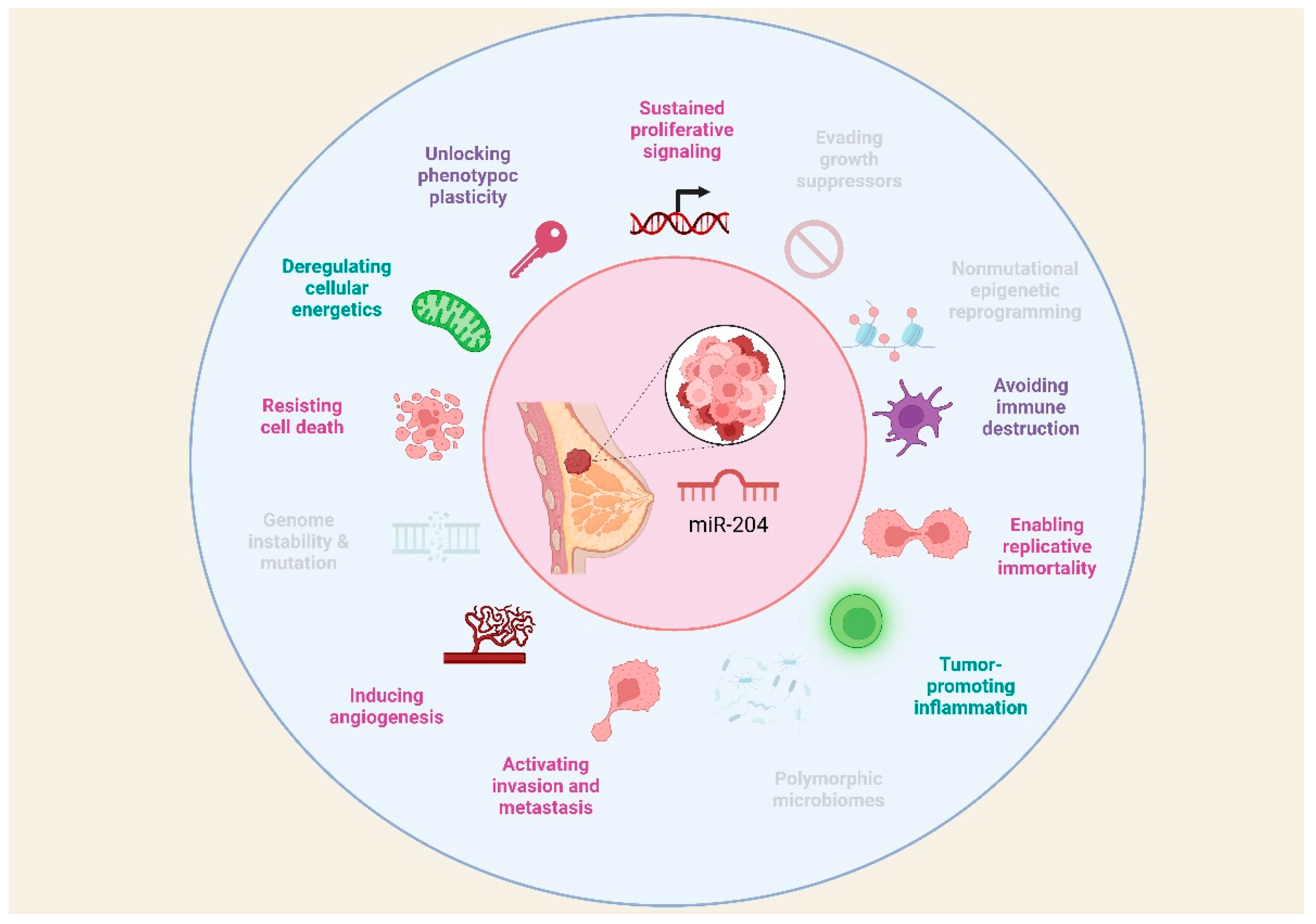
3.1. Cell Proliferation
Proliferation plays a crucial role in the advancement of BC, as it does in all types of cancers. Uncontrolled cell proliferation is a defining hallmark of cancer and contributes to the growth, invasion, and metastasis of tumors [
52]. miR-204 has an important role in inhibiting proliferation in different cell types [
53]. Target genes of miR-204 involved in proliferation in BC are shown in
Table 1. miR-204 downregulation in various types of cancer is related to an increase in cellular division and instauration of tumors [54-56]. For instance, it was found that overexpression of miR-204 in MCF7 BC cells inhibited their proliferation. The results showed that the upregulation of miR-204 triggered apoptotic cell death in MCF7 BC cells. Moreover, the analysis of the cell cycle revealed that miR-204 overexpression caused G2/M cell cycle arrest in these cancer cells. Also, it was shown that PTEN is a target of miR-204. Since PTEN regulates the PI3K/AKT signaling pathway, the effect of miR-204 overexpression was also assessed on this pathway and showed that miR-204 overexpression inhibits the expression of p-AKT and p-PI3K significantly in MCF7 [
35] and MDA-MB-231 [
57] BC cells. In the same way, T3 stimulation greatly enhanced the proliferation of MCF7 and T47D cells by decreasing the levels of miR-204 leading to increased expression of AREG, which in turn activates the AKT signaling pathway and promotes cell proliferation [
58].
