1. Introduction
Osteoarthritis (OA) is a common and debilitating whole-joint disease affecting millions of people worldwide [
1]. For knee osteoarthritis (KOA), the prevalence is estimated around 23% in people aged over 40, which is expected to increase due to ageing, obesity and the lack of disease-modifying OA drugs [
2]. Furthermore, this whole-joint disease has various personal, social and economic consequences for patients, their environment and society.
While there is a variety of possible treatments for KOA involving braces, exercise therapy, oral or topical non-steroidal anti-inflammatory drugs, infiltrations and total knee arthroplasty (TKA), it can be stated that not every patient benefits from a similar intervention [
3]. While TKA is a common and effective treatment for KOA, especially in elderly with typically higher Kellgren-Lawrence scores, it is found less suitable for younger, more active patients [
4]. As the decision for TKA strongly depends on the patients’ remaining functionality, quality of life (QoL) and structural degradation, surgery is often not the preferred method of treatment in the onset of KOA [
5,
6]. Nevertheless, a significant amount of time is often spent with a diminished QoL, meanwhile aiming to maintain a certain level of physical activity, albeit accompanied with pain, discomfort, and frustration. To tackle these problems and improve the QoL, many of these patients might benefit more from customized exercise therapy, infiltrations, and weight loss in order to maintain a healthy lifestyle and reduce work abstinence while postponing primary TKA’s and preventing revision TKA’s, thus simultaneously reducing the social economic burden [
3,
7,
8].
As supervised exercise therapy has proven to be effective in tackling pain and disability caused by KOA, it is often promoted as the first line of treatment [
3,
7,
8,
9]. Within the relatively young KOA population, the need for customized exercise therapy to strengthen the lower limb muscles and prevent the weakened joint structures from being loaded excessively, is indisputable. By improving muscle force and patient function, respective exercise therapy can improve the patient’s QoL substantially [
10,
11].
However, while conservative exercise therapy is strongly encouraged within those individuals diagnosed with KOA, low load resistance training (LLRT) is often the only feasible method of treatment as this does not exacerbate joint pain and inflammation. However, high load strength training (HLRT) effectively enables both strength and hypertrophic muscle adaptations [
12]. Therefore, the American College of Sports Medicine (ASCM) guidelines recommend training intensities of at least 70% of 1 Repetition Maximum (RM) when wanting to induce strength increments [
13].
Unfortunately, these levels of exercise intensity also imply substantial loading of the degenerated intra- and peri-articular structures, making its implementation in OA-associated exercise therapy often not feasible. However, due to its significant correlation with function, lower limb strength training, specifically targeting the Quadriceps, remains crucial in the rehabilitation of KOA. By improving both muscle mass and strength of the Quadriceps, the stability of the knee joint increases whilst also improving shock absorbing capabilities.
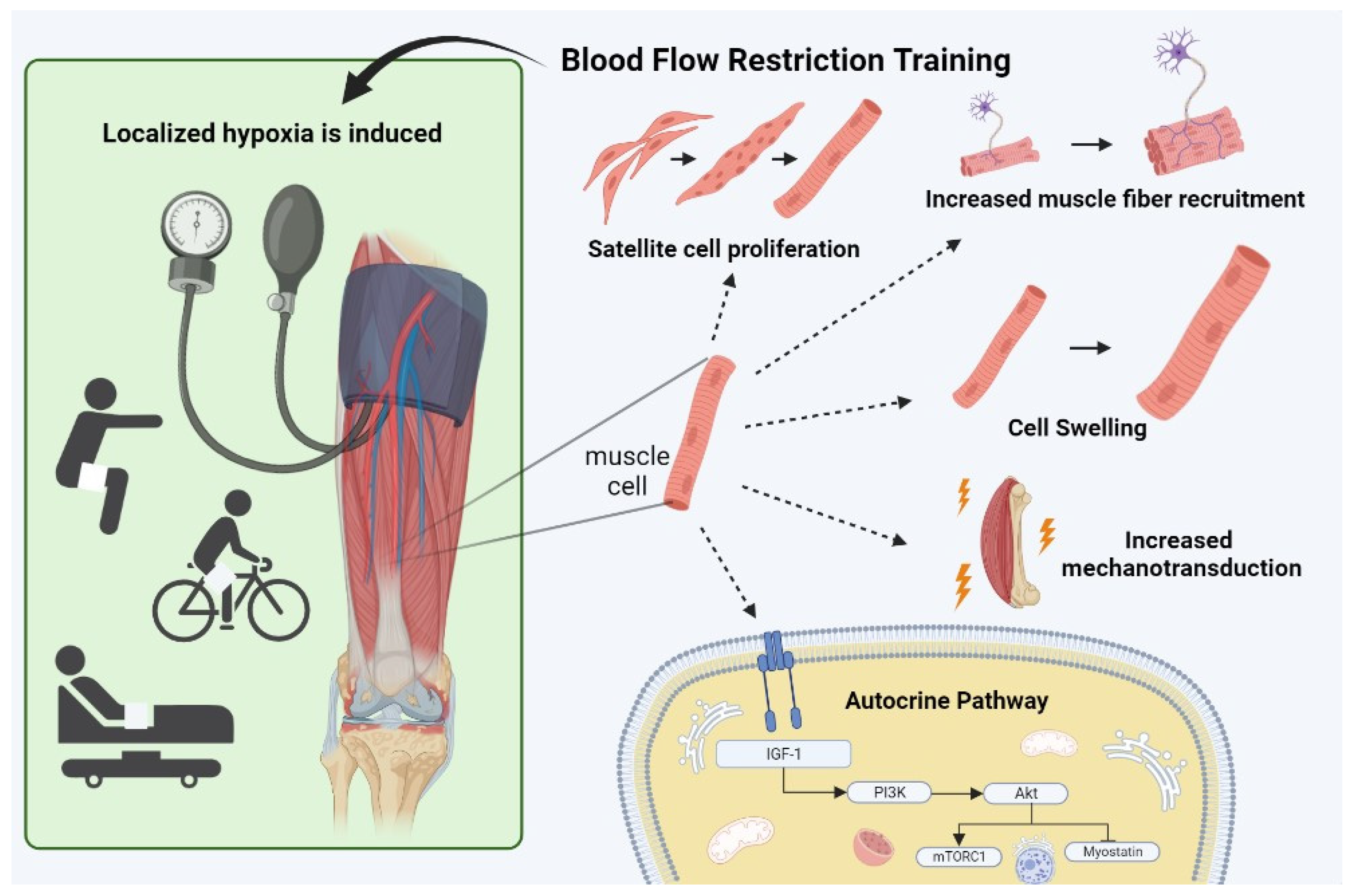
Recently, the use of blood flow restriction training (BFRT) combined with low load resistance training began to emerge as a possible substitute for high load strength training. With BFRT, a cuff is applied around the proximal aspect of the affected limb, causing partial arterial and full venous occlusion, thereby inducing localized hypoxia and accumulation of metabolites, mimicking the effects of high load resistance training, albeit without heavy loads. Consequently, BFRT might offer a suitable and more effective alternative for KOA patients who are not (yet) eligible for TKA, compared to the traditional exercise therapy. Therefore, this review aims to summarize the current evidence as regards the application of BFRT in exercise therapy of KOA patients, with particular consideration of the underlying mechanisms and its safety, as well as general guidelines for practical implementation into clinical practice. In doing so, this narrative review aims to create a framework allowing the translation from theory to practice.
