Submitted:
27 June 2024
Posted:
27 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Method
2.1. Identification of TRM Genes in Cucumber
2.2. Protein Length, Motif Composition and Gene Structure Analysis
2.3. Gene Duplication and Synteny Analysis
2.4. Transcriptome Analysis of CsTRM Genes in Friut
2.5. Transcriptome Analysis of CsTRMs in Response to Abiotic and Biotic Stresses
3. Result
3.1. Identification of CsTRM Genes Based on the Cucumber Pan-Genome
3.2. Analysis of Protein Length and Amino Acid Variations in the CsTRM Proteins
3.3. Synteny Analysis of CsTRM Genes
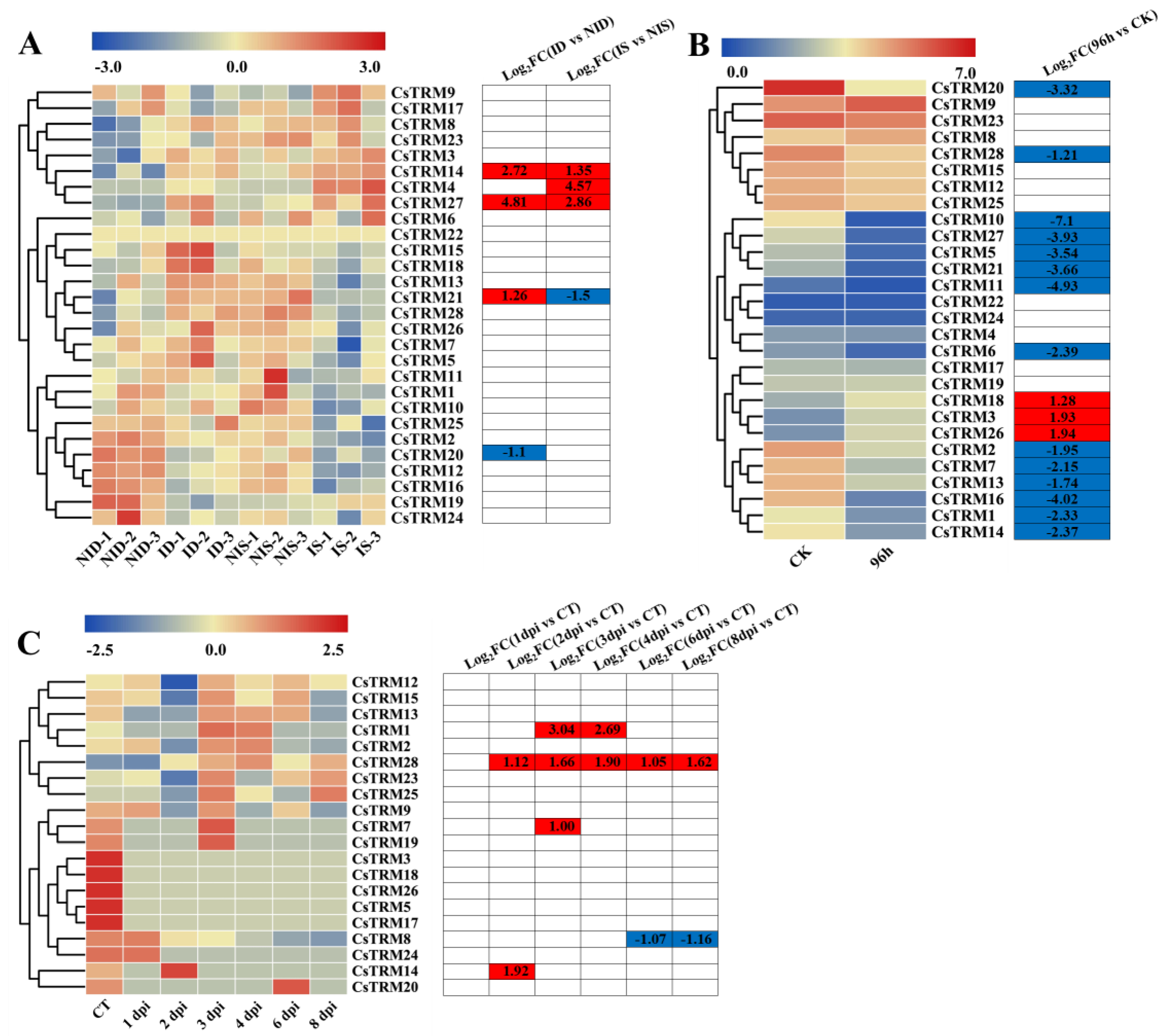
3.4. Expression Profiles of CsTRM Genes in the Fruit
3.5. Expression Profiles of CsTRM Genes under Abiotic and Biotic Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drevensek, S.; Goussot, M.; Duroc, Y.; Christodoulidou, A.; Steyaert, S.; Schaefer, E.; Duvernois, E.; Grandjean, O.; Vantard, M.; Bouchez, D.; Pastuglia, M. The Arabidopsis TRM1-TON1 interaction reveals a recruitment network common to plant cortical microtubule arrays and eukaryotic centrosomes. The Plant Cell 2012, 24, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Kim, G.T.; Kim, I.J.; Park, J.; Kwak, S.S.; Choi, G.; Chung, W.I. LONGIFOLIA1 and LONGIFOLIA2, two homologous genes, regulate longitudinal cell elongation in Arabidopsis. Development 2006, 133, 4305–4314. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.Q.; Ng, P.Q.; Shi, S.S.; Fan, D.; Li, J.; Zhao, J.; Wang, H.; David, R.; Mittal, P.; Do, T.; Bock, R.; Zhao, M.; Zhou, W.B.; Searle, I. Arabidopsis TRM5 encodes a nuclear-localised bifunctional tRNA guanine and inosine-N1-methyltransferase that is important for growth. PLoS ONE 2019, 14, e0225064. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jia, P.; Xin, P.; Chu, J.; Shi, D.Q.; Yang, W.C. The Arabidopsis TRM61/TRM6 complex is a bona fide tRNA N1-methyladenosine methyltransferase. Journal of experimental botany 2020, 71, 3024–3036. [Google Scholar] [CrossRef] [PubMed]
- Drevensek, S.; Goussot, M.; Duroc, Y.; Christodoulidou, A.; Steyaert, S.; Schaefer, E.; Duvernois, E.; Grandjean, O.; Vantard, M.; Bouchez, D.; Pastuglia, M. The Arabidopsis TRM1-TON1 interaction reveals a recruitment network common to plant cortical microtubule arrays and eukaryotic centrosomes. The Plant Cell 2012, 24, 178–191. [Google Scholar] [CrossRef]
- Camilleri, C.; Azimzadeh, J.; Pastuglia, M.; Bellini, C.; Grandjean, O.; Bouchez, D. The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. The Plant Cell 2002, 14, 833–845. [Google Scholar] [CrossRef]
- Azimzadeh, J.; Nacry, P.; Christodoulidou, A.; Drevensek, S.; Camilleri, C.; Amiour, N.; Parcy, F.; Pastuglia, M.; Bouchez, D. Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. The Plant Cell 2008, 20, 2146–2159. [Google Scholar] [CrossRef]
