Submitted:
28 June 2024
Posted:
01 July 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Origin of the samples
2.2. Colour analysis
2.3. Moisture and pH determination
2.4. Fat content determination
2.5. Extraction of the phenolic fraction
2.5.1. Polyphenols conventional Liquid-Liquid extraction
2.5.2. Dimethylsulfoxide-based polyphenols fast extraction
2.6. Phenolic compounds evaluation
2.6.1. Folin-Ciocalteau assay
2.6.2. AuNPs-based assay
2.6.3. Electrochemical measurement of catechins
2.7. Biogenic amines determination
2.8. Statistical analyses
3. Results and discussion
3.1. Colour indices and pH
3.2. Fat and moisture
3.3. Polyphenols evaluation
3.3. Biogenic amines
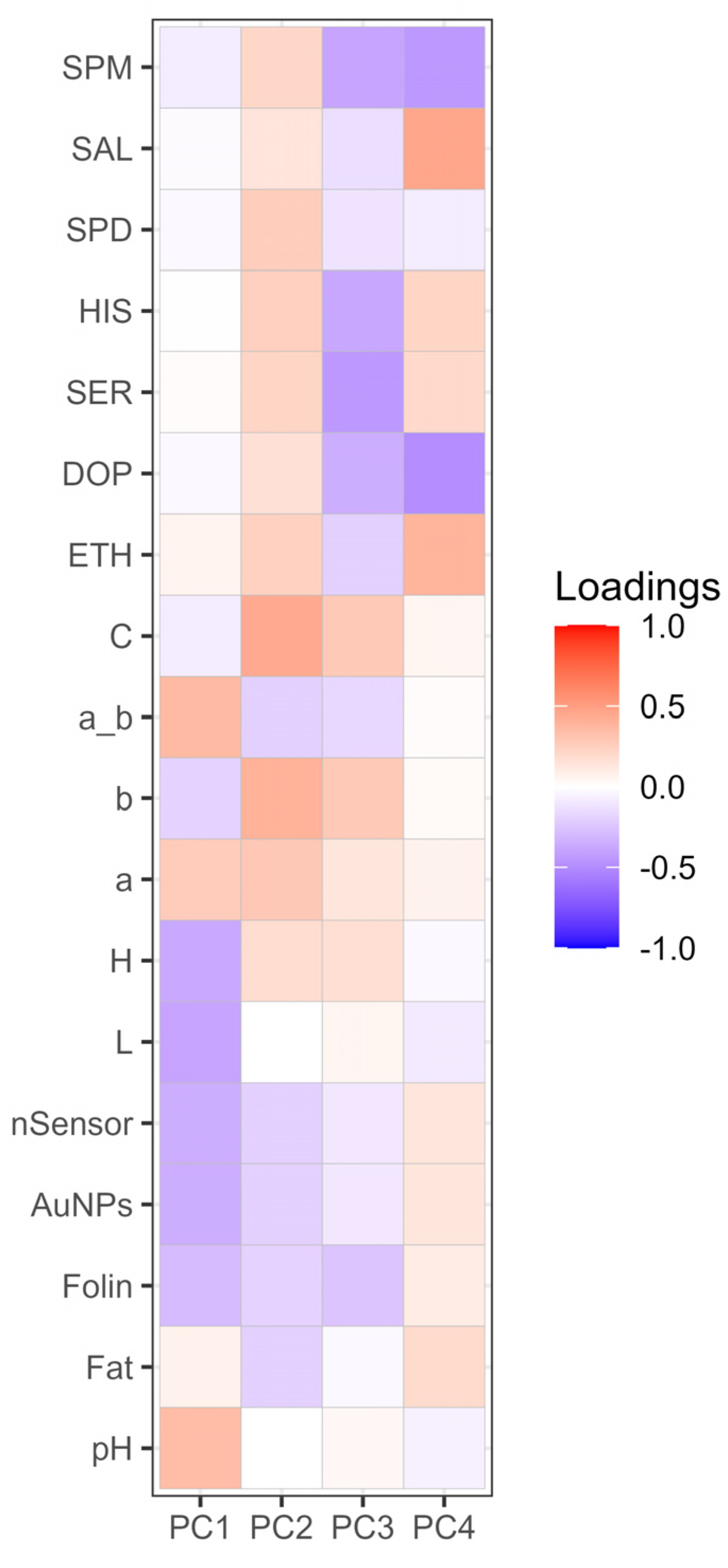
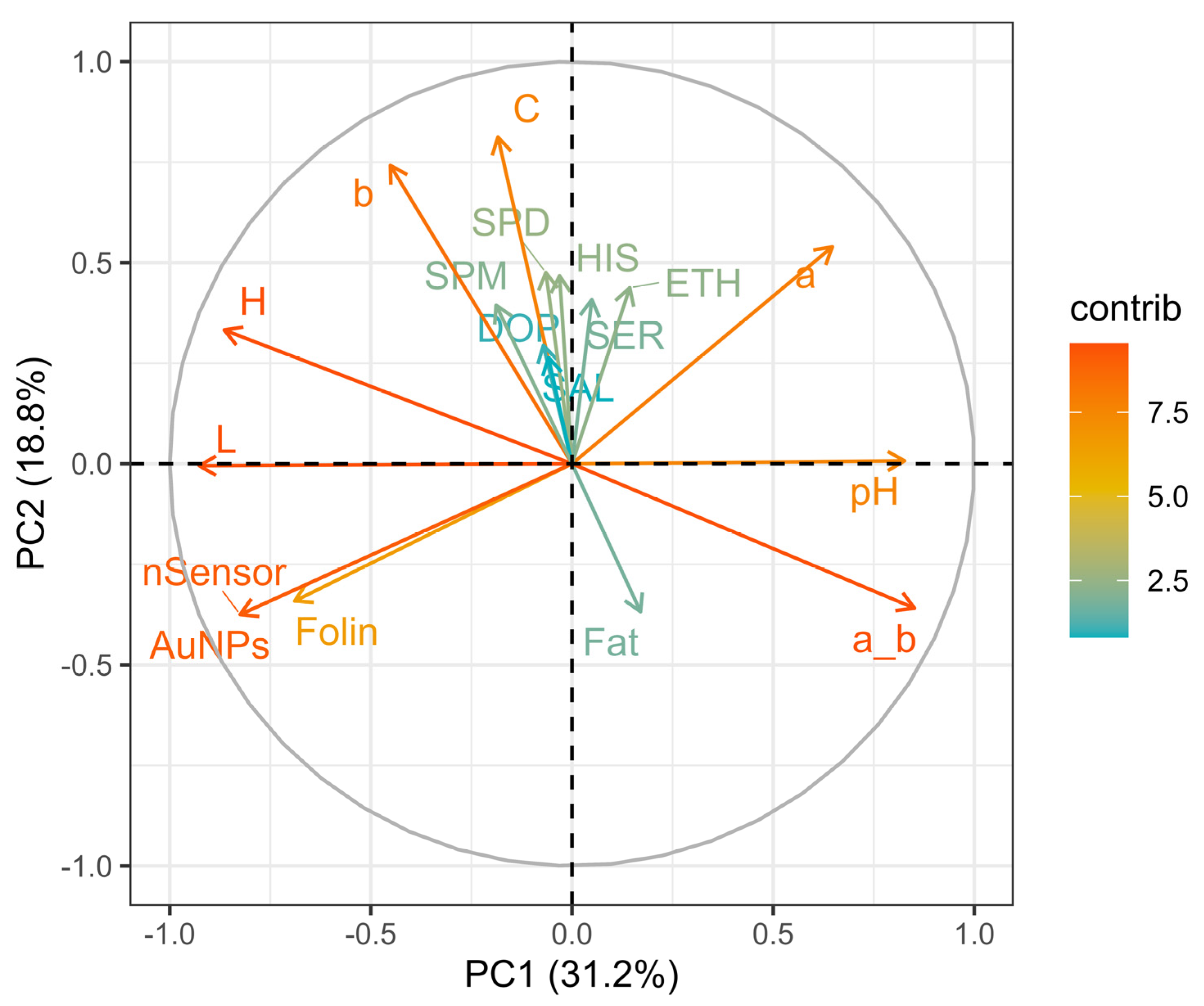
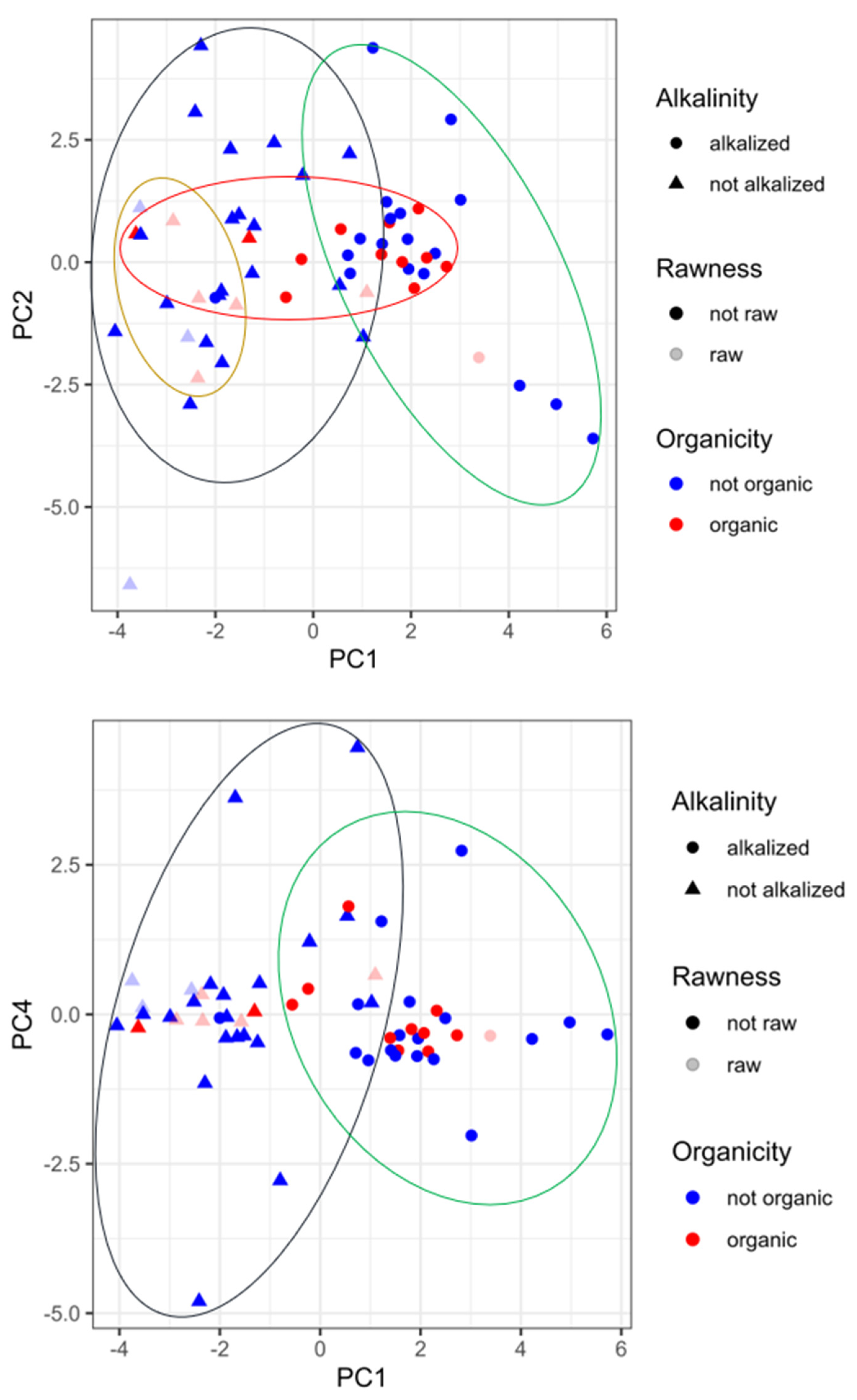
3.4. Principal component analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sioriki, E.; Tuenter, E.; Van de Walle, D.; Lemarcq, V.; Cazin, C.S.J.; Nolan, S.P.; Pieters, L.; & Dewettinck, K. The effect of cocoa alkalization on the non-volatile and volatile mood-enhancing compounds. Food Chem. 2022, 132082. [CrossRef]
- Tuenter, E.; Foubert, K.; & Pieters, L. Mood components in cocoa and chocolate: the mood pyramid. Planta Med., 2018, 839-844 . [CrossRef]
- Cooper, K.A.;Donovan, J.L.; Waterhouse, A.L.; Williamson G. Cocoa and health: A decade of research. Br J Nutr 2008, 99. 1–11 . [CrossRef]
- Delgado-Ospina, J.; Esposito, L.; Molina-Hernandez, J.B.; Pérez-Álvarez, J. A.; Martuscelli, M.; & Chaves-Lopez, C. Cocoa Shell Infusion: A Promising Application for Added-Value Beverages Based on Cocoa’s Production Coproducts. Foods 2023, 12, 2442. [CrossRef]
