Submitted:
25 June 2024
Posted:
26 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ascidian Collection and Experimental Design
2.2. Immunohistochemistry
2.3. Extract Preparation and Protein Concentration
2.4. Phenoloxidase (PO)
2.5. Glutathione Peroxidase (GPx)
2.6. Lysozyme (LYS)
2.7. Alkaline Phosphatase (ALP) and Esterase (EST)
2.8. Statistical Analyses
3. Results
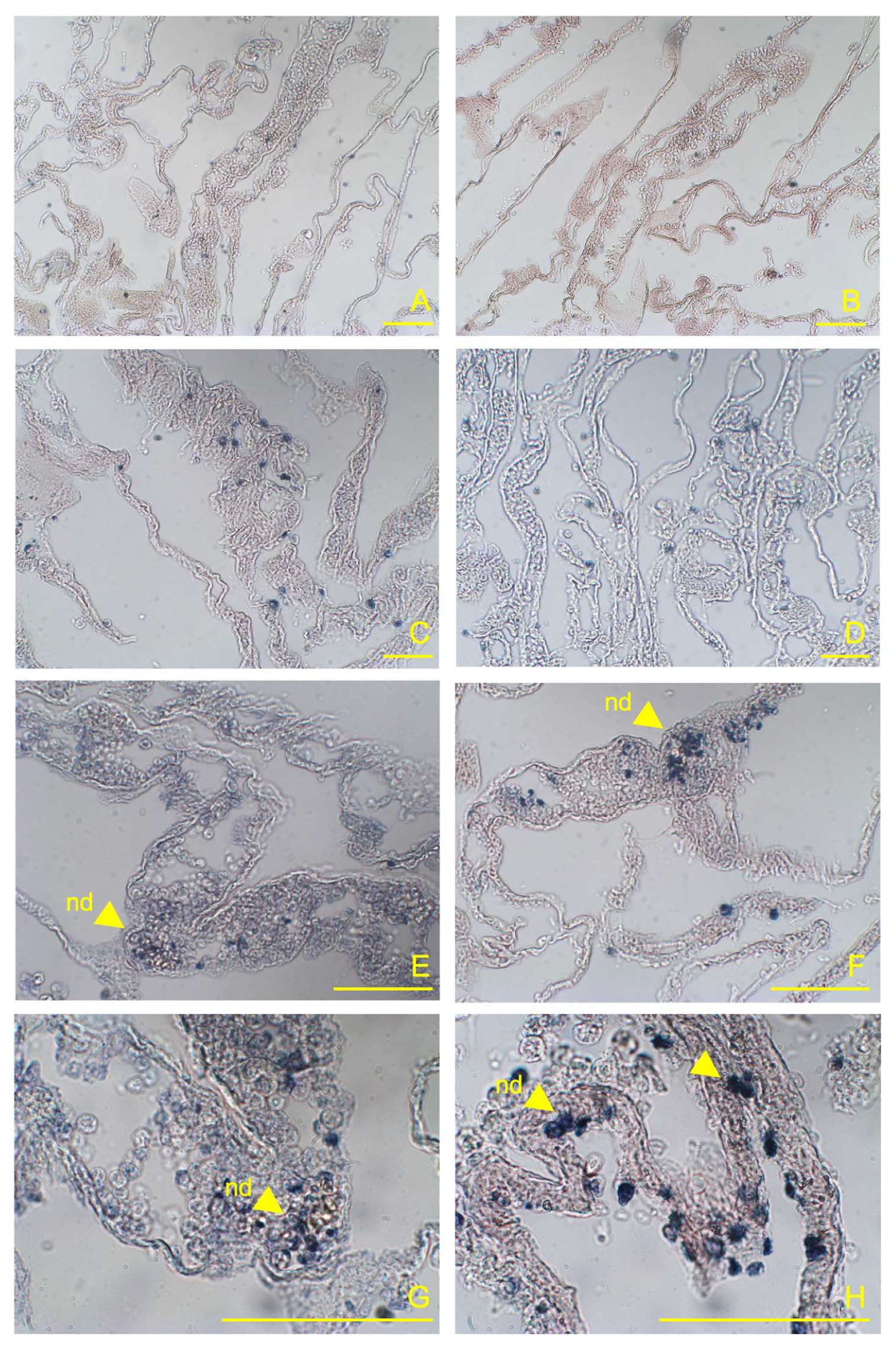
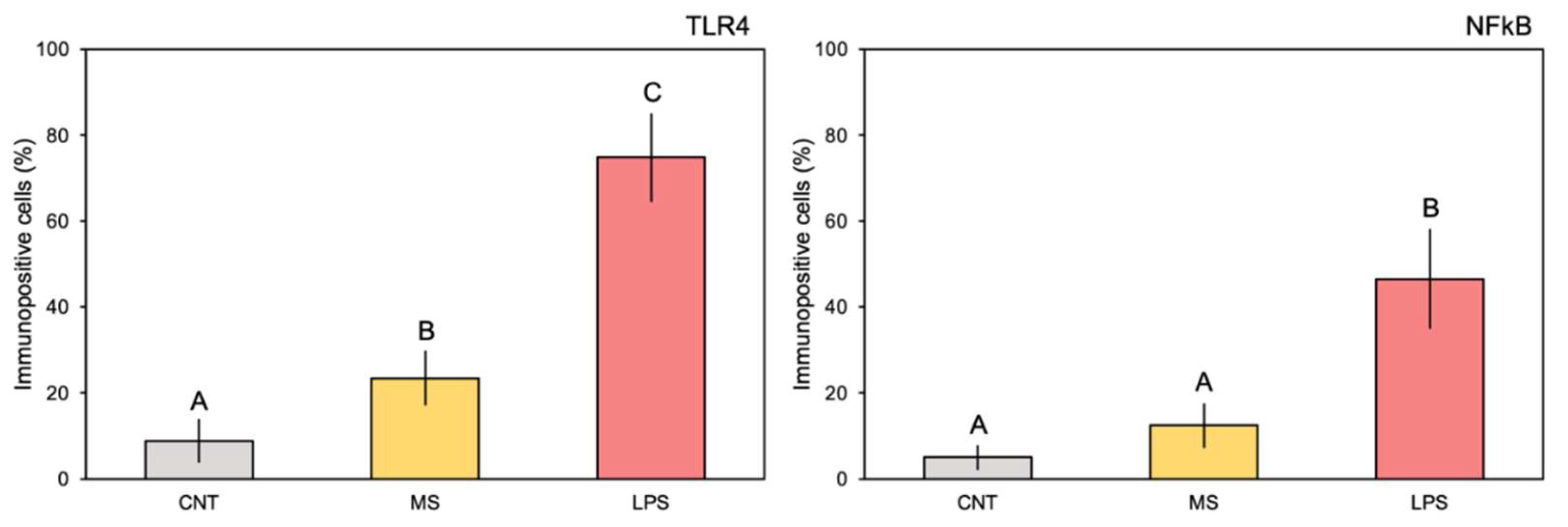
3.1. TLR4 and NFκB Immunolocalization
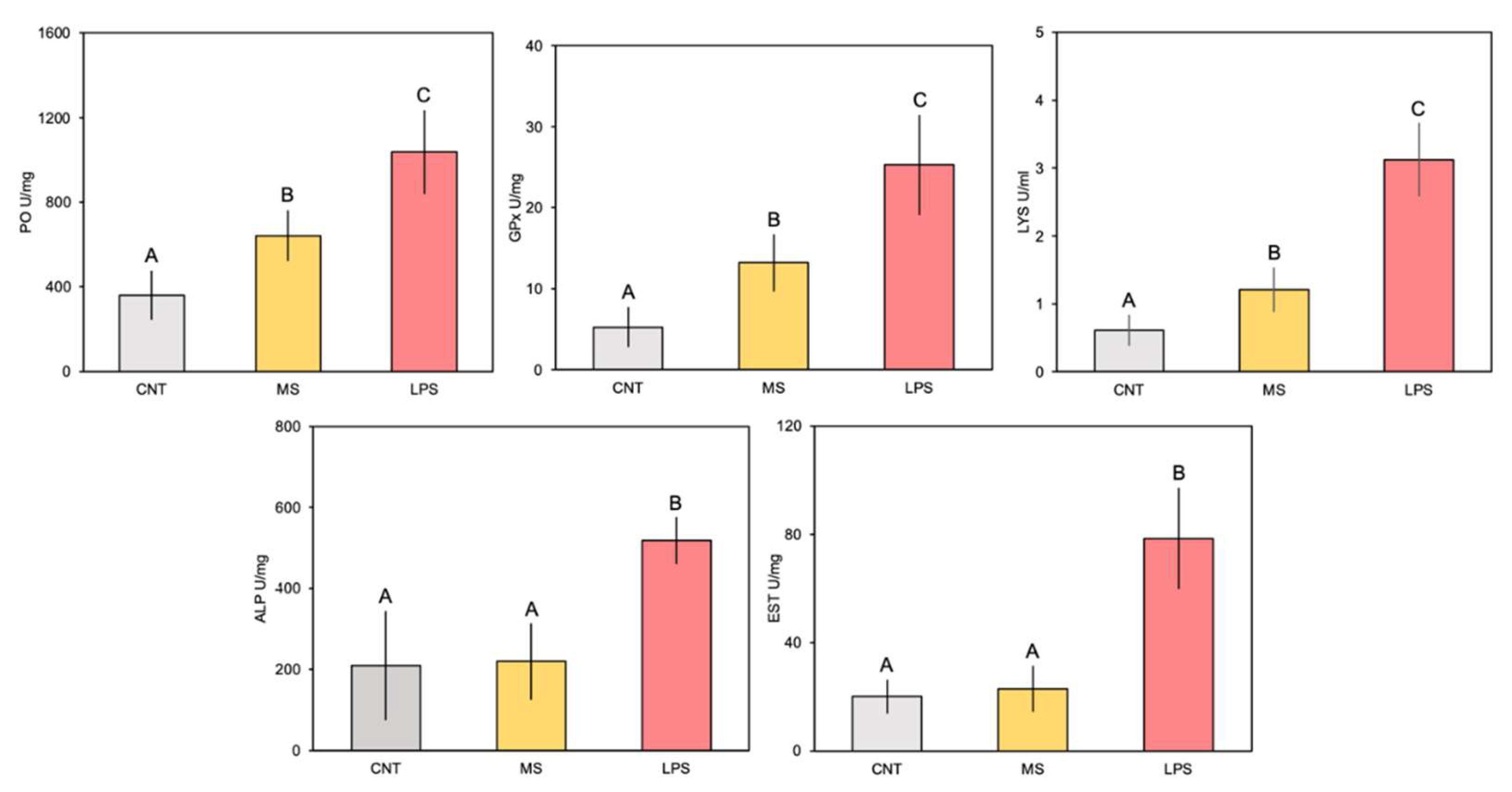
3.2. Enzymatic Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 2006, 439, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Delsuc, F.; Tsagkogeorga, G.; Lartillot, N.; Philippe, H. Additional molecular support for the new chordate phylogeny. Genesis 2008, 46, 592–604. [Google Scholar] [CrossRef]
- Vizzini, A. Gene expression and regulation of molecules involved in pharynx inflammatory response induced by LPS in Ciona intestinalis. Invertebrate Survival Journal 2017, 14, 119–128. [Google Scholar] [CrossRef]
- Longo, V.; Parrinello, D.; Longo, A.; Parisi, M.G.; Parrinello, N.; Colombo, P.; Cammarata, M. The conservation and diversity of ascidian cells and molecules involved in the inflammatory reaction: The Ciona robusta model. Fish & Shellfish Immunology 2021, 119, 384–396. [Google Scholar] [CrossRef]
- Parrinello, N.; Vizzini, A.; Salerno, G.; Sanfratello, M.A.; Cammarata, M.; Arizza, V.; Vazzana, M.; Parrinello, D. Inflamed adult pharynx tissues and swimming larva of Ciona intestinalis share Ci TNFα-producing cells. Cell and tissue research 2010, 341, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Arizza, V.; Bonura, A.; La Paglia, L.; Urso, A.; Pinsino, A.; Vizzini, A. Transcriptional and in silico analyses of MIF cytokine and TLR signalling interplay in the LPS inflammatory response of Ciona robusta. Scientific Reports 2020, 10, 11339. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Ogasawara, M.; Sekiguchi, T.; Kusumoto, S.; Satake, H. Toll-like receptors of the ascidian Ciona intestinalis: prototypes with hybrid functionalities of vertebrate Toll-like receptors. Journal of biological chemistry 2009, 284, 27336–27343. [Google Scholar] [CrossRef] [PubMed]
