Submitted:
25 June 2024
Posted:
26 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
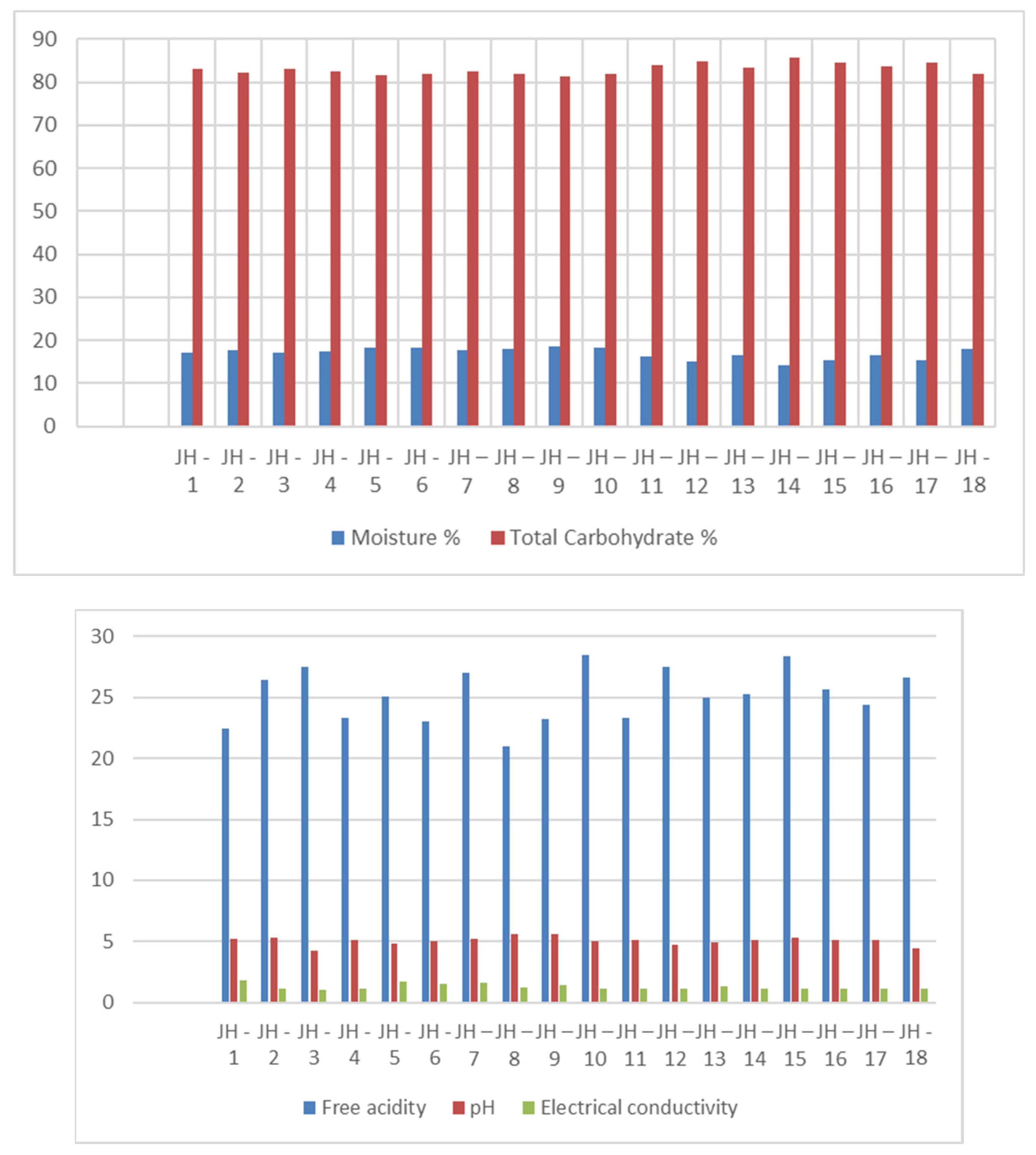
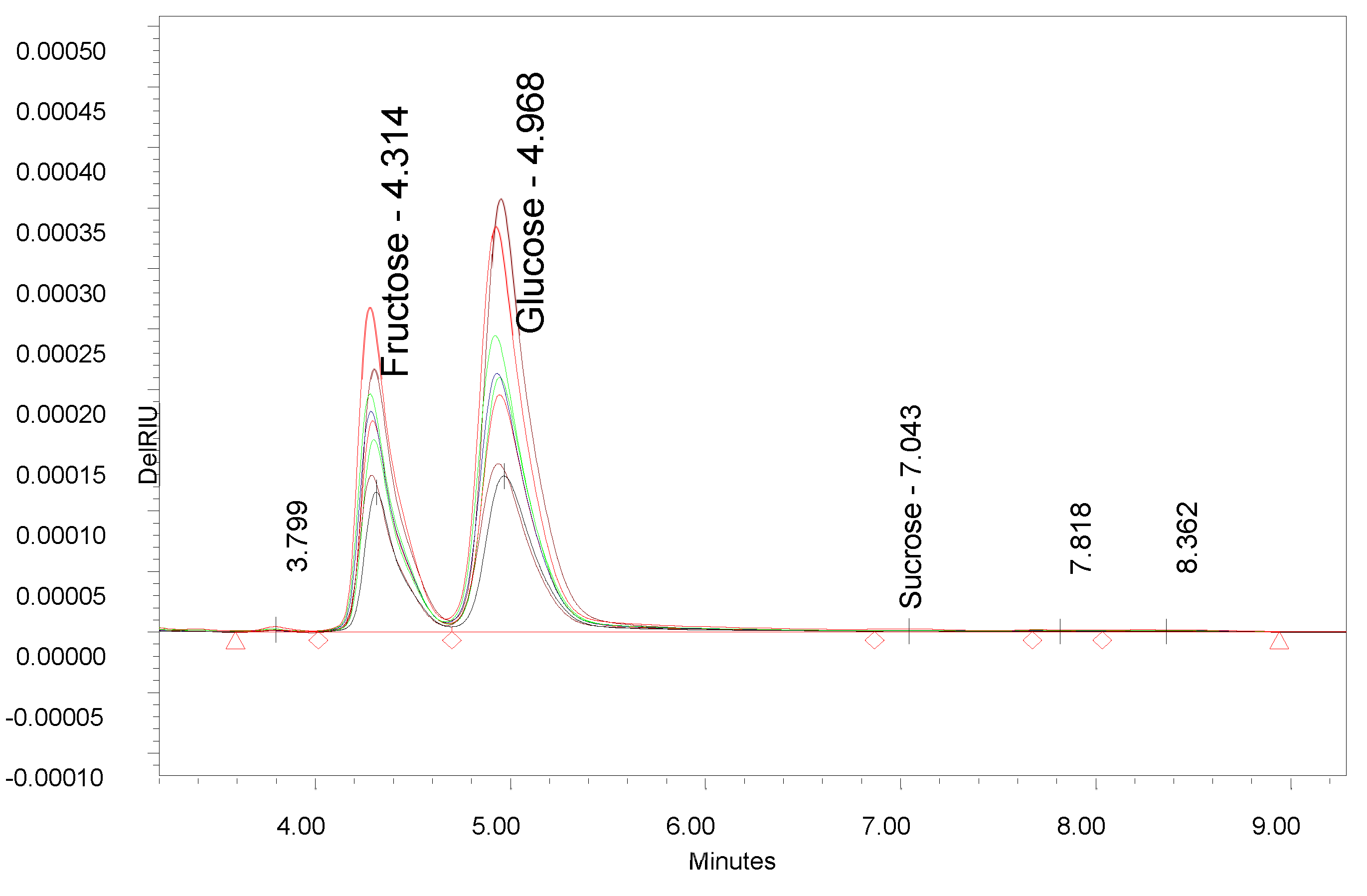
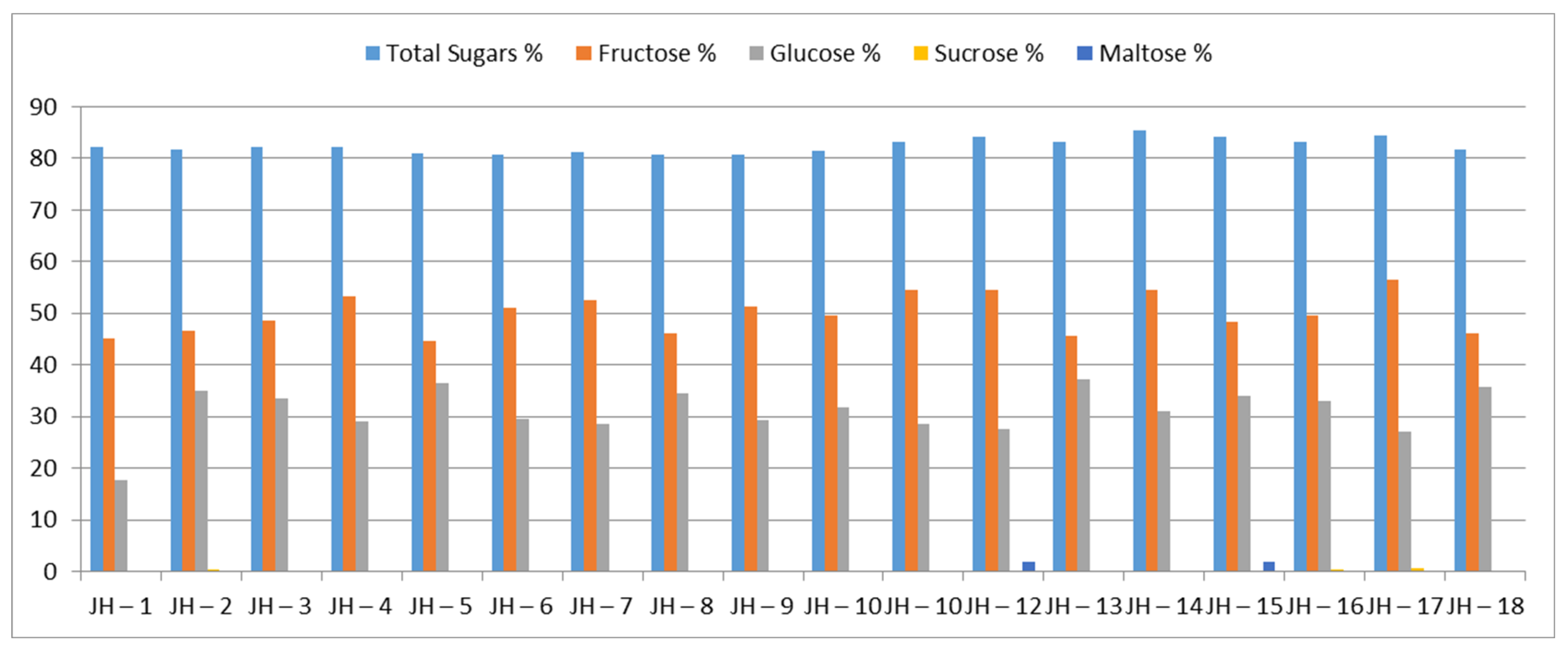
3. Results
4. Discussion
5. Conclusions
References
- www.jarahoney.com.
