Submitted:
01 July 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
Introduction
The Composition and Role of the Oral Microbiome
Factors Influencing Oral Microbiome
Diet
Smoking
Alcohol Consumption
Other Factors That Influence the Oral Microbiome
Oral Microbiome Dysbiosis
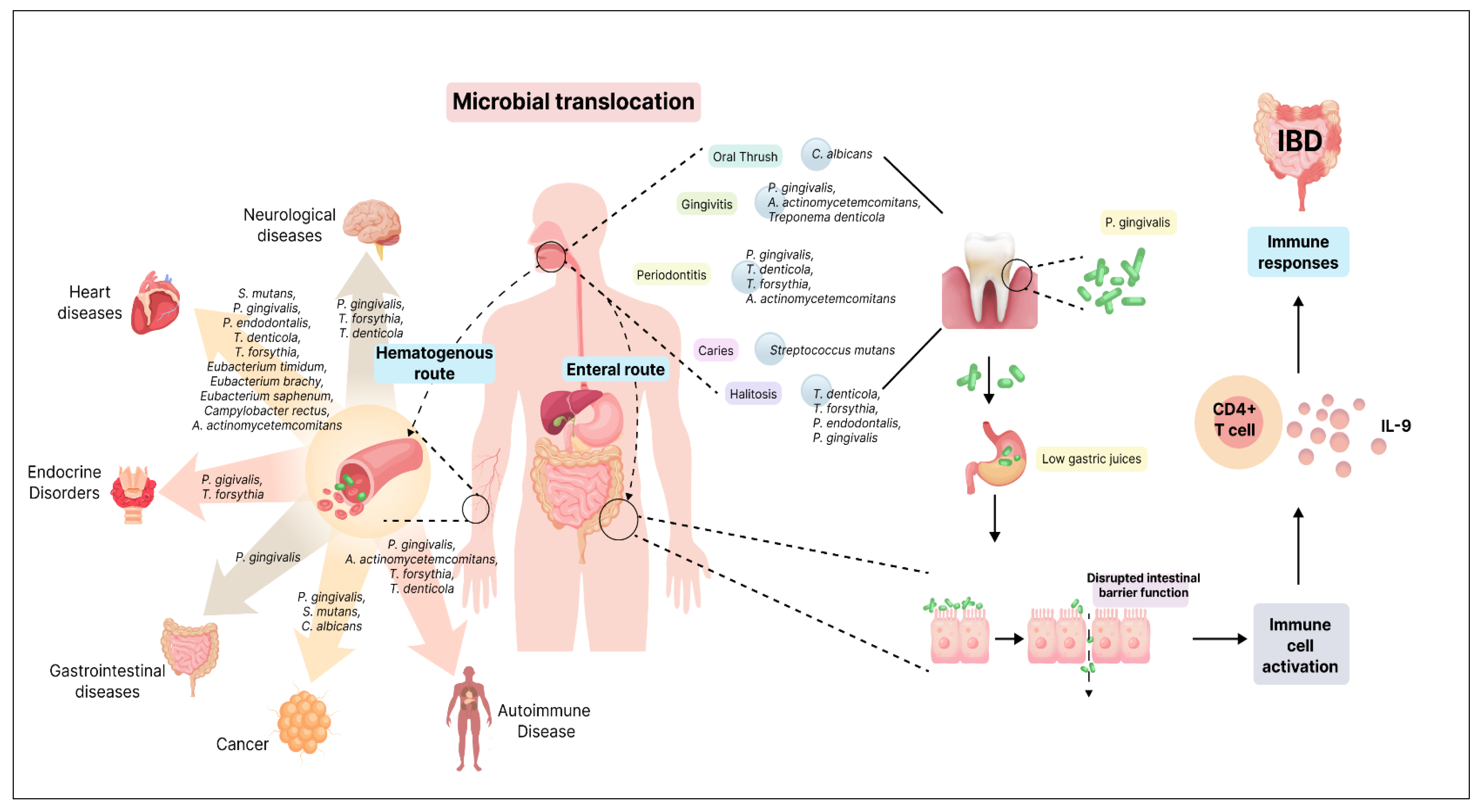
Oral-Gut Axis
Oral Microbiome and Oral Diseases
Dental Caries
Gingivitis
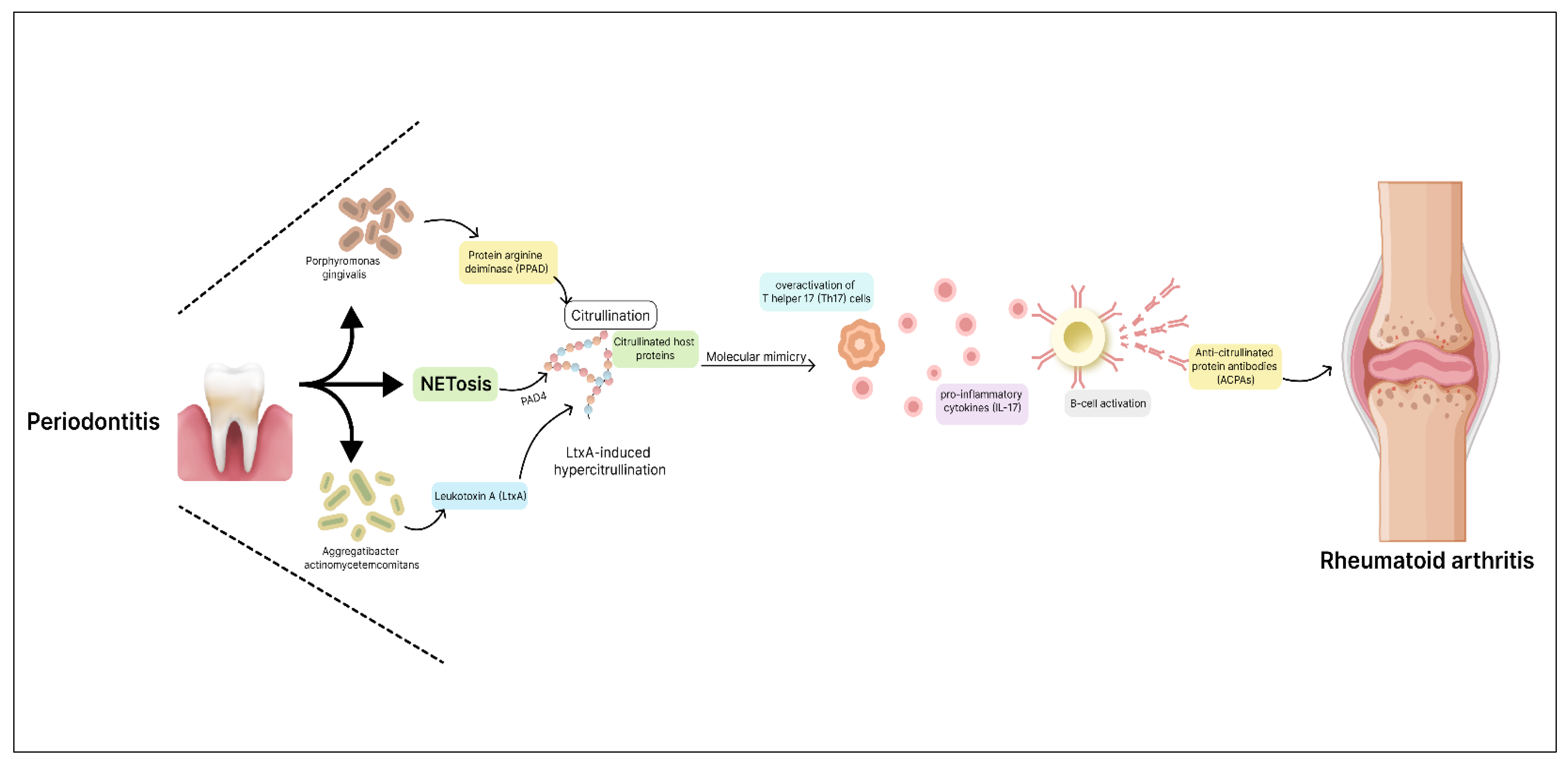
Periodontitis
Halitosis
Taste Impairment
Burning Mouth Syndrome
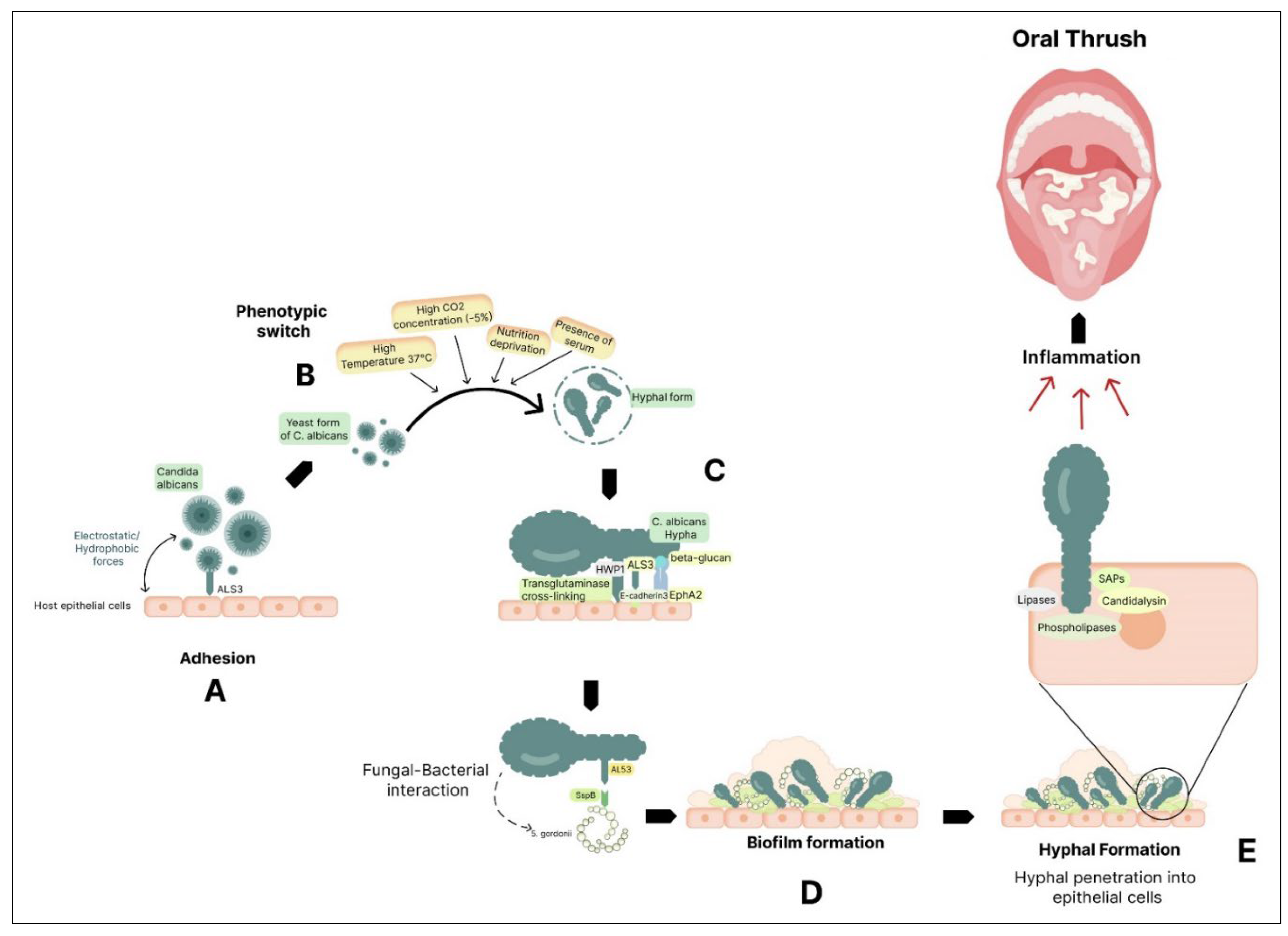
Oral Thrush
Oral Microbiome and Systemic Diseases
Gastrointestinal Disorder: Inflammatory Bowel Disease
Cardiovascular Diseases: Atherosclerosis
Endocrine Disorders: Diabetes Mellitus
Obesity
Neurological Disorders: Alzheimer’s Disease
Parkinson’s Disease
Autoimmune Conditions: Rheumatoid Arthritis
Systemic Lupus Erythematosus
Cancer
Oral Health and Dental Care Practices
Probiotics
Peptides in Oral Health and Their Role in Oral Care
Personalised Oral Care Approaches
Conclusion
Authors’ contributions
Funding
Availability of data and material
Consent for publication
Acknowledgement
Competing interests
Abbreviations
| ACPAs | Anticitrullinated Protein Antibodies |
| α-synuclein | Alpha-synuclein |
| ALS | Agglutinin-like sequence |
| AD | Alzheimer’s Disease |
| BBB | Blood-Brain Barrier |
| CaF2 | Calcium Fluoride |
| CD | Crohn’s Disease |
| CH3SH | Methyl mercaptan |
| CH3SSCH3 | Dimethyl sulfide |
| CPC | Cetylpyridinium chloride |
| CVD | Cardiovascular Diseases |
| EPS | Extracellular polymers |
| F- | Fluoride ions |
| FAP | Fluorapatite |
