Submitted:
27 June 2024
Posted:
27 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
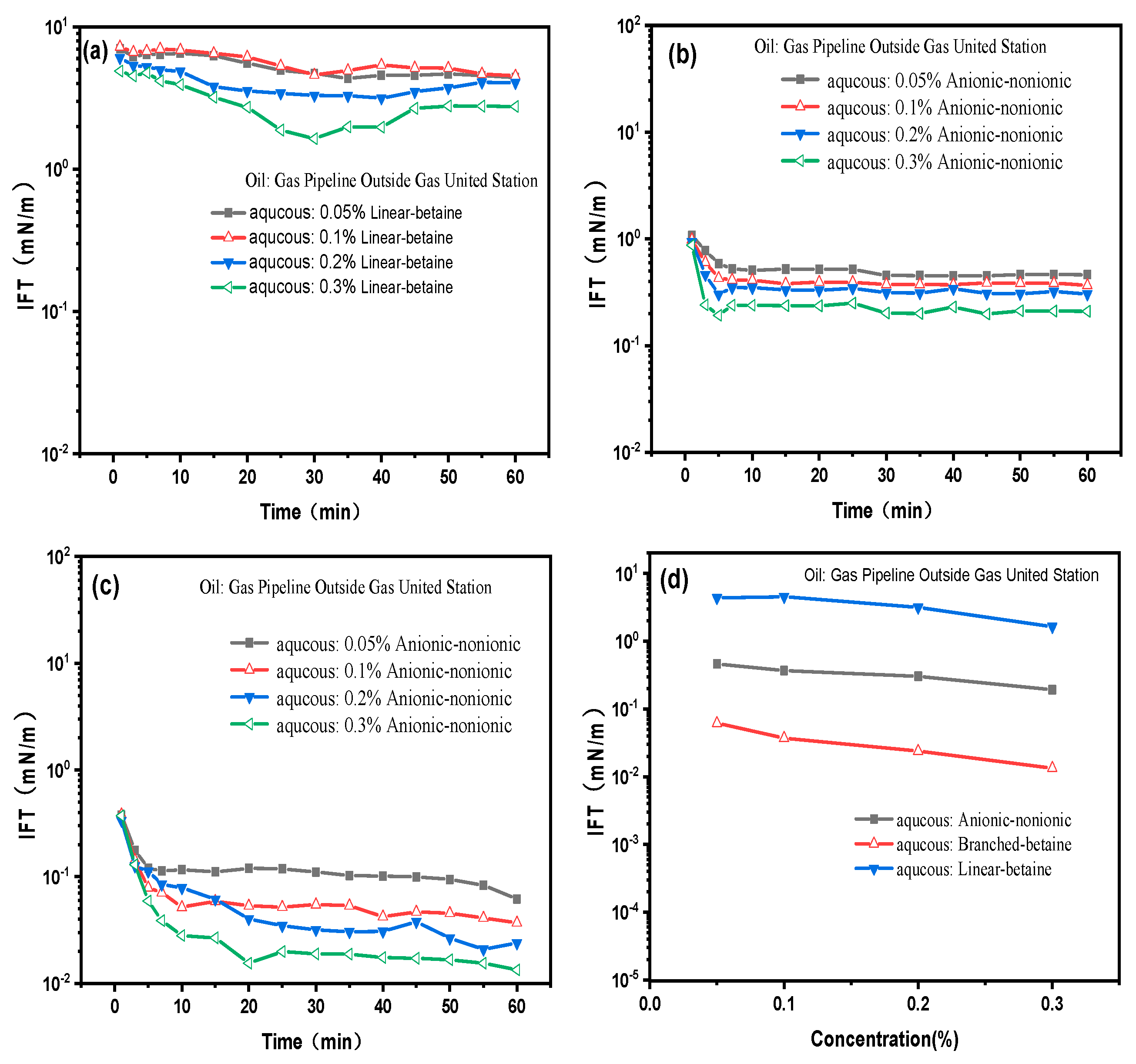
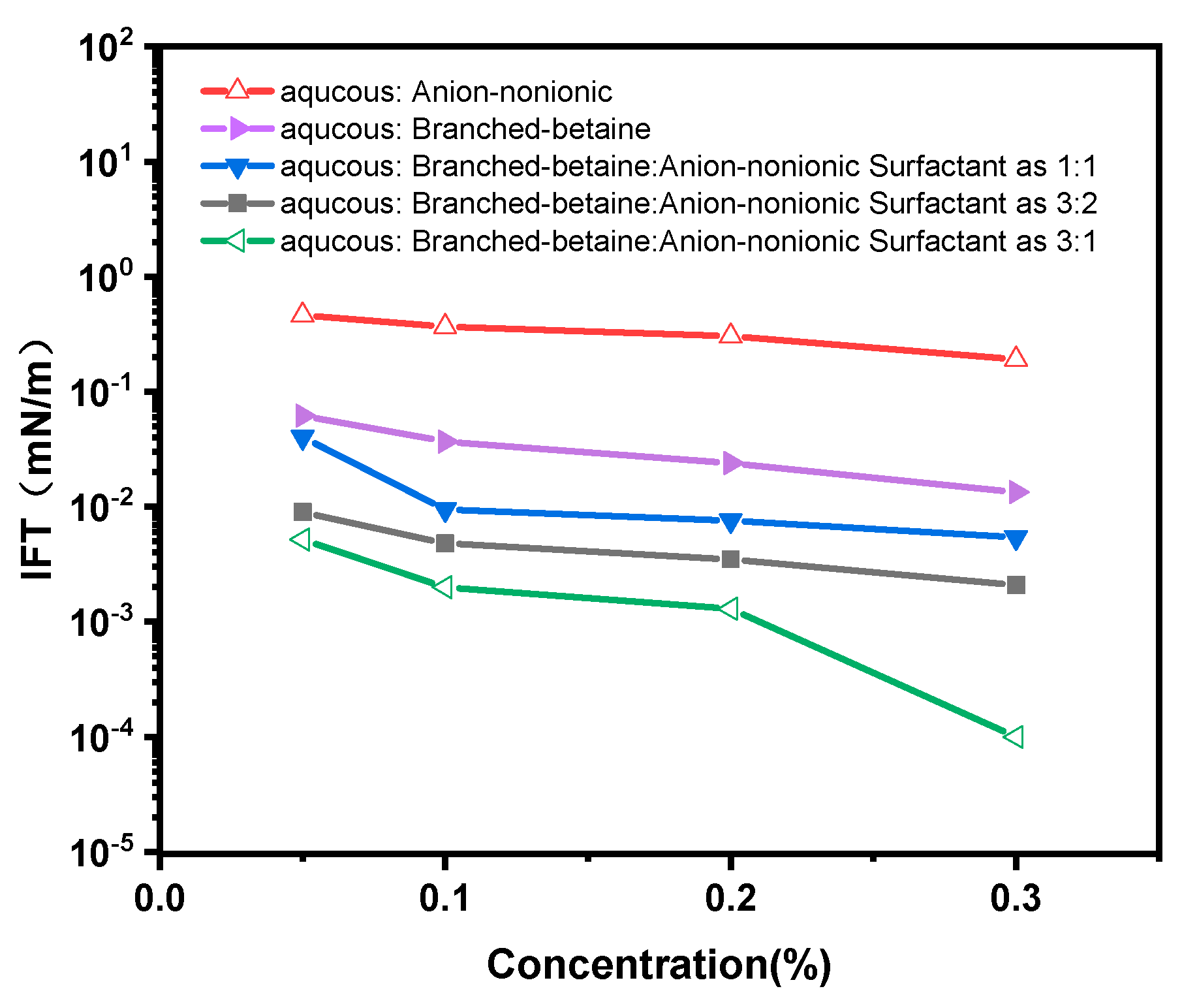
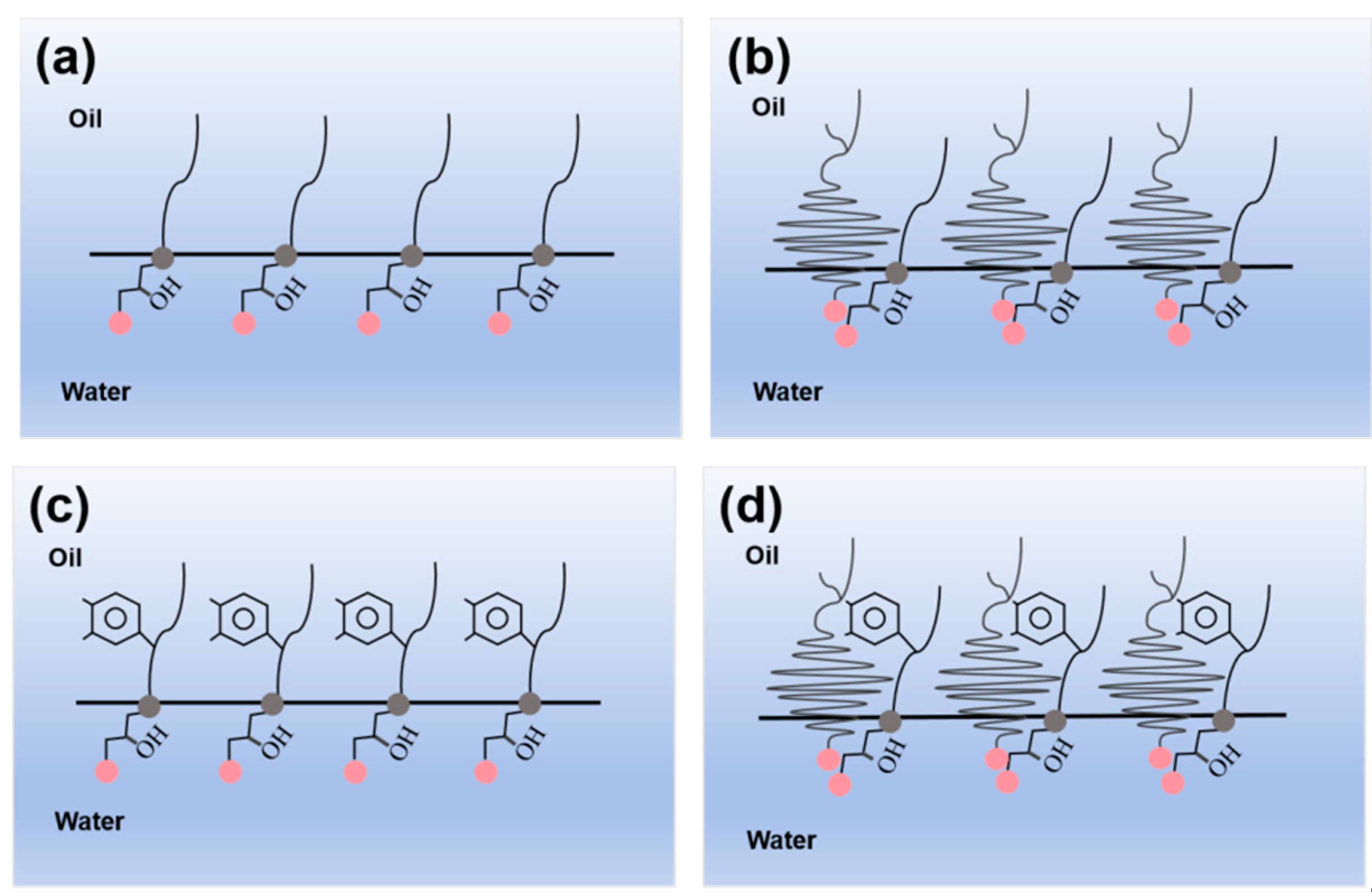
2.1. Evaluation of Surfactant Ability to Reduce Interfacial Tension in Situ Emulsification Formulations
2.2. Anionic vs. Nonionic Surfactant Effects on Emulsification of Bet
2.3. Control Factors of Enhanced Oil Recovery Systems
2.3.1. Microscopic Visualization of Enhanced Oil Recovery
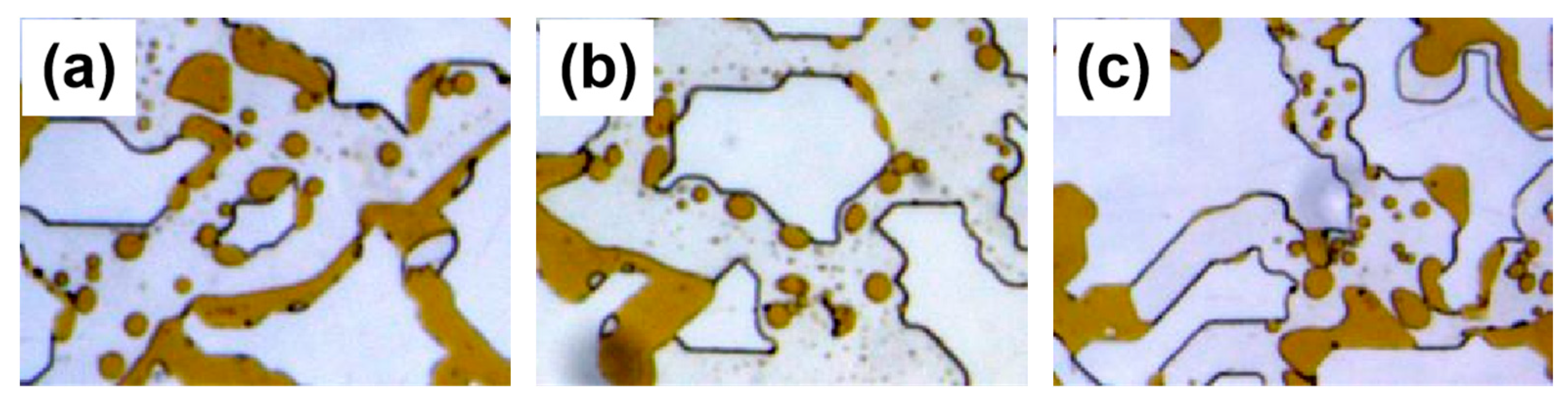
2.3.2. Study on In Situ Emulsification Transport and Transport Performance
