Submitted:
27 June 2024
Posted:
01 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
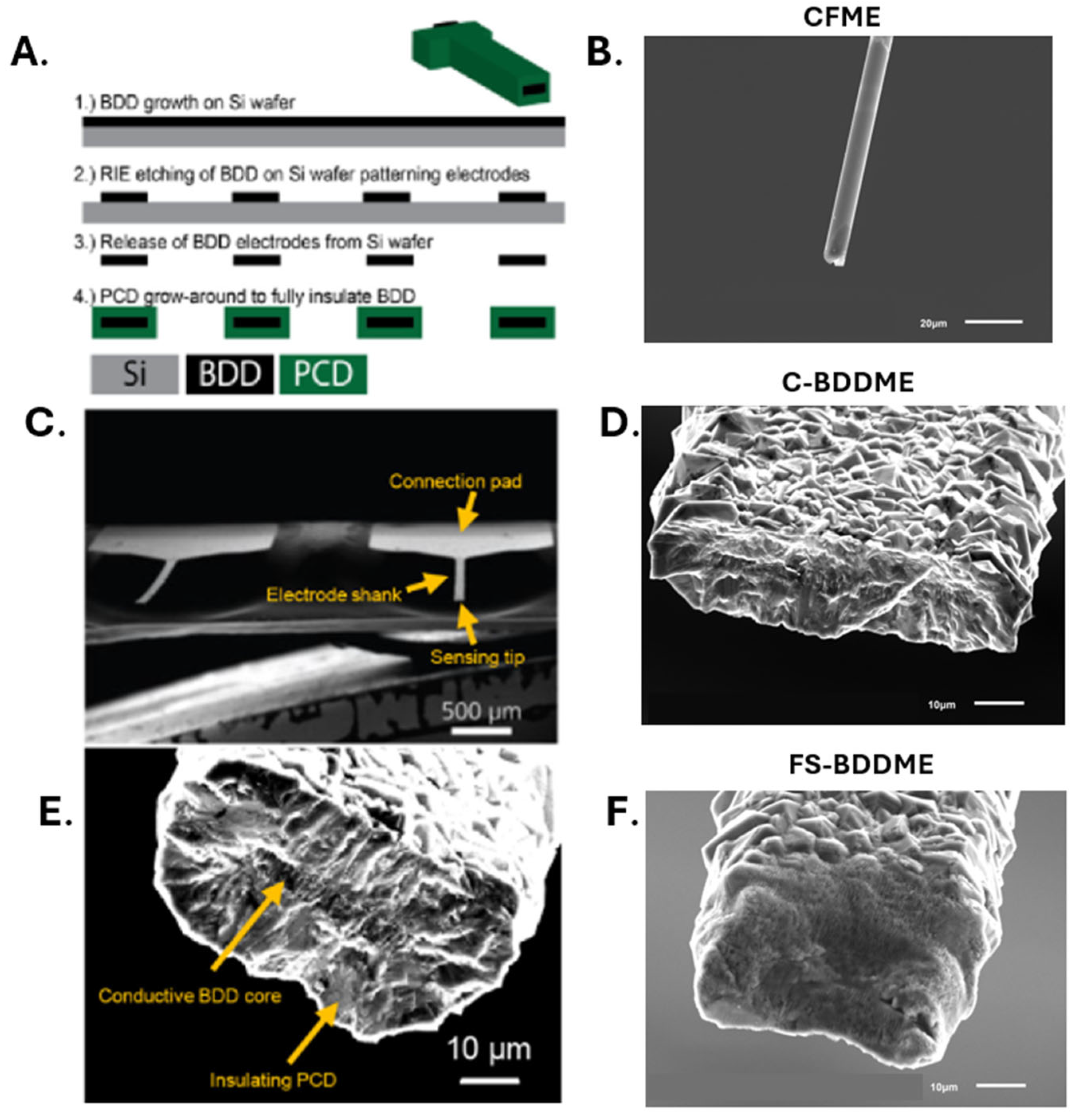
2.2. Carbon Fiber Microelectrode (CFME) Fabrication
2.3. Boron Doped Diamond Fabrication
2.4. Fast-Scan Cyclic Voltammetry (FSCV)
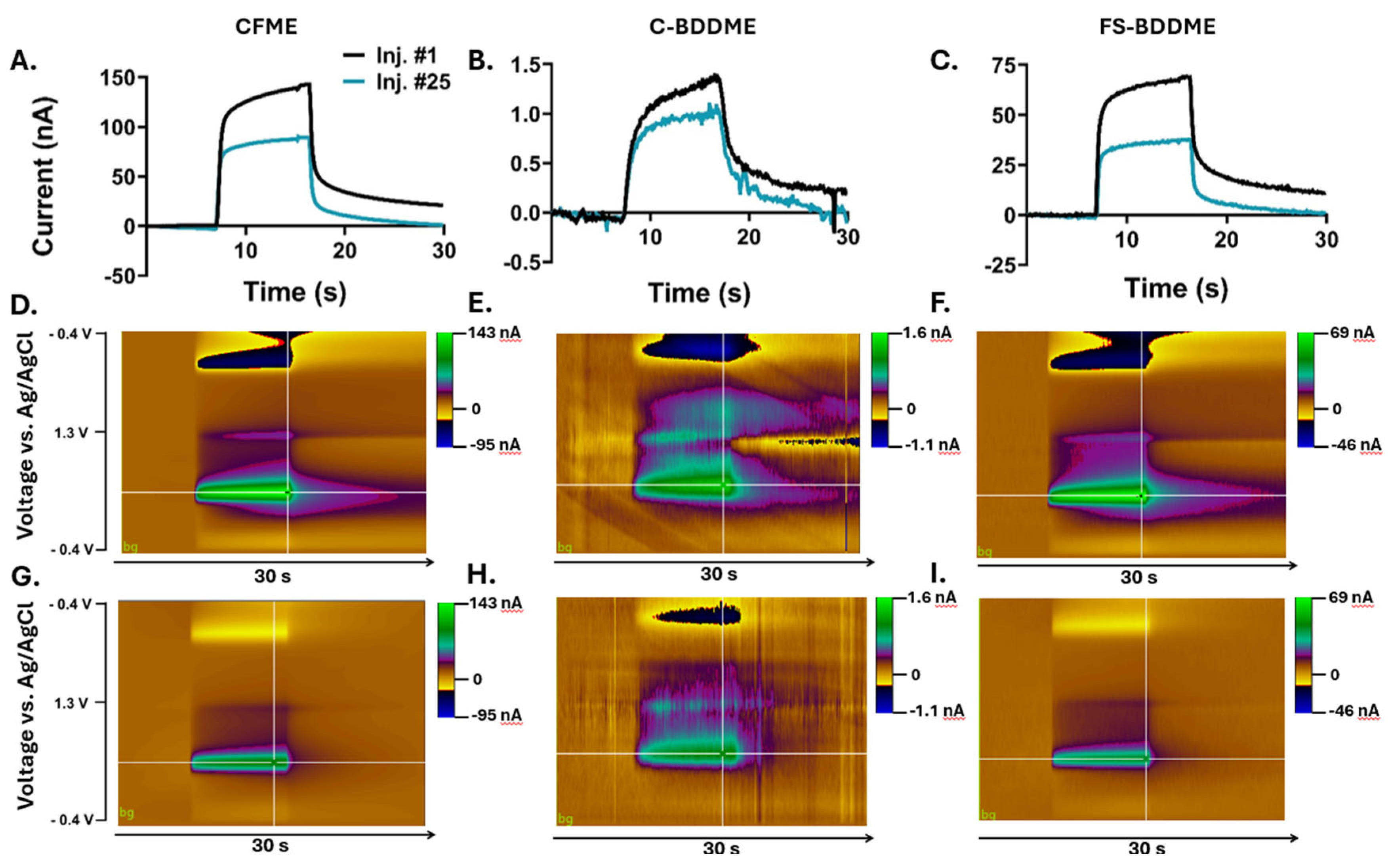
2.5. Electrochemical Fouling Protocol
2.6. Electrode Stability Analysis
2.7. SEM Imaging
2.8. Statistics
2.9. Hot-Acid Boiling
3. Results
3.1. Serotonin Response
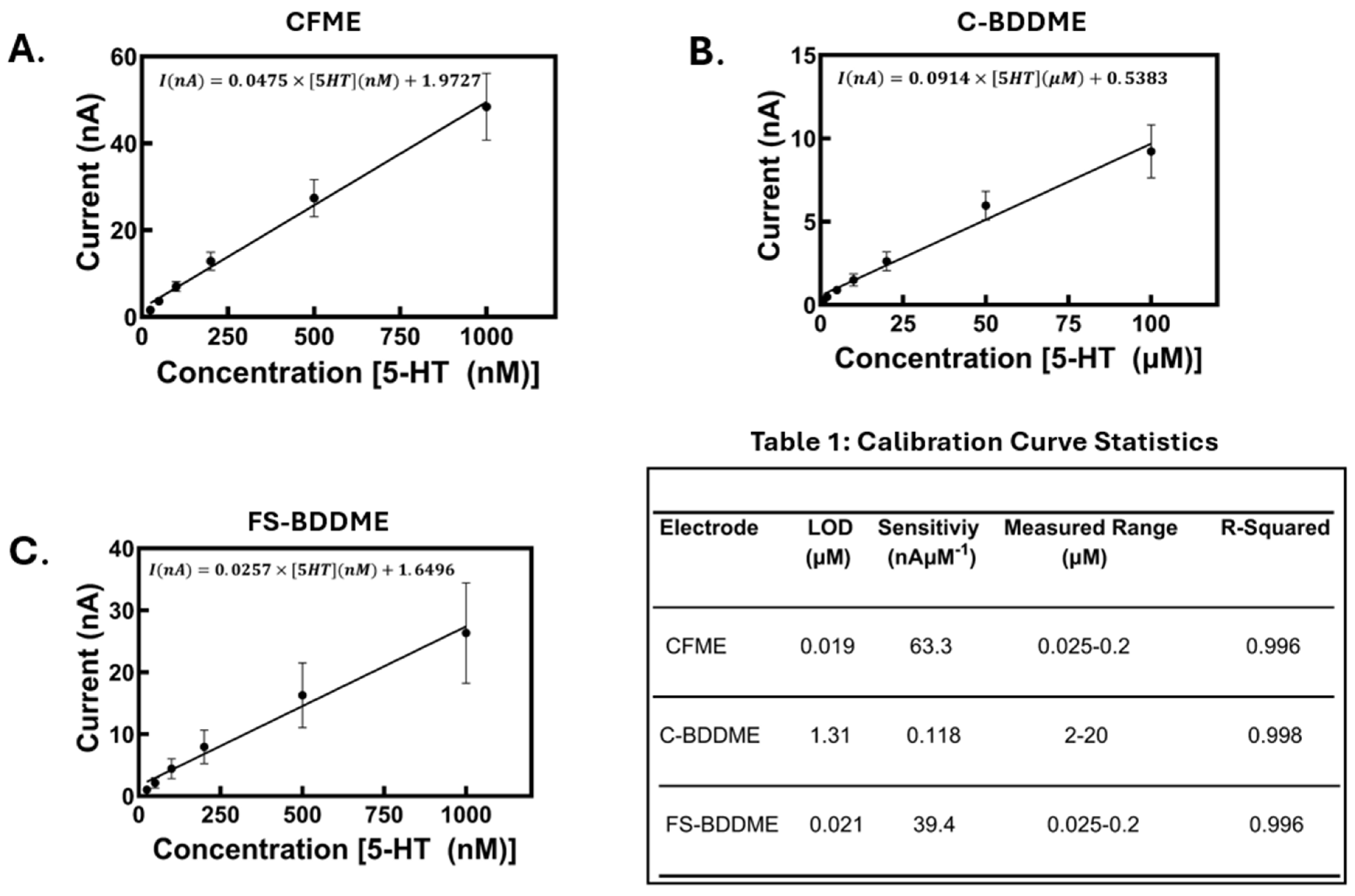
3.2. Electrode Calibrations
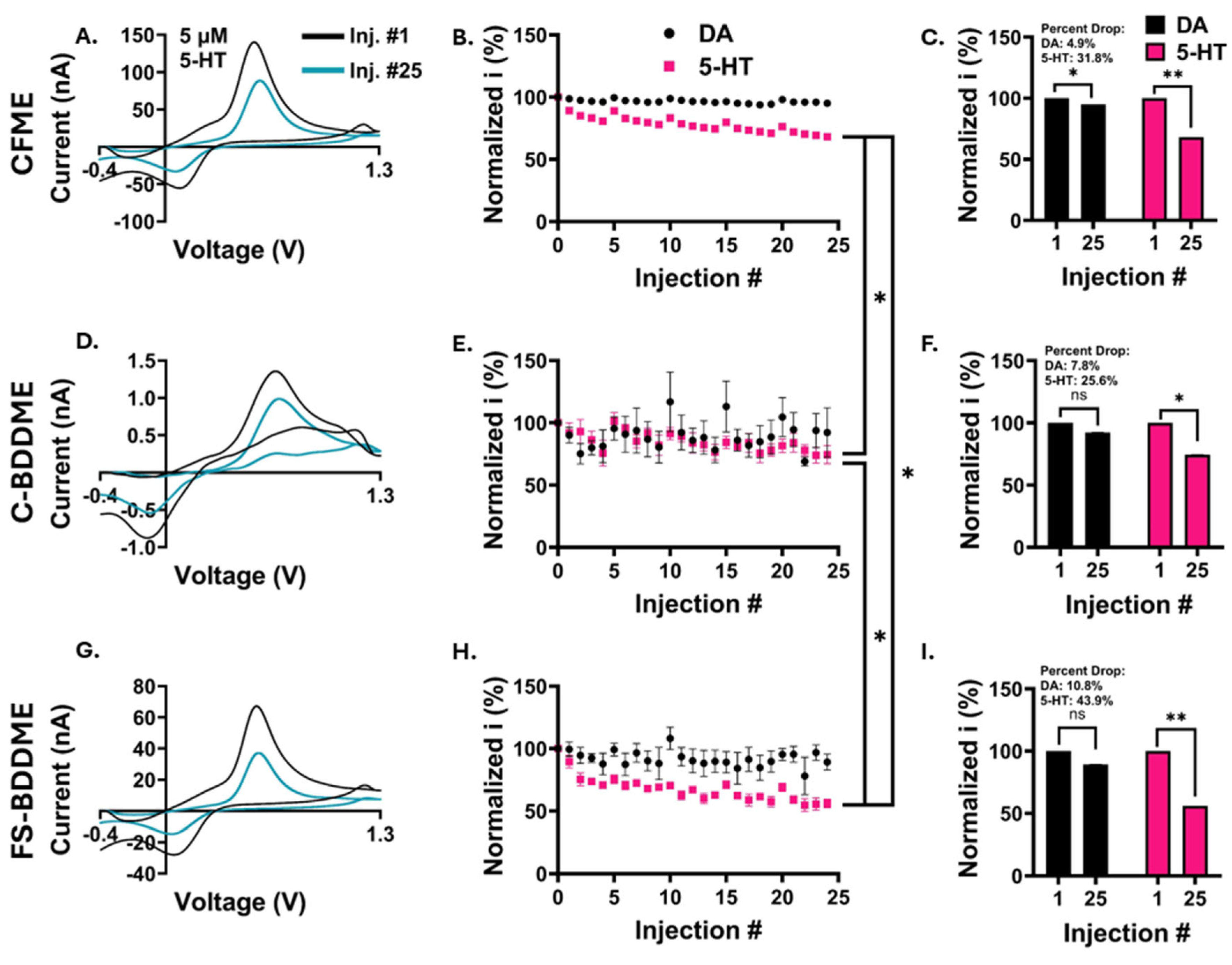
3.3. Electrochemical Fouling with 5 µM DA and 5-HT
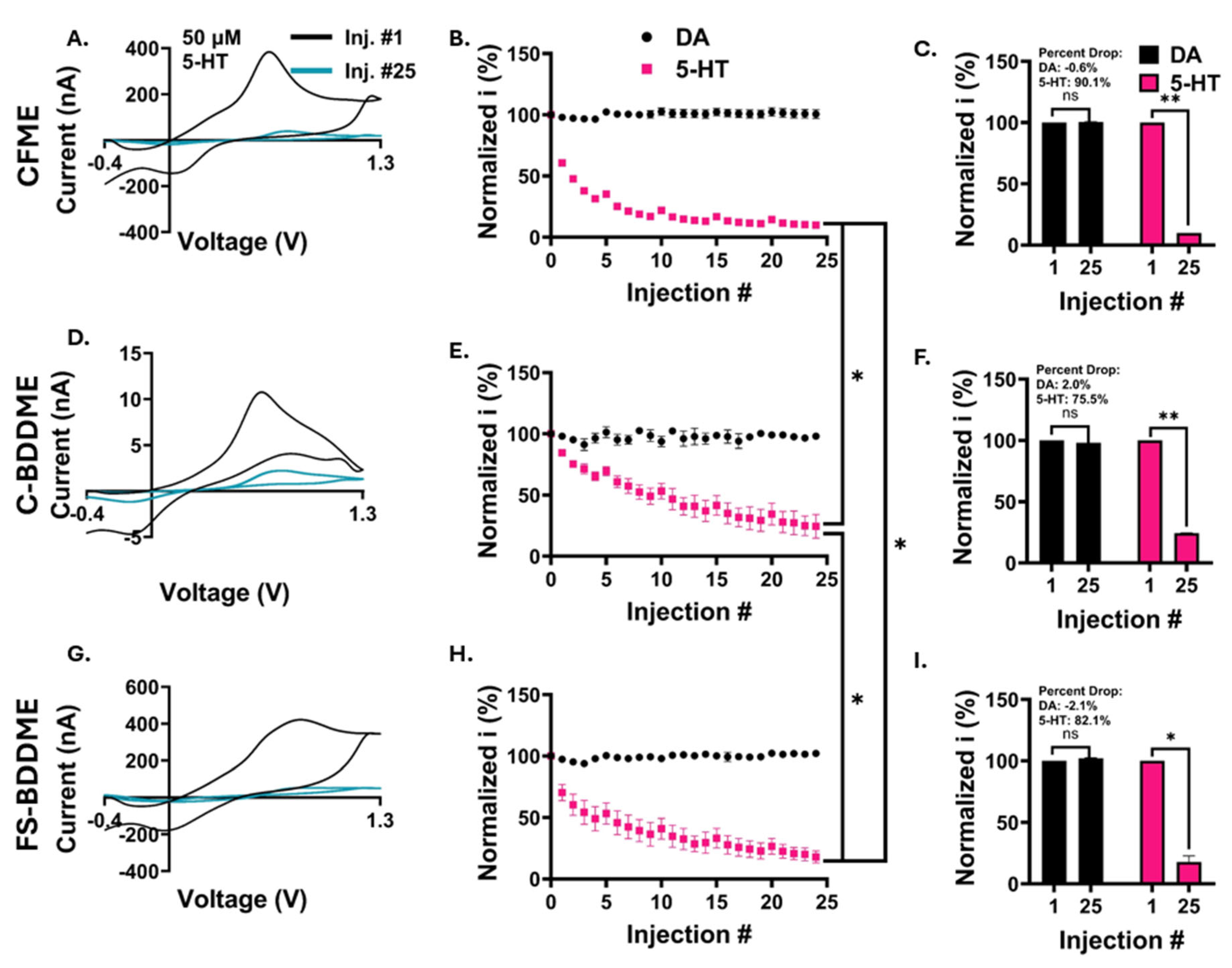
3.4. Electrochemical Fouling with 50 µM DA and 5-HT
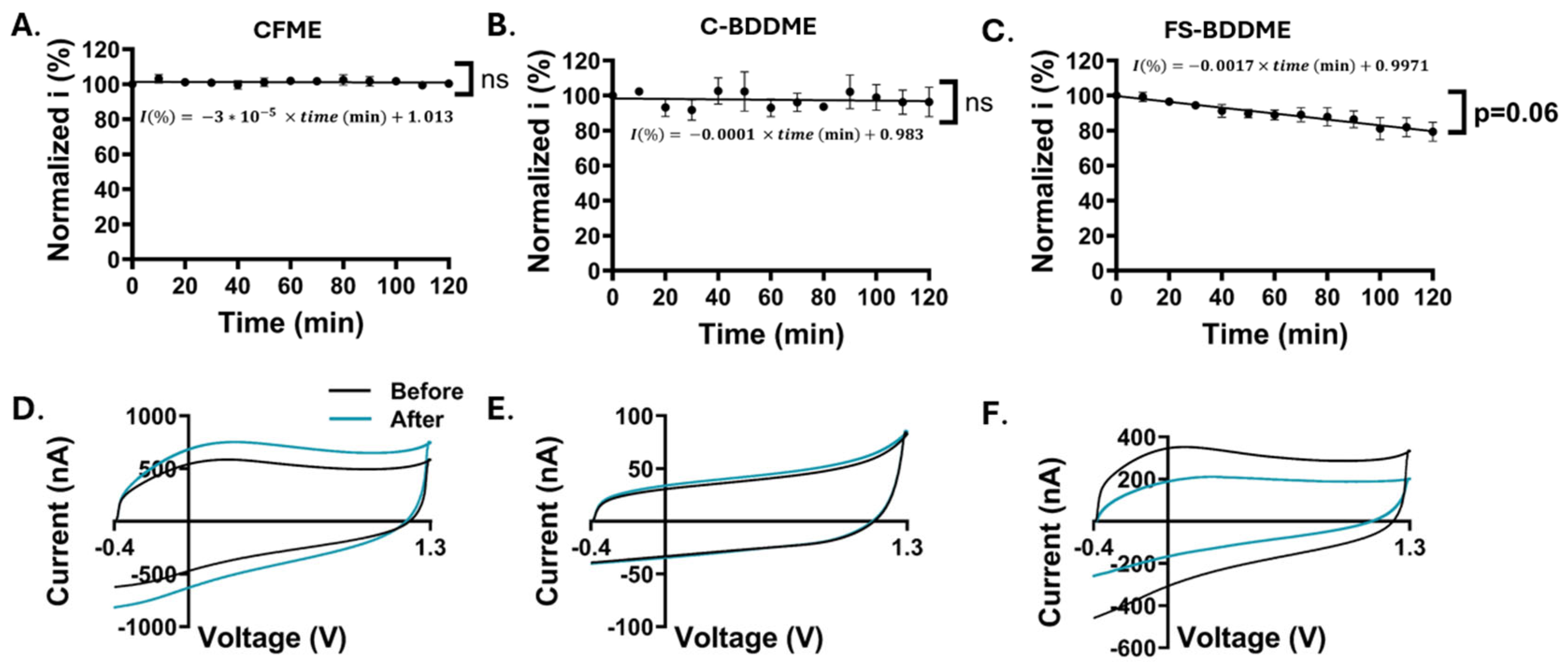
3.5. Electrode Stability Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyman SE. Neurotransmitters. Curr Biol. 2005;15(5):R154-R158. [CrossRef]
- Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524-532. [CrossRef]
- Wise RA, Jordan CJ. Dopamine, behavior, and addiction. J Biomed Sci. 2021;28(1):83. [CrossRef]
- Cooper S, Robison AJ, Mazei-Robison MS. Reward Circuitry in Addiction. Neurotherapeutics. 2017;14(3):687-697. [CrossRef]
- Stahl SM. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: Dopamine, serotonin, and glutamate. CNS Spectr. 2018;23(3):187-191. [CrossRef]
- Kishida KT, Saez I, Lohrenz T; et al. Subsecond dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward. Proc Natl Acad Sci U S A. 2016;113(1):200-205. [CrossRef]
