1. Introduction
Infantile Neuroaxonal Dystrophy (INAD, NBIA2A; MIM#256600) is a rapidly progressive neurodegenerative disorder. It was first described by Seitelberger in 1952, granting its name as Seitelberger disease. INAD is very rare, estimated to affect 1:1,000,000 children worldwide [
1]. It is characterized by psychomotor regression presenting in early infancy, with symptoms reflecting the involvement of peripheral nerves, the central nervous system, and the autonomic nervous system.
INAD results from pathogenic variants in the PLA2G6 gene located on chromosome 22q13.1 and is inherited as autosomal recessive [
2]. It is a subtype of PLA2G6-associated neurodegeneration (PLAN). Other conditions in this disease group include Karak syndrome, Juvenile-adult-onset dystonia-parkinsonism (PARK14; MIM# 612953), and atypical neuroaxonal dystrophy (NAD, NBIA2B; MIM#610217) [
1,
2]. As a subtype of PLAN, the pathophysiology of INAD has been described to be related to pathogenic variations in the PLA2G6 gene, which encodes calcium-independent phospholipase A2. This enzyme hydrolyses glycerophospholipids, contributing an essential step in axonal and synaptic phospholipid membrane remodeling. Pathogenic variations lead to the inability to repair oxidative damage to membranes [
2].
The natural progression of the disease has been described by multiple studies, with the onset of symptoms between 6 months and 3 years and an average at 15 months [
3,
4]. while another describes a range of 12 to 22 months [
4]. The initial presentation of the disease usually is a loss of balance and developmental delay [
3]. Developmental regression initially affects speech and gross motor functions, followed later by affection of fine motor and bulbar functions. This usually manifests in truncal hypotonia, spastic quadriparesis, progressive cognitive decline, losing vocalization, and bulbar dysfunctions. Seizures may be present early or late in the disease course, or not at all. Atypical INAD presents with similar but more subtle clinical features to INAD, and with a slower progression to its classic counterpart [
5]. The disease has a poor prognosis. Patients most often succumb to respiratory function decline secondary to bulbar affection, with an average age at death of 9.9 years (range 6.5-14 years) [
3,
4]. There are no specific curative treatments available for patients with INAD. They are treated conservatively with analgesics and physiotherapy with vigilance for complications. However, newer therapeutic options are currently being explored.
We report a rare case of INAD in a 9-year-old girl presenting with overlapping features, posing significant diagnostic challenges. We aim to spread awareness regarding this condition among practitioners, facilitate early identification, and outline the importance of appropriate genetic counseling.
2. Case Presentation
A 9-year-old Pakistani girl presented with recurrent pneumonia, with a prior known history of global developmental delay and psychomotor regression, since the age of 15 months. The child was born to parents with first-degree consanguineous marriage. The pregnancy was planned with an uncomplicated antenatal period, vaginal delivery at term, and a birth weight of 2.7 kg. The immediate postnatal course was uneventful without neonatal intensive care unit (ICU) admissions. She was breastfed till the age of 1 year, with received all age appropriate vaccinations.
She was diagnosed with Developmental Dysplasia of the Hip (DDH), and bilateral dislocation of the hips by an X-ray, and ultrasonography during early infancy and was managed nonsurgically with Pavlik harness. Her developmental milestones in gross motor, fine motor, social, and language skills were normal until 15 months of age. Then she presented with tip-toe walking and gross motor delay followed by progressive loss of various developmental milestones in other domains. Her paternal cousin is suspected to be suffering from a similar illness due to developmental delays, without any confirmation of diagnosis so far due to the limited resources. All her other siblings have normal growth and development.
