1. Introduction
Supraventricular tachycardia (SVT) is the most common cardiac arrythmia among paediatric populations, affecting 1 in 250 to 1000 children. SVT is defined as rapid and regular heart rate, often exceeding 180 beats per minute (bpm) in children and 220 bpm in infants. SVT originates above the AV node [
1]. SVT is a broad group of tachyarrhythmias which includes atrioventricular (AV) node-dependant tachyarrhythmias, such as accessory pathway-mediated atrioventricular reciprocating tachycardia (AVNRT), as well as atrial tachycardias such as atrial fibrillation, atrial flutter and ectopic atrial tachycardia [
2]. Management of SVT depends on the patient’s condition [
3]. In stable SVT, treatment includes vagal manoeuvres, followed by adenosine if the vagal manoeuvres fail to convert SVT to NSR. Unstable SVT is treated with electrical cardioversion. Adenosine is not effective in the treatment of atrial flutter, atrial fibrillation, and ventricular tachycardia.
Pediatric patients may present different signs and symptoms of SVT depending on their age. Signs and symptoms of SVT in infants may include nonspecific indicators, such as poor feeding, vomiting, diaphoresis, hypersomia and irritability. Toddlers and school-aged children may present with classic cardiac symptoms, such as palpations, chest pain, shortness of breath, and syncope. Adolescents may present with this constellation of symptoms, accompanied by anxiety and decreased exercise tolerance. Before initiating appropriate SVT management, factors such as exertion, anxiety, stress, pain, respiratory failure, fever, infections, sepsis, hypoglycemia, poisoning, hypovolemia, pulmonary embolism, anaphylaxis, anemia, etc. must be ruled out [
4].
2. Case Report
The Helicopter Emergency Services (HEMS) was dispatched to a 3.5-year-old child with arrhythmia. The ground Emergency Medical Team (EMS) on site requested support in patient management and securing their transport to a specialist centre. The straight-line distance to the incident site from the HEMS base was 38 km. The nearest hospital with a paediatric cardiology department was 39 km away from the incident site. The medical crew spent the arrival time on a briefing, agreeing on the division of tasks between the team members, as well as planning management for a child with a body weight of 16 kg. As it was not possible to land directly at the site of the incident due to terrain obstacles (densely developed area, power lines and too steep terrain slope), it was recommended that the emergency medical personnel on site transport the patient to the helicopter landing site (approx. 