- Delgado-Ospina, J.; Acquaticci, L.; Molina-Hernández, J.B.; Rantsiou, K.; Martuscelli, M.; Kamgang-Nzekoue, A.F.; Vittori, S.; Paparella, A.; & Chaves-López, C. Exploring the capability of yeast isolated from Colombian fermented cocoa beans to form and degrade biogenic amines in a lab-scale model system for cocoa fermentation. Microrganisms, 2020, 9(1); 28. [CrossRef]
- Oracz, J.; & Nebesny; E. Influence of roasting conditions on the biogenic amine content in cocoa beans of different Theobroma cacao cultivars. Food Res. Int. 2014, 55(1–10). [CrossRef]
- Martuscelli, M.; Esposito, L.; & Mastrocola, D. Biogenic Amines’ Content in Safe and Quality Food. Foods 2021, 10, 100. [CrossRef]
- Esposito, L.; Mastrocola, D.; & Martuscelli, M. Who Cares about Biogenic Amines? Foods, 2023, 12(21), 3900. [CrossRef]
- Esposito, L.; Martuscelli, M.; & Mastrocola, D. Approaching to biogenic amines as quality markers in packaged chicken meat. Front. Nutr. section of Food Sci Technol, 2022, 9-966790. [CrossRef]
- Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-Lopez, C. Effect of Fermentation; Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells. Foods, 2020, 9(4); 520, . [CrossRef]
- Chaves-Lopez, C.; Serio, A.; Montalvo, C.; Ramirez, C.; Peréz Alvares, J.A.; Paparella, A.; Mastrocola, D.; & Martuscelli, M. Effect of nisin on biogenic amines and shelf life of vacuum packaged rainbow trout (Oncorhynchus mykiss) fillets”. J Food Sci. Technol. 2017, 54(10); 3268-3277 . [CrossRef]
- Kurnik-Łucka, M.; Latacz, G.; Martyniak, A.; Bugajski, A.; Kieć-Kononowicz, K.; Gil, K. Salsolinol neurotoxic or Neuroprotective? Neurotox. Res., 2020, 37(2): 286–297 . [CrossRef]