2.4. Optimization of In Situ Emulsification Displacement Parameters and Evaluation of Oil Recovery Effects
2.4.1. Optimization of In Situ Emulsification System Strength and Evaluation of Oil Recovery Effects
2.4.2. Evaluation of Plug Combination Effects in Heterogeneous Emulsification Systems
3. Materials and Methods
3.1. Materials
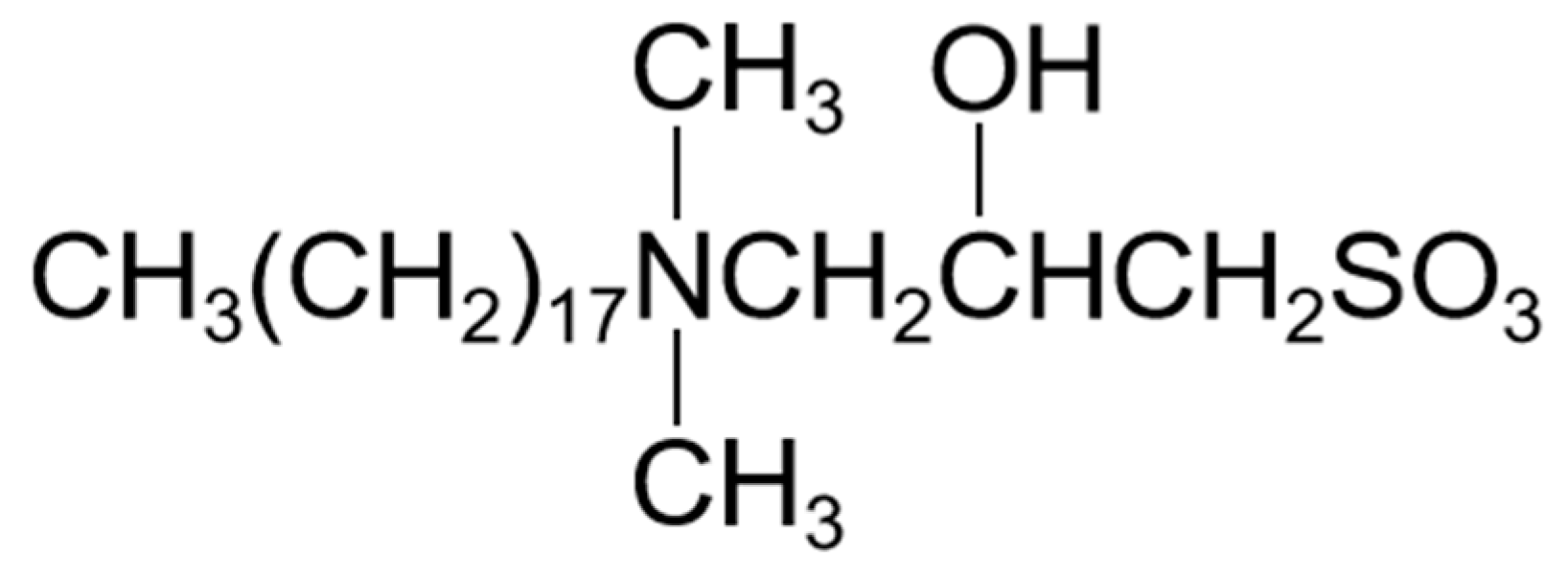
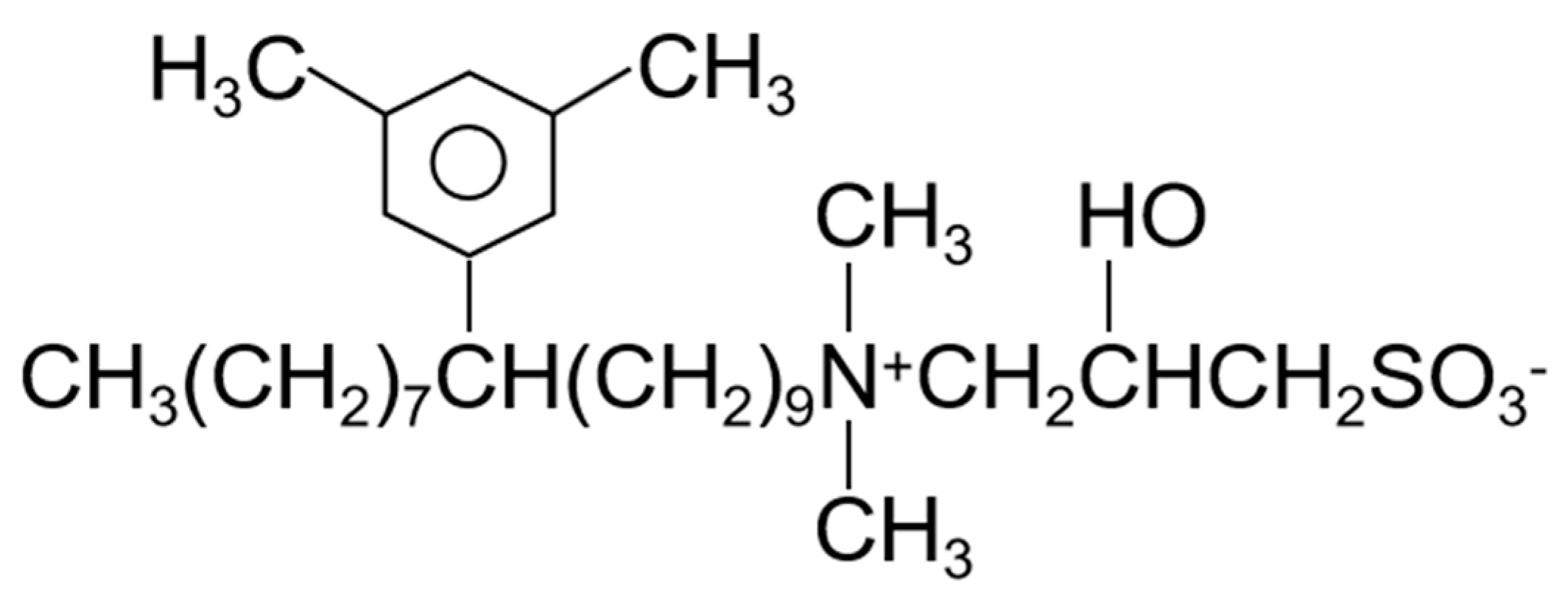
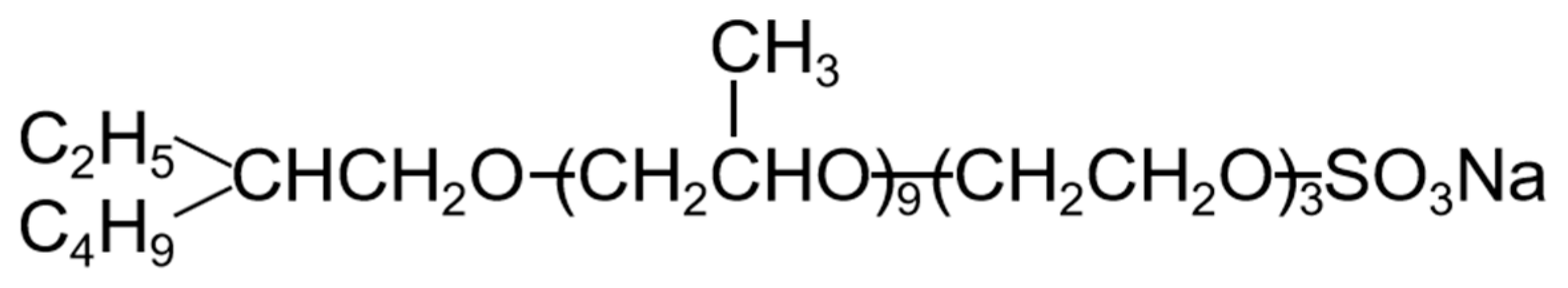
3.2. Methodology
3.2.1. Preparation of Surfactant Solutions
3.2.2. Measurement of Surfactant Interfacial Tension
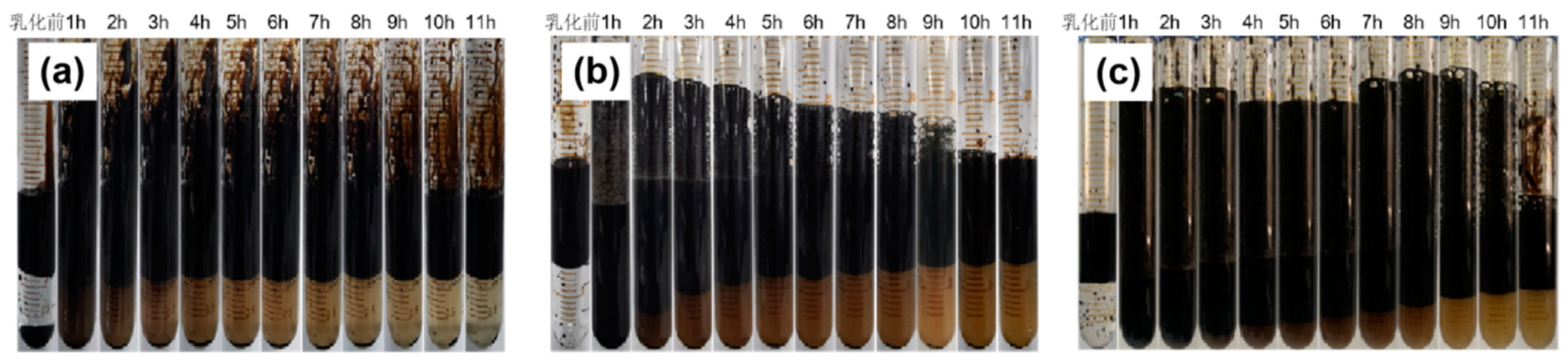
3.2.3. Emulsion Stability Determination
3.2.4. Microscopic Visualization Oil Displacement Experiment