- Ozinsky, A.; Underhill, D.M.; Fontenot, J.D.; Hajjar, A.M.; Smith, K.D.; Wilson, C.B.; Schroeder, L.; Aderem, A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proceedings of the National Academy of Sciences 2000, 97, 13766–13771. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Muzio, M.; Ni, J.; Feng, P.; Dixit, V.M. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science 1997, 278, 1612–1615. [Google Scholar] [CrossRef]
- Wesche, H.; Henzel, W.J.; Shillinglaw, W.; Li, S.; Cao, Z. ; MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity 1997, 7, 837–847. [Google Scholar] [CrossRef]
- Häcker, H.; Redecke, V.; Blagoev, B.; Kratchmarova, I.; Hsu, L.C.; Wang, G.G.; Kamps, M.P.; Raz, E.; Wagner, H.; Häcker, H.; Mann, M.; Karin, M. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 2006, 439, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harbor perspectives in biology 2009, 1, a000034. [Google Scholar] [CrossRef]
- Sun, S.C.; Chang, J.H.; Jin, J. Regulation of nuclear factor-κB in autoimmunity. Trends in immunology 2013, 34, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Beinke, S.; Ley, S.C. Functions of NF-κB1 and NF-κB2 in immune cell biology. Biochemical Journal 2004, 382, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Vallabhapurapu, S.; Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annual review of immunology 2009, 27, 693–733. [Google Scholar] [CrossRef]
- Sun, S.C. Non-canonical NF-κB signaling pathway. Cell research 2011, 21, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Ley, S.C. New insights into NF-κB regulation and function. Trends in immunology 2008, 29, 469–478. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.C. NF-κB in inflammation and renal diseases. Cell Biosci 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Satoh, N.; Yokosawa, H. Involvement of Rel/NF-κB in regulation of ascidian notochord formation. Development, growth & differentiation 2001, 43, 145–154. [Google Scholar] [CrossRef]
- Kawai, N.; Takahashi, H.; Nishida, H.; Yokosawa, H. Regulation of NF-κB/Rel by IκB is essential for ascidian notochord formation. Developmental biology 2005, 277, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, N.; Arizza, V.; Chinnici, C.; Parrinello, D.; Cammarata, M. Phenoloxidases in ascidian hemocytes: characterization of the pro-phenoloxidase activating system. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 2003, 135, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, M.; Arizza, V.; Cianciolo, C.; Parrinello, D.; Vazzana, M.; Vizzini, A.; Salerno, G.; Parrinello, N. The prophenoloxidase system is activated during the tunic inflammatory reaction of Ciona intestinalis. Cell and Tissue Research 2008, 333, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Immesberger, A.; Burmester, T. Putative phenoloxidases in the tunicate Ciona intestinalis and the origin of the arthropod hemocyanin superfamily. Journal of Comparative Physiology B 2004, 174, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Amparyup, P.; Sutthangkul, J.; Charoensapsri, W.; Tassanakajon, A. Pattern recognition protein binds to lipopolysaccharide and β-1, 3-glucan and activates shrimp prophenoloxidase system. Journal of Biological Chemistry 2012, 287, 10060–10069. [Google Scholar] [CrossRef]
- Cammarata, M.; Parrinello, N. The ascidian prophenoloxidase activating system. Invertebrate Survival Journal 2009, 1 (Suppl)), S67–S76. [Google Scholar]
- Vizzini, A.; Parrinello, D.; Sanfratello, M.A.; Trapani, M.R.; Mangano, V.; Parrinello, N.; Cammarata, M. Upregulated transcription of phenoloxidase genes in the pharynx and endostyle of Ciona intestinalis in response to LPS. Journal of invertebrate pathology 2015, 126, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Free radicals in biology and medicine. Oxford university press 2015, USA. [Google Scholar]
- Lesser, M.P.; Bythell, J.C.; Gates, R.D.; Johnstone, R.W.; Hoegh-Guldberg, O. Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. Journal of experimental marine biology and ecology 2007, 346, 36–44. [Google Scholar] [CrossRef]
- Jollès, P.; Jollès, J. What's new in lysozyme research? Always a model system, today as yesterday. Molecular and cellular biochemistry 1984, 63, 165–189. [Google Scholar] [CrossRef]
- Li, H.; Parisi, M.G.; Toubiana, M.; Cammarata, M.; Roch, P. Lysozyme gene expression and hemocyte behaviour in the Mediterranean mussel, Mytilus galloprovincialis, after injection of various bacteria or temperature stresses. Fish & shellfish immunology 2008, 25, 143–152. [Google Scholar] [CrossRef]
- Di Falco, F.; Cammarata, M.; Vizzini, A. Molecular characterisation, evolution and expression analysis of g-type lysozymes in Ciona intestinalis. Developmental & Comparative Immunology 2017, 67, 457–463. [Google Scholar] [CrossRef]