- Spinner, L.; Gadeyne, A.; Belcram, K.; Goussot, M.; Moison, M.; Duroc, Y.; Eeckhout, D.; De Winne, N.; Schaefer, E.; Van De Slijke, E.; Persiau, G.; Witters, E.; Gevaert, K.; Jaeger, G.D.; Bouchez, D.; Van Damme, D.; Pastuglia, M. A protein phosphatase 2A complex spatially controls plant cell division. Nature Communications 2013, 4, 1863. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.; Belcram, K.; Uyttewaal, M.; Duroc, Y.; Goussot, M.; Legland, D.; Laruelle, E.; Tauzia-Moreau, M.D.; Pastuglia, M.; Bouchez, D. The preprophase band of microtubules controls the robustness of division orientation in plants. Science 2017, 356, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Chen, B.Q.; Dang, X.; Zhu, L.L.; Rao, J.Q.; Ren, H.B.; Lin, C.T.; Qin, Y.; Lin, D.S. Arabidopsis IPGA1 is a microtubule-associated protein essential for cell expansion during petal morphogenesis. Journal of Experimental Botany 2019, 70, 5231–5243. [Google Scholar] [CrossRef]
- Van der Knaap, E.; Chakrabarti, M.; Chu, Y.H.; Clevenger, J.P.; Illa-Berenguer, E.; Huang, Z.J.; Keyhaninejad, N.; Mu, Q.; Sun, L.; Wang, Y.P.; Wu, S. What lies beyond the eye: the molecular mechanisms regulating tomato fruit weight and shape. Frontiers in Plant Science 2014, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, B.Y.; Keyhaninejad, N.; Rodríguez, G.R.; Kim, H.J.; Chakrabarti, M.; Illa-Berenguer, E.; Taitano, N.K.; Gonzalo, M.J.; Díaz, A.; Pan, Y.P.; Leisner, C.P.; Halterman, D.; Buell, C.R.; Weng, Y.Q.; Jansky, S.H.; van Eck, H.; Willemsen, J.; Monforte, A.J.; Meulia, T.; van der Knaap, E. A common genetic mechanism underlies morphological diversity in fruits and other plant organs. Nature Communications 2018, 9, 4734. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, M.D.; Wu, S.; Snouffer, A.; Wang, Y.P.; Van der Knaap, E. Plant organ shapes are regulated by protein interactions and associations with microtubules. Front Plant Science 2018, 9, 1766. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Q.; Keyhaninejad, N.; Taitano, N.; Sapkota, M.; Snouffer, A.; van der Knaap, E. A combinatorial TRM-OFP module bilaterally fine-tunes tomato fruit shape. New Phytologist 2023, 238, 2393–2409. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, B.; Keyhaninejad, N.; Rodríguez, G.R.; Kim, H.J.; Chakrabarti, M.; Illa-Berenguer, E.; Taitano, N.K.; Gonzalo, M.J.; Díaz, A.; Pan, Y.; Leisner, C.P.; Halterman, D.; Buell, C.R.; Weng, Y.; Jansky, S.H.; van Eck, H.; Willemsen, J.; Monforte, A.J.; Meulia, T.; van der Knaap, E. A common genetic mechanism underlies morphological diversity in fruits and other plant organs. Nature communications 2018, 9, 4734. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.Q.; Wang, S.S.; Wang, Y.; Chen, X.B.; Zhang, Y.; Gao, C.X.; Wang, F.; Huang, H.X.; Fu, X.D. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nature Genetics 2015, 47, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Xiong, G.S.; Hu, J.; Jiang, L.; Yu, H.; Xu, J.; Fang, Y.X.; Zeng, L.J.; Xu, E.; Xu, J.; Ye, W.J.; Meng, X.B.; Liu, R.F.; Chen, H.Q.; Jing, Y.H.; Wang, Y.H.; Zhu, X.D.; Li, J.Y.; Qian, Q. