1. Introduction
Endometriosis is defined as the presence of active endometrial glands and stroma outside the uterine cavity. It is a benign, chronic, and debilitating condition, the progression depends on a series of risk factors and individual factors. The true incidence of endometriosis is not yet known due to the polymorphism of the clinical picture, which makes diagnosis difficult. There are situations where symptomatic patients present with forms of microscopic lesions that are unidentifiable by imaging, and conversely, asymptomatic patients despite having macroscopic lesions. According to the latest publications from the World Health Organization (WHO), endometriosis affects approximately 10% of women of reproductive age, making it one of the main causes of infertility [
1,
2,
3,
4].
Over time, a series of hypotheses have sought to explain pathophysiology of endometriotic lesions development, but none have succeeded in fully explaining the process. Among the most appealing theories is that of retrograde menstruation, which explains predominance of endometriotic lesions in pelvic region, but cannot account for unusual endometriotic lesions such as those in the lungs or brain [
5,
6,
7,
8,
9,
10]. Existence of a local immune deficit complements the first theory [
11,
12,
13,
14,
15]. Other authors attribute appearance of endometriotic lesions to lymphatic or vascular dissemination of endometrial tissue or celomic metaplasia. [
16,
17,
18,
19].
Most common symptom of endometriosis that leads patients to seek medical attention is pain. Pain can manifest in various forms: menstrual pain, premenstrual pain, dyspareunia, non-menstrual abdominal pain, with its localization also varying depending on location of endometriotic lesions [
20,
21,
22,
23]. A very good quantification of pain is found within the questionnaire proposed by the British Society for Gynaecological Endoscopy (BSGE) [
24]. This questionnaire includes questions about nature, severity, duration and localization of pelvic pain, as well as any associated symptoms or factors that may exacerbate or alleviate the pain. Additionally, it assesses the impact of pelvic pain on daily activities, emotional well-being, and overall quality of life. Also, endometriosis is among the most significant causes of infertility, being implicated in 30-50% of cases of female infertility [
25,
26,
27].
Severity of endometriosis can be classified using systems such as revised American Society for Reproductive Medicine (rASRM) staging system or Enzian classification [
28,
29,
30,
31]. In our study, revised ASRM classification was applied. This system takes into account factors such as lesion size, extent and involvement of pelvic structures to classify endometriosis into stages ranging from minimal (Stage I) to severe (Stage IV).
Diagnosis of endometriosis is sometimes challenging. Transvaginal ultrasound followed by magnetic resonance imaging (MRI) to map the lesions and extent of endometriosis most commonly enables accurate diagnosis. Laparoscopy allows direct visualization of lesions, allows biopsy for histopathological confirmation and safe surgical treatment when necessary [
32,
33,
34]. The importance of biomarkers has gained special interest in recent years. They can play a crucial role in early diagnosis, establishing prognosis of endometriosis, monitoring treatment, identifying new therapeutic targets and personalizing treatment [
35,
36,
37].
Treatment of endometriosis can vary depending on severity of symptoms, patient's age, desire for pregnancy and other individual factors. It includes either medical management or surgical excision [
38,
39,
40,
41,
42].
Prognosis of endometriosis depends on several factors such as severity of the disease, response to treatment, patient's age, associated complications and desire for conception. Its assessment is not strictly limited to the risk of lesion recurrence or the number of visits to doctor required but encompasses a more detailed analysis of quality of life of individuals diagnosed with endometriosis. It can be partially assessed through questionnaires proposed by BSGE. Current efforts are aimed at assessing prognosis based on serum or tissue levels of certain biomarkers [
27,
30,
35,
37,
42,
43].
1.1. Endometriosis Development and Biomarkers
In most circumstances, despite menstrual reflux into peritoneal cavity, macrophages play an important role in eliminating these cellular debris, thus providing local protection. Macrophages concentration in peritoneal fluid seems to fluctuate during the menstrual cycle and is highest during menstrual bleeding. Estrogen (E2) has been found to be a regulator of type 2 macrophages phenotype (M2) which is responsible for their efficiency. These will secrete matrix metalloproteinase-9 (MMP-9) to break down the extracellular matrix, thus breaking down refluxed tissues into small pieces. Additionally, CD-36 receptor (cluster of differentiation 36) is expressed on cell membrane of macrophages to facilitate phagocytosis of these small fragments of endometrial debris. In case of endometriotic lesions, increased concentration of prostaglandin E2 (PGE2) will suppress expression of MMP-9 and CD36. This significantly inhibits phagocytic function of macrophages, favoring development of endometriotic lesions [
65,
66,
67,
68].
At the same time, macrophages, activated T lymphocytes, and even the endometriotic lesions themselves have the capacity to release a series of growth factors and pro-inflammatory and angiogenic cytokines that contribute to development of endometriotic lesions:
IL-8 (interleukin 8), known as an α-chemokine with chemotactic activity and acting as a strong angiogenic agent, primarily produced by peripheral macrophages, has been detected in high concentrations in peritoneal fluid of women with endometriosis. In addition to its chemotactic and activating properties for granulocytes, IL-8 has recently been found to stimulate the proliferation of endometrial cells [
65,
66,
67,
68].
Vascular endothelial growth factor (VEGF), one of the main angiogenic factors with ability to stimulate mitogenesis, migration, and differentiation of endothelial cells, is strongly expressed in endometriotic tissue as well as in peritoneal macrophages. Peritoneal activated macrophages are major source of VEGF in endometriosis, and this expression is directly regulated by ovarian steroids. E2 acts on various macrophage signaling pathways, especially those related to the support of inflammatory cell recruitment and the remodeling of inflamed tissues, such as mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase/protein kinase B, and Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). Consequently, dysregulation of ovarian steroid hormone homeostasis could influence survival of ectopic endometrial cells and promote lesion vascularization. It is well known that hypoxia induces expression of VEGF. Effects of hypoxia are mainly mediated by a protein complex called hypoxia-inducible factor-1 (HIF-1), which consists of two subunits, HIF-lα, the inducible unit, and HIF-1β, the constitutive unit. In endometriotic lesions, abnormally high levels of HIF-lα complex have been found, clearly proving that hypoxia is involved in production of VEGF in endometriosis [
67,
68].
IL-10 (interleukin 10), secreted largely by macrophages, is known for inhibiting T cell activation, but also for reducing expression of co-stimulatory molecules (CD-80 and CD-86) and indoleamine 2,3-dioxygenase. Interestingly, macrophages secrete IL-15 (interleukin 15), a chemoattractant for uNK (uterine natural killer) cells and downregulate the cytotoxicity of uNK cells [
66,
67].
Nerve growth factors (NGF), especially BDNF (Brain-derived neurotrophic factor), are abnormally synthesized and released by activated macrophages, mast cells, NK (natural killer) cells, and leukocytes within endometriotic formations, close to sensitive nerve fibers, and in peritoneal fluid. These sensitize or stimulate endings of sensitive nerve fibers, leading to a vicious cycle characterized by nociceptor sensitization, focal neoneurogenesis, and activation of sensitive nerve fibers, resulting in hyperalgesia [
68].
1.2. Objectives
The primary objective of this study was to investigate the role of IL-8, IL-10, VEGF-A, BDNF in assessing and determining endometriosis severity. On the otherside, we aimed to assess the relationship between severity of endometriosis and average pain score obtained through the application of BSGE questionnaire to patients. Another objective is to evaluate severity of endometriosis in relation to physical activity performed by patient.
Measuring IL-8 levels can help identify the inflammatory and angiogenic activity associated with endometriosis, aiding in early detection. IL-10's role in immune modulation highlights its potential as a marker for immune response in endometriosis, guiding therapeutic strategies. BDNF's involvement in nerve sensitization and pain underscores its importance in diagnosing pain-related symptoms of endometriosis and developing targeted pain management treatments. VEGF is crucial for assessing the extent of angiogenesis and lesion vascularization, making it a key target for treatments aimed at reducing blood supply to endometriotic lesions.
2. Results
2.1. Patient Characteristics
In our study, a total of 46 Caucasian patients diagnosed with endometriosis who underwent surgical treatment at our clinic between January 2022 and December 2023 were included. In all 46 patients, diagnosis of endometriosis was confirmed through histopathological examination. All patients in this study were initially informed about their participation. After signing the informed consent and GDPR agreement (The General Data Protection Regulation), patients responded to the questionnaire proposed by BSGE, and general data, family and personal medical history, symptomatology and physical activity practiced by patient was collected.
The mean age of patients included in study was 28.25 years with a standard deviation of 7.14. Severity of endometriosis, assessed by rASRM classification, had an average score of 26.50, corresponding to stage III severity: 5 cases were stage 2, 38 cases were stage 3 and 3 cases were stage 4. Average BMI of the patients was 25.31.