- Parisi, M.G.; Lentini, A.; Cammarata, M. Seasonal changes in morpho-functional aspects of two Anemonia sulcata (Pennant, 1777) wild populations. Marine Biodiversity 2017, 47, 561–573. [Google Scholar] [CrossRef]
- La Corte, C.; Dara, M.; Bertini, F.; Parrinello, D.; Piazzese, D.; Parisi, M.G. Response of Sabella spallanzanii to multiple stressors. The combined effect of infection and copper sulphate. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2023, 263, 109475. [Google Scholar] [CrossRef]
- Bisanti, L.; La Corte, C.; Dara, M.; Bertini, F.; Parrinello, D.; Chemello, R.; Cammarata, M.; Parisi, M.G. How does warmer sea water change the sensitivity of a Mediterranean thermophilic coral after immune-stimulation? Coral Reefs 2024, 43, 137–150. [Google Scholar] [CrossRef]
- Stamatis, H.; Christakopoulos, P.; Kekos, D.; Macris, B.J.; Kolisis, F.N. Studies on the synthesis of short-chain geranyl esters catalysed by Fusarium oxysporum esterase in organic solvents. Journal of Molecular Catalysis B: Enzymatic 1998, 4, 229–236. [Google Scholar] [CrossRef]
- Copeland, R.A. Enzymes: a practical introduction to structure, mechanism, and data analysis; John Wiley & Sons, 2023. [Google Scholar]
- Lopes, D.B.; Fraga, L.P.; Fleuri, L.F.; Macedo, G.A. Lipase and esterase: to what extent can this classification be applied accurately? Food Science and Technology 2011, 31, 603–613. [Google Scholar] [CrossRef]
- Vizzini, A.; Pergolizzi, M.; Vazzana, M.; Salerno, G.; Di Sano, C.; Macaluso, P.; Arizza, V.; Parrinello, D.; Cammarata, M.; Parrinello, N. FACIT collagen (1α-chain) is expressed by hemocytes and epidermis during the inflammatory response of the ascidian Ciona intestinalis. Developmental & Comparative Immunology 2008, 32, 682–692. [Google Scholar] [CrossRef]
- Parrinello, N.; Vizzini, A.; Arizza, V.; Salerno, G.; Parrinello, D.; Cammarata, M.; Giaramita, F.T.; Vazzana, M. Enhanced expression of a cloned and sequenced Ciona intestinalis TNFα-like (CiTNFα) gene during the LPS-induced inflammatory response. Cell and tissue research 2008, 334, 305–317. [Google Scholar] [CrossRef]
- Parisi, M.G.; Baranzini, N.; Dara, D.; La Corte, C.; Vizioli, J.; Cammarata, M. AIF-1 and RNASET2 are involved in the inflammatory response in the Mediterranean mussel Mytilus galloprovincialis following Vibrio infection. Fish & Shellfish Immunology 2022, 127, 109–118. [Google Scholar] [CrossRef]
