Submitted:
27 June 2024
Posted:
02 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
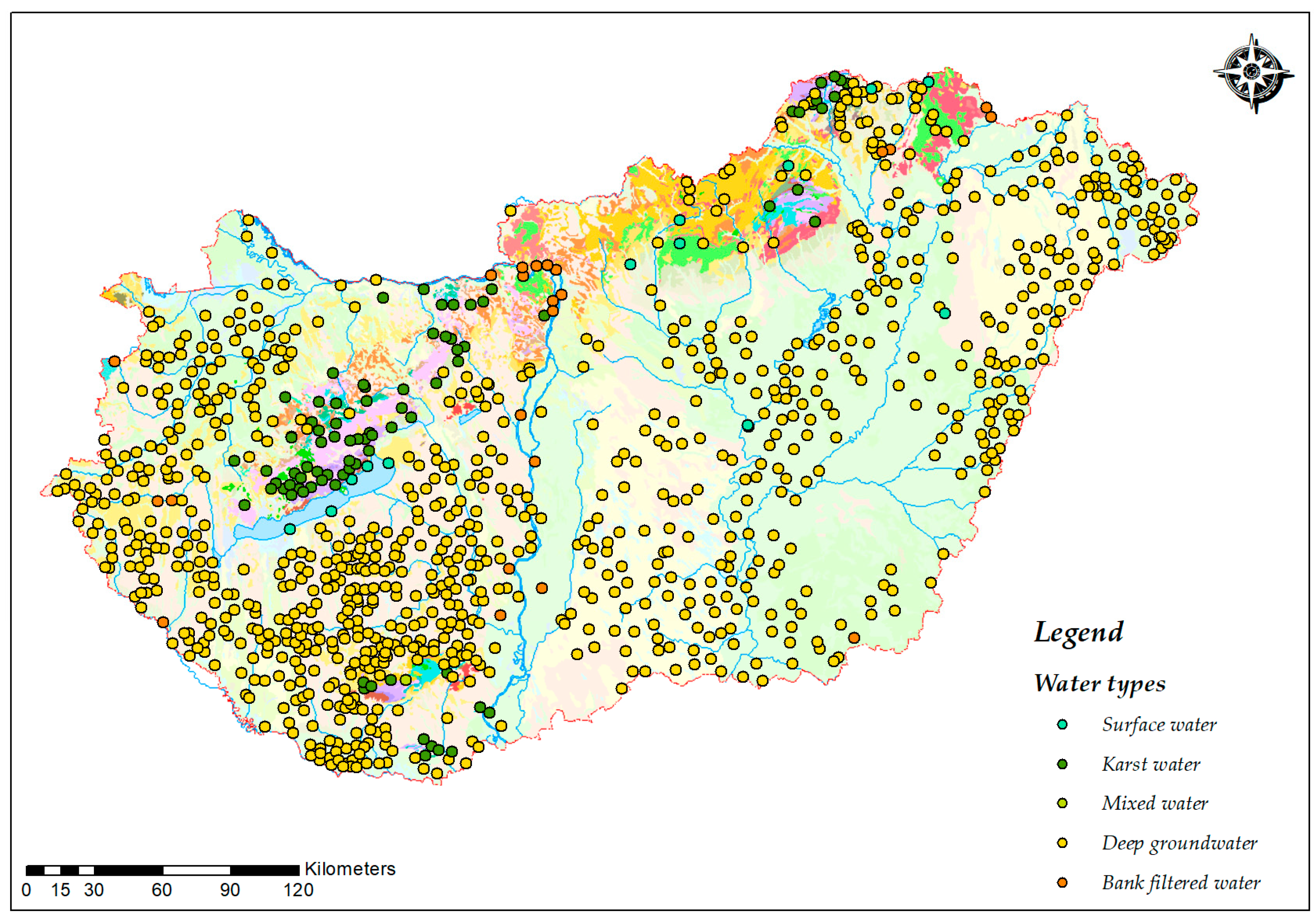
2.1. Study Design
2.2. Sampling
2.3. Chemical Analysis
2.4. Statistical and Geospatial Methods
3. Results
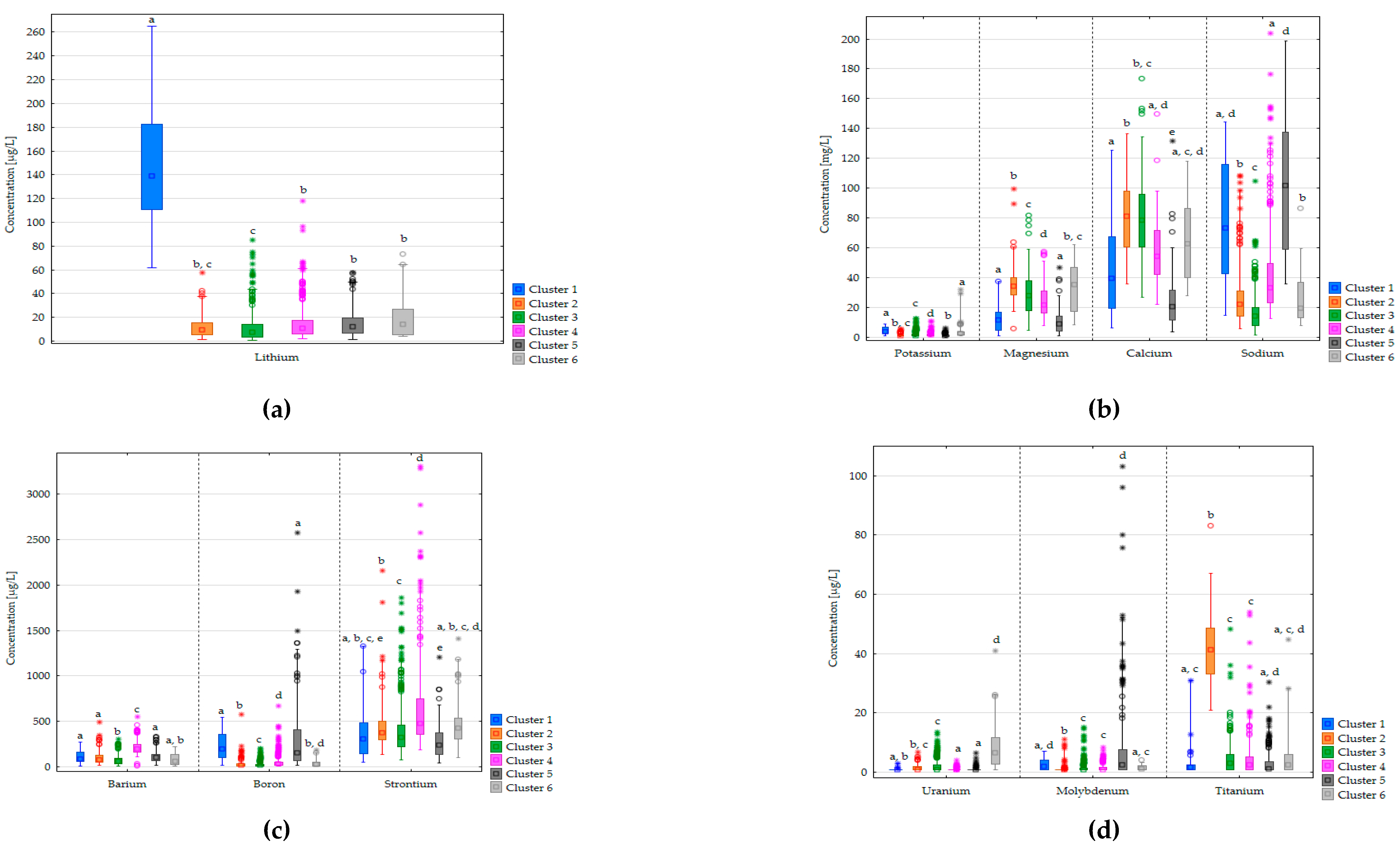
3.1. Prevalence and Distribution of Natural Elements
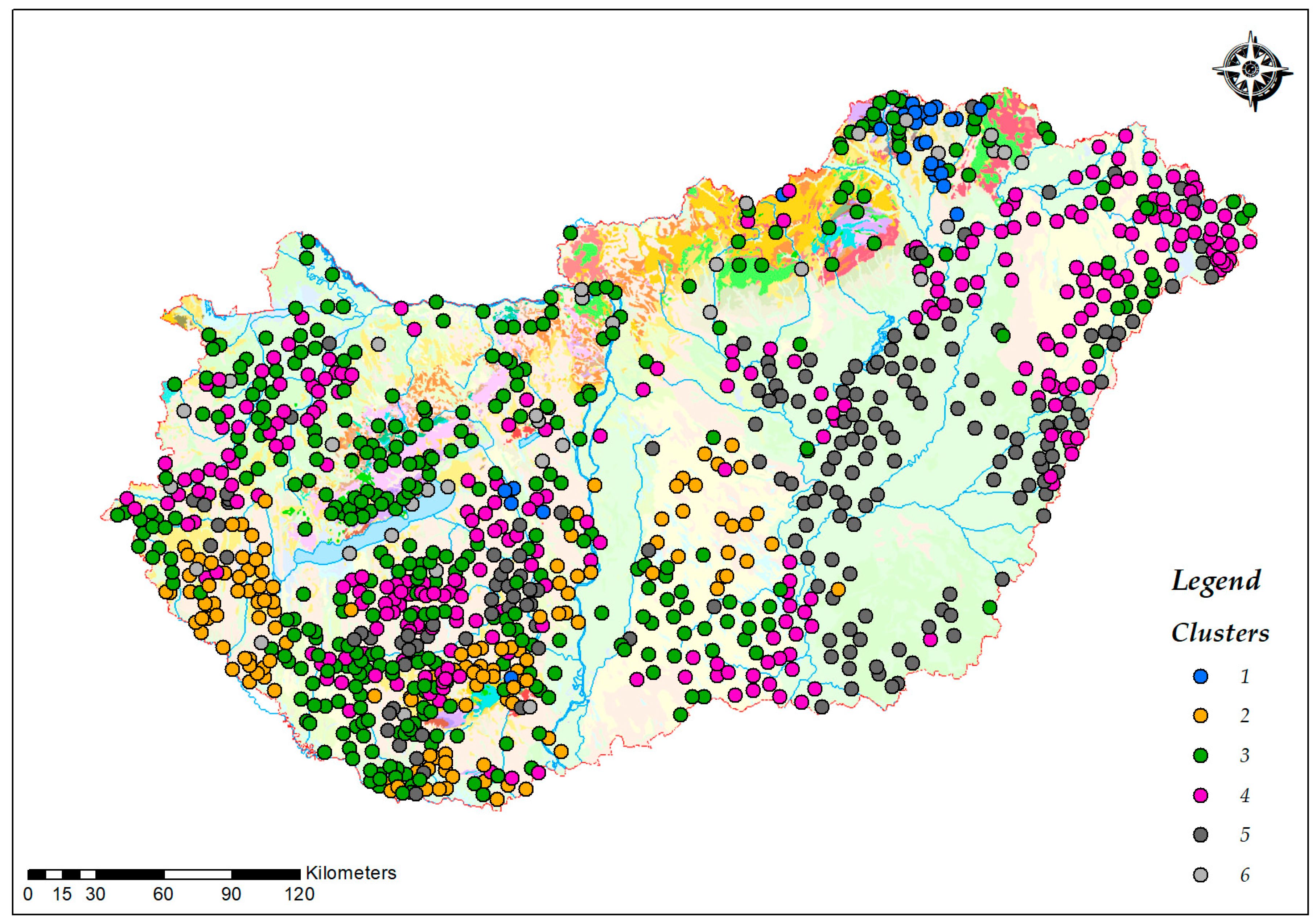
3.2. Water Supply System Clusters by Water Composition
3.3. Spatial Distribution
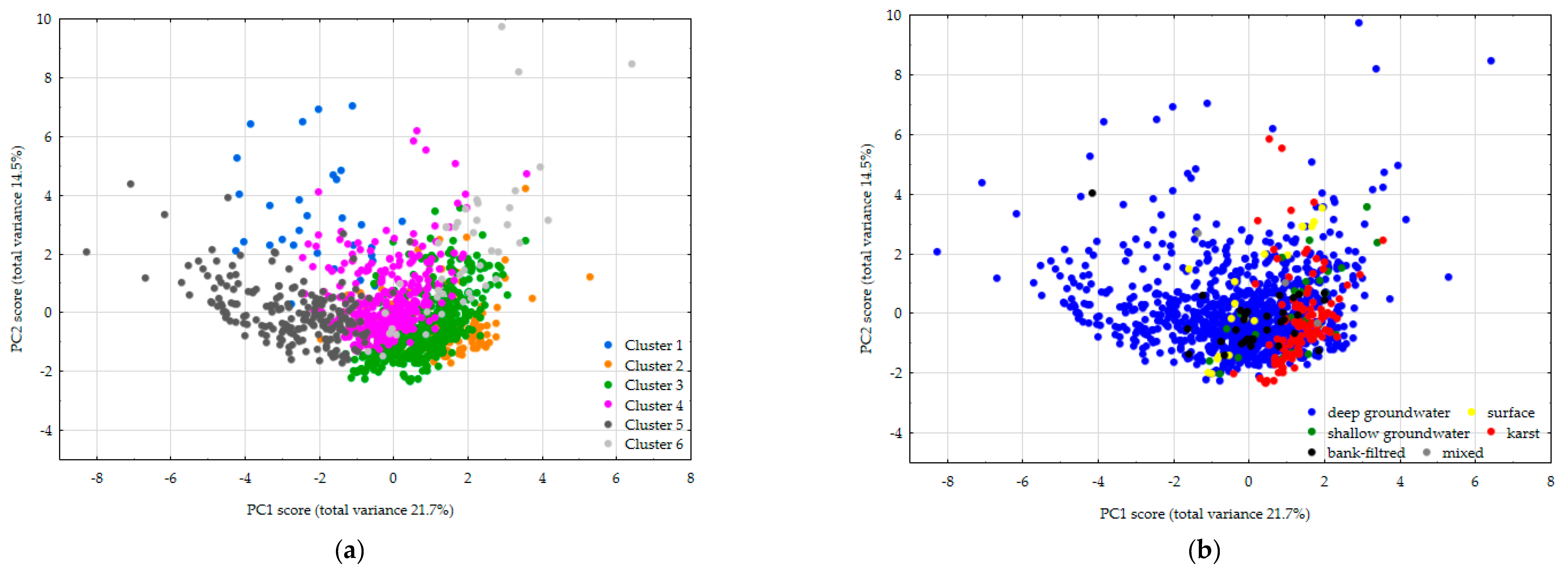
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The European Parliament and the Council of the European Union, ‘Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption.‘ 2020 [Online]. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184.
- World Health Organization, ‘Water safety plan manual Step-by-step risk management for drinking-water suppliers Second edition’. World Health Organization, 2023 [Online]. Available: https://iris.who.int/bitstream/handle/10665/366148/9789240067691-eng.pdf?sequence=1.
- Council of the European Union, ‘Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption.‘ 1998 [Online]. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31998L0083.
- World Health Organization, ‘Guidelines for drinking-water quality‘, Fourth edition incorporating the first and Second addenda. Geneva: World Health Organization, 2022 Available: https://iris.who.int/bitstream/handle/10665/352532/9789240045064-eng.pdf?sequence=1.
- European Food Safety Authority (EFSA), ‘Uranium in foodstuffs, in particular mineral water’, EFS2, vol. 7, no. 4, 0 2009. [CrossRef]