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nature Genetics 2015, 47, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Miao, J.; Gu, H.Y.; Peng, X.R.; Leburu, M.; Yuan, F.H.; Gu, H.W.; Gao, Y.; Tao, Y.J.; Zhu, J.Y.; Gong, Z.Y.; Yi, C.D.; Gu, M.H.; Yang, Z.F.; Liang, G.H. Natural Variations in SLG7 Regulate Grain Shape in Rice. Genetics 2015, 201, 1591–1599. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, X.F.; Sun, C.Z.; Song, X.F.; Li, X.L.; Cui, H.N.; Guo, J.Y.; Liu, L.; Ying, A.; Zhang, Z.Q.; Zhu, X.Y.; Yan, L.Y.; Zhang, X.L. CsTRM5 regulates fruit shape via mediating cell division direction and cell expansion in cucumber. Horticulture Research 2023, 10, uhad007. [Google Scholar] [CrossRef]
- Wade, R.H. On and around microtubules: an overview. Mol Biotechnol 2009, 43, 177–191. [Google Scholar] [CrossRef]
- Landrein, B.; Hamant, O. How mechanical stress controls microtubule behavior and morphogenesis in plants: history; experiments and revisited theories. Plant Journal 2013, 75, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Nick, P. Microtubules, signalling and abiotic stress. Plant Journal 2013, 75, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A.; Yan, A.; Krupinski, P.; Meyerowitz, E.M. Physical forces regulate plant development and morphogenesis. Curr Biol 2014, 24, R475–R483. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W. Regulation of developmental and environmental signaling by interaction between microtubules and membranes in plant cells. Protein Cell 2016, 7, 81–88. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, Q.; Sun, Y.; Li, Y. Histone H2B monoubiquitination regulates salt stress-induced microtubule depolymerization in Arabidopsis. Plant Cell Environ 2017, 40, 1512–1530. [Google Scholar] [CrossRef]
- Yang, P.Z.; Jin, J.W.; Zhang, J.R.; Wang, D.; Bai, X.C.; Xie, W.F.; Hu, T.M.; Zhao, X.; Mao, T.L.; Qin, T. MDP25 mediates the fine-tuning of microtubule organization in response to salt stress. Journal of integrative plant biology 2022, 64, 1181–1195. [Google Scholar] [CrossRef]
- McNally, F.J.; Roll-Mecak, A. Microtubule-severing enzymes: From cellular functions to molecular mechanism. Journal of Cell Biology 2018, 217, 4057–4069. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.R.; Xu, Z.J.; Zang, J.Z.; Bürstenbinder, K.; Wang, P.W. The morphological diversity of plant organs: manipulating the organization of microtubules may do the trick. Frontiers in Cell and Developmental Biology 2021, 9, 691. [Google Scholar] [CrossRef]
- Bao, Z.R.; Guo, Y.; Deng, Y.L.; Zang, J.Z.; Zhang, J.H.; Deng, Y.T.; Ouyang, B.; Qu, X.L.; Bürstenbinder, K.; Wang, P.W. Microtubule-associated protein SlMAP70 interacts with IQ67-domain protein SlIQD21a to regulate fruit shape in tomato. The Plant Cell 2023, 35, 4266–4283. [Google Scholar] [CrossRef]
- Gantet, P.; Masson, F.; Domergue, O.; Marquis-Mention, M.; Bauw, G.; Inze, D.; Rossignol, M.; de la Serve, B.T.; et al. Cloning of a cDNA encoding a developmentally regulated 22 kDa polypeptide from tobacco leaf plasma membrane. Biochemistry and molecular biology international 1996, 40, 469–477. [Google Scholar] [CrossRef]