miR-204 might also have an anti-oncogenic impact on BC cells by suppressing the TGFβ pathway since restoring miR-204 expression using RNA mimics in MDA-MB-231 and MCF-7 cells led to decreased cell proliferation [
50]. Transcriptome analysis of MDA-MB-231 showed reduced expression of genes related to cell proliferation, such as ANGPT1 and TGβR2. Knocking down TGFβR2, but not ANGPT1, slowed down cell proliferation [
59].
Besides, it has been shown that miR-204 directly targets FOXA1, binding to a complementary region. As a result, miR-204 controls the biological activities of BC cells by influencing cell proliferation through its interaction with FOXA1 [
60]. These findings indicate that miR-204 acts as a tumor suppressor in BC by hindering proliferation and inducing apoptosis in MCF7 cells. In the same manner, miR-204 targets and negatively regulates the function of COX5A. indicating that by targeting and downregulating COX5A, miR-204 could inhibit the proliferation of MCF7 and T-47D cells in BC [
61].
The modulation of mir-204 can be caused by the action of lncRNAs such as DSCAM-AS1 which is upregulated in BC tissue samples meanwhile mir-204 is downregulated. DSCAM-AS1 was discovered to be increased in HCC1937 BC cells, leading to enhanced cell proliferation and apoptosis evasion by blocking miR-204-5p and raising RRM2 expression [
62].
Conversely, the proliferation of MCF-7 and MDA-MB-231 cells is induced by miR-204 and miR-211, as assessed by cell proliferation and colony-forming assay, showing that tumor suppressors MX1 and TXNIP are direct targets of these miRs. Moreover, a strong association between miR-204 and miR-211 expression in BC tissue was observed. These results suggest that miR-204/211 contribute to increased cell proliferation, particularly in MCF-7 and MDA-MB-231 BC cells, through the downregulation of tumor suppressor genes [
50].
3.2. Cell Death Resistance
Programmed cell death constitutes a natural mechanism that acts as a barrier against cancer development. Apoptosis, a type of programmed cell death, is triggered by the imbalance between pro- and anti-apoptotic molecules. This process involves the activation of caspases, which induce cell disintegration. Consequently, resistance to programmed cell death emerges as one of the most significant abilities that malignant cells acquire during oncogenesis, thereby facilitating successful tumor formation [
52,
63].
The balance between pro- and anti-apoptotic molecules can be modulated by intracellular and extracellular signals. Various miRNAs have been identified as regulators of programmed cell death. The role of miR-204 in BC is subject to controversy, as it has been associated with both anti-tumor and pro-tumor effects, due to its influence on modulating various aspects of cancer, particularly in promoting or resisting tumor cell death [
64]. Target genes of miR-204 involved in cell death resistance in BC are shown in
Table 2.
On one hand, it is reported that miR-204 promotes the death of BC cells by regulating target genes involved in cell survival. Both cell lines and BC tissue samples tend to exhibit decreased expression of miR-204 [65-67]. This reduction has been related to a worse prognosis [
68]. Interestingly, increased expression of miR-204 inhibits cell proliferation, leads to cell cycle arrest in the G2/M phase, and increases apoptosis [
65,
67,
69], an effect that has been reversed when using miR-204 inhibitors [
66].