- Jerome Nriagu, Dong-Ha Nam, Titilayo A. Ayanwola, Hau Dinh, Erdenebayar Erdenechimeg, Chimedsuren Ochirb and Tsend-Ayush Bolormaa ., ‘High levels of uranium in groundwater of Ulaanbaatar, Mongolia’, Science of The Total Environment, vol. 414, pp. 722–726, Jan. 2012. [CrossRef]
- D. Cicchella, S. Albanese, B. De Vivo, E. Dinelli, L. Giaccio, A. Lima, P. Valera, ‘Trace elements and ions in Italian bottled mineral waters: Identification of anomalous values and human health related effects’, Journal of Geochemical Exploration, vol. 107, no. 3, pp. 336–349, Dec. 2010. [CrossRef]
- B. Frengstad, A. K. Midtgård Skrede, D. Banks, J. Reidar Krog, and U. Siewers, ‘The chemistry of Norwegian groundwaters: III. The distribution of trace elements in 476 crystalline bedrock groundwaters, as analysed by ICP-MS techniques’, Science of The Total Environment, vol. 246, no. 1, pp. 21–40, Jan. 2000. [CrossRef]
- Y. Wu, Y. Wang, and X. Xie, ‘Occurrence, behavior and distribution of high levels of uranium in shallow groundwater at Datong basin, northern China’, Science of The Total Environment, vol. 472, pp. 809–817, Feb. 2014. [CrossRef]
- D. Cinti, P. P. Poncia, L. Brusca, F. Tassi, F. Quattrocchi, and O. Vaselli, ‘Spatial distribution of arsenic, uranium and vanadium in the volcanic-sedimentary aquifers of the Vicano–Cimino Volcanic District (Central Italy)’, Journal of Geochemical Exploration, vol. 152, pp. 123–133, May 2015. [CrossRef]
- L. Jiang, Pengcheng He, Jiyan Chen, Yong Liu, Dehui Liu, Genggeng Qin and Ning Tan, ‘Magnesium Levels in Drinking Water and Coronary Heart Disease Mortality Risk: A Meta-Analysis’, Nutrients, vol. 8, no. 1, p. 5, Jan. 2016. [CrossRef]
