1. Introduction
In the 21st century, research on microbial compounds and their favorable impact on immune system has gathered pace, as it is now presumed to be one of the crucial factors that stimulate immune system development [
1]. That is the reason why the use of probiotics and prebiotics has attracted recent interest. Probiotics are live bacteria strains that present health benefits on the host, compete with pathogens, promote microbial antagonism, and inhibit bacteria toxin production [
2]. Prebiotics as specific dietary ingredients affect gut microbiota composition and support probiotics [
3]. Strikingly, in many cases, the beneficial role of probiotics results from the effects of postbiotics which are inanimate microorganisms and/or their components that confer a health benefit on the host [
4]. Postbiotics’ advantages over probiotics contain aspects such as the absence of bacteria translocation and a lesser risk of infection deterioration. Moreover, they promote immune system development, inhibit inflammation, prevent infections, regulate lipid metabolism, and stabilize gut microbiota composition [
5]. Among them, the most common are bacterial lysates (BLs), short-chained fatty acids (SCFAs), exopolysaccharides (EPSs), and heat-killed Lactobacilli [
6]. Although SCFAs and EPSs cannot be classified as postbiotics in and of themselves, they are still to be found in the presence of bacterial biomass and therefore present beneficial outcomes on the host [
4]. For this reason, we decided to discuss their great immunomodulatory potential in atopic asthma within this article.
Asthma, the most common of allergic diseases, affecting approximately 14% of children and young adults worldwide is a disease with strikingly prominent diversity of ontogenetic and environmental factors modulating its course [
7,
8]. At its core lies recognizing and processing internal and external stimuli by the innate immune system, leading to various different ways in which the adaptive response is triggered [
9]. Previously, asthma was considered to be a single diagnosis with standardized treatment for all patients. However, now being known as a heterogenous, multifactorial disorder, new approach acknowledging the modulation of the immune response with various specific molecules redirects focus from traditional treatment to additional immunomodulation. This publication aims to summarize and explain the immunomodulatory effects of postbiotics in the prevention of exacerbations and treatment of allergic asthma.
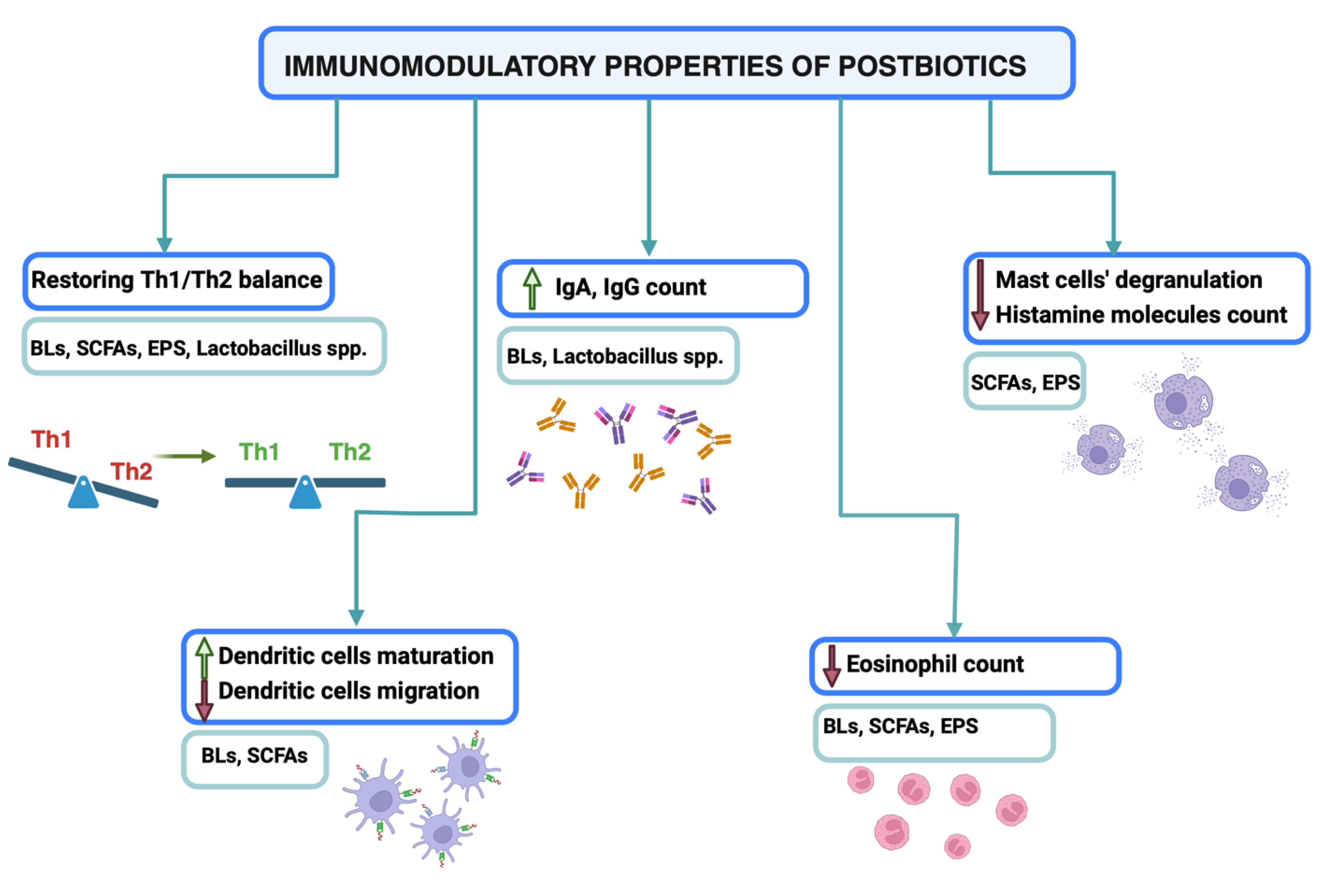
Figure 1.
