Introduction
Breast cancer remains a common and heterogeneous disease, encompassing different molecular subtypes with different clinical characteristics and responses to treatment modalities. Mortality rates associated with breast cancer have shown a declining trend [
1], but it remains a significant cause of morbidity and mortality among women worldwide. An important element in the multidisciplinary treatment of breast cancer is radiation therapy, used after breast-saving surgery or as an adjunct to mastectomy in certain clinical scenarios [
2]. Its purpose is to eliminate residual tumor cells, minimize local recurrence and improve overall survival rates [
3]. Deep inhalation breath holding (DIBH) has become a promising technique in left breast cancer radiotherapy to minimize radiation exposure to critical organs, particularly the heart [
4,
5,
6,
7,
8]. DIBH involves patients holding their breath during irradiation to temporarily remove the heart from the radiation field, reducing potential cardiac complications and those in other sensitive organs - organs at risk (OARs) [
7,
9,
10,
11]. Comparative studies show significant differences in radiation dose to the heart between DIBH and free breathing (FB) techniques, indicating a reduced cardiac risk with DIBH in breast cancer radiotherapy [
12,
13]. Advantages of DIBH include the physical separation of breast tissue from the heart, resulting in a reduced cardiac radiation dose [
5,
7,
8,
14]. Studies highlight the minimization of radiation dose to the lungs to prevent side effects, underscoring the potential of DIBH to reduce the risk of radiation-induced lung damage [
9,
10]. Controlled deep inhalation allows precise targeting of the tumor while sparing healthy heart tissue and protecting other structures, which is particularly important for patients with potential cardiotoxicity undergoing systemic treatment [
6,
7,
15]. However, successful implementation of DIBH depends on a variety of critical factors detailed in recent literature [
9,
16,
17,
18]. Challenges remain regarding patient comfort, the repeatability of breath-holding and the need for specialized equipment [
19,
20]. Eligibility criteria for DIBH in radiotherapy mainly focus on left breast cancer due to its proximity to the heart [
9,
21,
22,
23]. The purpose of this review article is to present the factors affecting the efficacy and side effects of DIBH in radiotherapy for left breast cancer, serving to optimize its clinical application while minimizing side effects, which requires further research and prospective studies [
13].
Materials and Methods
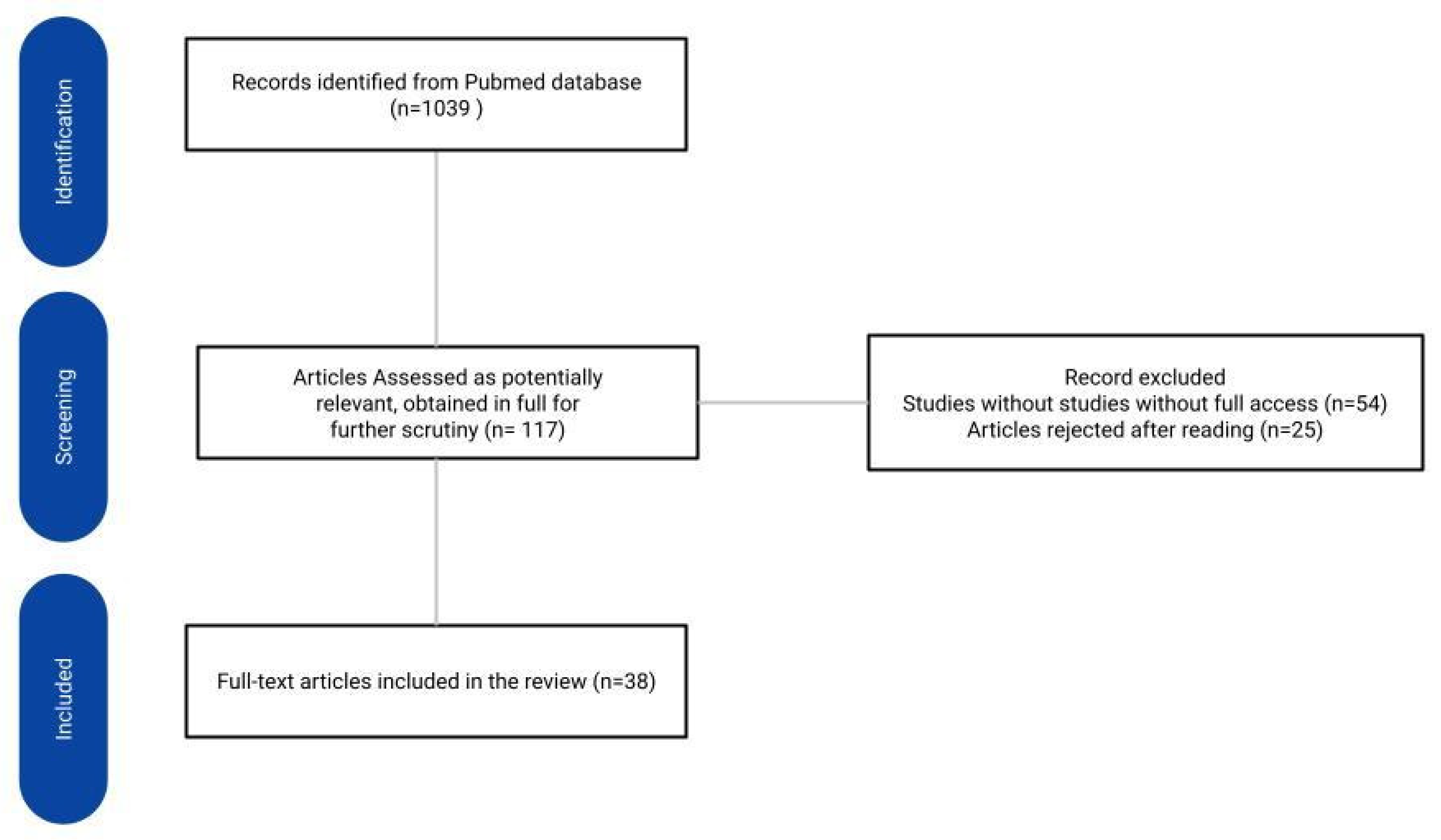
To determine potential factors affecting the efficacy of DIBH in patients with left-sided breast cancer, the PubMed database was searched according to the guidelines included in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The search was conducted on February 15, 2024 and limited to the last 5 years, using the following search terms: ‘DIBH’ or ‘left breast cancer radiotherapy’. Inclusion criteria included full-text articles with free access. The review included Clinical Trials, Meta-Analysis, Randomized Controlled Trials, Systematic Reviews and Reviews papers. Considering the heterogeneity of the included studies, only those that focused on factors determining the effectiveness of the DIBH technique were selected [
Figure 1].