- Nagasaki-Takeuchi, N.; Miyano, M.; Maeshima, M. A plasma membrane-associated protein of Arabidopsis thaliana AtPCaP1 binds copper ions and changes its higher order structure. J Biochem 2008, 144, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Takada, N.; Kobayashi, A.; Takahashi, H.; Kamiya, T.; Kinoshita, T.; Maeshima, M. Plasma Membrane-Associated Ca2+-Binding Protein PCaP1 is Involved in Root Hydrotropism of Arabidopsis thaliana. Plant Cell Physiol 2019, 60, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, M.; Marti, L.; Ferrari, S.; Tanaka-Takada, N.; Maeshima, M.; Ott, T.; De Lorenzo, G.; Mattei, B. The plasma membrane-associated Ca2+ -binding protein, PCaP1, is required for oligogalacturonide and flagellin-induced priming and immunity. Plant Cell Environment 2021, 44, 3078–3093. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Theerawitaya, C.; Kageyama, H.; Cha-Um, S.; Takabe, T. Expression of developmentally regulated plasma membrane polypeptide (DREPP2) in rice root tip and interaction with Ca(2+)/CaM complex and microtubule. Protoplasma 2015, 252, 1519–1527. [Google Scholar] [CrossRef]
- Su, C.; Klein, M.L.; Hernández-Reyes, C.; Batzenschlager, M.; Ditengou, F.A.; Lace, B.; Keller, J.; Delaux, P.M.; Ott, T. The Medicago truncatula DREPP Protein Triggers Microtubule Fragmentation in Membrane Nanodomains during Symbiotic Infections. The Plant Cell 2020, 32, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; He, K.; Higaki, T.; Wang, X.; Mao, T. Ethylene Signaling Modulates Cortical Microtubule Reassembly in Response to Salt Stress. Plant Physiol 2018, 176, 2071–2081. [Google Scholar] [CrossRef]
- Yang, J.; An, B.; Luo, H.; He, C.; Wang, Q. AtKATANIN1 Modulates Microtubule Depolymerization and Reorganization in Response to Salt Stress in Arabidopsis. Int J Mol Sci 2019, 21, 138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jeevaraj, T.; Yunus, M.H.; Chakraborty, S.; Chakraborty, N. The plant cytoskeleton takes center stage in abiotic stress responses and resilience. Plant Cell Environ 2023, 46, 5–22. [Google Scholar] [CrossRef]
- Smirnova, E.A.; Bajer, A.S. Microtubule converging centers and reorganization of the interphase cytoskeleton and the mitotic spindle in higher plant Haemanthus. Cell Motility and the Cytoskeleton 1994, 27, 219–233. [Google Scholar] [CrossRef]
- Parrotta, L.; Faleri, C.; Cresti, M.; Cai, G. Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta 2016, 243, 43–63. [Google Scholar] [CrossRef]
- Parveen, S.; Rahman, A. Actin isovariant ACT7 modulates root thermomor-phogenesis by altering intracellular auxin homeostasis. International Journal of Molecular Sciences 2021, 22, 7749. [Google Scholar] [CrossRef] [PubMed]
