1. Introduction
In Twin Anemia Polycythemia Sequence (TAPS), unequal red blood cell passage across intertwin anastomoses of the monochorionic twin placenta predisposes the donor to anemia and the recipient to polycythemia. Unlike Twin-to-Twin Transfusion Syndrome, the placental vascular anastomoses responsible for TAPS may not be fetoscopically accessible, necessitating a wider range of management approaches [
1]. Temporizing treatment of both twins in TAPS requires intrauterine transfusion (IUT) of the anemic donor and hemodilution of the polycythemic recipient achieved through a partial exchange transfusion (pET). This combined treatment is rarely described, potentially due to lower utilization or procedural complexity [
2].
In contrast to ultrasound guided IUTs, which are among the widest offered fetal interventions with a standardized procedural approach, fetal pET is infrequently performed (
Table 1) [
3,
4]. Historically, fetal exchange transfusions were performed prior to the 1990s for anemic fetuses with immune hydrops. These procedures intended to exchange blood volume for donor cells compatible with the maternal blood type to decrease the risk of ongoing hemolysis [
5,
6,
7]. While these reports describe the basic technique of sequential removal and transfusion of blood aliquots, these procedures were not intended to achieve hemodilution to a specific target hemoglobin. Robyr et al. was the first to describe partial fetal hemodilution in the polycythemic recipient twin for postlaser TAPS [
8]. Subsequent reports that describe sequential IUT and pET vary in detail and the procedural approach (
Table 1) [
9,
10,
11,
12,
13]. It was the goal of this study to present our outcomes with a detailed description of the procedural approach and success with sequential donor transfusion and recipient exchange in TAPS and compare findings with the existing literature.
2. Materials and Methods
We retrospectively reviewed all monochorionic twins referred to the Johns Hopkins Center for Fetal Therapy between July 2014 and September 2023. Patients underwent a detailed ultrasound assessment including an anatomic survey, fetal echocardiography, fetal biometry, amniotic fluid volume, fetal and maternal Doppler studies, using high-resolution ultrasound (GE Voluson E10). The diagnosis of TAPS was made when the middle cerebral artery peak systolic velocity (MCA PSV) in the donor exceeded 1.5 multiples of the median (MoM) and was below 0.8 MoM in the recipient. Patients meeting criteria for TAPS were staged by severity [
14]. Patients were offered Solomon laser, IUT and pET, selective twin reduction, expectant management or pregnancy termination based on the severity of the condition and the feasibility of surgical treatment. This study was focused on patients receiving IUT with pET as the primary treatment.
The IUT and pET procedure was performed in the ultrasound office or on labor and delivery after viability. We always transfused the donor twin first followed by the partial exchange of the recipient. This sequence was chosen to treat the anemic fetus first and for the potential advantage of addressing any intraprocedural red cell transfer to the recipient by adjusting the hemodilution volume. Fetal paralysis was achieved by ultrasound guided intramuscular or intravascular fetal injection of Rocuronium (0.5-1 mg/Kg sonographically estimated fetal weight). Bedside point of care testing of fetal blood samples was performed to verify that target hemoglobin values were achieved (HemoCue HB 201 DM analyzer; HemoCue USA, Brea, California).