- Escuredo Olga and Seijo, M. Carmen. ,, Honey: Chemical Composition, Stability and Authenticity”. Foods 2019, 8, 577; 15 November 2019. [Google Scholar] [CrossRef]
- Buba, Abubakar Gidado and Aliyu Shugaba,,Analysis of Biochemical Composition of Honey Samples from North-East Nigeria” Biochem Anal Biochem 2013, 2. [CrossRef]
- Biochemical Profiling and Physicochemical and Biological Valorization of Iraqi Honey: A Comprehensive Analysis-Omar Mohammed Hameed,Ohood Mzahim Shaker,Ahlem Ben Slima,Mohamed Makni. Molecules, 2024; 29, 671. [CrossRef]
- ,,Review of chemistry associated with honey” by Jamie Ayton, Paul Prenzler, Harsh Raman, Amanda Warren-Smith and Richard Meyer June 2019, Publication No. 19-031, Project No. PRJ-011685.
- Hüsne Akalın, Mustafa Bayram and Rahmi Ertan Anlı,, Determination of some individual phenolic compounds and antioxidant capacity of mead produced from different types of honey” Institute of Brewing & Distilling 15 March 2017, DOI 10.1002/jib.396.
- Semih Otles Editors, Kristbergsson Kristberg,,Functional Properties of Traditional Foods” Springer ISBN 978-1-4899-7662-8 DOI 10.1007/978-1-4899-7662-8.
- Ya-Qin Wen, Jinzhen Zhang, Yi Li, Lanzhen Chen, Wen Zhao, Jinhui Zhou and Yue Jin ,,Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis”, Molecules 2017, 22, 735. [CrossRef]
- Shi Shen, Jingbo Wang, Qin Zhuo, Xi Chen, Tingting Liu and Shuang-Qing Zhang ,, Quantitative and Discriminative Evaluation of Contents of Phenolic and Flavonoid and Antioxidant Competence for Chinese Honeys from Different Botanical Origins”, Molecules 2018, 23, 1110. [CrossRef]
- Ewa Makowicz, Izabela Jasicka-Misiak,*, Dariusz Teper and Paweł Kafarski. ,, HPTLC Fingerprinting—Rapid Method for the Differentiation of Honeys of Different Botanical Origin Based on the Composition of the Lipophilic Fractions”, Molecules 2018, 23, 1811; [CrossRef]
- Anguebes-Franseschi, F. , Abatal M., Pat Lucio, Flores A., Córdova Quiroz A. V., Ramírez-Elias M. A., San Pedro L., May Tzuc O. and Bassam A. ,, Raman Spectroscopy and Chemometric Modeling to Predict Physical-Chemical Honey Properties from Campeche, Mexico.
- Almasaudi, Saad B. Al-Nahari, Alaa A.M. Abd El-Ghany, El Sayed M. Barbour, Elie Al Muhayawi, Saad M Al-Jaouni, Soad Azhar, Esam Qari, Mohamad Qari, Yousef A. Harakeh, Steve,,Antimicrobial effect of different types of honey on Staphylococcus aureus” Saudi Journal of Biological Sciences, 2017-09, Crossref DOI link, 2017; -09. [CrossRef]
- Trautvetter Sophie, Koelling-Speer Isabelle, Speer Karl, Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS”, Apidologie, Springer Verlag, 2009, 40 (2), pp.140-150. <hal-00892006>HAL Id: hal-00892006 https://hal.archives-ouvertes.fr/hal-00892006.
- Missio da Silva Priscila, Gauche, Cony Gonzaga, Luciano Valdemiro Costa, Ana Carolina Oliveira Fett, Roseane,, Honey: Chemical composition, stability and authenticity” Journal Food Chemistry, 2015 Elsevier Ltd. All rights reserve DOI link to publisher maintained version. [CrossRef]
- Mouhoubi-Tafinine, Zina, Ouchemoukh, Salim, Tamendjari, Abderezak. ,, Antioxydant activity of some algerian honey and propolis” Journal Title: Industrial Crops and Products, 2016-10, Crossref DOI link: https://doi.org/10.1016/J.INDCROP.2016.02.033, Update policy: https://doi.org/10.1016/ELSEVIER_CM_POLICY.
- Martos Isabel, Ferreres Federicoand Tomas-Barberan Francisco A.,, Identification of Flavonoid Markers for the Botanical Origin of Eucalyptus Honey” J. Agric. Food Chem. No. 5 2000, Vol 48, 1498−1502.
- Sónia Soares, Diana Pinto, Francisca Rodrigues, Rita C. Alves and M. Beatriz P. P. Oliveira,, Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity”, Molecules 2017, 22, 1338; 11 August 2017. [CrossRef]
- Kadri, Samir Moura, Zaluski, Rodrigo, Pereira Lima, Giuseppina Pace, Mazzafera, Paulo, de Oliveira Orsi, Ricardo,, Characterization of Coffea arabica monofloral honey from Espírito Santo, Brazil” Journal Food Chemistry, 2016. CrossRef DOI link to publisher maintained version. [CrossRef]
- Nyuk Ling Chin and Kandhasamy Sowndhararajan ,,A Review on Analytical Methods for Honey Classification, Identification and Authentication” DOI: http://dx.doi.org/10.5772/intechopen.90232. [CrossRef]
- Franciane Marquele-Oliveira, Daniel Blascke Carrão, Rebeca Oliveira de Souza, Nathalia Ursoli Baptista, Andresa Piacezzi Nascimento, Elina Cássia Torres, Gabriela de Padua Moreno, Andrei Felipe Moreira Buszinski, Felipe Galeti Miguel, Gustavo Luis Cuba, Thaila Fernanda dos Reis, Joelma Lambertucci, Carlos Redher and Andresa A. Berretta.,,Fundamentals of Brazilian Honey Analysis: An Overview”. DOI.org/10.5772/67279.
- Samsonova, I. , Gryazkin A. Smirnov A. MannapovA. BeljaevV.,, Bioresource potential of forest lands as the source of honey yield in steppe area of the river Don”. I Samsonova et al 2019 IOP Conf. Ser.: Earth Environ. Sci. 316 012057. [CrossRef]
- Lowore Janet. Meaton Julia. Wood Adrian.,, African Forest Honey: an Overlooked NTFP with Potential to Support Livelihoods and Forests”. 8 March 2018 Environmental Management (2018) 62:15–28, Doi.org/10.1007/s00267-018-1015-8.
- www.jarahoney.com.
- Alliances Caucasus Programme (ALCP), Prospects for the export of Georgian Honey, November 2017.
- Abashdze, N. Vanidze M. Kharadze M.Djafaridze I. Kalandia A.,,WEST GEORGIAN HONEY CATIONS" 2018.
- Abashidze N, Jafaridze I.Chikovani D. Kalandia A. Vanidze M. ,,Antibiotics and heavy metals in Georgian honey" 2019.
- Noori Al-Waili, Khelod Salom, Ahmed Al-Ghamdi, and Mohammad Javed Ansari, ,,Antibiotic, Pesticide, and Microbial Contaminantsof Honey: Human Health Hazards” The Scientific World Journal Volume 2012, Article ID 930849, 9 pages , 10.1100/2012/930849. [CrossRef]
- Gökalp Dilek Fulya, Rasgele Pınar Goc, Kekecoglu Meral Salih Tunç Kaya ,,Rhododendron honey and active substance Grayanotoxin III induced chromosome abnormalities in mice bone marrow cells” Abstracts / Toxicology Letters 258S (2016) S62–S324.
- http://dx.doi.org/10.1016/j.toxlet.2016.06.1704.
- Shen Chongyu, Guo Siyan, Ding Tao, Liu Yun, Chen Lei, Fei Xiaoqing, Zhang Rui, Wu Bin, Shen Weijian, Chen Lei, Zhang Feng, Feng Feng, Deng Xiaojun, Yi Xionghai, Yang Gongjun, CHEN Guoqiang,, Determination of characteristic compound in Manuka honey by automatic on-line solid phase extraction-liquid chromatography-high resolution mass spectrometry” Chinese Journal of Chromatography Vol. 35 No. 10 1068-1072, October 2017. [CrossRef]
- Silici Sibel , Sahin Hüseyinc, Atayoğlu Ali Timucind, Enis Yonar Muhammet ,,Analysis of grayanatoxin in Rhododendron honey and effect on antioxidant parameters in rats” September 2014. [CrossRef]
- Harmonized Methods of the international Honey Commission, https://www.bee-hexagon.net/en/network.htm.
- A: Profiling and Physicochemical and Biological Valorization of Iraqi Honey: A Comprehensive Analysis-Omar Mohammed Hameed,Ohood Mzahim Shaker,Ahlem Ben Slima,Mohamed Makni,Molecules 2024, 29(3), 671; https://doi.org/10.3390/molecules29030671. [CrossRef]
- Sarmento Silva Tania Maria, Santos dos Francyana Pereira, Evangelista-rodrigues Adriana, Sarmento da Silva Eva Monica, Sarmento da Silva Gerlania, de Novais Jailson Santos, de Assis Ribeiro dos Santos Francisco, Camara Amorim Celso.,,Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant aCtivity of jandaira( Melipona subnitida) honey 0889-1575, 2012 Elsevier Inc. [CrossRef]
- Organic Honey from the Middle Atlas of Morocco: Physicochemical Parameters, Antioxidant Properties, Pollen Spectra, and Sugar Profiles- Toufik Bouddine,Hassan Laaroussi,Meryem Bakour,Ibtissame Guirrou,Farid Khallouki,Hamid Mazouz,Hassan Hajjaj,Lhoussain Hajji,, Foods 2022, 11, 3362. [CrossRef]
- Advanced Characterization of Monofloral Honeys from Romania- Daniela Pauliuc,Florina Dranca,Sorina Ropciuc,Mircea Oroian. Agriculture, 2022; 12, 526. [CrossRef]
- Geană, Elisabeta-Irina Ciucure, Corina Teodora Costinel, Diana Ionete, Roxana Elena ,, Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature” Journal Food Control, 2020-03-01, Cross Ref DOI link to publisher maintained version. [CrossRef]
- Sunflower Honey—Evaluation of Quality and Stability during Storage-Milica, Živkov Baloš,Nenad Popov,Sandra Jakšić,Željko Mihaljev,Miloš Pelić,Radomir Ratajac,Dragana Ljubojević Pelić. Foods 2023, 12, 2585. [CrossRef]
- Sakac Nikola, Sak-Bosnar Milan.,, A rapid method for the determination of honey diastase activity” 2012, Elsevier B.V. All rights reserved. [CrossRef]
- Al-Farsi, Mohamed, Al-Amri, Abeer, Al-Hadhrami, Ahlam, Al-Belushi, Sharifa,, Color, flavonoids, phenolics and antioxidants of Omani honey” Journal Heliyon, 2018 The Authors. CrossRef DOI link to publisher maintained version. [CrossRef]
- Marshall Sara, M. ,,Identipication and concentration of phenolic and carbonyl compounds in Florida honey” Universiti of Florida 2013.