Regarding the control group, 44 patients met the criteria and were included. Among these, 36.36% (n=16) had a histopathological diagnosis of uterine leiomyoma, 38.64% (n=17) had a diagnosis of non-endometriotic ovarian cyst (serous cystadenoma, mucinous cystadenoma, luteal cyst), 20.45% (n=9) had a diagnosis of mature ovarian teratoma, and 4.55% (n=2) had a diagnosis of uterine septum. The mean age of the control group was 29.39 years.
2.2. Expression of Biomarkers at Serum and Tissue Levels
Analysis of biomarkers IL-8, IL-10, BDNF and VEGF-A was conducted in both endometriosis group and control group with non-endometriotic gynecological pathology.
Expression of studied biomarkers in endometriosis group is shown in
Table 1, demonstrating their mean and standard deviation. Regarding IL-8 and IL-10, we observe a higher expression of these biomarkers at tissue level compared to their serum level. On the other hand, BDNF and VEGF-A exhibit a higher expression in serum compared to their tissue level.
The same biomarkers were studied in control group both at serum and tissue levels. Results are summarized in
Table 2. In case of BDNF and VEGF-A, the trend persists in control group, with higher serum values compared to tissue expression. For IL-8 and IL-10, the trend also persists, but with a relatively smaller increase compared to endometriosis group.
In case of the control group, we analyzed mean values based on the pathology confirmed by histopathological examination.
Table 3 summarizes expression of biomarkers according to pathology confirmed by histopathological examination.
Analysis of biomarker values by diagnostic categories shows a certain pattern for each pathology, as follows:
Uterine fibroid: exhibits elevated IL-8 values similar to endometriosis, with a similar trend for IL-10 but less elevated tissue values compared to endometriosis, higher BDNF values both serum and tissue, and lower serum VEGF-A values but higher tissue values.
Non-endometriotic ovarian cyst: tissue expression of IL-8 and IL-10 had significantly lower values in cases of non-endometriotic ovarian cysts (43.15 vs 114.36 pg/mL and 29.53 vs 71.88 pg/mL, respectively). Regarding BDNF and VEGF-A, only the latter showed higher tissue values.
Mature ovarian teratoma: shows reduced tissue values of IL-8 and IL-10, but higher values for BDNF and VEGF-A.
2.3. Results of BSGE Questionnaire
Regarding pain described by patient, for statistical analysis, average pain score was calculated based on patients' responses to questions in questionnaire. The mean pain score was 4.21 points on a numerical scale from 0 to 10, where 0 represents absence of pain and 10 signifies most severe pain imaginable, with a standard deviation of 1.24. Urinary tract involvement was identified in only 4 cases (8.7%), all of which reported suprapubic pain perceived at level of the bladder, and severity grade of endometriosis according to rASRM was 3. Intestinal function impairment was described in 17 cases (36.96%), with 5 patients reporting constipation and 12 patients reporting a sensation of incomplete bowel emptying. Intestinal involvement was predominantly encountered in patients with a more severe grade of endometriosis. Regarding medical treatment, 25 patients were using combined oral contraceptive (COC) or progestin medications for symptom control, and no patients were under treatment with Gonadotropin Releasing Hormones (GnRH) agonists, aromatase inhibitors, or other medications. Two patients were using levonorgestrel intrauterine devices (IUD) as a contraceptive method.
Patients' history revealed that 34.78% of patients (n=16) had a history of surgical interventions for endometriosis, and 6 patients presented non-gynecological comorbidities, including Sinus tachycardia, bronchial asthma in 2 cases, chronic gastritis, hypertension, and type I diabetes. Regarding analgesic medication, 86.96% of patients reported using it, with 32.60% reporting chronic use outside of menstruation or premenstrual periods. Patients' self-assessment of health on a scale from 0 to 100 showed a mean score of 77.07.
2.4. Correlation and Regression of Severity Determinants in Endometriosis
Correlation between values of studied biomarkers and severity of endometriosis according to rASRM classification was analyzed. For a more detailed analysis, we used the score obtained within the classification, rather than severity grade. The correlation between these variables and severity is shown in
Table 4.
A statistically significant positive correlation was found between variables IL-8 tissue expression, IL-10 tissue expression, FSFI, and average pain score according to BSGE, with p-values below 0.05 (0.003, 0.005, and <0.001, respectively). A statistically significant correlation was also found in case of hormonal treatment with combined oral contraceptives or progesterone and physical activity. In both cases, severity symptoms were lower with physical activity and hormonal medication use, with both having a p-value below 0.05 (0.003 and 0.042, respectively).
2.5. Analysis of Correlations between IL-8, IL-10 and the Severity of Endometriosis
Variables analyzed as determinants of endometriosis severity have a variable importance described according to
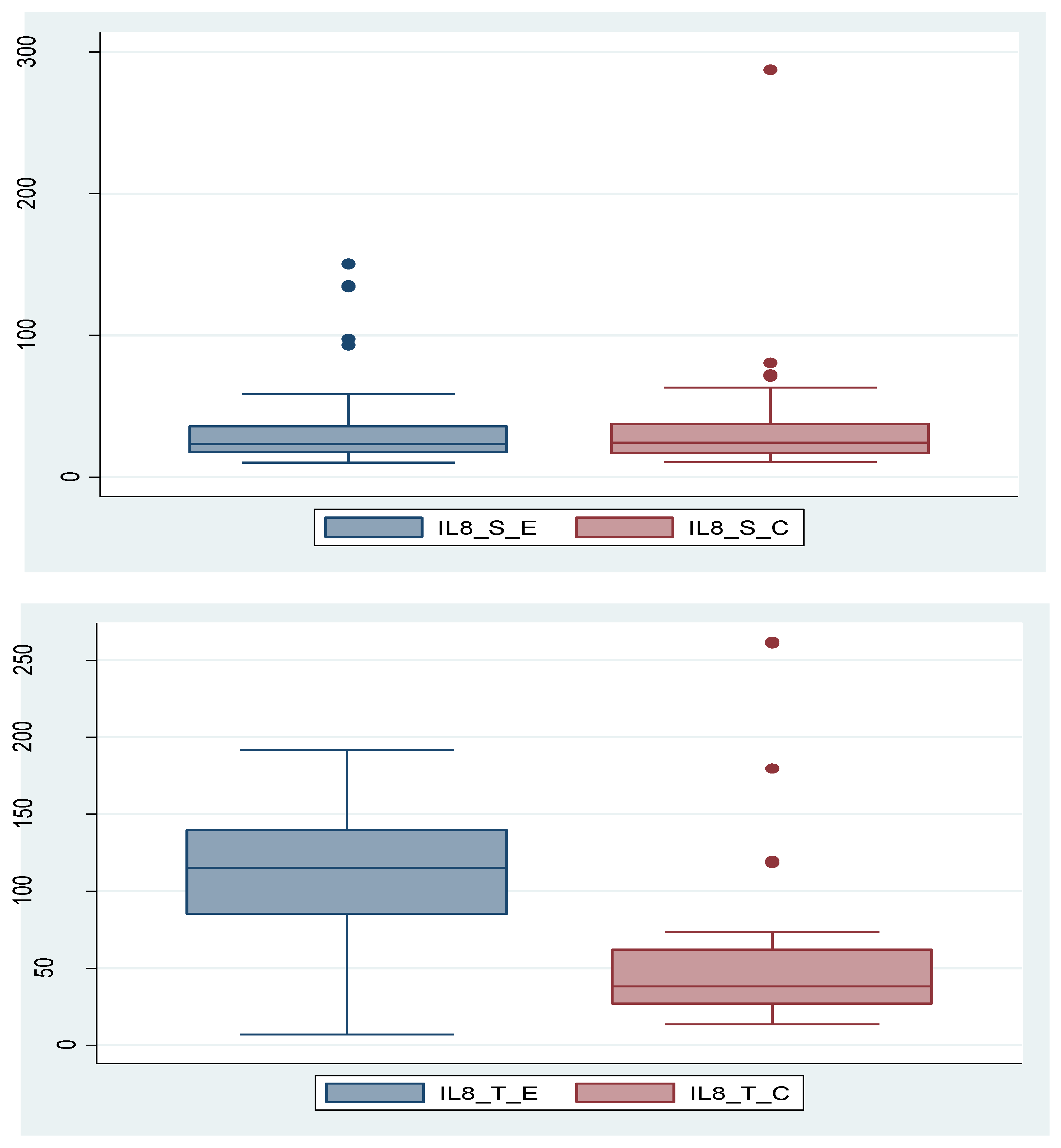
Table 1. Furthermore, we analyzed the association between certain variables and endometriosis severity using different functions. Analyzing IL-8, we found that it is not statistically significant (p=0.370) as a serum value, but its tissue expression is significantly higher in endometriosis group compared to control group (p=0.003). In the control group, there is a patient with a very high IL-8 serum level. If we consider this value as an outlier and remove the patient from the group, the conclusion remains unchanged; the IL-8_S is not statistically significantly different in the endometriosis group compared to the control group (p=0.713). These data are depicted in
Figure 1a,b and
Figure 2.