Immunomodulatory properties of postbiotics. Postbiotics act as immunomodulators and promote antiallergic immune responses. They provide the maintenance of violated Th1/Th2 balance, stimulate IgA and IgG production and provoke DCs maturation. Moreover, they reduce asthma symptoms by decreasing mast cells degranulation and eosinophils’ count. Created with biorender.com.
Figure 1.
Immunomodulatory properties of postbiotics. Postbiotics act as immunomodulators and promote antiallergic immune responses. They provide the maintenance of violated Th1/Th2 balance, stimulate IgA and IgG production and provoke DCs maturation. Moreover, they reduce asthma symptoms by decreasing mast cells degranulation and eosinophils’ count. Created with biorender.com.
2. Methods of Acquiring Data
The authors searched biomedical databases (PubMed, Scopus, Web of Science) for articles concerning the use of postbiotics in the asthma treatment. In all the databases the following keywords “asthma”, “postbiotics”, “bacterial lysates”, “short-chained fatty acids”, “exopolysaccharides” and “heat-killed Lactobacillus” were searched. The research was performed by two independent persons. All the relevant studies were identified by title and abstract reading. Duplicated articles were initially excluded. A careful analysis of full texts was done.
Studies to be included in the chapter regarding bacterial lysates had to meet all of the following inclusion criteria: clinical trial (double-blind RCT or open-label RCT or sequential trial or cohort study); study on humans (children or/and adults) with asthma; BLs (PMBL or PCBL) as an intervention (alone or combined with standard care); control group receiving only standard asthma treatment or placebo or both. All of the studies had to be written in English. Study size was not included as criteria. Studies were excluded if the study design was ineligible, if they included tests performed on animals or evaluated the use of BLs for asthma prevention.
Two authors independently extracted the following data from the eligible studies: study design, sample size, participants’ characteristics, interventions, comparators, clinical and other outcomes.
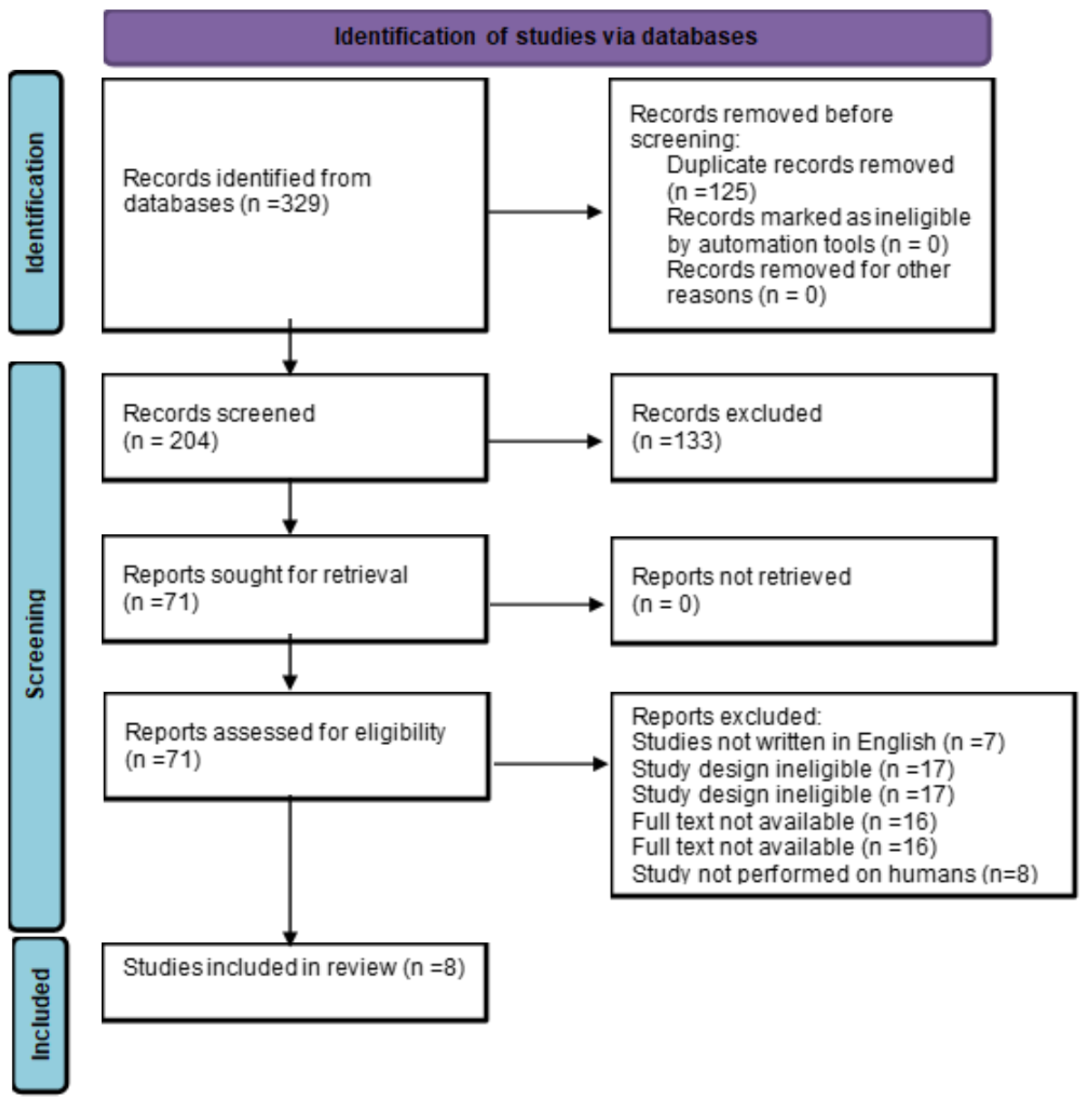
Figure 2.
