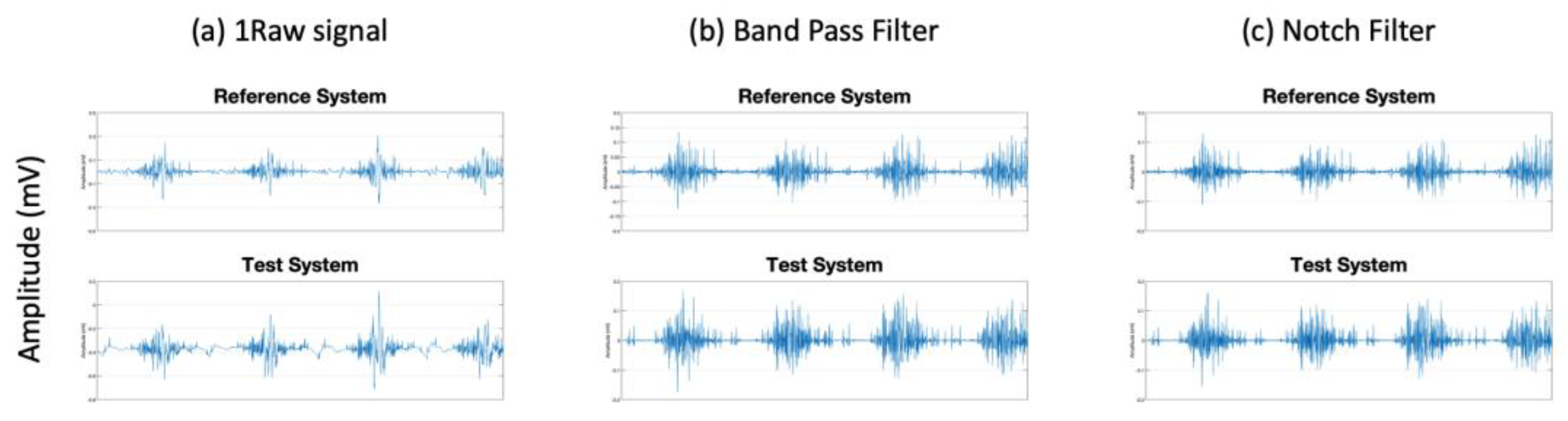
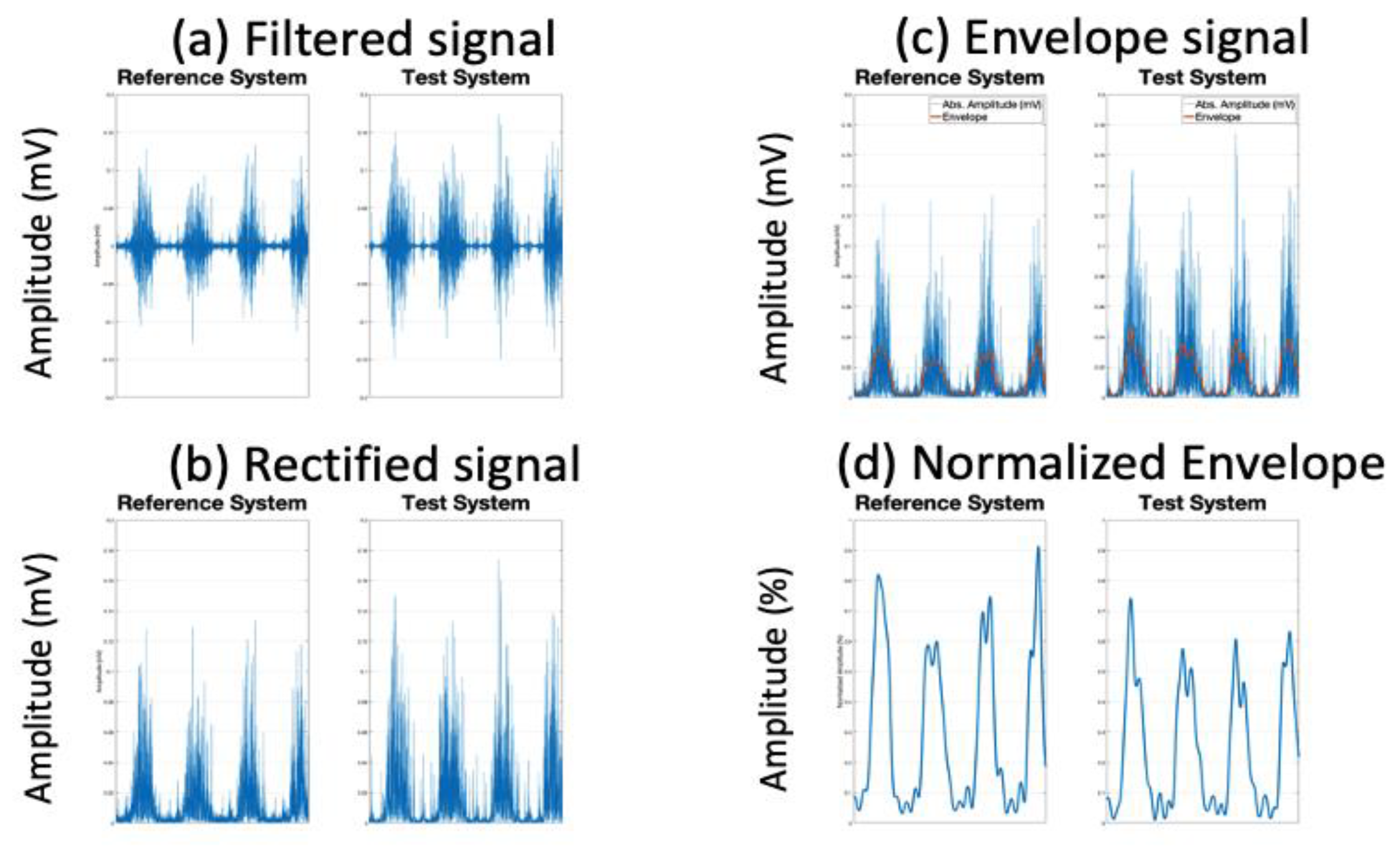
1. Introduction
The quantification of electromyographic (EMG) activity using surface electrodes (sEMG) is invaluable for understanding gait disorders in patients with central nervous system lesions. Several objectives underpin the need to understand these gait disorders. Firstly, EMG quantification aids in the technological identification of muscles activated during walking. Numerous studies have detailed the contributions and activation levels of lower limb muscles [
1,
2]. For instance, [
2] one has indicated the timing of different muscles’ activation during a normalized gait cycle. The authors observed that while timing may be similar, the intensity expressed as a percentage of maximal voluntary force differs between these muscles. Therefore, EMG activity quantification is particularly useful for identifying the nuances in muscle recruitment patterns. Quantifying these activation patterns by their temporal and/or intensity characteristics is also helpful to identify changes in muscle activation patterns, such as hyperactivity of certain muscles, co-contraction of agonist or antagonist muscle groups, or to a lesser extent, muscle paresis.
This quantification, which identifies the timing and/or intensity of muscle activation, is essential for characterizing gait disorders and quantifying the impact of therapeutic interventions. The therapeutic management of gait disorders in patients with central nervous system lesions can be pharmacological, rehabilitative, surgical, or combined with walking aids [
3,
4,
5,
6]. For example, pharmacologically, [
3] showed that the injection of botulinum toxin into the rectus femoris muscle reduced its abnormal activity observed during the swing phase via sEMG, leading to an improvement of peak knee flexion and gait quality. In consequence, quantifying muscle activity via sEMG plays a clinical key role for understanding and managing gait disorders in patients with central nervous system lesions. This tool plays a crucial role in clinical evaluation, monitoring progress, and optimizing treatment strategies for these patients.