Mechanistically, FOXA1 has been identified as a target of miR-204 [
65]. FOXA1 is a transcription factor that promotes cell growth and inhibits apoptosis [
70]. Therefore, by inhibiting FOXA1, miR-204 acts as a tumor suppressor promoting cell death [
65]. Additionally, miR-204 downregulates Bcl-2 [
66], and Bcl-2 specifically binds to FOXA1 [
71], suggesting the existence of a positive feedback loop between miR-204, FOXA1, and Bcl-2, which together could modulate apoptosis through the STAT3/Bcl-2/survivin pathway [
65].
A study conducted with BC tissue samples and cell lines revealed that another target gene of miR-204 is JAK2, whose signaling is associated with proliferation and apoptosis in cancer [
72,
73]. STAT3 is the main downstream target of phosphorylated JAK2 and is constitutively activated in various tumors, exerting oncogenic and anti-apoptotic functions [
74]. When STAT3 dimerizes and translocate to the nucleus, it regulates the transcription of anti-apoptotic genes Bcl-2 and survivin [
75,
76]. Therefore, when miR-204 suppresses JAK2, it inhibits the activation of STAT3, Bcl-2, and survivin [
66].
Consistent with reports supporting the role of miR-204 in inducing cancer cell death, a research group used bioinformatics tools to identify PTEN as a target of miR-204. However, in this case, the overexpression of miR-204 also induces an increase in PTEN expression. PTEN is the major brake of the PI3K/Akt signaling pathway [
77], therefore the overexpression of miR-204 significantly reduces p-Akt and p-PI3K, affecting cell survival [
67]. Complementarily, it has also been reported that PIK3CB, the main regulator of the PI3K/Akt pathway, is a direct target of miR-204-5p, the major strand of mature miR-204 [
19]. Accordingly, the overexpression of miR-204-5p limits the viability of BC cells by decreasing the expression of PI3KCB [
69].
Although apoptosis was considered the main form of programmed cell death some years ago, it is now recognized that cell death can occur through various programmed mechanisms, many of which have been identified to be involved in carcinogenesis [
78]. Recently, it has been described that intracellular copper accumulation contributes to the aggregation of mitochondrial lipoylated proteins, triggering a novel form of programmed cell death called cuproptosis [
79]. However, there is evidence that deregulation in copper levels promotes the development and progression of cancer, although more studies on this type of cell death are needed [
80]. An analysis conducted with online database information identified that overexpression of miR-204-5p in BC was associated with better overall survival. Additionally, it has been proposed that miR-204-5p could be an upstream regulator of SLC31A1, a gene associated with cuproptosis in BC [
81].
Contrary to findings suggesting that miR-204 induces programmed cell death in cancer cells, certain research has linked increased miR-204 expression with resistance to tumor cell death. A study conducted with BC cell lines and tissue samples identified tumor suppressors MX1 and TXNIP as targets of miR-204, suggesting that the functions of this miRNA are directed toward cell death suppression and proliferation stimulation [
64].
In the same context, with
in vitro and
in vivo models of estrogen receptor (ER)-negative and ER-positive BC, downregulation of miR-204 has been observed to promote ERα expression. Consequently, it has been proposed that miR-204 regulates ERα [
82], an important regulatory axis since ERs play a crucial role in BC evolution and treatment response. Loss of these receptors is associated with increased tumor aggressiveness and worse prognosis [
83]. Notably, miR-204 is considered to affect Akt phosphorylation by ERα [
84]. It was observed that when miR-204 decreased or ERα increased, Akt phosphorylation decreased, exerting an inhibitory effect on Mcl-1 expression. Since Mcl-1 contributes to regulating the balance between cell survival and death signals, tumor cells can increase their expression to avoid apoptosis and proliferate uncontrollably [
82].
Additionally, it has been demonstrated that miR-204 significantly inhibits caspase-3 activity after Tamoxifen treatment [
82], a chemotherapy drug that inhibits ERα target gene expression, regulating the cell cycle and apoptosis [
85]. Conversely, decreased miR-204 expression increases the sensitivity of BC cells to Tamoxifen treatment and enhances caspase-3 activity [
82]. Consequently, miR-204 is implicated in resistance to cell death directly and in response to chemotherapy.
Taken together, it has been experimentally demonstrated that miR-204 can exert a dual effect on BC cells, both inducing and inhibiting programmed cell death. As previously proposed by some authors [
64], these divergent findings could indicate that miR-204 may play a dual role in BC development and progression, where the anti-tumorigenic or pro-tumorigenic effect could depend on the specific type and status of the tumor.