3.2.5. Emulsion Flow Resistance Experiment
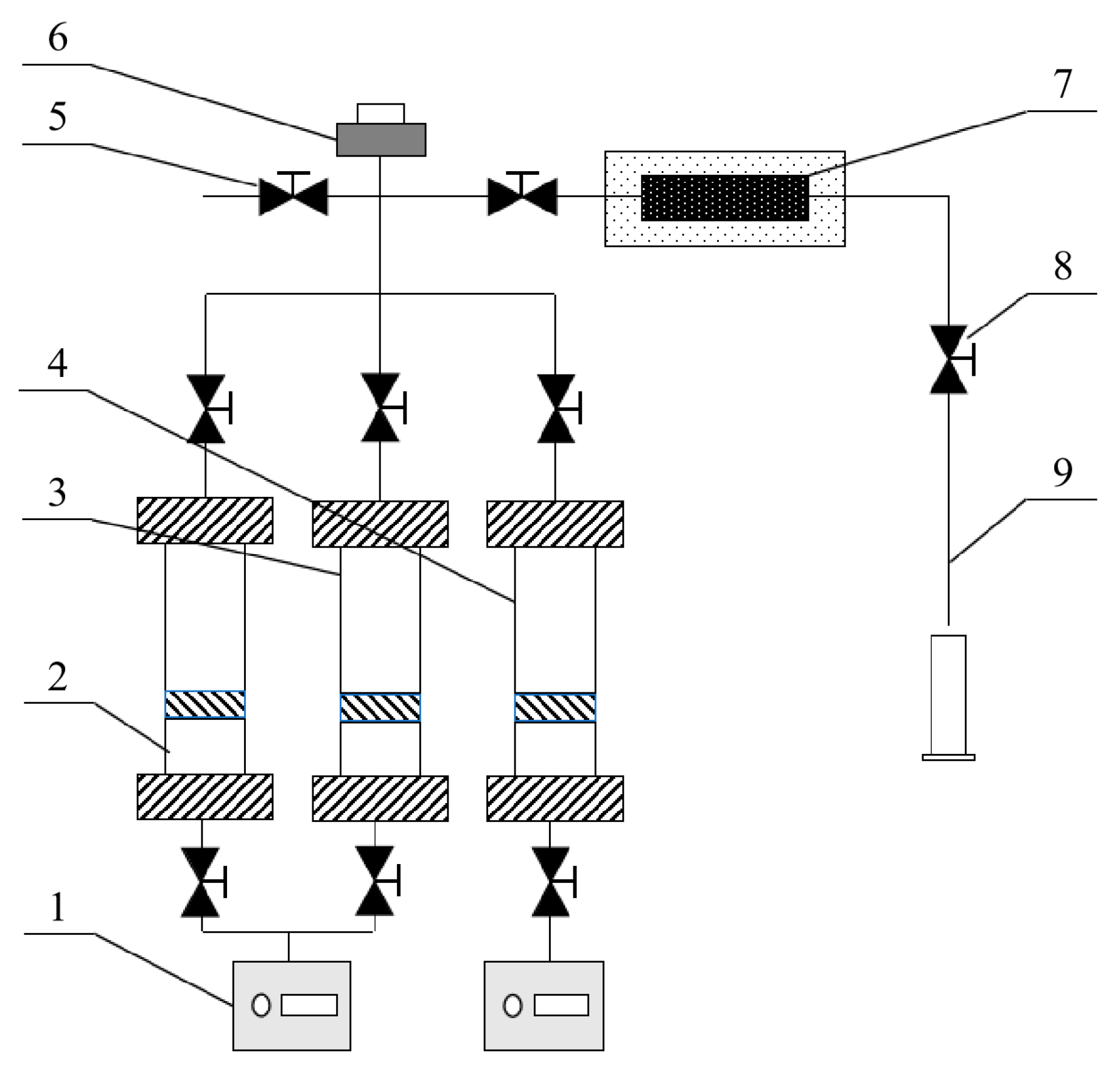
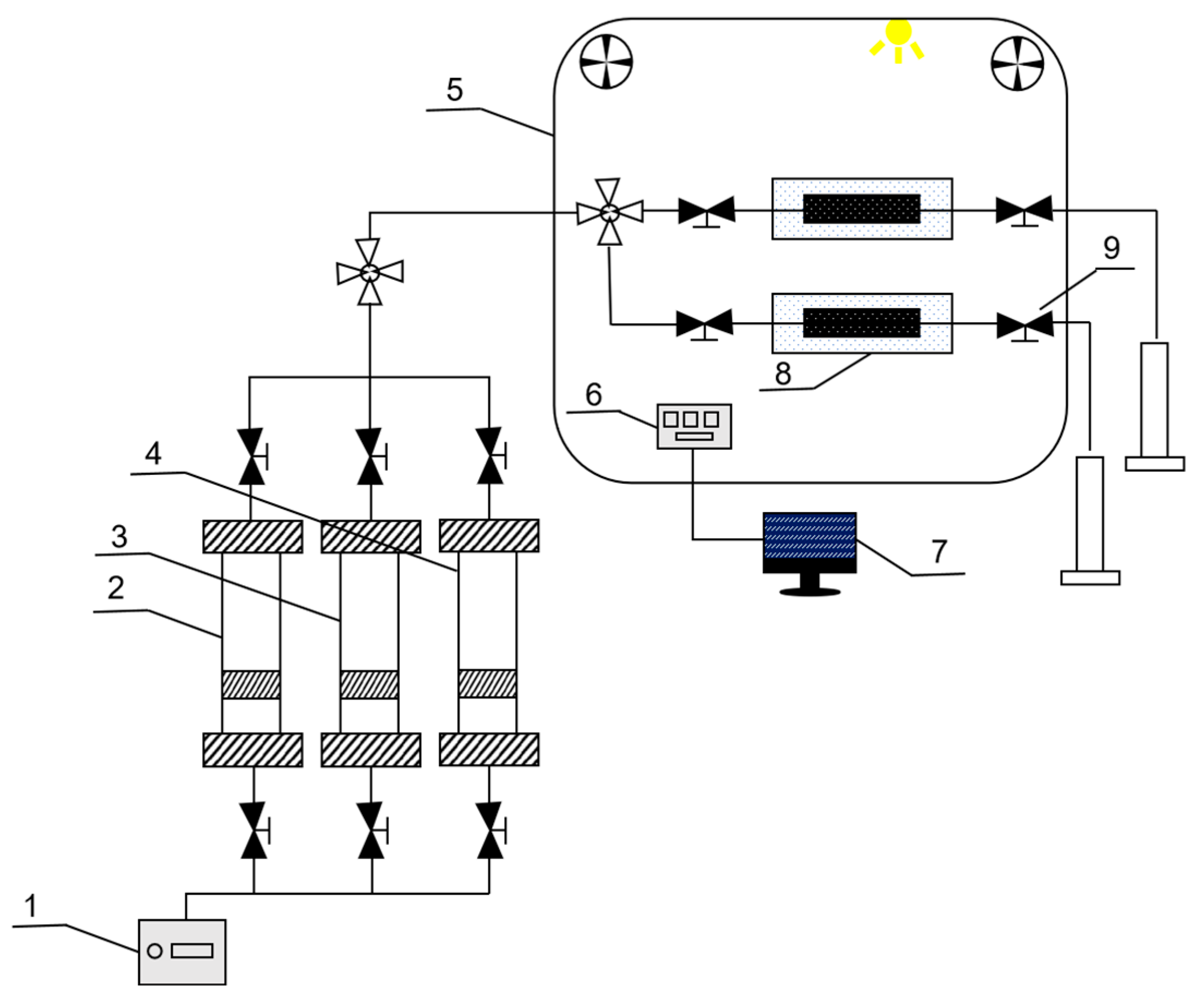
3.2.6. One-Dimensional Rock Core Chemical Flooding Experiment
3.2.7. Parallel Rock Core Oil Displacement Experiment
4. Conclusion
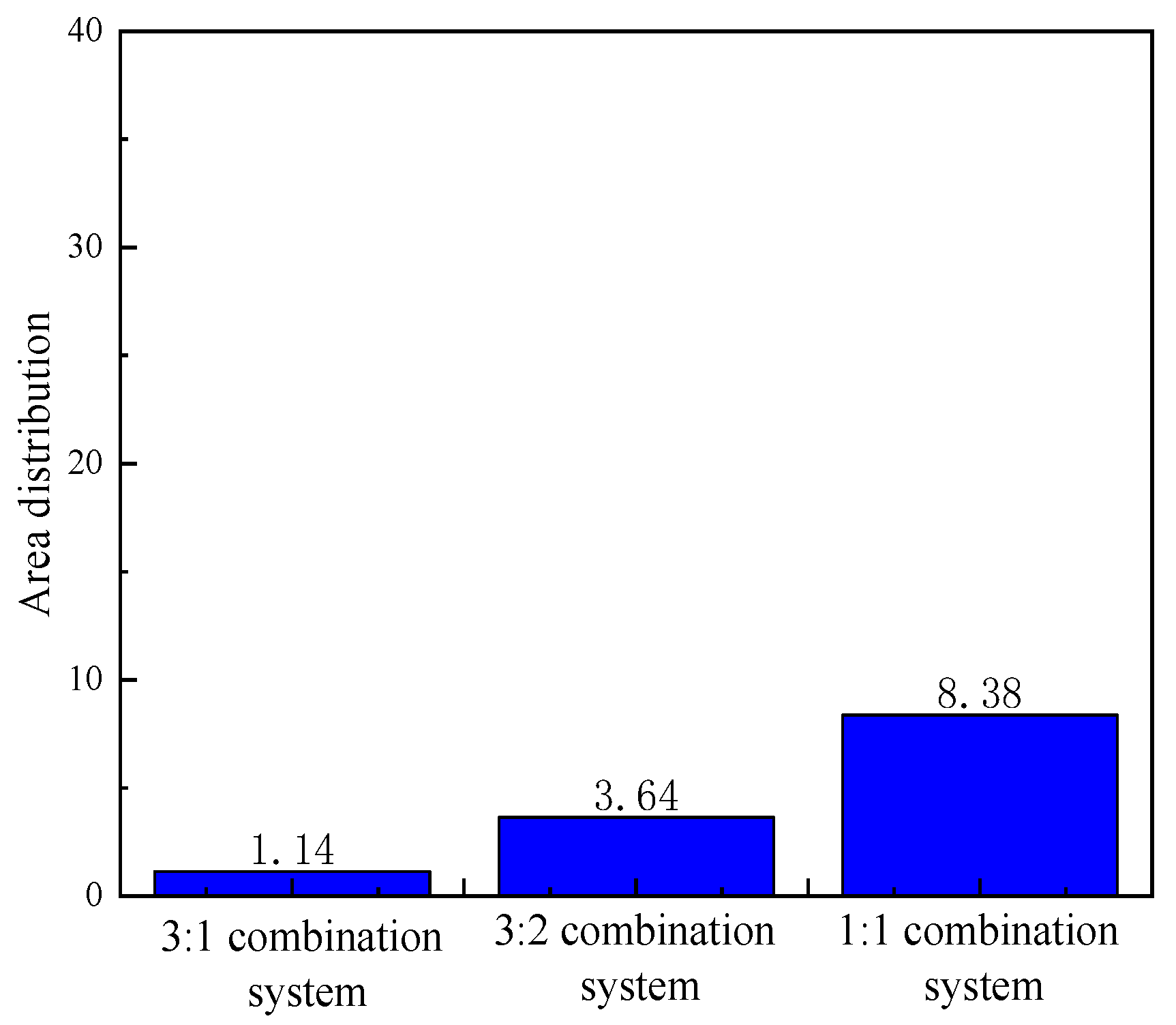
- The mixed solution of branched-chain betaine and non-ionic surfactant can achieve an extremely low interfacial tension, as low as 10 -4 mN/m. Moreover, the stability of the branched-chain betaine and non-ionic surfactant at a ratio of 3:1 is the strongest. With an increase in the ratio of betaine to surfactant, the viscosity of the aqueous phase film also