1. Introduction
Decline of fossil fuels and soil degradation are a pressing issue, impacting the livelihoods of millions, especially those living in developing countries, and it contributes to global food insecurity, biodiversity loss, and climate change [
1]. Multi-cropping enables crop producers to obtain more yields throughout the cropping season, which generates higher incomes for the marketing of production and the provision of food for humanity [
2,
3,
4,
5].
In recent years, maize production has increased particularly due to its use for biofuel production [
6]. Hemp is often grown not only for fiber, oil, and seeds but also for its high biomass yields that can be used for energy production [
7]. Several studies have been carried out to investigate the potential for fuel production from faba bean residual biomass due to its high biomass productivity of between 3.7 and 5.7 t ha
-1 [
8]. Faba beans are particularly important for maintaining the sustainability of agricultural systems, as they fix atmospheric nitrogen very efficiently, increasing soil fertility and protecting it from erosion and degradation [
9]. Harvesting faba bean, hemp, and maize seeds or biomass separately and removing the harvesting residues from the field leads to a rapid deterioration of soil properties, such as a decrease in humus and nutrients, a deterioration in soil structure and bioactivity, and an increase in hardness [
10]. This sets the stage for soil degradation. Studies have shown that growing multi-crops improves soil properties [
11,
12]. Multi-crops stabilize and restore soil fertility by reducing nutrient leaching into deeper soil layers and protecting soil from wind and water erosion [
13].
Soil aggregate-size distribution (structure) is one of the mains soils health indicators, and depends on the dynamic interactions between plants, microorganisms, and soil components (e.g., organic matter). Here, plant roots are the key factor in all size classes of soil aggregates. Roots also change the pore size distribution and pore geometry of the soil, thereby altering the hydraulic properties of the soil. The type and extent of structural effects of roots vary depending on the type of rooting, timing (alive or decomposed), environmental conditions (e.g. granulometric composition, moisture, temperature), and plant family [
14]. Exactly multi-crops grow higher root biomass compared to single-crops. This is due to interspecific synergy between plants [
15]. The roots of multi-crops are located at different soil depths and take up water and nutrients from different parts of the soil. The sorption capacity of roots varies between different crops: some plants are more able to provide water when growing together in agrocenosis, while others are more able to provide specific nutrients, such as nitrogen for faba bean crops [
16,
17]. The faba bean root system establishes a symbiosis with specific
Rhizobium bacteria, and the resulting biological nitrogen fixation is associated with a reduced fertilizer requirement in arable soils and increased soil biological activity [
18,
19]. The nitrogen content that can be fixed by faba bean depends mainly on the variety, local farming practices (e.g. nitrogen and phosphorus fertilization), soil properties, and symbiont content [
20,
21].
Multi-crops affect the whole soil biota by increasing the abundance, diversity, and activity of soil microorganisms [
22]. Generally, soil nutrient content, enzyme activity, and microbial biomass are higher in multi-cropping than in single-crops [
23]. Song et al. [
24] found that plant diversity is a key determinant affecting soil microbial biomass and activity. Hemp has been observed to improve soil quality through its ability of heavy metal phytoremediation [
25,
26,
27]. In addition, hemp can reduce greenhouse gas emissions by absorbing CO
2 [
28]. This may have important practical implications for sustainable and healthy soil use through the inclusion of hemp in the composition of multi-crops.
In Lithuania, faba bean (Vicia faba L.), hemp (Cannabis sativa L.), maize (Zea mays L.), and sugar beet (Beta vulgaris L.) have the highest biomass productivity, nutritional and energy potential and have a good theoretical potential for growing together. There is little practical or scientific data on the mixed cultivation of maize, hemp, and faba bean in a multi-crop, the biomass produced, and its use for food, feed, and energy. Also, there is little information about the effects of such multi-cropping on soil properties. The aim of this study was to assess the impact of single-crops and multi-crops on soil agrophysical properties, agrochemical parameters, and enzyme activity.
2. Materials and Methods
2.1. Site Description
The stationary field experiment was conducted in 2020–2022 at the Experimental Station of Vytautas Magnus University, Agriculture Academy (coordinates 54°53′7.5″N 23°50′18.11″E). The soil of the experimental field was a deeper gleyic saturated loam (45.6% sand, 41.7% silt, 12.7% clay) Planosol (
Endohypogleyic-Eutric Planosol-Ple-gln-w) [
29]. Soil pH
HCl varies from 7.3 to 7.8, total nitrogen content was 0.08–0.13%, humus was from 1.5 to 1.7%, available potassium was from 97 to 118 mg·kg
-1, available phosphorus was from 189 to 280 mg·kg
-1, available sulfur was from 1.2 to 2.6 mg·kg
-1, and available magnesium varies from 436 to 790 mg·kg
-1.
Lithuania is the country with surplus precipitation rates, however during las 156 years the precipitation rates became uneven with plenty short or long drought periods. For example, in 2020, the lack of moisture after sowing stopped seeds sprouting and negatively affected crops growth and development (
Table 1). In 2021, the meteorological conditions were suitable for crop germination. This allowed a strong root system to form properly in the initial stages of crop growth. However, the distribution of moisture was uneven. The lack of moisture and the high temperatures in July retarded crops biomass development. In 2022, as in 2020, there was a lack of moisture till crops germination. However, sufficient precipitation rates and suitable air temperatures in later stages helped to compensate for the initial lack of moisture.
To summarize, the precipitation rates during May-July were closer to long-term average. The riskiest periods of the season were April and August. In those months plants felt lack or surplus effects of precipitation, uneven distribution of precipitation had a negative impact on both the crop productivity indices and the soil physical properties.
2.2. Experimental Design and Agricultural Practice
Maize (
Zea mays L.), hemp (
Cannabis sativa L.) and faba bean (
Vicia faba L.) were sown in the experimental plots according to a predetermined sowing scheme as single, binary and ternary crops. A total of 21 plots were investigated in the experiment and the initial size of the plots was 8 m
2.
Table 2 shows experimental treatments and their abbreviations.
In the spring, when the soil reached the right moisture content, it was cultivated to a depth of 3–4 cm with a germinator-type cultivator. After the weeds had germinated in abundance, the interrows mellowed twice (
Table 3).
Synthetic pesticides were not used in the agrotechnics of the experiment.
2.3. Methods and Analysis
Samples for the soil agrochemical properties tests were taken in each year of the experiment after harvest. In each experimental plot, soil samples from 0–25 cm layers were taken from at least 10 spots with a Nekrasov auger. An average sample was made, in which the levels of the main macro-elements (N, P, K, Mg) and soil pH were determined based on laboratory analyses. The analyses were carried out at the Agrochemical Research Laboratory of the Lithuanian Research Centre for Agriculture and Forestry in Kaunas.
Samples (~300 g of soil) for soil structure and its stability were taken with a spade after sowing the crop, before interrow mellowing, and at the end of the faba bean growing season, from 0–25 cm layers in at least 4 spots in each experimental plot. Average samples were formed. The soil was dried in the laboratory. Soil samples of 200 g each were sieved for 2 min with a sieving amplitude of 60%. A Retsch sieving machine (Retsch Lab Equipment, VERDER Group, The Netherlands) and a set of sieves were used to determine the soil structure. The stability of the soil aggregates was determined by wet sieving with the Retsch wet sieving apparatus only on a previously dry-sieved soil fraction of 1–2 mm. The results of the tests are expressed as a percentage.
Soil samples for soil enzyme activity tests were taken after harvest from at least 10 spots in each experimental plot with a soil auger at a depth of 0–25 cm. Samples were dried in laboratory conditions. The activity of soil hydrolases (urease and sucrase) was determined: urease—according to the methods of Hofmann and Schmidt, saccharase—according to the methods of Hofmann and Seegerer, modified by A. I. Chunderova [
30]. The studies were carried out in the Laboratory of Food Raw Materials, Agronomic, and Zootechnical Research of Vytautas Magnus University Agriculture Academy.
For the statistical evaluation of data, a one-factor analysis of variance was used. The limits of significant difference for the probability levels R05 and R01 were determined by the computer program ANOVA according to the F criterion. Correlation and regression analyses were performed using STAT and SIGMA PLOT, and calculations were carried out at Vytautas Magnus University Agriculture Academy.
3. Results
3.1. Changes in Soil Aggregate-Size Distribution and Its Stability
Structured soil should contain at least 50% macroaggregates and no more than 5% microaggregates. The macrostructure is the most suitable for plants to grow, because the oxygen and water regime is optimal in such soil [
31].