- Jung, Y.J.; Youn, J.Y.; Ryu, J.C.; Surh, Y.J. Salsolinol, a naturally occurring tetrahydroisoquinoline alkaloid; induces DNA damage and chromosomal aberrations in cultured Chinese hamster lung fibroblast cells. 2001, Mutat. Res- Fundam. Rol, 474 (1–2); 25-33; [CrossRef]
- Yuan, Y.; Zhao, Y.; Yang, J.; Jiang, Y.; Lu, F.; Jia, Y.; Yang, B. Metabolomic analyses of banana during postharvest senescence by 1H-high resolution-NMR. Food Chem. 2017, 218, 406-412, . [CrossRef]
- Melzig, M.F.; Putscher, I.; Henklein, P.; Haber, H. In vitro pharmacological activity of the tetrahydroisoquinoline salsolinol present in products from Theobroma cacao L. like cocoa and chocolate. J. Ethnopharmacol, 2000, 73 153 – 159 . [CrossRef]
- Porter, L.J.; Ma, Z.; Chan, B.G. Cacao procyanidins: major flavanoids and identification of some minor metabolites; Phytochemistry, 1991, 30 (5); 1657-1663; [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D.; Budryn, G.; Luzak, B. Bioavailability and metabolism of selected cocoa bioactive compounds: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60(12); 1847–1985. [CrossRef]
- Alasti, F.M.; Asefi, N.; Maleki, R.; Sadegh, S.; Heris, S. The influence of three different types and dosage of alkaline on the inherent properties in cocoa powder. J. Food Sci. Technol., 2020, 57, 2561–2571. [CrossRef]
- Afoakwa, E.O. Chocolate Science and Technology 2nd Edition. 201,0 Wiley-Blackwell (chapter 5).
- Aprotosoaie, A. C.; Luca, S. V.; Miron, A. Flavor chemistry of cocoa and cocoa products-An overview.CRFSFS, 2016, 15(1); 73–91. [CrossRef]
- Moser, A. Alkalizing Cocoa and Chocolate. Manufacturing Confectioner, 2015, 95; 31–38.