2. Methodological Considerations
This review article encompasses a literature search on four main aspects: (1) The effects of BFRT in KOA, (2) mechanisms thought to be responsible for the effects of BFRT on muscle hypertrophy, -strength and pain reduction, (3) considerations towards safety and (4) practical implementation within a KOA-population. PubMed was searched from database inception to April 2024, and included previous review articles and consensus statements regarding the mechanisms, safety, and application of BFR, as well as literature focusing on KOA (
Table 1). Additionally, reference lists of articles obtained from this search were also examined for additional relevant literature. Only English-written papers that made a significant contribution to the body of knowledge on this topic were included for review. Ethical approval was not required for this study as no data collection involving human subjects occurred. Therefore, no institutional review board (IRB) or ethics committee approval was sought. The study solely relied on existing literature and secondary data sources.
3. Effect of BFRT in KOA
Research in strength training physiology has shown overwhelming that hypertrophic muscle adaptations can be induced at much lower exercise intensities than the intensities assumed to be crucial to result in muscle mass gains by combining LLRT with blood-flow restriction [
14]. For example, the systematic review (SR) and meta-analysis (MA) from Lixandrao and colleagues [
15] demonstrated that BFR with LLRT results in similar increases in muscle mass compared to HLRT, which is supported by the SR from Hughes et al. in 2017 [
16]. In line with these studies, a recent study of Hu and colleagues showed the additional effect of BFR in 112 patients with KOA, as the BFR group significantly improved in terms of strength, range of motion (ROM), quality of life (QoL) and ability of daily living (ADL) compared to a control group [
17].
Beside resistance training, which is the preferred exercise modality to induce muscle hypertrophy and -strength, it appears that enhanced levels of metabolic accumulation and ischemia during BFRT, combined with aerobic activities at low intensities (e.g., walking, cycling, rowing) also increase muscle volume and strength compared to aerobic exercises without BFR [
18,
19,
20]. For example, the study of Abe et al. (2010) indicated a significant increase in strength (11%) and thigh muscle mass volume (10.7%) after 6 weeks of walking 20 minutes 5x/week whereas no significant increases in the group without BFR were found [
19]. This is particularly interesting for the KOA population, which are encouraged to perform joint-friendly aerobic activities like walking and cycling on a regular basis, to safeguard joint load bearing capacity and a healthy body mass index (BMI). As these activities alone have little effect on muscle strength and size, the implementation of BFR would allow KOA patients to achieve strength and muscle mass increments whilst maintaining joint loading tolerance and a healthy cardiovascular profile.
In terms of pain, Ferraz et al. (2018) showed that the pain score according to the WOMAC subscale within KOA-patients was reduced significantly in both the LLRT-group and low load-BFRT group compared to HLRT, although positive muscle adaptations in terms of hypertrophy and strength were only found in the latter two [
21]. In line with these findings, Bryk et al. (2016) found that in patients with KOA, LL-BFRT resulted in similar benefits in function and quadriceps strength compared to HLRT, with HLRT inducing higher levels of anterior knee pain during training sessions compared to LL-BFRT [
22]. Therefore, evidence suggests that BFRT is a more effective training strategy than LLRT alone in individuals with KOA, as it appears to replicate the improvements in strength and muscle growth seen with HLRT, without the need for high intensities which might increase pain, discomfort and reduced adherence to therapy.
4. Mechanisms of BFRT
4.1. Hypertrophy and Strength
By limiting the arterial inflow whilst fully occluding the venous outflow using a personalized limb occlusion pressure (LOP), localized hypoxia is created enhancing the accumulation of metabolites such as lactate, hydrogen ions, inorganic and dihydrogen phosphate [
23,
24]. This metabolic stress has been suggested to be a primary factor responsible for anabolic/anticatabolic muscle adaptations by activating numerous other mechanisms such as increased type II muscle fiber recruitment, mechanotransduction, cell swelling
, muscle damage as well as satellite cell activation [
16,
23,
25,
26]. Important to note is that although these mechanisms might all induce anabolic muscle adaptations separately, it is assumed they contribute to a complex network altogether mediating a positive muscle protein synthesis (MPS), and concomitant muscle mass and muscle strength gains. Lastly, other mechanisms such as reactive oxygen species (ROS) and increase in growth hormone are often mentioned to explain increases in muscle mass and strength. However, due to the low graded and contradictive evidence, these proposed mechanisms are not further discussed within this paper.
4.1.1. Muscle Fiber Recruitment
Muscle fiber recruitment has been shown to play an essential role in the observed effects of BFRT. The recruitment of type II muscle fibers seems to be important for gaining muscle hypertrophy as these have a higher occurrence of signaling proteins compared to type I muscle fibers [
27,
28]. Muscle fibers are recruited according to the ‘size principle’, in which the smaller motor units associated with type I muscle fibers are activated initially at lower intensities, whereas the larger motor units related to type II muscle fibers are recruited at higher exercise intensities. Since the level of MPS is determined by the amount of muscle fibers activated, it is generally accepted that higher intensities (>70% of 1 Repetition Maximum (1RM)) are required to induce positive muscle adaptations in terms of hypertrophy and strength, as HLRT recruits both type I and type II muscle fibers (whereas LLRT mostly recruits type I fibers) [
13]. Interestingly, previous research using integrated electromyography (iEMG) has demonstrated that during BFRT, recruitment of type II muscle fibers happens even at low intensities (~20% 1RM), due to the low oxygen levels and metabolite accumulation induced by vascular occlusion, which causes the type I fibers to fatigue more rapidly [
26,
29,
30,
31]. Furthermore, group III and IV afferents located within a muscle are also stimulated by these metabolic processes. Stimulation of these afferents causes inhibition of the alpha motor neuron, thereby further enhancing muscle fiber recruitment to maintain adequate muscle force and protect against conduction failure [
26].
MPS occurs independently of exercise intensity, if fatigue is reached. As BFRT effectively induces muscle fatigue due to significantly decreased venous return and arterial inflow, it enables optimal fiber recruitment for muscle hypertrophy purposes [
26].