Results
A study by A. Kim et al. confirms that there was less cardiac irradiation after DIBH in patients who practiced the breath-holding technique at home about a week before treatment [
19]. Participants in the study were instructed exactly according to the regimen by specialists what exercises to perform in order to have the desired effect. The training mainly focused on controlling breath holding in the treatment position for 40 seconds while immobilizing the chest. Patients received 6Gy less dose to the heart after such training compared to patients who did not train before treatment. It has been noted that the reduction in irradiation mainly involves the part of the heart closest to the chest (i.e., coronary vessels, left ventricle) [
19]. The effect of individual patient training carried out over a short period of time (1-2 weeks) may be influenced by psychological, neurocognitive factors and abdominal muscle strengthening [
24]. This was confirmed by Puntiwa Oonsiri et al. by training 112 patients one week before DIBH irradiation with the type and technique of breathing exercises. They noted that in patients who trained, the duration of the procedure was reduced by two times compared to patients who did not train. The level of satisfaction after training was high [
25]. These results are consistent with the study by Feng Zhao et al. The researchers enrolled 20 patients in their training on how to correctly perform breathing maneuvers. The training that had been carried out by the researchers was both visual and spoken. They assessed that the abdominal maneuver achieved significantly lower doses on the heart during DIBH compared to the thoracic maneuver. This provides an opportunity for further research and training using both maneuvers in patients prior to breath-hold radiation therapy. Ingrid Romera-Martínez et al. stressed that the integration of DIBH into routine clinical procedures creates a difficulty in ensuring consistent patient configuration during treatment due to the need to train them. In order to achieve reproducibility of position, the rate of sparing normal tissue and feasibility of administration for voluntary DIBH compared to DIBH with a dedicated system, such as the Active Breathing Cooridnator (ABC) [
11].
The effectiveness of treatment and patient selection for DIBH is affected by training and patient education [
19,
23,
24,
25]. Patient training helps reduce cardiac complications after DIBH. An additional advantage has been the reduction in therapy time. Thus, the results of heart-sparing therapy will be better [
26]. Intra-fractional and inter-fractional patient reproducibility is important to confirm that the estimated dose to the heart is consistent with the dose actually administered. These challenges require a comprehensive evaluation of the factors affecting both the efficacy and potential drawbacks of radiotherapy.
3.1. DIBH in Left Breast Cancer Irradiation
During radiation therapy, the patient must be focused on breath control. Among patients undergoing treatment, an average of 30% suffer from anxiety, 19% suffer from anxiety combined with depression, and as many as 25% have psychiatric disorders that impact the effectiveness of treatment [
24]. During the study, Szilvia Gaál and colleagues excluded patients with mental illnesses and those suffering from hypoxia [
5]. The study shows that despite proper education and a 30-minute training of 135 female patients on how to perform the CT (computed tomography) DIBH technique, as many as 38 were rejected due to their high body mass index, medical condition and lack of motivation to pursue treatment [
9]. Christina Schröder et al. performed a study in which 20% of 130 patients were excluded from the radiotherapy analysis taking into account lack of cooperation, consent and patient anxiety. In the remaining 12% of patients, the use of the DIBH method would have been associated with dose overruns in OAR (Organ at Risk) [
9]. Correct performance of DIBH requires coordinated respiratory muscle work during the treatment schedule, so those who could not hold their breath for at least 15s [
9] and 20s [
16] were excluded during patient selection. Patients who are excluded from the DIBH method are very often smokers who have respiratory dyspnea. Such individuals are included in studies using alternative methods with respiratory support, such as CPAP (continuous positive airway pressure), so that the physical changes in the chest are comparable to healthy individuals [
27].
A crucial element affecting the effectiveness of the treatment is the awareness and motivation of patients to successfully implement the deep inhalation irradiation method (DIBH) up to 6 weeks of planned treatment. Factors that exclude patient selection mentioned in the literature are respiratory diseases, including asthma, obesity, poor physical stamina and lack of patient cooperation [
9,
16,
27]. It is important to tailor DIBH techniques individually according to the specifics of each case, taking into account the patient’s ability to tolerate the method and the individual patient’s cooperation and determination of the safety of the technique are what make it limiting or inapplicable.