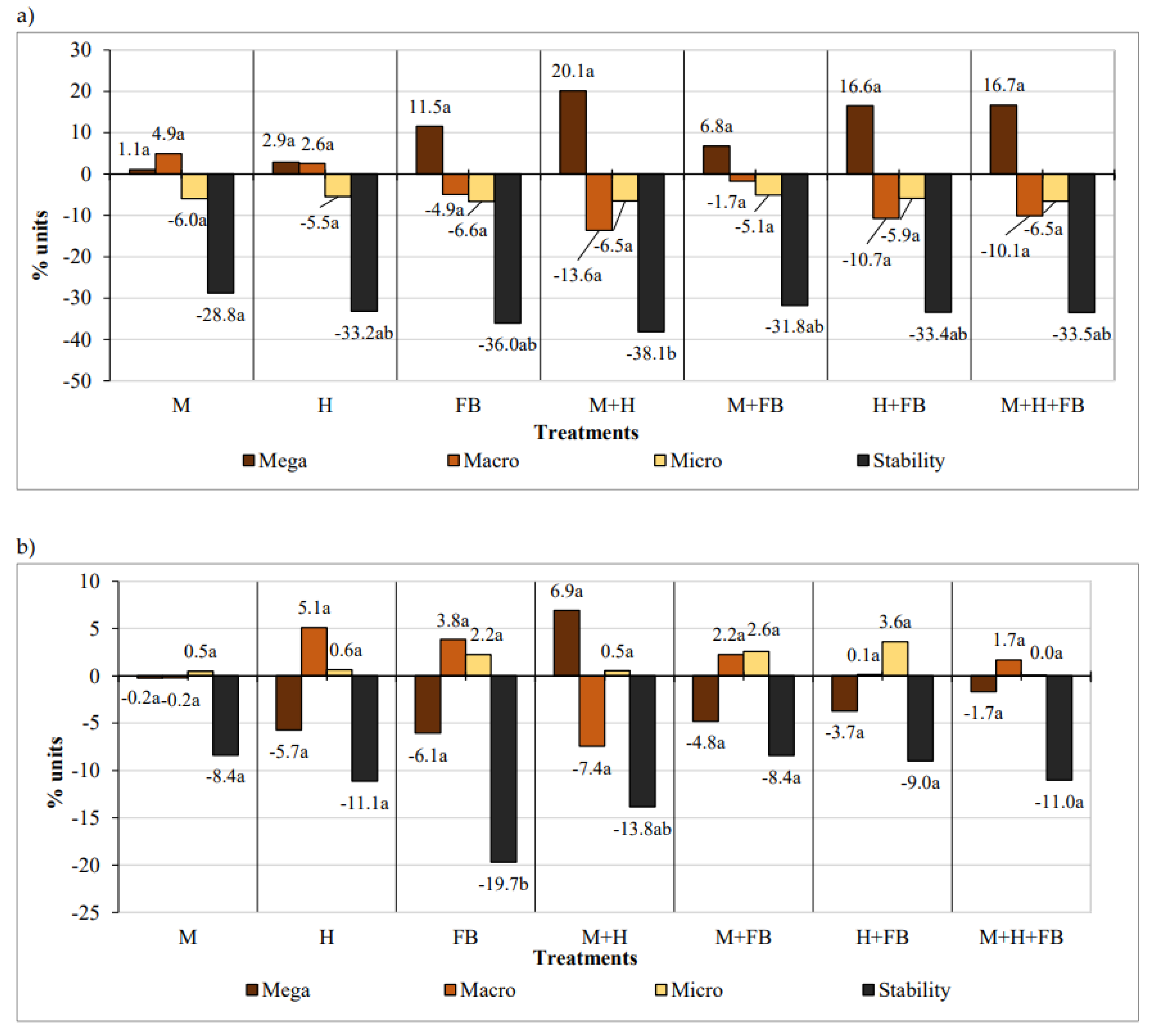
During the two years of experimentation from 2020 spring to 2021 autumn, the amount of soil megastructure increased, while the macro and microstructures decreased (
Figure 1, a). The differences between the treatments were non-significant. The water stability of structure was significantly reduced in the binary M+H crop (38.1% units) compared to the single maize crop. The differences between the other treatments were insignificant.
During the three years of experimentation from 2020 spring to 2022 autumn, different diversification of the maize did not have a significant impact on the soil aggregate-size distribution, but in the M+H binary crop, the largest increase in megaaggregates (6.9% units) and the largest decrease in macroaggregates (7.4% units) were determined. The stability of the soil structure significantly decreased in the single faba bean crop (19.7% units). In other plots, the decrease in structure stability ranged from 8.4% units up to 13.8% units and did not differ significantly (
Figure 1, b).
Figure 1.
Impact of crop diversification on the soil aggregate-size distribution and its stability changes in 2020–2021 (a) and 2020–2022 (b). Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 1.
Impact of crop diversification on the soil aggregate-size distribution and its stability changes in 2020–2021 (a) and 2020–2022 (b). Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
We hypothesized that for three years the intercrops in the maize cultivation would improve the proportions of the soil structural aggregates and increase the water stability of the structure, but this did not happen. It can be assumed that the aggregate-size distribution and stability of the soil structure worsened due to uneven distribution of precipitation during the growing seasons, as dry periods alternated with wet periods several times per vegetative season. Crops growing in the same pots for three years also worsened soil quality.
3.2. Changes in Soil Agrochemical Properties
3.2.1. Soil pH
The pH of the soil is one of the most important indicators, on which both the biological, physical and agrochemical properties of the soil, as well as the growth and development of plants, depend [
32].
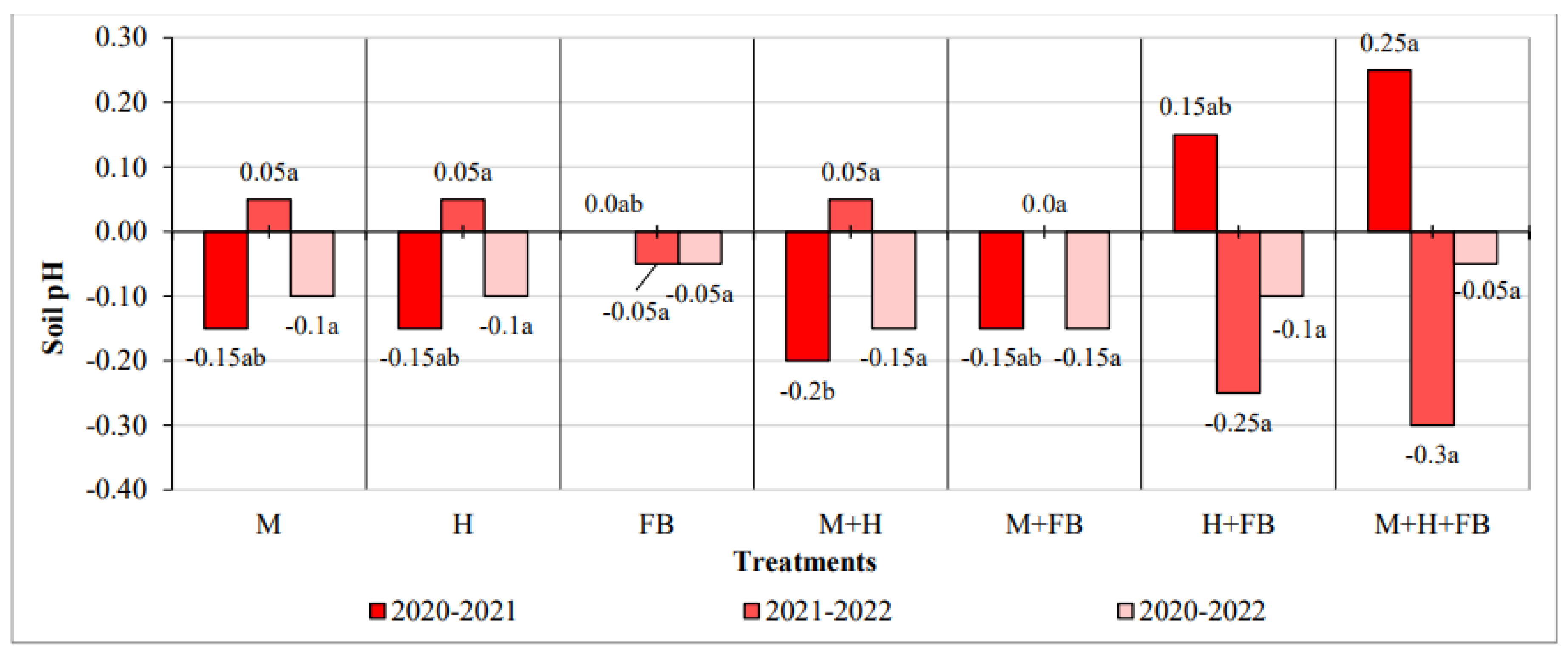
Maize crop diversification did not significantly affect soil pH in most cases. Only in 2020-2021 after evaluating the change in pH, it was found that the binary M+H crop differed significantly from the ternary crop. Paying attention to the regularity of the change, although slightly, the pH tended to decrease in all experimental fields for three years (
Figure 2).