Despite her normal growth, concerns arose when she started tip-toe walking at 15 months of age, with complaints of aching in her legs, and slowly causing her to avoid herself from walking around. At the age of 2 years, her symptoms rapidly deteriorated causing her to lose her ability to walk steadily, sit upright, and ultimately resorting to crawling as her only means of movement. Her cognitive capacity to perceive and process motor tasks was diminishing, resulting in the loss of her pincer and palmar grasp, as well as an inability to reach for objects. However, her hearing and visual senses remained partially intact, enabling her to react to loud sounds and perceive bright lights. She struggled to respond to conversations, and her social interactions were often negative. Moreover, she progressively showed signs of mental retardation. Beyond the usual symptoms, she experienced prolonged seizure attacks, recurrent pneumonia, and resistant hypertension. In addition to these, she had frequent medical encounters for multiple reasons, including diaper rashes, recurrent bed sores, skin abrasions, and chronic constipation. There was a history of multiple admissions into different hospitals and ICU with problems including but not limited to recurrent seizures, hypertension, chronic gastroesophageal reflux disease, recurrent urinary and respiratory infections, obstructive sleep apnea, and respiratory distress throughout her childhood.
On examination, she had elevated blood pressure. Neurological examination revealed spastic quadriplegia, augmented tendon reflexes, and Bilateral positive Babinski sign signifying symmetric pyramidal tract involvement. Her lower limb sensations were completely absent, but the reaction to painful stimuli was present in both the upper limbs. Other systemic examinations were unremarkable. Her investigations included complete blood count (CBC), urinalysis, electrolytes, renal function tests (RFT), liver function tests, C-reactive protein (CRP), and procalcitonin, with results within normal limits for age. EEG revealed nonspecific findings. Additional investigations such as arterial blood gas (ABG) analysis, urine cultures, and cerebrospinal fluid (CSF) analysis from a lumbar puncture (LP) showed no significant abnormalities, ruling out systemic infections or metabolic derangements. Further investigations such as nerve biopsy and EMG, were not performed.