- Quality Assessment of Raw Honey Issued from Eastern Romania- Aida Albu,Cristina-Gabriela Radu-Rusu,Ioan Mircea Pop,Gabriela Frunza,Gherasim Nacu. Agriculture 2021, 11, 247. [CrossRef]
- Norul Liza A-Rahaman, Lee Suan Chua, Mohamad Roji Sarmidi, Ramlan Aziz ,, Physicochemical and radical scavenging activities of honey samples from Malaysia” Vol.4, No.5B, 46-51 (2013). [CrossRef]
- Boussaid Amel, Chouaibi Moncef, Rezig Leila, Hellal Raoudha, Donsı` Francesco, Ferrari Giovanna, Hamdi Salem ,, Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia” Arabian Journal of Chemistry, 2018-02. [CrossRef]
- . A Preliminary Investigation of Special Types of Honey Marketed in Morocco- Rania Mehdi, Saadia Zrira, Rossella Vadalà,Vincenzo Nava,Concetta Condurso,Nicola Cicero,Rosaria Costa, J. Exp. Theor. Anal. 2023; 1, 1–20. [CrossRef]
- ,,Review of chemistry associated with honey” by Jamie Ayton, Paul Prenzler, Harsh Raman, Amanda Warren-Smith and Richard Meyer June 2019, Publication No. 19-031 , Project No. PRJ-011685.
- Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania - Silvia Pătruică, Ersilia Alexa, Diana Obiștioiu, Ileana Cocan, Isidora Radulov, Adina Berbecea, Roxana Nicoleta Lazăr, Eliza Simiz, Nicoleta Maria Vicar, Anca Hulea, Dragoș Moraru. Molecules 2022, 27, 4179. [CrossRef]
- Suze, A. Jansen,Iris Kleerekooper, Zonne L. M. Hofman, Isabelle F. P. M. Kappen, Anna Stary-Weinzinger, Marcel A. G. van der Heyden,, Grayanotoxin Poisoning: ‘Mad Honey Disease’ and Beyond Cardiovascular toxicology, April 2012. DOI 10.1007/s12012-012-9162-2.
- . [CrossRef]
- Ewa Majewska, Beata Dru_zyn ´ska, Rafał Wołosiak ,,Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays” Food Sci Biotechnol. 2019. [CrossRef]
- Mohammed Moniruzzaman, Chua Yung An, Pasupuleti Visweswara Rao, Mohammad Nurul Islam Hawlader, Siti Amirah Binti Mohd Azlan, Siti Amrah Sulaiman, and Siew Hua Gan ,,Identification of Phenolic Acids and Flavonoids in Monofloral Honey from Bangladesh by High Performance Liquid Chromatography: Determination of Antioxidant Capacity” Hindawi Publishing Corporation Bio Med Research International Volume 2014, Article ID 737490, 11 pages. [CrossRef]
- Fechner, Diana C., Hidalgo, Melisa J., Ruiz Díaz, Juan D., Gil, Raúl A., Pellerano, Roberto G.,, Geographical origin authentication of honey produced in Argentina” Journal Food Bioscience, 2019 Elsevier Ltd. All rights reserved, DOI link to publisher maintained version. [CrossRef]
- Sultan Ayoub Meoa, Saleh Ahmad Al-Asirib, Abdul Latief Mahesarc, Mohammad Javed Ansarid ,,Role of honey in modern medicine” Volume 24, Issue 5, July 2017, Pages 975-978, 24 December 2016.
- Khalil Ibrahim, Md. , Mahaneem Mohamed, Jamalullail Syed Mohsin Sahil, Alam Nadia and Sulaiman Siti Amrah,, Evaluation of Radical Scavenging Activity and Colour Intensity of Nine Malaysian Honeys of Different Origin” Journal of ApiProduct and ApiMedical Science 3 (1): 04 - 11 (2011), IBRA 2011 DOI 10.3896/IBRA.4.03.1.02.
- Elisabetta Schievano, Marco Sbrizza, Valentina Zuccato, Lucia Piana, Marco Tessari,,NMR carbohydrate profile in tracing acacia honey authenticity” Food Chemistry. www.elsevier.
- Pasias Ioannis, Proestos Charalampos, ,,HMF and diastase activity in honeys: A fully validated approach and a Chemometric analvsis for identification of honey freshness and adulteration” Food Chemistry · August 2017. [CrossRef]
- Özkök Duran, Silici Sibel,, Effects of crystallization on antioxidant property of honey”, 2018 VOL 3, NO. 2, PAGE 24–30 10.5455/ja.20180607113134.
- Stefan Bogdanov, Honey Composition” The Honey Book, Chapter 5, 16 June 2016.
- Kaspar Ruoff, Werner Luginbühl, Verena Kilchenmann, Jacques Olivier Bosset, Katharina Von Der Ohe, Werner Von Der Ohe, Renato Amadò,, Authentication of the botanical origin of honey using profiles of classical measurands and discriminant analysis” HAL Id: hal-00892278, Submitted on 1 Jan 2007,https://hal.archives-ouvertes.fr/hal-00892278.
- Yun Ma, Bing Zhang, Hongyan Li, Yulu Li, Jiangning Hu, Jing Li, Hongming Wang & Zeyuan Deng,, Chemical and molecular dynamics analysis of crystallization properties of honey” 2017, VOL. 20, NO. 4, 725–733. [CrossRef]
- Hagr, T.E. , Mirghani, M.E.S., Elnour, A.A.H.M. and Bkharsa, B.E. ,, Antioxidant capacity and sugar content of honey from Blue Nile State, Sudan” , International Food Research Journal 24(Suppl): S452-S456 (December 2017).
- François Ezin Azonwade, Armand Paraso, Cokou P.A gbangnanDossa, Victorien T.Dougnon , ChristineNtcha, WassiyathMousse, andLamineBaba-Moussa ,,Physicochemical Characteristics and Microbiological Quality of Honey Produced in Benin” Journal of Food Quality Volume 2018, Article ID 1896057, 13 pages. [CrossRef]
- Kharadze Maia, Abashidze Nona, Djaparidze Indira, Vanidze Maia, Kalandia Aleko,,Antioxidant Activity of Chestnut Honey Produced in Western Georgia” Bulletin of the Deorgian Nationel Academy of Sciences, vol. 12, no.2, 2018.
- Elisabeta-Irina Ciucure, Corina Teodora Costinel, Diana Ionete, Roxana Elena,, Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature” Journal Food Control, 2020-03-01, Cross Ref DOI link to publisher maintained version. [CrossRef]
- Karabagiasa Ioannis, K. , Karabourniotib Sofia, Karabagiasa Vassilios K., Badekaa Anastasia V.,,Palynological, physico-chemical and bioactivity parameters determination, of a less common Greek honeydew honey: “dryomelo” Journal Food Control, 2019 Elsevier Ltd. All rights reserved.2020-03. [CrossRef]
- Khalil Ibrahim, Md. , Mohammed Moniruzzaman, Ladï Boukraâ, Mokhtar Benhanifia, Md. Asiful Islam, Md. Nazmul Islam, Siti Amrah Sulaiman and Siew Hua Gan,, Physicochemical and Antioxidant Properties of Algerian Honey” Molecules 2012, 17, 11199–11215; [Google Scholar] [CrossRef]
- Antonio Iglesias, Xesus Feás, Sandra Rodrigues, Julio A. Seijas, M. Pilar Vázquez-Tato, Luís G. Dias and Leticia M. Estevinho,,Comprehensive Study of Honey with Protected Denomination of Origin and Contribution to the Enhancement of Legal Specifications” Molecules 2012, 17, ISSN 1420–3049; [CrossRef]
- Everaldo Attard and Adrian Bugeja Douglas. Physicochemical Characterization of Maltese Honey, IntechOpen, 2017. [CrossRef]
- Moniruzzaman Mohammed, Sulaiman Siti Amrah, Khalil Md Ibrahim and Gan Siew Hua,,Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: a comparison with manuka honey” Moniruzzaman et al. Chemistry Central Journal 2013, 7:138 http://journal.chemistrycentral.com/content/7/1/138.
- Pavlova Tatjana, Stamatovska Viktorija, Kalevska Tatjana.,, Q uality Characteriteristy of Honey: A Review”, PROCEEDINGS OF UNIVERSITY OF RUSE - 2018, volume 57, book 10.2.
- Baloš Milica Živkov, Popov Nenad, Vidaković Suzana, Pelić Dragana Ljubojević, Pelić Miloš, Mihaljev Željko, Jakšić Sandra,, Electrcal conductivity and acidity of honey” Arhiv veterinarske medicine, Vol. 11, No. 1, 91 - 101, 2018, UDK: 638.162:577.1.
- Wieczorek Jolanta, Pietrzak Monika, Pomianowski Janusz, Wieczorek Zbigniew,,Honey as a source of bioactive compounds”, Abbrev.: Pol. J. Natur. Sc., Vol 29(3): 275–285, Y. 2014.
- Sabo Mirjana, POTOČNJAK Mirjana, BANJARI Ines, PETROVIĆ Danijela,, Pollen analysis of honeys from Varaždin County, Croatia”, Turk J Bot 35 (2011) 581-587 © TÜBİTAK, 08.05.2011. [CrossRef]
- Lorena Salvador, Michelle Guijarro, Daniela Rubio, Bolívar Aucatoma, Tanya Guillén, Paul Vargas Jentzsch, Valerian Ciobotă, Linda Stolker, Sonia Ulic, Luis Vásquez, Patricia Garrido, Juan Bravo and Luis Ramos Guerrero,,Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador”. J Food MDPL, 2019, 8, 105; [CrossRef]
- Siok Peng Keka, Nyuk Ling China, Yus Aniza Yusofa, Sheau Wei Tanb, Lee Suan Chua,, Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis spp. and Trigona spp. Bees” Agriculture and Agricultural Science Procedia 2 ( 2014 ) 150 – 155. [CrossRef]
- Jaromír Lachman, Nková alena heJtmá, Sýkora Jan, karban Jindřich, orSák matyáš and rygerová barbora,, Contents of Major Phenolic and Flavonoid Antioxidants in Selected Czech Honey” Vol. 28, 2010, No. 5: 412–426.