However, while sEMG quantification is easier to use than fine-wire EMG, it presents several limitations that must be considered. In clinical practice in order to at identify gait disorders resulting from abnormal muscle activities, following electrode placement recommendations for each superficial muscle is essential. Whatever, patient-specific morphological or pathological characteristics, such as changes in soft tissue properties due to surgical interventions, the clinician are constrained to strictly adher to these placement recommendations. In consequence, sometimes these constraints may require the development of specific calculation codes from raw sEMG system data to combine scientific rigor with clinical utility.
Therefore, studies focusing on tool comparison are as essential as studies characterizing the EMG activity of primary muscles activated during gait in patients with central nervous system lesions. Several studies have validated new sEMG systems by comparison with representative sEMG systems in their application domain, such as analyzing muscle activity in industrial settings [
7,
8] or, in our case, clinical settings [
9,
10]. Regarding the comparison of new systems with reference systems in industrial settings, [
7] have quantified parameters characterizing muscle fatigue to validate signal quality. More recently, [
8] have also focused on similar applications with the same objective of fatigue quantification but using additional parameters. Concerning clinical applications, [
9] have compared an innovative system with a reference system using maximal voluntary contraction of arm flexor and extensor muscles by quantifying the correlation level of the same variables between the two systems. Regarding lower limb muscle activity, [
10] have compared a low-cost system with a reference system using functional movements such as jump, squat, lunge, and knee extension, using validation indicators based on signal similarity.