| GPI | Glycosylphosphatidylinositol |
| HA | Hydroxyapatite |
| HF | Hydrogen Fluoride |
| HWP1 | Hyphal wall protein |
| HSPs | Heat Shock Proteins |
| IBD | Inflammatory Bowel Disease |
| IL | Interleukin |
| LDH | Lactate Dehydrogenase |
| LPS | Lipopolysaccharides |
| LtxA | Leukotoxin A |
| MMP9 | Matrix Metalloproteinase 9 |
| nAChRs | Nicotine Acetylcholine Receptors |
| NETs | Neutrophil Extracellular Traps |
| NF-κB | Nuclear Factor Kappa-B |
| NGS | Next-Generation Sequencing |
| OH- | Hydroxyl ions |
| PD | Parkinson’s Disease |
| PPAD | Porphyromonas gingivalis peptidylarginine deiminase |
| RA | Rheumatoid Arthritis |
| RS. | Thiol radicals |
| RSH | Thiol compounds |
| RSSR | Disulfides |
| RT-PCR | quantitative Real-Time PCR |
| SAPs | Secreted Aspartyl Proteinases |
| SLE | Systemic Lupus Erythematosus |
| SspB | Surface protein SspB |
| Th | T helper |
| VSCs | Volatile Sulfur Compounds |
References
- Deo, P.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- The oral microbiome in health and disease - PubMed [Internet]. [cited 2023 Sep 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/23201354/.
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome – an update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Forshaw, R.J. Dental health and disease in ancient Egypt. Br. Dent. J. 2009, 206, 421–424. [Google Scholar] [CrossRef] [PubMed]
- The oral microbiome in health and disease and the potential impact on personalized dental medicine - PubMed [Internet]. [cited 2023 Sep 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/21902769/.