Identification of studies via databases.
Figure 2.
Identification of studies via databases.
3. Bacterial Lysates
BLs are immunomodulatory preparations consisting of antigens derived from the most common respiratory tract bacteria species [
10]. They can be obtained with chemical or mechanical lysis, which indicates their different biological effects [
11]. BLs can be administered orally, sublingually, or intranasally in various forms [
12]. Each route of administration provides slightly different effects; thus, it should be chosen considering the type of allergic disease and patients’ preferences [
13]. Patients with asthma usually receive the oral form, which mainly affects mucosal immune cells in the intestine [
14].
The fundamental mechanism of BLs’ action is based on the natural immune response provoked by pathogens. Bacterial antigens continuously stimulate the lymphoid tissue in the mucosa to produce cytokines that modulate the immune response, both locally and generally [
15]. These immunological changes provide a beneficial switch in the cytokines’ classes and affect the clinical course of the disease [
16].
Toll-like receptors (TLRs) expressed on dendritic cells (DCs) and monocytes are the first-line proteins that recognize and respond to antigens. They stimulate DCs to maturate and influence cytokines release [
17]. Studies suggest that BLs’ activity mainly depends on TLR 2 and TLR4 signaling which suppresses airway hyperreactivity, mucus production and Th2-type immune response in the lungs and lowers the risk of asthma development [
18,
19,
20]. BLs promote the production of IL-10, IL-12 and IFN-gamma characteristic for Th1-type immune response [
21,
22] and suppress the secretion of Th2-type cytokines: IL-4, IL-5, IL-13 IL-17, which maintenances the disturbed Th1/Th2 balance [
23,
24,
25]. Sublingually administered BLs increase the level of NK cells in asthmatic children [
24,
26]. They are also known to activate peripheral blood macrophages and increase serum levels of IgA, IgG and human beta-defensin 1 (hBD-1) [
27,
28]. Furthermore, they seem effective in lowering eosinophil counts in bronchoalveolar fluid and blood [
29]. The in depth mechanism of BLs’ action was previously described in our recent publication [
30].
Nine human studies evaluated the efficacy of BLs in the prevention of exacerbations and treatment of asthma. The very first clinical trial was conducted in 1987 by Weiss et al., who investigated the impact of Broncho-Vaxom (PCBL) on patients with asthma or chronic obstructive pulmonary disease (COPD). The research showed that PCBL reduces IgE and increases IgG levels in atopic patients. Although the results did not attain statistical significance, they pointed out the possible beneficial effects of BLs and encouraged scientists to conduct further research [
31]. The effectiveness of BLs in reducing asthma exacerbations and symptom severity was confirmed in 5 clinical trials [
23,
32,
33,
34,
35]. Both PCBLs and PMBLs were more effective in facilitating the clinical course of the disease than standardized care (SC) [
23,
33,
34]. Only the study by de Boer et al. did not show any difference in the number of exacerbations in the PCBL and SC groups. However, the FEV1 was increased in the PCBL group; thus, some improvements were observed [
21]. On the other hand, Roßberg et al., who evaluated the effect of BLs administered in infancy on the risk of developing atopic dermatitis (AD), allergic rhinitis (AR) and asthma, concluded that they do not reduce the risk of allergic diseases. Nevertheless, patients received a mixture of heat-killed Escherichia coli and Enterococcus faecalis; thus, the effects probably resulted from the specific antigen properties and the results should not be clinching [
36].Therefore, further research on the efficacy of BLs in asthma prevention should be conducted.
Table 1.
Characteristics of included studies.
Table 1.
Characteristics of included studies.
| Author, year |
Study design |
Subject (BLs/control) |
Mean Age |
Treatment regimen |
Clinical outcomes |
Immunological and other outcomes |
Emeryk et al.
2018
(32) |
RCT |
150 (74/76) |
6-16 years |
Ismigen (PMBL) vs Placebo |
The number of asthma exacerbations was lower in the PMBL group. |
|
de Boer et al.
2021
(21) |
RCT |
75 (38/37) |
16-60 years |
OM-85 (PCBL) vs SC |
Exacerbations were not different between groups after 18 months. |
FEV1 increased in the PCBL group. |
Lu et al.
2015
(28)
|
RCT |
60 (24/36) |
5-15 years |
OM-85 (PCBL) vs SC |
|
Increase serum IFN-γ/IL-4 ratio was observed |
Li et al.
2022
(33)
|
Retrospective PS-matched cohort study |
795 (337/458) |
6 months - 14 years |
QIPIAN (PMBL) vs SC |
Less exacerbations were observed in the PMBL group.
|
|
Bartkowiak - Emeryk et al.