3.3. Epithelial-Mesenchymal Transition
EMT in BC is a biological process that results in increased invasiveness and metastatic potential. miR-204 has been found to play a crucial role in regulating EMT in BC [
86]. Several studies have demonstrated that miR-204 acts as a tumor suppressor in BC by targeting key molecules involved in the EMT process (
Table 3), for instance, miR-204 has been shown to directly target and inhibit ZEB2, a transcription factor that promotes EMT, and it is also suggested that MALAT1 might enhance the EMT phenotype via the miR-204/ZEB2 axis. According to this proposal, MALAT1 acts as an endogenous sponge to negatively regulate miR-204 expression. Subsequently, miR-204 suppresses ZEB2 expression by binding to the uncoding region of ZEB2 3-UTR [
87]. Therefore, MALAT1 regulates the miR-204/ZEB2 axis in BC. Also, miR-204 directly targets the gene Six1, which is upregulated in BC specimens. Overexpression of Six1 leads to the downregulation of miR-204, which contributes to the promotion of EMT in BC. This regulatory circuit between miR-204 and Six1 constitutes a feedback loop that influences the progression of EMT in BC cells [
88]. In BC cell lines, it has been also reported that the lncRNA ARNILA can sequester miR-204, acting as a competing endogenous RNA (ceRNA) and promoting EMT by competitively binding to miR-204 while up-regulates Sox4 [
89].
In an integrative analysis, the expression patterns of several miRNAs related to processes linked to metastasis were evaluated. Dysregulation was observed in miR-204, miR-200c, miR-34a, and miR-10b, potentially resulting in a reduced survival rate in BC. Furthermore, these miRNAs could be modulated through the overexpression of OCT4, SOX2, KLF4, c-MYC, NOTCH1, SNAI1, ZEB1, and CDH2, genes directly associated with EMT [
90].
3.4. Stemness
Cancer stem cells (CSCs) are transformed cells with self-renewal capacity, the ability to initiate tumor formation, the capability to disseminate to distant sites, clonal long-term repopulation potential, and phenotypic plasticity to differentiate into other cell types, both stem and non-stem states [
91,
92]. Considering that stemness involves self-renewal and differentiation capacity in tumor cells, vital processes in carcinogenesis, it has been proposed as a hallmark of cancer [
93].
The recurrence of BC in patients treated with chemotherapy or surgical interventions is a significant challenge in managing this disease [
94]. BC stem cells (BCSCs) are believed to be primarily responsible for this phenomenon because, due to their potential for self-renewal and differentiation in multiple directions, they act as seed cells producing new malignant cells [
95]. Surprisingly, most deaths associated with this type of cancer are not attributable to the primary tumor but to metastasis to other organs [
96], a process closely associated with the presence and activity of BCSCs. Additionally, these cells exhibit unique resistance to chemotherapy, underlining their crucial role in BC progression and recurrence [
97].
Stemness is a process that can be post-transcriptionally regulated by miRNAs. In this regard, it has been reported that miR-204 may promote the presence of BCSCs (target genes of miR-204 involved in cell stemness in BC are shown in
Table 4). A study conducted bioinformatics analysis and
in vitro assays to analyze this hallmark in BC and identified miR-204 targets including CD44, FOXC1, HOTTIP, MYC, NOTCH1, SOX2, STAT3, and VIM1, genes involved in pathways associated with self-renewal [
90].
Conversely, another research has suggested that miR-204 may suppress stemness. One target of miR-204 is SAM68, a molecule that positively correlates with the self-renewal potential of BC cells by activating the Wnt/β-catenin pathway [
95]. Consistently, a study with tumor specimens, cell lines, and a murine model of BC reported that miR-204 can bind to the 3’-UTR of TCF4. The downregulation of miR-204 then upregulates TCF4, leading to the activation of the Wnt/β-catenin signaling pathway [
97]. This effect is explained given that TCF4, along with the coactivator β-catenin, functions as the main transcriptional mediators of the Wnt pathway [
98]. It is important to note that aberrant activation of the Wnt/β-catenin signaling pathway plays a crucial role in the origin and maintenance of CSCs [
99].
These findings highlight that the role of miR-204 in inducing CSCs remains controversial [
90]. Most studies consider miR-204 as a tumor suppressor, accordingly its reduced expression is associated with a more aggressive phenotype of BC [
100]. Nevertheless, some research suggests that miR-204 could be an onco-miR in BC [
101].