4.1.2. Mechanotransduction
When mechanical tension is applied to a muscle during exercise, a process called mechanotransduction is triggered. This process involves mechanosensors such as integrins and focal adhesions on the muscle cell membrane, which convert mechanical force into chemical signals. These signals activate anabolic and catabolic pathways within the muscle cell, leading to a shift in the balance of muscle protein synthesis and breakdown in favor of MPS [
32,
33]. Research has indeed shown that high load resistance training, involving the recruitment of abundant type I and II fibers can increase the phosphorylation of P70S6K-protein complex, hence increase MPS to a higher degree compared to LLRT alone [
34,
35]. As BFRT mimics this HLRT [
29], this novel training method could similarly enhance mechanotransduction and therefore increases MPS [
23].
4.1.3. Cell Swelling
Another mechanism which has been proposed to enhance MPS by applying BFRT is cell swelling. This phenomenon results from an increase in intracellular hydration and has been reported to stimulate protein synthesis and decrease proteolysis in various types of cells [
36]. The increased accumulation of metabolites through venous occlusion creates a pressure gradient, thereby enhancing reperfusion and subsequent intracellular swelling. This swelling, which is often referred to as ‘the pump’, is believed to initiate a signaling response within muscle cells in order to reinforce the muscle’s structural integrity over time [
37]. While some studies have reported positive effects of passive blood flow restriction on inducing anabolic muscle adaptations and reducing atrophy [
38,
39], other studies have not found significant differences in MPS between BFR with rest and rest alone [
40]. Therefore, more research is needed to fully understand the potential benefits of cellular swelling on muscle adaptations.
4.1.4. Muscle Damage
Exercise induced muscle damage (EIMD) is a common consequence of strength training, which from a mechanical perspective is thought to be induced by overstretching the sarcomere with subsequent extracellular matrix degradation as well as disruption of the cytoskeletal matrix and z-disk streaming [
41]. However, as BFRT is performed with low levels of mechanical stress, other mechanisms such as ischemia and subsequent reperfusion are likely responsible for muscle damage [
42]. Furthermore, activation of stretch-activated calcium channels or transient receptor potential channels may also contribute to muscle damage by increasing intracellular calcium levels which ultimately lead to muscle damage and necrosis [
43,
44].
Although EIMD is often associated with high levels of discomfort, it simultaneously triggers positive muscle adaptations as EIMD has been claimed to be an important regulator of satellite cell (SC) proliferation. Following EIMD, satellite cells located under the basal lamina rapidly proliferate and subsequently contribute to muscle remodeling and muscle growth [
23]. Indeed, several studies with HLRT already showed to produce significant levels of muscle damage due to the high loads and concomitant high mechanical stress placed upon muscles, compared with LLRT to which KOA-patients are often referred.
While the evidence for the effects of HLRT and LLRT on muscle damage is well established, the impact of BFRT on muscle damage appears to be more enigmatic. Although some studies found BFRT to induce only minimal levels of muscle damage [
45,
46], expressed in terms of delayed onset muscle soreness (DOMS) [
45], creatine kinase [
47] or interleukin (IL)-6 after [
34] 24 hours, other studies reported a larger degree of EIMD following BFRT, up to 48 hours after exercise [
48,
49]. However, elevated levels of muscle soreness, CK and IL-6 levels as a result of BFRT have especially been reported in individuals who are not accustomed to high metabolic stress associated with BFR, particularly when performing BFRT training until volitional failure [
50].
Despite the inconclusive evidence on the extent of EIMD caused by BFRT, literature suggests that muscle degeneration and regeneration occurs in response to exercise [
51], resulting in an increased proliferation of satellite cells in both acute and chronic BFRT [
52,
53]. This proliferation could be explained by increase in stretch-, hypoxia- and/or contraction induced nitric oxide (NO) secretion [
53]. Furthermore, other mechanisms such as increased levels of vascular endothelial growth factor (VEGF) and Hepatocyte Growth Factor (HGF) are associated with increased satellite cell proliferation and concomitant increase in MPS and muscle hypertrophy [
51,
54].
4.1.5. Autocrine pathway
Muscular hypertrophy and strength gains are primarily achieved through autocrine and paracrine actions, which stimulate protein synthesis by modulating anabolic and catabolic signaling pathways. The IGF-1/P13K/Akt signaling pathway is crucial in this process, promoting protein synthesis and suppressing proteolysis [
23,
55]. Within skeletal muscle, insulin growth factor (IGF-1) activates PI3K and Akt, triggering protein translation via mTORC1 induction, which regulates mRNA translation initiation and elongation [
55].
Moreover, the upregulation of the IGF/PI3K/Akt pathway seems to inhibit myostatin expression, a negative regulator of muscle growth that hampers myoblast and myotube differentiation through Smad2/3 phosphorylation [
55,
56]. Previous studies have demonstrated a decrease in myostatin expression and significant increases in muscle mass and strength after 8 weeks of LL-BFRT, comparable to the effects of HLRT [
57].
Nonetheless, further research is necessary to gain a comprehensive understanding of the underlying mechanisms responsible for these BFRT-induced effects.
4.2. The Effect of BFRT on Pain Reduction
Pain symptoms are associated with increased physical disability in patients with knee osteoarthritis [
58,
59]. Therefore, the primary objective in managing KOA is to alleviate pain while minimizing treatment-related adverse events. To control pain and improve physical function, oral non-steroidal anti-inflammatory drugs (NSAIDs) and paracetamol are the two most prescribed analgesics as 10% to 35% of the OA-patients report frequently using these drugs [
60,
61]. However, as these drugs involve an increased risk for gastrointestinal or cardiovascular complications, international guidelines as well as the National Institution for Health and Care Excellence strongly recommend exercise as a core therapy for reducing pain in KOA [
7,
8].
Indeed, resistance training has been found to be effective in reducing pain through the mechanism of exercised-induced hypoalgesia (EIH). The magnitude of this EIH appears to be greater with higher loads or prolonged exercise, as previous studies have shown that using an external load of >75% of 1RM resulted in significant pain reduction [
62]. Unfortunately, regardless of its benefits for pain, joint function and general (physical) health and well-being, HLRT is not feasible in many KOA patients due to their limitations in load bearing capacity, pain threshold and training background, making it very difficult to persevere and potentially harmful for some patients.
