Submitted:
26 June 2024
Posted:
26 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants and Design
2.2. Ultrasound Imaging
2.3. Ankle Isometric Muscle Strength Testing
2.4. Toe Grasp Force Measurement
2.5. Superficial Sensory Measurements
2.6. Dynamic Balance Measurement
2.7. Surface Muscle Activity Analysis
2.8. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Ankle Isometric Muscle Strength Measurements
3.3. Toe Grasp Strength
3.4. Plantar Superficial Sensation
3.5. Muscle Activity
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Booth, D. Surf lifesaving: the development of an Australasian "sport". Int J Hist Sport 2000, 17, 167–187. [Google Scholar] [CrossRef]
- Binnie, M.J.; Dawson, B.; Arnot, M.A.; Pinnington, H.; Landers, G.; Peeling, P. Effect of sand versus grass training surfaces during an 8-week pre-season conditioning programme in team sport athletes. J Sports Sci 2014, 32, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Binnie, M.J.; Dawson, B.; Pinnington, H.; Landers, G.; Peeling, P. Sand training: a review of current research and practical applications. J Sports Sci 2014, 32, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Pinnington, H.C.; Lloyd, D.G.; Besier, T.F.; Dawson, B. Kinematic and electromyography analysis of submaximal differences running on a firm surface compared with soft, dry sand. Eur J Appl Physiol 2005, 94, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, S.; Kumai, T.; Okunuki, T.; Maemichi, T.; Matsumoto, M.; Yabiku, H.; Liu, Z.; Yamaguchi, R.; Iwayama, A.; Ayukawa, G.; et al. Comparison of foot posture and foot muscle morphology between lifesaver athletes and healthy adults. Res Sports Med 2023, 31, 506–516. [Google Scholar] [CrossRef]
- Giatsis, G.; Panoutsakopoulos, V.; Kollias, I.A. Biomechanical differences of arm swing countermovement jumps on sand and rigid surface performed by elite beach volleyball players. J Sports Sci 2018, 36, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Hrysomallis, C. Balance ability and athletic performance. Sports Med 2011, 41, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Smith, R. Movement in the sand: training implications for beach volleyball. Strength and Conditioning Journal 2006, 28, 19–21. [Google Scholar] [CrossRef]
- Matsumoto, M.; Maemichi, T.; Wada, M.; Niwa, Y.; Inagaki, S.; Okunuki, T.; Ichikawa, S.; Kumai, T. Ultrasonic evaluation of the heel fat pad under weight-bearing conditions using a polymethylpentene resin plate: part 1. Ultrasound Med Biol 2022, 48, 358–372. [Google Scholar] [CrossRef]
- Matsumoto, M.; Maemichi, T.; Wada, M.; Niwa, Y.; Inagaki, S.; Taguchi, A.; Okunuki, T.; Tanaka, H.; Kumai, T. Ultrasonic evaluation of the heel fat pad under loading conditions using a polymethylpentene resin plate: part 2. reliability and agreement study. Ultrasound Med Biol 2023, 49, 460–472. [Google Scholar] [CrossRef]
- Chamorro, C.; Armijo-Olivo, S.; De la Fuente, C.; Fuentes, J.; Javier Chirosa, L. Absolute reliability and concurrent validity of hand held dynamometry and isokinetic dynamometry in the hip, knee and ankle joint: systematic review and meta-analysis. Open Med (Wars) 2017, 12, 359–375. [Google Scholar] [CrossRef]
- Bell-Krotoski, J.A.; Buford, W.L. The force/time relationship of clinically used sensory testing instruments. J Hand Ther 1997, 10, 297–309. [Google Scholar] [CrossRef]
- Powden, C.J.; Dodds, T.K.; Gabriel, E.H. The reliability of the star excursion balance test and lower quarter y-balance test in healthy adults: a systematic review. Int J Sports Phys Ther 2019, 14, 683–694. [Google Scholar] [CrossRef] [PubMed]
- SENIAM project. Recommendations for sensor locations on individual muscles. Available online: http://www.seniam.org/ (accessed on 10 June 2024).