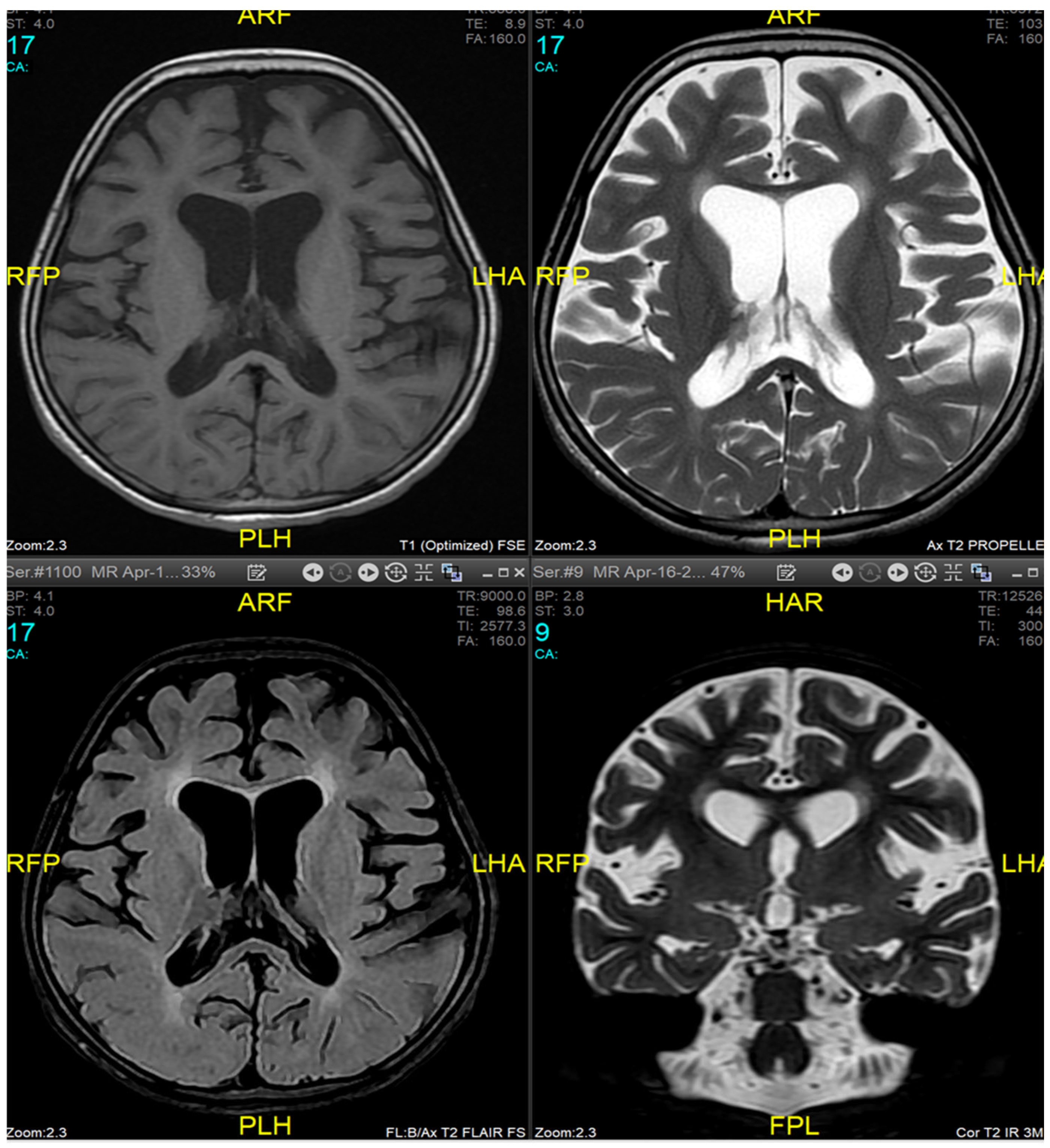
Magnetic resonance imaging (MRI) of the brain and genetic analysis were done to support the diagnosis. Her MRI now showed newly developed generalized atrophic changes of the brain, characterized by supra and infratentorial ventricular dilatation, prominent cortical sulci, and extra-axial CSF spaces such as Sylvian fissures and basal cistern. The diffuse deep periventricular white matter showed high signal intensities on T2/FLAIR (Fluid Attenuated Inversion Recovery) sequences. Additionally, there was global thinning out of the corpus callosum [
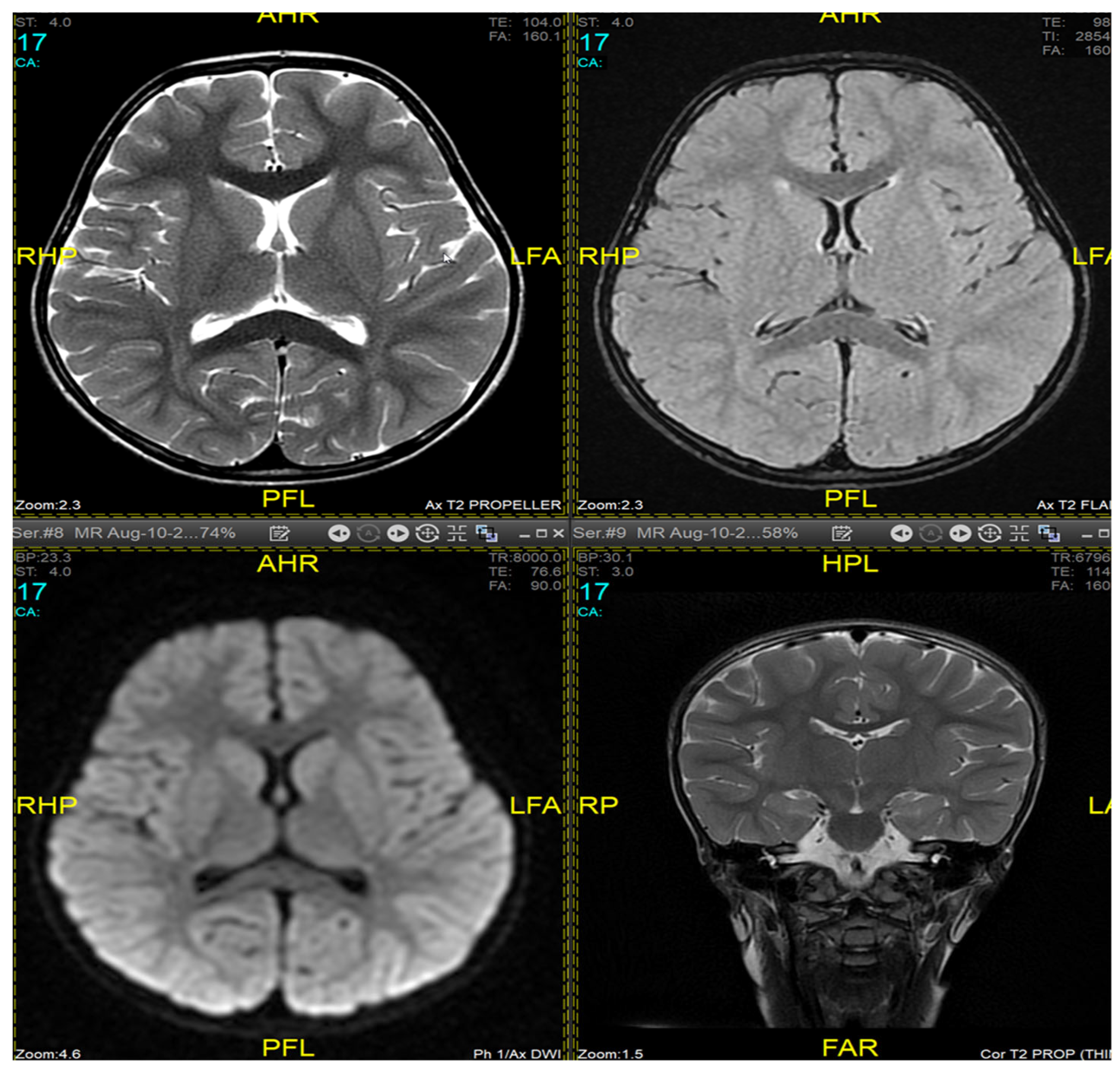
Figure 1]. This was in contrast to her prior brain MRI at the age of 2.5 years which showed no evidence of atrophic brain changes or iron deposition [
Figure 2].
Her molecular genetic analysis of whole exome sequencing (WES) at the age of 3.5 years identified the homozygous variant c.68G>A p.(Arg23Gln) chr22:38565366 on the PLA2G6 gene, which is predicted to have a pathogenic effect. Parallel analysis of parental WES revealed that both parents are heterozygous carriers.
She received supportive and targeted treatment for her problems involving a multidisciplinary team (MDT) aimed to prevent complications resulting from INAD. The received treatment included surgical interventions like PEG tube insertion, adenoidectomy, antiseizure medication Levetiracetam to control her seizures, topical/ systemic antibiotics with wound care for bed sores, Amlodipine for hypertension, and Pantoprazole for her gastrointestinal complaints. She then underwent tracheostomy to address her respiratory complications.
3. Discussion
Confusing clinical findings, along with the social and environmental factors, resulted in delayed diagnosis in our case [
6]