However, by applying BFR combined with LLRT, patients with a reduced load capacity might experience pain reduction while simultaneously benefiting from effects similar to HLRT, albeit without the need to implement heavy loads and high training intensities. Indeed, several studies have found that BFRT with 80% of the limb occlusion pressure resulted in a greater EIH response compared to light and heavy load resistance, which was prolonged for 24 hours in the exercising limb [
63] .Furthermore, in contrast to LLRT alone, Korakakis et al. (2018) found significant pain reduction after LLRT + BFR during functional testing in patients with anterior knee pain, which was also sustained after a 45-min physiotherapy session [
64]. In line with this, KOA-patients reported less knee pain and discomfort in the BFR-group compared to traditional HLRT [
22]. Interestingly, while a recent study by Ogrezeanu and colleagues found and increase in pain pressure thresholds (PPTs) following leg extension with BFR at 80% Limb Occlusion Pressure (LOP) in end stage KOA patients, their self reported pain significantly worsened [
65]. While this is in contrast to previous research claiming a pain reducing effect with BFR, it can be argued that leg extensions with such high occlusion pressures are not suitable within end-stage KOA patients without gradual exposure to BFR, and the visual analogue scale (VAS) does not differentiate between muscle pain or knee pain and does not take into account possible confounders such as the discomfort caused by the cuff pressure, mechanical load or the nocebo effect of physiological stress [
66].
Furthermore, the study of Hughes and Patterson (2020) found that BFRT led to a significant increase in peripheral blood beta-endorphin (BE) concentration, suggesting that BFRT may involve an opioid-mediated mechanism in EIH [
63]. The activation of the endogenous opioid system and stimulation of BE production may contribute to EIH by inhibiting noxious-evoked activity, as BFRT evokes a high level of metabolic stress which subsequently activates type III and IV afferents, thereby leading to a greater perception of intensity and discomfort. Additionally, other mechanisms have been proposed to explain the occurrence of EIH, including the recruitment of type II muscle fibers, a link between baroreceptors and pain pathways, ischemically- and metabolically-induced pain as well as the conditioned pain modulation (CPM) [
67].
5. Safety of Blood Flow Restriction
BFRT has gained popularity in recent years for its ability to increase both muscle mass [
15] and strength [
68], improve physical function [
18] and cardiorespiratory endurance capabilities within both resistance and aerobic training [
20]. Based on the available literature, BFRT appears to be a safe exercise modality when used according to evidence based guidelines [
69]. In their study in 2006, Nakajima and colleagues reported serious adverse event rates of 0.055%, 0.008% and 0.008% for deep venous thrombosis (DVT), pulmonary embolism (PE) and rhabdomyolysis, respectively [
70]. Despite these low adverse event rates, it is necessary to consider its safety, especially when applied in a clinical population with altered perceptual, cardiovascular or hemodynamic responses [
71]. Within BFRT literature, three primary areas of concern in terms of safety are often reported; venous thromboembolism (VTE), excessive hemodynamic/cardiovascular response and rhabdomyolysis [
72].
5.1. Risk for Venous Thromboembolism
The potential risk of VTE formation associated with BFRT has received considerable attention, particularly among individuals recovering from orthopedic surgery [
73]. During the initial 6 weeks following orthopedic surgery, there is a significantly elevated risk for VTE [
73]. However, current evidence indicates that the use of a tourniquet during surgery, which similarly induces “stasis”, albeit by applying much higher pressures over a prolonged amount of time, does not appear to increase this risk for VTE by itself [
74]. Therefore, the prospect of a brief (5-10 minutes per exercise), sub-occlusive pressure applied within KOA-patients should alleviate concerns regarding VTE risk [
69,
72,
73]. Furthermore, as studies showed no elevated levels of coagulation markers [
75] but instead even provided preliminary evidence of elevated fibrinolytic markers such as tissue plasminogen activator (tPA), it can be stated that the risk for VTE is not higher with BFRT (incidence rates of 0.055% and 0.008% for DVT and PE respectively) compared to traditional exercise [
70].
5.2. Excessive Cardiovascular Response
A second area of concern is the excessive cardiovascular response through increased stimulation of type III and IV afferents which might evoke the exercise pressor reflex (EPR). This EPR plays a strong role in the regulation of blood pressure and heart rate during exercise but appears to be dysregulated within patients with comorbidities such as hypertension (HTN), obesity and/or diabetes [
76,
77]. As these systemic conditions (e.g., obesity, HTN) are commonly associated with KOA, exaggerated sympathetic nerve activity could manifest itself, leading to abnormal elevations in mean arterial pressure and coronary vasomotor tone, thereby increasing the risk of adverse cardiovascular events during exercise [
76]. However, while BFRT has the capacity to increase the cardiovascular response to a similar degree compared to HLRT in both healthy and hypertensive individuals [
78], this increase appears to be within normal ranges, despite medical comorbidities [
79]. Furthermore, while comorbidities such as HTN should be taken into account, literature suggests that BFRT is capable to reduce postexercise systolic blood pressure to a larger degree compared to moderate intensity resistance training in hypertensive women [
80].
5.3. Rhabdomyolysis
Lastly, despite the low loads applied and the absence of mechanical disruption of myofibers, a few cases of rhabdomyolysis have been reported following BFRT [
81,
82,
83], which is characterized by the excessive release of CK and muscle myoglobin into the bloodstream [
84], although this not always appears to be present [
85]. While this is a serious side effect of resistance training, irrespective of the use of BFRT, its occurrence risk can likely be mitigated by gradually exposing the patient to BFRT, taking into account personal characteristics and previous experience with strength training [
72].
5.4. Practical Guidelines to Enhance Safety and Optimize Training Effectiveness
To prevent potential adverse events from occurring and make this type of training accessible for a diverse (patient) population, the use of personalized limb occlusion pressures (LOP)—which is the minimal pressure necessary to fully occlude both arterial and venous system—is recommended to limit excessive stress on the vascular system [
25]. The calculation of this personalized LOP allows practitioners for a selection of a pressure at a certain percentage of this LOP to standardize the level of occlusion across patients with different body characteristics. However, it should be noted that individualized LOP’s are determined at rest and do not consider muscle contractions, thereby producing a higher-than-anticipated pressure compared to resting conditions [
86]. Therefore, next to the use of individualized LOP’s, devices capable of autoregulation are recommended as they consider the contraction-related pressure and thus further enhance safety of BFRT [
86]. These devices ensure a constant LOP during resistance training sessions, as they adjust limb occlusion level in function of muscle contraction and relaxation phase throughout BFRT.
Despite blood flow restriction training has shown to be effective and well-tolerated in clinical settings, current literature often lacks individualized prescription of BFRT, especially regarding occlusive pressures, which should be tailored to ensure safe and effective application. For example, in the study of Segal et al. in patients with KOA risk factors [
87], a low load BFRT protocol was administered in a cohort of women, which was replicated in a cohort of men using similar modalities. While the women demonstrated an increase in muscle strength, no significant improvement in strength was observed in the male cohort, despite the same BFR protocol being applied [
88]. As men tend to have greater thigh circumference than women, it is conceivable that the same BFR pressure induced an insufficient BFR stimulus to enhance muscle strength.