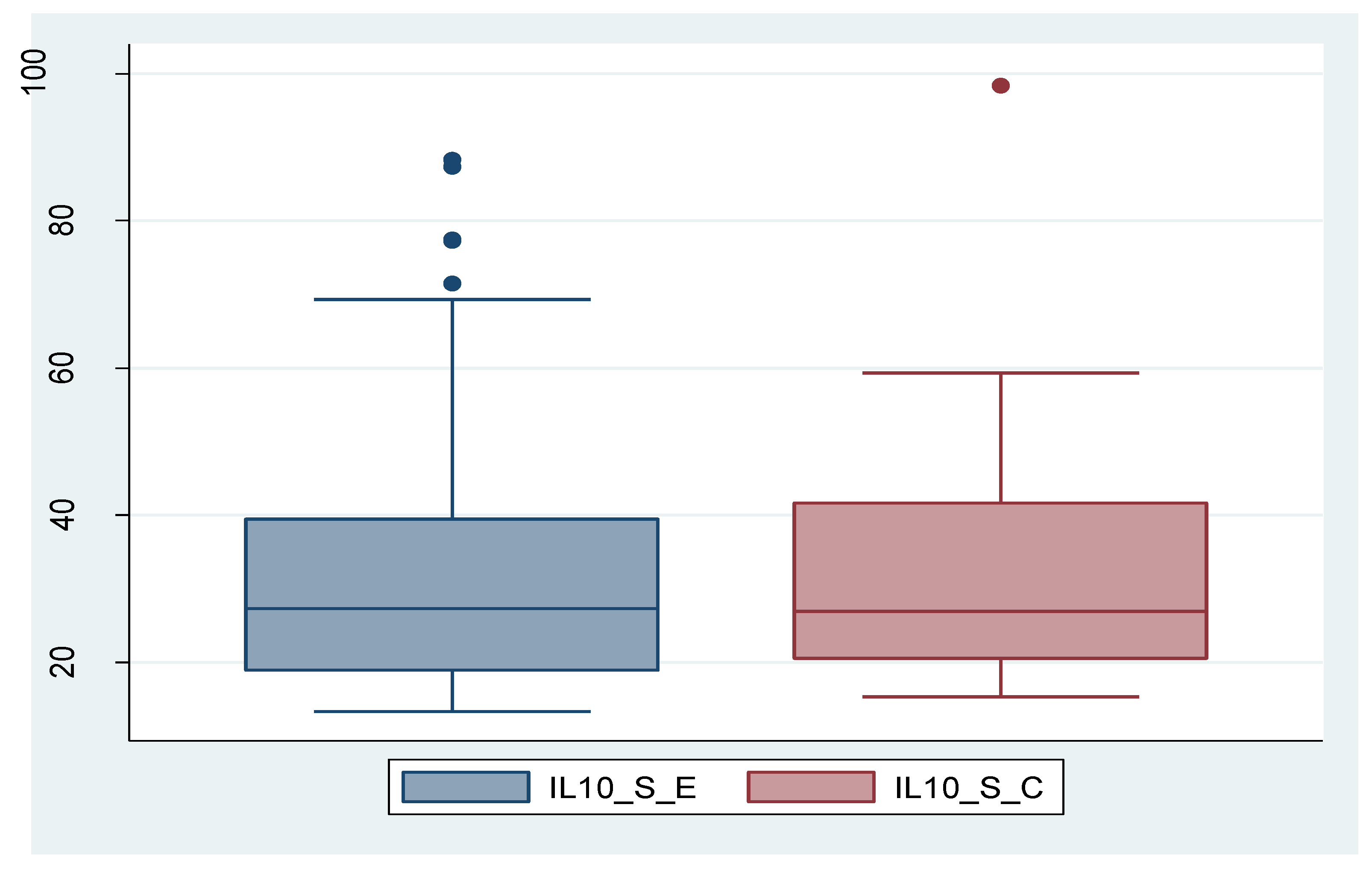
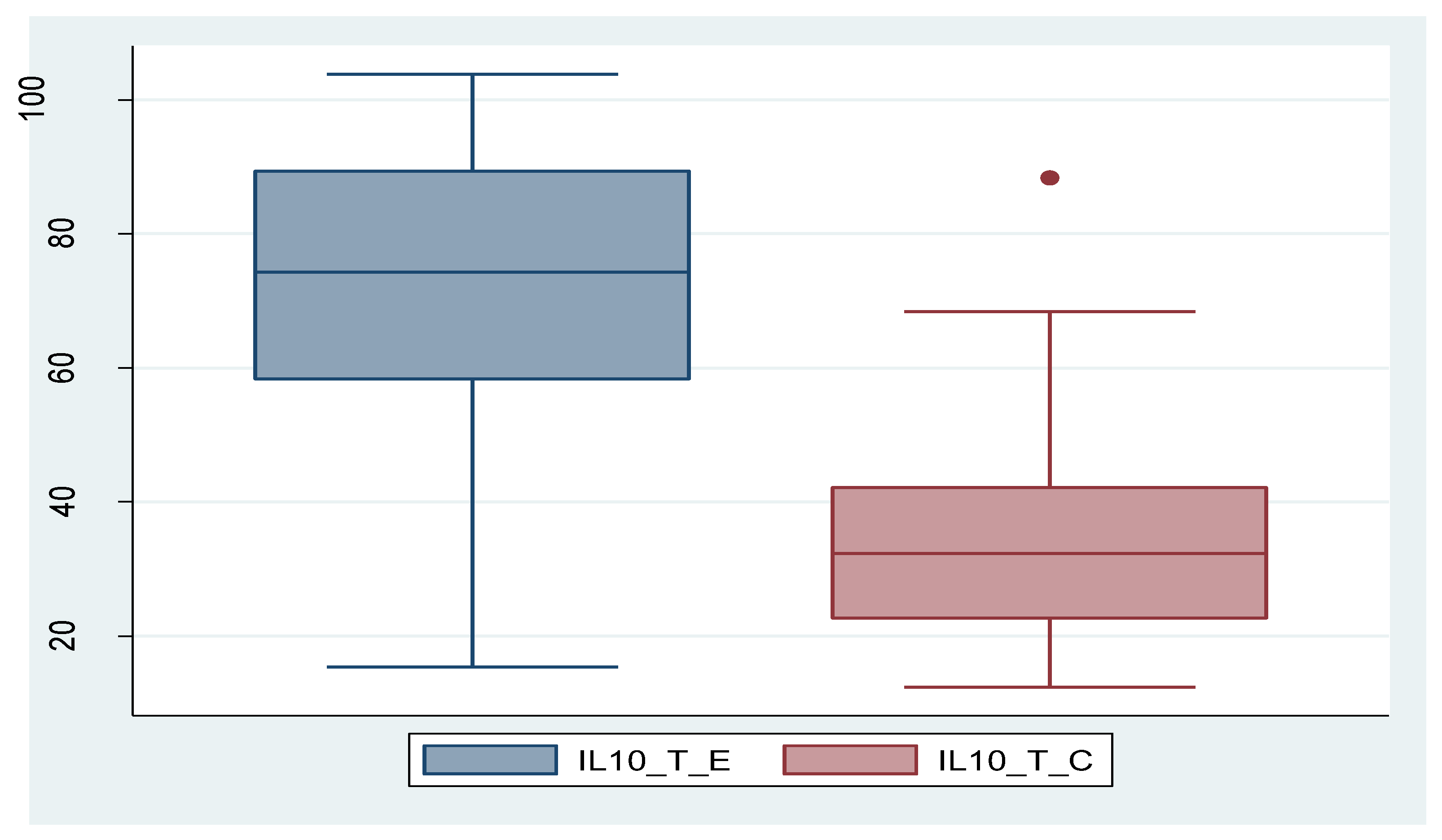
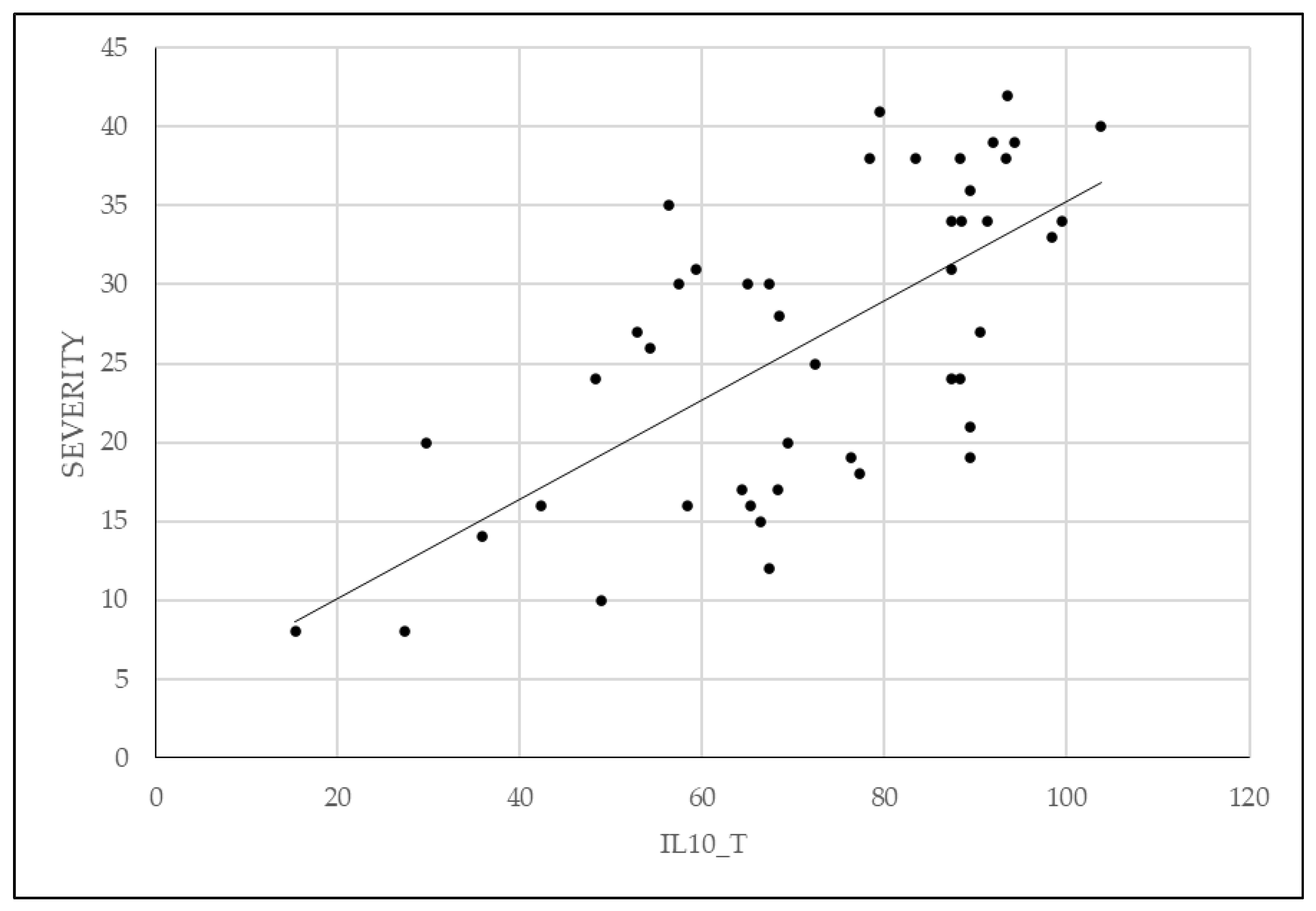
And in case of IL-10, its expression was significantly higher in tissue in endometriosis group compared to control group, while serum level did not show statistically significant values between the two groups. In the case of IL-10_S as well, there is a patient with an exceptionally high value, which can be considered an outlier. By removing this patient, the outcome remains unchanged; the difference between the two groups is not statistically significant (p=0.473). The data are presented in
Figure 3a,b and