The plasticity of CSCs is particularly important in cancer evolution, allowing them to adapt and survive the stressful conditions of the tumor microenvironment (TME), even in the face of alterations induced by oncologic therapy. Therefore, CSCs can also induce treatment resistance by promoting metabolic reprogramming in the TME [
91].
3.5. Metabolic Reprogramming
The basis of malignant neoplasia lies in the capacity for uncontrolled proliferation and migration acquired by tumor cells, which requires adjustments in energy metabolism to drive this process. This metabolic reprogramming is orchestrated mainly by proteins involved in other cancer hallmarks [
102], leading to an understanding of how the integration of all these distinctive characteristics acquired by cancer cells allows for cancer development and progression.
This metabolic reprogramming involves energy changes in both tumor and non-tumor cells, implying complex pathways of cellular regulation as well as intercellular communication, in which miRNAs may participate. Through studies with cell cultures and murine models, it has been identified that the overexpression of miR-204 alters genes involved in the metabolism of BC cells, achieving a significant metabolic suppression in them (target genes of miR-204 involved in metabolic reprogramming in BC are shown in
Table 5) [
69].
Additionally, it has also been reported that the presence of miR-204 in blood is associated with hypermetabolism and energy consumption in BC patients since miR-204-5p secreted in exosomes by tumor cells targets the VHL gene in adipose tissue, inducing the expression of the HIF1A protein and, in turn, the activation of the leptin signaling pathway [
103]. Leptin derived from adipocytes regulates the expression of genes associated with tumor progression, such as those involved in adhesion, invasion, angiogenesis, and apoptosis [
104,
105]. Furthermore, leptin signaling increases lipolysis in white adipose tissue, promoting cachexia [
103], a multiorgan wasting syndrome characterized by systemic inflammation accompanied by the loss of adipose tissue and skeletal muscle [
106]. Therefore, increased leptin signaling in the body is associated with BC aggressiveness [
103].
It is crucial to consider that changes in metabolism also involve the metabolic reprogramming of non-tumor cells present in the TME, which is the result of an adaptation process to factors derived from tumor cells. It has been reported that BC cells secrete extracellular vesicles containing miR-204. These vesicles are subsequently taken up by nearby cancer-associated fibroblasts (CAFs), where miR-204 acts on RAGC, a component of Rag GTPases that regulates mTORC1 signaling. mTORC1 is a protein kinase that coordinates cell growth in response to available nutrients. Consequently, by educating CAFs to reduce mRNA translation, BC cells remodel the amino acid metabolic flux and regulate proteins produced by the stroma during periods of nutrient fluctuation [
107]. As a result of this suppression in protein synthesis, CAFs could utilize intracellular amino acids to produce energy and possibly transfer energy to cancer cells through the secretion of metabolites. Additionally, suppression of mTORC1 signaling induces an increase in the synthesis of certain proteins associated with autophagy, lipid metabolism, survival, and intercellular communication, contributing to the survival of CAFs to continue favoring the growth of tumor cells [
107]. In this way, CAFs participate in shaping tumor metabolism by providing nutrients to cancer cells and modulating their metabolic pattern [
108,
109], highlighting the importance of the interaction between TME cell populations to sustain this and other cancer hallmarks.
3.6. Tumor Microenvironment Remodeling
Cancer involves the formation of a complex ecosystem called the TME, where tumor cells interact with other non-cancerous cell populations, collectively driving tumor formation, progression, and treatment response [
110,
111]. The TME promotes a state of chronic inflammation that contributes to multiple hallmark capabilities by providing bioactive molecules such as growth factors that sustain tumor proliferation, survival factors that limit cell death, matrix remodeling enzymes that facilitate invasion and metastasis, as well as cytokines that modulate various cellular programs [
102].
The interaction between tumor cells and their microenvironment is a crucial determinant of the abilities acquired by cancer [
112]. Therefore, intercellular communication in the TME is of great relevance and involves complex networks in which molecules such as miRNAs can participate. Models of BC
in vivo show that miR-204 shapes the TME by regulating the expression of key cytokines involved in monocyte and lymphocyte infiltration (target genes of miR-204 involved in tumor microenvironment modeling in BC are shown in
Table 5) [
69].