Indeed, besides blood pressure and cuff width, previous studies used thigh circumference to determine limb occlusion pressure, with larger limbs requiring higher pressures to reach a similar degree of occlusion compared to smaller limbs [
25]. These arbitrary pressures will have highly variable effects within heterogenous (patient or athletic) populations, possibly increasing the risk for adverse events [
89] or reducing the effectiveness, depending on the level of occlusion which cannot be estimated on the basis of thigh circumference. Although more research is necessary, individualized pressures between 40-80% LOP are generally recommended, with some studies showing the pressure to be inversely proportional to the load applied (i.e., when exercising at the low end of the load spectrum (~20% 1RM), higher pressures around 80% LOP should be applied) [
90]. However, from a perceptual perspective, greater degrees of (muscle) discomfort have been linked to higher pressures, especially during the initial application period [
91]. Therefore, while higher pressures might be advantageous for a given goal (e.g., pain reduction), it is important for practitioners to consider the associated perceptual demands. Employing lower, perhaps physiologically suboptimal pressures around 40% LOP at the outset of care may serve as a valuable approach to gradually acclimate KOA-patients to the perceptual demands required for exercise adaptation. Furthermore, individual assessment of (relative)-contra indications using questionnaires, medical history, Virchow’s Triad and physical examination remains important, especially in post-surgical patients or patients with comorbidities such as hypertension, obesity or diabetes [
72,
73].
Comparable to the heterogeneity in terms of LOP pressures applied, a lot of variety exists in terms of volume necessary to enhance muscular adaptations. However, according to Scott et al. and Loenneke et al. [
92,
93], two to three low load BFRT training sessions per week is sufficient for enhanced strength adaptations in clinical populations.
6. Practical Implementation
To maximize the benefits of this novel training method, Loenneke et al. [
94] proposed a progressive model through which BFR can be applied in various stages of rehabilitation, including both voluntary resistance and aerobic exercise as well as passively, without exercise. While the latter can be applied during bedrest to diminish atrophy for some patients [
38,
39], it might simultaneously serve as entry level for sedentary patients who are unaccustomed to BFRT. Next, following periods of (relative) rest, aerobic activities such as cycling or rowing are often recommended for patients with KOA to maintain their ROM and cardiovascular fitness whilst simultaneously maintaining muscle mass, as these non-weight bearing activities are associated with lower levels of pain and discomfort. As the addition of BFR in cycling or walking activities has been proven to increase both muscle mass and strength [
18,
19,
95], aerobic capacity [
19,
96] and the functional capacity compared to aerobic exercise without BFR [
97], it should be promoted and facilitated in patients with KOA. Similarly, as the training loads applied during BFR combined with resistance training, the intensities applied during aerobic BFR should be generally low, around 45% heart rate reserve or 40% VO2 max [
19,
25,
97].
BFR training guidelines, based on the evidence as regards the implementation of BFR in training and musculoskeletal rehabilitation, for patients with KOA are summarized in
Table 2.
| |
RESTING STATE BFR ACCOMMODATION |
AEROBIC TRAINING |
LOW LOAD RESISTANCE TRAINING |
| PATIENT PROFILE* |
Sedentary patients; Patients starting to rehabilitate after a period of bedrest; Patients with anxiety for BFRT |
All KOA patients |
All KOA patients |
| LOP |
70%-100% LOP |
<50% VO2max or Heart Rate Reserve (HRR) |
40-80% LOP (depending on the patient’s training status) |
| TRAINING FREQUENCY |
1-2x/day during supine position |
2-3x/week |
2-3x/week |
| RESTRICTION TIME |
5min |
5-20min per exercise |
5-10 min per exercise |
|
3-5x/5min, 3-5 min passive recovery |
2-4x/5min up to 2x10min,
1-2 min active or passive recovery |
75 reps—30/15/15/15 or sets until failure, 30-60 s rest |
| RESTRICTION FORM |
Intermittent |
Continuous or intermittent |
Continuous or intermittent |
| TYPE OF EXERCISE |
None;
Electrostimulation |
Walking; Cycling; rowing |
Quadriceps dominant Hamstring dominant Calf dominant |
| EXPECTED TRAINING RESULTS |
Prevention of muscle atrophy |
Optimization of cardiovascular response to aerobic stimuli; muscle volume and strength gains |
Muscle volume and strength gains |
| SAFETY GUIDELINES |
Use individualized LOP and autoregulated BFR devices, guaranteeing safe occlusion pressure levels |
Use individualized LOP and autoregulated BFR devices, guaranteeing safe occlusion pressure levels |
Use individualized LOP and autoregulated BFR devices, guaranteeing safe occlusion pressure levels |
7. Conclusion
As surgery is often not the preferred method of treatment in many KOA patients, conservative alternatives such as exercise therapy are generally promoted and facilitated. However, the intensity required to induce positive muscle adaptations with this exercise therapy implies substantial loading of the degenerated intra- and peri-articular structures, making its implementation in KOA patients often not possible. Instead, individualized blood flow restriction training might offer a feasible alternative acting as a surrogate for high load strength training while using low loads and promote training responses during low load aerobic or resistance activities, thereby improving muscle strength and mass, functional capacities and ultimately quality of life in patients with KOA. Based on the exponentially growing literature, 2-3 BFR sessions per week with low loads or low intensities and individualized LOP’s in a fixed or failure protocol appears optimal to maximize training effects, taking into account personal characteristics and safety guidelines.
Author Contributions
EJ, EW, EW and JS conceived and designed the study, and were responsible for developing the research question. EJ, EW and JS wrote the first draft of the manuscript. All authors contributed to critical revision of the manuscript and approval of the final draft prior to submission.
Funding
This research was funded by Flanders Research Foundation (Fonds voor Wetenschappelijk Onderzoek Vlaanderen (FWO)). The funding source had no involvement in the study design, interpretation, or writing of the manuscript.
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29-30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L., Osteoarthritis of the Knee. The New England journal of medicine, 2021. 384(1): p. 51-59.
- Aggarwal, V.K., et al., Revision total knee arthroplasty in the young patient: is there trouble on the horizon? The Journal of bone and joint surgery. American volume, 2014. 96(7): p. 536-42.
- Vince, K.G., You can do arthroplasty in a young patient, but...: Commentary on articles by John P. Meehan, MD, et al.: “Younger age is associated with a higher risk of early periprosthetic joint infection and aseptic mechanical failure after total knee arthroplasty,” and Vinay K. Aggarwal, et al.: “Revision total knee arthroplasty in the young patient: is there trouble on the horizon?”. The Journal of bone and joint surgery. American volume, 2014. 96(7): p. e58.
- Carr, A.J., et al., Knee replacement. Lancet (London, England), 2012. 379(9823): p. 1331-40.
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L., et al., 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis care & research, 2020. 72(2): p. 149-162.