2021
(30) |
RCT |
49 (21/28) |
6-15 years |
Ismigen (PMBL) vs placebo |
|
Increased serum T lymphocyte, CD4+CD25+FOXP3+, CD8+, CD3−CD16+CD56+
Decreased serum CD69+ and CD25+ subset of CD3+ |
Koatz et al.
2016
(34) |
open-label, prospective, sequential |
28 |
16-65 |
1st year SC
2nd year OM-85 (PCBL) |
Decreased symptom severity and the number of exacerbations |
Increased serum and salivary secretory IgA |
Han et al.
2016
(23) |
RCT |
136 (74/62) |
7 months-5 years |
OM-85 (PCBL) vs inhaled corticosteroids/aminophylline/antibiotics |
Decreased frequency and duration of capillary bronchitis and asthma |
Decreased serum IL-4, IL-17 levels
Increased serum IL-10 and IFN-g levels |
Abdou et al.
1993
(35) |
RCT |
50(25/25) |
Not applicable |
OM-85 vs SC |
Reduction in the duration and number of asthma attacks
|
Increased FEV1/FVC% ratio
Increased serum IgA, IgM and IgG levels
Decreased serum IgE level
Decreased eosinophil count in broncho-alvelolar fluid
Increased IgA/albumin ratio |
4. Short-Chained Fatty Acids
SCFAs are found in the human gut as a product of anaerobic fermentation of non-digestible dietary fiber and aminoacids by saprophytical bacteria, as well as, in marginal amounts, derived directly from the diet [
37]. Although the most abundant SCFA in the human gut tends to be acetate, the most beneficial in regard to health are found to be propionate and butyrate, being produced mostly by Bacteroidetes and Firmicutes, respectively [
38,
39].
SCFAs can be either absorbed by the gut lining endothelium cells to serve as an energy source or enter the bloodstream and modulate the immune response [
37]. Said immunomodulating effects are enabled via transporters: proton-coupled monocarboxylate transporter isoform 1 (MCT1) and the Na+-coupled monocarboxylate transporter 1 (SMCT1), which can be found on the apical surface of colonocytes as well as on the surface of immune system cells [
40,
41]. Mentioned uptake is accompanied by other butyrate-sensing mechanisms: G protein-coupled receptor (GPCR) class: GPR41/FFAR3 (free fatty acid receptor 3), GPR43/FFAR2, and GPR109A/HCAR2, Peroxisome proliferator-activated receptors (PPARs), and Zn2+-dependent class I, II, and IV histone deacetylases (HDACs). The essence of SCFA’s immunomodulation potency lies in deacetylation from histone complexes’ lysine. As a result, chromatin formation becomes denser and firmer so that gene expression becomes suppressed, inhibiting the immune system cells’ natural functions [
37]. In various studies, knockout of GPR41 and GPR43 has been proven to exacerbate asthma responses in mice, leading to assumptions that SCFAs can have a substantial effect on soothing asthmatic symptoms [
42].
Th2 cell induction was shown to be suppressed in mice with high SCFA levels [
43]. Moreover, DCs were proven to polarise naïve CD4+ T cells away from type 2 maturation and instead lean toward type 1 maturation [
44]. Furthermore, in the in vitro model, SCFAs decreased DCs’ migration abilities, resulting in decreased asthmatic symptoms [
45].
Jong-Hwa et al. showed in a study on AD that in mice individuals with higher SCFA levels, the eosinophilic percentage and eosinophilic count were decreased [
46]. Given the atopic nature of asthma and AD, this correlation of high SCFA level to low eosinophils seems promising in discovering new therapeutic possibilities in asthma. In other studies, butyrate has been revealed to induce eosinophil apoptosis and reduce their adhesion to endothelial cells. Mentioned before Zn2+-dependent class I, II, and IV HDACs have also been proven to inhibit eosinophilic survival and migration after exposure to propionate and butyrate [
47]. In this light, SCFA’s might contribute to alleviating symptoms of atopic asthma.
In allergic response, B cells after being stimulated by IL-4 and IL-13 mature into IgE - producing plasma cells. This ability of maturation and class-switching has been reported to be lower in mice with high fiber diet through epigenetic alterations [
48]. Propionate and butyrate also decreased IL-4 level, which is essential to differentiate B cells into IgE-producing cells [
49]. Moreover, SCFAs were shown to inhibit mast cell degranulation and release airway contractiveness [
50].
The imbalance of gut microbiota is a profound factor contributing to immunological diseases. It has been shown that amongst children suffering from asthma, the levels of SCFAs were significantly lower than in their healthy peers [
51].
5. Exopolysaccharydes
EPSs are carbohydrate polymers forming the external coating of the bacterial cell wall. They have diverse health effects, such as calcium and magnesium absorption, glycemic control, and anticarcinogenic and antioxidant effects [
52]. Notably, EPSs produced by commensal bacteria, like Lactobacillus or Bifidobacterium, present immunomodulatory properties [
53]. Schiavi et al. revealed that EPSs derived from Bifidobacterium longum inhibit eosinophilic migration to the airways, which was connected to Th2-associated interleukins IL-4 and IL-13 decrease [
54]. A recent study showed that EPSs isolated from Bacillus subtilis divested asthmatic inflammation, linked to the concentration-dependent decrease of IL-4 and IL-5 levels, leading to reduced eosinophilic count [
55]. Moreover, EPSs strongly bind the histamine molecules to the surface of bacterial wall and lower their blood count, mitigating the asthmatic response [
56]. In a randomized, double-blind, placebo-controlled clinical trial where patients with airborne allergy were administered EPS derived from Lactobacillus paracasei for 12 weeks, there was a significant alleviation of allergic symptoms reported by patients, which also correlated with the decrease in biochemical signs of allergic inflammation [
57].