- Suzan zein Alabdeen Makawi, Elrasheed Ahmed Gadkriem and Saad Mohamed Hussein Ayoub,, Determination of Antioxidant Flavonoids in Sudanese Honey Samples by Solid Phase Extraction and High Performance Liquid Chromatography” E-Journal of Chemistry ISSN: 0973-4945; CODEN ECJHAO 2009, 6(S1), S429-S437.
- Trautvetter Sophie, Koelling-Speer Isabelle, Speer Karl, Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS”, Apidologie, Springer Verlag, 2009, 40 (2), pp.140-150. <hal-00892006>HAL Id: hal-00892006 https://hal.archives-ouvertes.fr/hal-00892006.
- 1: thibaut, Jacquet nicolas, Berchem thomas, haubruge eric, Nguyen Bach Kim and Richel Aurore,,,Extraction of Honey Polyphenols: Method Development and Evidence of Cis Isomerization”, Analytical Chemistry Insights 2016:11 49–57. [CrossRef]
- Tu, J.-Q. , Zhang Z.-Y., Cui C.-X., ang M. Li Y, Y. and Zhang Y.-P. ,, Fast Separation and Determination of Flavonoids in Honey Samples by Capillary Zone Electrophoresis” KUI-10/2017, February 17, 2017. [CrossRef]
- Jibril Fatima Ibrahim, Hilmi Abu Bakar Mohd and Manivannan Lavaniya,,Isolation and characterization of polyphenols in natural honey for the treatment of human diseases”, ibril et al. Bulletin of the National Research Centre (2019) 43:4.
- . [CrossRef]
- Predescu Corina, Papuc Camelia, N icorscu Valentin.,, Aantioxidant activity of Sanflower and Meadow Honey” Scientific Works. Series C. Veterinary Medicine. Vol. LXI (1) ISSN 2065-1295; ISSN 2343-9394 (CD-ROM); ISSN 2067-3663 (Online); ISSN-L 2065-1295.
- Ozkoki Asli, Ozenirler Cigdem , Canli Deniz, Mayda Nazlı,Sorkun Kadriye ,, Monofloral Features of Turkish Honeys According to Melissopalynologic, Total Phenolic and Total Flavonoid Content “ GU J Sci 31(3): 713-723.
- Uroš, M. Gašić, DUšanka M. Milojković-opsenica, and Živoslav lj. Tešić, Polyphenols as Possible Markers of Botanical Origin of Honey” Journal of AoAC International Vol. 100, No. 4, 2017.
- Mohammed Moniruzzaman, Md Ibrahim Khalil, Siti Amrah Sulaiman and Siew Hua Gan,,Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera” Moniruzzaman et al. BMC Complementary and Alternative Medicine 2013, 13:43 http://www.biomedcentral.com/1472-6882/13/43.
- Rosane Gomes de Dliveira, Sona Jain, Alexandre Cândido Luna, Lisiane dos Santos Freitas, Edilson Divino de Araujd,, Screening for quality indicators and phenolic compounds of biotechnological interest in honey samples from six species of stingless bees (Hymenoptera: Apidae)” Food Science and Technology, ISSN 0101-2061, Doi httpI://dx.doi.org/10.1590/1678-457X.25716.
- Aziz end Pehlivan, Tuba,, Antioxidant activities of some monofloral honey types produced across Turkey” Saudi Journal of Biological Sciences, Crossref DOI link. [CrossRef]
- . [CrossRef]
- Lee Suan Chua, Norul Liza A. Rahaman, Nur Ardawati Adnan, and Ti Tjih Eddie Tan ,,Antioxidant Activity of Three Honey Samples in relation with Their Biochemical Components” JournalofAnalyticalMethodsinChemistry Volume2013, ArticleID313798, 8 pages. [CrossRef]
- Czipa, N. , BorBély M. and Győri Z.,, Proteine content of different Honey types” Acta Alimentaria, Vol. 41 (1), pp. 26–32 (2012) published online 4 August 2011. [CrossRef]
- Zhenxiong Huang, Lang Liu, Guojian Li, Hong Li, Dapeng Ye and Xiaoli Li ,, Nondestructive Determination of Diastase Activity of Honey Based on Visible and Near-Infrared Spectroscopy” Molecules 2019, 24, 1244. [CrossRef]
- Bogdanow Stefan, D’Arcy Bruce Robert, Mossel Brenda, Marcazzan Glan Luigi.,, Honey quality and international regulatory standards:review by the International Honey Commission”, Jacob Kerkvliet on 12 May 2014, Bee World 80(2):61–69.
- Tosi, E. , Martinet R., Ortega M, Lucero H. Re E. ,, Honey diastase activity modified by heating” Food Chemistry 106 (2008) 883–887. [CrossRef]
- Sudhanshu Saxena and Satyendra Gautam. ,, A Preliminary Cost Effective Qualitative Assay for Diastase Analysis in Honey” Insights in Enzyme Research ISSN 2573-4466, March 09, 2017. [CrossRef]
- Murtaja Reza Linkon Khan, Md. , Prodhan Utpal Kumar, Hakim Md. Abdul, Alim Md. Abdul ,, Study on the Physicochemical and Antioxidant Properties of Nigella Honey” International Journal of Nutrition and Food Sciences 2015; 4(2): 137-140. [CrossRef]
- Uršulin-Trstenjak Natalija, Puntaric Dinko, Levanic Davor, Gvozdic Vlatka, Pavlek Celjka, Puntaric Ada, Puntaric Eda, Puntaric Ida, Vidosavljevic Domagoj, Lasic Dario and Vidosavljevic Marina ,, Pollen, Physicochemical, and Mineral Analysis of Croatian Acacia Honey Samples: Applicability for Identification of Botanical and Geographical Origin” Journal of Food Quality Volume 2017, Article ID 8538693, 11 pages. [CrossRef]
- Sniderman J., M. Kale, Matley Kia A., Haberle Simon G., Cantrill David J.,, Pollen analysis of Australian honey“, PLoS ONE 13(5):e0197545, May 16, 2018. [CrossRef]
- Ponnuchamy Raja, Bonhomme Vincent Prasad Srinivasan, Das Lipi, Patel Prakas, Gaucherel Cédric, Pragasam Arunachalam ,Anupama Krishnamurthy ,, Honey Pollen: Using Melissopalynology to Understand Foraging Preferences of Bees in Tropical South India” PLOS ONE, July 2014 ,Volume 9 ,Issue 7 , e101618. [CrossRef]
- Selvaraju, Kirthiga, Vikram, Paritala, Soon, Jan Mei ORCID: 0000 0003 0488 1434, Krishnan, Kumara Thevan and Mohammed, Arifullah (2019) Melissopalynologica, physicochemica and antioxidant properties of honey from West Coast of Malaysia. Journal of Food Science and Technology, 56 (5). pp. 2508 2521. [CrossRef]
- Ruoff, K.; Luginbühl, W.; Künzli, R.; Iglesias, M. T.; Bogdanov, S.; Bosset, J. O.; von der Ohe, K.; von der Ohe, W.; Amadò, R. Authentication of the Botanical and Geographical Origin of Honey by Mid-Infrared Spectroscopy. J. Agric. Food Chem. 2006, 54, 6873–6880 Copyright 2006 American Chemical Society. [Google Scholar] [CrossRef] [PubMed]
- Ana Paula Conceição Silva & Francisco de Assis Ribeiro Dos Santos (2014) Pollen diversity in honey from Sergipe, Brazil, Grana, 53:2, 159-170. [CrossRef]
- Danielsen Randi and Mendes Patrícia, M. ,,Pollen Atlas of Portugal” Laboratório de Arqueociências, LARC/CIBIO/InBIO, Direção Geral do Património Cultural (DGPC) http://www.patrimoniocultural.gov.pt/static/data/patrimonio_arqueologico/larc/pollenatlasofportugal.pdf.
- Ashwini S Dhawan, Kulbhushan W Pawar and Ashwini A Lokhande,, Analysis of pollen grains in different honey samples from the region of Newasa tehsil in Maharashtra” Journal of Pharmacognosy and Phytochemistry 2018; 7(3): 3438-3442.
- Kıvrak Şeyda & Kıvrak İbrahim (2017),, Assessment of phenolic profile of Turkish honeys”, International Journal of Food Properties, 20:4, 864-876. [CrossRef]
- Goran saric, Ksenija Markovic, Nikola Major, Marina Krpan, Natalija Ur{ulin-Trstenjak, Mirjana Hruckar1 and Nada Vahcic,, Changes of Antioxidant Activity and Phenolic Content in Acacia and Multifloral Honey During Storage”, Food Technol. Biotechnol. 50 (4) 434–441 (2012), ISSN 1330-9862.