1 km away). Once they arrived at the HEMS landing site by ambulance, the patient was lying on a stretcher, was verbally responsive (AVPU), drowsy, breathing on their own at a rate of approximately 30 breaths per minute on passive oxygen therapy (straight mask) with a flow of 5 litres per minute, pulse palpated at the carotid artery was approximately 200 beats per minute. No monitoring nor IV access was established. In repeated assessment, the patient remained verbally responsive (AVPU – V), airways were patent, not at risk - no secretions in the nasal cavity and oral cavity, respiration was efficient, 24 breaths per minute, no shortness of breath or respiratory effort, no central cyanosis, slight cyanosis on the periphery (phalanges), SpO
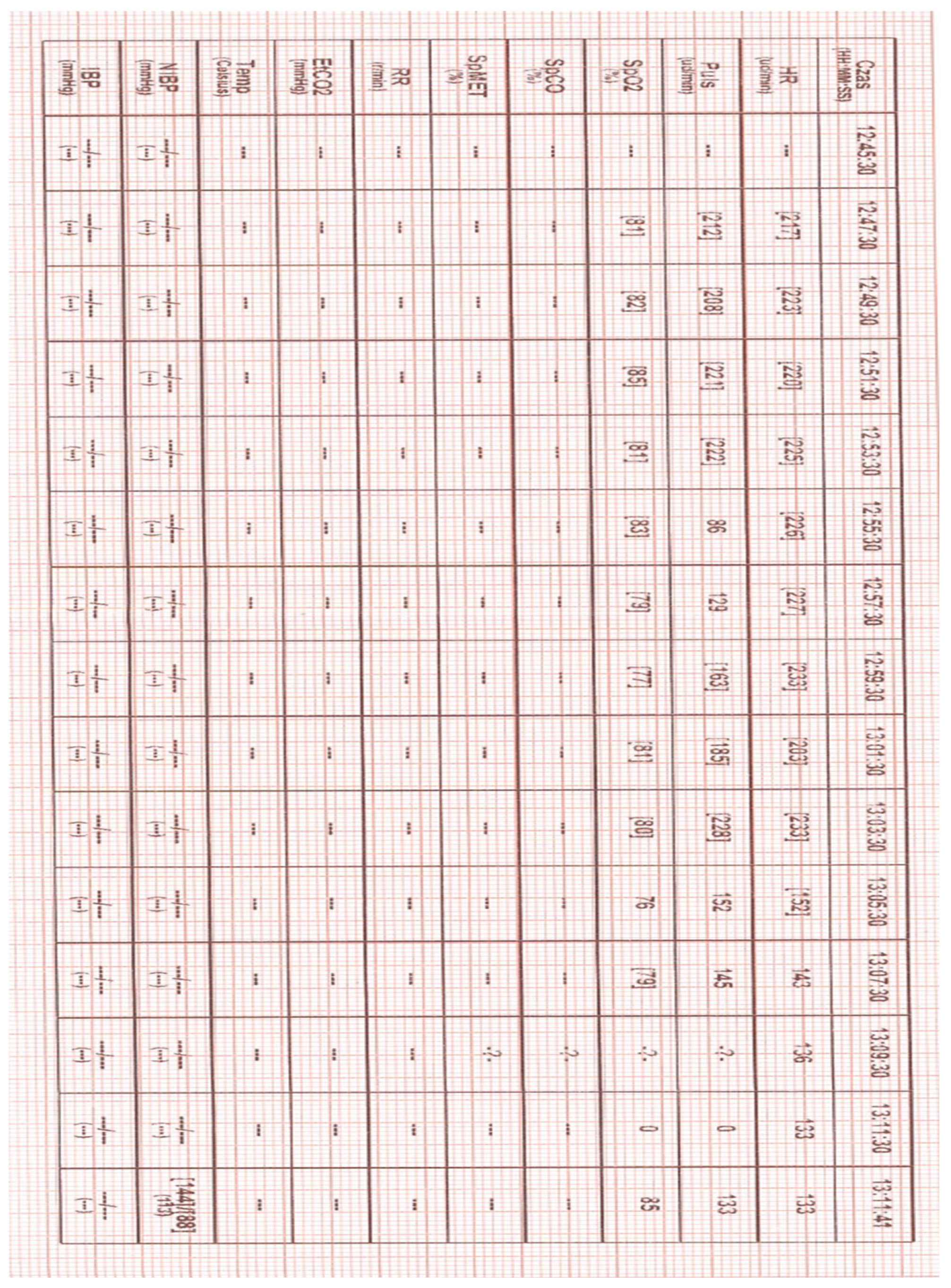
2 81 - 85% (HEMS increased oxygen flow to 10 L/min), auscultatory vesicular murmur symmetrical bilaterally, SVT with approx. 235 bpm, NBP 144/88, capillary refill normal, GCS 12 (E-4, V-4, M-5), glycemia normal, pupils equal - reactive to light, no meningeal symptoms, no convulsions, no visible injuries, no skin lesions, temperature normal. According to the information provided by the mother, the child had cardiological problems and was treated at a specialist clinic. For about 40 minutes the child presented with a very rapid heart rate, at first crying, then apathetic. During the ABCDE examination, two attempts to establish peripheral vascular access were unsuccessful. Due to the distance from the specialist center and air transport conditions (limited space and the no possibility of performing some procedures during a flight), after consultation with the specialist center, a decision was made to try to stabilize the patient on site. For this purpose, IO access was established by means of the EZ-IO system [Teleflex Medical Triangle Park, NC] with a 15 mm needle inserted into the right tibia near the tibial tuberosity. Once IO access was confirmed, 5 mL of 0.9% NaCl was administered first, followed by lidocaine 2% (without adrenaline and preservatives) at a dose of 0.5 mg/kg body weight (8 mg IO). As this failed to alleviate the patient’s pain, a second dose of lidocaine 2% was administered, constituting 1/2 of the first dose (4 mg IO). Adenosine was then prepared at a dose of 0.1 mg/kg body weight (1.5 mg), which was administred at 12:59 (event recorded in the attached heart monitor tracing) [
Figure 1]. the rhythm temporarily went from 233 bpm to 203 bpm. At. 13:05 it was decided to administer the second dose of 0.2 mg/kg body weight (3 mg) of adenosine. After about 2 minutes, the heart rate slowed down to 143 bpm and after another 10 minutes the patient's heart rate normalized (to 128 bpm) [
Figure 2]. In both administrations, adenosine was not diluted. For faster administration, a fluid bolus of 5 mL of 0.9% NaCl was then pushed. After reassessing the patient's condition, was transferred from the ambulance to the helicopter. During transport, the patient was stable, with no recurrence of SVT. The patient was transferred in a good general condition to the staff of the Hospital Emergency Department (ED).