Knowing the effect of EPS on various elements of the immune system and promising results on inhalant allergy in humans, those studies shine a new light on the potential use of EPSs, amongst other postbiotics, in controlling atopic asthma symptoms.
6. Heat-Killed Lactobacillus
The genus Lactobacillus is represented by almost 250 species of gram-positive, anaerobic bacteria colonizing multiple, diverse habitats, which can be used in many industrial and healthcare applications [
58]. For years, beneficial effects of
Lactobacillus spp. were observed in preventing gut dysbiosis and allergy development [
59,
60]. Additionally, a recent airway microbiome profiling revealed the presence of Lactobacillus spp. in the nasopharynx and pointed out its remarkable role in local immune changes and inhibition of respiratory tract pathogens’ growth and virulence [
61]. However, there is still insufficient data establishing whether the mentioned abilities result from live bacteria activity or bacterial products and particles that influence the host’s immunity.
In an allergy model, mice fed with heat-killed L. casei showed significantly lower IgE and IgG1 levels and suppressed T cells production of Th2 type (IL-4, IL-5, IL-10, IL-13) and proinflammatory (IFN-γ, TNF- α) cytokines compared with the placebo group. Moreover, the histological evidence showed the attenuation of lung inflammation and reduced proinflammatory cytokines in bronchoalveolar fluid [
62]. Choi et al. observed similar phenomena when examining the impact of heat-killed Lactobacillus spp. on dust-mite-induced AD in mice. Moreover, the mentioned supplementation ameliorated symptoms and reduced the number of mast cells and eosinophils in lesions. Among 18 Lactobacillus strains, L. brevis NS1401 induced the greatest IFN-γ and IL-12 secretion and the least IL-4 production [
63]. It suggests that immunomodulatory abilities depend on lactobacilli species and are unequal for the whole genus. Hong et al. compared three heat-killed Lactobacilli’s effects on airway hyper-responsiveness in a murine asthma model. The research showed that airway inflammation was suppressed in L. plantarum and L. curvatus treated mice, and lower IL-4 and IL-5 levels were observed. On the contrary, in L. sakei subsp. sakei. group, no differences were found [
64]. Similar results were shown by Lee et al., who compared cytokine regulatory effects of three Lactobacilli strains in in vitro study. Notably, Lactobacilli lysates were more likely to stimulate cytokines production than heat-killed bacteria, cell supernatants and live strains. Lipoteichoic acid isolated from bacteria promoted TNF-alpha production via TLR2-mediated NF-κB and extracellular-signal-regulated kinase (ERK) activation [
65]. Heat-killed Lactobacilli strains stimulate DCs to produce IL-12 p70 and switch T cells to Th1 type immune response [
66]. Moreover, they suppress the production of IL-6 and IL-17A which results in Treg/Th17 balance maintenance [
67]. Mentioned studies suggest that tyndallization do not reduce the immunomodulatory abilities of Lactobacillus spp. and the results of their use are comparable with those from live bacteria. Furthermore, heat-killed bacteria offer increased safety; thus, their usage in clinical practice should be considered.
Currently, there is insufficient data concerning their use in asthmatic patients. Nevertheless, promising effects of tyndallized Lactobacillus spp. are shown in research on other allergic diseases; thus, maybe in the future, extensive sample studies will be performed [
68].
7. Conclusions
As described above, postbiotics seem to be promising therapeutic approaches in asthma treatment. They undoubtedly affect the immune system and promote various changes in cytokine production, T-cell differentiation, immunoglobulin release, and eosinophil infiltration. Lowering the number of asthma exacerbations as the effect of postbiotics’ immunomodulating qualities could insinuate their appropriate use in asthma treatment. However, their underlying mechanisms of action are not fully elucidated, and it is difficult to determine whether they should be applied in clinical practice. Thus, further, large sample studies should be carried out. Health benefits, side effects and cost-effectiveness should be examined.