Tumors with higher expression of miR-204 show reduced infiltration of CD11b+ myeloid cells, fewer myeloid-derived suppressor cells (MDSCs), macrophages, and NK cells. Nevertheless, the increase in miR-204 expression is associated with an enhancement in infiltrating CD8+ and CD4+ T cells, including regulatory T cells [
69]. This phenomenon is related to the fact that the overexpression of miR-204 induces dysregulation in cytokine production [
69]. This modulation of immune cell infiltration is relevant, as the reduction in myeloid cell chemotaxis in the TME may be associated with decreased metastasis, as myeloid cells tend to promote tumor invasion and migration [
113].
Regarding this dysregulation in cytokine production in the TME, it has been identified that miR-204 induces a decrease in the expression of genes involved in TGF-β signaling, including PTGS2, which is implicated in the expression of IL-11. Additionally, miR-204 directly binds to the IL11 3’-UTR, exacerbating the reduction in IL-11 production by BC cells [
114]. Complementarily, miR-204 has been observed to inhibit the lncRNA DGUOK-AS1, a molecule that promotes cell migration, angiogenesis, and macrophage migration by inducing an increase in IL-11 production [
115].
IL-11 is an IL-6 family cytokine that can be secreted by various cells in the TME in response to inflammatory stimuli [
116,
117]. In this regard, IL-11 plays an important role in promoting angiogenesis, invasion, and migration of tumor cells, as well as in proinflammation and differentiation of tumor-associated macrophages (TAMs) [
118,
119]. Cancer cells can recruit monocytes to the TME through the secretion of cytokines and chemokines [
120], which subsequently differentiate into TAMs and, together with other cells in the microenvironment, modulate tumor behavior. Thus, TAMs infiltration has been significantly associated with clinical behavior and chemoresistance in BC [
115]. Consequently, high expression of IL-11 has been related to high histological grade, poor survival [
121], and BC bone metastasis development [
122].
Interestingly, miR-204 not only shapes the TME through the regulation of cytokine production but also by modulating the expression of immune checkpoints. It is reported that miR-204 is a potential upstream regulator of SLC31A1, which is positively correlated with the expression of immune checkpoints, notably CD274 and CTLA4, regulating the effective response of T lymphocytes. Therefore, the expression of miR-204 could also impact the efficacy of immunotherapy [
81]. The available information highlights the importance of miR-204 in shaping the TME in BC, which impacts tumor progression, treatment response, and consequently, the clinical evolution of patients. Regardless, additional studies are needed to evaluate the effect of this miRNA on the behavior of other populations present in the TME, as well as the effect of factors produced by non-tumor cells on the expression of miR-204 in BC cells.
3.7. Angiogenesis and Vasculogenic Mimicry
In a normal physiological context, angiogenesis plays a vital role in facilitating the growth of new blood vessels from pre-existing ones. This process is essential for the transportation of oxygen and nutrients, thereby ensuring the proper functioning of tissues and organs [
123]. However, in a tumor context, the microenvironment is primarily composed of endothelial cells, which play a significant role in the formation of new blood vessels that not only supply oxygen and nutrients to the tumor but also facilitate the creation of conduits that direct blood flow, thereby maintaining tissue perfusion [123-125]. In the tumor microenvironment, there exists an imbalance between proangiogenic and antiangiogenic factors, which ultimately favors tumor progression [123-125].
Several miRNAs function as either oncoRNAs or tumor suppressors, modulating molecules involved in angiogenesis, a critical process for tumor progression. These miRNAs are referred to as angiomiRNAs [
126], as they regulate angiogenic mechanisms in both normal physiological and pathological contexts. Recently, miR-204 has been related to this process modulating the expression of key molecules (target genes of miR-204 involved in angiogenesis and VM in BC are shown in
Table 6). For instance, in BC cell lines, where vasculogenic mimicry (VM) formation (
in vitro model simulating the generation of three-dimensional channels facilitating oxygen and blood supply) was induced, transfection with miR-204 inhibited VM formation under hypoxic conditions. This inhibition led to a reduction of over 80% in the formation of branch points and capillary tubes compared to non-transfected cells. Additionally, the study evaluated hypoxia-inducible factor-1α (HIF-1α), one of the principal regulators of angiogenesis. A decrease in HIF-1α protein expression was observed in cells transfected with miR-204 [
127].Besides, it was reported that in cancer stem cells CD44
+ CD29
- positive BC cell lines, under hypoxic conditions and following transfection with miR-204, vasculogenic mimicry (VM) formation was reduced, leading to a decrease in branch points. Additionally, a decrease in the protein expression of β-catenin and VEGFA was observed [
128].