increases, thereby slowing down the thinning rate of the film and reducing water separation. Consequently, the stability of the emulsion is enhanced accordingly
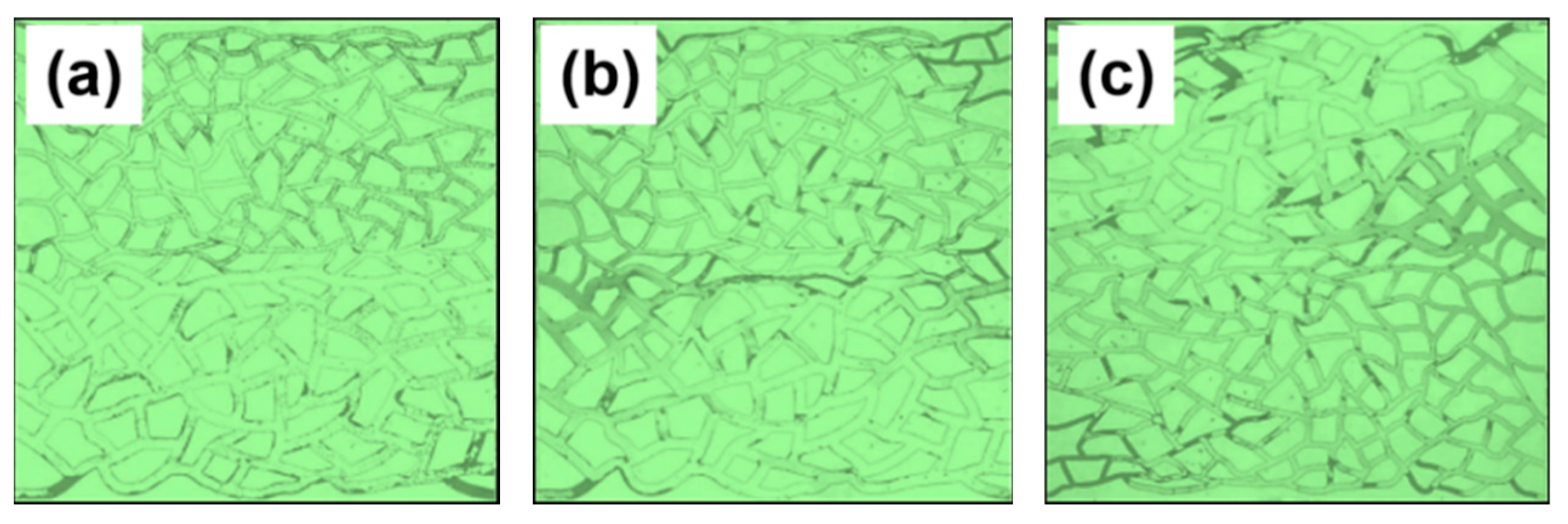
- In the microscopic visualization experiments, the oil displacement mechanisms of the emulsification systems were elucidated. All three compound systems effectively expanded the influence of droplet trapping, thereby enhancing oil recovery. Among them, the branched-chain betaine and non-ionic surfactant at a ratio of 3:1 exhibited significant displacement of residual oil over a large area, with notably improved effects on both large and small pore sections.
