.19 |
| SVNT | ||||||
|---|---|---|---|---|---|---|
| S-RBD SARS-CoV-2 IgG CLIA (BAU/mL) | Protective (≥30%) | Non-Protective (<30%) | Sensitivity (%) |
Specificity (%) |
Positive Predictive Value (%) |
Negative Predictive Value (%) |
| ≥37.29 | 55 | 2 |
90.5 |
|||
| <37.29 | 2 | 20 | 96.5 | 96.5 | 90.9 | |
| Total | 57 | 22 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