3.1.1. Positioning Patients on Their Prone Position Compared to the Supine Positioning
Uma Goyal et al. conducted a pilot study in which they proved the beneficial effect of the DIBH technique on mean and maximum cardiac radiation doses in patients in the supine position [
10]. The researchers determined that the mean dose and maximum dose for OAR in DIBH on the abdomen compared to free-breathing patients appeared to be lower by 0.21Gy and 8.51 Gy, respectively, for the heart but higher by 0.23Gy and 8.7 Gy for the left lung. These doses were comparable for the LAD [
10]. The largest meta-analysis involving as many as 19 studies and 751 hospitalized patients conducted by Junming Lai et al. draws the conclusion that DIBH treatment on the prone position (P-DIBH) guarantees better OAR protection than the same lying on the back (S-DIBH) or free breathing lying on the prone position (P-FB) [
12]. In the recommendations of the German Society for Radiation Oncology (DEGRO), Marciana-Nona Duma et al. concluded that the abdominal DIBH method is mainly performed in patients with increased breast volume, and the results of organ-sparing studies in this position are not consistent. Junming Lai et al. suggest that patients with smaller breasts report more benefit from DIBH lying in the prone position [
17]. Comparing nationwide trends in DIBH practice, Nina Desai et al. determined that in a group of 530 U.S. physicians, 244 of whom had more than 15 years of seniority, as many as 440 survey respondents reported using abdominal and/or DIBH patient positioning in clinical practice, with Varian RPM being the most common system (286 respondents) [
14]. In contrast, Xinzhuo Wang et al. reported higher dosimetric doses in 0.62 patients with left-sided breast cancer treated on the abdomen [
29].
The use of both techniques depends on the facilities of the center and the physicians performing the treatment, and the superiority of one technique over the other is debatable [
10,
29]. The positive effect on sparing the heart during DIBH radiotherapy in the abdominal position is explained by a gravity-induced increase in the distance between the breast and heart [
4,
7,
30]. Marcian-Non Dum et al.confirm the protective effect on the LAD of the abdominal DIBH method [
6]. In the supine position, in the vast majority of cases, the radiation dose to the heart is lower than in patients treated in the supine position with free breathing [
7]. Treatment of patients on the abdomen also has positive effects in patients with small breasts [
17]. A study by Xinzhuo Wang et al. shows that the change in radiation dose dependent on positioning is influenced by the plasticity or breast depth ratio of patients, among other factors [
29]. Undoubtedly, the technique of treating patients in the abdominal position has promising results. Despite the above studies, there is a further need to isolate patients who will benefit most from either technique.
3.1.2. Individual Anatomical Characteristics of the Patient
After reviewing the existing literature, Carmen Bergom et al. indicate that in order to minimize the radiation dose to the heart, the maximum heart distance (MHD) should be as far away from the treatment area as possible, while larger ciliary contact distances (CCD ps) and axial contact distances (CCD ax) to the heart are associated with increased radiation dose to the heart [
4]. A literature review of a retrospective dosimetry study by Mikael Dell’Oro et al. confirms the relevance of measuring cardiac contact distance and shows correlations between a greater difference in inspiratory volume and an increase in cardiac protection during DIBH [
21]. The study by Ning Cao et al. only demonstrates the relevance of larger CCD ps and lateral heart-to-chest distance (HCD) measured during CT scans with patients breathing freely. Moreover, one unit increase in the ratio of the two measurements relative to each other results in a reduction in cardiac dose in the range of 0.93-1.15 Gy. [
31]. The reduction in irradiation of organs at risk is influenced by breast size [
32]. Lisa Cunningham et al. conducted the most extensive retrospective study to date, in which 27% of prospective subjects with stage (T1/T2, N0/M1mic, M0) left breast cancer underwent breast-sparing surgery and received DIBH radiation therapy, 40.05 Gy, 15 doses. Using Kendall’s tau-B probability difference, the researchers showed that an increase in patients’ breast size [clinical radiation area expressed cm3 (CTV)] was associated with a decrease in planned radiation volume (PTV) for critical organs (heart, L and P lung, left anterior interventricular branch of the left coronary artery - LAD and P breast. ) in radiological procedures. In addition, in contrast to intensity-modulated proton therapy (IMPT), researchers have shown a positive correlation between CTV and mean right lung dose in intensity-modulated hybrid radiotherapy (h-IMRT) [
32]. In a meta-analysis, Junming Lai et al. described similar findings indicating an increase in radiation in a critical organ such as the heart depending on the breast size of patients [< 750 cm3-(0.4-2.1 Gy), 750-1500 cm3-(0-1.4 Gy), >1500 cm3-(0-0.8 Gy)][
17]. A study by Uma Goyal et al. shows no difference in MHD dependent on breathing technique ( DIBH and FB) while the use of the DIBH technique in a group of patients with large breasts (> 2028.92 cm3) observed significantly lower average doses [
10].
Studies show that patients’ anatomical differences have a significant impact on minimizing the radiation dose delivered to the heart during a radiotherapy session [
4,
7,
10,
17,
21,
31,
32]. When selecting patients, it is important to determine MHD, CCD ps, CCD ax, HCD, sagging, and breast size. The values of these parameters affect dose reductions for OAR. Measurement of the aforementioned values in free-breathing patients is a key predictor considered in patients qualified for DIBH. Other anatomical aspects can also affect radiation doses reaching the heart. For instance, a sagging breast can lower the lower limit of the contact area, resulting in a potential increase in radiation dose to the heart.