. Neuroaxonal dystrophies are a group of neurodegenerative diseases with autosomal recessive inheritance pattern. Intrauterine infections can result in structural and functional abnormalities of central nervous system [
7,
8]. Events in early neonatal life have not been found to affect the onset or clinical picture of INAD, however, postnatal complications were found to occur more frequently in INAD patients when compared to the general population [
3]. Our patient did not have any complications in the antenatal, intranatal, or postnatal period, and presented for the first time at 15 months of age. This is in accordance with another descriptive study involving 28 cases from different countries where the average age of presentation was reported to be 15 months (range 6 months- 3 years) [
3]. The natural history of the disease was reported in a study where speech impairment and loss of gross motor milestones were the earliest signs of the disease [
2,
3]. Nystagmus, seizure, gastrointestinal symptoms, skeletal deformities, and strabismus were the other reported symptoms [
2,
3]. Similarly, features of autonomic nervous system involvement like constipation, urinary retention or incontinence, reduced tear production, and temperature dysregulation were reported in patients of INAD by another study [
2]. In our patient, speech and hearing impairment presented later. Although our patient showed signs of motor impairment and seizures, there were no facial dyskinesia, tongue fasciculations, microcephaly, ataxia, tremors, strabismus, and nystagmus. Our patient exhibited hypertension, unlike other case reports. The differential diagnosis in children with similar features of severe psychomotor retardation, seizures, optic atrophy, blindness, and marked long-tract signs, at the age of around 3 years can be a lysosomal storage disorder like Schindler disease type I, which is caused by mutations in a-N-acetylgalactosaminidase (NAGA) [
9,
10,
11]. The MRI may also show diffuse white matter changes and demyelination. This condition is diagnosed by estimation of oligosaccharide in urine and assays of NAGA activity in leukocytes or fibroblasts [
9,
11,
12,
13,
14]. Urinary oligosaccharide was normal in our patient.