- L. A. Catling, I. Abubakar, I. R. Lake, L. Swift, and P. R. Hunter, ‘A systematic review of analytical observational studies investigating the association between cardiovascular disease and drinking water hardness’, Journal of Water and Health, vol. 6, no. 4, pp. 433–442, Dec. 2008. [CrossRef]
- World Health Organization, ‘Calcium and magnesium in drinking water : Public health significance’, Calcium and magnesium in drinking water : Beneficial impacts on health, p. 180, 2009 Available: https://iris.who.int/bitstream/handle/10665/43836/9789241563550_eng.pdf?sequence=1.
- V. Gianfredi, N. L. Bragazzi, D. Nucci, M. Villarini, and M. Moretti, ‘Cardiovascular diseases and hard drinking waters: Implications from a systematic review with meta-analysis of case-control studies’, Journal of Water and Health, vol. 15, no. 1, pp. 31–40, Feb. 2017. [CrossRef]
- F. Kozisek, ‘Regulations for calcium, magnesium or hardness in drinking water in the European Union member states’, Regulatory Toxicology and Pharmacology, vol. 112, p. 104589, Apr. 2020. [CrossRef]
- R. Russo, S. Sciacca, D. I. La Milia, A. Poscia, and U. Moscato, ‘Vanadium in drinking water: Toxic or therapeutic?! Systematic literature review and analysis of the population exposure in an Italian volcanic region’, European Journal of Public Health, vol. 24, no. suppl_2, Oct. 2014. [CrossRef]
- M. Ferrante, G. Oliveri Conti, Z. Rasic-Milutinovic, and D. Jovanovic, ‘Health effects of metals and related substances in drinking water‘ London: IWA publishing, 2014.
- Y. Vasseghian, S. Sadeghi Rad, J. A. Vilas–Boas, and A. Khataee, ‘A global systematic review, meta-analysis, and risk assessment of the concentration of vanadium in drinking water resources’, Chemosphere, vol. 267, p. 128904, Mar. 2021. [CrossRef]