Figure 4.
The analysis of BDNF and VEGF-A between the two groups did not show statistically significant differences in endometriosis group compared to control group.
2.6. Analysis of Linear Regression of Patient-Reported Pain and Physical Activity on Severity of Endometriosis
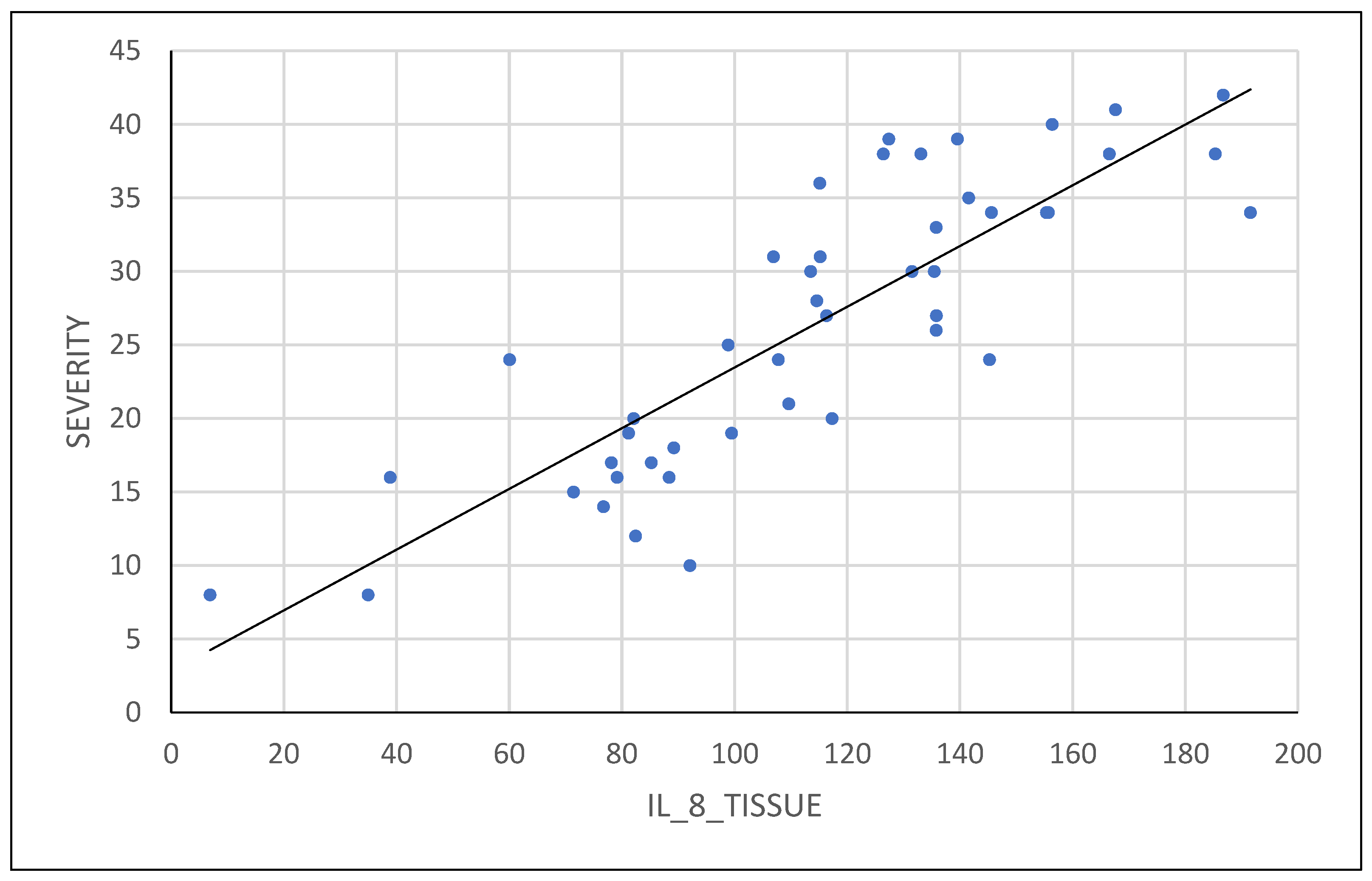
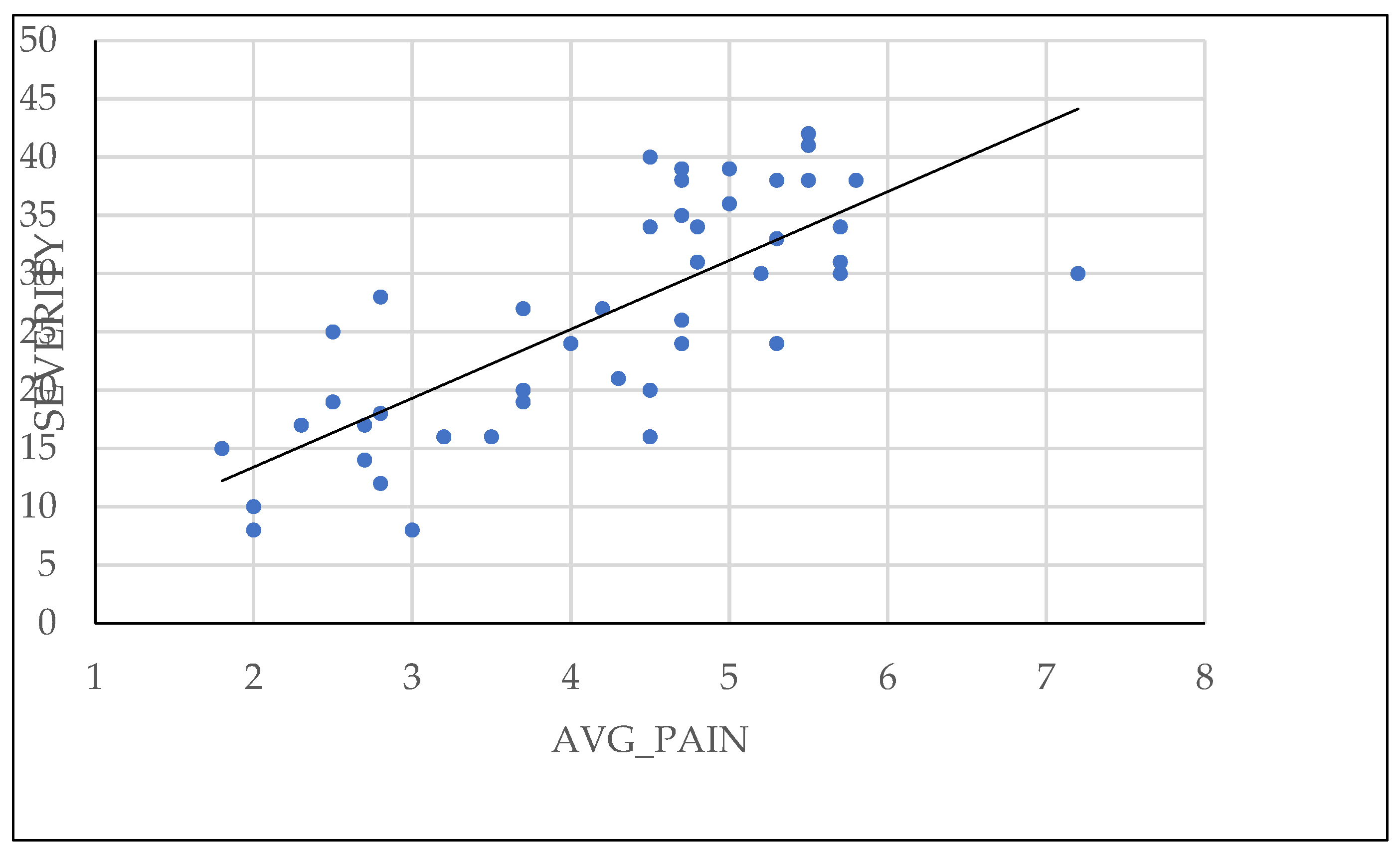
The pelvic pain questionnaire proposed by BSGE was found to be directly correlated with severity of endometriosis, as shown in
Figure 5. Additionally, patients who used combined oral contraceptive or progestin medication had less extensive lesions at the time of surgical intervention.