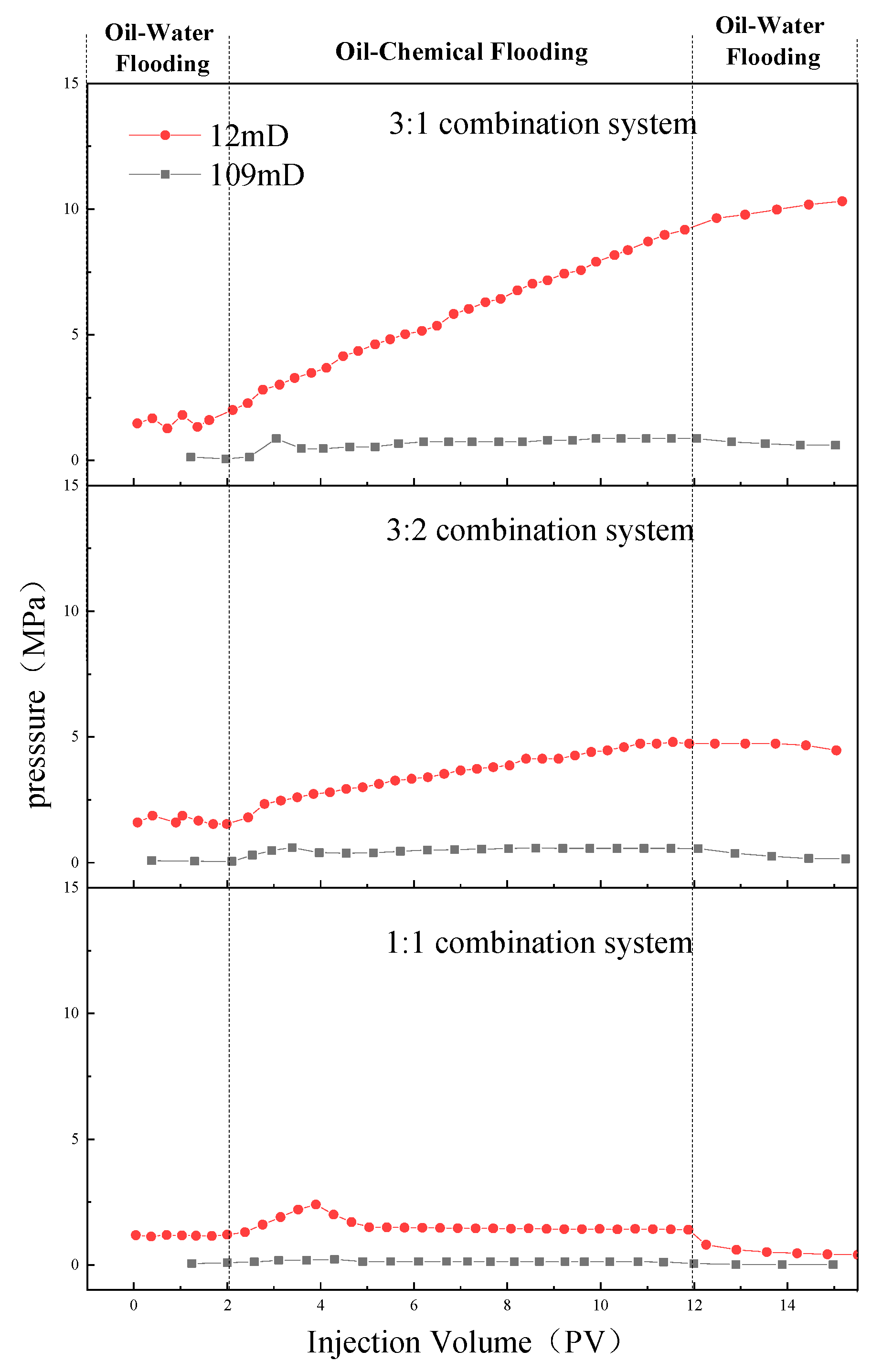
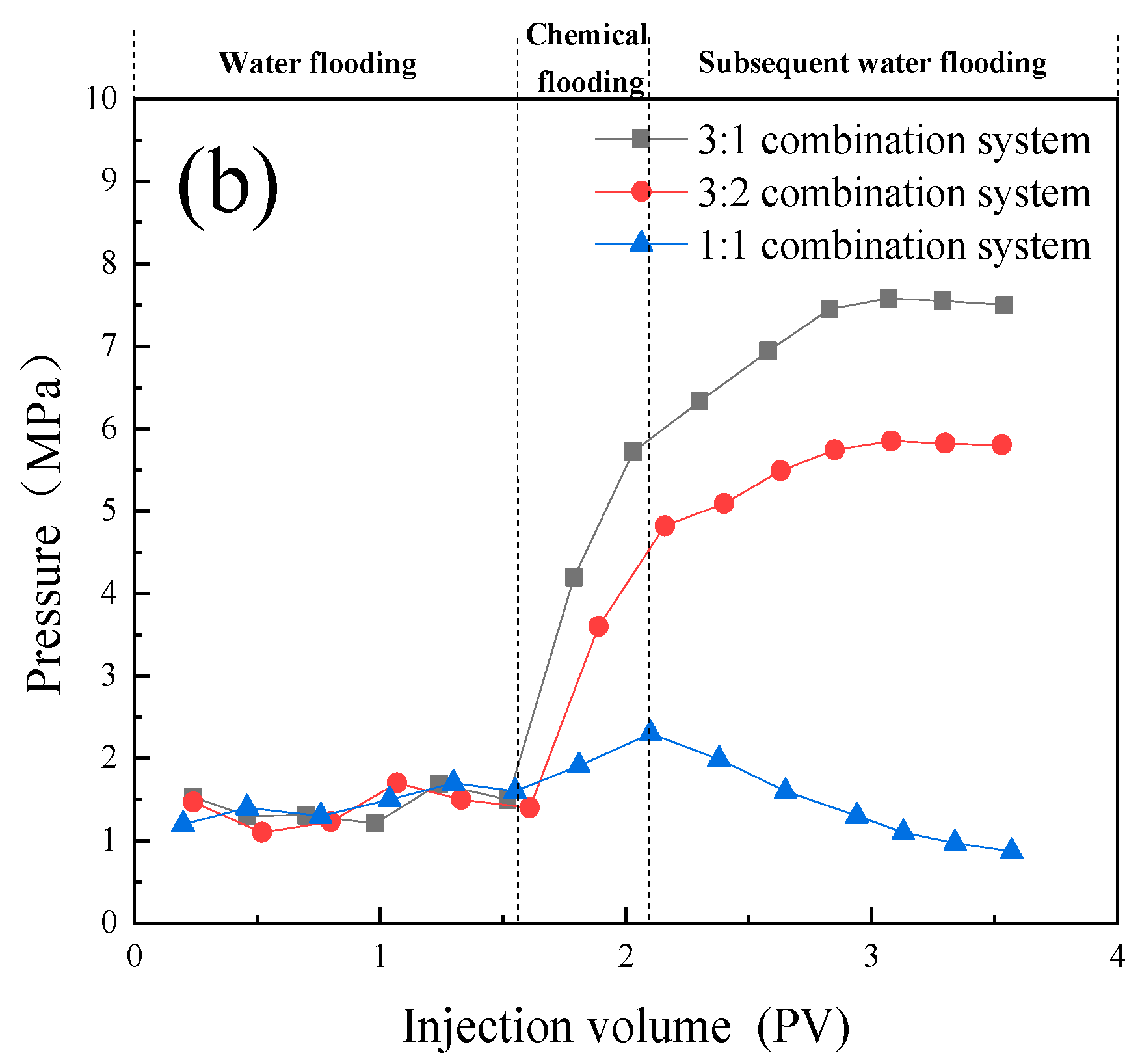
- In the emulsion flow resistance experiments, all three compound systems, post in-situ emulsification, demonstrated the capability to pass through both 109 × 10 -3μm -2 and 12 × 10 -3μm -2 permeability cores. However, in the 109 × 10 -3μm -2 cores, characterized by larger pore throats, the 3:1 compound system displayed superior stability, effectively plugging the pores and displacing oil, thereby enhancing oil recovery. It exhibited the best compatibility. In the 12 × 10 -3μm -2 cores, with smaller pore throats, the 1:1 compound system could smoothly pass through the pores, engaging in in-situ emulsification with residual oil, thus enhancing oil recovery. It also demonstrated optimal compatibility.
- The one-dimensional core flooding experiments validated that the 3:1 compound system was best suited for the 109 × 10 -3μm -2 permeability cores, yielding a chemical flooding recovery rate of 17.33%. Similarly, the 1:1 compound system was found to be most compatible with the 12 × 10 -3μm -2 permeability cores, resulting in a chemical flooding recovery rate of 8.87%. This further confirmed the performance disparities of different compound systems under varying core permeabilities. It also affirmed that emulsions with higher stability are more suitable for cores with larger permeabilities, while those with lower stability are better suited for cores with smaller permeabilities.