- Saddoris MP, Wang X, Sugam JA, Carelli RM. Cocaine Self-Administration Experience Induces Pathological Phasic Accumbens Dopamine Signals and Abnormal Incentive Behaviors in Drug-Abstinent Rats. J Neurosci. 2016;36(1):235-250. [CrossRef]
- Park J, Aragona BJ, Kile BM, Carelli RM, Wightman RM. In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell. Neuroscience. 2010;169(1):132-142. [CrossRef]
- Buchanan AM, Mena S, Choukari I; et al. Serotonin as a biomarker of toxin-induced Parkinsonism. Mol Med Camb Mass. 2024;30(1):33. [CrossRef]
- Venton BJ, Cao Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. The Analyst. 2020;145(4):1158-1168. [CrossRef]
- Robinson DL, Venton BJ, Heien MLAV, Wightman RM. Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry in Vivo. Clin Chem. 2003;49(10):1763-1773. [CrossRef]
- Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem. 2011;119(5):932-944. [CrossRef]
- Samaranayake S, Abdalla A, Robke R, Wood KM, Zeqja A, Hashemi P. In vivo histamine voltammetry in the mouse premammillary nucleus. Analyst. 2015;140(11):3759-3765. [CrossRef]
- Dunham KE, Venton BJ. Improving serotonin fast-scan cyclic voltammetry detection: New waveforms to reduce electrode fouling. The Analyst. 2020;145(22):7437-7446. [CrossRef]
- Jackson BP, Dietz SM, Wightman RMark. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal Chem. 1995;67(6):1115-1120. [CrossRef]