3.2.2. Total Nitrogen
Nitrogen is one of the most important nutritional elements for plants. Plant growth, productivity and quality depend on it. Nitrogen is involved in various physiological processes, such as tissue formation, protein synthesis, etc. [
33].
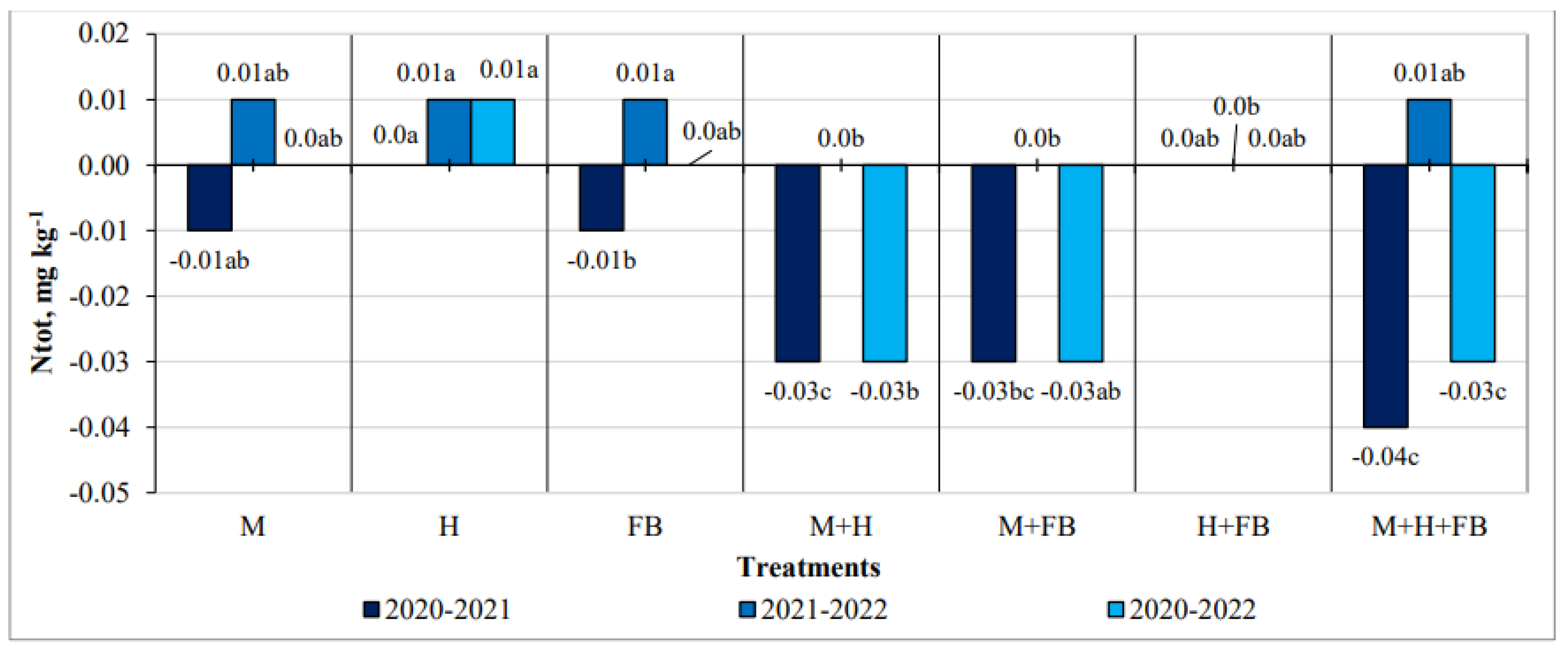
Crops in individual cases had a significant influence on the amount of total nitrogen, but these results were not consistent throughout the years of the experiment. The change of total nitrogen in multiple crops was negative in most cases (
Figure 3).
3.2.3. Available Phosphorus
This element is most important at the beginning of vegetation because it is involved in cell division and ensures optimal root development [
34]. However, the lack of phosphorus often becomes a factor that prevents plants from reaching their fertility potential, because mineral fertilizers are quite expensive, and phosphorus in the soil often passes into forms unavailable to plants [
35]. In case of lack of available phosphorus and drought, it has been observed that plants grown in mixtures directly absorb phosphorus released in the soil from leguminous plants, thus creating conditions for plant resistance [
36].
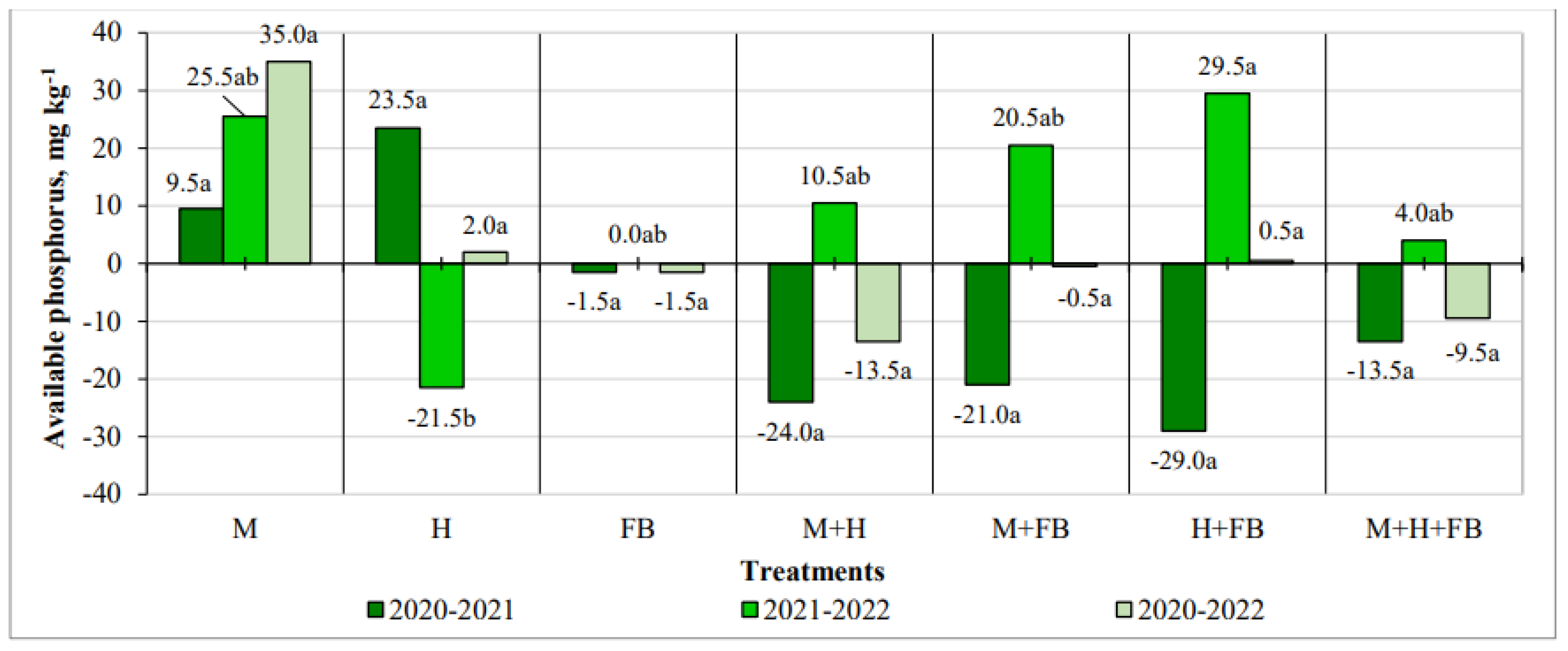
In 2020-2021, most multicrops tended to decrease the amount of this element. In 2021-2022, significantly the highest increase in phosphorus content was found in the hemp and faba bean binary crop (29.5 mg kg
-1) compared to the single hemp crop. Evaluating the change over the three years of the experiment, it was noticed that in the single maize crop, although insignificant, the amount of phosphorus in the soil increased throughout the years of the experiment, while in the multicrops it decreased, except for the years 2021-2022, when due to droughts, part of the phosphorus was not absorbed by the plants (
Figure 4).