- Bisanti, L.; La Corte, C.; Dara, M.; Bertini, F.; Parisi, M.G.; Chemello, R.; Cammarata, M.; Parrinello, D. Global warming-related response after bacterial challenge in Astroides calycularis, a Mediterranean thermophilic coral. Scientific Reports 2024, 14, 8495. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Winder, A.J.; Harris, H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. European Journal of Biochemistry 1991, 198, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ross, N.W.; Firth, K.J.; Wang, A.; Burka, J.F.; Johnson, S.C. Changes in hydrolytic enzyme activities of naive Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Diseases of aquatic organisms 2000, 41, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Parry Jr, R.M.; Chandan, R.C.; Shahani, K.M. A rapid and sensitive assay of muramidase. Proceedings of the Society for Experimental Biology and Medicine 1965, 119, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Swalla, B.J. Molecular phylogeny of the protochordates: chordate evolution. Canadian Journal of Zoology 2005, 83, 24–33. [Google Scholar] [CrossRef]
- Tsagkogeorga, G.; Turon, X.; Hopcroft, R.R.; Tilak, M.K.; Feldstein, T.; Shenkar, N.; Loya, Y.; Huchon, D.; Douzery, E.J.P.; Delsuc, F. An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models. BMC evolutionary biology 2009, 9, 1–16. [Google Scholar] [CrossRef]
- Ermak, T.H. The renewing cell populations of ascidians. American Zoologist 1982, 22, 795–805. [Google Scholar] [CrossRef]
- Sawada, T.; Zhang, J.; Cooper, E.L. Sustained viability and profileration of hemocytes from the cultured pharynx of Styela clava. Marine Biology 1994, 119, 597–603. [Google Scholar] [CrossRef]
- Peddie, C.M.; Smith, V.J. “Lymphocyte-like” cells in ascidians: Precursors for vertebrate lymphocytes? Fish & Shellfish Immunology 1995, 5, 613–629. [Google Scholar] [CrossRef]
- Peddie, C.M.; Richest, A.C.; Smith, V.J. Proliferation of undifferentiated blood cells from the solitary ascidian, Ciona intestinalis in vitro. Developmental & Comparative Immunology 1995, 19, 377–387. [Google Scholar] [CrossRef]
- Trapani, M.R.; Sanfratello, M.A.; Mangano, V.; Parrinello, D.; Vizzini, A.; Cammarata, M. Phenoloxidases of different sizes are modulated by LPS inoculation into Ciona intestinalis tunic and pharynx. Invertebrate Survival Journal 2015, 12, 75–81. [Google Scholar]