In addition, various models have demonstrated the significant role of miR-204 in tumor angiogenesis. Its downregulation has been identified in BC samples, cell lines, and
in vivo models. An inverse correlation has been established between miR-204 expression and the miRNA-204/
ANGPT1/TGFβR2 axis, wherein lower miR-204 expression is associated with higher expression levels of ANGPT1 and TGFβR2 proteins [
59].
Lastly, long non-coding RNAs have been discovered to act as sponges, sequestering specific miRNAs, such as HOTAIR. HOTAIR is up-regulated in BC tumors and is believed to sequester miR-204. It has been identified that HOTAIR contains a conserved potential binding site for miR-204. Consequently, cell lines exhibiting high miR-204 expression demonstrated low expression levels of HOTAIR. Also, in a bioinformatics analysis, it was found that miR-204 can bind to the 3' UTR region of focal adhesion kinase 1
(FAK) which has even been found to be related to processes of migration and vasculogenic mimicry [
129].
3.8. Invasion, Migration and Metastasis
Some of the fundamental processes for malignant progression are migration, invasion, and metastasis. These processes are essential for tumor cells to spread successfully. Migration depends on the morphology of the cells that will migrate, thus relying on various factors such as genetic and molecular cell-cell junctions, rearrangement of the cytoskeleton, and adhesion to the matrix [
130,
131]. On the other hand, invasion and metastasis can occur through a series of steps. This process starts with local invasion, followed by the intravasation of cancer cells into blood vessels. Subsequently, extravasation takes place, involving the migration of cancer cells to distant tissues. Then, micrometastasis develops, forming small nests of cancer cells. Finally, colonization occurs, as these tumor nests or micrometastatic lesions grow into tumors [
102,
132].
It has been shown that low expression of miR-204 enhances key processes such as migration, invasion, and metastasis. Therefore, in BC samples, the microdeletion of genomic loci specifically containing miR-204 has been directly linked to activation pathways in tumor progression [
100]. Besides, transfection with miR-204 inhibits migration and invasion. Additionally, a bioinformatics analysis revealed that the
PTEN gene is a target of miR-204, which consequently negatively modulates signaling pathways such as PI3K/AKT. As a result, miR-204 inhibits metastasis in BC cell lines by targeting the PI3K/AKT signaling pathway [
35].
Down-regulation of miR-204 has been observed in cell lines enhancing overexpression of
FOXA1. Consequently, increasing miR-204 expression leads to the suppression of cell invasion and metastasis processes through FOXA1 downregulation [
65]. Another target of these miRNAs is the adaptor protein complex 1, sigma subunit 3 (
AP1S3), which is also overexpressed in BC. However, upregulation of miR-204 significantly block cancer cell migration and invasion by downregulating
AP1S3 at the protein level [
133]. Also, the role of miR-204 in regulating a member of the atypical right open reading frame (RIO) protein kinase family,
RIOK1, was investigated. It was observed that there was a significant reduction in migration
in vitro assays, thereby impacting tumor progression [
134].
In addition, significant overexpression of the long non-coding RNA DSCAM-AS1 (Down syndrome antisense cell adhesion molecule) has been observed in BC samples and a specific cell line. This lncRNA functions as an endogenous competitor (ceRNA) of miR-204. A negative association between DSCAM-AS1 and miR-204 has been shown to enhance BC cell migration. However, when co-transfection of DSCAM-AS1 and miR-204 is carried out, the
RRM2 Ribonucleotide reductase M2 (
RRM2) gene is suppressed in BC cells, thus DSCAM-AS1/miR-204-5p/
RRM2 is an important pathway in BC to support cell migration and invasion is observed [
135].
It has been observed that high expression of exosomal circRHOT1 lead to down-regulation of miR-204 expression and the gene that regulate this miRNA is Protein arginine methyltransferase 5 (
PRMT5) so miR-204-5p/
PRMT5 axis could participate in cancer progression. However, when circRHOT1 is suppressed, miR-204 expression is significantly increased. In the same study, an
in vivo model was utilized, where a miR-204 inhibitor was combined with circRHOT1 suppression. This combination facilitated the recovery of cell function and promoted migration, invasion, and other processes related to tumor progression. These effects were achieved by increasing the expression of E-cadherin and N-cadherin while decreasing vimentin, thus inhibiting the EMT process [
136].