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol. 2014, 60, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Minty, M.; Vinel, A.; Canceill, T.; Loubières, P.; Burcelin, R.; Kaddech, M.; Blasco-Baque, V.; Laurencin-Dalicieux, S. Oral Microbiota: A Major Player in the Diagnosis of Systemic Diseases. Diagnostics 2021, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment - PubMed [Internet]. [cited 2023 Sep 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/30666248/.
- Lee, Y.-H.; Chung, S.W.; Auh, Q.-S.; Hong, S.-J.; Lee, Y.-A.; Jung, J.; Lee, G.-J.; Park, H.J.; Shin, S.-I.; Hong, J.-Y. Progress in Oral Microbiome Related to Oral and Systemic Diseases: An Update. Diagnostics 2021, 11, 1283. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef]
- The human oral microbiome - PubMed [Internet]. [cited 2023 Sep 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/20656903/.
- Bacterial interactions in dental biofilm - PubMed [Internet]. [cited 2023 Sep 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/21778817/.
- Genetic and physiologic characterization of urease of Actinomyces naeslundii - PubMed [Internet]. [cited 2023 Sep 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/9916052/.
- Zhang, Y.; Ding, Y.; Guo, Q. Probiotic Species in the Management of Periodontal Diseases: An Overview. Front. Cell. Infect. Microbiol. 2022, 12, 806463. [Google Scholar] [CrossRef] [PubMed]
- Marsh, PD. Role of the Oral Microflora in Health. Microb Ecol Health Dis. 2000 Jan 1;12(3):130–7.
- Bradshaw, D.J.; Marsh, P.D.; Watson, G.K.; Allison, C. Role of Fusobacterium nucleatum and Coaggregation in Anaerobe Survival in Planktonic and Biofilm Oral Microbial Communities during Aeration. Infect. Immun. 1998, 66, 4729–32. [Google Scholar] [CrossRef]
- Könönen, E.; Fteita, D.; Gursoy, U.K.; Gursoy, M. Prevotella species as oral residents and infectious agents with potential impact on systemic conditions. J. Oral Microbiol. 2022, 14, 2079814. [Google Scholar] [CrossRef]
- Ecology of the Oral Microbiome: Beyond Bacteria - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/28089325/.
- Diaz, P.; Dongari-Bagtzoglou, A. Critically Appraising the Significance of the Oral Mycobiome. J. Dent. Res. 2020, 100, 133–140. [Google Scholar] [CrossRef]
- Candida and other fungal species: forgotten players of healthy oral microbiota - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/24487378/.
- Diversity and quantitative analysis of Archaea in aggressive periodontitis and periodontally healthy subjects - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/21539593/.
- Methanogenic Archaea and human periodontal disease - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/15067114/.
- Moye, Z.D.; Zeng, L.; Burne, R.A. Fueling the caries process: carbohydrate metabolism and gene regulation byStreptococcus mutans. J. Oral Microbiol. 2014, 6. [Google Scholar] [CrossRef]
- A Cross-Talk between Diet and the Oral Microbiome: Balance of Nutrition on Inflammation and Immune System’s Response during Periodontitis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/35745156/.
- Schlagenhauf, U. On the Role of Dietary Nitrate in the Maintenance of Systemic and Oral Health. Dent. J. 2022, 10, 84. [Google Scholar] [CrossRef]
- Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/29807159/.
- Antioxidant-Rich Natural Raw Materials in the Prevention and Treatment of Selected Oral Cavity and Periodontal Diseases - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/34829719/.
- Cigarette smoking and the oral microbiome in a large study of American adults - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/27015003/.
- Jia, Y.-J.; Liao, Y.; He, Y.-Q.; Zheng, M.-Q.; Tong, X.-T.; Xue, W.-Q.; Zhang, J.-B.; Yuan, L.-L.; Zhang, W.-L.; Jia, W.-H. Association Between Oral Microbiota and Cigarette Smoking in the Chinese Population. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Wagenknecht, D.R.; BalHaddad, A.A.; Gregory, R.L. Effects of Nicotine on Oral Microorganisms, Human Tissues, and the Interactions between Them. Curr. Oral Heal. Rep. 2018, 5, 78–87. [Google Scholar] [CrossRef]
- Beghini, F.; Renson, A.; Zolnik, C.P.; Geistlinger, L.; Usyk, M.; Moody, T.U.; Thorpe, L.; Dowd, J.B.; Burk, R.; Segata, N.; et al. Tobacco exposure associated with oral microbiota oxygen utilization in the New York City Health and Nutrition Examination Study. Ann. Epidemiology 2019, 34, 18–25. [Google Scholar] [CrossRef]
- Yussof, A.; Yoon, P.; Krkljes, C.; Schweinberg, S.; Cottrell, J.; Chu, T.; Chang, S.L. A meta-analysis of the effect of binge drinking on the oral microbiome and its relation to Alzheimer’s disease. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alcohol-related diseases of the mouth and throat - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/16508283/.
- Enberg, N.; Alho, H.; Loimaranta, V.; Lenander-Lumikari, M. Saliva flow rate, amylase activity, and protein and electrolyte concentrations in saliva after acute alcohol consumption. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2001, 92, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.; A Born, I.; Mall, G. Effect of chronic ethanol and nicotine consumption on the function and morphology of the salivary glands.. 1988, 140–50.
- Consequences of alcohol consumption on host defence - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/10659718/.
- Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin Exp Res. 1996 Aug;20(5):900–7.
- Pepersack, T.; Fuss, M.D.; Otero, J.; Bergmann, P.; Valsamis, J.; Corvilain, J. Longitudinal study of bone metabolism after ethanol withdrawal in alcoholic patients. J. Bone Miner. Res. 1992, 7, 383–387. [Google Scholar] [CrossRef]
- Ogden, G.R.; Wight, A.J.; Rice, P. Effect of alcohol on the oral mucosa assessed by quantitative cytomorphometry. J. Oral Pathol. Med. 1999, 28, 216–220. [Google Scholar] [CrossRef]
- Fan, X.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Freedman, N.D.; Alekseyenko, A.V.; Wu, J.; Yang, L.; Pei, Z.; et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- The oral microbiome in alcohol use disorder: a longitudinal analysis during inpatient treatment - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/34880965/.
- Diurnal changes of the oral microbiome in patients with alcohol dependence - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/36579346/.
- Alcohol and tobacco consumption affects bacterial richness in oral cavity mucosa biofilms - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/25278091/.
- The Effects of Alcohol Drinking on Oral Microbiota in the Chinese Population - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/35565124/.
- Gm A. Oral Biofilm and Its Impact on Oral Health, Psychological and Social Interaction. [cited 2023 Sep 20]; Available from: https://clinmedjournals.org/articles/ijodh/international-journal-of-oral-and-dental-health-ijodh-7-127.php?jid=ijodh.
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Ryan, C.A.; Dempsey, E.; Ross, R.P.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Lynch, N.; McCullough, M. Oral fungal infections: an update for the general practitioner. Aust. Dent. J. 2010, 55, 48–54. [Google Scholar] [CrossRef] [PubMed]
- The impact of horizontal gene transfer on the adaptive ability of the human oral microbiome - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/25250243/.
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/22698087/.
- Gut microbiome and aging: Physiological and mechanistic insights - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/29951588/.
- Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/30723224/.
- Neonatal oral fluid as a transmission route for bifidobacteria to the infant gut immediately after birth - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/31213639/.
- Proton pump inhibitors affect the gut microbiome - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/26657899/.
- Park SY, Hwang BO, Lim M, Ok SH, Lee SK, Chun KS, et al. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers. 2021 Apr 28;13(9):2124.