- N. Kabacs, A. Memon, T. Obinwa, J. Stochl, and J. Perez, ‘Lithium in drinking water and suicide rates across the East of England’, Br J Psychiatry, vol. 198, no. 5, pp. 406–407, May 2011. [CrossRef]
- V. Liaugaudaite, N. Mickuviene, N. Raskauskiene, R. Naginiene, and L. Sher, ‘Lithium levels in the public drinking water supply and risk of suicide: A pilot study’, Journal of Trace Elements in Medicine and Biology, vol. 43, pp. 197–201, Sep. 2017. [CrossRef]
- M. Pompili, Maurizio Pompili, Monica Vichi, Enrico Dinelli, Roger Pycha, Paolo Valera, Stefano Albanese, Annamaria Lima, Benedetto De Vivo, Domenico Cicchella, Andrea Fiorillo, Mario Amore, Paolo Girardi, Ross J Baldessarini, ‘Relationships of local lithium concentrations in drinking water to regional suicide rates in Italy’, The World Journal of Biological Psychiatry, vol. 16, no. 8, pp. 567–574, Nov. 2015. [CrossRef]
- Giotakos, P. Nisianakis, G. Tsouvelas, and V.-V. Giakalou, ‘Lithium in the Public Water Supply and Suicide Mortality in Greece’, Biol Trace Elem Res, vol. 156, no. 1–3, pp. 376–379, Dec. 2013. [CrossRef]
- Nestor D. Kapusta, Nilufar Mossaheb, Elmar Etzersdorfer, Gerald Hlavin, Kenneth Thau, Matthaus Willeit, Nicole Praschak-Rieder, Gernot Sonneck and Katharina Leithner-Dziubas, ‘Lithium in drinking water and suicide mortality’, Br J Psychiatry, vol. 198, no. 5, pp. 346–350, May 2011. [CrossRef]
- Balint Izsak, Anna Hidvegi, Lajos Balint, Tibor Malnasi, Marta Vargha, Tamas Pandics, Zoltan Rimer, and Peter Dome ‘Investigation of the association between lithium levels in drinking water and suicide mortality in Hungary’, Journal of Affective Disorders, vol. 298, pp. 540–547, Feb. 2022. [CrossRef]