- Herrington, L.; Hatcher, J.; Hatcher, A.; McNicholas, M. A comparison of Star Excursion Balance Test reach distances between ACL deficient patients and asymptomatic controls. Knee 2009, 16, 149–152. [Google Scholar] [CrossRef]
- Olmsted, L.C.; Carcia, C.R.; Hertel, J.; Shultz, S.J. Efficacy of the star excursion balance tests in detecting reach deficits in subjects with chronic ankle instability. J Athl Train 2002, 37, 501–506. [Google Scholar]
- Özkal, Ö.; Kara, M.; Topuz, S.; Kaymak, B.; Bakı, A.; Özçakar, L. Assessment of core and lower limb muscles for static/dynamic balance in the older people: An ultrasonographic study. Age Ageing 2019, 48, 881–887. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, X.; Mao, M.; Sun, W.; Zhang, C.; Chen, Y.; Li, L. Relationship of proprioception, cutaneous sensitivity, and muscle strength with the balance control among older adults. J Sport Health Sci 2021, 10, 585–593. [Google Scholar] [CrossRef]
- Menz, H.B.; Morris, M.E.; Lord, S.R. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J Gerontol A Biol Sci Med Sci 2005, 60, 1546–1552. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Oyama, Y.; Tanaka, M.; Sakamoto, A. Toe functions have little effect on dynamic balance ability in elderly people. J Phys Ther Sci 2017, 29, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kamikura, S.; Sakuraba, K.; Miura, T. Effects of reach balance exercise on toe grip strength and balance in college basketball players. Prog Rehabil Med 2018, 3, 20180008. [Google Scholar] [CrossRef]
- Inglis, J.T.; Kennedy, P.M.; Wells, C.; Chua, R. The role of cutaneous receptors in the foot. Adv Exp Med Biol 2002, 508, 111–117. [Google Scholar] [PubMed]