- Interconnections Between the Oral and Gut Microbiomes: Reversal of Microbial Dysbiosis and the Balance Between Systemic Health and Disease - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/33652903/.
- Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/29051379/.
- Kitamoto, S.; Nagao-Kitamoto, H.; Hein, R.; Schmidt, T.M.; Kamada, N. The Bacterial Connection between the Oral Cavity and the Gut Diseases. J. Dent. Res. 2020, 99, 1021–1029. [Google Scholar] [CrossRef]
- Walker, M.Y.; Pratap, S.; Southerland, J.H.; Farmer-Dixon, C.M.; Lakshmyya, K.; Gangula, P.R. Role of oral and gut microbiome in nitric oxide-mediated colon motility. Nitric Oxide 2018, 73, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Extensive transmission of microbes along the gastrointestinal tract - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/30747106/.
- Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/29097091/.
- Identification of bistable populations of Porphyromonas gingivalis that differ in epithelial cell invasion - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/20576685/.
- Tanner, A.; Kressirer, C.; Rothmiller, S.; Johansson, I.; Chalmers, N. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef]
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet Lond Engl. 2007 Jan 6;369(9555):51–9.
- Caries ecology revisited: microbial dynamics and the caries process - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/18832827/.
- The oral microbiota: dynamic communities and host interactions - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/30301974/.
- Seminario, A.; Broukal, Z.; Ivancaková, R. Mutans streptococci and the development of dental plaque. 2005, 106, 349–58. [Google Scholar]
- Madléna, M.; Dombi, C.; Gintner, Z.; Bánóczy, J. Effect of amine fluoride/stannous fluoride toothpaste and mouthrinse on dental plaque accumulation and gingival health. Oral Dis. 2004, 10, 294–297. [Google Scholar] [CrossRef]
- Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/32674310/.
- Structure-Based Discovery of Small Molecule Inhibitors of Cariogenic Virulence - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/28729722/.
- Simón-Soro, A.; Guillen-Navarro, M.; Mira, A. Metatranscriptomics reveals overall active bacterial composition in caries lesions. J. Oral Microbiol. 2014, 6, 25443–25443. [Google Scholar] [CrossRef] [PubMed]
- Solving the etiology of dental caries - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/25435135/.
- Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/23091642/.
- Association between Bifidobacteriaceae and the clinical severity of root caries lesions - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/19121067/.
- Badet, C.; Thebaud, N.B. Ecology of Lactobacilli in the Oral Cavity: A Review of Literature. Open Microbiol. J. 2008, 2, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Microbial geography of the oral cavity - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/23674263/.
- Intan Suhana MA, Farha A, Hassan BM. Inflammation of the Gums. Malays Fam Physician Off J Acad Fam Physicians Malays. 2020;15(1):71–3.
- Rathee M, Jain P. Gingivitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 May 3]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK557422/.
- Oral microbiomes: more and more importance in oral cavity and whole body - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/29736705/.
- Lu, M.; Xuan, S.; Wang, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Moore, W.E.C.; Moore, L.V.H. The bacteria of periodontal diseases. Periodontol. 2000 1994, 5, 66–77. [Google Scholar] [CrossRef] [PubMed]
- The role of the microbiota in periodontal disease - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/32385883/.
- Kistler, J.O.; Booth, V.; Bradshaw, D.J.; Wade, W.G. Bacterial Community Development in Experimental Gingivitis. PLOS ONE 2013, 8, e71227. [Google Scholar] [CrossRef] [PubMed]
- Clinical, Immune, and Microbiome Traits of Gingivitis and Peri-implant Mucositis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/28033066/.
- Abdulkareem, A.A.; Al-Taweel, F.B.; Al-Sharqi, A.J.; Gul, S.S.; Sha, A.; Chapple, I.L. Current concepts in the pathogenesis of periodontitis: from symbiosis to dysbiosis. J. Oral Microbiol. 2023, 15, 2197779. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- The Role of Oral Pathobionts in Dysbiosis during Periodontitis Development - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/24646638/.
- Kapoor, U.; Sharma, G.; Juneja, M.; Nagpal, A. Halitosis: Current concepts on etiology, diagnosis and management. Eur. J. Dent. 2016, 10, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Halitosis: a review of associated factors and therapeutic approach - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/19838550/.
- Hampelska, K.; Jaworska, M.M.; Babalska, Z.Ł.; Karpiński, T.M. The Role of Oral Microbiota in Intra-Oral Halitosis. J. Clin. Med. 2020, 9, 2484. [Google Scholar] [CrossRef]
- Microbial basis of oral malodor development in humans - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/24101743/.
- Nakano, Y.; Yoshimura, M.; Koga, T. Correlation between oral malodor and periodontal bacteria. Microbes Infect. 2002, 4, 679–683. [Google Scholar] [CrossRef]
- The relationship between oral malodor, gingivitis, and periodontitis. A review - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/10368052/.
- Porter SR, Scully C. Oral malodour (halitosis). BMJ. 2006 Sep 23;333(7569):632–5.
- Persson, S.; Edlund, M.; Claesson, R.; Carlsson, J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 1990, 5, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Suzuki, N.; Nakano, Y.; Yasui, M.; Yoneda, M.; Shimazaki, Y.; Hirofuji, T.; Yamashita, Y. Discrimination of the oral microbiota associated with high hydrogen sulfide and methyl mercaptan production. Sci. Rep. 2012, 2, 215. [Google Scholar] [CrossRef] [PubMed]
- Washio, J.; Sato, T.; Koseki, T.; Takahashi, N. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J. Med Microbiol. 2005, 54, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Solemdal, K.; Sandvik, L.; Willumsen, T.; Mowe, M.; Hummel, T. The Impact of Oral Health on Taste Ability in Acutely Hospitalized Elderly. PLOS ONE 2012, 7, e36557. [Google Scholar] [CrossRef] [PubMed]
- A Finkelstein, J.; Schiffman, S.S. Workshop on Taste and Smell in the Elderly: An Overview. Physiol. Behav. 1999, 66, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A. Interaction of Saliva and Taste. J. Dent. Res. 1990, 69, 838–843. [Google Scholar] [CrossRef]
- Matsuo, R. Role of Saliva in the Maintenance of Taste Sensitivity. Crit. Rev. Oral Biol. Med. 2000, 11, 216–229. [Google Scholar] [CrossRef]
- Nederfors T, Isaksson R, Mörnstad H, Dahlöf C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population--relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997 Jun;25(3):211–6.
- Impact of primary Sjogren’s syndrome on smell and taste: effect on quality of life - PubMed [Internet]. [cited 2024 May 3]. Available from: https://pubmed.ncbi.nlm.nih.gov/19752179/.
- Weifenbach, J.M.; Schwartz, L.K.; Atkinson, J.C.; Fox, P.C. Taste performance in Sjogren's syndrome. Physiol. Behav. 1995, 57, 89–96. [Google Scholar] [CrossRef]
- Aravindhan, R.; Vidyalakshmi, S.; Kumar, M.; Satheesh, C.; Balasubramanium, A.; Prasad, V. Burning mouth syndrome: A review on its diagnostic and therapeutic approach. J. Pharm. Bioallied Sci. 2014, 6, S21–S25. [Google Scholar] [CrossRef]
- Lee, B.-M.; Park, J.W.; Jo, J.H.; Oh, B.; Chung, G. Comparative analysis of the oral microbiome of burning mouth syndrome patients. J. Oral Microbiol. 2022, 14, 2052632. [Google Scholar] [CrossRef] [PubMed]
- Singh A, Verma R, Murari A, Agrawal A. Oral candidiasis: An overview. J Oral Maxillofac Pathol JOMFP. 2014 Sep;18(Suppl 1):S81-85.