- Fateme Barjasteh-Askari, Mojtaba Davoudi, Homaon Amini, Moamad Ghorbni, Medi Yaseri, Masoud Yunesian, Amir Hossein Mahvi and David Lester, ‘Relationship between suicide mortality and lithium in drinking water: A systematic review and meta-analysis’, Journal of Affective Disorders, vol. 264, pp. 234–241, Mar. 2020. [CrossRef]
- Anjum Memon, Imogen Rogers, Sophie M D D Fitzsimmons, Ben Cater, Rebecca Strawbridge, Diego Hidalgo-Mazzei, and Allan H Young, ‘Association between naturally occurring lithium in drinking water and suicide rates: Systematic review and meta-analysis of ecological studies’, Br J Psychiatry, vol. 217, no. 6, pp. 667–678, Dec. 2020. [CrossRef]
- B. Eyre-Watt, E. Mahendran, S. Suetani, J. Firth, S. Kisely, and D. Siskind, ‘The association between lithium in drinking water and neuropsychiatric outcomes: A systematic review and meta-analysis from across 2678 regions containing 113 million people’, Aust N Z J Psychiatry, vol. 55, no. 2, pp. 139–152, Feb. 2021. [CrossRef]
- R. Abejón, ‘A Bibliometric Analysis of Research on Selenium in Drinking Water during the 1990–2021 Period: Treatment Options for Selenium Removal’, IJERPH, vol. 19, no. 10, p. 5834, May 2022. [CrossRef]
- E. Barron, V. Migeot, S. Rabouan, M. Potin-Gautier, F. Se ́ by, P. Hartemann, Y. Lévi, and B. Legube, ‘The case for re-evaluating the upper limit value for selenium in drinking water in Europe’, Journal of Water and Health, vol. 7, no. 4, pp. 630–641, Dec. 2009. [CrossRef]