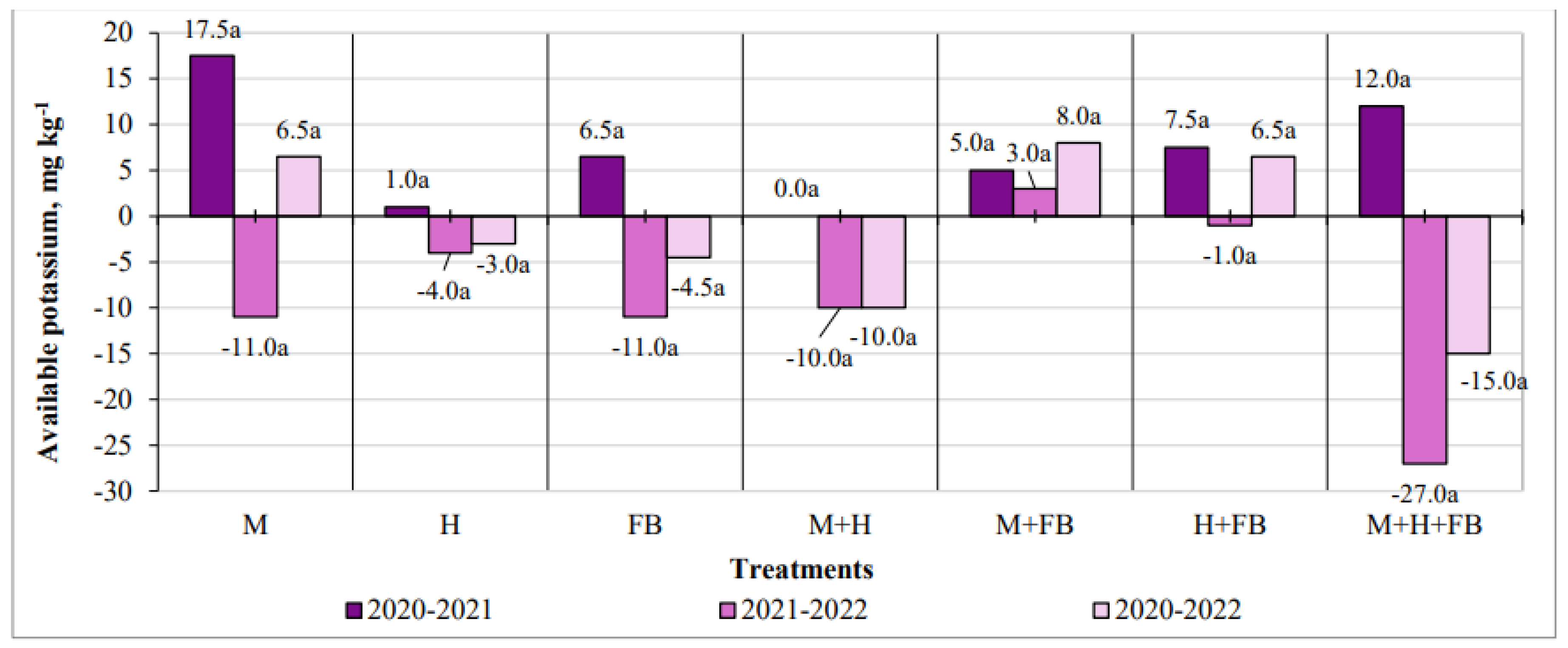
3.2.4. Available Potassium
It is a macro element that promotes the transport of nutrients and helps the plant to protect itself from various stressful situations, such as: drought, frost, plant diseases or pests. Unfortunately, the lack of this element often results in yield and quality losses [
37].
Crop diversification had no significant impact on changes in the amount of this element in the soil (
Figure 5). The largest negative change was found in ternary cultivation, as it produced the highest plant biomass [
38].
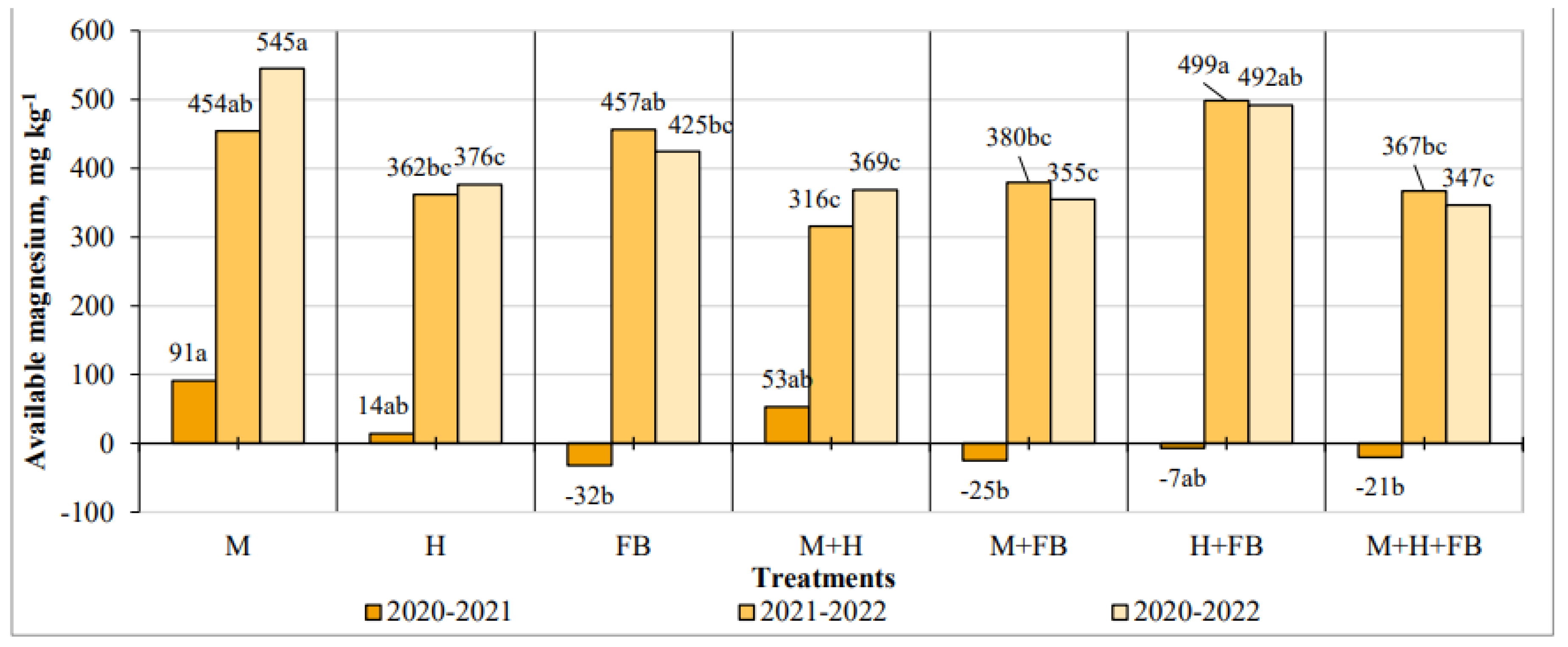
3.2.5. Available Magnesium
Magnesium is included in the composition of chlorophyll, so it ensures the proper activity of photosynthesis, which is closely related to plant productivity [
39].
The diversification of crops in some cases had a significant impact on the amount of available magnesium. It is important to note that the amount of this element increased significantly in all crops of the treatments (
Figure 6). After three years of experimentation, the largest positive change was found in the single maize crop (545 mg kg
-1). The amount of available magnesium in this treatment regularly increased in all years of the study. The least change was found in ternary cultivation. This could have been influenced by the largest amount of biomass removed, as this crop was also the most productive (
Figure 6).
3.3. Changes in Soil Enzymatic Activity
Soil enzymes are responsible for nutrient cycling and their availability [
40]. The enzyme sacharase is responsible for organic carbon conversion processes in the soil [
41]. Urease is the main source of nitrogen in the soil, which promotes the conversion of organic nitrogen into ammonium nitrogen, necessary for plant growth and metabolism [
42].