The anemic donor was transfused based on the opening hemoglobin with O-Rhesus negative blood with a hemoglobin concentration of 25 mg/dl at a rate of 1-5 cc/minute with the goal to achieve a normal hemoglobin value. Transfusion volume was estimated based on the fetal weight and further adjusted based on intraprocedural bedside testing [
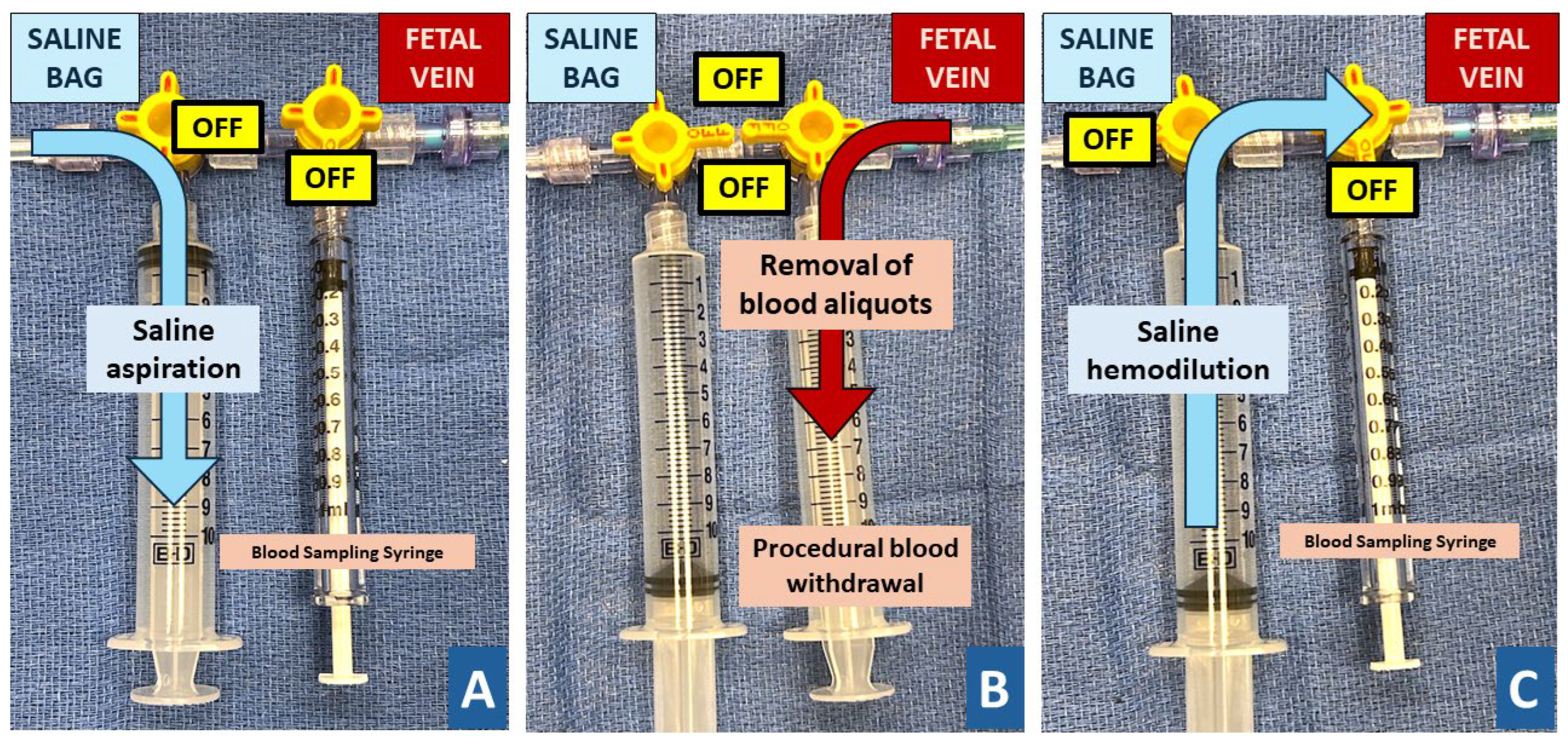
15]. Once the donor transfusion was completed, the blood sampling was performed in the recipient and paralysis administered if fetal movement was still present. After the opening sample, a double syringe setup was attached to the needle that allowed alternating blood withdrawal and saline infusion without detachment (
Figure 1). Guided by the opening hemoglobin value, blood was withdrawn in 5-20 cc aliquots followed by infusion of matching aliquots of 0.9% sterile saline solution with the goal to dilute to a high-normal hemoglobin concentration. The exchange volume was estimated using formulas originally published for partial volume exchange in neonates and further adjusted, guided by intraprocedural samples and bedside testing [
16]. For all procedures, the opening and closing blood samples were also analyzed in the central laboratory with additional determination of the fetal hemoglobin content by Kleihauer-Betke (KB) testing. Patients were discharged for outpatient ultrasound monitoring after the procedure, after return of fetal activity and reassuring fetal heart rate for procedures performed after viability.