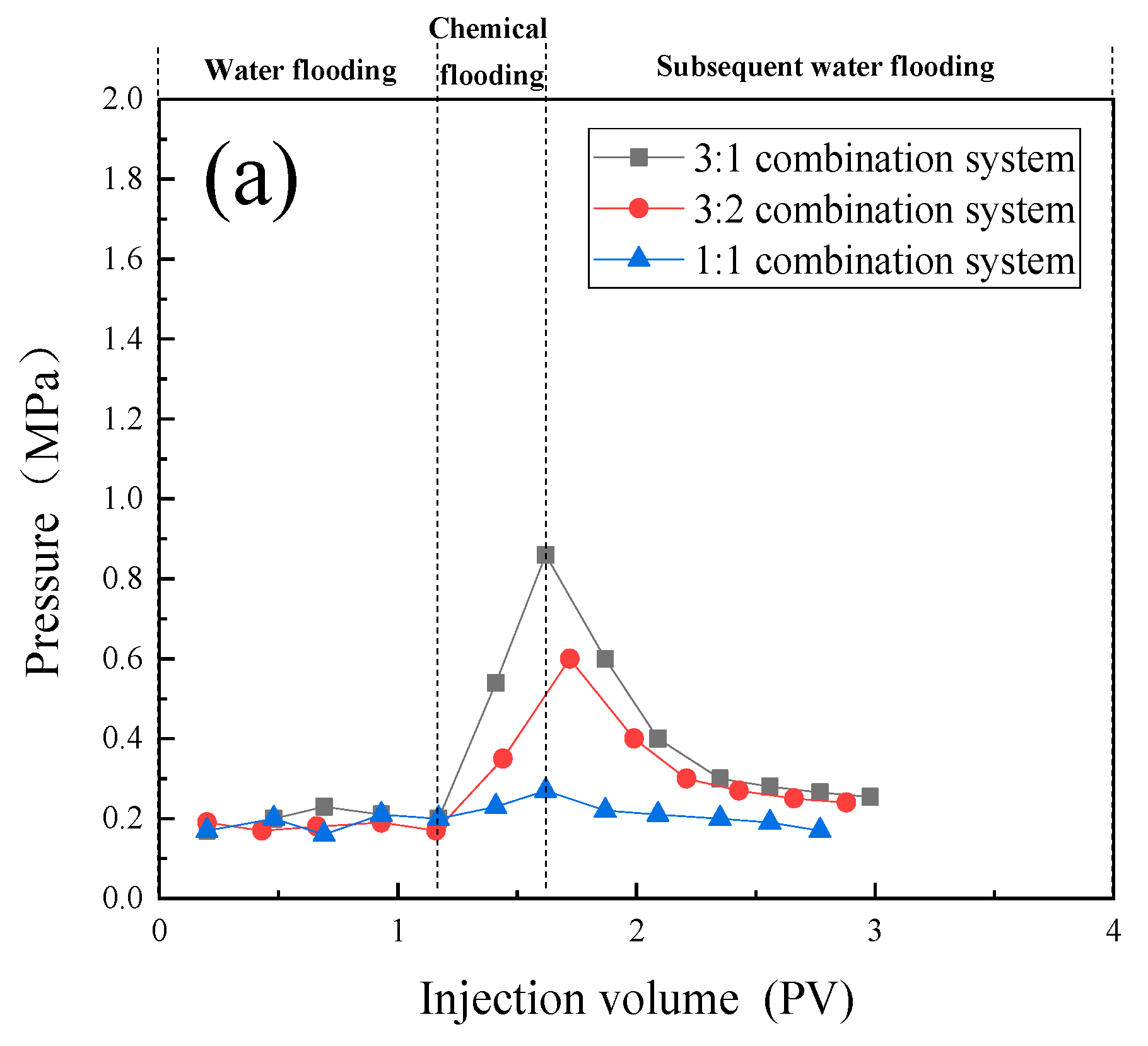
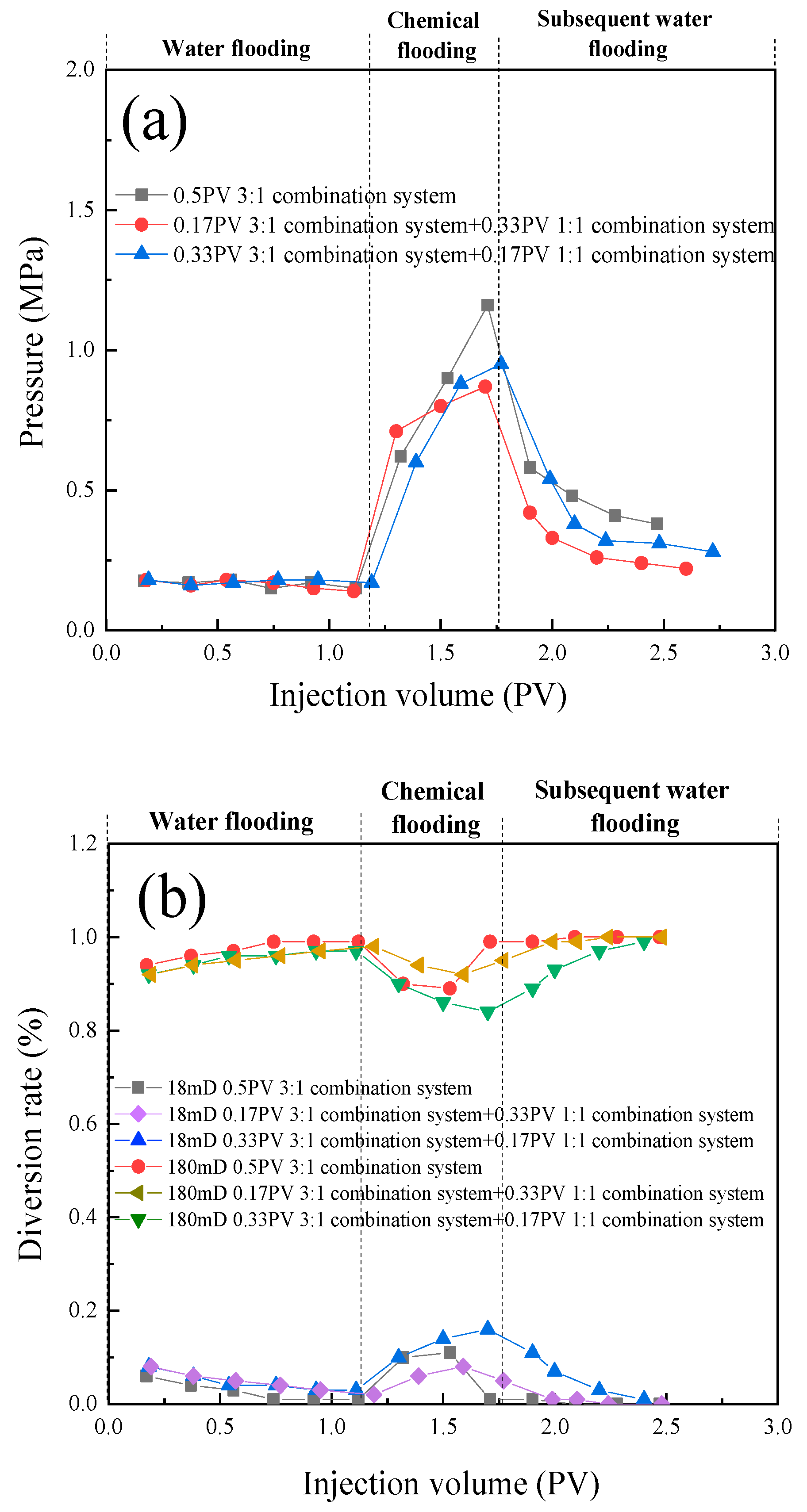
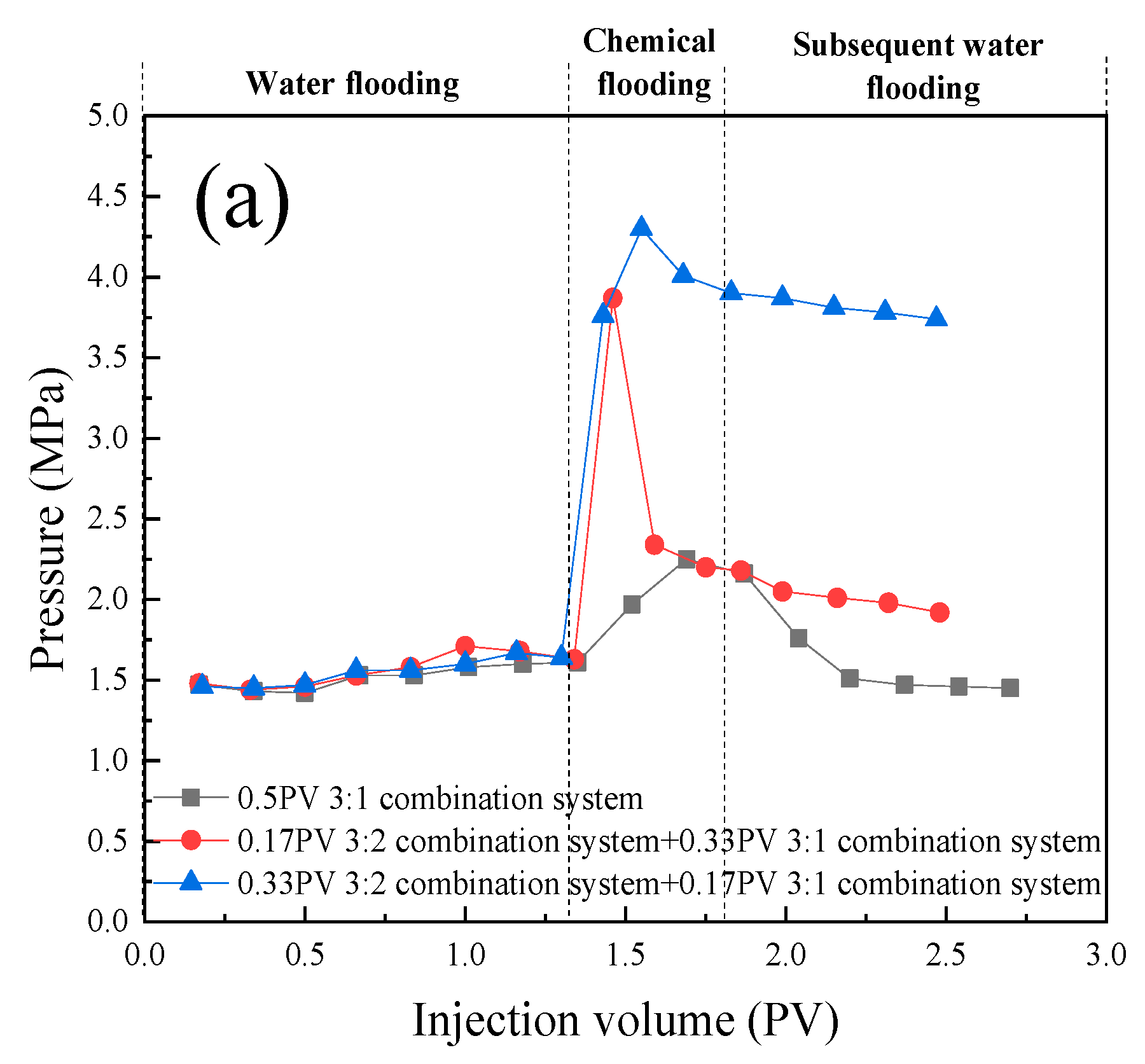
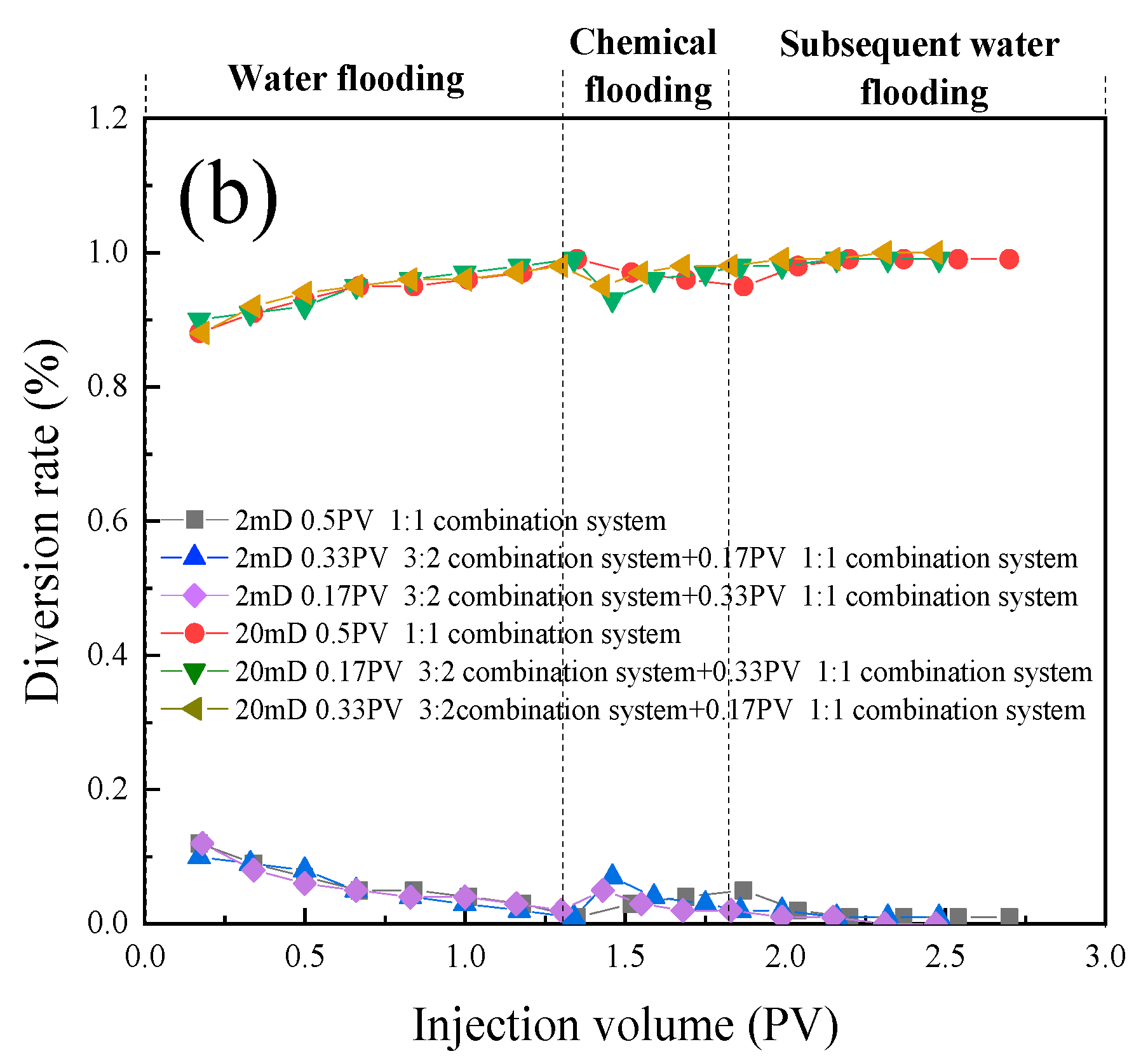
- In the parallel oil displacement experiments, optimal combinations were further selected for different core permeabilities. For the core with a permeability of 180 × 10 -3μm -2 + 18 × 10 -3μm -2, the best combination was determined to be a sequential injection of 0.33PV of the 3:1 compound system (branched-chain betaine and non-ionic surfactant) followed by 0.17PV of the 1:1 compound system. Similarly, for the core with a permeability of 20 × 10 -3μm -2 + 2 × 10 -3μm -2, the best combination was found to be injecting only 0.5PV of the 1:1 compound system (branched-chain betaine and non-ionic surfactant). Further validation was conducted on the intrinsic relationship between the stability of emulsions and the permeability of rock cores. In rock cores with larger pores, emulsion systems with higher stability demonstrate superior plugging effects and possess greater spreading capabilities, thereby enhancing oil recovery rates. Conversely, in rock cores with smaller pores, emulsion systems with lower stability are more prone to penetration, leading to in-situ emulsification with residual oil. However, this process does not cause damage or retention to the rock cores or residual oil, and likewise contributes to increased oil recovery rates. Therefore, an optimal combination of emulsion stability exists for specific heterogeneous rock cores.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, L.A. Physico–Chemical Environment of Petroleum Reservoirs in Relation to Oil Recovery Systems. In Improved Oil Recovery by Surfactant and Polymer Flooding; Elsevier, 1977; 1–26. [Google Scholar]
- Pal, N.; Vajpayee, M.; Mandal, A. Cationic/Nonionic Mixed Surfactants as Enhanced Oil Recovery Fluids: Influence of Mixed Micellization and Polymer Association on Interfacial, Rheological, and Rock-Wetting Characteristics. Energy Fuels 2019, 33, 6048–6059. [Google Scholar] [CrossRef]
- Mohsenatabar Firozjaii, A.; Akbari, M.; Zargar, G. Sensitivity Analysis and Optimization on Effective Parameters during Chemical Enhanced Oil Recovery (CEOR) Using Experimental Design and Numerical Simulation. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 2019, 41, 1847–1861. [Google Scholar] [CrossRef]
- Su, L.; Sun, J.; Ding, F.; Gao, X.; Zheng, L. Effect of Molecular Structure on Synergism in Mixed Zwitterionic/Anionic Surfactant System: An Experimental and Simulation Study. Journal of Molecular Liquids 2021, 322, 114933. [Google Scholar] [CrossRef]
- Zheng, J.; Luo, J.; Zhou, D.; Shen, T.; Li, H.; Liang, L.; Lu, M. Preparation and Properties of Non-Ionic Polyurethane Surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2010, 363, 16–21. [Google Scholar] [CrossRef]
- Mcclements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Critical Reviews in Food Science and Nutrition 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.-L.; Cao, X.-L.; Zhu, Y.-W.; Ma, B.-D.; Xu, Z.-C.; Zhang, L.; Ma, G.-Y.; Zhang, L. Studies on Interfacial Tensions of Betaine and Anionic-Nonionic Surfactant Mixed Solutions. Journal of Molecular Liquids 2020, 311, 113262. [Google Scholar] [CrossRef]
- Mohamad-Aziz, S.N.; Mishra, P.; Zularisam, A.W.; Sakinah, A.M.M. Isooctane-Based Anionic and Zwitterionic Surfactant: Synergistic Interaction of Mixed Reverse Micelle and Solubilisation of Erythromycin. Journal of Molecular Liquids 2019, 286, 110882. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Liu, J.; Zhu, Y.; Hu, J.; Feng, R.; Fu, C. Adaptability of a Hydrophobically Associating Polyacrylamide/Mixed-surfactant Combination Flooding System to the Shengli Chengdao Oilfield. J of Applied Polymer Sci 2014, 131. [Google Scholar] [CrossRef]
- Xiao, Z.; Dexin, L.; Yue, L.; Lulu, L.; Jie, Y. Synergistic Effects between Anionic and Amphoteric Surfactants on Promoting Spontaneous Imbibition in Ultra-Low Permeability Reservoirs: Study of Mechanism and Formula Construction. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 625, 126930. [Google Scholar] [CrossRef]