In 1979, a set of diagnostic criteria for INAD were defined based on an analysis of clinicopathological findings in 50 patients summarized as, (i) onset before 3 years, (ii) progressive course of diffuse central nervous system disorder with psychomotor deterioration and increasing neurological involvement comprising symmetrical pyramidal tract signs and marked hypotonia, and finally (iii) presence of axonal spheroids on biopsy of central or peripheral nervous system tissue [
6]. The role of MRI was not initially considered for this analysis. With the inclusion of imaging results, MRI features were considered by another study [
15]. Finally, with the advancement of molecular diagnostic techniques, skin biopsy is no longer used regularly and WES is currently playing a vital role in diagnosing patients with INAD by identifying pathological variants of the PLA2G6 gene [
5]. It is to be noted that various mechanisms for genetic changes in the gene exist and all of them may not have been explored yet. The mutations reported in the literature are homozygous mutations (MIM *603604), the gene encoding phospholipase A2 (PLA2) group VI (cytosolic, calcium-independent [Entrez Protein accession number NP_003551.2]) [
16], in the position C.2370 T>G (p. Y790X), and C. T2208G in the PLA2G6 gene [
6].Compound heterozygous mutations, c.1798C>T; p.R546W (rs368008077) + c.2357C>T; p.R741W (rs530348521) was also reported in another case [
5]. Furthermore, a study of 22 Indian families with INAD, ANAD, and DPC failed to detect any mutation in the PLA2G6 gene coding region in 45% of cases [
17].
MRI findings are variable depending on the disease stage at which imaging was performed [
1,
3,
9,
11]. Imaging may be completely normal in some patients, as was the case in our patient during the first presentation, making it insufficient to rule out INAD based on radiological findings only and necessitating follow up imaging later. Typical findings described include cerebral atrophy, white matter changes, and iron deposits in the globus pallidus, substantia nigra, and dentate nucleus [
18,
19]. Due to the presence of iron deposits, INAD can be classified as a subtype of neurodegeneration with brain iron accumulation diseases (NBIA). However, iron deposits are not specific to INAD [
20]. Our patient exhibited typical MRI features, on a follow-up study, but did not have any evidence of iron accumulation in the brain.
Management of INAD remains largely supportive, with no cure available yet. Possible curative therapies explored for a rare disease like INAD involving a defective enzyme are enzyme replacement, gene replacement, and gene correction [
21]. There are specific procedural challenges to enzyme replacement in the brain, to reach the mitochondria, and efficacy of the treatment. A recent research reported the successful use of gene therapy in patient-derived neural progenitor cells, in flies and mice in slowing the progress of INAD [
22]. However, human studies are underway and results are awaited. With the advancement of whole genome sequencing, newer genes are likely to be identified as a cause of INAD and can be targeted for gene therapy. For parents at risk of having an INAD-affected child, it is important to provide preconception counseling and parental carrier testing to accurately predict recurrence risk [
23]. Subsequently, offering parents preimplantation genetic testing [
24], or prenatal testing [
25,
26], which have been previously performed successfully. With the development of medical technology capable of detecting carriers, and easily performing genetic testing, at-risk couples may be identified and appropriately counseled on the disease before conception [
26]. It is necessary to report observations and disease progress of rare diseases for the development of novel therapeutic and investigative approaches in the future, and ultimately improve patient outcomes. We urge medical practitioners to consider conditions such as INAD as a possible cause of developmental regression in patients presenting such as described in our case.
4. Conclusions
INAD is a very rare progressive neurodegenerative disorder presenting in early childhood, with reduced life expectancy. Awareness about the disease will help in early diagnosis of this condition, prevention of complications, and appropriate parental counseling. There is no curative treatment available for INAD yet. There is a need for research to formulate targeted treatment and care plans for patients with this disease. Future research should also be targeted at identifying all genes responsible for INAD by whole genome sequencing and success of human trials for specific gene therapy.