3.1.2.1. Age
A review of the literature by Mikael Dell’Oro et al. suggests that younger patients receive a lower mean dose of myocardial irradiation (MHD) during DIBH [
21]. Cristoforo Simonetto et al. conducted a comparative study determining the modeled effect of DIBH on post-treatment mortality attributable to ischemic heart disease (IHD) risk according to the age of patients at the time of treatment. Comparing the increasing risk of exposure with advancing age and the association of IHD risk with patient age, poor cancer prognosis is associated with a very low risk of IHD, which instead increases with patient age [
16]. Unclear evidence regarding the timing of coronary complications from the time of irradiation suggests that if radiation-induced processes were to last, for example, 10 years before appearing through an increase in the risk of IHD, the expected reduction in life years for patients undergoing treatment after reaching about 70 years of age would be reduced more significantly [
16]. As noted by Amr A. Mahmoud et al., in patients undergoing DIBH as opposed to free-breathing patients, age is an important predictor of cardiovascular events [
33].
The younger age of patients undergoing DIBH promotes better efficacy of the technique [
5]. Cristoforo Simonetto et al. found that the age at which patients begin treatment is a low predictor of the risk of death [
16]. The younger the patient, the greater the chance of her performing the breath-hold technique more accurately and thus saving OAR [
21]. There is a need to take this factor into account when planning and evaluating radiation therapy. Understanding these correlations may contribute to a more personalized approach to radiation therapy, while increasing the effectiveness of treatment and minimizing potential side effects.
3.1.2.2. Cardiovascular Risk Factors
A clinically significant complication after DIBH is the development of ischemic heart disease. As a result of irradiation, myocardial micro- and macrovessels can be damaged. Patients undergoing DIBH with comorbidities that severely burden the heart and an unhealthy lifestyle have a significantly increased risk of cardiovascular disease.
3.1.2.3. Diabetes
Diabetics are a special group of patients who have an increased risk of myocardial dysfunction after radiation therapy [
34]. A study by Gasch et al. evaluated the EAR [10-year absolute risk of cardiovascular disease (CVD)] after left breast radiotherapy [
35]. In the first group of 200 patients without diabetes, the baseline risk was 0.031 - 0.035; after irradiation, the excess relative risk (ERR) increased to 0.11. This allows a final estimate of the EAR over the next 10 years of 0.003 - 0.004 in this group of patients. In contrast, 10 patients with left breast cancer and diabetes had a baseline risk of 0.1-0.2, so the EAR over the next 10 years is 0.01 - 0.03. Moreover, A.Mahmoud et al. describe and point to diabetes as a factor that causes an increase in cardiac events in patients (2.2% of study subjects) after left breast complementary radiotherapy by DIBH [
33]. In a study carried out by Moon-Sing Lee et al. distinguished that, patients with diabetes should be closely monitored by multidisciplinary teams of physicians after irradiation [
34].
Diagnosed diabetes mellitus was considered an important factor that can significantly affect the risk of CVD in study subjects. The aforementioned studies show that patients with diabetes will have a 10-year excess absolute risk of cardiovascular disease, on average, 5 times higher [
35].
3.1.2.4. Smoking
A study by A. Mahmoud et al., where the smoking group accounted for 19.2% of all patients undergoing DIBH, showed that this is a risk group with a significantly increased incidence of late cardiac complications [
33]. Gasch et al. identify this as the most important factor affecting the risk of cardiovascular disease after using the DIBH method in female patients. From a group of 210 patients, 28 smokers were selected who had a cumulative cardiovascular disease risk (taking into account the initial risk and excess absolute risk EAR) of 6.07%. Compared to non-smokers (182 patients), this risk is 3.55% [
35]. Marc D. Piroth et al. also identify cigarette smoking as a very important factor with toxic effects on the heart after radiotherapy. They confirm that smokers have a higher mortality rate from radiation therapy than non-smokers [
36].
Smoking significantly increases the occurrence of ischemic heart disease (IHD) [
5]. At the same time, it is the most important factor affecting the risk of cardiovascular disease after DIBH in female patients. It is easy to see that compulsive cigarette smoking almost doubles the risk of CVD [
35].
3.1.2.5. Hypertension
A. Mahmoud et al. showed that hypertensive patients are more likely to have cardiac events after DIBH [
33]. Similar conclusions were reached by A. Gasch et al. who indicated that hypertensive patients have an increased incidence of coronary artery disease after receiving left-sided radiotherapy [
35]. Moon-Sing Lee and colleagues emphasize the importance of thorough diagnostics in patients with arterial hypertension prior to radiation therapy [
34]. Cristoforo Simonetto et al., in patients undergoing left-sided adjuvant radiotherapy for breast cancer, assessed the impact of arterial hypertension, smoking, and hypercholesterolemia on mortality due to ischemic heart disease (IHD). In the study, they evaluated two patients of the same age undergoing radiation therapy using DIBH. One was a smoker with systolic blood pressure above 140 mmHg and elevated cholesterol levels of 246 mg/dL, while the other was a non-smoker with a systolic blood pressure of 104 mmHg and cholesterol level of 165 mg/dL. The risk of ischemic heart disease in the future for the first patient is 9 times higher compared to the second patient [
16].
A risk factor for future cardiovascular disease after DIBH is hypertension, that is a persistent increase in blood pressure. Such patients can be treated with appropriate ACEIs (angiotensin-converting enzyme inhibitors) and antagonists for the angiotensin II receptor [
33]. Well-chosen treatment can reduce the risk of cardiovascular disease after DIBH [
34].
In addition to the above factors, the authors also mention other potentially influencing factors on the cardiac risk in patients after DIBH [
Table 1].