Author Contributions
Conceptualization, KW and AJ; Data curation, KW and AJ; Data analysis, AJ; Writing - rough preparation, KW and AJ; Writing - review and editing WF, KW and AJ; Visualization WF; Supervision, WF and KJ; Project administration, KW and WF. All authors have read and agreed with the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Weinberger M. Can We Prevent Exacerbations of Asthma Caused by Common Cold Viruses? Journal of Allergy and Clinical Immunology (2010) 126(4):770-1. [CrossRef]
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat Rev Gastroenterol Hepatol (2014) 11(8):506-14. [CrossRef]
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (Isapp) Consensus Statement on the Definition and Scope of Prebiotics. Nat Rev Gastroenterol Hepatol (2017) 14(8):491-502. [CrossRef]
- Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. The International Scientific Association of Probiotics and Prebiotics (Isapp) Consensus Statement on the Definition and Scope of Postbiotics. Nat Rev Gastroenterol Hepatol (2021) 18(9):649-67. [CrossRef]
- Wegh CAM, Geerlings SY, Knol J, Roeselers G, Belzer C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int J Mol Sci (2019) 20(19). [CrossRef]
- Zolkiewicz J, Marzec A, Ruszczynski M, Feleszko W. Postbiotics-a Step Beyond Pre- and Probiotics. Nutrients (2020) 12(8). [CrossRef]
- Naja AS, Permaul P, Phipatanakul W. Taming Asthma in School-Aged Children: A Comprehensive Review. J Allergy Clin Immunol Pract (2018) 6(3):726-35. [CrossRef]
- Gans MD, Gavrilova T. Understanding the Immunology of Asthma: Pathophysiology, Biomarkers, and Treatments for Asthma Endotypes. Paediatr Respir Rev (2020) 36:118-27. [CrossRef]
- Nobs SP, Zmora N, Elinav E. Nutrition Regulates Innate Immunity in Health and Disease. Annu Rev Nutr (2020) 40:189-219. [CrossRef]
- Bessler WG, Vor dem Esche U, Masihi N. The Bacterial Extract Om-85 Bv Protects Mice against Influenza and Salmonella Infection. Int Immunopharmacol (2010) 10(9):1086-90. [CrossRef]
- Suarez N, Ferrara F, Rial A, Dee V, Chabalgoity JA. Bacterial Lysates as Immunotherapies for Respiratory Infections: Methods of Preparation. Front Bioeng Biotechnol (2020) 8:545. [CrossRef]
- Cazzola M, Anapurapu S, Page CP. Polyvalent Mechanical Bacterial Lysate for the Prevention of Recurrent Respiratory Infections: A Meta-Analysis. Pulm Pharmacol Ther (2012) 25(1):62-8. [CrossRef]
- Pivniouk V, Gimenes-Junior JA, Ezeh P, Michael A, Pivniouk O, Hahn S, et al. Airway Administration of Om-85, a Bacterial Lysate, Blocks Experimental Asthma by Targeting Dendritic Cells and the Epithelium/Il-33/Ilc2 Axis. J Allergy Clin Immunol (2022) 149(3):943-56. [CrossRef]
- Rossi GA, Pohunek P, Feleszko W, Ballarini S, Colin AA. Viral Infections and Wheezing-Asthma Inception in Childhood: Is There a Role for Immunomodulation by Oral Bacterial Lysates? Clin Transl Allergy (2020) 10:17. [CrossRef]
- Ver Heul A, Planer J, Kau AL. The Human Microbiota and Asthma. Clin Rev Allergy Immunol (2019) 57(3):350-63. [CrossRef]
- Kearney SC, Dziekiewicz M, Feleszko W. Immunoregulatory and Immunostimulatory Responses of Bacterial Lysates in Respiratory Infections and Asthma. Ann Allergy Asthma Immunol (2015) 114(5):364-9. [CrossRef]
- Kirtland ME, Tsitoura DC, Durham SR, Shamji MH. Toll-Like Receptor Agonists as Adjuvants for Allergen Immunotherapy. Front Immunol (2020) 11:599083. [CrossRef]
- Haapakoski R, Karisola P, Fyhrquist N, Savinko T, Lehtimaki S, Wolff H, et al. Toll-Like Receptor Activation During Cutaneous Allergen Sensitization Blocks Development of Asthma through Ifn-Gamma-Dependent Mechanisms. J Invest Dermatol (2013) 133(4):964-72. [CrossRef]
- Coviello S, Wimmenauer V, Polack FP, Irusta PM. Bacterial Lysates Improve the Protective Antibody Response against Respiratory Viruses through Toll-Like Receptor 4. Hum Vaccin Immunother (2014) 10(10):2896-902. [CrossRef]
- Li Y, Tu C, Chen M, Tan C, Zheng X, Wang Z, et al. Establishing a High Microbial Load Maternal-Offspring Asthma Model in Adult Mice. Int Immunopharmacol (2020) 83:106453. [CrossRef]
- de Boer GM, Braunstahl GJ, van der Ploeg EK, van Zelst CM, van Bruggen A, Epping G, et al. Bacterial Lysate Add-on Therapy to Reduce Exacerbations in Severe Asthma: A Double-Blind Placebo-Controlled Trial. Clin Exp Allergy (2021) 51(9):1172-84. [CrossRef]
- Han L, Zheng CP, Sun YQ, Xu G, Wen W, Fu QL. A Bacterial Extract of Om-85 Broncho-Vaxom Prevents Allergic Rhinitis in Mice. Am J Rhinol Allergy (2014) 28(2):110-6. [CrossRef]
- Han RF, Li HY, Wang JW, Cong XJ. Study on Clinical Effect and Immunologic Mechanism of Infants Capillary Bronchitis Secondary Bronchial Asthma Treated with Bacterial Lysates Broncho-Vaxom. Eur Rev Med Pharmacol Sci (2016) 20(10):2151-5.
- Lu Y, Li Y, Xu L, Xia M, Cao L. Bacterial Lysate Increases the Percentage of Natural Killer T Cells in Peripheral Blood and Alleviates Asthma in Children. Pharmacology (2015) 95(3-4):139-44. [CrossRef]
- Rodrigues A, Gualdi LP, de Souza RG, Vargas MH, Nunez NK, da Cunha AA, et al. Bacterial Extract (Om-85) with Human-Equivalent Doses Does Not Inhibit the Development of Asthma in a Murine Model. Allergol Immunopathol (Madr) (2016) 44(6):504-11. [CrossRef]
- Bartkowiak-Emeryk M, Emeryk A, Rolinski J, Wawryk-Gawda E, Markut-Miotla E. Impact of Polyvalent Mechanical Bacterial Lysate on Lymphocyte Number and Activity in Asthmatic Children: A Randomized Controlled Trial. Allergy Asthma Clin Immunol (2021) 17(1):10. [CrossRef]
- Luan H, Zhang Q, Wang L, Wang C, Zhang M, Xu X, et al. Om85-Bv Induced the Productions of Il-1beta, Il-6, and Tnf-Alpha Via Tlr4- and Tlr2-Mediated Erk1/2/Nf-Kappab Pathway in Raw264.7 Cells. J Interferon Cytokine Res (2014) 34(7):526-36. [CrossRef]
- Liao JY, Zhang T. [Influence of Om-85 Bv on Hbd-1 and Immunoglobulin in Children with Asthma and Recurrent Respiratory Tract Infection]. Zhongguo Dang Dai Er Ke Za Zhi (2014) 16(5):508-12.