- Fitzpatrick, R.; McCloskey, D.I. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol 1994, 478 Pt 1, 173–186. [Google Scholar] [CrossRef]
- Kavounoudias, A.; Roll, R.; Roll, J.P. The plantar sole is a 'dynamometric map' for human balance control. Neuroreport 1998, 9, 3247–3252. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Almeida, Y.; Black, M.L.; Christou, E.A.; Clark, D.J. Site-specific differences in the association between plantar tactile perception and mobility function in older adults. Front Aging Neurosci 2014, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, G.; Thoumie, P.; Do, M.C.; Schieppati, M. Cutaneous and muscular afferents from the foot and sensory fusion processing: Physiology and pathology in neuropathies. J Peripher Nerv Syst 2021, 26, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Karagiannakis, D.N.; Iatridou, K.I.; Mandalidis, D.G. Ankle muscles activation and postural stability with Star Excursion Balance Test in healthy individuals. Hum Mov Sci 2020, 69, 102563. [Google Scholar] [CrossRef]
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: a new paradigm for understanding intrinsic foot muscle function. Br J Sports Med 2015, 49, 290. [Google Scholar] [CrossRef]
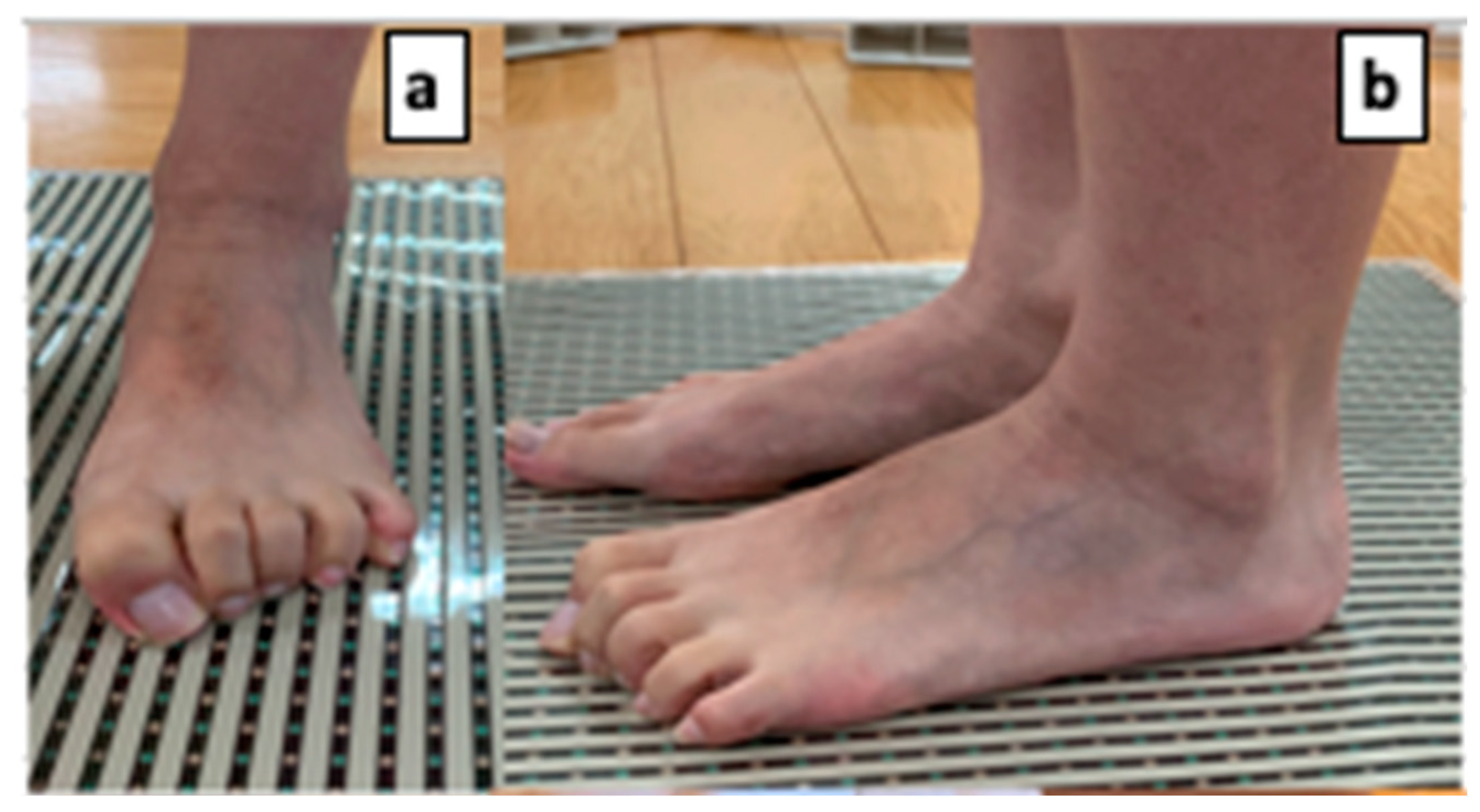
| Lifesavers | |
| (N=15; 12 males 3 females) | |
| Variable | Mean (SD) |
| Age (years) | 24.5 (5.4) |
| Height (cm) | 169 (7.1) |
| Weight (kg) | 66.1 (11.4) |
| BMI (kg/m2) | 23 (2.5) |
| Peformance Indicators | Mean±Standard Deviation (N=15) | ||
|---|---|---|---|
| muscle strength (N/Kg) | Dorsiflexion | 3.88 | 1.15 |
| planter flexion | 2.45 | 0.56 | |
| Eversion | 2.31 | 0.61 | |