- Rajendra Santosh AB, Muddana K, Bakki SR. Fungal Infections of Oral Cavity: Diagnosis, Management, and Association with COVID-19. SN Compr Clin Med. 2021;3(6):1373–84.
- Candidiasis - StatPearls - NCBI Bookshelf [Internet]. [cited 2023 Sep 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560624/.
- Oral thrush: Overview - InformedHealth.org - NCBI Bookshelf [Internet]. [cited 2023 Sep 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK367586/.
- Cotter, G.; Kavanagh, K. Adherence mechanisms of Candida albicans. 2000, 57, 241–9. [Google Scholar] [PubMed]
- The ALS gene family of Candida albicans - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/11286882/.
- Nikou, S.-A.; Kichik, N.; Brown, R.; Ponde, N.O.; Ho, J.; Naglik, J.R.; Richardson, J.P. Candida albicans Interactions with Mucosal Surfaces during Health and Disease. Pathogens 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family--a sticky pursuit - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/17852717/.
- Naglik JR, Fostira F, Ruprai J, Staab JF, Challacombe SJ, Sundstrom P. Candida albicans HWP1 gene expression and host antibody responses in colonization and disease. J Med Microbiol. 2006 Oct;55(Pt 10):1323–7.
- Sundstrom P, Balish E, Allen CM. Essential role of the Candida albicans transglutaminase substrate, hyphal wall protein 1, in lethal oroesophageal candidiasis in immunodeficient mice. J Infect Dis. 2002 Feb 15;185(4):521–30.
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.J.; Nobbs, A.H.; Vickerman, M.M.; Barbour, M.E.; Jenkinson, H.F. Interaction of Candida albicans Cell Wall Als3 Protein with Streptococcus gordonii SspB Adhesin Promotes Development of Mixed-Species Communities. Infect. Immun. 2010, 78, 4644–4652. [Google Scholar] [CrossRef] [PubMed]
- Lewis M, a. O, Williams DW. Diagnosis and management of oral candidosis. Br Dent J. 2017 Nov 10;223(9):675–81.
- Candida albicans Hyphae: From Growth Initiation to Invasion - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/29371503/.
- Candida albicans secreted aspartyl proteinases in virulence and pathogenesis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/12966142/.
- Candida albicans pathogenicity mechanisms - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/23302789/.
- Höfs, S.; Mogavero, S.; Hube, B. Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J. Microbiol. 2016, 54, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Candidalysin Is Required for Neutrophil Recruitment and Virulence During Systemic Candida albicans Infection - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/31401652/.
- andidalysin is a fungal peptide toxin critical for mucosal infection - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/27027296/.
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 Is a Candida albicans Invasin That Binds to Cadherins and Induces Endocytosis by Host Cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef] [PubMed]
- Bacteria from diverse habitats colonize and compete in the mouse gut - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/25284151/.
- Peeters, M.; Geypens, B.; Claus, D.; Nevens, H.; Ghoos, Y.; Verbeke, G.; Baert, F.; Vermeire, S.; Vlietinck, R.; Rutgeerts, P. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology 1997, 113, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/9922310/.
- Fusobacterium nucleatum - symbiont, opportunist and oncobacterium - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/30546113/.
- Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017 Oct 20;358(6361):359–65.
- Porphyromonas gingivalis indirectly elicits intestinal inflammation by altering the gut microbiota and disrupting epithelial barrier function through IL9-producing CD4+ T cells - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/34958712/.
- The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/23303375/.
- Li, Y.; Zhu, M.; Liu, Y.; Luo, B.; Cui, J.; Huang, L.; Chen, K.; Liu, Y. The oral microbiota and cardiometabolic health: A comprehensive review and emerging insights. Front. Immunol. 2022, 13, 1010368. [Google Scholar] [CrossRef]
- Lucchese, A. Streptococcus mutans antigen I/II and autoimmunity in cardiovascular diseases. Autoimmun. Rev. 2017, 16, 456–460. [Google Scholar] [CrossRef]
- Xie, M.; Tang, Q.; Nie, J.; Zhang, C.; Zhou, X.; Yu, S.; Sun, J.; Cheng, X.; Dong, N.; Hu, Y.; et al. BMAL1-Downregulation Aggravates Porphyromonas Gingivalis -Induced Atherosclerosis by Encouraging Oxidative Stress. Circ. Res. 2020, 126, E15–E29. [Google Scholar] [CrossRef]
- Stinson, M.W.; Alder, S.; Kumar, S. Invasion and Killing of Human Endothelial Cells by Viridans Group Streptococci. Infect. Immun. 2003, 71, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Wong, L.-Y.; Jia, L.T.; Kuklenyik, Z.; Calafat, A.M. Trends in Exposure to Polyfluoroalkyl Chemicals in the U.S. Population: 1999−2008. Environ. Sci. Technol. 2011, 45, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Ramos, H.C.; Rumbo, M.; Sirard, J.-C. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 2004, 12, 509–517. [Google Scholar] [CrossRef]
- Zindel, J.; Kubes, P. DAMPs, PAMPs, and LAMPs in Immunity and Sterile Inflammation. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 493–518. [Google Scholar] [CrossRef]
- Aggregatibacter actinomycetemcomitans leukotoxin (LtxA; Leukothera) induces cofilin dephosphorylation and actin depolymerization during killing of malignant monocytes - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/25169107/.
- Aarabi, G.; Heydecke, G.; Seedorf, U. Roles of Oral Infections in the Pathomechanism of Atherosclerosis. Int. J. Mol. Sci. 2018, 19, 1978. [Google Scholar] [CrossRef] [PubMed]
- Linkages between oral commensal bacteria and atherosclerotic plaques in coronary artery disease patients - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/28649401/.
- Oral Microbiome in Relation to Periodontitis Severity and Systemic Inflammation - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/34070915/.
- Lourenço, T.G.B.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P.V. Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I. Cells in Stress: Transcriptional Activation of Heat Shock Genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef]
- Goulhen, F.; Grenier, D.; Mayrand, D. Oral Microbial Heat-shock Proteins and Their Potential Contributions to Infections. Crit. Rev. Oral Biol. Med. 2003, 14, 399–412. [Google Scholar] [CrossRef]
- Xiao, E.; Mattos, M.; Vieira, G.H.A.; Chen, S.; Corrêa, J.D.; Wu, Y.; Albiero, M.L.; Bittinger, K.; Graves, D.T. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe 2017, 22, 120–128. [Google Scholar] [CrossRef]
- Mashimo, P.A.; Yamamoto, Y.; Slots, J.; Park, B.H.; Genco, R.J. The Periodontal Microflora of Juvenile Diabetics: Culture, Immunofluorescence, and Serum Antibody Studies. J. Periodontol. 1983, 54, 420–430. [Google Scholar] [CrossRef]
- Diabetes and periodontal disease: a case-control study - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/15857077/.
- da Cruz, G.A.; de Toledo, S.; Sallum, E.A.; Sallum, A.W.; Ambrosano, G.M.B.; Sardi, J.d.C.O.; da Cruz, S.E.B.; Gonçalves, R.B. Clinical and Laboratory Evaluations of Non-Surgical Periodontal Treatment in Subjects With Diabetes Mellitus. J. Periodontol. 2008, 79, 1150–1157. [Google Scholar] [CrossRef]
- The Oral Microbiota Is Modified by Systemic Diseases - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/30359170/.
- Wu, Y.-Y.; Xiao, E.; Graves, D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int. J. Oral Sci. 2015, 7, 63–72. [Google Scholar] [CrossRef]
- Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/18390927/.