- Li, Z.; Wu, H.; Hu, Y.; Chen, X.; Yuan, Y.; Luo, Y.; Hou, J.; Bai, B.; Kang, W. Ultra-Low Interfacial Tension Biobased and Catanionic Surfactants for Low Permeability Reservoirs. Journal of Molecular Liquids 2020, 309, 113099. [Google Scholar] [CrossRef]
- Ludwig, M.; Geisler, R.; Prévost, S.; Von Klitzing, R. Shape and Structure Formation of Mixed Nonionic–Anionic Surfactant Micelles. Molecules 2021, 26, 4136. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.M.; Pitt, A.R. Rheology and Stability of Oil-in-Water Nanoemulsions Stabilised by Anionic Surfactant and Gelatin 1) Addition of Nonionic, Cationic and Ethoxylated-Cationic Co-Surfactants. Advances in Colloid and Interface Science 2008, 144, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Akanno, A.; Guzmán, E.; Fernández-Peña, L.; Ortega, F.; G. Rubio, R. Surfactant-Like Behavior for the Adsorption of Mixtures of a Polycation and Two Different Zwitterionic Surfactants at the Water/Vapor Interface. Molecules 2019, 24, 3442. [Google Scholar] [CrossRef] [PubMed]
- Gerola, A.P.; Costa, P.F.A.; Nome, F.; Quina, F. Micellization and Adsorption of Zwitterionic Surfactants at the Air/Water Interface. Current Opinion in Colloid & Interface Science 2017, 32, 48–5. [Google Scholar]
- Zhang, R.; Somasundaran, P. Advances in Adsorption of Surfactants and Their Mixtures at Solid/Solution Interfaces. Advances in Colloid and Interface Science 2006, 123–126, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Gerola, A.P.; Costa, P.F.A.; Quina, F.H.; Fiedler, H.D.; Nome, F. Zwitterionic Surfactants in Ion Binding and Catalysis. Current Opinion in Colloid & Interface Science 2017, 32, 39–47. [Google Scholar]
- Jurjevec, S.; Žagar, E.; Kovačič, S. Functional Macroporous Amphoteric Polyelectrolyte Monoliths with Tunable Structures and Properties through Emulsion-Templated Synthesis. Journal of Colloid and Interface Science 2020, 575, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.T.M.; Quina, F.H. Ion–Micelle Interactions and the Modeling of Reactivity in Micellar Solutions of Simple Zwitterionic Sulfobetaine Surfactants. Current Opinion in Colloid & Interface Science 2019, 44, 168–176. [Google Scholar]
- Nome, F.; Romsted, L. Reactivity in Colloidal Systems and at Interfaces. Current Opinion in Colloid & Interface Science 2013, 18, 1–2. [Google Scholar]
- Kamal, M.S.; Sultan, A.S.; Hussein, I.A. Screening of Amphoteric and Anionic Surfactants for cEOR Applications Using a Novel Approach. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2015, 476, 17–23. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, G.; Brewer, M.; Lv, Q.; Sudhölter, E.J.R. Comprehensive Review on Surfactant Adsorption on Mineral Surfaces in Chemical Enhanced Oil Recovery. Advances in Colloid and Interface Science 2021, 294, 102467. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.R.; Sharma, T. Rheological Analysis and EOR Potential of Surfactant Treated Single-Step Silica Nanofluid at High Temperature and Salinity. Journal of Petroleum Science and Engineering 2021, 196, 107704. [Google Scholar] [CrossRef]
- Lv, W.; Bazin, B.; Ma, D.; Liu, Q.; Han, D.; Wu, K. Static and Dynamic Adsorption of Anionic and Amphoteric Surfactants with and without the Presence of Alkali. Journal of Petroleum Science and Engineering 2011, 77, 209–218. [Google Scholar] [CrossRef]
- Llinares, R.; Ramírez, P.; Carmona, J.; Carrillo, F.; Munñoz, J. Formulation and Optimization of Emulsions Based on Bitter Fennel Essential Oil and EO/BO Block Copolymer Surfactant. Colloids & Surfaces A Physicochemical & Engineering Aspects 2017, 536, 142–147. [Google Scholar]
- Santos, J.; Trujillo-Cayado, L.A.; Calero, N.; Alfaro, M.C.; Muñoz, J. Development of Eco-Friendly Emulsions Produced by Microfluidization Technique. Journal of Industrial & Engineering Chemistry 2016, 36, 90–95. [Google Scholar]
- Suleimanov, B.A.; Ismailov, F.S.; Veliyev, E.F. Nanofluid for Enhanced Oil Recovery. Journal of Petroleum Science & Engineering 2011, 78, 431–437. [Google Scholar]
- Adibhatla, B.; Mohanty, K. Oil Recovery From Fractured Carbonates by Surfactant-Aided Gravity Drainage: Laboratory Experiments and Mechanistic Simulations. Spe Reservoir Evaluation & Engineering 2006, 11, 119–130. [Google Scholar]