| toe grasp strength | %BW | 47.4 | 14.4 |
| cross section area(muscles) | Abductor hallucis(ABH) | 3.16 | 0.65 |
| Adductor digiti minimi (ADD) | 1.58 | 0.44 | |
| Flexor digitorum brevis( FDB) | 2.61 | 0.7 | |
| Flexor hallucis brevis( FHB) | 2.52 | 0.7 | |
| Tibialis anterior (TA) | 6.88 | 1.3 | |
| Peroneal longus and brevis(PL) | 4.94 | 1.14 | |
| Posterior tibialis (PT) | 5.32 | 1.03 | |
| Extensor digitorum longus (EDL) | 3.72 | 0.89 | |
| Flexor hallucis longus (FHL) | 2.75 | 0.9 | |
| Flexor digitorum longus (FDL) | 2.83 | 0.64 | |
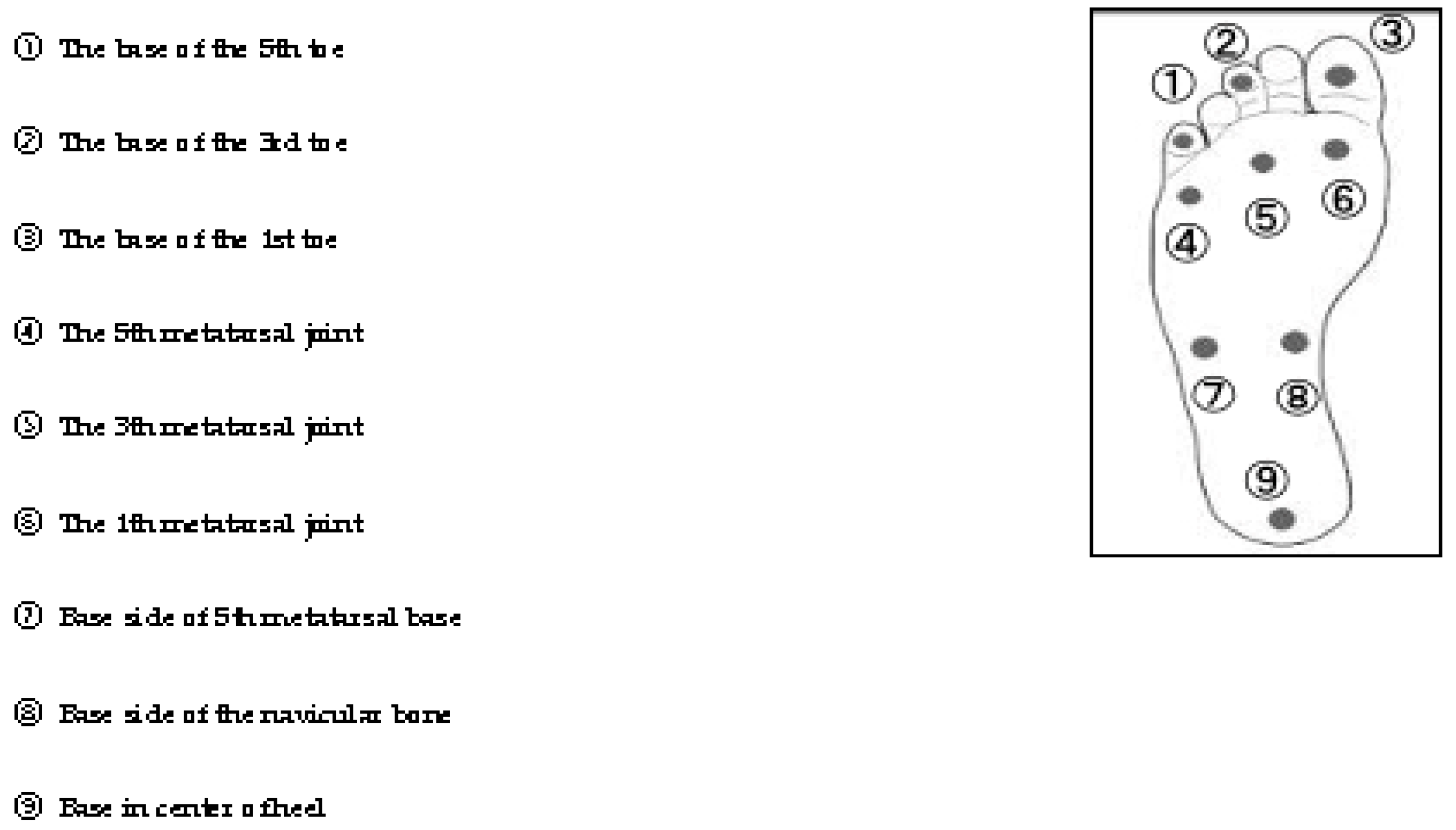
| plantar superficial sensation | ①The base of the 5th toe | 3.42 | 0.66 |
| ②The base of the 3rd toe | 3.44 | 0.59 | |
| ③The base of the 1st toe | 3.64 | 0.56 | |
| ④The 5th metatarsal joint | 3.71 | 0.36 | |
| ⑤The 3th metatarsal joint | 3.65 | 0.44 | |
| ⑥The 1th metatarsal joint | 3.58 | 0.47 | |
| ⑦Base side of 5th metatarsal base | 3.70 | 0.52 | |
| ⑧Base side of the navicular bone | 3.42 | 0.50 | |
| ⑨Base in center of heel | 4.17 | 0.66 | |
| Y balance test | Reach distance(anterior) | 65.3 | 4.2 |
| Reach distance(posteromedial) | 112.4 | 6.5 | |
| Reach distance(posterolateral) | 110.4 | 6.3 | |
| Composite | 96.0 | 4.9 | |
| Mean±Standard Deviation (N=15) | |||
|---|---|---|---|
| Y balance test | Reach distanc(anterior) | 65.3 | 4.2 |
| Electromyography | ABH | 50.2 | 20.3 |
| ADD | 33.8 | 39.6 | |
| TA | 40.7 | 16.9 | |
| PL | 38.4 | 18.4 | |
| PB | 32.0 | 12.0 | |
| GM | 11.6 | 8.3 | |
| SOL | 52.8 | 33.5 | |
| Reach distance (posteromedial) |
112.4 | 6.5 | |
| ABH | 45.4 | 20.9 | |
| ADD | 26.1 | 25.3 | |
| TA | 45.3 | 15.0 | |
| PL | 41.3 | 16.2 | |
| PB | 33.3 | 10.9 | |