- Benahmed, A.G.; Gasmi, A.; Doşa, A.; Chirumbolo, S.; Mujawdiya, P.K.; Aaseth, J.; Dadar, M.; Bjørklund, G. Association between the gut and oral microbiome with obesity. Anaerobe 2020, 70, 102248. [Google Scholar] [CrossRef]
- Schamarek, I.; Anders, L.; Chakaroun, R.M.; Kovacs, P.; Rohde-Zimmermann, K. The role of the oral microbiome in obesity and metabolic disease: potential systemic implications and effects on taste perception. Nutr. J. 2023, 22, 1–13. [Google Scholar] [CrossRef]
- Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/33178196/.
- Goodson JM, Groppo D, Halem S, Carpino E. Is obesity an oral bacterial disease? J Dent Res. 2009 Jun;88(6):519–23.
- Metabolic endotoxemia initiates obesity and insulin resistance - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/17456850/.
- Salivary inflammatory markers and microbiome in normoglycemic lean and obese children compared to obese children with type 2 diabetes - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/28253297/.
- Profiling the Oral Microbiome and Plasma Biochemistry of Obese Hyperglycemic Subjects in Qatar - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/31816998/.
- Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/28532414/.
- Mathur R, Barlow GM. Obesity and the microbiome. Expert Rev Gastroenterol Hepatol. 2015;9(8):1087–99.
- Experimental periodontitis induces gene expression of proinflammatory cytokines in liver and white adipose tissues in obesity - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/20367095/.
- Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/34769677/.
- Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/11929559/.
- Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/23666172/.
- Miklossy, J. Emerging roles of pathogens in Alzheimer disease. Expert Rev. Mol. Med. 2011, 13, e30. [Google Scholar] [CrossRef]
- Miklossy, J. Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J Neuroinflammation. 2011 Aug 4;8:90.
- Sureda A, Daglia M, Argüelles Castilla S, Sanadgol N, Fazel Nabavi S, Khan H, et al. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol Res. 2020 Jan;151:104582.
- Microbial involvement in Alzheimer disease development and progression - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/32709243/.
- Amyloid beta is an early responder cytokine and immunopeptide of the innate immune system - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/33163614/.
- Liu S, Butler CA, Ayton S, Reynolds EC, Dashper SG. Porphyromonas gingivalis and the pathogenesis of Alzheimer’s disease. Crit Rev Microbiol. 2023 Jan 4;1–11.
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PloS One. 2010 Mar 3;5(3):e9505.
- von Campenhausen, S.; Bornschein, B.; Wick, R.; Bötzel, K.; Sampaio, C.; Poewe, W.; Oertel, W.; Siebert, U.; Berger, K.; Dodel, R. Prevalence and incidence of Parkinson's disease in Europe. Eur. Neuropsychopharmacol. 2005, 15, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Weintraub D, Comella CL, Horn S. Parkinson’s disease--Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Manag Care. 2008 Mar;14(2 Suppl):S40-48.
- Parkinson disease - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/28332488/.
- Parkinson’s Disease: A Systemic Inflammatory Disease Accompanied by Bacterial Inflammagens - PMC [Internet]. [cited 2024 Apr 12]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6718721/.
- Chen, C.-K.; Wu, Y.-T.; Chang, Y.-C. Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: a population-based retrospective matched-cohort study. PeerJ 2017, 5, e3647. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Park, J.-B.; Park, Y.-G. Evaluation of the association between periodontitis and risk of Parkinson’s disease: a nationwide retrospective cohort study. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Oral P. gingivalis impairs gut permeability and mediates immune responses associated with neurodegeneration in LRRK2 R1441G mice - PMC [Internet]. [cited 2024 Apr 12]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7677837/.
- The Potential of LPS-Binding Protein to Reverse Amyloid Formation in Plasma Fibrin of Individuals With Alzheimer-Type Dementia - PMC [Internet]. [cited 2024 Apr 12]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6113936/.
- The oral microbiome in autoimmune diseases: friend or foe? - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/36949458/.
- Exploring the Oral Microbiome in Rheumatic Diseases, State of Art and Future Prospective in Personalized Medicine with an AI Approach - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/34209167/.
- Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/16245073/.
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Microbial complexes in subgingival plaque - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/9495612/.
- The possible role of oral microbiome in autoimmunity - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/33898698/.
- Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 Cells in Autoimmunity and Immunodeficiency: Protective or Pathogenic? Front Immunol. 2012;3:129.
- Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/20961415/.
- Periodontal disease and influence of periodontal treatment on disease activity in patients with rheumatoid arthritis and spondyloarthritis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/31701185/.
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans –induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176–369ra176. [Google Scholar] [CrossRef] [PubMed]
- Rooney, C.M.; Mankia, K.; Emery, P. The Role of the Microbiome in Driving RA-Related Autoimmunity. Front. Cell Dev. Biol. 2020, 8, 538130. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Cheng, Z.; Zhu, F.; Bi, C.; Shi, Q.; Chen, X. The Oral Microbiome and Its Role in Systemic Autoimmune Diseases: A Systematic Review of Big Data Analysis. Front. Big Data 2022, 5, 927520. [Google Scholar] [CrossRef] [PubMed]
- Host-Microbial Interactions in Systemic Lupus Erythematosus and Periodontitis - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/31781106/.
- Liu, F.; Ren, T.; Li, X.; Zhai, Q.; Xu, X.; Zhang, N.; Jiang, P.; Niu, Y.; Lv, L.; Shi, G.; et al. Distinct Microbiomes of Gut and Saliva in Patients With Systemic Lupus Erythematous and Clinical Associations. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Salivary dysbiosis and the clinical spectrum in anti-Ro positive mothers of children with neonatal lupus - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/31677965/.
- Interleukin-17 in systemic lupus erythematosus: A comprehensive review - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/25894789/.
- IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/22180658/.
- The paradigm of IL-6: from basic science to medicine - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/12110143/.
- Yamamura K, Izumi D, Kandimalla R, Sonohara F, Baba Y, Yoshida N, et al. Intratumoral Fusobacterium Nucleatum Levels Predict Therapeutic Response to Neoadjuvant Chemotherapy in Esophageal Squamous Cell Carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2019 Oct 15;25(20):6170–9.
- Sun, J.; Tang, Q.; Yu, S.; Xie, M.; Xie, Y.; Chen, G.; Chen, L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020, 9, 6306–6321. [Google Scholar] [CrossRef]
- Sarao, L.K.; Arora, M. Probiotics, prebiotics, and microencapsulation: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 344–371. [Google Scholar] [CrossRef] [PubMed]
- A concerted probiotic activity to inhibit periodontitis-associated bacteria - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/33667279/.
- Cosseau, C.; Devine, D.A.; Dullaghan, E.; Gardy, J.L.; Chikatamarla, A.; Gellatly, S.; Yu, L.L.; Pistolic, J.; Falsafi, R.; Tagg, J.; et al. The Commensal Streptococcus salivarius K12 Downregulates the Innate Immune Responses of Human Epithelial Cells and Promotes Host-Microbe Homeostasis. Infect. Immun. 2008, 76, 4163–4175. [Google Scholar] [CrossRef]
- Streptococcus salivarius inhibits immune activation by periodontal disease pathogens - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/33962608/.
- Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/24271166/.
- Keller MK, Bardow A, Jensdottir T, Lykkeaa J, Twetman S. Effect of chewing gums containing the probiotic bacterium Lactobacillus reuteri on oral malodour. Acta Odontol Scand. 2012 May;70(3):246–50.