We recorded pre-procedure characteristics including placental location, MCA Doppler for both twins, estimated fetal weights and TAPS stage. Procedural characteristics included gestational age, maternal anesthesia, fetal paralysis and tocolytic treatment. The details on needle gauge; fetal vascular access; procedure duration; opening and closing hematocrit, hemoglobin, and KB values for each fetus and any procedural complications were recorded. For transfusions we noted the administered blood volume and for pETs the aliquot size of removed blood and administered saline. Pregnancy outcomes were ascertained after delivery from the respective delivery sites.
For blood transfusions we compared the recorded transfusion volume and observed change in donor hemoglobin to the estimated transfusion volume based on published formulas [
9,
15,
17,
18,
19]. The same approach was taken for the volume of blood partially exchanged with normal saline during recipient exchange transfusions [
9,
16,
20]. We used descriptive statistics for pre-, intraprocedural and outcome details. Comparisons between observed to expected transfusion and exchange volumes used Mann-Whitney U or Kruskal Wallis tests as appropriate. Pooled statistic of this case series and published literature are present for datapoints where the corresponding details could be ascertained (
Table 1). Statistical analyses were performed using via IBM SPSS (26.02).
3. Results
Five of 78 patients (6.4%) presenting with spontaneous TAPS were treated with concurrent donor transfusion and partial exchange transfusions of the recipient. The median time at diagnosis was 27.6 [interquartile range (IQR) 25.1-28.9] weeks and the majority had stage II TAPS. The cohort underwent a total of 20 IUTs; in one procedure, only fetal blood sampling was performed on the TAPS recipient (
Table 1). Two of 5 patients had anterior placentas, and median gestational age at first procedure was 28.3 [IQR 27.1-30.0] weeks. All procedures were performed under local anesthesia, with 3 of 20 (15%) with supplementary maternal intravenous sedation. Most patients received a tocolytic pre-procedure (80%). The median interval between serial IUTs with pET was 8 [IQR 7-10] days and the interval from the last procedure to delivery was 5.7 [IQR 1.3-6.9] weeks. The median gestational age at delivery was 33.0 [IQR 31.9-33.3] weeks, a median of 5.6 [IQR 1.9-6.0] weeks after first procedure.
All IUT and pET procedures were performed with a 20-gauge needle utilizing placental cord insertion, free loop or intrahepatic umbilical vein for access based on operator preference. Three IUT procedures required a second attempt to achieve vascular access. One patient underwent IUT with pET and an amnioreduction in the setting of stage I TTTS at 29.1 GA. In one IUT, an additional intraperitoneal transfusion was performed. During one pET, procedure fetal heart rate decelerations occurred after withdrawal of 5 cc of blood in a recipient with 54.3% hematocrit. After cessation of the procedure and successful intrauterine resuscitation no subsequent procedures were attempted, and the patient subsequently delivered two weeks later at 34.3 GA with two live births. We encountered no other complications.
Although the median number of samples during procedure types was comparable, three or more samples were utilized in more than 30% of pETs (
Table 2). A median of 48 [IQR 39-63] cc of blood was transfused to the anemic fetus in IUTs, and 32 [IQR 20-50] cc of blood was exchanged for 32 [IQR 15-20] cc of saline in pETs. Aliquot size ranged from 5 cc to 20 cc and varied during the procedure with the most common aliquot size being 10 cc (
Table 2). The magnitude of hemoglobin change was greater for transfusions (7.2 [IQR 5.25-8.27] g/dl versus 2.5 [IQR 1.4-3] g/dl than partial exchanges (Mann Whitney U, p<0.05).
Following transfusion treatments delivery indications were unrelated to TAPS and all patients had concordant MCAs at their last ultrasound prior to delivery. Every patient had two live births with neither baby requiring neonatal intervention for anemia or polycythemia.
In general, IUT volumes were higher than pET volumes (p=0.021,
Table 2). Formulas assuming a blood volume of 150 ml per Kg sonographically estimated fetal weight underestimated the transfusion non-significantly (p=0.258,
Table 3). A lower estimated fetal blood volume underestimated the transfusion volume to achieve the target hemoglobin by 15 [IQR 13.5-21.5] ml (p=0.002,
Table 3). For partial exchange transfusions the removed volume aliquots required to achieve the desired hemoglobin value differed from the predicted values for all formulas. Formulas based on a blood volume of 150 ml/kg fetal weight overestimated the volume of blood to be removed by 8.5 milliliters [IQR 11.6 to 6.84], while lower blood volume assumptions underestimated the amount by 6.2 – 22.3 milliliters (p<0.05 for all,
Table 3).