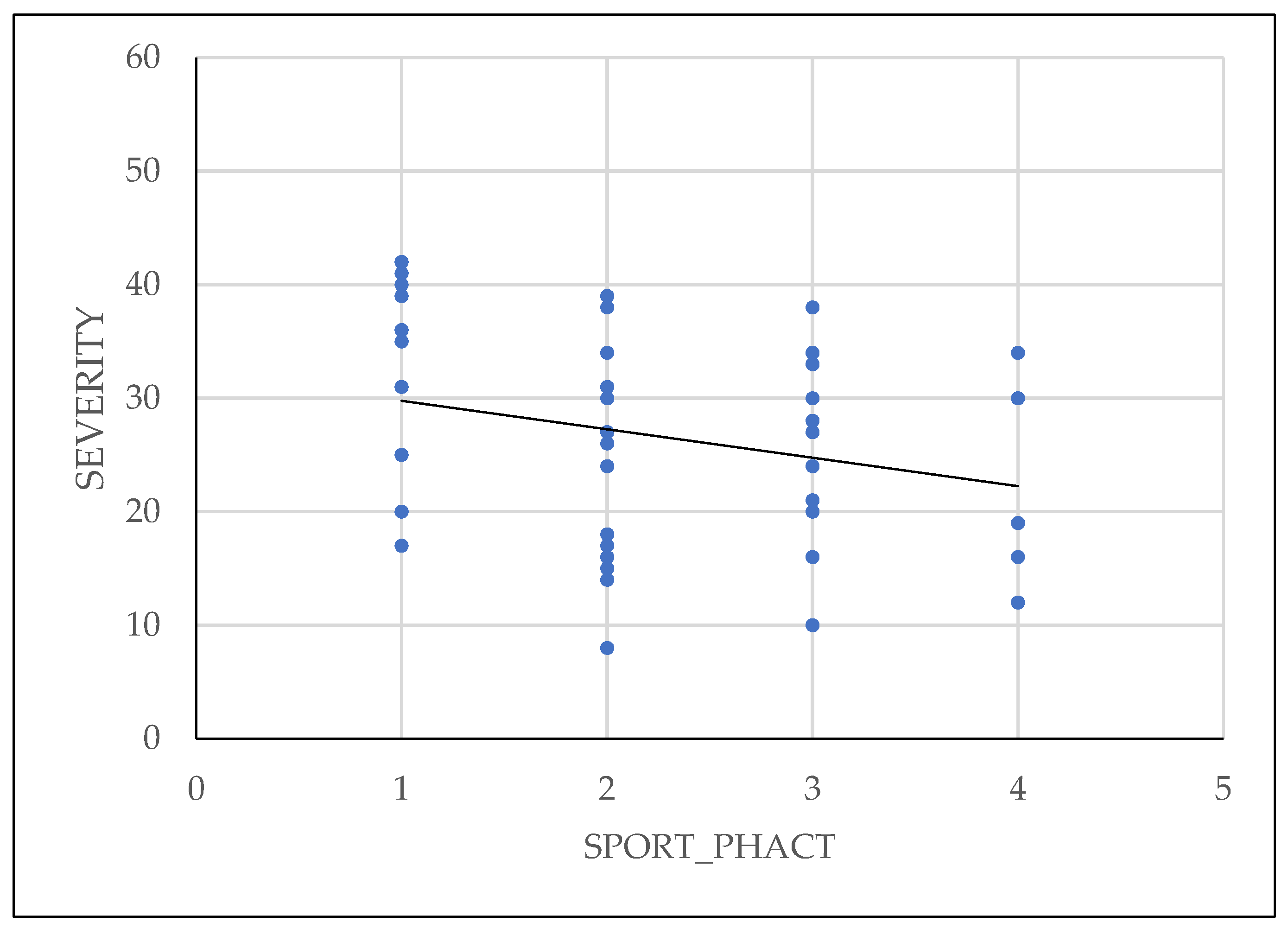
Physical activity of patients has been shown to reduce the symptoms and also to have influence on the severity of the disease, as shown in
Figure 6.
3. Discussion
Severity of endometriosis should be viewed beyond assessment through a numerical scale. While this scale allows for a concrete diagnosis and application of sequential treatment for the patient, variability of the clinical picture makes it difficult to assess patient's prognosis. Therefore, a more detailed analysis of the factors contributing to severity of endometriosis or those correlated with severity of the disease is necessary. RASRM classification takes into account lesions present at level of the peritoneum, ovaries, Douglas pouch, and also presence of adhesions at level of the ovaries or uterine tubes. The advantages of using rASRM are that it is the most widely used classification score for endometriosis, it is easy to use, and the four severity grades are easily understandable by patients. Disadvantages of using rASRM compared to ENZIAN are that it does not consider deep endometriosis, intestinal involvement or retroperitoneal lesions [
28,
29,
30,
31].
Existence of chronic inflammation in endometriosis is well-known and studied over the years. Studies by Taylor et al., Khorram et al., Orisaka et al. and many others have shown that expression of cytokines such as IL-5, RANTES, or interferon gamma (IFN-γ) is elevated and plays an important role in maintaining a state of chronic local inflammation [
44,
45,
46,
47,
48,
49].
IL-8 is a pro-inflammatory chemokine involved in recruiting and activating leukocytes at the site of inflammation and is produced by endothelial cells, monocytes, macrophages, and fibroblasts. However, specificity of this interleukin is relatively low, as it is elevated in other diseases such as rheumatoid arthritis, psoriasis, inflammatory bowel disease, or cancer. This fact is also confirmed in our study by the fact that the serum IL-8 levels do not differ significantly between the two study groups. However, IL-8 can still be useful when analyzing tissue expression, as values are statistically significantly higher in endometriosis group compared to control group, demonstrating that tissue levels of IL-8 are directly proportional to severity of endometriosis, a fact clearly visible in
Figure 1 of our study. Pathophysiological implications of increased tissue expression of IL-8 include presence of chronic inflammation leading to neovascularization, stimulation of pain receptors, decreased egg quality and subsequent infertility.
These findings are corroborated by other studies in specialized literature who concluded that IL-8 plays a central role in development of endometriosis through its pro-inflammatory function and neutrophil chemotaxis, being involved in all stages of lesion development (adhesion, invasion, implantation, and proliferation of ectopic endometrial cells), even protecting these cells against programmed cell death through apoptosis [
50,
51,
52,
53,
54].