- ‘Magyarország ivóvizének minősége, 2022.’, National Center for Public Health and Pharmacy, 2023 [Online]. Available: https://www.nnk.gov.hu/attachments/article/726/2023_9_Ivo%CC%81vi%CC%81zmino%CC%8Bse%CC%81g%202022.pdf.
- Erőss, K. Csondor, B. Izsák, M. Vargha, Á. Horváth, and T. Pándics, ‘Uranium in groundwater – The importance of hydraulic regime and groundwater flow system’s understanding’, Journal of Environmental Radioactivity, vol. 195, pp. 90–96, Dec. 2018. [CrossRef]
- K. Csondor, P: Baják, H. Sureck, B. Izsák, Á. Horváth, M. Vagha, A. Erőss, ‘Transient nature of riverbank filtered drinking water supply systems - A new challenge of natural radioactivity assessment’, Journal of Environmental Radioactivity, vol. 211, p. 106072, Jan. 2020. [CrossRef]
- Petra Baják, Katalin Hegedűs-Csondor, Mia Tiljander, Kirsti Korkka-Niemi, Heinz Surbeck, Bálint Izsák, Márta Vargha, Ákos Horváth, Tamás Pándics and Anita Erőss, ‘Integration of a Shallow Soda Lake into the Groundwater Flow System by Using Hydraulic Evaluation and Environmental Tracers’, Water, vol. 14, no. 6, p. 951, Mar. 2022. [CrossRef]
- Petra Baják, Bence Molnár, Katalin Hegedűs-Csondor, Mia Tiljander, Viktor Jobbágy, Viktória Kohuth-Ötvös, Bálint Izsák, Márta Vargha , Ákos Horváth, Emese Csipa, Mihály Óvári, Csaba Tóbi, Péter Völgyesi, Krzysztof Pelczar, Mikael Hult, and Anita Erőss, ‘Natural Radioactivity in Drinking Water in the Surroundings of a Metamorphic Outcrop in Hungary: The Hydrogeological Answer to Practical Problems’, Water, vol. 15, no. 9, p. 1637, Apr. 2023. [CrossRef]
- C. W. Croghan and P. P. Egeghy, ‘Methods of Dealing with Values Below the Limit of Detection using SAS’. US-EPA, 2003 Record ID: 64046.