A literature review identified five manuscripts describing variable procedural details on 9 patients with postlaser or spontaneous TAPS undergoing a total of 16 IUTs with pET [
8,
9,
10,
11,
12]. Combined with the current series diagnosis was at a median gestational age of 27.1 [IQR 25-27.6] weeks and first treatment instituted at 27.3 [IQR 25.4-28.6] weeks. With a median interval of 4.7 [IQR 2.1-5.6] weeks after initiation of transfusions, patients delivered at 31.8 [IQR 30.0-33.3] weeks gestation (
Table 1).
4. Discussion
Intrauterine transfusion of the anemic donor twin and partial exchange transfusion of the polycythemic recipient is an infrequently reported temporizing management for patients with twin anemia polycythemia sequence. With most patients presenting in the late second trimester, this management approach potentially delayed delivery by almost 5 weeks across all reported cases, with no additional postnatal transfusion required in the 5 cases presented in this series. Our procedural approach of sequentially targeting the anemic donor and then polycythemic recipient twin was successful in almost all procedures. While formula estimation of anticipated transfusion volume worked well for the donor, dilution volumes in the recipient were inadequately predicted by formulas derived from neonatal partial exchange transfusions. This stresses the importance of bedside testing to achieve the target hemoglobin values during partial exchange transfusions.
Because TAPS has differing presentations, management approaches can vary [
1]. Prior reports on exchange transfusion treatments have not described the procedural approach with the same degree of detail as presented here. We use larger aliquots and perform IUT prior to pET, addressing the more compromised donor first [
21]. While performing IUT only will address the more clinically pressing needs of the anemic, donor fetus, an additional recipient exchange allows for monitoring of the hemoglobin values of the polycythemic fetus and dilution of the blood volume that may have passed over during the donor transfusion. Kleihauer-Betke tests in the opening sample of the recipient suggests that this indeed does happen. While our sample size is too small to analyze for dynamics of intraprocedural blood transfer, additional measurements of recipient KB following donor transfusion may provide insight into the impact of donor IUT on recipient polycythemia. This testing could also be utilized clinically to identify cases with accelerated intertwin blood transfer that are unlikely to benefit from fetal transfusions as the primary management strategy.
While the ability to perform bedside hemoglobin testing of samples may be advantageous to capture intertwin transfusion of donor blood to the recipient, it is important during both interventions in the combined procedure. All published IUT and pET formulas are modifications of the neonatal formula, differing with their evaluation of circulating blood volume per kilogram of estimated fetal weight [
20]. The placental contribution to fetal circulating blood volume has been studied [
22,
23], but the intraprocedural transfusion of blood between fetuses and the monochorionic physiology may further complicate formulaic estimation of transfusion and exchange volumes. Ultimately, our practice is to use formulas to guide the beginning of a procedure, and then rely on bedside testing of intraoperative sampling to adjust transfusion and exchange volumes.
Our study is limited by a small number of cases. Our cohort is also difficult to compare to the literature because we report spontaneous TAPS cases, which may have better outcomes than postlaser TAPS [
4]. Furthermore, while the bedside hemoglobin and hematocrits presented in our analysis inform procedural decision-making, they may be less accurate than laboratory testing. Finally, our comparison to the existing literature is limited by the lack of published detail on previously reported cases. While pET was initially described in 2006 by Robyr, the absence of any description precludes us including cases in
Table 1 [
8].
Our study benefits from the largest number of procedures detailed so far, doubling the case size in existing literature, and our thorough description of our procedural approach. The intraprocedural sampling hematocrits and KB values contribute to investigations of TAPS disease processes, our outcomes encourage clinicians to consider pET when treating TAPS with IUT, and our procedural description aids other groups in refining their pET procedural approach.