- Keller MK, Hasslöf P, Stecksén-Blicks C, Twetman S. Co-aggregation and growth inhibition of probiotic lactobacilli and clinical isolates of mutans streptococci: an in vitro study. Acta Odontol Scand. 2011 Sep;69(5):263–8.
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Lactobacillus plantarum Disrupts S. mutans-C. albicans Cross-Kingdom Biofilms - PubMed [Internet]. [cited 2023 Sep 20]. Available from: https://pubmed.ncbi.nlm.nih.gov/35392605/.
- Biochemistry, Peptide - StatPearls - NCBI Bookshelf [Internet]. [cited 2024 May 2]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562260/.
- Frontiers | Bioactive Synthetic Peptides for Oral Tissues Regeneration [Internet]. [cited 2024 May 2]. Available from: https://www.frontiersin.org/articles/10.3389/fmats.2021.655495/full.
- The antimicrobial peptides secreted by the chromaffin cells of the adrenal medulla link the neuroendocrine and immune systems: From basic to clinical studies - PMC [Internet]. [cited 2024 May 2]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9452953/.
- Alkilzy, M.; Santamaria, R.; Schmoeckel, J.; Splieth, C. Treatment of Carious Lesions Using Self-Assembling Peptides. Adv. Dent. Res. 2018, 29, 42–47. [Google Scholar] [CrossRef]
- The antimicrobial peptides and their potential clinical applications - PMC [Internet]. [cited 2024 May 2]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6684887/.
- Cathelicidins, multifunctional peptides of the innate immunity - PubMed [Internet]. [cited 2024 May 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/12960280/.
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2016, 25, 25–31. [Google Scholar] [CrossRef]
- Oppenheim, F.G.; Xu, T.; McMILLIAN, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477. [Google Scholar] [CrossRef] [PubMed]
- Anticandidal activity of major human salivary histatins. - PMC [Internet]. [cited 2024 May 2]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC258054/.
- Wang, Q.-Q.; Wang, S.; Zhao, T.; Li, Y.; Yang, J.; Liu, Y.; Zhang, H.; Miao, L.; Sun, W. Biomimetic oligopeptide formed enamel-like tissue and dentin tubule occlusion via mineralization for dentin hypersensitivity treatment. J. Appl. Biomater. Funct. Mater. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Physico-chemical properties of crystal surfaces in matrix–mineral interactions during mammalian biomineralisation - ScienceDirect [Internet]. [cited 2024 May 3]. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1359029402000171?via%3Dihub.
- Effects of xylitol chewing gum and candies on the accumulation of dental plaque: a systematic review - PubMed [Internet]. [cited 2024 Jan 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/34677696/.
- Nayak, P.A.; Nayak, U.A.; Khandelwal, V. The effect of xylitol on dental caries and oral flora. Clin. Cosmet. Investig. Dent. 2014, 6, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Drake, D.; Villhauer, A.L. An in vitro comparative study determining bactericidal activity of stabilized chlorine dioxide and other oral rinses. . 2011, 22, 1–5. [Google Scholar] [PubMed]
- Shinada, K.; Ueno, M.; Konishi, C.; Takehara, S.; Yokoyama, S.; Zaitsu, T.; Ohnuki, M.; Wright, F.A.C.; Kawaguchi, Y. Effects of a mouthwash with chlorine dioxide on oral malodor and salivary bacteria: a randomized placebo-controlled 7-day trial. Trials 2010, 11, 14–14. [Google Scholar] [CrossRef]
- C V, S CH, E H, A AA. A Preliminary Investigation on the Antimicrobial Activity of Listerine®, Its Components, and of Mixtures Thereof. Phytother Res PTR [Internet]. 2015 Oct [cited 2024 Jan 8];29(10). Available from: https://pubmed.ncbi.nlm.nih.gov/26104602/.
- Al-Ani, E.; Heaselgrave, W. The Investigation of Thymol Formulations Containing Poloxamer 407 and Hydroxypropyl Methylcellulose to Inhibit Candida Biofilm Formation and Demonstrate Improved Bio-Compatibility. Pharmaceuticals 2022, 15, 71. [Google Scholar] [CrossRef]
- Wińska K, Mączka W, Łyczko J, Grabarczyk M, Czubaszek A, Szumny A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules. 2019 Jun 5;24(11):2130.
- The use of mouthwash containing essential oils (LISTERINE®) to improve oral health: A systematic review - PubMed [Internet]. [cited 2024 Jan 2]. Available from: https://pubmed.ncbi.nlm.nih.gov/30166864/.
- Current concept on the anticaries fluoride mechanism of the action - PubMed [Internet]. [cited 2024 Jan 8]. Available from: https://pubmed.ncbi.nlm.nih.gov/11811302/.
- Vranić, E.; Lačević, A.; Mehmedagić, A.; Uzunović, A. Formulation ingredients for toothpastes and mouthwashes. Bosn. J. Basic Med Sci. 2004, 4, 51–58. [Google Scholar] [CrossRef]
- Zhang, J.; Sardana, D.; Li, K.Y.; Leung, K.C.M.; Lo, E.C.M. Topical Fluoride to Prevent Root Caries: Systematic Review with Network Meta-analysis. J. Dent. Res. 2020, 99, 506–513. [Google Scholar] [CrossRef]
- O’hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2021, 110, 223–230. [Google Scholar] [CrossRef]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335–40. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.K.L. Oil pulling for maintaining oral hygiene – A review. J. Tradit. Complement. Med. 2016, 7, 106–109. [Google Scholar] [CrossRef] [PubMed]
- The effect of oil pulling with coconut oil to improve dental hygiene and oral health: A systematic review - PMC [Internet]. [cited 2024 Jan 8]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7475120/.