- Di Mattia, C.; Martuscelli, M.; Mastrocola, D.; Neri, L.; Pittia, P; Sacchetti G. Technological parameters and antioxidant activity of cocoa powders. Progress Nutr. 2011, 13(1); 39-47.
- Deeth, H. C.; Lewis; M. J. High temperature processing of milk and milk products (Illustrate). John Wiley & Sons, 2017.
- [24]AOAC. Official Methods of Analysis of AOAC International; Methods 2009.01; and 2011.25; 19th ed.; AOAC International: Rockville,MD, USA; 2012.
- Di Mattia, C.; Martuscelli, M.; Sacchetti, G.; Scheirlinck, I.; Beheydt, B.; Mastrocola, D.; Pittia, P. Effect of fermentation and drying on procyanidins; antiradical activity and reducing properties of cocoa beans. Food Bioprocess. Technol.; 2013, 6; 3420 . [CrossRef]
- Della Pelle, F.; Blandón-Naranjo; L., Alzate; M.; Del Carlo, M.; Compagnone, D. Cocoa powder and catechins as natural mediators to modify carbon-black based screen-printed electrodes. Application to free and total glutathione detection in blood. Talanta; 2020, 207; 120349 . [CrossRef]
- Rojas, D.; Hernández-Rodríguez, J. F.; Della Pelle, F.; Del Carlo; M.; Compagnone, D.; Escarpa, A. Oxidative stress on-chip: Prussian blue-based electrode array for in situ detection of H2O2 from cell populations. Biosens Bioelectron; 2020, 170; 112669. [CrossRef]
- Della Pelle, F.; González, M. C.; Sergi, M.; Del Carlo, M.; Compagnone, D.; Escarpa, A. Gold nanoparticles-based extraction-free colorimetric assay in organic media: an optical index for determination of total polyphenols in fat-rich samples. Anal. Chem., 2015, 87(13); 6905-6911 . [CrossRef]
- Della Pelle F.; Rojasa D.; Scroccarello A.; Del Carlo M. ; Ferraro G.; Di Mattia C.; Martuscelli M.; Escarpa A.; Compagnone D. High-performance carbon black/molybdenum disulfide nanohybrid sensor for cocoa catechins determination using an extraction-free approach. Sens. Actuators B. Chem.; 2019, 296; 126651 . [CrossRef]
- Esposito, L.; Mascini, M.; Silveri, F.; Pepe, A.; Mastrocola, D.; Martuscelli, M. A machine learning approach to uncover nicotinamide and other antioxidants as novel markers for chicken meat quality assessment. Food Biosci 2024, 58;103577 1-10 . [CrossRef]
- Mann, H. B.; Whitney, D. R. On a test of whether one of two random variables is stochastically larger than the other. Annals Math Stat; 1947, 18,50–60.
- Jolliffe, I.T. Principal Component Analysis. 2002, Springer New York; NY. [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Retrieved 2022, https://www.R-project.org/ accessed on December 12; 2023.
- Sacchetti, G.; Ioannone, F.; De Gregorio, M.; Di Mattia, C.; Serafini, M.; Mastrocola, D. Non enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng.; 2016, 169: 44-52. [CrossRef]
- Miller, K. B.; Hurst, W. J.; Payne, M. J.; Stuart, D. A.; Apgar, J.; Sweigart, D. S.; Ou; B. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J. Agric. Food Chem. 2008, 56. 8527–8533 . [CrossRef]
- Martins; S. I. F. S.; Jongen; W. M. F.; & van Boekel; M. A. J. S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Techn., 2001, 11; 364–373. [CrossRef]
- Germann; D.; Stark, T. D.; Hofmann; T. Formation and Characterization of Polyphenol-Derived Red Chromophores. Enhancing the Color of Processed Cocoa Powders: Part 1. J.Agric. Food Chem, 2019, 67, 4632–4642. [CrossRef]
- Germann, D.; Stark T. D.; Hofmann, T. Formation and Characterization of Polyphenol-Derived Red Chromophores. Enhancing the Color of Processed Cocoa Powders: Part 2. J.Agric. Food Chem., 2019, 67; 4643–4651. [CrossRef]
- Sharma, G. Digital Color Imaging Handbook. 2003, CRC Press.