We demonstrate that the double procedural set-up can be effectively performed to prolong pregnancy. While we do not offer an analysis of serial IUTs without pET, the concordant MCAs prior to delivery, our neonatal outcomes, and previous modeling by Slaghekke et al. suggest that our combined approach was effective in preventing serial IUTs from exacerbating the polycythemia of the recipient twin [
13]. Combined pET/IUT for TAPS requires further study but is a promising temporizing treatment for prolonging pregnancy and mitigating poor neonatal outcomes.
Author Contributions
Conceptualization, C.S, M.R., M.K., J.M., and A.B.; methodology, C.S, M.R., M.K., J.M., and A.B.; software, C.S. and A.B.; validation, C.S. and A.B.; formal analysis, C.S. and A.B.; investigation, C.S., M.R., M.K., J.M., and A.B.; resources, C.S., M.R., M.K., J.M., and A.B.; data curation, C.S., M.R., M.K., J.M., and A.B.; writing—original draft preparation, C.S. and A.B.; writing—review and editing, C.S., M.R., M.K., J.M., and A.B.; visualization, C.S., M.R., M.K., J.M., and A.B.; supervision, M.R., M.K., J.L., A.B.; project administration, C.S. and A.B.; funding acquisition, M.R., M.K., J.M., and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
Albert W. Turner Foundation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Johns Hopkins (IRB00404129, 9/11/2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is available upon reasonable request of the authors.
Acknowledgments
We thank Albert W. Turner Foundation and Kelly family for their ongoing support of research and the TWIN Lab students working together with the Johns Hopkins Center for Fetal Therapy.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Baschat, A.A.; Miller, J.L. Pathophysiology, diagnosis, and management of twin anemia polycythemia sequence in monochorionic multiple gestations. Best Park Res Clin Obstet and Gynaecol. 2022, 84, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, L.S.A.; Enrico, L.; Oepkes, D.; Haak, M.C.; Klumper, F.J.C.M.; Middeldorp, J.M.; van Klink, J.M.M.; Slaghekke, F. Twin Anemia Polycythemia Sequence: Knowledge and Insights After 15 Years of Research. Maternal-Fetal Medicine. [CrossRef]
- Zwiers, C.; Lindenburg, I.T.M.; Klumper, F.J.; de Haas, M.; Oepkes, D.; van Kamp, I.L. Complications of intrauterine intravascular blood transfusion: lessons learned after 1678 procedures. Ultrasound Obstet Gynecol, 2017, 50, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Giorgione, V; D’Antionio, F; Manji, A; Reed, K; Khalil, A. Perinatal outcome of pregnancy complicated by twin-anemia polycythemia sequence: systemic review and meta-analysis. Ultrasound Obstet Gynecol 2021, 58, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Freda, V. J; Adamsons, K, Jr. Exchange transfusion in utero. Am J Obst & Gynec, 1964, 89, 817–821. [Google Scholar] [CrossRef]
- Asensio, S.H; Figueroa-Longo, J.G; Pelegrina, I.A. Intrauterine Exchange Transfusion. Am J Obst & Gynec, 1966, 95, 1129–1133. [Google Scholar] [CrossRef]
- Grannum, P.A.; Copel, J.A.; Plaxe, S.C.; Scioscia, A.L.; Hobbins, J.C. In utero exchange transfusion by direct intravascular injection in severe erythroblastosis fetalis. The New England Journal of Medicine, 1986, 314, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Robyr, R.; Lewi, L.; Salomon, L.J.; Yamamoto, M.; Bernard, J.; Deprest, J.; Ville, Y. Prevalence and management of late fetal complications following successful selective laser coagulation of chorionic plate anastomoses in twin-to-twin transfusion syndrome. Am J Obst & Gynec, 2006, 194, 796-803. [CrossRef]