- Pressman, E.; Harel, D.; Zamski, E.; Shaked, R.; Althan, L.; Rosenfeld, K.; Firon, N. The effect of high temperatures on the expression and activity of sucrose-cleaving enzymes during tomato (Lycopersicon esculentum) anther development. The Journal of Horticultural Science and Biotechnology 2006, 81, 341–348. [Google Scholar] [CrossRef]
- Zheng, Y.; Anderson, S.; Zhang, Y.; Garavito, R.M. The structure of sucrose synthase-1 from Arabidopsis thaliana and its functional implications. Journal of Biological Chemistry 2011, 286, 36108–36118. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Jayakodi, M.; Stein, N.; Mascher, M. Plant pangenomes for crop improvement; biodiversity and evolution. Nature reviews Genetics 2024, 10, 1038. [Google Scholar] [CrossRef]
- Wang, C.; Han, J.; Wang, T.; Chen, C.; Liu, J.; Xu, Z.; Zhang, Q.; Wang, L.; Ren, Z. Pan-Genome-Wide Identification and Transcriptome-Wide Analysis of DREB Genes That Respond to Biotic and Abiotic Stresses in Cucumber. Agriculture 2022, 12, 1879. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; Kissinger, J.C.; Paterson, A.H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Che, G.; Gu, R.; Zhao, J.; Liu, X.; Song, X.; Zi, H.; Cheng, Z.; Shen, J.; Wang, Z.; Liu, R.; Yan, L.; Weng, Y.; Zhang, X. Gene regulatory network controlling carpel number variation in cucumber. Development 2020, 147, dev184788. [Google Scholar] [CrossRef]
- Jing, L.; Yan, S.; Yang, W.; Li, Y.; Xia, M.; Chen, Z.; Wang, Q.; Yan, L.; Song, X.; Liu, R.; Zhang, X. Transcriptomic analysis reveals the roles of microtubule-related genes and transcription factors in fruit length regulation in cucumber (Cucumis sativus L.). Sci. Rep. 2015, 5, 8031. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, L.; Che, G.; Pan, Y.; Li, Y.; Hou, Y.; Zhao, W.; Zhong, Y.; Ding, L.; Yan, S.; Sun, C.; Liu, R.; Yan, L.; Wu, T.; Li, X.; Weng, Y.; Zhang, X. A Functional Allele of CsFUL1 Regulates Fruit Length through Repressing CsSUP and Inhibiting Auxin Transport in Cucumber. The Plant cell 2019, 31, 1289–1307. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, J.; Liang, Y.; Liu, J.; Jia, J. Transcriptomic dynamics provide an insight into the mechanism for silicon-mediated alleviation of salt stress in cucumber plants. Ecotoxicol Environ Saf 2019, 174, 245–254. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Tang, R.; Wang, L.; Chen, C.; Ren, Z. Genome-Wide identification and expression analysis of Hsf and Hsp gene families in cucumber (Cucumis sativus L.). Plant Growth Regul 2021, 95, 223–239. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, X.; Shi, Y.; Qi, X.; Chen, X. Elucidation of the molecular responses of a cucumber segment substitution line carrying Pm5.1 and its recurrent parent triggered by powdery mildew by comparative transcriptome profiling. BMC Genom 2017, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Chen, N.; Liu, T.; Zhu, J.; Wang, J.; He, X.; Jin, Y. Large-Scale Transcriptome Analysis of Cucumber and Botrytis cinerea during Infection. PLoS One 2015, 10, e0142221. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.N.; Savory, E.A.; Vaillancourt, B.; Childs, K.L.; Hamilton, J.P.; Day, B.; Buell, C.R. Expression Profiling of Cucumis sativus in Response to Infection by Pseudoperonospora cubensis. PLoS ONE 2012, 7, e34954. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Chai, S.; Yang, Z.; Zhang, Q.; Xin, H.; Xu, Y.; Lin, S.; Chen, X.; Yao, Z.; Yang, Q.; Fei, Z.; Huang, S.; Zhang, Z. Graph-based pan-genome reveals structural and sequence variations related to agronomic traits and domestication in cucumber. Nat Commun 2022, 13, 682. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, Y.J.; Zhang, Q.X.; Wang, L.N.; Ren, Z.H. Identification and Analysis on TRM Family in Cucumber. Journal of Shandong Agricultural University 2021, 52, 358–363. [Google Scholar]
- Yin, S.; Zhao, L.; Liu, J.; Sun, Y.; Li, B.; Wang, L.; Ren, Z.; Chen, C. Pan-genome Analysis of WOX Gene Family and Function Exploration of CsWOX9 in Cucumber. Int. J. Mol. Sci. 2023, 24, 17568. [Google Scholar] [CrossRef]
- Bai, S.L.; Peng, Y.-B.; Cui, J.X.; Gu, H.T.; Xu, L.Y.; Li, Y.Q.; Xu, Z.H.; Bai, S.N. Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 2004, 220, 230–240. [Google Scholar] [CrossRef]
- Ma, H.; Liu, M. The microtubule cytoskeleton acts as a sensor for stress response signaling in plants. Mol Biol Rep 2019, 46, 5603–5608. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Gu, C.; Wang, S.; Xue, Y.; Wang, Z.; Han, L.; Song, W.; Liu, X.; Zhang, J.; Li, M.; Li, C.; Wang, L.; Zhang, X.; Zhou, Z. The exocyst subunit CsExo70B promotes both fruit length and disease resistance via regulating receptor kinase abundance at plasma membrane in cucumber. Plant Biotechnol J 2024, 22, 347–362. [Google Scholar] [CrossRef] [PubMed]
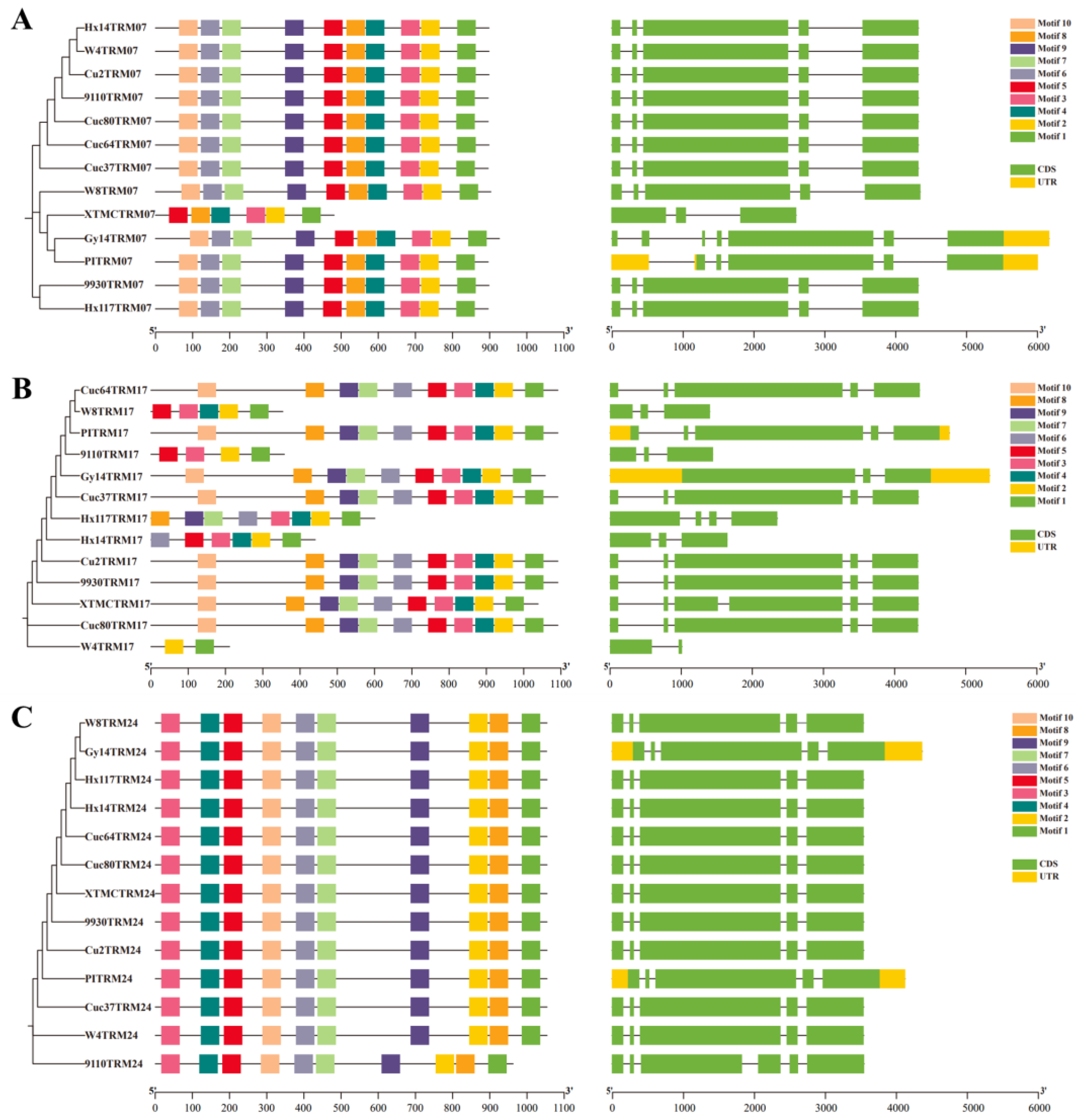
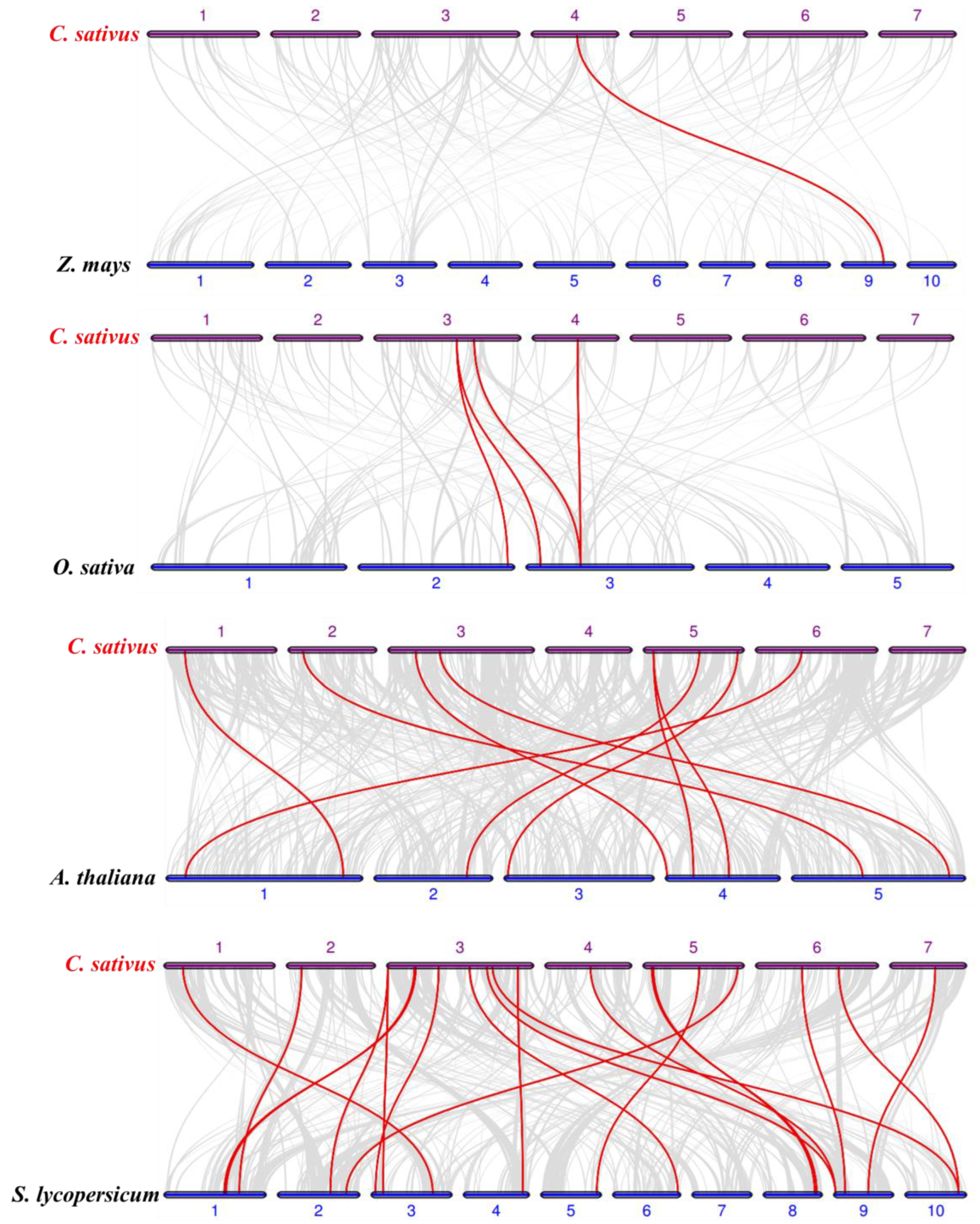
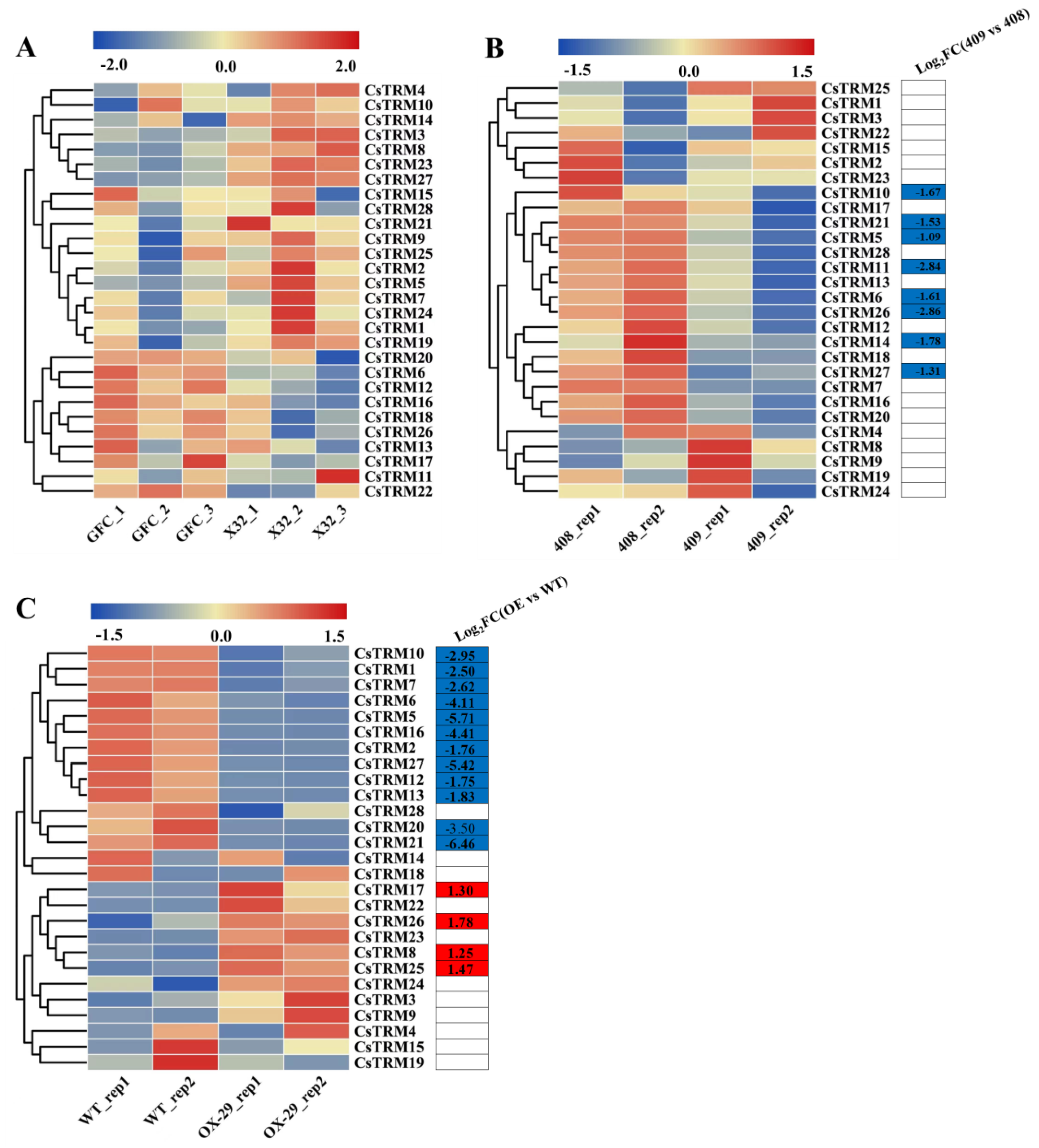
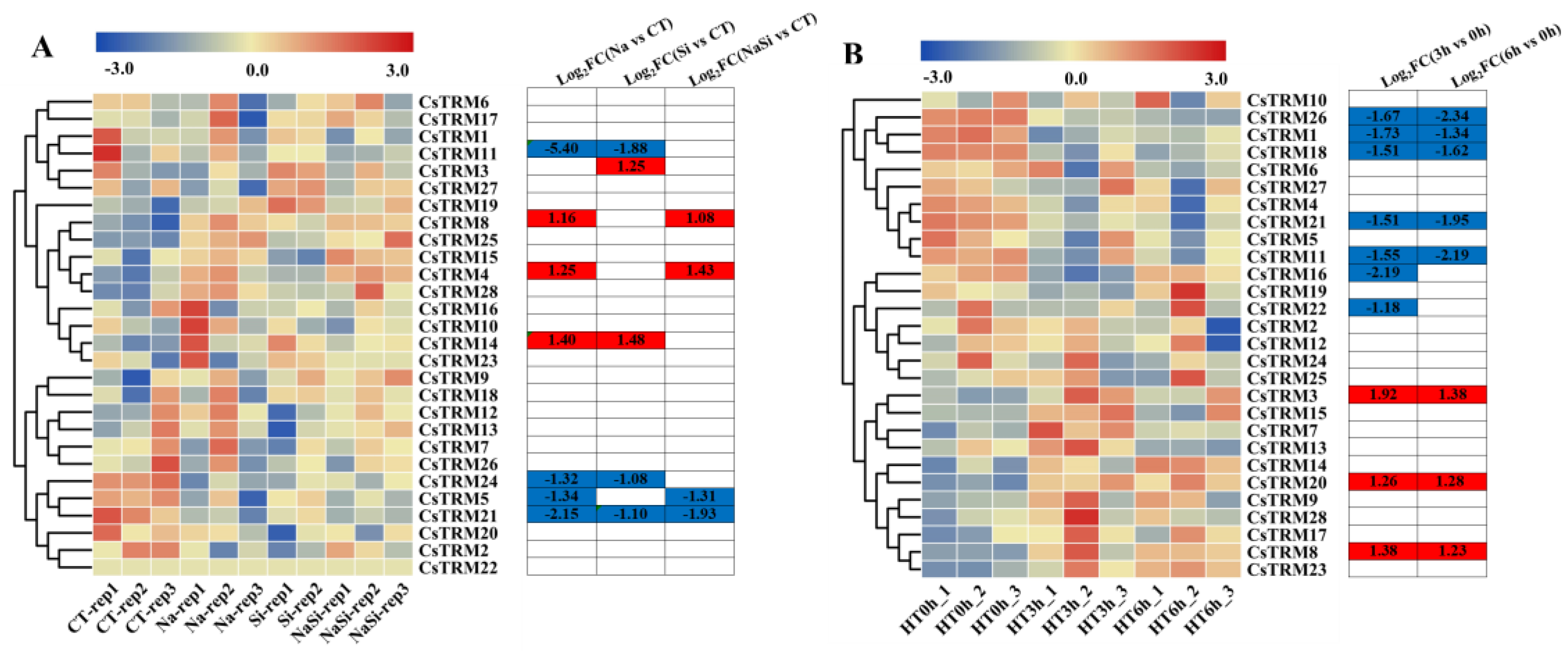
| Gene name | 9930 | XTMC | Cu2 | Cuc80 | PI | Cuc64 | W4 | W8 | Hx14 | Hx117 | Cuc37 | Gy14 | 9110gt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CsTRM01 | 1G003000 | 1G002960 | 1G003190 | 1G003020 | 1G02950 | 1G003020 | 1G003020 | 1G003050 | 1G008150 | 1G003070 | 1G003050 | 1G002970 | 1G003150 |
| CsTRM02 | 1G006080 | 1G006040 | 1G006230 | 1G006090 | 1G06220 | 1G006110 | 1G006160 | 1G006130 | 1G012280 | 1G009330 | 1G006130 | 1G005930 | 1G006390 |
| CsTRM03 | 1G024390 | 1G023100 | 1G019380 | 1G034690 | 1G022250 | 1G022640 | 1G033380 | 1G035450 | 1G025870 | 1G016710 | 1G021460 | ||
| CsTRM04 | 1G036240 | 1G038300 | 1G031670 | 1G23930 | 1G045200 |
1G039460 1G050980 |
1G048780 | 1G052690 | 1G036240 | 1G023410 | 1G034790 | ||
| CsTRM05 | 2G002210 | 2G001130 | 2G002170 | 2G01120 | 2G001150 | 2G001150 | 2G002120 | 2G002150 | 2G001120 | 2G001120 | 2G002200 | ||
| CsTRM06 | 2G006910 | 2G004780 | 2G004780 | 2G005680 | 2G04550 | 2G004690 | 2G004760 | 2G005690 | 2G006680 | 2G005730 | 2G004660 | 2G004680 | 2G005790 |