| GM | 9.9 | 7.6 | |
| SOL | 47.8 | 36.0 | |
| Reach distance (posterolateral) |
110.4 | 6.3 | |
| ABH | 31.8 | 13.3 | |
| ADD | 29.8 | 21.1 | |
| TA | 46.9 | 11.0 | |
| PL | 36.6 | 15.3 | |
| PB | 28.4 | 10.0 | |
| GM | 9.8 | 8.2 | |
| SOL | 52.8 | 32.6 | |
| Y balance test | Reach distance | anterior | posteromedial | posterolateral | Composite | ||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | ||
| cross section area(muscles) | Abductor hallucis(ABH) | -0.085 | 0.762 | 0.246 | 0.377 | 0.206 | 0.461 | 0.174 | 0.535 |
| Adductor digiti minimi (ADD) | -0.006 | 0.983 | 0.311 | 0.259 | 0.148 | 0.599 | 0.201 | 0.474 | |
| Flexor digitorum brevis( FDB) | 0.140 | 0.618 | -0.006 | 0.984 | -0.006 | 0.983 | 0.035 | 0.902 | |
| Flexor hallucis brevis( FHB) | 0.184 | 0.512 | -0.033 | 0.907 | -0.163 | 0.563 | -0.032 | 0.909 | |
| Tibialis anterior (TA) | 0.064 | 0.821 | -0.243 | 0.384 | -0.302 | 0.274 | -0.22 | 0.432 | |
| Peroneal longus and brevis(PL) | 0.068 | 0.81 | -0.139 | 0.621 | -0.359 | 0.188 | -0.197 | 0.481 | |
| Posterior tibialis (PT) | -0.032 | 0.908 | -0.251 | 0.366 | -0.351 | 0.2 | -0.272 | 0.327 | |
| Extensor digitorum longus (EDL) | 0.089 | 0.752 | -0.322 | 0.242 | -0.455 | 0.089 | -0.313 | 0.255 | |
| Flexor hallucis longus (FHL) | 0.06 | 0.831 | 0.244 | 0.382 | 0.133 | 0.636 | 0.183 | 0.514 | |
| Flexor digitorum longus (FDL) | 0.069 | 0.806 | -0.021 | 0.94 | -0.007 | 0.979 | 0.007 | 0.98 | |
| muscle strength | Dorsiflexion | 0.438 | 0.103 | 0.428 | 0.112 | 0.443 | 0.098 | 0.505 | 0.055 |
| planter flexion | 0.276 | 0.319 | 0.572* | 0.026 | 0.695* | 0.004 | 0.632* | 0.012 | |
| Eversion | -0.051 | 0.857 | 0.097 | 0.731 | -0.038 | 0.892 | 0.012 | 0.965 | |