- Bahtiyar M.O..; Ekmekci, E.; Demirel, E.; Irani, R.A.; Copel, J.A. In utero Partial Exchange Transfusion Combined with in utero Blood Transfusion for Prenatal Management of Twin Anemia-Polcythemia Sequence. Fetal Diagn Ther, 2019, 45, 28-35. 45. [CrossRef]
- Genova, L.; Slaghekke, F.; Klumper, F.J.; Middeldorp, J.M. Management of Twin-Anemia Polycythemia Sequence Using Intrauterine Blood Transfusion for the Donor and Partial Exchange Transfusion for the Recipient. Fetal Diagn Ther, 2013, 34, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Gucciardo, L.; Lewi, L.; Vaast, P.; Debska, M.; De Catte, L.; van Mieghem, T.; Done, E.; Devliger, R; Deprest, J. Twin anemia polycythemia sequence from a prenatal perspective. Prenat Diagn, 2010, 30, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Moaddab, A.; Nassr, A.A; Espinoza, J.; Ruano, R.; Bateni, Z.H.; Shamshirsaz, A.A.; Mandy, G.T.; Welty, S.E.; Erfani, H.; Popek, E.J.; et al. Twin anemia polycythemia sequence: a single center experience and literature review. Eur J Obstet Gynecol Reprod Biol, 2016, 205, 158–164. [Google Scholar] [CrossRef]
- Slaghekke, F.; van den Wijngaard, J.P.H.M; Akkermans, J.; van Gemert, M.J.C.; Middeldorp, J.M.; Klumper, F.J.; Oepkes, D.; Lopriore, E. Intrauterine transfusion combined with partial exchange transfusion for twin anemia polycythemia sequence: modeling a novel technique. Placenta, 2015, 36, 599-602. [CrossRef]
- Slaghekke, F.; Kist, W.J.; Oepkes, D.; Pasman, S.A.; Middeldorp, J.M.; Klumper, F.J.; Walther, F.J.; Vandenbussche, F.P.H.A.; Lopriore, E. Twin Anemia-Polycythemia Sequence: Diagnostic Criteria, Classification, Perinatal Management and Outcome. Fetal Diagn Ther 2010; 27 (4), 181–190. [CrossRef]
- Giannina, G.; Moise, K.J. Jr; Dorman, K. A simple method to estimate volume for fetal intravascular transfusions. Fetal Diagn Ther. 1998 Mar-Apr, 13 (2), 94-97. [CrossRef]
- UCSF NCNC (Northern California Neonatology Consortium). Consensus Guidelines for Partial Exchange Transfusion for Polycythemia in Neonates. UCSF Benioff Children’s Hospital. July 5, 2023. Available Online At: https://medconnection.ucsfbenioffchildrens.org/polycythemia-guidelines. Accessed May 30 2024.
- Socol, M.L.; MacGregor, S.N.; Pielet, B.W.; Tamura, R.K.; Sabbagha, R.E. Percutaneous umbilical transfusion in severe rhesus isoimmunization: resolution of fetal hydrops. Am J Obstet Gynecol. 1987, 57(6), 1369–75. [Google Scholar] [CrossRef] [PubMed]
- Rodeck, C.H.; Nicolaides, K.H.; Warsof, S.L.; Fysh, W.J.; Gamsu, H.R.; Kemp, J.R. The management of severe rhesus isoimmunization by fetoscopic intravascular transfusions. Am J Obstet Gynecol. 1984, 150, 769–74. [Google Scholar] [CrossRef] [PubMed]
- Perinatology.com: Intravascular Fetal Transfusion. Available online: https://www.perinatology.com/protocols/rhc.htm. Accessed May 15 2024.
- Maertzdorf, W.J.; Aldenhuyzen-Dorland, W.; Slaaf, D.W.; Tangelder, G.J.; Blanco, C.E. Circulating Blood Volume in Appropriate and Small for Gestational Age Full Term and Preterm Polycythaemic Infants. Acta Paediatr Scan. 1991, 80, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Knijnenburg, P.J.C.; Lopriore, E.; Slaghekke, F.; van Klink, J.M.M. Long-term follow-up of complicated monochorionic twin pregnancies: Focus on neurodevelopment. Best Pract Res Clin Obstet Gynaecol. 2022, 84, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, K.H.; Clewell, W.H.; Rodeck, C.H. Measurement of human fetoplacental blood volume in erythroblastosis fetalis. Am J Obstet Gynecol. 1987, 157(1), 50–3. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A; Hustead, R.F.; Robinson, R.G; Haswell, G.L. Measurement of fetoplacental blood volume in the human previable fetus. Am J Obstet Gynecol. 1987, 157, 1369–75. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).