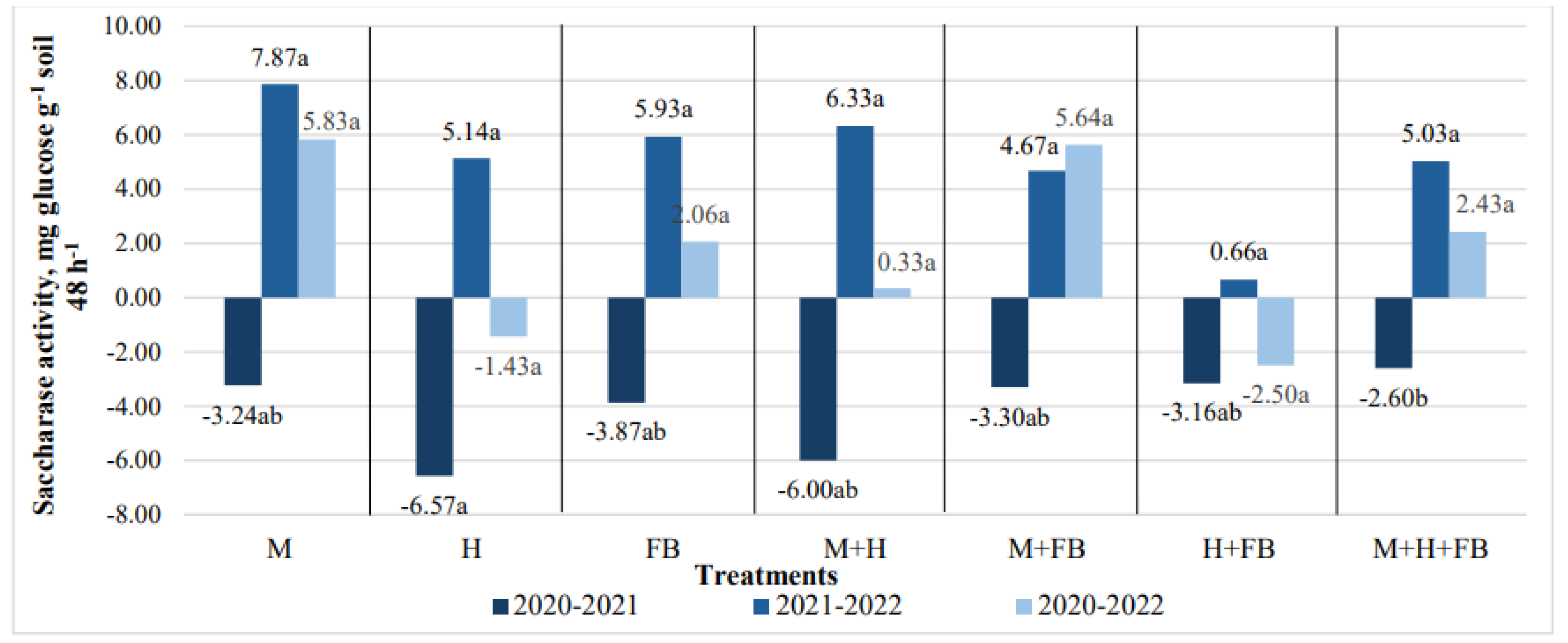
3.3.1. Saccharase Activity
In 2020-2021, a decrease in the saccharase activity was found in the plots of all tested treatments. The activity was significantly lowest in the single hemp crop and the binary maize and hemp crop (-6.57 and -6.00 mg glucose g
-1 soil 48 h
-1). In 2021-2022, the opposite results were obtained. In all cultivations, a positive change in the activity of this enzyme was determined, although it was not significant (
Figure 7).
In 2020-2022, saccharase activity did not differ significantly between treatments. The highest increase of the activity was found in single maize and binary maize and faba bean cultivations.
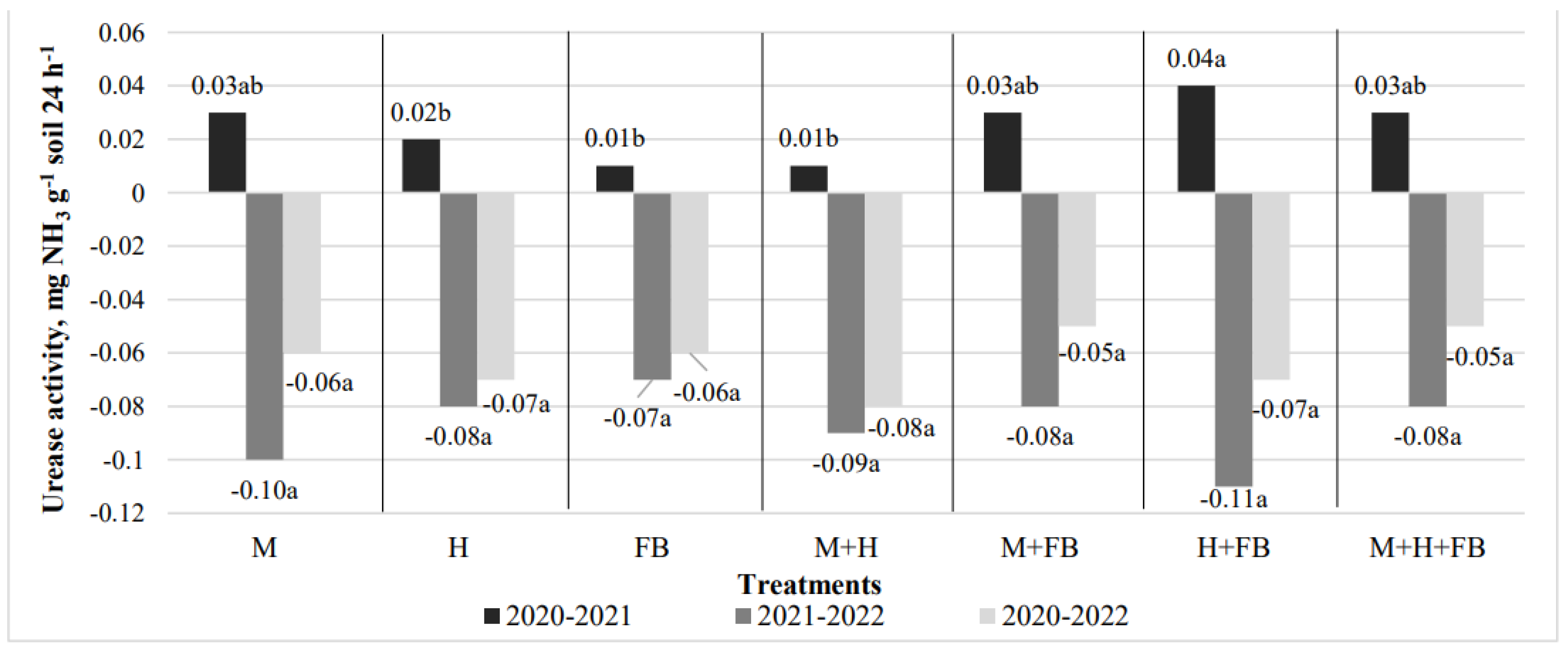
3.3.2. Urease Activity
A positive change in the activity of the enzyme urease was determined only at the end of the 2021 vegetative period (Figure 10). The highest increase in activity was found in the binary crop of hemp and faba bean (0.04 mg NH
3 g
-1 soil 24 h
-1). After the second and third year of crops growing, urease activity decreased in all cultivations, but the differences between treatments were insignificant (
Figure 8).
4. Discussion
4.1. Changes in Soil Aggregate-Size Distribution and Its Stability
During 3 years of experimentation, the amount of soil megastructure in all experimental plots decreased on average 1.5, while the amount of macro- and microstructure, on the contrary, increased by 1.1 and 1.6 times, respectively. The increase in the amount of soil macrostructure could have been influenced by the fact that the dense roots of plants formed by multiple crops. It increased the amount of nutrients in the soil, more efficient activity of microorganisms, and the formation of stable soil aggregates. Mikučionienė et al., [
43] and Duchicela et al., [
44] found that the more intense activity of soil microorganisms led to the abundance of by-products released into the environment. They acted as a binding agent, sticking microaggregates together and thus forming macroaggregates, the presence of which improves soil structure. In our experiment, the increase in the amount of macroaggregates after three years was also determined in the binary M+FB crop (2.2 percent units) and the ternary crop (1.7 percent units). We found that the amount of available magnesium in the soil related to the amount of soil macrostructure (r = 0.50, P ≤ 0.05). George et al. [
45] also claims that multicrops have a positive effect on soil functioning, improve soil structure by increasing the amount of macroaggregates, and reducing the amount of micro- and megaaggregates in the soil. Mottin et al. [
46] found that crops of the
Poaceae family promote higher levels of macroaggregates compared to plants of the
Fabaceae family.