3.1.3. Other Factors
Yongkai Lu et al. indicate that the efficacy of IMRT in synergy with DIBH is somewhat limited by the potential impact of respiratory motion [
37]. In contrast, VMAT according to V. Salvestrini et al. and Marciana-Nona Duma et al. uses its rotational delivery and adaptive modulation capabilities to harmonize with DIBH, showing better target coverage and reduced irradiation of healthy tissues during specific respiratory phases [
6,
20]. Proton therapy, discussed by Chirayu G. Patel and Ashlyn S. Everett MD et al. is distinguished by its inherent physical properties, particularly the Bragg peak, which allows for precise dose deposition, minimizing the integral dose to surrounding tissues [
38,
39]. According to Chirayu G. Patel et al. proton therapy shows promise in reducing total dose exposure; however, its compatibility with DIBH and management of respiratory motion present challenges that require further research and optimization to maximize its benefits in combination with DIBH [
38].
Te choice between intensity-modulated radiation therapy (IMRT), volumetric modulated arc therapy (VMAT) and proton therapy in combination with deep breath-holding (DIBH) reveals clear advantages and differences between the above techniques. IMRT highlighted in studies by authors such as Mazen Sakka, Yongkai Lu and Montserrat Pazos et al. show significant compliance with tumor volumes while sparing critical organs due to its ability to modulate beam intensity (conformal dose distribution) [
8,
37,
40]. The ability to adapt to motion dynamics is a significant advantage over IMRT in combination with DIBH, as it minimizes uncertainties associated with breathing-induced organ motion.
Results from the study by Liang et al. have yielded significant advances in the combined use of continuous positive airway pressure (CPAP) and DIBH in MR-guided radiation therapy for thoracic tumours. The study was volunteer-based and evaluated the practicality of using CPAP both in combination and independently of DIBH techniques to control respiratory motion during MR linear gas pedal (MR-linac) radiotherapy. Integration of CPAP during DIBH showed a significant reduction in respiratory motion amplitude, resulting in minimized intra-fractional tumour motion during treatment sessions, as well as improved accuracy in target localization and reduced discrepancies between planned and delivered doses [
18].
These findings demonstrate the potential of CPAP as an effective adjunctive tool in the context of DIBH-guided MR radiotherapy. Further large-scale studies and clinical trials are warranted to confirm and extend these encouraging results, paving the way for sophisticated and precise radiotherapy treatments for DIBH-guided thoracic oncology.
The modern Active Breathing Coordinator (ABC) system greatly improves and ensures reproducible chest movements during DIBH. Sean All et al. confirm the precision, feasibility and safety of the chest irradiation field for patients treated with DIBH along with ABC, and indicate that this system should be used as often as possible in combination with DIBH [
41].
In a study by A.Mkanne et al. it was noted that greater benefit after DIBH is achieved by patients after mastectomy than after BCS [
42]. Similar conclusions were reached by S.Misre et al. who in their study compared patients after BCS and after modified radical mastectomy (MRM). In both cases, the doses administered to the heart were reduced during DIBH radiotherapy; however, in MRM, significantly lower doses were applied to the lungs and LAD. This confirms that this technique may be particularly beneficial for patients undergoing mastectomy [
13,
43].
Lung restrictions were less common in the group of MRM patients requiring lymph node irradiation. Further studies are needed between the effectiveness of DIBH therapy depending on the surgical treatment chosen [
13].
The relevance of higher values of the BMI on cardiac protection during radiation is confirmed by Mikael Dell’Oro et al. [
21]. BMI is another factor that affects the effectiveness of DIPH. Researchers Patricia Browne et al. showed a positive correlation between body mass index and MHD and maximum LAD dose, but did not determine which patients would have the best positive effects [
22]. A study by Abbas Mkanna et al. among many predictive factors shows the relevance of BMI, an increase of which correlated with a greater difference in mean heart dose (MHDD) between FB and DIBH treatment plans [
42].
Limitations of the Review
The limitations of this systematic review are primarily due to the relatively small number of recent authoritative studies focusing on individual patient characteristics that affect the effectiveness of DIBH treatment. This technique plays a key role in radiation therapy for breast cancer, and research is still ongoing to determine the predisposition of patients for whom this treatment would be the best choice. Our research method has no control over the quality of the primary studies to which it refers. Published scientific data and publications are based on a very large group of patients.
Conclusions
The primary factors influencing reproducibility and saving of normal tissues are adequate education, respiratory training and patient preparation prior to radiation therapy. Studies confirm that a patient’s awareness, motivation for treatment and mental state significantly affect the effectiveness of treatment. Lack of consent, fear, anxiety, depression and comorbid mental disorders disqualify some patients from the DIBH method. Many patients are unable to hold their breath properly due to obesity, poor physical condition or asthma. For respiratory diseases and smokers, alternative breathing methods should be considered. Individual patients’ anatomical characteristics (MHD, CCD ps, CCD ax, HCD, sagging, and breast size) may affect the effectiveness of treatment. Cardiovascular risk factors, such as diabetes, smoking and hypertension, affect the effectiveness of DIBH and potential complications. Additional factors include age, obesity, previous cardiovascular disease, high BMI and family history. Studies suggest that younger patients undergoing DIBH receive lower cardiac doses, and age is an important prognostic factor. Increased BMI values correlate with reduced MHD and maximal LAD. Comparative analysis between patient positioning is inconclusive, but indicates greater benefit of DIBH on the abdomen in patients with large breasts. Post-mastectomy patients compared to BCS may benefit more from DIBH. DIBH is influenced by enhancement techniques: IMRT-high compliance with breast volume and VMAT-better dose modification to respiratory motion, modern ABC-ensuring repetitive chest movements. There is a need for further research determining the predictive factors of patients, understanding of which will result in more effective and personalized treatment involving fewer side effects.