- Hersey M, Woodruff JL, Maxwell N; et al. High-fat diet induces neuroinflammation and reduces the serotonergic response to escitalopram in the hippocampus of obese rats. Brain Behav Immun. 2021;96:63-72. [CrossRef]
- Weese ME, Krevh RA, Li Y, Alvarez NT, Ross AE. Defect Sites Modulate Fouling Resistance on Carbon-Nanotube Fiber Electrodes. ACS Sens. 2019;4(4):1001-1007. [CrossRef]
- Wrona MZ, Dryhurst G. Electrochemical oxidation of 5-hydroxytryptamine in aqueous solution at physiological pH. Bioorganic Chem. 1990;18(3):291-317. [CrossRef]
- Hashemi P, Dankoski EC, Petrovic J, Keithley RB, Wightman RM. Voltammetric Detection of 5-Hydroxytryptamine Release in the Rat Brain. Anal Chem. 2009;81(22):9462-9471. [CrossRef]
- Singh YS, Sawarynski LE, Dabiri PD, Choi WR, Andrews AM. Head-to-head comparisons of carbon fiber microelectrode coatings for sensitive and selective neurotransmitter detection by voltammetry. Anal Chem. 2011;83(17):6658-6666. [CrossRef]
- Gupta B, Perillo ML, Siegenthaler JR; et al. In Vitro Biofouling Performance of Boron-Doped Diamond Microelectrodes for Serotonin Detection Using Fast-Scan Cyclic Voltammetry. Biosensors. 2023;13(6):576. [CrossRef]
- Seaton BT, Hill DF, Cowen SL, Heien ML. Mitigating the Effects of Electrode Biofouling-Induced Impedance for Improved Long-Term Electrochemical Measurements In Vivo. Anal Chem. 2020;92(9):6334-6340. [CrossRef]