Our study follows this evaluation trend of new systems against reference systems by comparing recorded signals in functional situations. We propose to evaluate a commercially available low-cost system compared to a reference system in participants with stroke-related movement disorders in functional situations such as comfortable and fast-paced walking and a sit-to-stand test.
3. Results
In the experimental tasks, data were collected from 3 subjects performing 3 different tasks: walking at self-selected comfortable and fast speeds, and one-minute sit-to-stand. The EMG signals were recorded from two muscles, the gastrocnemius medialis and the vastus lateralis. A total of 881 cycles were collected for the two muscles and 3 experimental conditions. For the walking at self-selected comfortable speed task, a total of 361 cycles were collected from the both muscles. In the walking at self-selected fast speed task, 443 cycles were collected. For the one-minute sit-to-stand task, 59 cycles were recorded from both muscles.
Table 2 summarizes the validation indicators, including the Cross-Correlation Coefficient (CCC), Linear Correlation Coefficient (LCC), and Spearman’s correlation (SC).
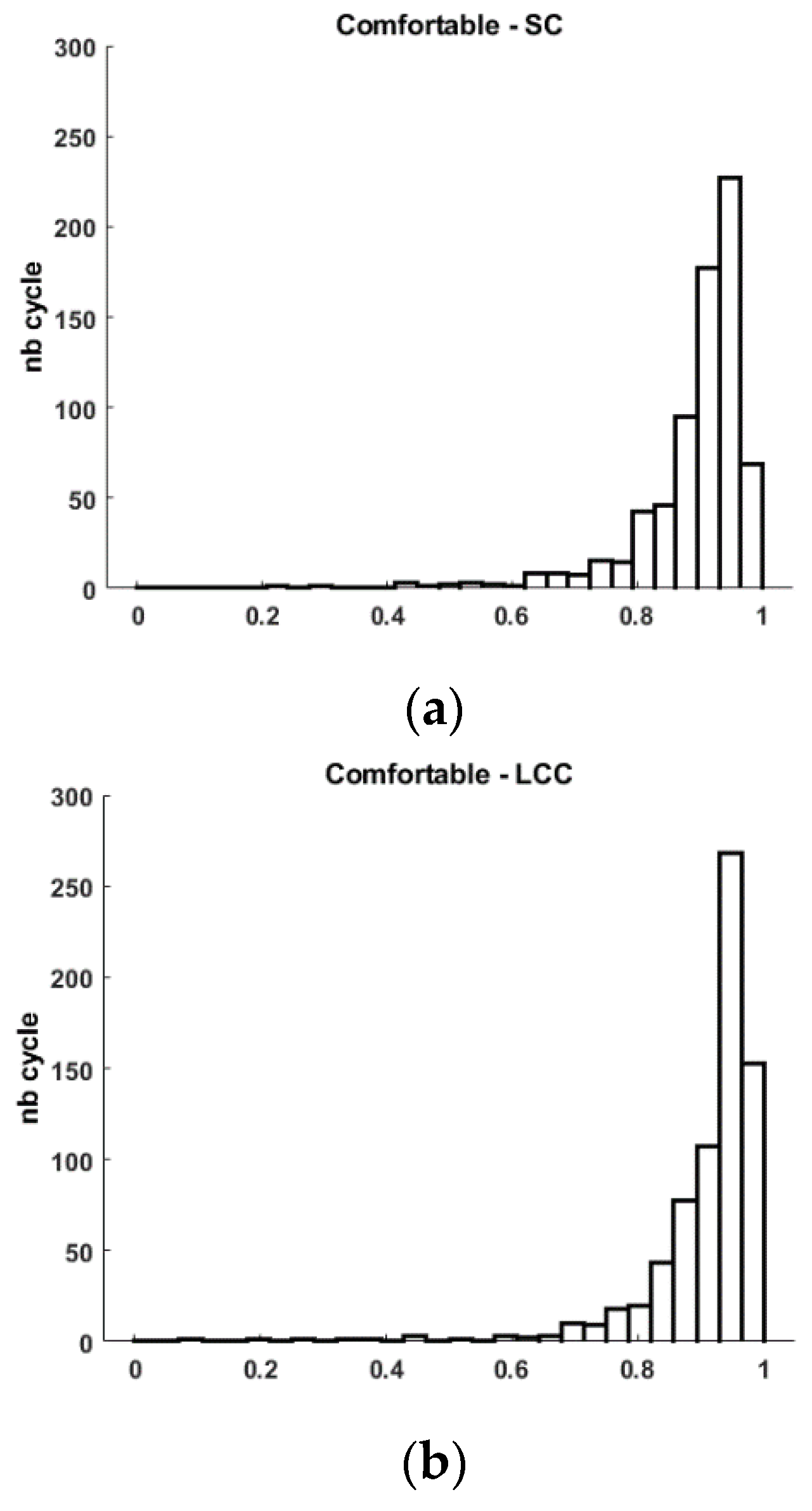
The analysis of the EMG signals during the walking at self-selected comfortable speed task showed that the CCC values ranged from 0.864 to 0.997, with a mean of 0.975 and a standard deviation of 0.017. The LCC values ranged from 0.088 to 0.991, with a mean of 0.909 and a standard deviation of 0.094. Spearman’s correlation values ranged from 0.232 to 0.990, with a mean of 0.894 and a standard deviation of 0.091 (
Table 2 Figure 3).
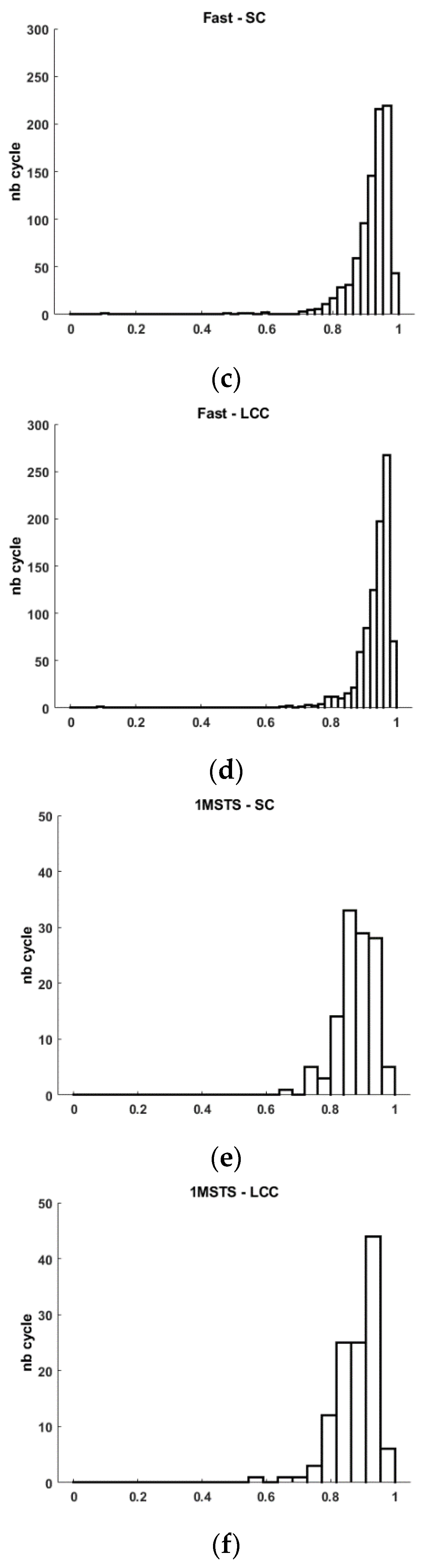
For the walking at self-selected fast speed task, the CCC values ranged from 0.876 to 0.997, with a mean of 0.978 and a standard deviation of 0.014. The LCC values ranged from 0.092 to 0.992, with a mean of 0.935 and a standard deviation of 0.056. Spearman’s correlation values ranged from 0.095 to 0.991, with a mean of 0.918 and a standard deviation of 0.064 (
Table 2 Figure 3)).
Figure 3.
Histogram of Spearman’s correlation (SC), and Linear Correlation Coefficient (LCC) for Comfortable (a-b), Fast (c-d) walking tasks and 1MSTS (e-f).
Figure 3.
Histogram of Spearman’s correlation (SC), and Linear Correlation Coefficient (LCC) for Comfortable (a-b), Fast (c-d) walking tasks and 1MSTS (e-f).