- Scroccarello, A.; Della Pelle, F.; Rojas, D.; Ferraro, G.; Fratini; E., Gaggiotti, S.; Cichelli, A.; Compagnone, D. Metal nanoparticles based lab-on-paper for phenolic compounds evaluation with no sample pretreatment. Application to extra virgin olive oil samples. Anal. Chim. Acta, 2021, 1183; 338971 . [CrossRef]
- Scroccarello, A.; Della Pelle, F.; Neri, L.; Pittia, P.; Compagnone, D. Silver and gold nanoparticles based colorimetric assays for the determination of sugars and polyphenols in apples. Food Res Int, 2019, 119; 359-368 . [CrossRef]
- Razola-Díaz, M.D.C.; Aznar-Ramos, M.J.; Verardo, V.; Melgar-Locatelli, S.; Castilla-Ortega, E.; Rodríguez-Pérez, C. Exploring the Nutritional Composition and Bioactive Compounds in Different Cocoa Powders. Antioxidants, 2023, 14;12(3):716. [CrossRef]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From Cocoa to Chocolate: The Impact of Processing on In Vitro Antioxidant Activity and the Effects of Chocolate on Antioxidant Markers In Vivo. Front. Immunol., 2017, 8;1207. [CrossRef]
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols; proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 1; 2015, 174:256-62. [CrossRef]
- Jiang, Z., Han, Z.; Zhu, M.; Wan, X.; Zhang, L. Effects of thermal processing on transformation of polyphenols and flavor quality. Curr. Opin. Food Sci., 2023, 101014 . [CrossRef]
- Todorovic, V.; Milenkovic, M.; Vidovic, B.; Todorovic, Z.; Sobajic, S. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J. Food Sci. 2017, 82 1020–1027 . [CrossRef]
- Spizzirri, U.G.; Ieri, F.; Campo, M.; Paolino, D.; Restuccia, D.; Romani, A. Biogenic Amines; Phenolic; and Aroma-Related Compounds of Unroasted and Roasted Cocoa Beans with Different Origin. Foods 2019, 8; 306. [CrossRef]
- Do Carmo Brito, B. N.; Chisté, R. C.; Pena, R. S.; Gloria, M. B. A.; Lopes, A. S. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem; 2017, 228; 484–490. [CrossRef]
- Sentellas, S.; Saurina, J. Authentication of Cocoa Products Based on Profiling and Fingerprinting Approaches: Assessment of Geographical; Varietal; Agricultural and Processing Features. Foods 2023, 12;3120. [CrossRef]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M. T.; Latorre-Moratalla, M.; Vidal-Carou, M. D. C Histamine intolerance: The current state of the art. Biomolecules, 2020, 10(8); 1181 . [CrossRef]
- EFSA (European Food Safety Authority). Scientific Opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006 following a request in accordance with Article 19 of Regulation (EC) No 1924/2006. EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). EFSA J., 2012, 10(7), 2809. [CrossRef]
- EFSA (European Food Safety Authority). Scientific opinion on risk-based control of biogenic amines formation in fermented foods. EFSA J., 2011; 9(10); 2392 . [CrossRef]
- Dala-Paula, B.M.; Deus, V.L.; Tavano, O.L.; Gloria, B.M.A. In vitro bioaccessibility of amino acids and bioactive amines in 70% cocoa dark chocolate: What you eat and what you get. Food Chem; 2021, 343; 128397 . [CrossRef]
- Navailles, S.; Lagière, M.; Contini; A.; De Deurwaerdère, P. Multisite Intracerebral Microdialysis to Study the Mechanism of L-DOPA Induced Dopamine and Serotonin Release in the Parkinsonian Brain. ACS Chem. Neurosci. 2013, 4; 5; 680–692. [CrossRef]
- Weng, J.; Paslaski, S.; Daly, J.; VanDam, C.; Brown, J. Modulation for emergent networks: Serotonin and dopamine. Neural Networks, 2013, 41; 225-239; [CrossRef]
- McCutcheon, J.E. The role of dopamine in the pursuit of nutritional value. Physiol Behav; 2015, 152, Part B; 408-415; [CrossRef]
- Sharmistha, S.; Tanmay, S.; Runu, C.; Maksim, R.; Mohammad, A.S.; Muthu, T.; Kannan R.R.R. Dark chocolate: An overview of its biological activity; processing; and fortification approaches. Curr. Res. Food Sci. 5; 2022, 1916-1943. [CrossRef]
- Xie, G.; Hipólito, L.; Zuo, W.; Polache, A.; Granero, L.; Krnjević, K.; Ye, J.H. Salsolinol Stimulates Dopamine Neurons in Slices of Posterior Ventral Tegmental Area Indirectly by Activating μ-Opioid Receptors. J. Pharmacol. Exp. Ther. 2012, 341(1): 43–50. [CrossRef]
- Wen, J.; Zhang, L.; Liu, H.; Wang, J.; Li, J.; Yang, Y.; Wang, Y.; Cai, H.; Li, R.; Zhao, Y. Salsolinol attenuates doxorubicin-induced chronic heart failure in rats and improves mitochondrial function in H9c2 cardiomyocytes. Front. Pharmacol. 2019, 10:1135 . [CrossRef]
- Muñoz-Esparza, N. C.; Latorre-Moratalla, M. L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M. T.; Vidal-Carou, M. C. Polyamines in food. Front. Nut. 2019, 6, 108. [CrossRef]
- Handa, A.K.; Fatima, T.; Mattoo; A.K. Polyamines: bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6-10. [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; De Luca, M.; Parisi, O.I.; Picci, N. Biogenic amines as quality marker in organic and fair-trade cocoa-based products. Sustainability; 2016, 8; 856.
- Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Picci, N. Determination of biogenic amine profiles in conventional and organic cocoa-based products. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk. Assess.; 2015, 32; 1156–1163. [CrossRef]
- Deus, V. L.; Bispo, E. S.; Franca, A. S.; Gloria, M. B. A. Influence of cocoa clones on the quality and functional properties of chocolate–Nitrogenous compounds. LWT; 2020, 134; 110202 . [CrossRef]
- Montoya, C. C.; Valencia, W. G.; Sierra, J. A.; Penagos, L. Enhanced pink-red hues in processed powders from unfermented cacao beans. LWT, 2021, 138; 110671 . [CrossRef]
| Provider code (N of samples collected) |
number code | Geographic origin |
|---|---|---|
| BC (N=15) | 1; 5; 8; 12-14; 38-46 | Ecuador; Sao Tomè; Dominican Republic; Peru |
| CA (N=1) | 6 | Colombia |
| CE (N=2) | 4; 10 | Ivory Coast |
| CG (N=8) | 7; 16; 19; 21-25 | Ivory Coast |
| CM (N=1) | 15 | Ivory Coast |
| DN (N=5) | 11; 28-31 | Ghana |
| IM (N=13) | 17; 18; 20; 32-37; 47-48; 50-51 | Ecuador; Peru |
| JR (N=3) | 26-27; 53 | Ecuador |
| ME (N=2) | 58-59 | Bali |
| PD (N=6) | 9; 49; 54-57 | Vietnam |
| UO (N=1) | 3 | Colombia |
| VV (N=1) | 2 | Bali |
| ZI (N=1) | 52 | Ivory Coast |
| L* | a* | b* | C | h* | pH | |
|---|---|---|---|---|---|---|
| not organic (N. 41) | 41.49±7.50 | 12.97±1.63 | 19.59±3.19 | 23.58±3.02 | 56.17±4.99 | 6.02±0.78 |
| (24.77-55.61) | (9.05-16.35) | (10.95-25.64) | (14.20-28.67) | (41.29-63.63) | (4.75-7.32) | |
| organic (N. 18) | 40.71±6.08 | 13.45±1.46 | 20.16±2.20 | 24.30±2.00 | 56.19±4.09 | 6.38±0.84 |
| (30.08-52.09) | (10.93-15.69) | (14.86-24.05) | (20.02-27.27) | (47.96-64.79) | (5.08-7.32) | |
| p-value | 0.675 | 0.320 | 0.499 | 0.363 | 0.985 | 0.121 |
| not raw (N. 50) | 40.32±7.29 | 19.34±1.54 | 19.87±2.61 | 24.00±2.42 | 55.93±4.62 | 6.24±0.81 |
| (24.77-55.61) | (9.71-16.35) | (11.17-24.12) | (16.93-27.61) | (41.29-64.79) | (4.75-7.32) | |
| raw (N. 9) | 46.46±5.39 | 11.92±1.30 | 19.22±4.43 | 22.69±4.18 | 57.50±5.19 | 5.55±0.58 |
| (33.17-51.25) | (9.05-13.41) | (10.95-25.64) | (14.20-28.67) | (47.96-63.41) | (5.08-6.87) | |
| p-value | 0.019 | 0.012 | 0.716 | 0.238 | 0.246 | 0.009 |
| not alkalized (N. 30) |
46.52±5.52 | 12.18±1.42 | 20.51±2.92 | 23.89±2.95 | 59.11±3.39 | 5.39±0.28 |
| (32.86-55.61) | (9.05-14.82) | (10.95-25.64) | (14.20-28.67) | (50.46-64.79) | (4.75-5.95) | |
| alkalized (N. 29) | 35.80±4.44 | 14.10±1.06 | 19.00±2.76 | 23.70±2.58 | 53.14±3.87 | 6.90±0.28 |
| (24.77-42.23) | (11.86-16.35) | (11.17-22.35) | (16.93-27.53) | (41.29-57.93) | (6.01-7.32) | |
| p-value | <0.001 | <0.001 | 0.055 | 0.806 | <0.001 | <0.001 |
| Total phenolic content (mg GAeq g-1) | |||||||
|---|---|---|---|---|---|---|---|
| sample | Label | alkalization | Fat (%) | Folin | AuNPs | nSensor | |
| 1 | not organic | raw | NA | 5.6 ± 0.9 | 141.0 ± 3.7 | 136.9 ± 7.1 | 57.1 ± 0.4 |
| 2 | organic | raw | NA | 15.1 ± 1.3 | 72.0 ± 1.4 | 79.1 ± 0.5 | 33.0 ± 0.9 |
| 3 | organic | raw | NA | 8.5 ± 0.7 | 60.8 ± 1.6 | 51.7 ± 0.2 | 21.6 ± 0.4 |
| 4 | not organic | alkalized | 24.6 ± 2.1 | 36.2 ± 2.3 | 43.8 ± 0.1 | 18.3 ± 0.8 | |
| 5 | organic | raw | NA | 7.0 ± 0.6 | 51.8 ± 1.1 | 71.0 ± 0.8 | 29.6 ± 0.8 |
| 6 | organic | R | NA | 8.5 ± 0.7 | 67.8 ± 1.0 | 52.0 ± 6.1 | 21.7 ± 2.0 |
| 7 | not organic | R | NA | 9.3 ± 1.2 | 48.1 ± 0.2 | 70.1 ± 7.2 | 29.3 ± 0.6 |
| 8 | not organic | R | NA | 9.1 ± 1.8 | 73.7 ± 1.2 | 39.5 ± 6.0 | 16.5 ± 2.2 |
| 9 | not organic | R | NA | 9.1 ± 0.8 | 53.5 ± 0.7 | 72.9 ± 2.3 | 30.5 ± 1.8 |
| 10 | not organic | R | alkalized | 20.7 ± 1.8 | 35.7 ± 1.6 | 39.0 ± 2.1 | 16.3 ± 1.7 |