- Güell AG, Meadows KE, Unwin PR, Macpherson JV. Trace voltammetric detection of serotonin at carbon electrodes: Comparison of glassy carbon, boron doped diamond and carbon nanotube network electrodes. Phys Chem Chem Phys. 2010;12(34):10108. [CrossRef]
- Puthongkham P, Venton BJ. Recent advances in fast-scan cyclic voltammetry. The Analyst. 2020;145(4):1087-1102. [CrossRef]
- Puthongkham P, Venton BJ. Nanodiamond Coating Improves the Sensitivity and Antifouling Properties of Carbon Fiber Microelectrodes. ACS Sens. 2019;4(9):2403-2411. [CrossRef]
- Melnikov PV, Alexandrovskaya AY, Naumova AO; et al. Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomater Basel Switz. 2021;11(11):2980. [CrossRef]
- Purcell EK, Becker MF, Guo Y; et al. Next-Generation Diamond Electrodes for Neurochemical Sensing: Challenges and Opportunities. Micromachines. 2021;12(2):128. [CrossRef]
- Fan B, Rusinek CA, Thompson CH; et al. Flexible, diamond-based microelectrodes fabricated using the diamond growth side for neural sensing. Microsyst Nanoeng. 2020;6(1):1-12. [CrossRef]
- Singh YS, Sawarynski LE, Michael HM; et al. Boron-Doped Diamond Microelectrodes Reveal Reduced Serotonin Uptake Rates in Lymphocytes from Adult Rhesus Monkeys Carrying the Short Allele of the 5-HTTLPR. ACS Chem Neurosci. 2010;1(1):49-64. [CrossRef]
- Dong H, Wang S, Galligan JJ, Swain GM. Boron-doped diamond nano/microelectrodes for biosensing and in vitro measurements. Front Biosci Sch Ed. 2011;3(2):518-540. [CrossRef]
- Bennet KE, Tomshine JR, Min HK; et al. A Diamond-Based Electrode for Detection of Neurochemicals in the Human Brain. Front Hum Neurosci. 2016;10:102. [CrossRef]