- Skou, S.T. and E.M. Roos, Physical therapy for patients with knee and hip osteoarthritis: supervised, active treatment is current best practice. Clinical and experimental rheumatology, 2019. 37 Suppl 120(5): p. 112-117.
- Fisher, N.M.; Pendergast, D.R.; E Gresham, G.; Calkins, E. Muscle rehabilitation: its effect on muscular and functional performance of patients with knee osteoarthritis. Arch. Phys. Med. Rehabil. 1991, 72, 367–74. [Google Scholar] [PubMed]
- Schilke, J.M.; Johnson, G.O.; Housh, T.J.; O'Dell, J.R. Effects of Muscle-Strength Training on the Functional Status of Patients with Osteoarthritis of the Knee Joint. Nurs. Res. 1996, 45, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J., et al., Muscular adaptations in low- versus high-load resistance training: A meta-analysis. European journal of sport science, 2016. 16(1): p. 1-10.
- American College of Sports, M. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and science in sports and exercise, 2009. 41(3): p. 687-708.
- Kim, K.-H.; Kang, S.-H.; Kim, N.; Choi, J.; Kang, S. Short-Term Impact of Low-Intensity Exercise with Blood Flow Restriction on Mild Knee Osteoarthritis in Older Adults: A Pilot Study. Healthcare 2024, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports Med. 2017, 48, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, B.; Wang, Y.; Yang, F.; Zhang, J.; Zhong, W.; Lu, S.; Luo, C. Effectiveness of blood flow restriction versus traditional weight-bearing training in rehabilitation of knee osteoarthritis patients with MASLD: a multicenter randomized controlled trial. Front. Endocrinol. 2023, 14, 1220758. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Sakamaki, M.; Fujita, S.; Ozaki, H.; Sugaya, M.; Sato, Y.; Nakajima, T. Effects of low-intensity walk training with restricted leg blood flow on muscle strength and aerobic capacity in older adults. J. Geriatr. Phys. Ther. 2010, 33, 34–40. [Google Scholar] [PubMed]
- Abe, T.; Fujita, S.; Nakajima, T.; Sakamaki, M.; Ozaki, H.; Ogasawara, R.; Sugaya, M.; Kudo, M.; Kurano, M.; Yasuda, T.; et al. Effects of Low-Intensity Cycle Training with Restricted Leg Blood Flow on Thigh Muscle Volume and VO2MAX in Young Men. J. Sports Sci. Med. 2010, 9, 452–8. [Google Scholar] [PubMed]
- Held, S., Behringer, and L. Donath, Low intensity rowing with blood flow restriction over 5 weeks increases VO2max in elite rowers: A randomized controlled trial. Journal of science and medicine in sport, 2020. 23(3): p. 304-308.
- Ferraz, R.B.; Gualano, B.; Rodrigues, R.; Kurimori, C.O.; Fuller, R.; Lima, F.R.; De Sá-Pinto, A.L.; Roschel, H. Benefits of Resistance Training with Blood Flow Restriction in Knee Osteoarthritis. Med. Sci. Sports Exerc. 2018, 50, 897–905. [Google Scholar] [CrossRef]
- Bryk, F.F.; dos Reis, A.C.; Fingerhut, D.; Araujo, T.; Schutzer, M.; Cury, R.d.P.L.; Duarte, A.; Fukuda, T.Y. Exercises with partial vascular occlusion in patients with knee osteoarthritis: a randomized clinical trial. Knee Surgery, Sports Traumatol. Arthrosc. 2016, 24, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Pearson, S.J.; Hussain, S.R. A Review on the Mechanisms of Blood-Flow Restriction Resistance Training-Induced Muscle Hypertrophy. Sports Med. 2014, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.D.S.; Karabulut, M.; Bemben, M.G. The Evolution of Blood Flow Restricted Exercise. Front. Physiol. 2021, 12. [Google Scholar] [CrossRef]
- Patterson, S.D., et al., Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Frontiers in physiology, 2019. 10: p. 533.
- Loenneke, J.; Fahs, C.; Wilson, J.; Bemben, M. Blood flow restriction: The metabolite/volume threshold theory. Med Hypotheses 2011, 77, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Goreham, C.; Ouyang, J.; Ball-Burnett, M.; Ranney, D.; Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; et al. Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R591–R596. [Google Scholar] [CrossRef]
- Fry, A.C. The Role of Resistance Exercise Intensity on Muscle Fibre Adaptations. Sports Med. 2004, 34, 663–679. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y.; Ishii, N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Moore, D.R.; Burgomaster, K.A.; Schofield, L.M.; Gibala, M.J.; Sale, D.G.; Phillips, S.M. Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur. J. Appl. Physiol. 2004, 92, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Brechue, W.F.; Fujita, T.; Shirakawa, J.; Sato, Y.; Abe, T. Muscle activation during low-intensity muscle contractions with restricted blood flow. J. Sports Sci. 2009, 27, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Zou, K., et al., The alpha(7)beta(1)-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. Journal of applied physiology (Bethesda, Md.: 1985), 2011. 111(4): p. 1134-41.
- Schiaffino, S.; Reggiani, C.; Akimoto, T.; Blaauw, B. Molecular Mechanisms of Skeletal Muscle Hypertrophy. J. Neuromuscul. Dis. 2020, 8, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Glynn, E.L.; Drummond, M.J.; Timmerman, K.L.; Fujita, S.; Abe, T.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiol. 2010, 108, 1199–1209. [Google Scholar] [CrossRef]
- Fujita, S.; Abe, T.; Drummond, M.J.; Cadenas, J.G.; Dreyer, H.C.; Sato, Y.; Volpi, E.; Rasmussen, B.B. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J. Appl. Physiol. 2007, 103, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Busch, G.L.; Ritter, M.; Völkl, H.; Waldegger, S.; Gulbins, E.; Häussinger, D. Functional Significance of Cell Volume Regulatory Mechanisms. Physiol. Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.; Fahs, C.; Rossow, L.; Abe, T.; Bemben, M. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med Hypotheses 2012, 78, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Takazawa, H.; Ishii, N. Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Med. Sci. Sports Exerc. 2000, 32, 2035–2039. [Google Scholar] [CrossRef]
- Kubota, A.; Sakuraba, K.; Sawaki, K.; Sumide, T.; Tamura, Y. Prevention of Disuse Muscular Weakness by Restriction of Blood Flow. Med. Sci. Sports Exerc. 2008, 40, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Nyakayiru, J.; Fuchs, C.J.; Trommelen, J.; Smeets, J.S.J.; Senden, J.M.; Gijsen, A.P.; Zorenc, A.H.; VAN Loon, L.J.C.; Verdijk, L.B. Blood Flow Restriction Only Increases Myofibrillar Protein Synthesis with Exercise. Med. Sci. Sports Exerc. 2019, 51, 1137–1145. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Umbel, J.D.; Hoffman, R.L.; Dearth, D.J.; Chleboun, G.S.; Manini, T.M.; Clark, B.C. Delayed-onset muscle soreness induced by low-load blood flow-restricted exercise. Eur. J. Appl. Physiol. 2009, 107, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Whitehead, N.P.; Yeung, E.W. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J. Physiol. 2005, 567, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.W., et al., Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. The Journal of physiology, 2005. 562(Pt 2): p. 367-80.