Funding
This research received no external funding.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics. CA Cancer J Clin 2019, Volume 69(6), pp. 438-451. [CrossRef]
- Kunkler, I.H.; Chua, B.H. Postmastectomy radiotherapy: a review. Curr Opin Oncol 2021 Vol 33(6), pp. 547-552. [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG).; Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; Gray, R.; Pierce, L.; Whelan, T.; Wang, Y.; Peto, R. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials Lancet 2011, Volume 378(9804), pp. 1707-1716. [CrossRef]
- Bergom, C.; Currey, A.; Desai, N.; Tai, A.; Strauss, J.B. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front Oncol 2018 Volume 8:87. [CrossRef]
- Gaál, S.; Kahán, Z.; Paczona, V.; Kószó, R.; Drencsényi, R.; Szabó, J.; Rónai, R.; Antal, T.; Deák, B.; Varga, Z. Deep-inspirational breath-hold (DIBH) technique in left-sided breast cancer: various aspects of clinical utility. Radiat Oncol 2021, Volume 16(1),89. [CrossRef]
- Duma, M,N.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; Piroth, M.D.; Sedlmayer, F.; Souchon, R.; Sauer, R. Breast Cancer Expert Panel of the German Society of Radiation Oncology (DEGRO). Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther Onkol 2019, Volume 195(10), pp. 861-871. [CrossRef]
- Stowe, H.B.; Andruska, N.D.; Reynoso, F.; Thomas, M.; Bergom, C. Heart Sparing Radiotherapy Techniques in Breast Cancer: A Focus on Deep Inspiration Breath Hold. Breast Cancer (Dove Med Press) 2022, Volume 14, pp. 175-186. [CrossRef]
- Sakka, M.; Kunzelmann, L.; Metzger, M.; Grabenbauer, G.G. Cardiac dose-sparing effects of deep-inspiration breath-hold in left breast irradiation: Is IMRT more beneficial than VMAT? Strahlenther Onkol 2017, Volume 193(10), pp. 800-811. [CrossRef]
- Schröder, C.; Kirschke, S.; Blank, E.; Rohrberg, S.; Förster, R.; Buchali, A. Deep inspiration breath-hold for patients with left-sided breast cancer - A one-fits-all approach? A prospective analysis of patient selection using dosimetrical and practical aspects. Br J Radiol 2022, Volume 95(1135), pp. 20210295. [CrossRef]
- Goyal, U.; Saboda, K.; Roe, D.; Gonzalez, V. J. Prone Positioning With Deep Inspiration Breath Hold for Left Breast Radiotherapy. Clin Breast Cancer 2021 Volume 21(4):e295-e301. [CrossRef]
- Romera-Martínez, I.;, Muñoz-Montplet, C.; Jurado-Bruggeman, D.; Onsès-Segarra, A.; Fuentes-Raspall, R.; Buxó, M.; Vilanova, J.C. A Novel Device for Deep-Inspiration Breath Hold (DIBH): Results from a Single-Institution Phase 2 Clinical Trial for lPatients with Left-Sided Breast Cancer. Pract Radiat Oncol 2020 Volume 10(4):e290-e297. [CrossRef]
- Lai, J.; Zhong, F.; Deng, J.; Hu, S.; Shen, R.; Luo, H.; Luo, Y. Prone position versus supine position in postoperative radiotherapy for breast cancer: A meta-analysis. Medicine (Baltimore). 2021 Volume 100(20):e26000. [CrossRef]
- Lu, Y.; Yang, D.; Zhang, X.; Teng, Y.; Yuan, W.; Zhang, Y.; He, R.; Tang, F.; Pang, J.; Han, B.; Chen R, Li Y. Comparison of Deep Inspiration Breath Hold Versus Free Breathing in Radiotherapy for Left Sided Breast Cancer. Front Oncol 2022 Volume 12:845037. [CrossRef]
- Desai, N.; Currey,; A, Kelly, T.; Bergom, C. Nationwide Trends in Heart-Sparing Techniques Utilized in Radiation Therapy for Breast Cancer. Adv Radiat Oncol 2019 Volume 4(2), pp. 246-252. [CrossRef]
- Pandeli, C.; Smyth, L.M.L.; David, S.; See, A.W. Dose reduction to organs at risk with deep-inspiration breath-hold during right breast radiotherapy: a treatment planning study. Radiat Oncol 2019 Volume 14(1), p. 223. [CrossRef]
- Simonetto, C.; Eidemüller, M.; Gaasch, A.; Pazos, M.; Schönecker, S.; Reitz, D.; Kääb, S.; Braun, M.; Harbeck, N.; Niyazi, M.; Belka, C.; Corradini, S. Does deep inspiration breath-hold prolong life? Individual risk estimates of ischaemic heart disease after breast cancer radiotherapy. Radiother Oncol 2019, Volume 131, pp. 202-207. [CrossRef]
- Lai, J.; Hu, S.; Luo, Y.;, Zheng, R.; Zhu, Q.; Chen, P.; Chi, B.; Zhang, Y.; Zhong, F.; Long, X. Meta-analysis of deep inspiration breath hold (DIBH) versus free breathing (FB) in postoperative radiotherapy for left-side breast cancer. Breast Cancer 2020 Volume 27(2), pp. 299-307. [CrossRef]