- Park J, Quaiserová-Mocko V, Pecková K, Galligan JJ, Fink GD, Swain GM. Fabrication, characterization, and application of a diamond microelectrode for electrochemical measurement of norepinephrine release from the sympathetic nervous system. Diam Relat Mater. 2006;15(4-8):761-772. [CrossRef]
- Zhao H, Bian X, Galligan JJ, Swain GM. Electrochemical measurements of serotonin (5-HT) release from the guinea pig mucosa using continuous amperometry with a boron-doped diamond microelectrode. Diam Relat Mater. 2010;19(2-3):182. [CrossRef]
- Patel BA, Bian X, Quaiserová-Mocko V, Galligan JJ, Swain GM. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst. 2006;132(1):41-47. [CrossRef]
- Sarada BV, Rao TN, Tryk DA, Fujishima A. Electrochemical oxidation of histamine and serotonin at highly boron-doped diamond electrodes. Anal Chem. 2000;72(7):1632-1638. [CrossRef]
- Patel AN, Unwin PR, Macpherson JV. Investigation of film formation properties during electrochemical oxidation of serotonin (5-HT) at polycrystalline boron doped diamond. Phys Chem Chem Phys PCCP. 2013;15(41):18085-18092. [CrossRef]
- Cooper SE, Venton BJ. Fast-scan cyclic voltammetry for the detection of tyramine and octopamine. Anal Bioanal Chem. 2009;394(1):329-336. [CrossRef]
- Pyakurel P, Privman Champaloux E, Venton BJ. Fast-Scan Cyclic Voltammetry (FSCV) Detection of Endogenous Octopamine in Drosophila melanogaster Ventral Nerve Cord. ACS Chem Neurosci. 2016;7(8):1112-1119. [CrossRef]