IL-10 is an anti-inflammatory cytokine produced by a variety of immune system cells, including T helper lymphocytes, monocytes, or macrophages. Its main functions include inflammation suppression, immunosuppression by suppressing the activity of immune cells, and promoting immune tolerance and immunomodulation [
55]. IL-10 follows the same pattern both serologically and tissue-wise as IL-8, with non-significantly different serum levels between the two study groups, but significantly different tissue levels between them, showing higher expression in endometriosis. From this, we conclude that stimulation of the local immune system (at tissue level) is higher in cases of endometriotic lesions. Recalling the theories of endometriosis development as mentioned in the introductory part, we specify that retrograde menstruation alone is not sufficient to explain the development of endometriotic lesions and that a certain immune cell deficit may be necessary. IL-10 is known for inhibiting the activation of T cells, but also for reducing expression of co-stimulatory molecules (CD-80 and CD-86) and indoleamine 2,3-dioxygenase. Interestingly, macrophages secrete IL-15, a chemotactic factor for uNK cells and downregulate the cytotoxicity of uNK cells [
55,
56,
57,
58,
59,
60,
61].
Tissue overexpression of IL-10 in association with other interleukins can explain immune tolerance, the deficit in phagocytosis of these cellular debris, followed by adhesion and development of endometriotic lesions starting from these changes.
Surprisingly, the quantification of BDNF and VEGF-A in the two study groups did not show significant differences either serologically or in tissue. This could be explained either by the relatively small number of patients in the two study groups or by the variability of pathology in the control group.
It must be noted that control group consists of patients with gynecological pathology and not disease-free patients. This fact limits the comparison of studied biomarkers between the two groups. Comparative studies of biomarkers expression in endometriosis and other gynecological pathologies could be a subject of future investigations.
Continuing analysis of biomarkers in target group with endometriosis, we found high statistical significance for tissue values compared to their serum levels. Higher tissue values reflect a greater predictive accuracy and a direct correlation with severity of endometriosis. Elevated tissue IL-8 reflects more accurately local inflammatory process, providing an image of disease activity, combined with presence of a deficit in local immune system with immune tolerance to cellular debris reflected by the increased value of IL-10. It is worth mentioning again that serum levels of these biomarkers can be easily influenced by the presence of other systemic pathologies.
Patient reported pain using the BSGE questionnaire has proven to be a faithful factor in predicting the severity of endometriosis. In addition to quantifying the pain experienced by the patient, the BSGE questionnaire investigates symptoms of urinary or gastrointestinal system involvement, as well as impact on female fertility or daily routine activities. This can help us better understand the needs and concerns of patients and formulate more effective and personalized treatment strategies. Additionally, it allows monitoring the progression of the disease over time and evaluating the response to treatment.
Association between increased physical activity and a lower degree of severity of endometriosis confirms data from the literature. Regular physical activity on engaging in sports can reduce severity of endometriosis because it helps lower estrogen levels, which play a key role in development and progression of endometrial lesions. Exercise also enhance blood circulation, promoting removal of excess hormones and toxins that may contribute to endometriosis symptoms. Furthermore, consistent physical activity is know to improve pain tolerance and reduce inflammation, offering relief from the chronic pain associated with endometriosis[
62,
63,
64].
Our results are also supported by other authors in specialized literature. For example, Martire in 2023 study emphasizes the role of differential diagnosis of types of dysmenorrhea reported by patients, highlighting in the diagnostic chapter the role of investigations regarding inflammatory and possibly autoimmune status for detecting endometriosis in young patients [
69]. Of course, these investigations complement the classical diagnostic methods, with same author emphasizing the role of transvaginal ultrasound performed by an expert in detecting early lesions of endometriosis [
70].
The present study, however, has several limitations and weaknesses. Verifying the results is best done by comparing endometriosis group to a group of disease-free individuals without any other pathology. In lack of of patients without any pathology to use as a control group we decided that thus would be the best way to validate our endometriosis group results. Among these, we mention:
relatively small number of patients in the two study groups
sample of patients exclusively of Caucasian race
heterogeneity of the control group
4. Materials and Methods
The study design is a prospective case-control cross-sectional study, which includes patients with endometriosis investigated and treated at the Obstetrics and Gynecology Clinic II of the Cluj-Napoca Emergency County Hospital, Romania, from January 2022 to December 2023. Additionally, a control group was recruited consisting of reproductive-age patients with non-endometriotic gynecological pathology, also treated at the Obstetrics and Gynecology Clinic II of the Cluj-Napoca Emergency County Hospital, Romania, during same period. Informed consent was obtained from all subjects or their legal representatives by signing a detailed form regarding the investigated issue. Another consent form was signed by the subjects or their legal representatives for the use of personal data. The study was conducted following guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the "Iuliu Hatieganu" University of Medicine and Pharmacy, Cluj-Napoca, Romania (AVZ251 dated 25.02.2022).
Inclusion criteria for endometriosis group consisted of reproductive-age patients who met both clinical and paraclinical criteria for diagnosis of endometriosis, and after histopathological examination following surgical intervention, diagnosis of endometriosis was confirmed. As for the inclusion criteria for control group, reproductive-age patients with non-endometriotic gynecological pathology who underwent surgical intervention were included. Exclusion criteria included:
Patients who did not freely express their consent for enrollment in the study.
Patients who did not meet necessary conditions for study enrollment or who did not attend scheduled follow-up visits as per the protocol.
After obtaining informed consent, for each person included in the study, 3 forms were completed:
A form containing general data, family and personal medical history, symptomatology
Questionnaire regarding the physical activity practiced by the patient.
BSGE questionnaire.
Preoperatively, from each patient, regardless of group, a blood sample will be collected for analysis of certain biomarkers in serum of patients from endometriosis group and control group, respectively. The samples will be centrifuged for 15 minutes at 1000xg at a temperature ranging from 2 to 8°C. Obtained supernatant will be stored at a temperature of -80°C until processing. Additionally, intraoperatively, a tissue sample (ovarian cyst wall or leiomyoma fragment) will be obtained for analysis of biomarkers at this level. Tissue sample will be placed in saline solution and frozen at -80°C until processing.
4.1. Analysis of Patient Symptoms and Endometriosis Severity
Endometriosis severity is assessed according to rASRM classification. The rASRM classification is designed to classify endometriosis via direct visualisation of the pelvic organs at laparoscopy or laparotomy. This classification is based on assigning scores to endometriotic lesions found at the peritoneal and ovarian levels based on size of lesions. By analogy, points are also assigned for adhesions on the ovaries and uterine tubes. Additionally, points are given for partial or complete obliteration of the Douglas pouch. Finally, all assigned points are summed, and the resulting scores are classified into four severity classes.
Analysis of the patients' symptomatology was assessed using questionnaire proposed by the BSGE for pelvic pain. First part of the questionnaire details the pain intensity on a scale from 0 to 10 and type of pain: premenstrual, menstrual, non-cyclic pelvic, dyspareunia, abdominal pain related to the digestive tract during and outside menstruation, lower back pain, urinary bladder pain or pain during urination. Second part refers to impairment of intestinal function in absence of gastrointestinal pathology, with impairment assessed as a sensation of incomplete bowel emptying, constipation or traces of blood in stool. Next chapter investigates whether the patient underwent preoperative treatment (combined oral contraceptives, IUD, GnRH analogs, GnRH analogs + estrogen, progesterone, hormonal substitutes, or aromatase inhibitors). Fertility is also assessed within the questionnaire as:
unaffected,
patient trying to conceive for less than 18 months without success, or
patient trying to conceive for more than 18 months without success.
Following questions refer to analgesic medication required by patient (Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) or opioids) and history of surgical intervention for endometriosis. Last part of questionnaire assesses patient's perceived health status on a scale from 0 to 100 and their daily activity. In our analysis, to conduct a more accurate statistical analysis, we considered the average pain described by patient through the questions in first part of the questionnaire.
Another element investigated in the group of patients affected by endometriosis was related to physical activity practiced by patient. The variable is defined as the maximum value declared by the patient to 6 questions in the questionnaire regarding the frequency, duration and intensity of sports activities and physical activities outside of sports.
4.2. Analysis of Investigated Biomarkers
Both in the case of patients in endometriosis group and in the case of patients in control group, a blood sample was collected, centrifuged, and resulting serum was frozen. Additionally, a tissue sample was collected and frozen. Subsequently, both samples were analyzed for expression of certain biomarkers (serum and tissue) believed to influence endometriosis development and progression. Studied markers were:
IL 8 - Elabscience Human IL-8 (Interleukin 8) Elisa kit, catalog no: E-EL-H6008
IL 10 - Elabscience Human IL-10 (Interleukin 10) Elisa kit, catalog no: E-EL-H6154
VEGF - Elabscience Human VEGF-A (Vascular Endothelial Cell Growth Factor A) ELISA kit, catalog no: E-EL-H0111
BDNF - Elabscience Human BDNF (Brain Derived Neurotrophic Factor) ELISA kit, catalog No: E-EL-H0010.