3. Discussion
As mentioned previously, the choice of SVT management depends on the patient’s hemodynamic and clinical condition (stable or unstable). Clinical findings in SVT may differ depending on the age of the child and the duration of SVT. ERC and AHA guidelines recommend vagal manoeuvres and adenosine as first-line treatment for stable SVT [
5,
6].
Adenosine can serve as a diagnostic or therapeutic agent. In diagnostics, adenosine is used in myocardial perfusion stress imaging by virtue of its vasodilatory effects. In the therapeutic setting, adenosine is used for its antiarrhythmic properties in supraventricular tachycardia and can function as a diagnostic tool, depending on the type of SVT in both paediatric and adult patients [
7]. Adenosine is indicated as an adjunct to thallium-201 in myocardial perfusion scintigraphy, as well as for conversion of paroxysmal supraventricular tachycardia into sinus rhythm. Adenosine has a short duration of action (half-life <10 seconds) and a wide therapeutic window [
8]. Agonism of adenosine receptors A1 and A2 reduces conduction time in the atrioventricular node of the heart. Conduction time is decreased by inducing potassium efflux and inhibiting calcium influx through channels in nerve cells, leading to hyperpolarization and increased threshold for calcium-dependent action potentials. Decreased conduction time leads to an antiarrhythmic effect. Inhibition of calcium influx reduces the activity of adenylate cyclase, relaxing vascular smooth muscles. Relaxed vascular smooth muscles lead to increased blood flow through normal coronary arteries but not through stenotic arteries, allowing easier uptake of thallium-201 in normal coronary arteries [
8,
9]. The most frequently described side effects of adenosine administration are bronchoconstriction, seizures and hypersensitivity. Adenosine can also cause bradycardia leading to
torsade de pointes, especially in patients with a prolonged QT interval. A total of 6 cases of arrhythmia caused by the administration of adenosine in pediatric patients (aged 0-16 years with overt or hidden Wolf-Parkinson-White syndrome [WPW]) have been described in literature . The cases mentioned included: 3 cases of atrial fibrillation, 2 cases of atrial flutter and one case of ventricular fibrillation. In three cases, the arrhythmias resolved spontaneously, while in the remaining cases they required treatment with amiodarone or amiodarone administration and electrical cardioversion [
10]. Patients with an overdose of adenosine may present with asystole, heart block or cardiac ischemia. Those effects are generally short-lived. Patients with an overdose should receive symptomatic and supportive care, which may include a slow intravenous injection of theophylline [
7,
8].
Adenosine failure happens when its administration does not result in a definitive or sustained termination of tachycardia. There are three scenarios that cause this: administration errors (e.g. insufficient adenosine dose), failure to terminate the arrhythmia and insufficient duration of adenosine effect [
11].
Adenosine should be administered into a venous vessel as close as possible to the heart (vein in the antecubital bend). Literature describes two administration techniques. Standard guidelines recommended adenosine to be administered IV with an immediate bolus of normal saline solution (NSS; double-syringe technique [DST]). Only one syringe is used as part of the second technique (single-syringe technique [SST]). In this technique, adenosine is diluted with NSS. SST may be as safe as DST, equally effective for SVT termination, or even potentially more effective with the first dose. SST constitutes a simpler and more rapid approach, obviating the need for syringe switching or using a three-way stopcock (which was actually used to administer the drug in the described case) and reducing the margin of error in adenosine administration [
12,
13]. In the described case, DST was used. Literature formulates no recommendations regarding the selection of adenosine delivery technique when intraosseous access is used.