Author Contributions
Conceptualization, A.F, S.A., A.Fa., M.M. and S.S.K; methodology, S.S.K.,R.D.,B.G. and M.G.; software, B.G. and S.S.K. ; validation, S.S.K.,R.D.,B.G. and M.G.; formal analysis, S.S.K.,R.D.,B.G. and M.G.; investigation, A.F, S.A., A.Fa. and M.M.; resources, S.S.K.,R.D.,B.G. and M.G. ; data curation, S.S.K.,R.D.,B.G. and M.G ; writing—original draft preparation, A.F, S.A., A.Fa. and M.M.; writing—review and editing, , S.S.K.,R.D.,B.G. and M.G.; visualization, A.F, S.A., A.Fa. and M.M.; supervision, S.S.K.,R.D.,B.G. and M.G.; project administration, S.S.K; funding acquisition-None. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Ministry of Health and Prevention Research ethics committee/RAK subcommittee Approval No- MOHAP/REC/2024/8-2024-PG-M dated- 22/02/2024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Dr Aamenah Barakat.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gregory, A.; Kurian, M.A.; Maher, E.R.; Hogarth, P.; Hayflick, S.J. PLA2G6-Associated Neurodegeneration. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993 [cited 2024 June 13]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1675/.
- Iodice, A.; Spagnoli, C.; Salerno, G.G.; Frattini, D.; Bertani, G.; Bergonzini, P.; et al. Infantile neuroaxonal dystrophy and PLA2G6-associated neurodegeneration: An update for the diagnosis. Brain Dev. 2017, 39, 93–100. [Google Scholar] [CrossRef]
- Altuame, F.D.; Foskett, G.; Atwal, P.S.; Endemann, S.; Midei, M.; Milner, P.; et al. The natural history of infantile neuroaxonal dystrophy. Orphanet J Rare Dis. 2020, 15, 109. [Google Scholar] [CrossRef]
- Kurian, M.A.; Morgan, N.V.; MacPherson, L.; Foster, K.; Peake, D.; Gupta, R.; et al. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN). Neurology. 2008, 29, 1623–1629. [Google Scholar] [CrossRef]
- Jain, S.; Bhasin, H.; Romani, M.; Valente, E.M.; Sharma, S. Atypical Childhood-onset Neuroaxonal Dystrophy in an Indian Girl. J Pediatr Neurosci. 2019, 14, 90–93. [Google Scholar] [CrossRef]
- Ansari, B.; Nasiri, J.; Namazi, H.; Sedghi, M.; Afzali, M. Infantile Neuroaxonal Dystrophy in Two Cases: Siblings with Different Presentations. Iran J Child Neurol. 2022, 16, 193–198. [Google Scholar] [CrossRef]
- Dube, R.; Kar, S.S. COVID-19 in pregnancy: The foetal perspective—A systematic review. BMJ Paediatr. Open 2020, 4, e000859. [Google Scholar] [CrossRef]
- Ganguli, S.; Chavali, P.L. Intrauterine Viral Infections: Impact of Inflammation on Fetal Neurodevelopment. Front. Neurosci. 2021, 15, 771557. [Google Scholar] [CrossRef]
- Sahyouni, J.K.; Odeh, L.B.M.; Mulla, F.; Junaid, S.; Kar, S.S.; Almarri, N.M.J.A. Infantile Sandhoff disease with ventricular septal defect: a case report. J Med Case Reports. 2022, 16, 317. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Kar, S.S.; Al Ramah, A.K. Progressive Pseudo-Rheumatoid Dysplasia a Rare Genetic Musculoskeletal Condition Causing Crippling Disability in a Young Boy- a Case Report. OBM Genetics. 2023, 7, 202. [Google Scholar] [CrossRef]