- Gupta B, Perillo ML, Christensen IE; et al. Waveform Development for Neurotransmitter Detection on Novel Boron-Doped Diamond Microelectrodes. In: 2023 11th International IEEE/EMBS Conference on Neural Engineering (NER). ; 2023:1-5. [CrossRef]
- Roberts JG, Sombers LA. Fast-Scan Cyclic Voltammetry: Chemical Sensing in the Brain and Beyond. Anal Chem. 2018;90(1):490-504. [CrossRef]
- Takmakov P, Zachek MK, Keithley RB; et al. Carbon Microelectrodes with a Renewable Surface. Anal Chem. 2010;82(5):2020-2028. [CrossRef]
- Rusinek CA, Guo Y, Rechenberg R; et al. All-Diamond Microfiber Electrodes for Neurochemical Analysis. J Electrochem Soc. 2018;165(12):G3087-G3092. [CrossRef]
- Einaga Y. Diamond electrodes for electrochemical analysis. J Appl Electrochem. 2010;40(10):1807-1816. [CrossRef]
- Duo I, Fujishima A, Comninellis C. Electron transfer kinetics on composite diamond (sp3)–graphite (sp2) electrodes. Electrochem Commun. 2003;5(8):695-700. [CrossRef]
- Callou TP, Garcia R, Mukai A, Giacomin NT, de Souza RG, Bechara SJ. Advances in femtosecond laser technology. Clin Ophthalmol Auckl NZ. 2016;10:697-703. [CrossRef]
- Banna GMHU, Siegenthaler J, Benedict A; et al. Heavy metal sensing in plant and soil solutions using carbon fiber electrode. Sens Actuators Phys. 2024;370:115232. [CrossRef]
- Mitul AF, Zhou B, Zhao H, Han M. Micromachining & FBG fabrication using point by point technique utilizing femto-second laser.