- Nappi, A.J.; Ottaviani, E. Cytotoxicity and cytotoxic molecules in invertebrates. BioEssays 2000, 22, 469–480. [Google Scholar] [CrossRef]
- Sadd, B.M.; Schmid-Hempel, P. PERSPECTIVE: principles of ecological immunology. Evolutionary applications 2009, 2, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Hikima, J.I.; Minagawa, S.; Hirono, I.; Aoki, T. Molecular cloning, expression and evolution of the Japanese flounder goose-type lysozyme gene, and the lytic activity of its recombinant protein. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression 2001, 1520, 35–44. [Google Scholar] [CrossRef]
- Zheng, W.; Tian, C.; Chen, X. Molecular characterization of goose-type lysozyme homologue of large yellow croaker and its involvement in immune response induced by trivalent bacterial vaccine as an acute-phase protein. Immunology Letters 2007, 113, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Trapani, M.R.; Parisi, M.G.; Parrinello, D.; Sanfratello, M.A.; Benenati, G.; Palla, F.; Cammarata, M. Specific inflammatory response of Anemonia sulcata (Cnidaria) after bacterial injection causes tissue reaction and enzymatic activity alteration. Journal of invertebrate pathology 2016, 135, 15–21. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Orlando, P.; Pierobon, P.; De Falco, M.; Ruggiero, A.M.; Stefano, G.S.; Tino, A.; Grippo, P. Kelletinin A, from the marine mollusc Buccinulum corneum, promotes differentiation in Hydra vulgaris. Research communications in molecular pathology and pharmacology 1999, 103, 17–28. [Google Scholar]
- Parisi, M.G.; Grimaldi, A.; Baranzini, N.; La Corte, C.; Dara, M.; Parrinello, D.; Cammarata, M. Mesoglea extracellular matrix reorganization during regenerative process in Anemonia viridis (Forskål, 1775). International Journal of Molecular Sciences 2021, 22, 5971. [Google Scholar] [CrossRef]
| Ordinary one-way ANOVA |
F | P value | R square |
|---|---|---|---|
| TLR4 | 170.00 | < 0.0001 | 0.95 |
| NFκB | 51.24 | < 0.0001 | 0.87 |
| Ordinary one-way ANOVA |
F | P value | R square |
|---|---|---|---|
| PO | 15.29 | 0.0044 | 0.83 |
| GPx | 16.08 | 0.0039 | 0.91 |
| LYS | 30.52 | 0.0007 | 0.91 |
| EST | 42.11 | < 0.0001 | 0.84 |
| ALP | 12.09 | 0.0028 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