Contrary to expectations, the water-stability of the soil structure also decreased during the 3-year experimentation. The reason for this could be the drastically uneven distribution of precipitation during the vegetative periods. According to Sarah [
47], the amount of rainfall has a significant impact on the stability of the soil structure. In general, the average stability of the soil aggregates in the experiment was 43.5%, so the soil could be regarded as insufficiently structured. The greatest decrease in soil durability after three years of the experiment was found in the single faba bean crop (19.7 percent units) and the binary M+H crop (13.8 percent units). According to other researchers, structure stability was lower in single faba bean crop compared to multi-crops [
48], because interspecific competition between crops promotes plants rooting [
22]. Therefore, the cultivation of multi-crops has a positive effect on the durability of the soil structure. In our experiment, soil stability was partially dependent on megastructure content in the soil (r = 0.510, P ≤ 0.05).
4.2. Changes in Soil Agrochemical Properties
The soil pH varied between the years of the experiment, but during the three years it decreased in the plots of all treatments, although the differences between the treatments were not significant. Biological nitrogen fixation by faba bean plants also acidifies the rhizosphere [
49].
The positive balance of phosphorus was maintained in the single maize, hemp and the binary hemp and faba bean crop, while potassium - in the single maize, binary maize and faba bean and hemp and faba bean. According to Li et al. [
50,
51], faba bean plants grown in mixtures can secrete a larger amount of phosphorus-mobilizing root exudates, which support the phosphorus nutrition of other plant species in multiple crops. Similarly, in maize crops with peas and faba beans, higher levels of available phosphorus were found in the soil [
52,
53]. In addition, for example, white lupins absorb phosphorus from insoluble soil compounds and thereby improve their absorption in wheat [
54].
Wang et al., [
55] in their studies with multi-crops found that per year multi-crops remove about 200-300 kg ha
-1 of available potassium from the soil. One of the main means of balancing the amount of available potassium in the soil is to grow multiple systems of cereal-bean crops, which would significantly increase the amount of available potassium in the soil, compared to monocrops [
56]. Li et al. [
57] found that there was no significant difference in potassium accumulation in faba beans and maize in mixed crops compared to monocultures.
In our experiment, the amount of total nitrogen in the soil decreased the most in binary and multicrops, except for the binary crop of hemp and faba bean, where the amount of nitrogen did not change for three years. Rudinskienė [
58] found that nitrogen significantly increased in binary and ternary crops by alternating them. In our experiment, the crops grew in the same place for three years. According to Ofori et al. [
59], a legume crop capable of sequestering atmospheric N contributes to improving soil nutrient balance. However, maize and hemp consumed a lot of, and the crops practically were not fertilized. That reduced the amount of nitrogen in the soil, regardless of the services provided by faba beans.
The amount of available magnesium in the soil of the experiment increased significantly. We found positive relationships between the amount of available magnesium and the amounts of available phosphorus (r = 0.49, P ≤ 0.05) and available potassium (r = 0.45, P ≤ 0.05).
4.3. Changes in Soil Enzyme Activity
In the first year of research, the activity of the enzyme saccharase in the soil decreased in the plots of all treatments. On the contrary, the enzymatic activity in the soil increased in the second year. This means that multicrops can stimulate the growth and development of plant roots, improving the biological and chemical properties of the soil [
60]. Also, multicrops can improve soil nutrient metabolism by improving the activity of the soil enzyme sucrase [
61]. The increased activity of sucrase indicates the acceleration of the decomposition of hydrolytic carbohydrates and the intensification of the processes of mineralization of organic substances in the soil [
62]. Cui et al. [
63] found that in multicrops, the activity of the saccharase was reliably correlated with soil ammonia and nitrate nitrogen. After three years of research, most treatments maintained a positive saccharase balance, except for single hemp and binary hemp and faba bean crops. So, the cultivation of hemp was not favorable for the development of microorganisms and the accumulation of the enzyme saccharase due to the release of various phytoncide substances.
In the first years of the research, the amount of the enzyme urease increased, and in subsequent years it decreased in all treatments, as the amount of nitrogen in the soil usually decreased. Chen et al., [
64], Breza et al., [
65], Bian et al., [
66] also found correlation between urease activity and amount of nitrogen in the soil.
5. Conclusions
Crop diversification did not significantly affect the change in soil aggregate-size distribution. After three years of experimentation, a significant decrease in the soil stability was found in the single faba bean and binary maize-hemp crop. At the end of the experiment, the highest decrease in total nitrogen, available phosphorus and potassium was determined in the soil of the binary maize-hemp crop and the ternary crop, but only nitrogen decreased significantly. These crops were characterized by higher productivity, which is why the amount of material removed was higher. At the end of the experiment, crop diversification had no significant effect on the change in soil saccharase and urease activity. Among the diversified crops, the decrease in the activity of saccharase was determined in the binary of hemp-faba beans crop, and of urease - in the binary of maize-hemp crop. In general, when assessing the changes in the agrophysical, agrochemical and agrobiological properties of the soil, among the diversified crops in this study, the best indicators were obtained when growing a binary crop maize-faba bean crop. However, this study could be carried out over a longer period, since in our experiment the change in soil properties was more influenced by meteorological conditions than crop diversification.
Author Contributions
Conceptualization, K.R. and J.B.; methodology, K.R., J.B.; software, J.B. and U.G.; validation, J.B., R.K., A.S. and K.R.; formal analysis, J.B., U.G. and R.K.; investigation, J.B., A.Š., U.G., R.K., A.S., A.M. and K.R.; resources, A.Š., R.K. and A.S.; data curation, J.B. and U.G.; writing—original draft preparation, J.B., R.K., A.S., A.Š. and K.R.; writing—review and editing, K.R., J.B., R.K. and A.S.; visualization, J.B., R.K., K.R. and A.S.; supervision, K.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Most of the data generated or analyzed during this study are included in the present article.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study, analyses, writing of the manuscript.
References
- Ahakwa, I. Towards land degradation neutrality: Does green energy and green human capital matter? Renew. Sustain. Energy Rev. 2024, 197, 114396. [Google Scholar] [CrossRef]
- Aly, A.A.; Borik, Z.M. Chapter 1—Food crops, growth and productivity as an important focus for sustainable agriculture. Pl. S. Rna in Food Crops. 2023, 3–23. [Google Scholar]
- Lithourgidis, A.S.; Dordas, C.A.; Damalas, C.A.; Vlachostergios, D.N. Annual intercrops: an alternative pathway for sustainable agriculture. Aust. J. Crop Sci. 2011, 5, 396–410. [Google Scholar]
- Ehrmann, J.; Ritz, K. Plant: soil interactions in temperate multi-cropping production systems. Plant Soil. 2014, 376, 1–29. [Google Scholar] [CrossRef]
- Heuermann, D.; Gentsch, N.; Boy, J. Interspecific competition among catch crops modifies vertical root biomass distribution and nitrate scavenging in soils. Sci. Rep. 2019, 9, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; Ahmad, A.; Khaliq, T.; Habib-ur-rahman, M.; Niaz, S.; Gaiser, T.; Ghafoor, I.; Hassan, H.S.; Quasim, M.; Hoogenboom, G. Climate change; impact uncertainty assessment and adaptations for sustainable maize production using multi-crop and climate models. Environ. Sci. Pollut. Res. 2021, 29, 18967–18988. [Google Scholar] [CrossRef]