- Liang, E.; Dolan, J.L.; Morris, E.D.; Vono, J.; Bazan, L.F.; Lu, M.; Glide-Hurst, CK. Application of Continuous Positive Airway Pressure for Thoracic Respiratory Motion Management: An Assessment in a Magnetic Resonance Imaging-Guided Radiation Therapy Environment. Adv Radiat Oncol 2022 Volume 7(3):100889. [CrossRef]
- Kim, A.; Kalet, A.M.; Cao, N.; Hippe, D.S.; Fang, L.C.; Young, L.; Meyer, J.; Lang, E.V.; Mayr, N.A. Effects of Preparatory Coaching and Home Practice for Deep Inspiration Breath Hold on Cardiac Dose for Left Breast Radiation Therapy. Clin Oncol (R Coll Radiol) 2018 Volume 30(9), pp. 571-577. [CrossRef]
- Salvestrini, V.; Iorio, G.C,; Borghetti, P.; De Felice, F.; Greco, C.; Nardone, V.; Fiorentino, A.; Gregucci, F.; Desideri, I. The impact of modern radiotherapy on long-term cardiac sequelae in breast cancer survivor: a focus on deep inspiration breath-hold (DIBH) technique. J Cancer Res Clin Oncol 2022 Volume 148(2), pp. 409-417. [CrossRef]
- Dell’Oro, M.; Giles, E.; Sharkey, A.; Borg, M.; Connell, C.; Bezak, E. A Retrospective Dosimetric Study of Radiotherapy Patients with Left-Sided Breast Cancer; Patient Selection Criteria for Deep Inspiration Breath Hold Technique. Cancers (Basel) 2019 Volume 11(2):259. [CrossRef]
- Aznar, M.C.; Carrasco de Fez, P.; Corradini, S.; Mast, M.; McNair, H.; Meattini, I.; Persson, G.; van Haaren, P. ESTRO-ACROP guideline: Recommendations on implementation of breath-hold techniques in radiotherapy. Radiother Oncol 2023 Volume 185, p.109734. [CrossRef]
- Aznar, MC.; Carrasco de Fez, P.; Corradini, S.; Mast, M.; McNair, H.; Meattini, I.; Persson, G.; van Haaren, P. ESTRO-ACROP guideline: Recommendations on implementation of breath-hold techniques in radiotherapy. Radiother Oncol 2023, Volume 185, p. 109734. [CrossRef]
- Mayr, NA.; Borm, KJ.; Kalet, AM.; Wootton, LS.; Chadderdon, A.L.; Combs, S.E.; Wang, W.; Cao, N.; Lo, SS.; Sandison, G.A.; Meyer, J. Reducing Cardiac Radiation Dose From Breast Cancer Radiation Therapy With Breath Hold Training and Cognitive Behavioral Therapy. Top Magn Reson Imaging 2020, Volume 29(3), pp. 135-148. [CrossRef]
- Oonsiri, P.; Wisetrinthong, M.; Chitnok, M.; Saksornchai, K.; Suriyapee, S. An effective patient training for deep inspiration breath hold technique of left-sided breast on computed tomography simulation procedure at King Chulalongkorn Memorial Hospital. Radiat Oncol J 2019 Volume 37(3), pp. 201-206. [CrossRef]
- Zhao, F.; Shen, J.; Lu, Z.; Luo, Y.; Yao, G.; Bu, L.; Ge, J.; Yang, X.; Ning, L.; Yan, S. Abdominal DIBH reduces the cardiac dose even further: a prospective analysis. Radiat Oncol 2018 Volume 22;13(1), p. 116. [CrossRef]
- Kil, WJ.; Pham, T.; Kim, K. Heart sparing breast cancer radiotherapy using continuous positive airway pressure (CPAP) and conventional supine tangential fields: an alternative method for patients with limited accessibility to advanced radiotherapy techniques. Acta Oncol 2019 Volume 58(1), pp. 105-109. [CrossRef]
- Jacobse, JN.; Duane, F.K.; Boekel, N.B.; Schaapveld, M.; Hauptmann, M.; Hooning, M.J.; Seynaeve, C.M.; Baaijens, M.H.A.; Gietema, J.A.; Darby, S.C.; van Leeuwen, F.E.; Aleman, B.M.P.; Taylor, C.W. Radiation Dose-Response for Risk of Myocardial Infarction in Breast Cancer Survivors. Int J Radiat Oncol Biol Phys 2019, volume 103(3), pp. 595-604. [CrossRef]
- Wang, X.; Fargier-Bochaton, O.; Dipasquale, G.; Laouiti, M.; Kountouri, M.; Gorobets, O.; Nguyen, NP.; Miralbell, R.; Vinh-Hung, V. Is prone free breathing better than supine deep inspiration breath-hold for left whole-breast radiotherapy? A dosimetric analysis. Strahlenther Onkol 2021, Volume 197(4), pp. 317-331. [CrossRef]
- Alonso, C.; Janowski, E.; Libby, B.; Showalter, S. Comparison of heart dose in early-stage left-sided breast cancers treated with intraoperative radiation therapy or whole-breast irradiation with deep inspiratory breath hold. Brachytherapy 2018, Volume 17(5), pp. 831-836. [CrossRef]