| 11 | not organic | R | NA | 7.9 ± 0.7 | 31.1 ± 2.4 | 39.4 ± 1.5 | 16.5 ± 0.8 |
| 12 | not organic | R | NA | 8.8 ± 0.7 | 24.9 ± 3.0 | 31.9 ± 0.6 | 13.4 ± 1.5 |
| 13 | organic | R | alkalized | 10.4 ± 0.9 | 15.8 ± 0.2 | 13.9 ± 0.1 | 5.9 ± 0.7 |
| 14 | organic | R | alkalized | 16.5 ± 1.4 | 14.2 ± 0.1 | 11.9 ± 0.2 | 5.1 ± 0.8 |
| 15 | not organic | R | NA | 14.3 ± 1.2 | 20.3 ± 0.2 | 31.2 ± 0.9 | 13.1 ± 0.9 |
| 16 | not organic | R | NA | 8.7 ± 0.7 | 25.3 ± 1.7 | 40.1 ± 1.1 | 16.8 ± 0.6 |
| 17 | organic | R | alkalized | 17.5 ± 1.5 | 20.1 ± 1.7 | 20.4 ± 1.0 | 8.6 ± 1.5 |
| 18 | not organic | R | alkalized | 10.2 ± 0.9 | 25.9 ± 1.0 | 18.7 ± 0.9 | 7.9 ± 1.0 |
| 19 | organic | R | alkalized | 8.1 ± 0.7 | 9.2 ± 0.2 | 8.9 ± 0.3 | 3.8 ± 0.4 |
| 20 | not organic | R | alkalized | 12.4 ± 1.1 | 22.3 ± 0.2 | 21.1 ± 0.4 | 8.9 ± 0.9 |
| 21 | not organic | R | alkalized | 8.7 ± 0.9 | 16.0 ± 0.1 | 10.5 ± 0.2 | 4.5 ± 0.9 |
| 22 | not organic | R | alkalized | 19.9 ± 1.8 | 14.0 ± 0.0 | 6.6 ± 0.1 | 2.8 ± 0.8 |
| 23 | not organic | R | alkalized | 20.5 ± 1.7 | 9.5 ± 0.0 | 8.6 ± 0.5 | 3.7 ± 0.4 |
| 24 | not organic | R | alkalized | 11.5 ± 1.0 | 8.8 ± 0.1 | 4.5 ± 0.2 | 2.0 ± 0.7 |
| 25 | not organic | R | NA | 22.1 ± 1.9 | 33.3 ± 0.0 | 38.2 ± 5.2 | 16.0 ± 1.3 |
| 26 | organic | raw | NA | 9.7 ± 0.8 | 43.3 ± 0.3 | 63.9 ± 1.7 | 26.7 ± 1.7 |
| 27 | organic | raw | NA | 20.9 ± 1.8 | 32.8 ± 0.6 | 38.1 ± 0.9 | 15.9 ± 1.1 |
| 28 | not organic | R | NA | 9.0 ± 0.8 | 37.6 ± 2.7 | 48.9 ± 2.2 | 20.5 ± 2.3 |
| 29 | not organic | R | NA | 20.7 ± 1.8 | 27.3 ± 0.4 | 32.2 ± 0.3 | 13.5 ± 1.3 |
| 30 | not organic | R | alkalized | 7.9 ± 0.7 | 3.6 ± 0.4 | 13.5 ± 0.1 | 5.7± 0.8 |
| 31 | not organic | R | alkalized | 7.5 ± 0.6 | 16.9 ± 0.0 | 9.9± 0.4 | 4.2 ± 0.6 |
| 32 | not organic | R | NA | 9.3 ± 0.8 | 43.4 ± 1.2 | 49.2 ± 0.1 | 20.6 ± 0.8 |
| 33 | not organic | R | NA | 10.3 ± 0.9 | 44.2 ± 1.6 | 48.0 ± 1.1 | 20.1 ± 0.2 |
| 34 | not organic | R | alkalized | 6.4 ± 0.5 | 33.2 ± 0.0 | 12.4 ± 0.0 | 5.2 ± 0.1 |
| 35 | not organic | R | NA | 17.9 ± 1.5 | 39.9 ± 0.9 | 38.3 ± 1.9 | 16.0 ± 0.5 |
| 36 | organic | R | alkalized | 9.6 ± 0.8 | 45.2 ± 2.1 | 59.4 ± 1.4 | 24.8± 1.6 |
| 37 | organic | R | alkalized | 15.9 ± 1.4 | 49.8 ± 2.0 | 45.7 ± 0.4 | 19.1 ± 1.2 |
| 38 | not organic | R | NA | 9.5 ± 0.8 | 40.2 ± 1.9 | 50.2 ± 0.4 | 21.0 ± 1.8 |
| 39 | not organic | R | NA | 20.7 ± 1.8 | 67.6 ± 0.5 | 49.7 ± 5.2 | 20.8 ± 0.7 |
| 40 | not organic | R | alkalized | 8.0 ± 0.9 | 21.1 ± 0.2 | 7.2 ± 0.3 | 3.1 ± 0.6 |
| 41 | not organic | R | alkalized | 19.3 ± 1.6 | 22.5 ± 0.2 | 16.4 ± 0.2 | 6.9 ± 0.1 |
| 42 | not organic | R | alkalized | 8.9 ± 0.8 | 17.1 ± 0.1 | 9.6 ± 0.3 | 4.1 ± 0.3 |
| 43 | not organic | R | alkalized | 8.2 ± 0.6 | 30.9 ± 2.0 | 14.8 ± 1.0 | 6.2 ± 0.9 |
| 44 | not organic | R | alkalized | 7.6 ± 0.6 | 15.3 ± 0.2 | 10.9 ± 0.1 | 4.6 ± 0.6 |
| 45 | organic | R | NA | 9.3 ± 0.8 | 51.8 ± 1.5 | 55.5 ± 2.5 | 23.2 ± 0.2 |
| 46 | organic | R | alkalized | 7.3 ± 0.6 | 25.6 ± 1.9 | 35.5 ± 0.7 | 14.9 ± 0.5 |
| 47 | not organic | R | alkalized | 21.0 ± 1.8 | 26.0 ± 0.5 | 21.8 ± 1.8 | 9.2 ± 1.1 |
| 48 | organic | R | alkalized | 16.6 ± 1.4 | 35.0 ± 1.2 | 30.7 ± 0.4 | 12.9 ± 0.3 |
| 49 | not organic | R | NA | 27.3 ± 2.3 | 56.8 ± 2.0 | 63.8 ± 0.4 | 26.7 ± 2.1 |
| 50 | organic | R | alkalized | 17.5 ± 1.7 | 26.6 ± 2.6 | 14.6 ± 0.4 | 6.2 ± 1.2 |
| 51 | organic | R | alkalized | 17.4 ± 1.4 | 65.4 ± 1.8 | 58.2 ± 0.2 | 24.3 ± 1.6 |
| 52 | not organic | R | alkalized | 18.8 ± 1.7 | 14.9 ± 0.2 | 8.8 ± 0.1 | 3.7 ± 0.3 |
| 53 | organic | raw | alkalized | 17.1 ± 1.3 | 25.2 ± 1.5 | 13.3 ± 0.6 | 5.6 ± 0.8 |
| 54 | not organic | R | NA | 22.1 ± 1.9 | 46.2 ± 0.5 | 45.9 ± 0.3 | 19.2 ± 1.5 |