- Malayil, S.; Loughran, L.; Ulken, F.M.; Satyavolu, J. Exploring hemp seed hull biomass for an integrated c-5 biorefinery: Xylose and activated carbon. J. Bioresour. Bioprod. 2024, 4–38. [Google Scholar] [CrossRef]
- Gomez, L.D.; Amalfitano, C.; Andolfi, A.; Simister, R.; Somma, S.; Ercolando, M.R.; Borrelli, C.; McQueen-Mason, S.J.; Frusciante, L.; Cuciniello, A.; Caruso, G. Valorising Faba bean residual biomass: effect of farming system and planting time on the potential for biofuel production. Biomass Bioenergy. 2017, 107, 227–232. [Google Scholar] [CrossRef]
- Karkanis, A.; Ntatsi, G.; Lepse, L.; Fernandez, J.A.; Vagen, I.M.; Rawald, B.; Alsina, I.; Kronberga, A.; Balliu, A.; Olle, M.; Bodner, G.; Dubova, L.; Rosa, E.; Savvas, D. Faba Bean Cultivation–Revealing Novel Managing Practices for More Sustainable and Competitive European Cropping Systems. Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Luo, Ch.; Wang, X.; Li, Y.; Ding, H.; Liu, T.; Dong, Y. Enhancing soil properties, soil-borne diseases control, and quality through selecting high C/N agricultural waste during reductive soil disinfestation for continuous tobacco cropping. J. Crop Prot. 2024, 180, 106657. [Google Scholar] [CrossRef]
- Panda, S.K.; Panda, P.; Pramanick, B.; Shankar, T.; Praharaj, S.; Saren, B.K.; Gitari, H.I.; Brahmachari, K.; Hossain, A.; Maitra, S. Advantages of cotton based intercropping system: A review. Int. J. Biores. Sci. 2020, 7, 51–57. [Google Scholar] [CrossRef]
- Raza, M.A.; Gul, H.; Wang, J.; Yasin, H.S.; Qin, R.; Khalid, M.H.B.; Naeem, M.; Feng, L.Y.; Iqbal, N.; Gitari, H.; Ahmad, S.; Battaglia, M.; Ansar, M.; Yang, F.; Yang, W. Land productivity and water use efficiency of maize-soybean strip intercropping systems in semi-arid areas: A case study in Punjab Province, Pakistan. J. Clean. Prod. 2021, 308, 127–282. [Google Scholar] [CrossRef]
- Mu-chun, Y.; Ting-ting, X.; Peng-hui, S.; Jian-jun, D. Effects of Different Cropping Patterns of Soybean and Maize Seedlings on Soil Enzyme Activities and MBC and MBN. J. North. Agr. Univ. 2012, 19, 42–47. [Google Scholar] [CrossRef]
- Bodner, G.; Mentler, A.; Keiblinger, K. Plant Roots for Sustainable Soil Structure Management in Cropping Systems. In The Root Systems in Sustainable Agricultural Intensification; Rengel, Z., Djalovic, I., Eds.; Wiley–Blackwell, 2021; pp. 57–83. [Google Scholar]
- Kumar, R.; Pandey, S.; Pandey, A. Plant roots and carbon sequestration. Curr. Sci. 2006, 91, 885–890. [Google Scholar]
- Gill, S.; Abid, M.; Azam, F. Mixed cropping effects on growth of wheat (Triticum aestivum L.) and chickpea (Cicer arietenum L.). Pak. J. Bot. 2009, 41, 1029–1036. [Google Scholar]
- Salama, H.S.A.; Abdel-moneim, M.H. Maximizing land use efficiency and productivity of soybean and fodder maize intercrops 657 through manipulating sowing schedule and maize harvest regime. Agron. 2021, 11, 863. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, C.H.; Iannetta, P.; Jones, H.; Karley, A.; Li, L.; Mckenzie, B.; Pakeman, R.; Paterson, E.; Shob, Chr.; Shen, J.; Squire, G.; Watson, Chr.A.; Zhang, C.H.; Zhang, F.; Zhang, J.; White, P.J. Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Bailey, B.A. Role of cover crops in improving soil and row crop productivity. Commun. Soil Sci. Plan. 2005, 36, 2733–2757. [Google Scholar] [CrossRef]
- Adak, M.S.; Kibritci, M. Effect of nitrogen and phosphorus levels on nodulation and yield components in faba bean (Vicia faba L.). Legume Res. 2016, 39, 991–994. [Google Scholar]
- Argaw, A.; Mnalku, A. Effectiveness of native Rhizobium on nodulation and yield of faba bean (Vicia faba L.) in Eastern Ethiopia. Arch. Agron. Soil Sci. 2017, 63, 1390–1403. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Jensen, E.S. Facilitative root interactions in intercrops. Plant Soil. 2005, 274, 237–250. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, G.; Wu, F. Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur. J. Soil Biol. 2011, 47, 279–287. [Google Scholar] [CrossRef]
- Song, Y.N.; Zhang, F.S.; Marschner, P.; Fan, F.L.; Gao, H.M.; Bao, X.; Sun, J.; Li, L. Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol. Fertil. Soils. 2007, 43, 565–574. [Google Scholar] [CrossRef]
- Golaszewska, K.; Golaszewski, J. Hemp Production. Sust.Agr. Rev. 2020, 42, 1–36. [Google Scholar]
- Hernandez-Allica, J.; Becerril, J.M.; Garbisu, C. Assessment of the phytoextraction potential of high biomass crop plants. Environ. Pollut. 2008, 152, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Linger, P.; Mussig, J.; Fisher, H.; Kobert, J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential. Ind. Crops Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Shen, Z.; Tiruta-barna, L.; Hamelin, L. From hemp grown on carbon-vulnerable lands to long-lasting bio-based products: Uncovering trade-offs between overall environmental impacts, sequestration in soil, and dynamic influences on global temperature. Sci.Total Environ. 2022, 846, 157331. [Google Scholar] [CrossRef]
-
World Reference Base for Soil Resources, 3rd ed.; World Soil Resources Reports No.106; FAO: Rome, Italy, 2014; Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 09 June 2024).
- Chunderova, A.I. The Enzymatic Activity of Sod-Podzolic Soils of the North-Western Region. Doctoral Dissertation, Tallinn University, Tallinn, Estonia, 1973; 46p. [Google Scholar]
- Romaneckas, K.; Kimbirauskienė, R.; Adamavičienė, A.; Buragienė, S.; Sinkevičienė, A.; Šarauskis, E.; Jasinskas, A.; Minajeva, A. Impact of sustainable tillage on biophysical properties of Planosol and on faba bean yield. Agr. Food Sci. 2019, 28, 101–111. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; Hafeezlaghari, A.; Mustafabhabhan, G.; Hussaintalpur, K.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Richardson, A.E.; Barea, J.M.; Mcneill, A.M.; Prigent-combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil. 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphorus and plant nutrition: an overview. In Phosphorus: Agriculture and the environment; Sims, T., Sharpley, A.N., Eds.; 2005; Volume 46, pp. 353–378. [Google Scholar]
- Eichler-Löbermann, B.; Busch, S.; Jablonowski, N.D.; Kavka, M.; Brandt, C. Mixed cropping as affected by phosphorus and water supply. Agron. 2020, 10, 1506. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Balandaitė, J.; Romaneckas, K.; Švereikaitė, A.; Kimbirauskienė, R.; Sinkevičienė, A.; Romaneckas, A. The biomass productivity of maize, hemp and faba bean multi-crops. Agron. 2022, 12, 1–12. [Google Scholar]
- Cakmak, I.; Yazici, A.M. Magnesium: a forgotten element in crop production. Bet. Crops. 2010, 94, 23–25. [Google Scholar]
- Hazra, K.; Nath, C.P.; Singh, U.; Praharaj, C.S.; Kumar, N.; Singh, S.S.; Singh, N.P. Diversification of maize-wheat cropping system with legumes and integrated nutrient management increases soil aggregation and carbon sequestration. Geoderma. 2019, 353, 308–319. [Google Scholar] [CrossRef]
- Rao, S.; Grover, M.; Kundu, S.; Desai, S. Soil Enzymes. In Encyclopedia of Soil Science; Taylor and Francis, 2017; pp. 2100–2107. [Google Scholar]
- Acosta-Martínez, V.; Pérez-Guzmán, L.; Johnson, J.M. Simultaneous determination of β-glucosidase, β-glucosaminidase, acid phosphomonoesterase, and arylsulfatase activities in a soil sample for a biogeochemical cycling index. Appl. Soil Eco. 2019, 142, 72–80. [Google Scholar] [CrossRef]
- Mikučionienė, R.; Vaisvalavičius, R.; Aleinikovienė, J.; Smalstienė, V. Visuminės organinės anglies pasiskirstymo dirvožemio struktūriniuose agregatuose vertinimas. Žmogaus ir gamtos sauga. 2018, 1822–1823. [Google Scholar]
- Duchicela, J.; Sullivan, T.S.; Bontti, E.; Bever, J. Soil aggregate stability increase is strongly related to fungal community succession along an abandoned agricultural field chronosequence in the Bolivian Altiplano. J. Appl. Ecol. 2013, 50, 1266–1273. [Google Scholar] [CrossRef]
- George, S.J.; Harper, R.J.; Hobbs, R.J.; Tibbett, M. A sustainable agricultural landscape for Australia: a review of interlacing carbon sequestration, biodiversity and salinity management in agroforestry systems. Agr. Ecosyst. Environ. 2012, 163, 28–36. [Google Scholar] [CrossRef]
- Mottin, M.C.; Seidel, E.P.; Fey, E.; Vanelli, J.; Alves, A.L.; Richart, A.; Frandoloso, J.F.; Anschau, K.A.; Francziskowski, M.A. Biomass Productivity and Physical Properties of the Soil after Cultivation of Cover Plant in the Autumn and Winter. Am. J. Plant. Sci. 2018, 9, 775–788. [Google Scholar] [CrossRef]