4.3. Statistical Analysis
The study explored the differences in the values of certain variables (interleukins IL-8, IL-10, BDNF, and VEGF-A) between a sample of patients with endometriosis and a control group. These differences were tested using the Student's t-test, designed for independent samples with unequal variances. To visualize these differences, box plots were employed, offering a clear graphical representation of the data distribution. The study utilized Ordinary Least Squares (OLS) linear regression to validate the effects of IL-8, IL-10, patient-reported pain, and physical activity on the severity of endometriosis. To minimize the risk of Omitted Variable Bias (OVB) in the regression analysis, several control factors were included: FSFI score, age, BMI, and the biomarkers BDNF and VEGF. The research aimed to ensure the reliability of the results by testing whether IL-8 and IL-10 maintain their sign and statistical significance in the regression, both with and without the presence of other biomarkers. This approach was adopted to mitigate potential confounding effects of IL-8 and IL-10 taken from tissue samples. As a measure of robustness, the study also checked Pearson correlations between the severity of endometriosis and potential influencing factors. Additionally, scatter plot representations allowed for the observation of the linear shape of these correlations, further supporting the study’s findings.
5. Conclusion
In our study, we demonstrated that IL-8 and IL-10 can serve as a diagnostic and prognostic element of endometriosis, their value being directly proportional to the severity of endometriosis. These data confirm presence of a local chronic inflammation and existence of an immune deficit that contributes to development of endometriotic lesions. No relationship was identified between BDNF and VEGF-A serum or tissue levels and endometriosis severity. However, we cannot exclude their association with pathophysiology of endometriosis development and various symptoms, as this analysis was not the focus of the current study. Future development of a panel of biomarkers capable of assessing the diagnosis and prognosis of endometriosis can be extremely useful.
Pelvic pain questionnaire proposed by BSGE showed a good correlation with severity of endometriosis, and we believe that its introduction into clinical practice can enhance understanding of the disease, assessment of treatment response, and quality of life of the patient.
Physical activity once again demonstrates its extremely important role not only in promoting a healthy lifestyle but also as an alternative or adjunctive treatment in alleviating endometriosis symptoms and disease regression.
To validate the results and to further understand endometriosis pathology, we propose conducting future studies focusing on the pathophysiological mechanisms, early diagnosis, and personalized treatment of patients.
Author Contributions
Conceptualization, I.D.N. and D.M.; methodology, A.M.; software, R.C.; validation, M.R., I.D.N. and M.O.; formal analysis,C.B.; investigation, C.O.; resources, A.M.; data curation, D.M.; writing—original draft preparation, I.D.N.; writing—review and editing, A.M.; visualization, A.G.M.; supervision, D.M.; project administration, R.C.; funding acquisition, I.D.N. All authors have read and agreed to the published version of the manuscript. The autors contributed equally to this study.
Funding
This research was funded by Iuliu Hațieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania, grant number 881/34/12.01.2022.
Institutional Review Board Statement
The study was conducted following guidelines of the Helsinki Declaration and was approved by the Ethics Committee of the "Iuliu Hatieganu" University of Medicine and Pharmacy, Cluj-Napoca, Romania (AVZ251 dated 25.02.2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Della Corte, Luigi et al. “The Burden of Endometriosis on Women's Lifespan: A Narrative Overview on Quality of Life and Psychosocial Wellbeing.” International journal of environmental research and public health vol. 17,13 4683. 29 Jun. 2020. [CrossRef]
- Škegro, Bernarda et al. “Endometriosis, Pain and Mental Health.” Psychiatria Danubina vol. 33,Suppl 4 (2021): 632-636.
- Koninckx, Philippe R et al. “Pathogenesis Based Diagnosis and Treatment of Endometriosis.” Frontiers in endocrinology vol. 12 745548. 25 Nov. 2021. [CrossRef]
- Rahmioglu, N., Mortlock, S. et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat Genet 55, 423–436 (2023). [CrossRef]
- Bulun, Serdar E. “Endometriosis caused by retrograde menstruation: now demonstrated by DNA evidence.” Fertility and sterility vol. 118,3 (2022): 535-536. [CrossRef]
- D'Hooghe, Thomas M, and Sophie Debrock. “Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons.” Human reproduction update vol. 8,1 (2002): 84-8. [CrossRef]
- Guo, Sun-Wei et al. “From Retrograde Menstruation to Endometrial Determinism and a Brave New World of "Root Treatment" of Endometriosis: Destiny or a Fanciful Utopia?.” Biomolecules vol. 13,2 336. 9 Feb. 2023. [CrossRef]
- Liang, Yanchun et al. “Pro-endometriotic niche in endometriosis.” Reproductive biomedicine online vol. 38,4 (2019): 549-559. [CrossRef]
- Tong, Shan-Shan et al. “Case report of pulmonary endometriosis and review of the literature.” The Journal of international medical research vol. 47,4 (2019): 1766-1770. [CrossRef]
- Andres, Marina P et al. “Extrapelvic Endometriosis: A Systematic Review.” Journal of minimally invasive gynecology vol. 27,2 (2020): 373-389. [CrossRef]
- Fukui, Atsushi et al. “Pelvic endometriosis and natural killer cell immunity.” American journal of reproductive immunology (New York, N.Y. : 1989) vol. 85,4 (2021): e13342. [CrossRef]
- Szukiewicz, Dariusz. “Epigenetic regulation and T-cell responses in endometriosis - something other than autoimmunity.” Frontiers in immunology vol. 13 943839. 22 Jul. 2022. [CrossRef]
- Zutautas, Katherine B et al. “The dysregulation of leukemia inhibitory factor and its implications for endometriosis pathophysiology.” Frontiers in immunology vol. 14 1089098. 23 Mar. 2023. [CrossRef]
- Ho, H N et al. “Peritoneal cellular immunity and endometriosis.” American journal of reproductive immunology (New York, N.Y. : 1989) vol. 38,6 (1997): 400-12. [CrossRef]
- Wang, Linlin et al. “A History of Endometriosis Is Associated With Decreased Peripheral NK Cytotoxicity and Increased Infiltration of Uterine CD68+ Macrophages.” Frontiers in immunology vol. 12 711231. 31 Aug. 2021. [CrossRef]
- Vinatier, D et al. “Theories of endometriosis.” European journal of obstetrics, gynecology, and reproductive biology vol. 96,1 (2001): 21-34. [CrossRef]
- Lamceva, Jelizaveta et al. “The Main Theories on the Pathogenesis of Endometriosis.” International journal of molecular sciences vol. 24,5 4254. 21 Feb. 2023. [CrossRef]
- Hill, Christopher J et al. “Endometriosis and the Fallopian Tubes: Theories of Origin and Clinical Implications.” Journal of clinical medicine vol. 9,6 1905. 18 Jun. 2020. [CrossRef]
- Czyzyk, Adam et al. “Update on endometriosis pathogenesis.” Minerva ginecologica vol. 69,5 (2017): 447-461. [CrossRef]
- Gruber, Teresa Mira, and Sylvia Mechsner. “Pathogenesis of Endometriosis: The Origin of Pain and Subfertility.” Cells vol. 10,6 1381. 3 Jun. 2021. [CrossRef]
- Aredo, Jacqueline V et al. “Relating Chronic Pelvic Pain and Endometriosis to Signs of Sensitization and Myofascial Pain and Dysfunction.” Seminars in reproductive medicine vol. 35,1 (2017): 88-97. [CrossRef]
- Singh, Sukhbir S et al. “Endometriosis and Pelvic Pain for the Gastroenterologist.” Gastroenterology clinics of North America vol. 51,1 (2022): 195-211. [CrossRef]
- Nezhat, Camran et al. “Optimal Management of Endometriosis and Pain.” Obstetrics and gynecology vol. 134,4 (2019): 834-839. [CrossRef]
- Bastu, Ercan et al. “Improvement in quality of life and pain scores after laparoscopic management of deep endometriosis: a retrospective cohort study.” Archives of gynecology and obstetrics vol. 302,1 (2020): 165-172. [CrossRef]
- Tanbo, Tom, and Peter Fedorcsak. “Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options.” Acta obstetricia et gynecologica Scandinavica vol. 96,6 (2017): 659-667. [CrossRef]
- Bulletti, Carlo et al. “Endometriosis and infertility.” Journal of assisted reproduction and genetics vol. 27,8 (2010): 441-7. [CrossRef]
- de Ziegler, Dominique et al. “Endometriosis and infertility: pathophysiology and management.” Lancet (London, England) vol. 376,9742 (2010): 730-8. [CrossRef]