- Brian S. Everitt, Sabine Landau, Morven Leese, and Daniel Stahl, Cluster analysis, 5th ed. in Wiley series in probability and statistics. Chichester, West Sussex, U.K: Wiley, 2011. [CrossRef]
- S. Wold, K. Esbensen, and P. Geladi, ‘Principal component analysis’, Chemometrics and Intelligent Laboratory Systems, vol. 2, no. 1–3, pp. 37–52, Aug. 1987. [CrossRef]
- Ádám Tóth, Petra Baják , Márk Szijártó , Mia Tiljander, Kirsti Korkka-Niemi, Nina Hendriksson and Judit Mádl-Szőnyi, ‘Multimethodological Revisit of the Surface Water and Groundwater Interaction in the Balaton Highland Region—Implications for the Overlooked Groundwater Component of Lake Balaton, Hungary’, Water, vol. 15, no. 6, p. 1006, Mar. 2023. [CrossRef]
- J. Tóth, ‘Groundwater as a geologic agent: An overview of the causes, processes, and manifestations’, Hydrogeology Journal, vol. 7, no. 1, pp. 1–14, Feb. 1999. [CrossRef]
- G. Dura, P. Rudnai, M. Kádár, and M. Vargha, ‘The health risks of consuming drinking water with elevated arsenic content of geochemical origin’, Central European Geology, vol. 57, no. 3, pp. 307–316, Sep. 2014. [CrossRef]
- ‘Módszertani Útmutató Ivóvizek radiológiai paramétereinek vizsgálatához és értékeléséhez 2023’. National Center for Public Health and Pharmacy, 2023 [Online]. Available: https://www.nnk.gov.hu/attachments/article/726/2023_8_M%C3%B3dsz.%C3%BAtm._Iv%C3%B3viz_radiologiai%20vizsg..pdf.
- M. Ćurković, L. Sipos, D. Puntarić, K. Dodig-Ćurković, N. Pivac, and K. Kralik, ‘Arsenic, Copper, Molybdenum, and Selenium Exposure through Drinking Water in Rural Eastern Croatia’, Pol. J. Environ. Stud., vol. 25, no. 3, pp. 981–992, 2016. [CrossRef]
- P. L. Smedley, D. M. Cooper, and D. J. Lapworth, ‘Molybdenum distributions and variability in drinking water from England and Wales’, Environ Monit Assess, vol. 186, no. 10, pp. 6403–6416, Oct. 2014. [CrossRef]
| Variable | Dimension | LOQ | n | Minimum | Lower quartile | Mean | Std.Dev. | Median | Upper quartile | Maximum | % of samples <LOQ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | µg/L | 10 | 1256 | <LOQ | 13 | 84 | 185 | 25 | 62 | 2570 | 16 |
| Ba | µg/L | 10 | 1256 | <LOQ | 54 | 120 | 83 | 103 | 170 | 551 | 2 |
| Be | µg/L | 1.0 | 1256 | <LOQ | <LOQ | <LOQ | 0 | <LOQ | <LOQ | <LOQ | 100 |
| Ca | mg/L | 0.50 | 1256 | 2.5 | 40 | 64 | 31 | 63 | 85 | 174 | 0 |
| Co | µg/L | 1.0 | 1256 | <LOQ | <LOQ | <LOQ | 0.42 | <LOQ | <LOQ | 11 | 99 |
| K | mg/L | 0.50 | 1256 | <LOQ | 1.1 | 1.9 | 1.9 | 1.4 | 2.0 | 32 | 2 |