- Raikos, V. Encapsulation of Vitamin E in Edible Orange Oil-in-Water Emulsion Beverages: Influence of Heating Temperature on Physicochemical Stability during Chilled Storage. Food Hydrocolloids 2017, 72, 155–162. [Google Scholar] [CrossRef]
- Domian, E.; Brynda-Kopytowska, A.; Oleksza, K. Rheological Properties and Physical Stability of o/w Emulsions Stabilized by OSA Starch with Trehalose. Food Hydrocolloids 2015, 44, 49–58. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple Light Scattering Measurement for Concentrated Emulsion and Suspension Instability Analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Esfandyari, H.; Moghani Rahimi, A.; Esmaeilzadeh, F.; Davarpanah, A.; Mohammadi, A.H. Amphoteric and Cationic Surfactants for Enhancing Oil Recovery from Carbonate Oil Reservoirs. Journal of Molecular Liquids 2021, 322, 114518. [Google Scholar] [CrossRef]
| Type of injected chemicals | Gas permeability (10 -3μm -2) |
Porosity (%) |
Water flooding recovery factor (%) |
Chemical flooding recovery factor (%) |
Total recovery factor (%) |
|---|---|---|---|---|---|
| 3:1 combination system | 109 | 18.1 | 64.00 | 17.33 | 81.33 |
| 3:2 combination system | 109 | 18.2 | 62.67 | 10.31 | 72.97 |
| 1:1 combination system | 109 | 18.15 | 64.13 | 3.57 | 67.7 |
| 3:1 combination system | 12 | 17.1 | 36.29 | 3.23 | 39.52 |
| 3:2 combination system | 12 | 17.5 | 34.68 | 7.26 | 41.94 |
| 1:1 combination system | 12 | 17.95 | 35.48 | 8.87 | 44.35 |
| Injection mode | Permeability (10 -3μm -2) |
Porosity (%) |
Water drive recovery ratio (%) |
Chemical flooding recovery factor (%) |
Total recovery factor (%) |
|---|---|---|---|---|---|
| Only inject a 3:1 combination system. | 180 | 23.4 | 63.1 | 20.34 | 83.45 |
| 18 | 15.3 | 9.63 | 2.04 | 11.67 | |
| Sequential injection 1 (inject the 0.17PV 3:1 combination system first., then inject the 0.33PV 1:1 combination system) | 180 | 22.9 | 65.45 | 15.95 | 81.4 |
| 18 | 15.2 | 14 | 5.45 | 19.45 | |
| Sequential injection 2 (inject the 0.33PV 3:1 combination system first., then inject the 0.17PV 1:1 combination system) | 180 | 23.6 | 62.91 | 19.21 | 82.12 |
| 18 | 15.1 | 13.64 | 9.09 | 22.73 | |
| Only inject a system 3 | 20 | 17.95 | 46.67 | 11.67 | 58.33 |
| 2 | 10.9 | 8.24 | 5.47 | 13.71 | |
| Sequential injection 1 (inject the 0.17PV 3:2 combination system first., then inject the 0.33PV 1:1 combination system) | 20 | 17.5 | 49.23 | 13.85 | 63.08 |
| 2 | 10.8 | 8.33 | 2.78 | 11.11 | |
| Sequential injection 2 (inject the 0.33PV 3:2 combination system first., then inject the 0.17PV 1:1 combination system.) | 20 | 17.65 | 48.33 | 8.33 | 56.67 |
| 2 | 10.2 | 8.72 | 1.18 | 9.89 |
| Na ++K + | Ca 2+ | Mg 2+ | Cl - | SO4 2- | HCO 3- | Total dissolved salinity |
|---|---|---|---|---|---|---|
| 53574.06 | 1766.168 | 919.1179 | 87071.7 | 1444.128 | 490.7045 | 145265.9 |
| Injection mode | Core permeability |
|---|---|
| 0.5PV 3:1 combination system | 109 × 10 -3μm -2 |
| 0.5PV 3:2 combination system | 109 × 10 -3μm -2 |
| 0.5PV 1:1 combination system | 109 × 10 -3μm -2 |
| 0.5PV 3:1 combination system | 12 × 10 -3μm -2 |
| 0.5PV 3:2 combination system | 12 × 10 -3μm -2 |
| 0.5PV 1:1 combination system | 12 × 10 -3μm -2 |
| Injection mode and system | Gas permeability |
|---|---|
| Only inject a 3:1 combination system. | 180 × 10 -3μm -2 |
| 18 × 10 -3μm -2 | |
| Sequential injection 1 (inject the 0.17PV 3:1 combination system first., then inject the 0.33PV 1:1 combination system) | 180 × 10 -3μm -2 |
| 18 × 10 -3μm -2 | |
| Sequential injection 2 (inject the 0.33PV 3:1 combination system first., then inject the 0.17PV 1:1 combination system) | 180 × 10 -3μm -2 |
| 18 × 10 -3μm -2 | |
| Only inject a 1:1 combination system | 20 × 10 -3μm -2 |
| 2 × 10 -3μm -2 | |
| Sequential injection 1 (inject the 0.17PV 3:2 combination system first., then inject the 0.33PV 1:1 combination system) | 20 × 10 -3μm -2 |
| 2 × 10 -3μm -2 | |
| Sequential injection 2 (inject the 0.33PV 3:2 combination system first., then inject the 0.17PV 1:1 combination system.) | 20 × 10 -3μm -2 |
| 2 × 10 -3μm -2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