The IO technique has presented itself as a possible method of adenosine administration. It’s fast, reliable, needs minimal training and can be implemented rapidly. This technique is recommended by AHA guidelines [
5]. ERC indicates intravenous administration as the preferred method of adenosine administration (insufficient evidence supporting effective and safe IO adenosine administration) [
6]. The ERC indicates exactly when intraosseous access should be performed as part of Pediatric Advanced Life Support (PALS). An attempt to establish intraosseous access should be made when the chances of obtaining peripheral access are low, already at an early stage of treatment (alternative access) [
5]. Multiple studies have shown the importance and reliability of IO access in the pediatric population [
14]. IO access refers to the placement a specialized hollow bore needle through the cortex of a bone into the medullary space for infusion or collecting laboratory samples (before submitting the sample for laboratory testing, it must be properly marked). The distal femur, proximal tibia and distal tibia are recommended sites for IO placement in neonates and children. The preferred access site in infants and children is the anteromedial surface of the tibia, approximately 1 to 2 cm below the tibia tuberosity [
15]. The choice of where to perform intraosseous access depends mainly on the device used. It seems reasonable to assume that access, as in the case of venous access, should be performed as close to the heart as possible (humeral head) due to the short half-life of adenosine in the bloodstream. However, obtaining such access will depend on the device available. Currently, pre-hospital emergency services in Poland (emergency medical services, hospital emergency departments and HEMS) have access to both mechanical and manual devices for IO access. The mechanical devices include NIO-P (New Intraosseous – Pediatric, PerSys Medical, Houston, Tx), BIG-P (Bone Injection Gun – Pediatric, PerSys Medical, Houston, Tx) and Arrow IZ-IO (Teleflex Medical Triangle Park, NC – used by HEMS teams in Poland). When it comes to manual devices, the Jamshidi IO needle (Jamshidi, Baxter HealthCare Corporation, Deerfield, Ill) and Cook IO needle (Cook Medical, O’Halloran Road National Technology Park Limerick, Ireland) are available. Mechanical devices used to perform IO access are more reliable, take a shorter time to obtain vascular access and require less training before they can be used correctly.. An important element when selecting a device for intraosseous access is the chance of success in performing this type of access with the first attempt. Mechanical devices are much more advantageous also in this aspect [
16]. Regardless of whether intraosseous access is performed using a mechanical or manual device, a three-way tap will be an integral part of the access. Its use requires a certain amount of fluid inside, which may affect the therapeutic effect of adenosine, especially when SST is used (too low drug dose).
One of the elements of the intraosseous access procedure is proper analgesia. When using the IZ-IO device, the manufacturer recommends 0.5 ml/kg body weight of 2% lidocaine without adrenaline and preservatives in a slow bolus (120 seconds), followed by 2-5 ml NS push (60 seconds) [
17]. Although lignocaine has antiarrhythmic effects, it is not a pharmacological agent recommended by the AHA and ERC for interrupting an episode of SVT. However, its influence on the condition of the presented patient cannot be ruled out.
In literature on intraosseous adenosine administration, there is evidence both confirming and rejecting the effectiveness of adenosine administration via this route. In 1994, research was carried out on piglets to demonstrate whether adenosine used IO was effective or not. Conclusions from this research indicated that the intraosseous route is an effective way of administering adenosine. Moreover, the peripheral venous dose required to achieve atrioventricular block is higher than the central venous dose, while the intraosseous dose falls between the central venous and peripheral venous doses of adenosine [
18]. The effectiveness of adenosine in terminating supraventricular tachycardia in a pediatric patient was confirmed in the case of an 11-day-old boy described by Friedman (conversion to NSR with 100 mcg of adenosine, an early dose was given prior to attempting cardioversion, a saline flush was not reported) [
19]. In 2012, a paper by Goodman and Lu was published, presenting cases of a 2-month-old and a 4-month-old child diagnosed with SVT. In both cases, administration of adenosine did not achieve the intended result. In the first case, rhythm conversion occurred through the administration of adenosine via intravenous access (250 mcg IO, 100 mcg IV). In the second case central access was used to administer the drug (200 mcg IO), but with SVT recurrence [
20]. The cases described by Friedman and Goodman and Lu were compiled by Clark. Clark stated that intraosseous adenosine does not appear to be as effective as intravenous administration. However, he emphasizes that the reason for the failure of intraosseous administration may be the delivery technique (drug dose, NS bolus rate) [
21]. In the following years, papers were published, proving the effectiveness of intraosseous adenosine administration. Helleman et al. described the case of a 2-week-old boy who experienced an episode of SVT. Conversion to NSR was achieved with 0.5 mg adenosine diluted to 3 ml NS (SST technique) [
22]. İlknur et al. reported the case of a 28-day-old boy in whom conversion of SVT to NSR was achieved with adenosine (3450 grams - 0.1 mcg of adenosine per kg, single dose) [
23]. The described case concerns a child older than 12 months, which makes it unique in the context of previously described cases.