- Bakker, H.D.; de Sonnaville, M.L.C.S.; Vreken, P.; Abeling, N.G.G.M.; Groener, J.E.M.; Keulemans, J.L.M.; et al. Human alpha-N-acetylgalactosaminidase (alpha-NAGA) deficiency: no association with neuroaxonal dystrophy? Eur J Hum Genet. 2001, 9, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.; Kar, S.S.; Mahapatro, S.; Tripathy, R.; Mohanty, R. A rare cause of seizure-Dysgenesis of corpus callosum. Indian J. Pract. Paediatr. 2009, 11, 411–414. [Google Scholar]
- Morgan, N.V.; Westaway, S.K.; Morton, J.E.V.; Gregory, A.; Gissen, P.; Sonek, S.; et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006, 38, 752–754. [Google Scholar] [CrossRef]
- Aicardi, J.; Castelein, P. Infantile neuroaxonal dystrophy. Brain 1979, 102, 727–748. [Google Scholar] [CrossRef]
- Nardocci, N.; Zorzi, G.; Farina, l.; Binelli, S.; Scaioli, W.; Ciano, C.; et al. Infantile neuroaxonal dystrophy. Neurology. 1999, 52, 1472–1472. [Google Scholar] [CrossRef]
- Khateeb, S.; Flusser,H. ; Ofir, R.; Shelef, I.; Narkis, G. Am. J. Hum. Genet. 2006, 79, 942–948. [Google Scholar] [CrossRef]
- Kapoor, S.; Shah, M. H.; Singh, N.; Rather, M. I.; Bhat, V.; Gopinath, S.; et al. Genetic analysis of PLA2G6 in 22 Indian families with infantile neuroaxonal dystrophy, atypical late-onset neuroaxonal dystrophy and dystonia parkinsonism complex. PLoS One 2016, 11, e0155605. [Google Scholar] [CrossRef]
- Lehéricy, S.; Roze, E.; Goizet, C.; Mochel, F. MRI of neurodegeneration with brain iron accumulation. Curr Opin Neurol. 2020, 33, 462–473. [Google Scholar] [CrossRef]
- McNeill, A.; Birchall, D.; Hayflick, S.J.; Gregory, A.; Schenk, J.F.; Zimmerman, E.A.; et al. T2* and FSE MRI distinguishes four subtypes of neurodegeneration with brain iron accumulation. Neurology 2008, 70, 1614–1619. [Google Scholar] [CrossRef]
- Guo, Y.P.; Tang, B.S.; Guo, J.F. PLA2G6-Associated Neurodegeneration (PLAN): Review of Clinical Phenotypes and Genotypes. Front Neurol. 2018, 9, 1100. [Google Scholar] [CrossRef] [PubMed]
- Babin, P.L.; Rao, S.N.R.; Chacko, A.; Alvina, F.B.; Panwala, A.; Panwala, L.; et al. Infantile Neuroaxonal Dystrophy: Diagnosis and Possible Treatments. Front Genet. 2018, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Tepe, B.; McGrane, G.; Tipon, R.C.; Croft, G.; Panwala, L.; et al. Exploring therapeutic strategies for infantile neuronal axonal dystrophy (INAD/PARK14). Elife 2023, 12, e82555. [Google Scholar] [CrossRef] [PubMed]
- Solomons, J.; Ridgway, O.; Hardy, C.; Kurian, M.A.; Jayawant, S.; Hughes, S.; et al. Infantile neuroaxonal dystrophy caused by uniparental disomy. Dev Med Child Neurol. 2014, 56, 386–389. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, D.; Zhang, G.; Zhang, Z.; Liu, X.; Zhou, P.; et al. Successful clinical application of pre-implantation genetic diagnosis for infantile neuroaxonal dystrophy. Exp Ther Med. 2020, 19, 956–964. [Google Scholar] [CrossRef]
- Zou, Y.; Luo, H.; Yuan, H.; Xie, K.; Yang, Y.; Huang, S.; et al. Identification of a Novel Nonsense Mutation in PLA2G6 and Prenatal Diagnosis in a Chinese Family With Infantile Neuroaxonal Dystrophy. Front Neurol. 2022, 13, 904027. [Google Scholar] [CrossRef]
- Li, H.; Zou, Y.; Bao, X.; Wang, H.; Wang, J.; Jin, H.; et al. Monozygotic twins with infantile neuroaxonal dystrophy: A case report and literature review. Exp Ther Med. 2016, 12, 3387–3389. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).