In the one-minute sit-to-stand task, the CCC values ranged from 0.914 to 0.990, with a mean of 0.965 and a standard deviation of 0.018. The LCC values ranged from 0.576 to 0.973, with a mean of 0.881 and a standard deviation of 0.065. Spearman’s correlation values ranged from 0.649 to 0.966, with a mean of 0.880 and a standard deviation of 0.058 (
Table 2 Figure 3).
4. Discussion
This study aims to validate a low-cost sEMG system against a reference sEMG system to enable the analysis of surface muscle electromyographic activity during locomotor or functional tasks in patients with central nervous system lesions causing movement disorders. To achieve this goal, we compared the output signals of the two sEMG systems. Three chronic stroke patients with movement disorders performed two walking conditions and a functional task. In clinical evaluation activities of motor capabilities or difficulties, the quantification of muscle activities is necessary. However, although indirect quantification of the force produced by the analyzed muscle is possible from a signal obtained under a condition where the person develops maximal voluntary muscle force, it remains difficult for patients with central nervous lesion. Indeed, [
19] indicates that for hemiparetic patients following a central nervous system lesion of traumatic or vascular origin, a disturbance of motor control is observed [
19]. This disturbance of motor control is a result of damage to the corticospinal pathways, also known as pyramidal syndrome, which corresponds to a set of neurological manifestations affecting voluntary motor control [
19]. Thus, it is difficult to normalize the EMG signal collected for the analyzed muscles during locomotor tasks by performing a movement where the patient must voluntarily develop maximal force against resistance. However, to compare multiple tasks performed by the same patient, several studies have proposed using other situations than maximal voluntary isometric contraction, such as a movement at constant angular velocity or the studied motor task [
20,
21]. Given the population of our study, we integrated these works into our signal analysis steps. Additionally, in clinical evaluation activities of motor capabilities or difficulties, the quantification of temporal parameters of muscle contraction is pertinent information. Numerous studies highlight an association of movement disorders not by the amount of muscle force developed but by the timing of muscle activity during the performed motor task. For example, [
3] indicates that the knee flexion deficit during the swing phase observed in hemiparetic patients is concomitant with the EMG activity of the rectus femoris muscle in the middle of this phase. These authors attribute the activity of this muscle to the fact that its contraction at this moment in the gait cycle limits knee flexion since the rectus femoris has a knee extension action. Similarly, [
6] implicates the muscle activity of the triceps surae at the end of the swing phase as a potential cause of the plantar flexion strike. Or the absence of EMG activity of the tibialis anterior at the end of the swing phase as a cause of the lack of ankle dorsiflexion [
6].
For all these motor situations, the clinician needs to identify these muscle contractions. Therefore, the use of an sEMG system must be able to meet the constraints related to medical device regulations and enable this clinical analysis. This is why we chose the same similarity comparison criteria proposed by [
10]. These authors proposed several indicators, including the Spearman coefficient, the linear correlation coefficient, and the cross-correlation coefficient. The different gait or sit-to-stand cycles performed by the three participants on the two analyzed muscles allowed us to test the similarity of over 800 cycles. We specifically analyzed the similarity of 443 cycles for the comfortable walking condition, 361 cycles at a fast pace, and 59 cycles for the 1MSTS test. For these different conditions and these three comparison criteria, our results indicate that the low-cost commercial sEMG has good to very good similarity with the reference sEMG.
Firstly, in agreement with the work by [
10], the cross-correlation coefficient (CCC) indirectly indicates the quality of post-processing synchronization as we note a nearly zero time lag. The calculated CCCs for comfortable walking, fast walking, and 1MSTS are respectively 0.975±0.017, 0.978±0.014, 0.965±0.018 (
Table 2). Obtaining values of 1 is impossible due to the propagation of the electrical signal during muscle contraction. Although close, the EMG sensors are not exactly in front of the same muscle area. Thus, during muscle contraction, the electrical signal propagates, at different latency to different muscle localisation [
22]. This physiological constraint must be estimated, particularly by the cross-correlation coefficient, so that the lag does not impact the similarity calculation by other indicators (Spearman coefficient, linear correlation coefficient). Consequently, the closer the value is to 1, the more the interpretation of values obtained with other indicators will be related to the quality of the sEMG system.