- Liu C, Huang R, Yao R, Yang A. The Immunotherapeutic Role of Bacterial Lysates in a Mouse Model of Asthma. Lung (2017) 195(5):563-9. [CrossRef]
- Kaczynska A, Klosinska M, Janeczek K, Zarobkiewicz M, Emeryk A. Promising Immunomodulatory Effects of Bacterial Lysates in Allergic Diseases. Front Immunol (2022) 13:907149. [CrossRef]
- Weiss S, Fux T. [Effect of Broncho-Vaxom on Serum Ige and Igg Levels in Patients with Bronchial Asthma and Chronic Obstructive Lung Disease. A Placebo-Controlled Double-Blind Study]. Schweiz Med Wochenschr (1987) 117(39):1514-8.
- Emeryk A, Bartkowiak-Emeryk M, Raus Z, Braido F, Ferlazzo G, Melioli G. Mechanical Bacterial Lysate Administration Prevents Exacerbation in Allergic Asthmatic Children-the Eolia Study. Pediatr Allergy Immunol (2018) 29(4):394-401. [CrossRef]
- Li L, Li J, Hu C, Di Nardo M, Srinivasan V, Adamko DJ, et al. Effectiveness of Polyvalent Bacterial Lysate for Pediatric Asthma Control: A Retrospective Propensity Score-Matched Cohort Study. Transl Pediatr (2022) 11(10):1697-703. [CrossRef]
- Koatz AM, Coe NA, Ciceran A, Alter AJ. Clinical and Immunological Benefits of Om-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and Copd and Recurrent Respiratory Infections. Lung (2016) 194(4):687-97. [CrossRef]
- Abdou MA, Hanna KM, El Attar S, Abdel Nabi E, Hatem A, Abdel Ghaffar M. Influence of a Bacterial Extract, Broncho-Vaxom, on Clinical and Immunological Parameters in Patients with Intrinsic Asthma. International Journal of Immunotherapy (1993) 9(2):127-33.
- Rossberg S, Keller T, Icke K, Siedmann V, Lau I, Keil T, et al. Orally Applied Bacterial Lysate in Infants at Risk for Atopy Does Not Prevent Atopic Dermatitis, Allergic Rhinitis, Asthma or Allergic Sensitization at School Age: Follow-up of a Randomized Trial. Allergy (2020) 75(8):2020-5. [CrossRef]
- Yip W, Hughes MR, Li Y, Cait A, Hirst M, Mohn WW, et al. Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma. Front Immunol (2021) 12:628453. [CrossRef]
- Xiong RG, Zhou DD, Wu SX, Huang SY, Saimaiti A, Yang ZJ, et al. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods (2022) 11(18). [CrossRef]
- Louis P, Flint HJ. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ Microbiol (2017) 19(1):29-41. [CrossRef]
- Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. Butyrate: A Double-Edged Sword for Health? Adv Nutr (2018) 9(1):21-9. [CrossRef]
- Parada Venegas D, De la Fuente MK, Landskron G, Gonzalez MJ, Quera R, Dijkstra G, et al. Short Chain Fatty Acids (Scfas)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol (2019) 10:277. [CrossRef]
- Ang Z, Ding JL. Gpr41 and Gpr43 in Obesity and Inflammation - Protective or Causative? Front Immunol (2016) 7:28. [CrossRef]
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat Med (2014) 20(2):159-66. [CrossRef]
- Kaisar MMM, Pelgrom LR, van der Ham AJ, Yazdanbakhsh M, Everts B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells Via Both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109a Signaling. Front Immunol (2017) 8:1429. [CrossRef]
- Cait A, Hughes MR, Antignano F, Cait J, Dimitriu PA, Maas KR, et al. Microbiome-Driven Allergic Lung Inflammation Is Ameliorated by Short-Chain Fatty Acids. Mucosal Immunol (2018) 11(3):785-95. [CrossRef]
- Kim JH, Kim K, Kim W. Gut Microbiota Restoration through Fecal Microbiota Transplantation: A New Atopic Dermatitis Therapy. Exp Mol Med (2021) 53(5):907-16. [CrossRef]
- Theiler A, Barnthaler T, Platzer W, Richtig G, Peinhaupt M, Rittchen S, et al. Butyrate Ameliorates Allergic Airway Inflammation by Limiting Eosinophil Trafficking and Survival. J Allergy Clin Immunol (2019) 144(3):764-76. [CrossRef]
- Sanchez HN, Moroney JB, Gan H, Shen T, Im JL, Li T, et al. B Cell-Intrinsic Epigenetic Modulation of Antibody Responses by Dietary Fiber-Derived Short-Chain Fatty Acids. Nat Commun (2020) 11(1):60. [CrossRef]
- Shi Y, Xu M, Pan S, Gao S, Ren J, Bai R, et al. Induction of the Apoptosis, Degranulation and Il-13 Production of Human Basophils by Butyrate and Propionate Via Suppression of Histone Deacetylation. Immunology (2021) 164(2):292-304. [CrossRef]