- Sarah, P. Soil aggregation response to long-and short-term differences in rainfall amount under arid and Mediterranean climate conditions. Geomorph. 2005, 70, 1–11. [Google Scholar] [CrossRef]
- Sánchez-Navarro, V.; Zornoza, R.; Faz, Á.; Fernández, J.A. Comparing legumes for use in multiple cropping to enhance soil organic carbon, soil fertility, aggregates stability and vegetables yields under semi-arid conditions. Sci. Hortic. 2019, 246, 835–841. [Google Scholar] [CrossRef]
- Liu, E.; Teclemariam, S.G.; Yan, C.; Yu, J.; Gu, R.; Liu, S.; He, W.; LIu, Q. Long-term effects of no-tillage management practice on soil organic carbon and its fractions in the northern China. Geod. 2014, 213, 379–384. [Google Scholar] [CrossRef]
- Li, Z.; Gao, Q.; Liu, Y.; He, C.; Zhang, X.; Zhang, J. Overexpression of transcription factor ZmPTF1 improves low phosphate tolerance of maize by regulating carbon metabolism and root growth. Planta. 2011, 233, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F.S. Plant diversity and overyielding: insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Latati, M.; Blavet, D.; Alkama, N.; Laoufi, H.; Drevon, J.J.; Gerard, F.; Pansu, M.; Ounane, S.M. The intercropping cowpea-maize improves soil phosphorus availability and maize yields in an alkaline soil. Pl. Soil. 2014, 385, 181–191. [Google Scholar] [CrossRef]
- Liao, D.; Zhang, C.; Li, H.; Lambers, H.; Zhang, F. Changes in soil phosphorus fractions following sole cropped and intercropped maize and faba bean grown on calcareous soil. Pl. Soil. 2020, 448, 587–601. [Google Scholar] [CrossRef]
- Cu, S.T.T.; Hutson, J.; Schuller, K.A. Mixed culture of wheat (Triticum aestivum L.) with white lupin (Lupinus albus L.) improves the growth and phosphorus nutrition of the wheat. Plant Soil 2005, 272, 143–151. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Wu, P.; He, J.; Chen, X.; Gao, Y.; Cao, X. Radiation interception and utilization by wheat/maize strip intercropping systems. Agric. For. Meteorol. 2015, 204, 58–66. [Google Scholar] [CrossRef]
- Nasar, J.; Shao, Z.; Gao, Q.; Zhou, X.; Fahad, S.; Liu, S.; Li, C.; Banda, J.S.K.; Kgorutla, L.E.; Dawar, K.M. Maize-alfalfa intercropping induced changes in plant and soil nutrient status under nitrogen application. Arch. Agron. Soil Sci. 2022, 68, 151–165. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Shi, X.; Han, X.; Chen, X.; Wei, Y.; Xiong, F. Effects of belowground interactions on crop yields and nutrient uptake in maize-faba bean relay intercropping systems. Arch. Agron. Soil. Sci. 2023, 69, 314–325. [Google Scholar] [CrossRef]
- Rudinskienė, A.; Marcinkevičienė, A.; Velička, R.; Kosteckas, R.; Kriaučiūnienė, Z.; Vaisvalavičius, R. The Comparison of Soil Agrochemical and Biological Properties in the Multi-Cropping Farming Systems. Plants. 2022, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Ofori, E.; Oteng-Darko, P.; Berchie, J.N.; Nimako, F.O.; Yeboah, S.; Danquah, E.O. Monitoring of soil moisture regime and water 689 use efficiency under maize cowpea cropping system. Int. J. Curr.Microbiol. 2014, 3, 837–848. [Google Scholar]
- Rankoth, L.M.; Udawatta, R.P.; Veum, K.S.; Jose, S.; Alagele, S. Cover crop influence on soil enzymes and selected chemical parameters for a claypan corn–soybean rotation. Agron. 2019, 9, 125. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Sui, P.; Liu, X.; Zhang, T.; Li, X. Crop rotation to diversify the soil microbiome in the semi-arid area of Inner Mongolia, China. Arch. Agron. Soil Sci. 2022, 69, 1161–1176. [Google Scholar] [CrossRef]
- Saha, S.; Prakash, V.; Kundu, S.; Kumar, N.; Mina, B.L. Soil enzymatic activity as affected by long term application of farm yard manure and mineral fertilizer under a rainfed soybean–wheat system in NW Himalaya. E. J. Soil Biol. 2008, 4, 309–315. [Google Scholar] [CrossRef]
- Cui, T.; Fang, L.; Wang, M.; Jiang, M.; Shen, G. Intercropping of gramineous pasture ryegrass (Lolium perenne L.) and leguminous forage alfalfa (Medicago sativa L.) increases the resistance of plants to heavy metals. J. Chem. 2018, 1–11. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes: a meta-analysis. Agric Ecosyst Environ 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Breza, L.C.; Mooshammer, M.; Bowles, T.M.; Jin, V.L.; Schmer, M.R.; Thompson, B.; Grandy, A.S. Complex crop rotations improve organic nitrogen cycling. Soil Biol. and Biochem. 2023, 177, 108911. [Google Scholar] [CrossRef]
- Bian, X.; Yang, X.; Zhang, K.; Zhai, Y.; Li, Q.; Zhang, L.; Sun, X. Potential of Medicago sativa and Perilla frutescens for overcoming the soil sickness caused by ginseng cultivation. Front. Microbiol 2023, 14, 1134331. [Google Scholar] [CrossRef] [PubMed]
Figure 2.
Impact of crop diversification on soil pH changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 2.
Impact of crop diversification on soil pH changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 3.
Impact of crop diversification on total nitrogen changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 3.
Impact of crop diversification on total nitrogen changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 4.
Impact of crop diversification on available phosphorus changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 4.
Impact of crop diversification on available phosphorus changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 5.
Impact of crop diversification on available potassium changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Lower-case letter (a) indicates insignificant differences between treatments at P > 0.05.
Figure 5.
Impact of crop diversification on available potassium changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Lower-case letter (a) indicates insignificant differences between treatments at P > 0.05.
Figure 6.
Impact of crop diversification on available magnesium changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between crop diversification level at P < 0.05.
Figure 6.
Impact of crop diversification on available magnesium changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between crop diversification level at P < 0.05.
Figure 7.
Impact of crop diversification on saccharase activity changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 7.
Impact of crop diversification on saccharase activity changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between treatments at P < 0.05.
Figure 8.
Impact of crop diversification on urease activity changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between crop diversification level at P < 0.05.
Figure 8.
Impact of crop diversification on urease activity changes. Note: M—maize single crop, H—hemp single crop, FB—faba bean single crop, M+H—binary maize and hemp crop, M+FB—binary maize and faba bean crop, H+FB—binary hemp and faba bean crop, M+H+FB—ternary maize, hemp and faba bean crop. Different lower-case letters indicate significant differences between crop diversification level at P < 0.05.
Table 1.
The average air temperature (°C) and precipitation rate (mm) during crops vegetative season. Kaunas Meteorological Station.
Table 1.
The average air temperature (°C) and precipitation rate (mm) during crops vegetative season. Kaunas Meteorological Station.
| Month / Year |
2020 |
2021 |
2022 |
Long-term average |
| Air temperature |
| April |
6.9 |
6.2 |
6.2 |
6.9 |
| May |
10.5 |
11.4 |
11.0 |
13.2 |
| June |
19.0 |
19.5 |
17.7 |
16.1 |
| July |
17.4 |
22.6 |
17.9 |
18.7 |
| August |
18.7 |
16.5 |
20.9 |
17.3 |
| Precipitation rate |
| April |
4.0 |
33.7 |
38.4 |
41.3 |
| May |
94.4 |
121.6 |
84.0 |
61.7 |
| June |
99.3 |
40.3 |
77.6 |
76.9 |
| July |
60.5 |
48.4 |
100.5 |
96.6 |
| August |
92.8 |
122.2 |
38.7 |
88.9 |
Table 2.
Experimental treatments.
Table 2.
Experimental treatments.
| Biodiversity level |
Crops |
Abbreviation |
| Single crop |
Maize, hemp, faba bean (separate crops) |
M (1), H (2), FB (3) |
| Binary crop |
Maize + hemp |
M + H (4) |
| Maize + faba bean |
M + FB (5) |
| Hemp + faba bean |
H + FB (6) |
| Ternary crop |
Maize + hemp + faba bean |
M + H + FB (7) |
Table 3.
Agrotechnical measures.
Table 3.
Agrotechnical measures.
| Agrotechnical measures |
Execution time |
| |
2020 |
2021 |
2022 |
| Pre-seeding cultivation |
22 04 |
22 04 |
18 04 |
| Seeding |
30 04 |
28 04 |
22 04 |
| Interrows mellowing |
29 05 15 06 |
08 06 25 07 |
18 05 08 06 |
| Biomass harvesting |
03 09 |
24 08 |
29 08 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).