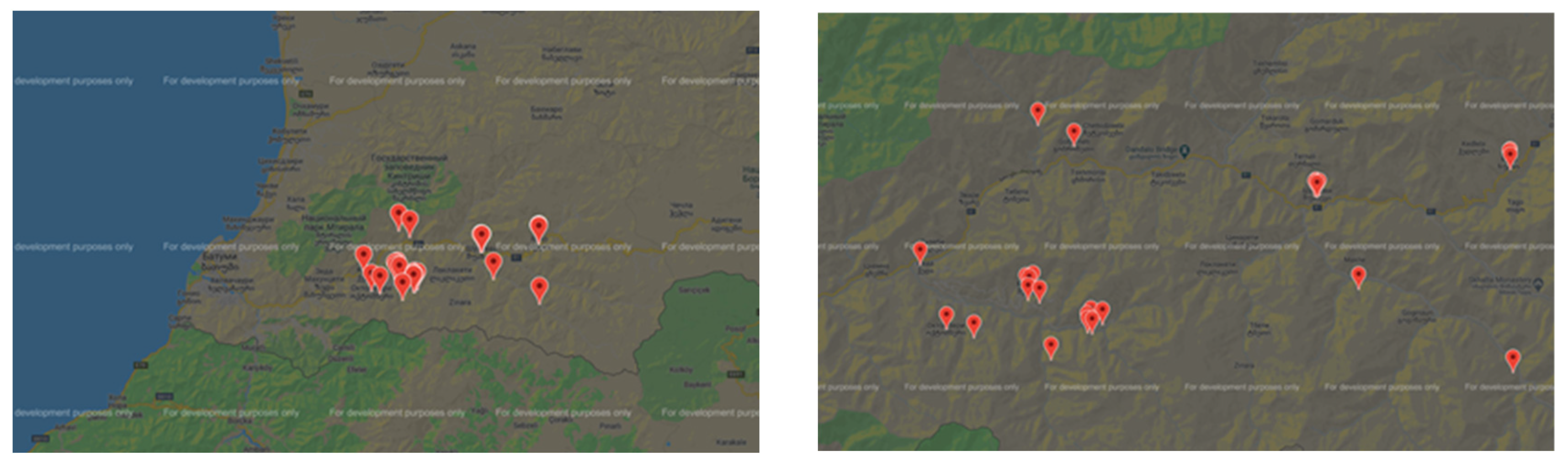
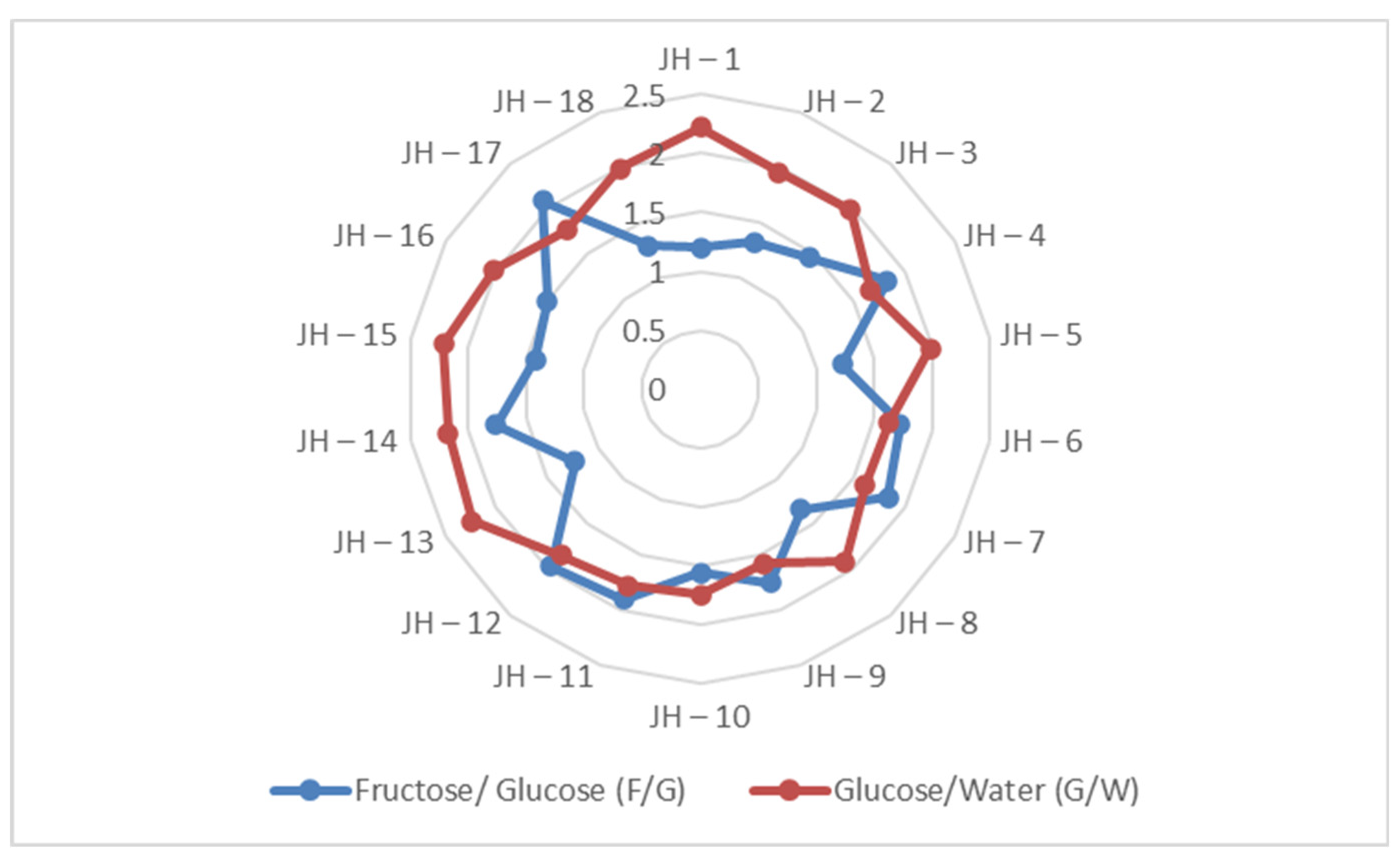
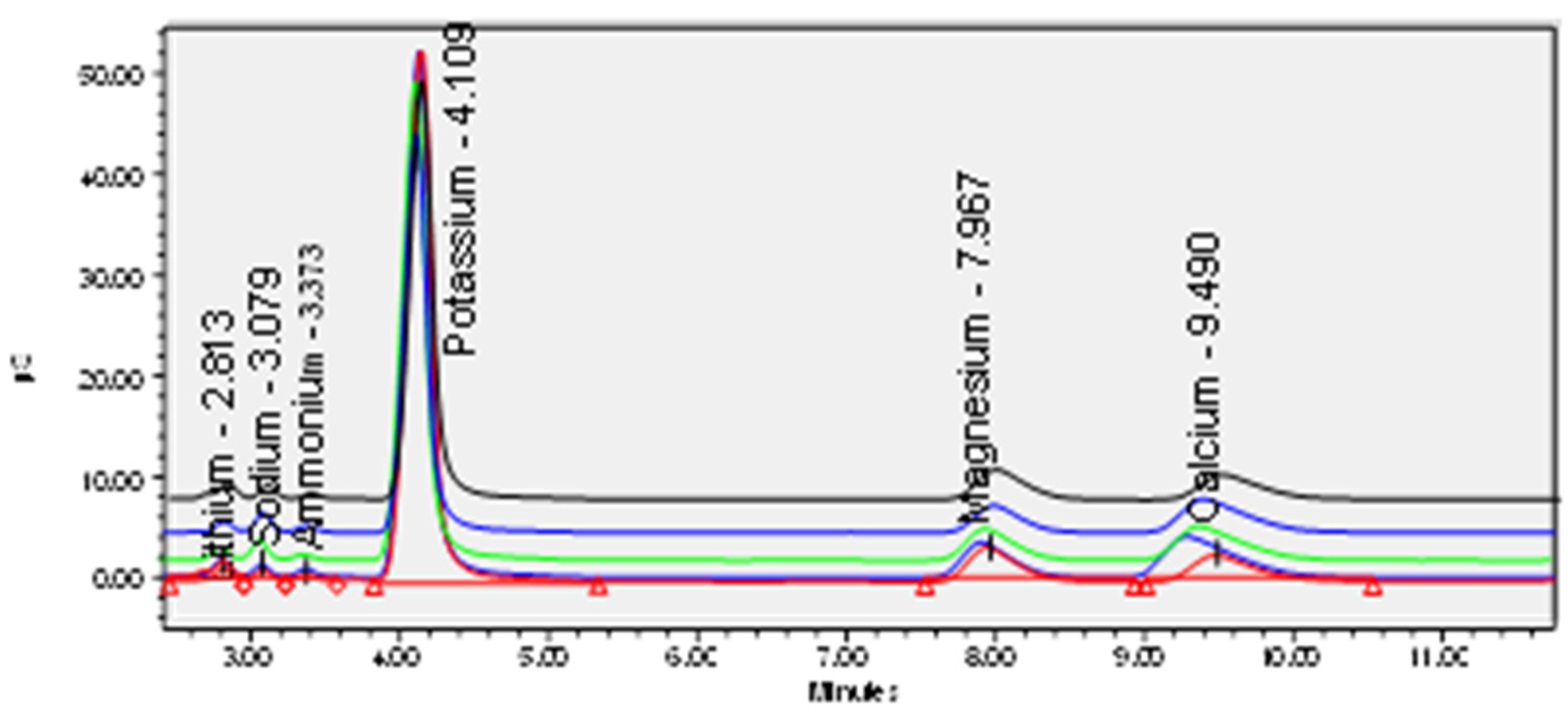
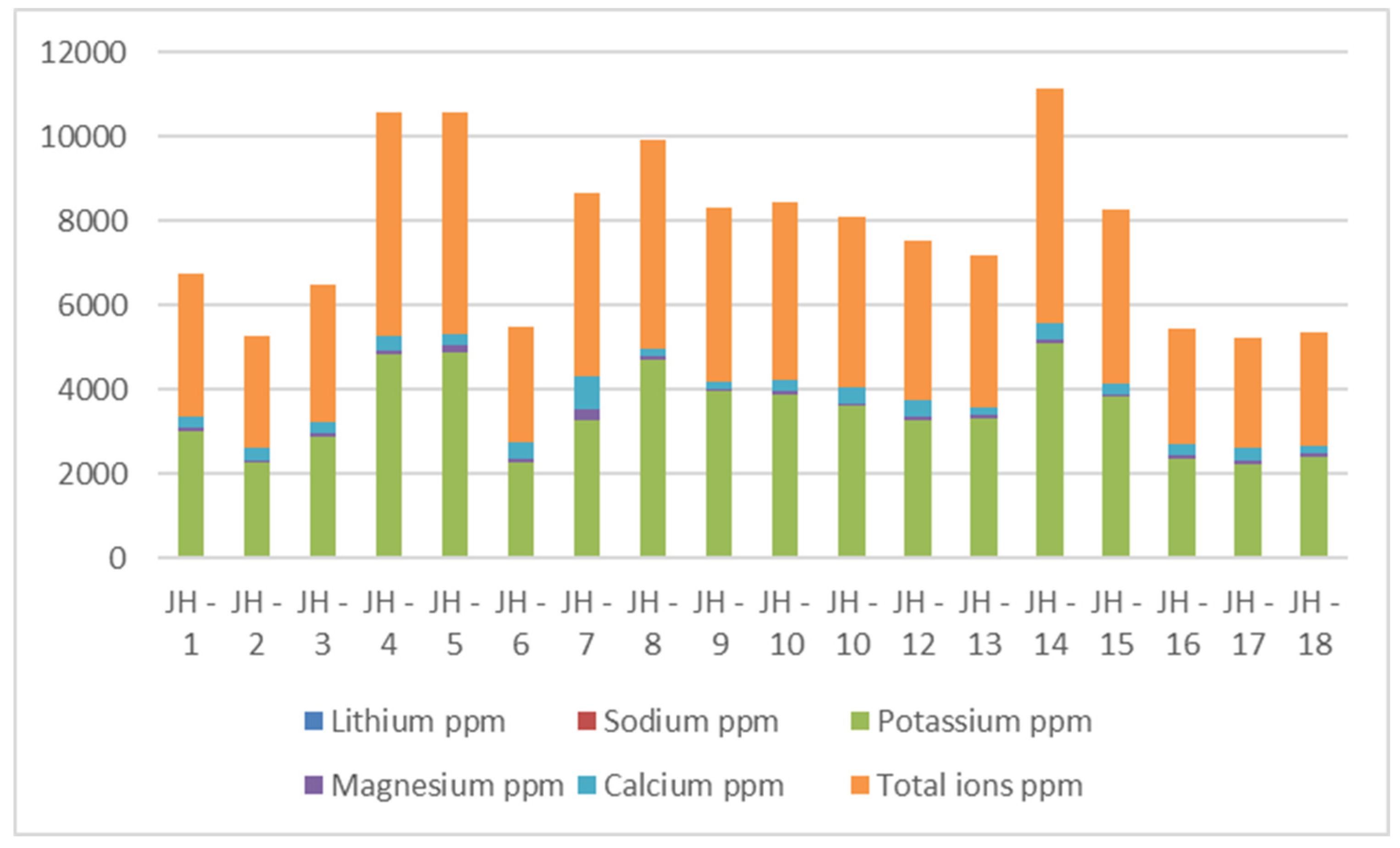
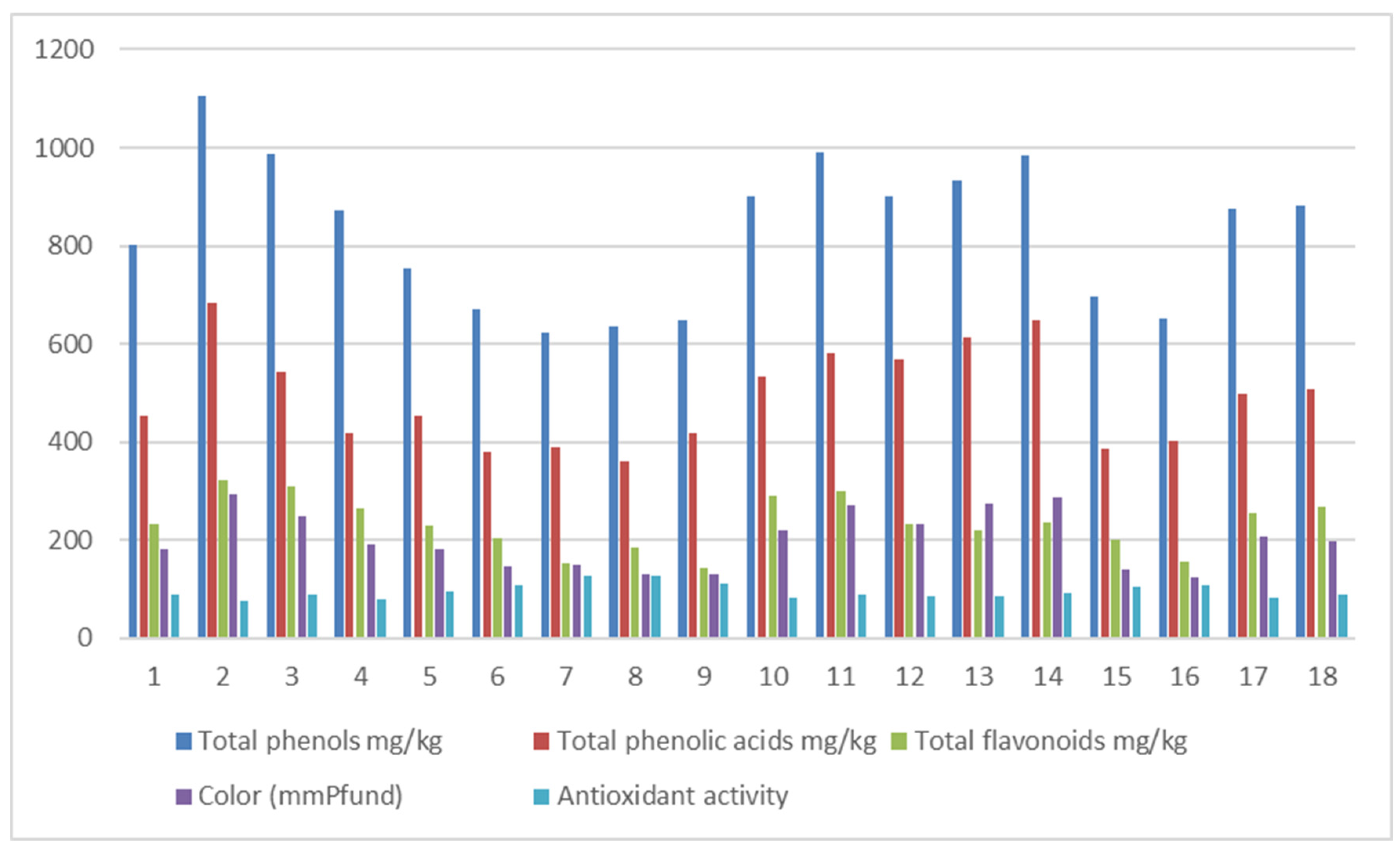
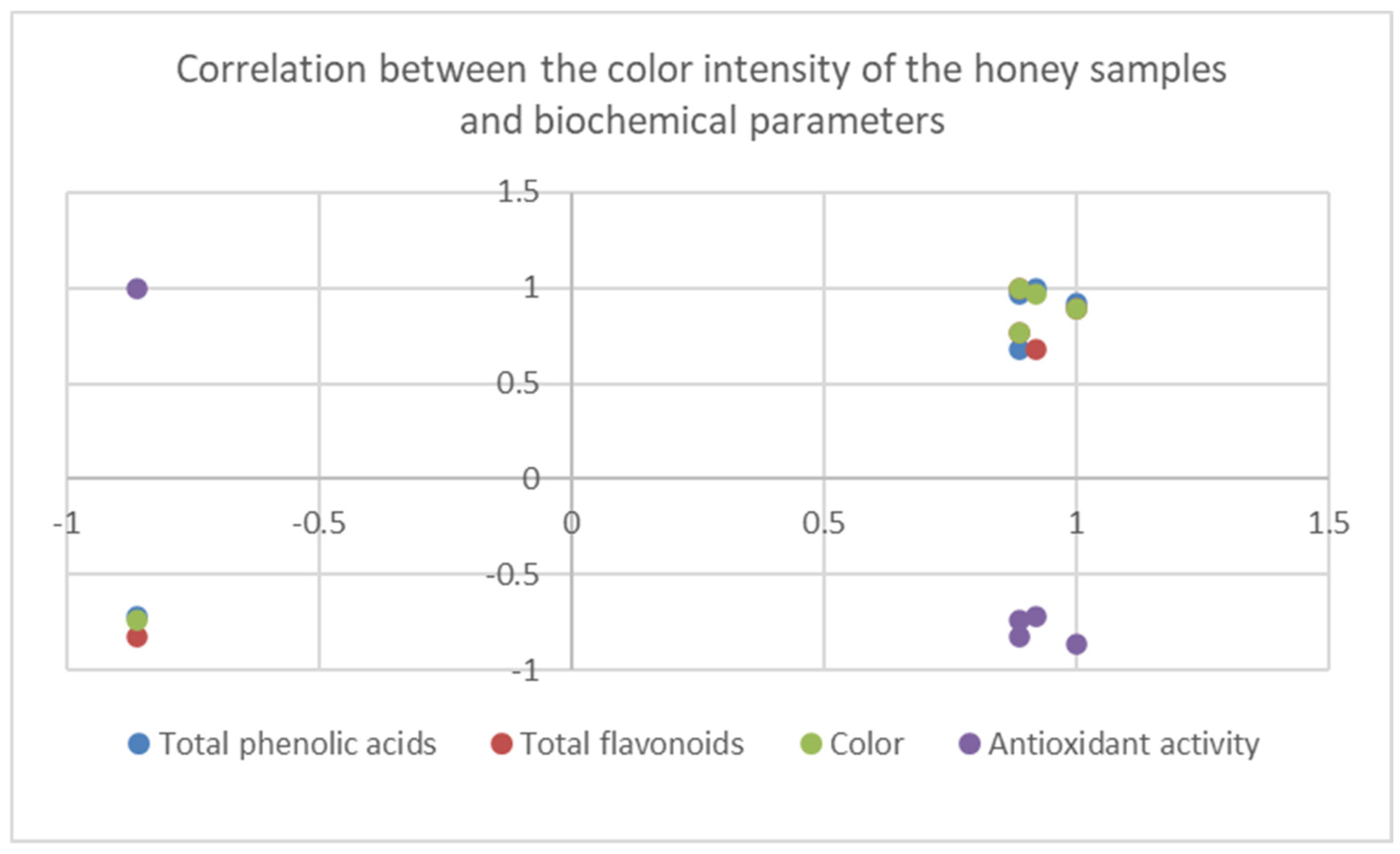
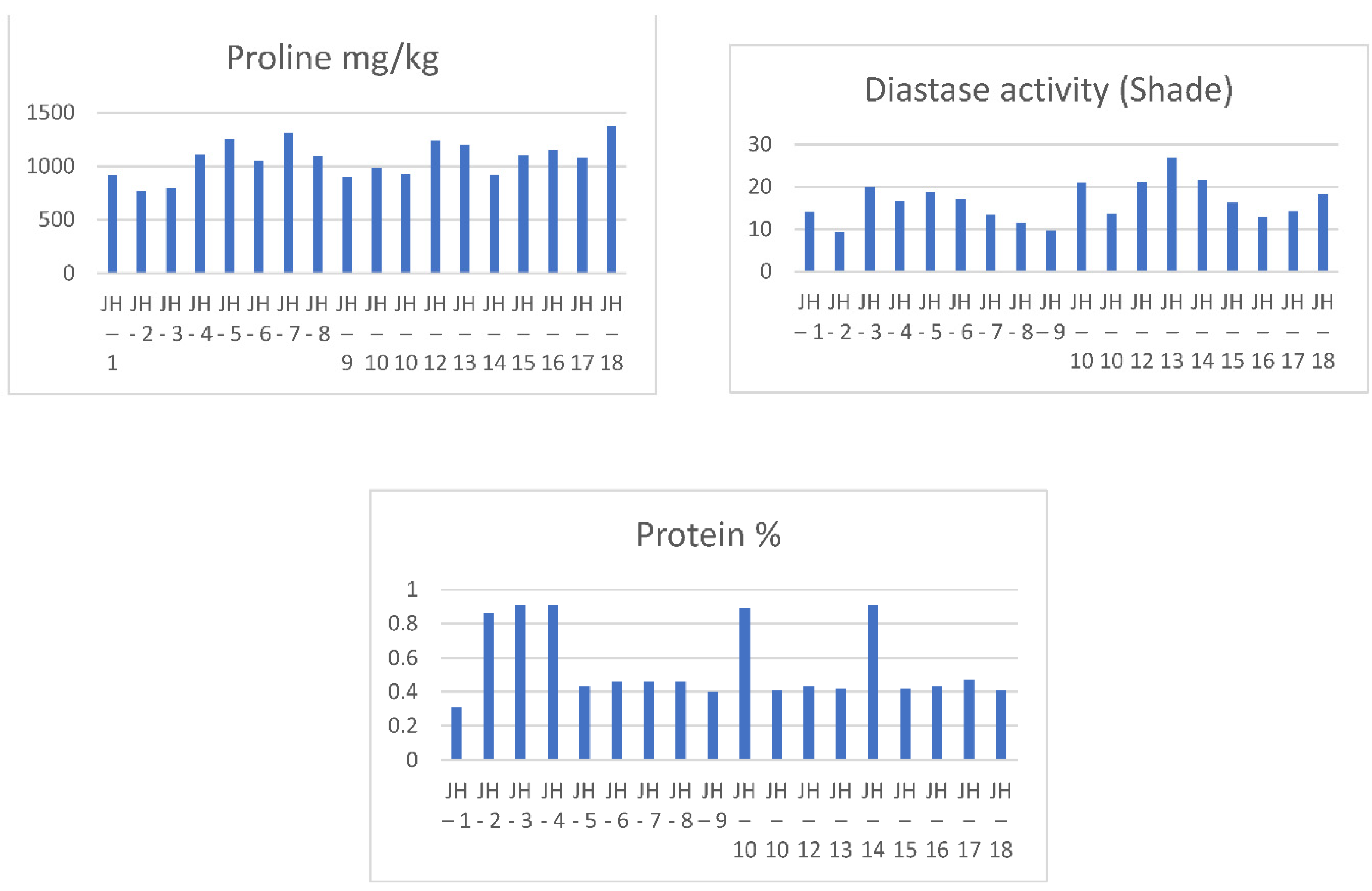
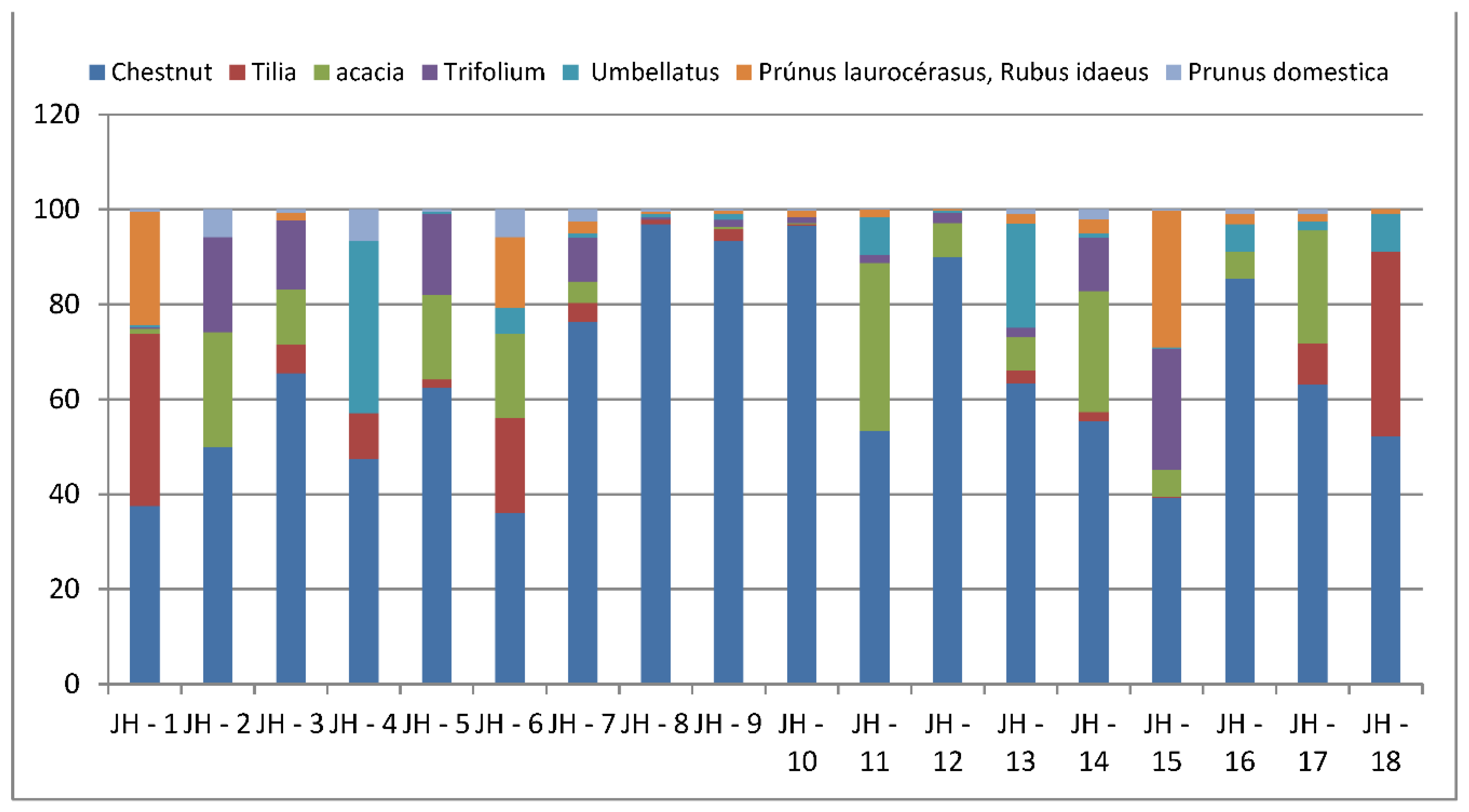
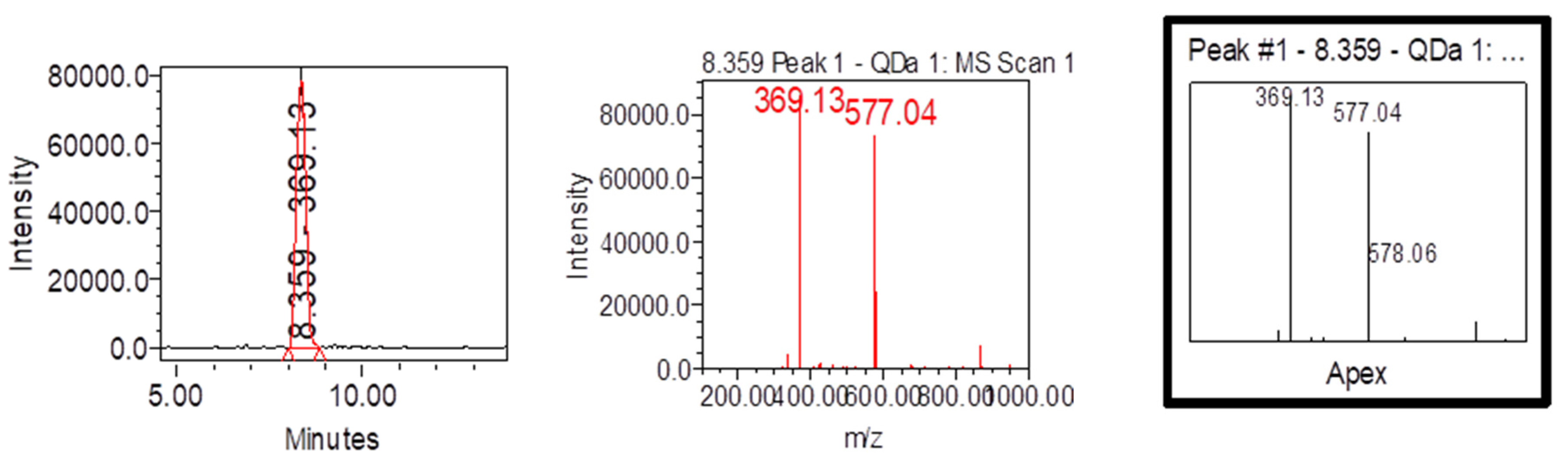
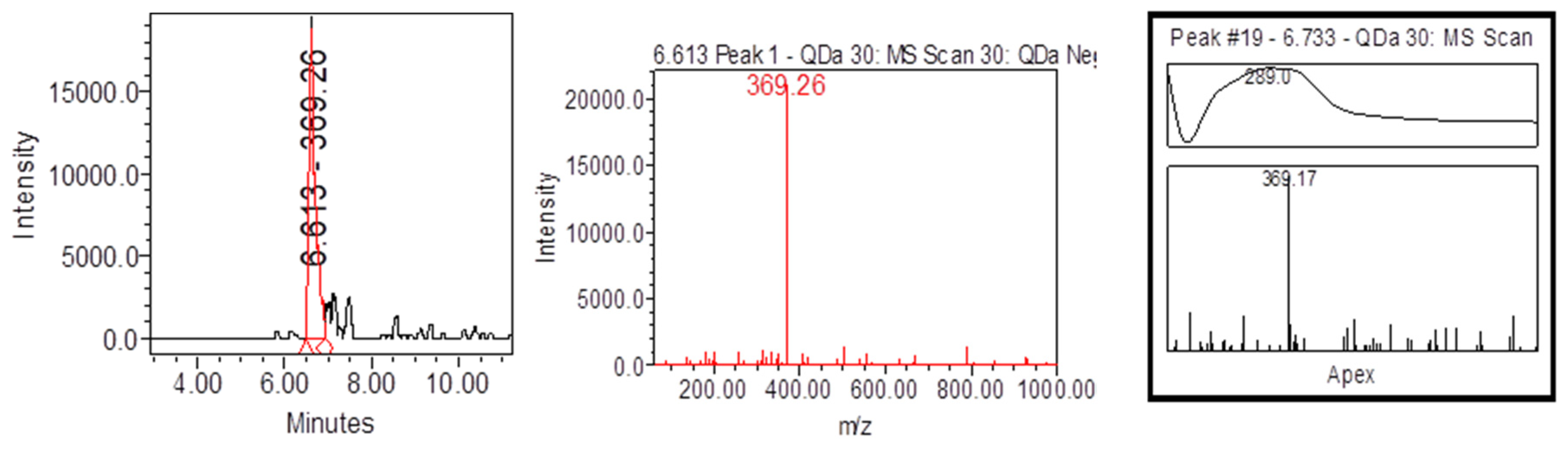
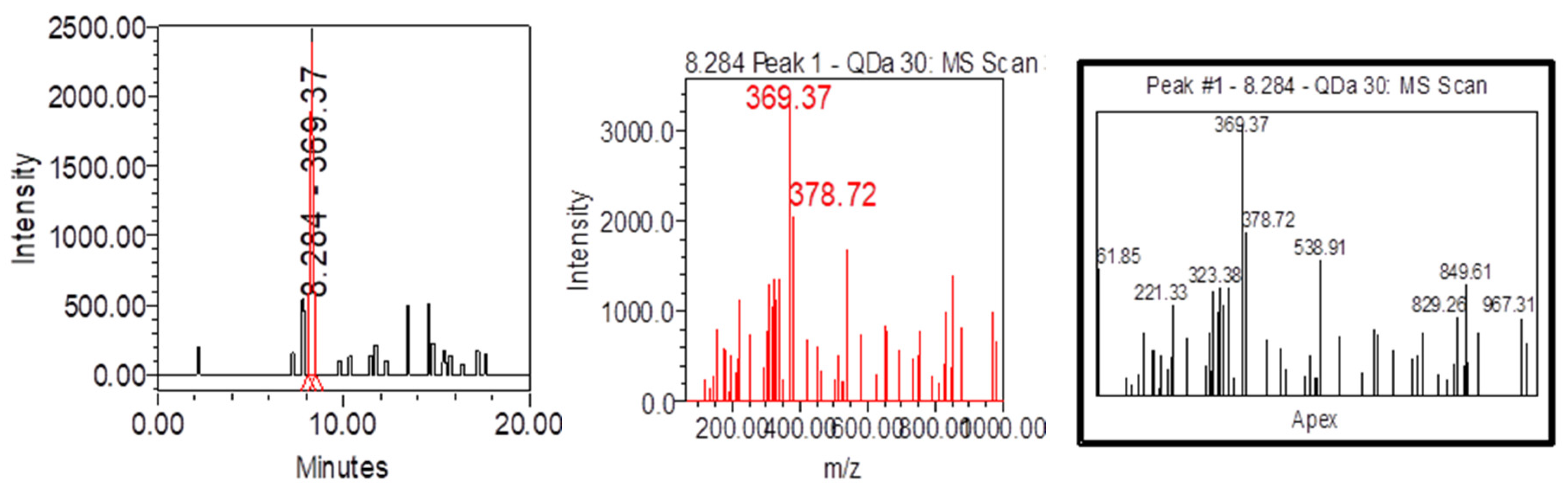
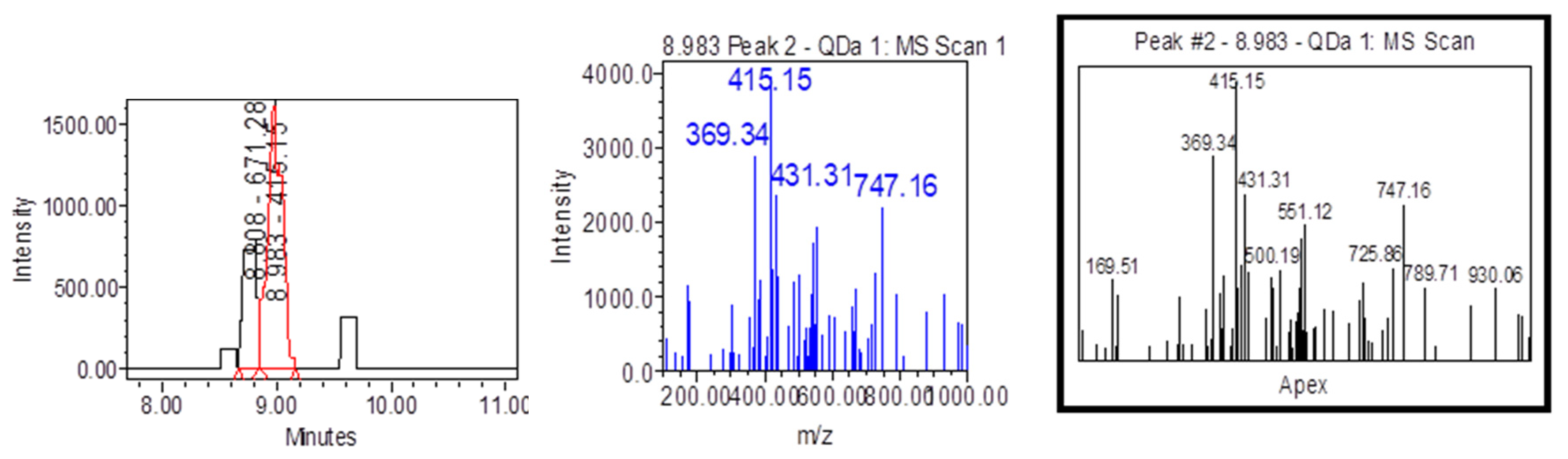
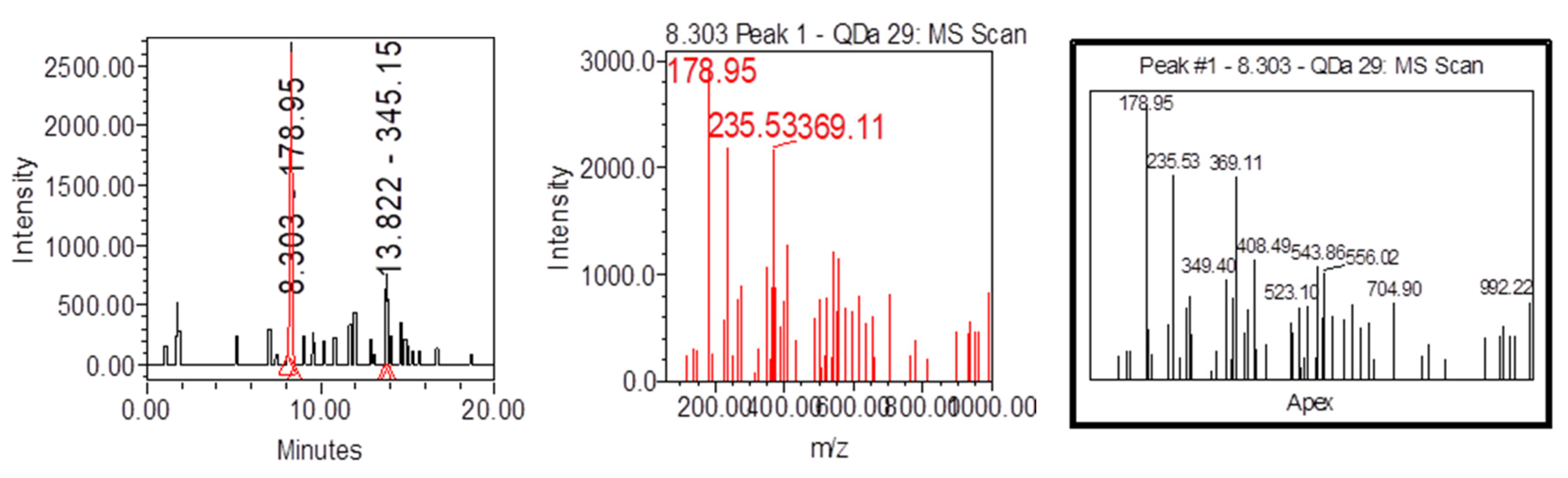