- Folkerts J, Redegeld F, Folkerts G, Blokhuis B, van den Berg MPM, de Bruijn MJW, et al. Butyrate Inhibits Human Mast Cell Activation Via Epigenetic Regulation of Fcepsilonri-Mediated Signaling. Allergy (2020) 75(8):1966-78. [CrossRef]
- Bottcher MF, Nordin EK, Sandin A, Midtvedt T, Bjorksten B. Microflora-Associated Characteristics in Faeces from Allergic and Nonallergic Infants. Clin Exp Allergy (2000) 30(11):1590-6. [CrossRef]
- Juraskova D, Ribeiro SC, Silva CCG. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods (2022) 11(2). [CrossRef]
- Kaur N, Dey P. Bacterial Exopolysaccharides as Emerging Bioactive Macromolecules: From Fundamentals to Applications. Res Microbiol (2023) 174(4):104024. [CrossRef]
- Schiavi E, Plattner S, Rodriguez-Perez N, Barcik W, Frei R, Ferstl R, et al. Exopolysaccharide from Bifidobacterium Longum Subsp. Longum 35624 Modulates Murine Allergic Airway Responses. Benef Microbes (2018) 9(5):761-73. [CrossRef]
- Zhang L, Yi H. An Exopolysaccharide from Bacillus Subtilis Alleviates Airway Inflammatory Responses Via the Nf-Kappab and Stat6 Pathways in Asthmatic Mice. Biosci Rep (2022) 42(1). [CrossRef]
- Kinoshita H, Hariu M, Nakashima Y, Watanabe K, Yasuda S, Igoshi K. Lactic Acid Bacterial Exopolysaccharides Strongly Bind Histamine and Can Potentially Be Used to Remove Histamine Contamination in Food. Microbiology (Reading) (2021) 167(1). [CrossRef]
- Noda M, Kanno K, Danshiitsoodol N, Higashikawa F, Sugiyama M. Plant-Derived Lactobacillus Paracasei Ijh-Sone68 Improves Chronic Allergy Status: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients (2021) 13(11). [CrossRef]
- Oberg TS, McMahon DJ, Culumber MD, McAuliffe O, Oberg CJ. Invited Review: Review of Taxonomic Changes in Dairy-Related Lactobacilli. J Dairy Sci (2022) 105(4):2750-70. [CrossRef]
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in Primary Prevention of Atopic Disease: A Randomised Placebo-Controlled Trial. Lancet (2001) 357(9262):1076-9. [CrossRef]
- Ni Y, Zhang Y, Zheng L, Rong N, Yang Y, Gong P, et al. Bifidobacterium and Lactobacillus Improve Inflammatory Bowel Disease in Zebrafish of Different Ages by Regulating the Intestinal Mucosal Barrier and Microbiota. Life Sci (2023) 324:121699. [CrossRef]
- Tonetti FR, Tomokiyo M, Fukuyama K, Elean M, Moyano RO, Yamamuro H, et al. Post-Immunobiotics Increase Resistance to Primary Respiratory Syncytial Virus Infection and Secondary Pneumococcal Pneumonia. Benef Microbes (2023):1-14. [CrossRef]
- Lim LH, Li HY, Huang CH, Lee BW, Lee YK, Chua KY. The Effects of Heat-Killed Wild-Type Lactobacillus Casei Shirota on Allergic Immune Responses in an Allergy Mouse Model. Int Arch Allergy Immunol (2009) 148(4):297-304. [CrossRef]
- Choi CY, Kim YH, Oh S, Lee HJ, Kim JH, Park SH, et al. Anti-Inflammatory Potential of a Heat-Killed Lactobacillus Strain Isolated from Kimchi on House Dust Mite-Induced Atopic Dermatitis in Nc/Nga Mice. J Appl Microbiol (2017) 123(2):535-43. [CrossRef]
- Hong HJ, Kim E, Cho D, Kim TS. Differential Suppression of Heat-Killed Lactobacilli Isolated from Kimchi, a Korean Traditional Food, on Airway Hyper-Responsiveness in Mice. J Clin Immunol (2010) 30(3):449-58. [CrossRef]
- Lee YD, Hong YF, Jeon B, Jung BJ, Chung DK, Kim H. Differential Cytokine Regulatory Effect of Three Lactobacillus Strains Isolated from Fermented Foods. J Microbiol Biotechnol (2016) 26(9):1517-26. [CrossRef]
- Chuang L, Wu KG, Pai C, Hsieh PS, Tsai JJ, Yen JH, et al. Heat-Killed Cells of Lactobacilli Skew the Immune Response toward T Helper 1 Polarization in Mouse Splenocytes and Dendritic Cell-Treated T Cells. J Agric Food Chem (2007) 55(26):11080-6. [CrossRef]
- Li AL, Meng XC, Duan CC, Huo GC, Zheng QL, Li D. Suppressive Effects of Oral Administration of Heat-Killed Lactobacillus Acidophilus on T Helper-17 Immune Responses in a Bovine Beta-Lactoglobulin-Sensitized Mice Model. Biol Pharm Bull (2013) 36(2):202-7. [CrossRef]
- Jeong K, Kim M, Jeon SA, Kim YH, Lee S. A Randomized Trial of Lactobacillus Rhamnosus Idcc 3201 Tyndallizate (Rht3201) for Treating Atopic Dermatitis. Pediatr Allergy Immunol (2020) 31(7):783-92. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).