- Haas, Dietmar et al. “The rASRM score and the Enzian classification for endometriosis: their strengths and weaknesses.” Acta obstetricia et gynecologica Scandinavica vol. 92,1 (2013): 3-7. [CrossRef]
- Nicolaus, Kristin et al. “Extensive endometriosis surgery: rASRM and Enzian score independently relate to post-operative complication grade.” Archives of gynecology and obstetrics vol. 301,3 (2020): 699-706. [CrossRef]
- Fruscalzo, Arrigo et al. “Endometriosis and Infertility: Prognostic Value of #Enzian Classification Compared to rASRM and EFI Score.” Journal of personalized medicine vol. 12,10 1623. 1 Oct. 2022. [CrossRef]
- Zondervan, Krina T et al. “Endometriosis Classification Systems: An International Survey to Map Current Knowledge and Uptake.” Journal of minimally invasive gynecology vol. 29,6 (2022): 716-725.e1. [CrossRef]
- Horne, Andrew W, and Stacey A Missmer. “Pathophysiology, diagnosis, and management of endometriosis.” BMJ (Clinical research ed.) vol. 379 e070750. 14 Nov. 2022. [CrossRef]
- Pascoal, E et al. “Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research.” Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology vol. 60,3 (2022): 309-327. [CrossRef]
- Van den Bosch, Thierry, and Dominique Van Schoubroeck. “Ultrasound diagnosis of endometriosis and adenomyosis: State of the art.” Best practice & research. Clinical obstetrics & gynaecology vol. 51 (2018): 16-24. [CrossRef]
- Ahn, Soo Hyun et al. “Biomarkers in endometriosis: challenges and opportunities.” Fertility and sterility vol. 107,3 (2017): 523-532. [CrossRef]
- Jiang, Hong et al. “Bioinformatics identification and validation of biomarkers and infiltrating immune cells in endometriosis.” Frontiers in immunology vol. 13 944683. 29 Nov. 2022. [CrossRef]
- Králíčková, Milena et al. “The Search for Biomarkers in Endometriosis: a Long and Windy Road.” Reproductive sciences (Thousand Oaks, Calif.) vol. 29,6 (2022): 1667-1673. [CrossRef]
- Koninckx, Philippe R et al. “Deep endometriosis: definition, diagnosis, and treatment.” Fertility and sterility vol. 98,3 (2012): 564-71. [CrossRef]
- Kho, Rosanne M et al. “Surgical treatment of different types of endometriosis: Comparison of major society guidelines and preferred clinical algorithms.” Best practice & research. Clinical obstetrics & gynaecology vol. 51 (2018): 102-110. [CrossRef]
- França, Patricia Ribeiro de Carvalho et al. “Endometriosis: A Disease with Few Direct Treatment Options.” Molecules (Basel, Switzerland) vol. 27,13 4034. 23 Jun. 2022. [CrossRef]
- Mira, Ticiana A A et al. “Systematic review and meta-analysis of complementary treatments for women with symptomatic endometriosis.” International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics vol. 143,1 (2018): 2-9. [CrossRef]
- Brichant, Geraldine et al. “New Therapeutics in Endometriosis: A Review of Hormonal, Non-Hormonal, and Non-Coding RNA Treatments.” International journal of molecular sciences vol. 22,19 10498. 28 Sep. 2021. [CrossRef]
- Asghari, Samira et al. “Endometriosis: Perspective, lights, and shadows of etiology.” Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie vol. 106 (2018): 163-174. [CrossRef]
- Tseng, J F et al. “Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis.” The Journal of clinical endocrinology and metabolism vol. 81,3 (1996): 1118-22. [CrossRef]
- Khorram, O et al. “Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis.” American journal of obstetrics and gynecology vol. 169,6 (1993): 1545-9. [CrossRef]
- Taylor, Hugh S et al. “Endometriosis is a chronic systemic disease: clinical challenges and novel innovations.” Lancet (London, England) vol. 397,10276 (2021): 839-852. [CrossRef]
- Orisaka, Makoto et al. “Chronic low-grade inflammation and ovarian dysfunction in women with polycystic ovarian syndrome, endometriosis, and aging.” Frontiers in endocrinology vol. 14 1324429. 13 Dec. 2023. [CrossRef]
- Wei, Yajing et al. “Autonomic nervous system and inflammation interaction in endometriosis-associated pain.” Journal of neuroinflammation vol. 17,1 80. 7 Mar. 2020. [CrossRef]
- Velho, Renata Voltolini et al. “Neurogenic Inflammation in the Context of Endometriosis-What Do We Know?.” International journal of molecular sciences vol. 22,23 13102. 3 Dec. 2021. [CrossRef]
- Sikora, Justyna et al. “Abnormal peritoneal regulation of chemokine activation-The role of IL-8 in pathogenesis of endometriosis.” American journal of reproductive immunology (New York, N.Y. : 1989) vol. 77,4 (2017): 10.1111/aji.12622. [CrossRef]
- Malhotra, Neena et al. “Correlation of angiogenic cytokines-leptin and IL-8 in stage, type and presentation of endometriosis.” Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology vol. 28,3 (2012): 224-7. [CrossRef]
- Măluțan, Andrei Mihai et al. “Endometriosis-associated changes in serum levels of interferons and chemokines.” Turkish journal of medical sciences vol. 47,1 115-122. 27 Feb. 2017. [CrossRef]
- Rahmawati, Nanda Yuli et al. “IL-8 and IL-12p70 are associated with pelvic pain among infertile women with endometriosis.” Pain medicine (Malden, Mass.) vol. 24,11 (2023): 1262-1269. [CrossRef]
- Nishimoto-Kakiuchi, Ayako et al. “A long-acting anti-IL-8 antibody improves inflammation and fibrosis in endometriosis.” Science translational medicine vol. 15,684 (2023): eabq5858. [CrossRef]
- Suen, Jau-Ling et al. “IL-10 from plasmacytoid dendritic cells promotes angiogenesis in the early stage of endometriosis.” The Journal of pathology vol. 249,4 (2019): 485-497. [CrossRef]
- Zhong, Shulin et al. “Association between polymorphisms of cytokine genes and endometriosis: A comprehensive systematic review and meta-analysis.” Journal of reproductive immunology vol. 158 (2023): 103969. [CrossRef]
- Zhou, Wen-Jie et al. “Anti-inflammatory cytokines in endometriosis.” Cellular and molecular life sciences : CMLS vol. 76,11 (2019): 2111-2132. [CrossRef]
- Wang, X-M et al. “Inflammatory cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were associated with infertility in patients with endometriosis.” European review for medical and pharmacological sciences vol. 22,9 (2018): 2513-2518. [CrossRef]
- Huang, Xin et al. “Single-cell transcriptome analysis reveals endometrial immune microenvironment in minimal/mild endometriosis.” Clinical and experimental immunology vol. 212,3 (2023): 285-295. [CrossRef]
- Malutan, Andrei Mihai et al. “The association between interleukin-10 (IL-10) -592C/A, -819T/C, -1082G/A promoter polymorphisms and endometriosis.” Archives of gynecology and obstetrics vol. 295,2 (2017): 503-510. [CrossRef]
- Matsuzaki, Sachiko et al. “IL-10 is not anti-fibrotic but pro-fibrotic in endometriosis: IL-10 treatment of endometriotic stromal cells in vitro promotes myofibroblast proliferation and collagen type I protein expression.” Human reproduction (Oxford, England) vol. 38,1 (2023): 14-29. [CrossRef]
- Tennfjord, Merete Kolberg et al. “Effect of physical activity and exercise on endometriosis-associated symptoms: a systematic review.” BMC women's health vol. 21,1 355. 9 Oct. 2021. [CrossRef]
- Abril-Coello, Rebeca et al. “Benefits of physical therapy in improving quality of life and pain associated with endometriosis: A systematic review and meta-analysis.” International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics vol. 162,1 (2023): 233-243. [CrossRef]
- Ricci, Elena et al. “Physical activity and endometriosis risk in women with infertility or pain: Systematic review and meta-analysis.” Medicine vol. 95,40 (2016): e4957. [CrossRef]
- Haney, Muscato et al. “Peritoneal fluid cell populations in infertility patients.” Fertility and sterility vol. 35,6 (1981): 696-8. [CrossRef]
- van Furth, Raeburn et al. “Characteristics of human mononuclear phagocytes.” Blood vol. 54,2 (1979): 485-500.
- Dmowski, Steele et al. “Deficient cellular immunity in endometriosis.” American journal of obstetrics and gynecology vol. 141,4 (1981): 377-83. [CrossRef]
- Endometriosis Pathogenesis, Clinical Impact and Management; Volume 9: Frontiers in Gynecological Endocrinology – A. R. Genazzani, M. Nisolle, F. Petraglia, R. N. Taylor; Springer 2021; ISBN 978-3-030-57865-7.
- Martire, Francesco G et al. “Endometriosis and Adolescence: The Impact of Dysmenorrhea.” Journal of clinical medicine vol. 12,17 5624. 29 Aug. 2023. [CrossRef]
- Martire, Francesco Giuseppe et al. “Early noninvasive diagnosis of endometriosis: dysmenorrhea and specific ultrasound findings are important indicators in young women.” Fertility and sterility vol. 119,3 (2023): 455-464. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).