- Bucher ES, Brooks K, Verber MD; et al. Flexible software platform for fast-scan cyclic voltammetry data acquisition and analysis. Anal Chem. 2013;85(21):10344-10353. [CrossRef]
- Abraham J. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. In: Tietje C, Brouder A, eds. Handbook of Transnational Economic Governance Regimes. Brill | Nijhoff; 2010:1041-1053. [CrossRef]
- Uhrovčík J. Strategy for determination of LOD and LOQ values – Some basic aspects. Talanta. 2014;119:178-180. [CrossRef]
- Rodeberg NT, Sandberg SG, Johnson JA, Phillips PEM, Wightman RM. Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in Vivo Fast-Scan Cyclic Voltammetry. ACS Chem Neurosci. 2017;8(2):221-234. [CrossRef]
- Heien MLAV, Khan AS, Ariansen JL; et al. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci. 2005;102(29):10023-10028. [CrossRef]
- Roberts JG, Toups JV, Eyualem E, McCarty GS, Sombers LA. In Situ Electrode Calibration Strategy for Voltammetric Measurements In Vivo. Anal Chem. 2013;85(23):11568-11575. [CrossRef]
- Saylor RA, Hersey M, West A; et al. In vivo Hippocampal Serotonin Dynamics in Male and Female Mice: Determining Effects of Acute Escitalopram Using Fast Scan Cyclic Voltammetry. Front Neurosci. 2019;13:362. [CrossRef]
- Blier P, de Montigny C, Chaput Y. A role for the serotonin system in the mechanism of action of antidepressant treatments: Preclinical evidence. J Clin Psychiatry. 1990;51 Suppl:14-20; discussion 21.