| Samples | Samplers code | Sampling Place (municipality) | Height above mean sea level, m | Coordinates |
|---|---|---|---|---|
| Jara Honey 1 | JH - 1 | Keda - Gobroneti | 607 | 41° 39′ 8.5″ N, 42° 2′ 18.6″ E |
| Jara Honey 2 | JH - 2 | Keda - Zesopeli | 536 | 41° 37′ 14.2″ N, 41° 57′ 44.6″ E |
| Jara Honey 3 | JH - 3 | Keda - Tskhmorisi | 523 | 41° 38′ 22″ N, 42° 2′ 50″ E |
| Jara Honey 4 | JH - 4 | Keda - Zendidi | 336 | 41° 36′ 10″ N, 41° 55′ 55″ E |
| Jara Honey 5 | JH - 5 | Keda - Zundaga | 400 | 41° 34′ 42.4″ N, 41° 49′ 32.9″ E |
| Jara Honey 6 | JH - 6 | Keda – Namonastrevi | 820 | 41° 34′ 13″ N, 42° 3′ 10″ E |
| Jara Honey 7 | JH - 7 | Keda - Silibauri | 490 | 41° 34′ 46.3″ N, 42° 0′ 47.1″ E |
| Jara Honey 8 | JH - 8 | Keda - Medzibna | 640 | 41° 33′ 51.9″ N, 41° 58′ 29″ E |
| Jara Honey 9 | JH - 9 | Keda – Merisi | 700 | 41° 34′ 54″ N, 42° 0′ 18″ E |
| Jara Honey 10 | JH – 10 | Shuakhevi - Instkirveli | 1385 | 41° 42′ 57.4″ N, 42° 14′ 9.3″ E |
| Jara Honey 11 | JH - 11 | Shuakhevi - Khabelashvilebi | 766 | 41° 42′ 28″ N, 42° 10′ 45.7″ E |
| Jara Honey 12 | JH - 12 | Shuakhevi - Kidzinidzeebi | 1040 | 41° 35′ 6.1″ N, 42° 11′ 37.4″ E |
| Jara Honey 13 | JH - 13 | Shuakhevi - Karapeti | 1334 | 41° 33′ 8.1″ N, 42° 15′ 24.5″ E |
| Jara Honey 14 | JH - 14 | Khulo - Skhalta | 800 | 41° 35′ 4.84″ N, 42° 19′ 46.21″ E |
| Jara Honey 15 | JH - 15 | Khulo – Kvatia | 1090 | 41° 34′ 40″ N, 42° 24′ 17″ E |
| Jara Honey 16 | JH - 16 | Khulo - Pushrukauli | 1180 | 41° 33′ 41″ N, 42° 27′ 0″ E |
| Jara Honey 17 | JH - 17 | Khulo - Rakvta | 1350 | 41° 34′ 11″ N, 42° 28′ 58″ E |
| Jara Honey 18 | JH - 18 | Khulo - Bardnali | 570 | 42° 36′ 22″ N, 42° 42′ 50″ E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