| Li | µg/L | 1.0 | 1256 | <LOQ | 5.1 | 16 | 25 | 10 | 17 | 265 | 2 |
| Mg | mg/L | 0.50 | 1256 | 0.55 | 15 | 25 | 14 | 23 | 35 | 100 | 0 |
| Mo | µg/L | 1.0 | 1256 | <LOQ | <LOQ | 2.4 | 6.6 | <LOQ | 1.6 | 103 | 58 |
| Na | mg/L | 0.50 | 1256 | <LOQ | 15 | 40 | 40 | 25 | 50 | 252 | 0 |
| Se | µg/L | 1.0 | 1256 | <LOQ | <LOQ | <LOQ | 0.46 | <LOQ | <LOQ | 7.0 | 86 |
| Sr | µg/L | 10 | 1256 | 21 | 241 | 448 | 354 | 366 | 519 | 3310 | 0 |
| Ti | µg/L | 1.0 | 1256 | <LOQ | <LOQ | 8.4 | 14 | 2.9 | 7.3 | 83 | 31 |
| U | µg/L | 1.0 | 1256 | <LOQ | <LOQ | 1.5 | 2.4 | <LOQ | 1.3 | 41 | 72 |
| V | µg/L | 1.0 | 1256 | <LOQ | <LOQ | <LOQ | 0.85 | <LOQ | <LOQ | 17 | 91 |
| Variable | B | Ba | Ca | K | Li | Mg | Mo | Na | Se | Sr | Ti | U | V |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 1.0000 | ||||||||||||
| Ba | 0.0621 | 1.0000 | |||||||||||
| Ca | -0.3420 | -0.0809 | 1.0000 | ||||||||||
| K | 0.0875 | 0.0403 | 0.1315 | 1.0000 | |||||||||
| Li | 0.2236 | 0.0838 | -0.0813 | 0.4015 | 1.0000 | ||||||||
| Mg | -0.2747 | 0.0612 | 0.5562 | 0.1469 | -0.0243 | 1.0000 | |||||||
| Mo | 0.3175 | -0.0234 | -0.2026 | -0.0067 | 0.0415 | -0.1592 | 1.0000 | ||||||
| Na | 0.5370 | 0.2132 | -0.5792 | 0.0611 | 0.2606 | -0.3611 | 0.3008 | 1.0000 | |||||
| Se | -0.0614 | -0.0811 | 0.1124 | 0.0818 | 0.0401 | 0.1862 | -0.0333 | -0.0841 | 1.0000 | ||||
| Sr | -0.0984 | 0.3538 | 0.1649 | 0.1933 | 0.1898 | 0.4615 | -0.0911 | 0.0021 | 0.0619 | 1.0000 | |||
| Ti | -0.0796 | -0.0622 | 0.3296 | -0.0229 | -0.0475 | 0.2999 | -0.0709 | -0.1128 | -0.0696 | 0.0323 | 1.0000 | ||
| U | -0.0819 | -0.1528 | 0.1199 | 0.1362 | 0.0449 | 0.2978 | 0.0205 | -0.1064 | 0.4326 | 0.1069 | -0.0059 | 1.0000 | |
| V | -0.0353 | -0.1312 | 0.0167 | 0.1988 | -0.0467 | 0.0322 | 0.0213 | -0.0807 | 0.0463 | -0.0496 | -0.0232 | 0.1333 | 1.0000 |
| Cluster number | Characteristic element | Σn | Distribution by water type | |||||
|---|---|---|---|---|---|---|---|---|
| deep groundw. | karst w. | shallow groundw. | surface w. | bank filtered w. | mixed w. | |||
| Cluster 1 | Li, B, Na, Mo, K | 31 | 27 | 1 | 1 | 1 | 1 | 0 |
| Cluster 2 | Ti, Ca, Mg | 151 | 138 | 5 | 2 | 0 | 5 | 1 |
| Cluster 3 | Ca | 488 | 333 | 101 | 20 | 12 | 20 | 1 |
| Cluster 4 | Ba, Na, Sr | 343 | 340 | 3 | 0 | 0 | 0 | 0 |
| Cluster 5 | Mo, B, Na | 200 | 196 | 0 | 0 | 0 | 2 | 1 |
| Cluster 6 | Mg, K, U, Se, V | 43 | 27 | 1 | 6 | 5 | 4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