- Thiebaud, R.; Loenneke, J.; Fahs, C.; Kim, D.; Ye, X.; Abe, T.; Nosaka, K.; Bemben, M. Muscle damage after low-intensity eccentric contractions with blood flow restriction. Acta Physiol. Hung. 2014, 101, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, R.S.; Yasuda, T.; Loenneke, J.P.; Abe, T. Effects of low-intensity concentric and eccentric exercise combined with blood flow restriction on indices of exercise-induced muscle damage. Interv. Med. Appl. Sci. 2013, 5, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sieljacks, P.; Matzon, A.; Wernbom, M.; Ringgaard, S.; Vissing, K.; Overgaard, K. Muscle damage and repeated bout effect following blood flow restricted exercise. Eur. J. Appl. Physiol. 2015, 116, 513–525. [Google Scholar] [CrossRef] [PubMed]
- de Queiros, V.S., et al., Application and side effects of blood flow restriction technique: A cross-sectional questionnaire survey of professionals. Medicine, 2021. 100(18): p. e25794.
- de Queiros, V.S., et al., Effect of resistance training with blood flow restriction on muscle damage markers in adults: A systematic review. PloS one, 2021. 16(6): p. e0253521.
- Fu, X.; Wang, H.; Hu, P. Stem cell activation in skeletal muscle regeneration. Cell. Mol. Life Sci. 2015, 72, 1663–1677. [Google Scholar] [CrossRef] [PubMed]
- Wernbom, M.; Apro, W.; Paulsen, G.; Nilsen, T.S.; Blomstrand, E.; Raastad, T. Acute low-load resistance exercise with and without blood flow restriction increased protein signalling and number of satellite cells in human skeletal muscle. Eur. J. Appl. Physiol. 2013, 113, 2953–2965. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.L.; Aagaard, P.; Bech, R.D.; Nygaard, T.; Hvid, L.G.; Wernbom, M.; Suetta, C.; Frandsen, U. Proliferation of myogenic stem cells in human skeletal muscle in response to low-load resistance training with blood flow restriction. J. Physiol. 2012, 590, 4351–4361. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, A.C.; Anderson, J.E. Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev. Dyn. 2006, 236, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef] [PubMed]
- Laurentino, G.C.; Ugrinowitsch, C.; Roschel, H.; Aoki, M.S.; Soares, A.G.; Neves, M.; Aihara, A.Y.; Fernandes, A.D.R.C.; Tricoli, V. Strength Training with Blood Flow Restriction Diminishes Myostatin Gene Expression. Med. Sci. Sports Exerc. 2012, 44, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N., R. Arant, and R.F. Loeser, Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA, 2021. 325(6): p. 568-578.
- da Costa, B.R., et al., Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet (London, England), 2017. 390(10090): p. e21-e33.
- Zeng, C.; Doherty, M.; Persson, M.; Yang, Z.; Sarmanova, A.; Zhang, Y.; Wei, J.; Kaur, J.; Li, X.; Lei, G.; et al. Comparative efficacy and safety of acetaminophen, topical and oral non-steroidal anti-inflammatory drugs for knee osteoarthritis: evidence from a network meta-analysis of randomized controlled trials and real-world data. Osteoarthr. Cartil. 2021, 29, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R., et al., Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann. Intern. Med.
- Koltyn, K.F.; Arbogast, R.W. Perception of pain after resistance exercise. Br. J. Sports Med. 1998, 32, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Patterson, S.D. The effect of blood flow restriction exercise on exercise-induced hypoalgesia and endogenous opioid and endocannabinoid mechanisms of pain modulation. J. Appl. Physiol. 2020, 128, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Korakakis, V.; Whiteley, R.; Giakas, G. Low load resistance training with blood flow restriction decreases anterior knee pain more than resistance training alone. A pilot randomised controlled trial. Phys. Ther. Sport 2018, 34, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ogrezeanu, D.C.; López-Bueno, L.; Sanchís-Sánchez, E.; Suso-Martí, L.; López-Bueno, R.; Núñez-Cortés, R.; Cruz-Montecinos, C.; Pérez-Alenda, S.; Casaña, J.; Gargallo, P.; et al. Exercise-induced hypoalgesia with end-stage knee osteoarthritis during different blood flow restriction levels: Sham-controlled crossover study. PM&R 2023, 15, 1565–1573. [Google Scholar] [CrossRef]
- McGuire, D.B. Comprehensive and multidimensional assessment and measurement of pain. J. Pain Symptom Manag. 1992, 7, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Patterson, S.D. Low intensity blood flow restriction exercise: Rationale for a hypoalgesia effect. Med. Hypotheses 2019, 132, 109370. [Google Scholar] [CrossRef] [PubMed]
- Grønfeldt, B.M.; Nielsen, J.L.; Mieritz, R.M.; Lund, H.; Aagaard, P. Effect of blood-flow restricted vs heavy-load strength training on muscle strength: Systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Minniti, M.C.; Statkevich, A.P.; Kelly, R.L.; Rigsby, V.P.; Exline, M.M.; Rhon, D.I.; Clewley, D. The Safety of Blood Flow Restriction Training as a Therapeutic Intervention for Patients with Musculoskeletal Disorders: A Systematic Review. Am. J. Sports Med. 2019, 48, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Kurano, M.; Iida, H.; Takano, H.; Oonuma, H.; Morita, T.; Meguro, K.; Sato, Y.; Nagata, T. ; KAATSU Training Group Use and safety of KAATSU training:Results of a national survey. Int. J. KAATSU Train. Res. 2006, 2, 5–13. [Google Scholar] [CrossRef]
- Hughes, L.; Rosenblatt, B.; Gissane, C.; Paton, B.; Patterson, S.D. Interface pressure, perceptual, and mean arterial pressure responses to different blood flow restriction systems. Scand. J. Med. Sci. Sports 2018, 28, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R., et al., Perceived Barriers to Blood Flow Restriction Training. Frontiers in physiology, 2021.
- Bond, C.W.; Hackney, K.J.; Brown, S.L.; Noonan, B.C. Blood Flow Restriction Resistance Exercise as a Rehabilitation Modality Following Orthopaedic Surgery: A Review of Venous Thromboembolism Risk. J. Orthop. Sports Phys. Ther. 2019, 49, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, P.M., et al., Do thigh tourniquets contribute to the formation of intra-operative venous emboli? Acta orthopaedica Belgica, 2004. 70(3): p. 253-9.