| CsTRM07 | 2G013800 | 2G013420 | 2G014430 | 2G016150 | 2G11310 | 2G012220 | 2G015170 | 2G022290 | 2G022190 | 2G018160 | 2G012230 | 2G011350 | 2G015370 |
| CsTRM08 | 3G000320 | 3G000290 | 3G000270 | 3G00310 | 3G000310 | 3G000300 | 3G000290 | 3G000310 | 3G000300 | 3G000310 | 3G000260 | 3G000300 | |
| CsTRM09 | 3G008900 | 3G014120 | 3G011330 | 3G009320 | 3G08770 | 3G009130 | 3G011320 | 3G009390 | 3G013030 | 3G018440 | 3G009280 | 3G008870 | 3G011230 |
| CsTRM10 | 3G009320 | 3G014570 | 3G09200 | 3G009570 | 3G011790 | 3G009840 | 3G013470 | 3G018890 | 3G009740 | 3G009280 | 3G011660 | ||
| CsTRM11 | 3G016640 | 3G023990 | 3G019120 | 3G016980 | 3G16440 | 3G027380 | 3G019160 | 3G017460 | 3G023810 | 3G029290 | 3G017050 | 3G016550 | 3G018960 |
| CsTRM12 | 3G020250 | 3G028160 | 3G024300 | 3G021230 | 3G20290 | 3G031530 | 3G023320 | 3G021650 | 3G030910 | 3G038450 | 3G021080 | 3G020040 | 3G025120 |
| CsTRM13 | 3G028590 | 3G044970 | 3G034490 | 3G039640 | 3G27110 | 3G050790 | 3G034230 | 3G032590 | 3G049730 | 3G053340 | 3G043310 | 3G025270 | 3G034790 |
| CsTRM14 | 3G033690 | 3G052230 | 3G039760 | 3G045880 | 3G31210 | 3G055180 | 3G039400 | 3G038880 | 3G057040 | 3G059810 | 3G049600 | 3G029050 | 3G041170 |
| CsTRM15 | 3G035160 | 3G053700 | 3G041160 | 3G047320 | 3G32570 | 3G056620 | 3G040870 | 3G040290 | 3G058490 | 3G061290 | 3G050990 | 3G030380 | 3G042680 |
| CsTRM16 | 3G036950 | 3G056500 | 3G043950 | 3G049050 | 3G34290 | 3G032070 | 3G044470 | ||||||
| CsTRM17 | 3G045060 | 3G067760 | 3G055880 | 3G057270 | 3G42630 | 3G066680 | 3G051990 | 3G050610 | 3G070610 | 3G071560 | 3G061110 | 3G040150 | 3G052920 |
| CsTRM18 | 4G024630 | 4G030170 | 4G024030 | 4G078840 | 4G14290 | 4G027840 | 4G018900 | 4G021930 | 4G026900 | 4G030090 | 4G084710 | 4G013800 | 4G026010 |
| CsTRM19 | 4G031780 | 4G042910 | 4G034540 | 4G090510 | 4G21450 | 4G044030 | 4G027440 | 4G033570 | 4G040430 | 4G039790 | 4G095540 | 4G020000 | 4G035410 |
| CsTRM20 | 5G002760 | 5G003630 | 5G003640 | 5G002610 | 5G05360 | 5G003650 | 5G002590 | 5G005490 | 5G006540 | 5G003570 | 5G002660 | 5G003770 | |
| CsTRM21 | 5G003260 | 5G004130 | 5G004140 | 5G003110 | 5G05880 | 5G003020 | 5G003100 | 5G004990 | 5G007040 | 5G004090 | 5G003170 | 5G003160 | 5G004310 |
| CsTRM22 | 5G005590 | 5G007580 | 5G007530 | 5G005560 | 5G08200 | 5G000650 | 5G006510 | 5G001620 | 5G009400 | 5G009680 | 5G005630 | 5G005580 | |
| CsTRM23 | 5G026190 | 5G042130 | 5G041190 | 5G050680 | 5G17200 | 5G021470 | 5G028290 | 5G024190 | 5G042910 | 5G054980 | 5G043620 | 5G016900 | 5G034200 |
| CsTRM24 | 5G038540 | 5G060730 | 5G054610 | 5G063070 | 5G29400 | 5G043920 | 5G040890 | 5G049340 | 5G063180 | 5G067580 | 5G056820 | 5G028860 | 5G046740 |
| CsTRM25 | 6G016870 | 6G024320 | 6G018060 | 6G025320 | 6G14470 | 6G015450 | 6G015250 | 6G019390 | 6G025200 | 6G018200 | 6G015300 | 6G014340 | 6G017280 |
| CsTRM26 | 6G022550 | 6G035270 | 6G023850 | 6G053560 | 6G17180 | 6G022100 | 6G019950 | 6G023190 | 6G032860 | 6G030040 | 6G019540 | 6G016650 | 6G023980 |
| CsTRM27 | 6G040450 | 6G052040 | 6G035430 | 6G079450 | 6G25260 | 6G035100 | 6G032700 | 6G033070 | 6G052400 | 6G045570 | 6G036810 | 6G024690 | 6G035970 |