- Cao, N.; Kalet, A.M.; Young, L.A.; Fang, L.C.; Kim, J.N.; Mayr, N.A.; Meyer, J. Predictors of cardiac and lung dose sparing in DIBH for left breast treatment. Phys Med 2019, Volume 67, pp. 27-33. [CrossRef]
- Cunningham, L.; Penfold, S.; Giles, E.; Le, H.; Short, M. Impact of Breast Size on Dosimetric Indices in Proton Versus X-ray Radiotherapy for Breast Cancer. J Pers Med 2021, Volume 11(4), p. 282. [CrossRef]
- Mahmoud, A.A.; Sadaka, E.A.; Abouegylah, M.; Amin, S.A.; Elmansy, H.; Asal, M.F.; Köksal, M.A.; Gawish, A. Impact of breath-hold technique on incidence of cardiac events in adjuvant left breast cancer irradiation. J Cancer Res Clin Oncol 2023, Volume 149(9), pp. 5853-5859. [CrossRef]
- Lee, M.S.; Liu, D.W.; Hung, S.K.; Yu, C.C.; Chi, C.L.; Chiou, W.Y.; Chen, L.C.; Lin, R.I.; Huang, L.W.; Chew, C.H.; Hsu, F.C.; Chan, M.W.Y.; Lin, H.Y. Emerging Challenges of Radiation-Associated Cardiovascular Dysfunction (RACVD) in Modern Radiation Oncology: Clinical Practice, Bench Investigation, and Multidisciplinary Care. Front Cardiovasc Med 2020, Volume 7, p. 16. [CrossRef]
- Gaasch, A.; Schönecker, S.; Simonetto, C.; Eidemüller, M.; Pazos, M.; Reitz, D.; Rottler, M.; Freislederer, P.; Braun, M.; Würstlein, R.; Harbeck, N.; Niyazi, M.; Belka, C.; Corradini, S. Heart sparing radiotherapy in breast cancer: the importance of baseline cardiac risks. Radiat Oncol 2020, Volume 15(1), p. 117. [CrossRef]
- Piroth, M,D.; Baumann, R.; Budach, W.; Dunst, J.; Feyer, P.; Fietkau, R.; Haase, W.; Harms, W.; Hehr, T.; Krug, D.; Röser, A.; Sedlmayer. F.; Souchon, R.; Wenz, F.; Sauer, R. Heart toxicity from breast cancer radiotherapy: Current findings, assessment, and prevention. Strahlenther Onkol 2019, Volume 195(1), pp. 1-12. [CrossRef]
- Lu, Y.; Ma, Y.; Yang, D.; Li, Y.; Yuan, W.; Tang, F.; Xu, L.; Zhou, L.; Lin, H.; L,i B.; Chen, R.; He, C.; Zhao, D. Cardiorespiratory dose comparison among six radiotherapy regimens for patients with left-sided breast cancer. Sci Rep 2023, Volume 13(1), p. 13339. [CrossRef]
- Patel, C.G.; Peterson, J.; Aznar, M.; Tseng, Y.D.; Lester, S.; Pafundi, D.; Flampouri, S.; Mohindra, P.; Parikh, R.R.; Mailhot, Vega, R.; Konig, L.; Plastaras, J.P.; Bates, J.E.; Loap, P.; Kirova, Y.M.; Orlandi, E.; Lütgendorf-Caucig, C.; Ntentas, G.; Hoppe, B. Systematic review for deep inspiration breath hold in proton therapy for mediastinal lymphoma: A PTCOG Lymphoma Subcommittee report and recommendations. Radiother Oncol 2022, Volume 177, pp. 21-32. [CrossRef]
- Everett, A.S.; Hoppe, B.S.; Louis, D.; McDonald, A.M.; Morris, C.G.; Mendenhall. N.P.; Li, Z.; Flampouri, S. Comparison of Techniques for Involved-Site Radiation Therapy in Patients With Lower Mediastinal Lymphoma. Pract Radiat Oncol 2019, Volume 9(6), pp. 426-434. [CrossRef]
- Pazos, M.; Schönecker, S.; Reitz, D.; Rogowski, P.; Niyazi, M.; Alongi, F.; Matuschek, C.; Braun, M.; Harbeck, N.; Belka, C.; Corradini, S. Recent Developments in Radiation Oncology: An Overview of Individualised Treatment Strategies in Breast Cancer. Breast Care (Basel) 2018, Volume 13(4), pp. 285-291. [CrossRef]
- All, S.; Zhao, B.; Montalvo, S.; Maxwell, C.; Johns, C.; Gu, X.; Rahimi, A.; Alluri, P.; Parsons, D.; Chiu, T.; Schroeder, S.; Kim, D.N. Feasibility and efficacy of active breathing coordinator assisted deep inspiration breath hold technique for treatment of locally advanced breast cancer. J Appl Clin Med Phys 2023, Volume 24(2), e13893. [CrossRef]
- All, S.; Zhao, B.; Montalvo, S.; Maxwell, C.; Johns, C.; Gu, X.; Rahimi, A.; Alluri, P.; Parsons, D.; Chiu, T.; Schroeder, S.; Kim, DN. Feasibility and efficacy of active breathing coordinator assisted deep inspiration breath hold technique for treatment of locally advanced breast cancer. J Appl Clin Med Phys 2023, Volume 24(2), e13893. [CrossRef]
- Misra, S.; Mishra, A.; Lal, P.; Srivastava, R.; Verma, M.; Senthil Kumar, S.K.; Maria, Das, K.J. Cardiac dose reduction using deep inspiratory breath hold (DIBH) in radiation treatment of left sided breast cancer patients with breast conservation surgery and modified radical mastectomy. J Med Imaging Radiat Sci 2021, Volume 52(1), pp. 57-67. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).