| toe grasp strength | %BW | 0.501 | 0.057 | 0.579* | 0.024 | 0.588* | 0.021 | 0.652* | 0.008 |
| plantar superficial sensation | ①The base of the 5th toe | 0.083 | 0.769 | -0.16 | 0.569 | -0.193 | 0.49 | -0.131 | 0.642 |
| ②The base of the 3rd toe | -0.036 | 0.9 | -0.108 | 0.702 | -0.162 | 0.564 | -0.128 | 0.65 | |
| ③The base of the 1st toe | -0.164 | 0.56 | -0.375 | 0.168 | -0.331 | 0.228 | -0.356 | 0.193 | |
| ④The 5th metatarsal joint | -0.474 | 0.075 | -0.607* | 0.016 | -0.47 | 0.077 | -0.607* | 0.016 | |
| ⑤The 3th metatarsal joint | -0.522* | 0.046 | -0.431 | 0.109 | -0.262 | 0.346 | -0.452 | 0.091 | |
| ⑥The 1th metatarsal joint | -0.409 | 0.131 | -0.552* | 0.033 | -0.511 | 0.051 | -0.581* | 0.023 | |
| ⑦Base side of 5th metatarsal base | -0.265 | 0.341 | -0.219 | 0.433 | -0.186 | 0.508 | -0.252 | 0.365 | |
| ⑧Base side of the navicular bone | -0.18 | 0.521 | -0.277 | 0.317 | -0.285 | 0.303 | -0.297 | 0.282 | |
| ⑨Base in center of heel | -0.297 | 0.282 | -0.495 | 0.061 | -0.449 | 0.093 | -0.498 | 0.059 | |
| Electromyography | Abductor hallucis(ABH) | 0.07 | 0.805 | -0.224 | 0.422 | -0.515* | 0.05 | ||
| Adductor digiti minimi (ADD) | 0.150 | 0.593 | -0.145 | 0.607 | -0.041 | 0.884 | |||
| Tibialis anterior (TA) | 0.143 | 0.611 | 0.257 | 0.355 | 0.253 | 0.363 | |||
| Peroneal longus(PL) | -0.233 | 0.404 | 0.075 | 0.791 | -0.065 | 0.817 | |||
| Peroneal brevis(PB) | -0.214 | 0.444 | 0.117 | 0.678 | 0.127 | 0.652 | |||
| Medial Gastrocnemius(MG) | 0.075 | 0.79 | 0.47 | 0.077 | 0.195 | 0.487 | |||
| Soleus (SOL) | 0.046 | 0.869 | -0.116 | 0.681 | -0.195 | 0.487 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