| CsTRM28 | 7G025430 | 7G031600 | 7G024250 | 7G035470 | 7G13640 | 7G025890 | 7G021920 | 7G034950 | 7G031600 | 7G031470 | 7G037050 | 7G012470 | 7G023340 |
| CsTRM29 | UnG00530 |
| Protein number | 9930 | XTMC | Cu2 | Cuc80 | PI | Cuc64 | W4 | W8 | Hx14 | Hx117 | Cuc37 | Gy14 | 9110gt |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CsTRM01 | 1048 | 1048 | 1048 | 1048 | 1048 | 1048 | 1043 | 1048 | 1048 | 1048 | 1048 | 1063 | 1048 |
| CsTRM02 | 1040 | 1067 | 1067 | 1067 | 1067 | 1067 | 1067 | 1067 | 1067 | 1067 | 1067 | 1067 | 1067 |
| CsTRM03 | 780 | 788 | 788 | 788 | 781 | 781 | 781 | 781 | 781 | 788 | 781 | ||
| CsTRM04 | 402 | 402 | 402 | 402 | 402 | 402/402 | 402 | 402 | 402 | 402 | 402 | ||
| CsTRM05 | 776 | 803 | 803 | 803 | 803 | 803 | 803 | 803 | 803 | 803 | 803 | 803 | 803 |
| CsTRM06 | 722 | 722 | 722 | 722 | 722 | 722 | 722 | 750 | 722 | 722 | 722 | ||
| CsTRM07 | 893 | 478 | 893 | 891 | 891 | 893 | 893 | 899 | 893 | 891 | 891 | 922 | 891 |
| CsTRM08 | 893 | 879 | 879 | 893 | 879 | 879 | 879 | 893 | 879 | 879 | 904 | 879 | |
| CsTRM09 | 930 | 933 | 933 | 933 | 932 | 933 | 933 | 933 | 933 | 933 | 933 | 932 | 933 |
| CsTRM10 | 346 | 346 | 344 | 344 | 344 | 344 | 346 | 344 | 346 | 305 | 346 | ||
| CsTRM11 | 616 | 616 | 616 | 616 | 616 | 616 | 616 | 616 | 616 | 616 | 616 | 616 | 616 |
| CsTRM12 | 953 | 953 | 954 | 954 | 953 | 953 | 953 | 953 | 953 | 952 | 953 | 954 | 953 |
| CsTRM13 | 963 | 963 | 963 | 963 | 963 | 963 | 963 | 963 | 963 | 963 | 963 | 927 | 963 |
| CsTRM14 | 353 | 353 | 353 | 353 | 353 | 353 | 353 | 353 | 353 | 353 | 353 | 353 | 353 |
| CsTRM15 | 888 | 888 | 888 | 888 | 888 | 888 | 888 | 888 | 888 | 888 | 888 | 888 | 888 |
| CsTRM16 | 472 | 472 | 550 | 550 | 472 | 472 | 550 | ||||||
| CsTRM17 | 1091 | 1038 | 1091 | 1091 | 1091 | 1091 | 210 | 353 | 440 | 600 | 1091 | 1058 | 357 |
| CsTRM18 | 961 | 961 | 961 | 961 | 961 | 922 | 961 | 961 | 961 | 961 | 961 | 961 | 961 |
| CsTRM19 | 903 | 903 | 987 | 987 | 906 | 987 | 906 | 906 | 906 | 906 | 906 | 906 | 906 |
| CsTRM20 | 785 | 785 | 785 | 785 | 781 | 781 | 781 | 781 | 785 | 785 | 745 | 785 | |
| CsTRM21 | 476 | 476 | 476 | 476 | 476 | 476 | 476 | 476 | 476 | 476 | 476 | 476 | 476 |
| CsTRM22 | 495 | 495 | 495 | 495 | 495 | 495 | 495 | 495 | 495 | 495 | 495 | 449 | |
| CsTRM23 | 794 | 794 | 794 | 848 | 795 | 795 | 794 | 795 | 794 | 794 | 795 | 794 | 736 |
| CsTRM24 | 1049 | 1049 | 1049 | 1049 | 1049 | 1049 | 1049 | 1049 | 1049 | 1049 | 1049 | 1049 | 958 |
| CsTRM25 | 1011 | 1011 | 1011 | 1011 | 902 | 959 | 1009 | 1011 | 1022 | 1009 | 1011 | 940 | 1011 |
| CsTRM26 | 936 | 936 | 936 | 936 | 936 | 936 | 938 | 936 | 936 | 936 | 936 | 936 | 936 |
| CsTRM27 | 505 | 505 | 505 | 505 | 505 | 505 | 505 | 736 | 505 | 473 | 505 | 505 | 505 |
| CsTRM28 | 960 | 960 | 960 | 959 | 959 | 994 | 995 | 1047 | 895 | 995 | 995 | 976 | 978 |
| CsTRM29 | 788 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