| Microbial Component | Predominant Phyla/Genera | Other Notable Genera |
|---|---|---|
| Bacteria | Actinobacteria, Bacteroidetes, Chlamydia, Euryarchaeota, Fusobacteria, Firmicutes, Proteobacteria, Spirochaetes, Tenericutes |
- Gram-positive cocci: Abiotrophia, Peptostreptococcus, Streptococcus, Stomatococcus - Gram-positive rods: Actinomyces, Bifidobacterium, Corynebacterium, Eubacterium, Lactobacillus, Propionibacterium, Pseudoramibacter, Rothia - Gram-negative cocci: Moraxella, Neisseria, Veillonella - Gram-negative rods: Campylobacter, Capnocytophaga, Desulfobacter, Desulfovibrio, Eikenella, Fusobacterium, Hemophilus, Leptotrichia, Prevotella, Selemonas, Simonsiella, Treponema, Wolinella |
| Fungi | Candida | Cladosporium, Aureobasidium, Saccharomycetales, Aspergillus, Fusarium, Cryptococcus |
| Archaea | Euryarchaeota | - |
| Disease | Commensals | Pathogens | Directionality | Mechanisms | References |
|---|---|---|---|---|---|
| Oral Diseases | |||||
| Dental Caries |
Lactobacillus spp., Veillonella, Propionibacterium, Bifidobacterium, Corynebacterium, Capnocytophaga |
S. mutans | ↑ | Acidogenic bacteria produce acidic by-products, leading to demineralization and cavitation; Dysbiosis-driven disorder | [64,65,66,71,72,73,74,75,76,77] |
| Gingivitis | Streptococcus sp., Actinomyces sp., Veillonella sp. |
P. gingivalis, T. denticola, A. actinomycetemcomitans, Fusobacterium sp., P. intermedia |
↑ | Caused by the accumulation of microbial plaque on the tooth surface, which penetrates the gingival tissue and leads to inflammation. | [79] |
| Periodontitis | Prevotella melaninogenica |
P. gingivalis, T. denticola, T. forsythia, F. nucleatum ss. polymorphum, P. intermedia |
↑ | Pathogenic bacteria induce inflammation, oxidative stress, immune activation, and tissue damage; Potential systemic implications | [11,83,84,85] |
| Halitosis |
Prevotella melaninogenica, Veillonella spp. Peptostreptococcus Actinomyces spp. Eubacterium, Megasphaera, Selenomonas, Leptotrichia, Fusobacterium, Eikenella corrodens |
Treponema denticola, Porphyromonas gingivalis, Porphyromonas endodontalis, Tannerella forsythensis, Bacteroides loescheii, Centipeda periodontii |
↑ | Anaerobic bacteria produce VSCs causing malodor; Bacterial degradation of sulfur-containing amino acids | [92,93,94,95] |
| Taste impairment | Lactobacilli | - | ↑ | High levels of acid produced by the bacteria impair taste, affecting taste perception. | [99] |
| Burning mouth syndrome (BMS) |
Streptococcus, Rothia, Bergeyella, Granulicatella |
- | ↑ | Alteration in bacterial strains may contribute to the development of BMS influencing pathways involved in inflammation, immune responses, and sensory perception | [108] |
| Oral Thrush |
Candida parapsilosis, Candida krusei, Candida stellatoidea, Candida tropicalis |
Candida albicans, Candida glabrata, Candida dubliniensis, Candida guilliermondii |
↑ | Overgrowth of Candida species due to factors like poor oral hygiene, weakened immune system, or underlying medical conditions; Adhesion to host surfaces and tissue invasion through various mechanisms; Hyphal formation, biofilm production, and secretion of enzymes that degrade host immune factors | [109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128] |
| Systemic diseases | |||||
| IBD | - |
P. gingivalis, F. nucleatum |
↑ | Oral-resident bacteria may infiltrate the gut microbiome; Gut dysbiosis due to altered gut epithelial permeability | [59,132,134] |
| Atherosclerosis |
Prevotella nigrescens, Parvimonas micra, |
S. mutans, P. gingivalis, P. endodontalis, T. denticola, T. forsythia, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Eubacterium timidum, Eubacterium brachy, Eubacterium saphenum, Campylobacter rectus, |
↑ | Oral dysbiosis triggers local inflammation, systemic inflammatory responses, oxidative stress, immune activation, platelet aggregation | [135,136,137,138,145,146,147] |
| Diabetes | Capnocytophaga, |
P. gingivalis, T. forsythia |
↑ | Hyperglycemia and oxidative stress create a conducive environment for microbial dysbiosis; Elevated inflammatory reactions |
[151,152,153,154,155,156] |
| Obesity |
Proteobacteria, Chloroflexi, Firmicutes |
- | ↑ | Potential migration of oral bacteria to the gut; Alterations in bacterial populations impact metabolic homeostasis | [162,163,164,165,166] |
| AD | - |
P. gingivalis, T. forsythia, T. denticola, |
↑ | Oral microbiota’s production of inflammatory agents potentially contributes to neuroinflammation and AD progression | [168,169,170,171,173,176] |
| PD | - | P. gingivalis | ↑ | P. gingivalis infection is correlated with PD, with studies demonstrating the presence of gingipain R1 (RgpA) in the bloodstream, indicating systemic dissemination. P. gingivalis may contribute to PD pathogenesis by inducing systemic inflammation, promoting hypercoagulability, and exacerbating neurodegeneration. | [180,183,184] |
| RA |
Prevotella, Veillonella, Lactobacillus salivarius |
P. gingivalis, A. actinomycetemcomitans, |
↑ | P. gingivalis implicated in RA onset via citrullination and ACPA production; Dysbiosis of oral microbiota exacerbates joint inflammation. | [186,188,189,190] |
| SLE |
Veillonella, Streptococcus, Prevotella |
T. forsythia, T. denticola |
↑ | Oral microbial dysbiosis and periodontitis may exacerbate SLE via immune activation; Potential contribution to autoimmune responses | [197,198,199,200,201,202] |
| Cancer |
S. oralis, S. mitis, S. sanguinis, Lactobacillus fermentum, Lactobacillus acidophilus, Bifidobacterium adolescentis |
P. gingivalis, F. nucleatum, |
↑ | Specific bacteria infiltrate cells, initiate tumor development, produce cancer-promoting substances like lipopolysaccharide | [203,204] |
| Component | Role/Mechanism | Formulation | References |
|---|---|---|---|
| Probiotics | Support balance of oral microbiota, produce specialized metabolites for maintaining microbiota equilibrium, promote healthy immunity |
Probiotic supplements containing strains such as Streptococcus salivarius M18, Streptococcus salivarius K12, Lactobacillus plantarum, Bifidobacterium lactis, Lactobacillus reuteri, Lactobacillus salivarius, help maintain oral microbiota balance, combat bad breath and gum inflammation, and enhance immune responses. | [205] |
| Xylitol | Rebalances mouth acidity, reduces S. mutans counts, and disrupts S. mutans energy production. | Xylitol mints and gums, contains xylitol, natural peppermint flavour, magnesium stearate, natural menthol |
[225,226] |
| Chlorine Dioxide Mouth Rinse | Neutralizes volatile sulfur compounds (VSCs), kills odour-producing bacteria, reduces plaque and F. nucleatum counts | Stabilized chlorine dioxide (ClO2), trisodium phosphate, citric acid; typically found in commercially available mouth rinses | [228] |
| Thymol Mouth Rinse | Antifungal properties, disrupts C. albicans hyphae production and adhesion to epithelial cells | Thymol, eucalyptol, menthol, methyl salicylate dissolved in ethanol (27%); available in various commercial mouthwash formulations | [230] |
| Fluoride Toothpaste | Strengthens enamel, prevents cavities, promotes oral hygiene | Sodium fluoride, hydrated silica, cellulose gum, glycerin, available in various brands and formulations of toothpaste | [233] |
| Hydroxyapatite Toothpaste | Replenishes lost minerals in enamel, restores enamel structure, alternative to fluoride-based products | Hydroxyapatite particles, available in various brands and formulations of toothpaste | [236] |
| Cetylpyridinium Chloride (CPC) | Antimicrobial properties, disrupts bacterial membranes and cellular functions, effective against plaque-forming bacteria | Found in various oral care formulations such as mouthwashes and toothpaste | [237] |
| Clove Oil | Antibacterial and antifungal effects, inhibits multi-resistant Staphylococcus species growth, reduces C. albicans ergosterol levels | Clove oil containing eugenol, eugenyl acetate, carvacrol, available in various oral care products such as mouth rinses and gels | [238] |
| Oil Pulling | Generates antioxidants to damage microbial cell walls, removes bacteria by attracting them to oil, hinders bacterial co-aggregation and plaque formation | Coconut, sesame, or sunflower oil, typically used as a standalone oral hygiene technique | [239] |
| Antimicrobial Peptides | Antimicrobial peptides (AMPs) such as defensins and cathelicidins disrupt microbial cell membranes through electrostatic interactions, forming pores that lead to ion leakage and cell death, providing natural defense against oral pathogens. | Antimicrobial peptides (AMPs) like defensins and cathelicidins are integrated into oral care products to combat oral bacteria, fungi, and viruses. | [218,219] |
| Enamel Strengthening Peptides | Enamel strengthening peptides, exemplified by peptide P11-4, mimic the mineral structure of enamel. They facilitate remineralization by attracting calcium ions, promoting the formation of hydroxyapatite essential for enamel regeneration and strengthening. | P11-4: dental gels, pastes, some toothpastes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