- Hrovatin K, Kunej T, Dolžan V. Genetic variability of serotonin pathway associated with schizophrenia onset, progression, and treatment. Am J Med Genet B Neuropsychiatr Genet. 2020;183(2):113-127. [CrossRef]
- Bang D, Kishida KT, Lohrenz T; et al. Sub-second Dopamine and Serotonin Signaling in Human Striatum during Perceptual Decision-Making. Neuron. 2020;108(5):999-1010.e6. [CrossRef]
- Hill DF, Parent KL, Atcherley CW, Cowen SL, Heien ML. Differential release of dopamine in the nucleus accumbens evoked by low-versus high-frequency medial prefrontal cortex stimulation. Brain Stimulat. 2018;11(2):426-434. [CrossRef]
- Hermans A, Keithley RB, Kita JM, Sombers LA, Wightman RM. Dopamine Detection with Fast-Scan Cyclic Voltammetry Used with Analog Background Subtraction. Anal Chem. 2008;80(11):4040-4048. [CrossRef]
- Clark JJ, Sandberg SG, Wanat MJ; et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7(2):126-129. [CrossRef]
- Huffman ML, Venton BJ. Carbon-fiber microelectrodes for in vivo applications. Analyst. 2008;134(1):18-24. [CrossRef]
- Lin J, Peng Z, Liu Y; et al. Laser-induced porous graphene films from commercial polymers. Nat Commun. 2014;5(1):5714. [CrossRef]
- Su S, Li J, Lee GCB, Sugden K, Webb D, Ye H. Femtosecond laser-induced microstructures on diamond for microfluidic sensing device applications. Appl Phys Lett. 2013;102(23):231913. [CrossRef]
- Kononenko VV, Kononenko TV, Pimenov SM, Sinyavskii MN, Konov VI, Dausinger F. Effect of the pulse duration on graphitisation of diamond during laser ablation. Quantum Electron. 2005;35(3):252-256. [CrossRef]
- Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Subsecond Adsorption and Desorption of Dopamine at Carbon-Fiber Microelectrodes. Anal Chem. 2000;72(24):5994-6002. [CrossRef]
- Heien MLAV, Phillips PEM, Stuber GD, Seipel AT, Wightman RM. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst. 2003;128(12):1413-1419. [CrossRef]
- Clark JJ, Collins AL, Sanford CA, Phillips PEM. Dopamine Encoding of Pavlovian Incentive Stimuli Diminishes with Extended Training. J Neurosci. 2013;33(8):3526-3532. [CrossRef]
- Cobb SJ, Laidlaw FHJ, West G; et al. Assessment of acid and thermal oxidation treatments for removing sp2 bonded carbon from the surface of boron doped diamond. Carbon. 2020;167:1-10. [CrossRef]
- Klauser F, Ghodbane S, Boukherroub R; et al. Comparison of different oxidation techniques on single-crystal and nanocrystalline diamond surfaces. Diam Relat Mater. 2010;19(5):474-478. [CrossRef]
- Shinoda M, Gattass RR, Mazur E. Femtosecond laser-induced formation of nanometer-width grooves on synthetic single-crystal diamond surfaces. J Appl Phys. 2009;105(5):053102. [CrossRef]
- Thompson CH, Riggins TE, Patel PR, Chestek CA, Li W, Purcell E. Toward guiding principles for the design of biologically-integrated electrodes for the central nervous system. J Neural Eng. 2020;17(2):021001. [CrossRef]
- Schwerdt HN, Zhang E, Kim MJ; et al. Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a rodent model. Commun Biol. 2018;1(1):1-11. [CrossRef]
- Stiller A, Black B, Kung C; et al. A Meta-Analysis of Intracortical Device Stiffness and Its Correlation with Histological Outcomes. Micromachines. 2018;9(9):443. [CrossRef]
- Salatino JW, Ludwig KA, Kozai TDY, Purcell EK. Glial responses to implanted electrodes in the brain. Nat Biomed Eng. 2017;1(11):862-877. [CrossRef]
- Kozai TDY, Langhals NB, Patel PR; et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat Mater. 2012;11(12):1065-1073. [CrossRef]
- Kozai TDY, Jaquins-Gerstl AS, Vazquez AL, Michael AC, Cui XT. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem Neurosci. 2015;6(1):48-67. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