- Madarame, H.; Kurano, M.; Takano, H.; Iida, H.; Sato, Y.; Ohshima, H.; Abe, T.; Ishii, N.; Morita, T.; Nakajima, T. Effects of low-intensity resistance exercise with blood flow restriction on coagulation system in healthy subjects. Clin. Physiol. Funct. Imaging 2010, 30, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Spranger, M.D.; Krishnan, A.C.; Levy, P.D.; O'Leary, D.S.; Smith, S.A. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am. J. Physiol. Circ. Physiol. 2015, 309, H1440–H1452. [Google Scholar] [CrossRef] [PubMed]
- Cristina-Oliveira, M.; Meireles, K.; Spranger, M.D.; O’leary, D.S.; Roschel, H.; Peçanha, T. Clinical safety of blood flow-restricted training? A comprehensive review of altered muscle metaboreflex in cardiovascular disease during ischemic exercise. Am. J. Physiol. Circ. Physiol. 2019, 318, H90–H109. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Peiffer, J.J.; Thomas, H.J.; Marston, K.J.; Hill, K.D. Hemodynamic Responses to Low-Load Blood Flow Restriction and Unrestricted High-Load Resistance Exercise in Older Women. Front. Physiol. 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Neto, G.R.; Novaes, J.S.; Dias, I.; Brown, A.; Vianna, J.; Cirilo-Sousa, M.S. Effects of resistance training with blood flow restriction on haemodynamics: a systematic review. Clin. Physiol. Funct. Imaging 2016, 37, 567–574. [Google Scholar] [CrossRef]
- Araújo, J.P.; Silva, E.D.; Silva, J.C.G.; Souza, T.S.P.; Lima, E.O.; Guerra, I.; Sousa, M.S.C. The Acute Effect of Resistance Exercise with Blood Flow Restriction with Hemodynamic Variables on Hypertensive Subjects. J. Hum. Kinet. 2014, 43, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.M.A.B.; Slysz, J.T.M.; Burr, J.F. Risks of Exertional Rhabdomyolysis With Blood Flow–Restricted Training: Beyond the Case Report. Am. J. Ther. 2018, 28, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Suzuki, Y.; Azuma, K.; Matsumoto, H. Rhabdomyolysis After Performing Blood Flow Restriction Training: A Case Report. J. Strength Cond. Res. 2016, 30, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C. and T.M. Manini, Can KAATSU Exercise Cause Rhabdomyolysis? Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine, 2017. 27(1): p. e1-e2.
- Burr, J.F.; Hughes, L.; Warmington, S.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; Neto, G.R.; et al. Response: Commentary: Can Blood Flow Restricted Exercise Cause Muscle Damage? Commentary on Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2020, 11, 574633. [Google Scholar] [CrossRef] [PubMed]
- Sauret, J.M. Marinides, and G.K. Wang, Rhabdomyolysis. American family physician, 2002. 65(5): p. 907-12.
- Jacobs, E.; Rolnick, N.; Wezenbeek, E.; Stroobant, L.; Capelleman, R.; Arnout, N.; Witvrouw, E.; Schuermans, J. Investigating the autoregulation of applied blood flow restriction training pressures in healthy, physically active adults: an intervention study evaluating acute training responses and safety. Br. J. Sports Med. 2023, 57, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.A., et al., Efficacy of blood flow-restricted, low-load resistance training in women with risk factors for symptomatic knee osteoarthritis. PM & R: the journal of injury, function, and rehabilitation, 2015. 7(4): p. 376-84.
- Segal, N.; Davis, M.D.; Mikesky, A.E. Efficacy of Blood Flow-Restricted Low-Load Resistance Training For Quadriceps Strengthening in Men at Risk of Symptomatic Knee Osteoarthritis. Geriatr. Orthop. Surg. Rehabilitation 2015, 6, 160–167. [Google Scholar] [CrossRef]
- Jessee, M.B.; Buckner, S.L.; Mouser, J.G.; Mattocks, K.T.; Loenneke, J.P. Letter to the editor: Applying the blood flow restriction pressure: the elephant in the room. Am. J. Physiol. Circ. Physiol. 2016, 310, H132–H133. [Google Scholar] [CrossRef] [PubMed]
- Lixandrão, M.E.; Ugrinowitsch, C.; Laurentino, G.; Libardi, C.A.; Aihara, A.Y.; Cardoso, F.N.; Tricoli, V.; Roschel, H. Effects of exercise intensity and occlusion pressure after 12 weeks of resistance training with blood-flow restriction. Eur. J. Appl. Physiol. 2015, 115, 2471–2480. [Google Scholar] [CrossRef]
- Spitz, R.W.; Chatakondi, R.N.; Bell, Z.W.; Wong, V.; Viana, R.B.; Dankel, S.J.; Abe, T.; Yamada, Y.; Loenneke, J.P. Blood Flow Restriction Exercise: Effects of Sex, Cuff Width, and Cuff Pressure on Perceived Lower Body Discomfort. Percept. Mot. Ski. 2020, 128, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.P.; Wilson, J.M.; Marín, P.J.; Zourdos, M.C.; Bemben, M.G. Low intensity blood flow restriction training: a meta-analysis. Eur. J. Appl. Physiol. 2011, 112, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.R.; Loenneke, J.P.; Slattery, K.M.; Dascombe, B.J. Exercise with Blood Flow Restriction: An Updated Evidence-Based Approach for Enhanced Muscular Development. Sports Med. 2014, 45, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Loenneke, J.; Abe, T.; Wilson, J.; Thiebaud, R.; Fahs, C.; Rossow, L.; Bemben, M. Blood flow restriction: An evidence based progressive model (Review). Acta Physiol. Hung. 2012, 99, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Slysz, J.; Stultz, J.; Burr, J.F. The efficacy of blood flow restricted exercise: A systematic review & meta-analysis. J. Sci. Med. Sport 2015, 19, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, M.J.; Conway, L.; Warmington, S.A. Blood flow restriction walking and physical function in older adults: A randomized control trial. J. Sci. Med. Sport 2017, 20, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Domain-specific search strategy in Pubmed.
Table 1.
Domain-specific search strategy in Pubmed.
| Research Domain |
Search strategy |
Hits |
| Blood Flow Restriction |
(“blood flow restriction training” OR “BFR training” OR “blood flow restriction exercise” OR “BFR exercise” OR “blood flow restriction therapy” OR “BFR therapy” OR “blood-flow restriction” OR BFR OR “occlusion training” OR “occlusion therapy” OR “Blood Flow Restriction Therapy”[Mesh]) |
7277 |
| Knee Osteoarthritis |
“knee osteoarthr*” or “KOA” or “gonarthrosis” |
35349 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).