| 55 | not organic | R | NA | 26.1 ± 2.2 | 64.3 ± 0.7 | 59.7 ± 0.3 | 24.9 ± 0.7 |
| 56 | not organic | R | NA | 23.5 ± 2.0 | 37.0 ± 0.4 | 42.5 ± 2.4 | 17.8 ± 0.8 |
| 57 | not organic | R | NA | 18.9 ± 1.6 | 28.7 ± 1.1 | 63.6 ± 1.6 | 26.6 ± 2.1 |
| 58 | not organic | raw | NA | 16.2 ± 1.4 | 69.7 ± 0.1 | 71.5 ± 1.2 | 29.8 ± 2.1 |
| 59 | not organic | raw | NA | 11.2 ± 1.0 | 50.4 ± 0.5 | 68.8 ± 2.0 | 28.7 ± 0.8 |
| Phenolic compounds (mg GAEeq g-1DDW) |
||
|---|---|---|
| TPC | nSensor | |
| conventional (N. 41) | 42.18±27.45 | 18.13±12.57 |
| (3.94-149.2) | (2.21-60.47) | |
| organic (N. 18) | 45.39±22.27 | 19.21±10.51 |
| (9.97-84.71) | (4.11-38.86) | |
| p-value | 0.467 | 0.477 |
| not raw (N. 50) | 38.61±21.19 | 16.20±9.91 |
| (3.94-86.99) | (2.21-36.69) | |
| raw (N. 9) | 68.44±35.19 | 31.03±14.64 |
| (30.40-149.2) | (6.77-60.47) | |
| p-value | 0.003 | 0.003 |
| not alkalized (N. 30) | 57.48±25.38 | 26.57±9.32 |
| (23.67-149.2) | (14.65-60.47) | |
| alkalized (N. 29) | 28.35±16.38 | 10.07±7.73 |
| (3.94-79.23) | (2.21-29.44) | |
| p-value | <0.001 | <0.001 |
| ETH | DOP | SER | HIS | SPD | SAL | SPM | total BAs | |
|---|---|---|---|---|---|---|---|---|
| N of positive (%) | 9 (15%) | 3 (5%) | 13 (22%) | 11 (19%) | 5 (8%) | 4 (7%) | 3 (5%) | 18 (31%) |
| BAs (mg kg-1 DDW) | ||||||||
| Median | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| median (of positive samples) |
22.89 | 88.32 | 82.13 | 69.70 | 23.88 | 0.22 | 25.35 | 154.08 |
| Mean | 6.11 | 5.80 | 18.53 | 11.40 | 3.53 | 0.06 | 1.46 | 46.89 |
| dev st | 20.23 | 27.23 | 38.97 | 28.21 | 15.92 | 0.31 | 6.84 | 95.07 |
| Minimum | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Maximum | 91.54 | 176.95 | 181.81 | 139.28 | 105.96 | 1.86 | 43.66 | 480.87 |
| ETH | DOP | SER | HIS | SPD | SAL | SPM | total BAs | |
|---|---|---|---|---|---|---|---|---|
| conventional (N. 41) | 7.15±22.05 | 8.34±32.46 | 23.54±43.42 | 15.75±32.76 | 5.08±18.96 | 0.09±0.37 | 2.10±8.15 | 62.04±107.95 |
| (0.00-91.54) | (0.00-176.95) | (0.00-181.81) | (0.00-139.28) | (0.00-105.96) | (0.00-1.86) | (0.00-43.66) | (0.00-480.87) | |
| organic (N. 18) | 3.76±15.61 | 0.00±0.00 | 7.14±23.40 | 1.49±6.33 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 12.39±39.99 |
| (0.00-66.28) | (0.00-0.00) | (0.00-95.62) | (0.00-26.84) | (0.00-0.00) | (0.00-0.00) | (0.00-0.00) | (0.00-161.90) | |
| p-value | 0.464 | 0.455 | 0.573 | 0.158 | 0.199 | 0.107 | 0.174 | 0.832 |
| not raw (N. 50) | 7.21±21.82 | 6.84±29.51 | 21.87±41.51 | 13.45±30.23 | 4.16±17.24 | 0.07±0.34 | 1.72±7.41 | 55.33±101.10 |
| (0.00-91.54) | (0.00-176.95) | (0.00-181.81) | (0.00-139.28) | (0.00-105.96) | (0.00-1.86) | (0.00-43.66) | (0.00-480.87) | |
| raw (N. 9) | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 |
| (0.00-0.00) | (0.00-0.00) | (0.00-0.00) | (0.00-0.00) | (0.00-0.00) | (0.00-0.00) | (0.00-0.00) | (0.00-0.00) | |
| p-value | 0.246 | 0.152 | 0.372 | 0.174 | 0.461 | 0.838 | 0.178 | 0.159 |
| not alkalized (N. 30) | 3.82±13.39 | 8.84±35.61 | 19.93±44.54 | 15.70±29.40 | 4.42±19.67 | 0.12±0.43 | 2.87±9.46 | 55.71±108.08 |
| (0.00-69.61) | (0.00-176.95) | (0.00-181.81) | (0.00-86.25) | (0.00-105.96) | (0.00-1.86) | (0.00-43.66) | (0.00-480.87) | |
| alkalized (N. 29) | 8.48±25.50 | 2.65±14.25 | 17.08±32.96 | 6.95±26.71 | 2.61±11.08 | 0.00±0.02 | 0.00±0.00 | 37.77±80.33 |
| (0.00-91.54) | (0.00-76.73) | (0.00-107.60) | (0.00-139.28) | (0.00-57.48) | (0.00-0.08) | (0.00-0.02) | (0.00-296.41) | |
| p-value | 0.399 | 0.781 | 0.425 | 0.161 | 0.686 | 0.171 | 0.175 | 0.380 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