For the comfortable walking condition, we observe an average Spearman correlation coefficient of 0.894±0.091. This result is consistent with the results obtained by [
10]. A more in-depth analysis indicates that some cycles present a positive but weak similarity (SC min 0.232), while other cycles have very strong similarity (SC max 0.990). Graphically, we observe that the distribution of cycles with high similarity is much more significant than those with low similarity (
Figure 3). This graphical observation also helps to understand the low calculated standard deviation. This analysis is similar for the linear correlation coefficient. Indeed, we quantify an average LCC of 0.909±0.094 with a minimum value of 0.088 and a maximum of 0.991.
For the fast walking condition, we observe an average Spearman correlation coefficient of 0.918±0.064. A more in-depth analysis indicates that some cycles present a positive but very weak similarity (SC min 0.095), while other cycles have very strong similarity (SC max 0.991). As with comfortable walking, we observe graphically that the distribution of cycles with high similarity is much more significant than those with low similarity (
Figure 3). This graphical observation also helps to understand the low calculated standard deviation. This analysis is similar for the linear correlation coefficient. Indeed, we quantify an average LCC of 0.935±0.056 with a minimum value of 0.092 and a maximum of 0.992. For an industrial user, it is certain that obtaining the highest possible values is preferable, but it is also necessary to provide explanations when some cycles have positive but weak correlation coefficients. We believe that these few cycles with low similarity may result from motion artifacts as described by [
23]. These authors detail all the best practices necessary to obtain a high-quality EMG signal. Despite adhering to these recommendations, such as cleaning the skin before placing the sEMG electrodes, no recorded signal can be entirely perfect regardless of the commercialized EMG system. Thus, cycles with low similarity may correspond to motion artifacts contained in the signal of the low-cost system as well as the reference system. In clinical activities aimed at studying movement disorders, particularly those associated with disturbances in muscle activity, the possibility that the recorded signal from a commercialized system may be subject to artifacts is a guarantee of quality, as surprising as it may seem. Thanks to numerous works that have long described the elements affecting EMG signal quality related to recording, processing, and sEMG electronics, we can identify these artifacts during analysis and interpretation [
12,
22,
23]. Thus, in the context of analyzing movement disorders, especially for patients with central nervous system lesions, identifying these clinically considered disturbing muscle activities is essential. Numerous studies incriminate several muscles for different movement disorders, facilitating identification during the analysis of EMG signals obtained during clinical examination with the patient [36]. However, although these works greatly assist the clinician in understanding the specific movement disorders of their patient, interpretation remains specific for each patient to allow the clinician to consciously choose the therapeutic options they will propose. This clinical reality requires trust in the analysis equipment. This trust relies on regulatory and scientific validity, as well as the system’s sensitivity and, consequently, its exposure to motion artifacts and other sources of disturbance, which are increasingly and better reduced. On this point, we agree with the validation indicators used by [
10], as they quantify the level of similarity between two signals but also, by indicating the minimum similarity values, allow us to appreciate the system’s sensitivity to motion artifacts, among others.
These disturbances were only slightly present in the 1MSTS condition. Indeed, we obtain a minimum SC of 0.649 and a minimum LCC of 0.576. For the rest, all the validation indicators show good similarity, as we quantify an average SC of 0.880±0.058 and an average LCC of 0.881±0.065. However, these values, while considered good performance, are lower than in the two walking conditions. The sit-to-stand movement involves a greater range of hip and knee flexion/extension movements than walking. We can think that the skin motion artifact may be more significant in this condition than in the other two and thus impact the signal quality. This ultimately can increase the number of cycles with good rather than very good similarity (
Figure 3).
The comparison of new sEMG systems with reference systems in our user community of clinicians is essential. Whether we have usage objectives for guiding therapeutic choices or answering research questions, we cannot solely rely on compliance with medical device regulations. Our work contributes to the scientific community’s evaluation dynamics of these new devices. Thus, in line with the validation indicators proposed by [
10], we observed good to very good similarity of EMG signals collected between low-cost commercialized sEMG systems and reference systems for analyzing movement disorders in patients with central nervous system lesions.
Author Contributions
conceptualization, D.P., N.R.; methodology, D.P., L.T., N.R.; software, D.P. and N.R.; formal analysis, D.P. and L.T.; investigation, D.P. N.R.; resources, D.P., L.T. and N.R.; data curation, D.P. and L.T.; writing—original draft preparation, D.P. and L.T.; writing—review and editing, D.P., N.R., L.T. and C.C.; visualization, D.P. and L.T.; supervision, D.P., N.R. All authors have read and agreed to the published version of the manuscript.