Submitted:
01 July 2024
Posted:
01 July 2024
You are already at the latest version
Abstract
Keywords:
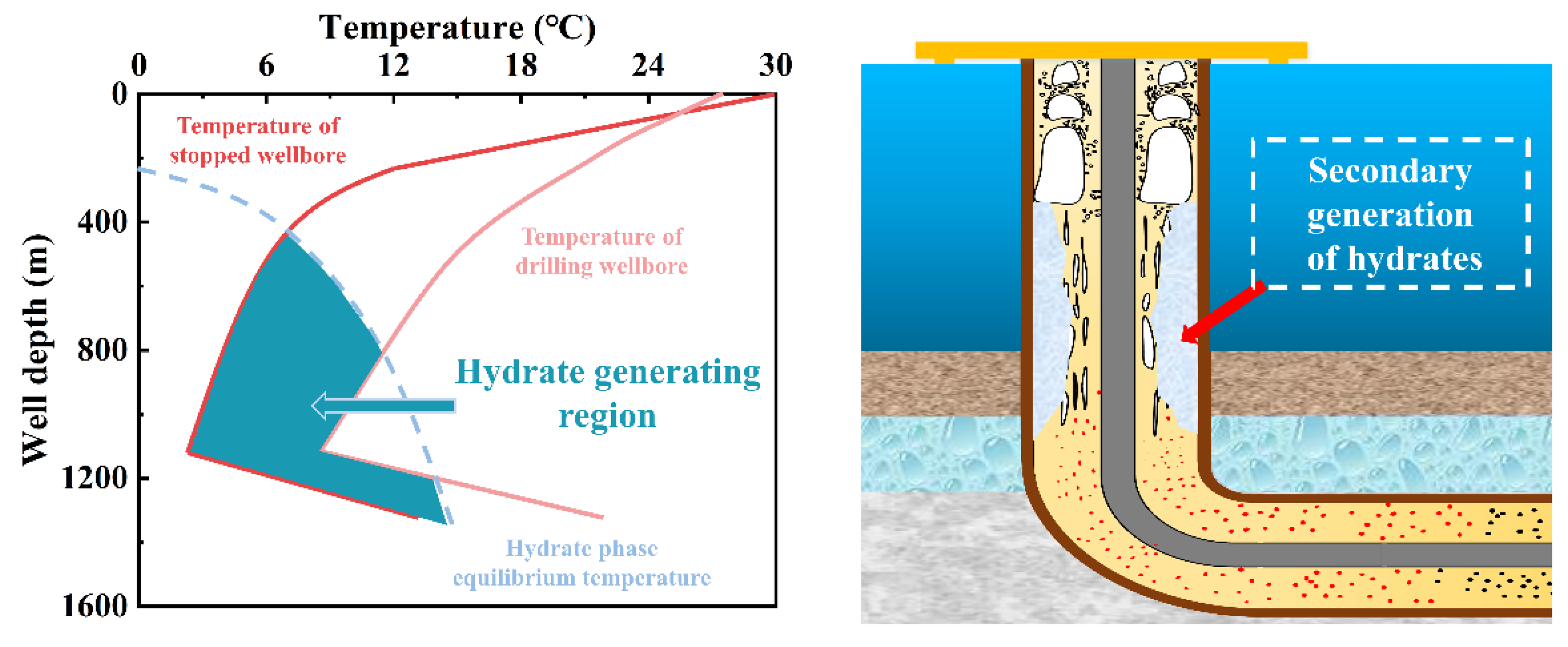
1. Introduction
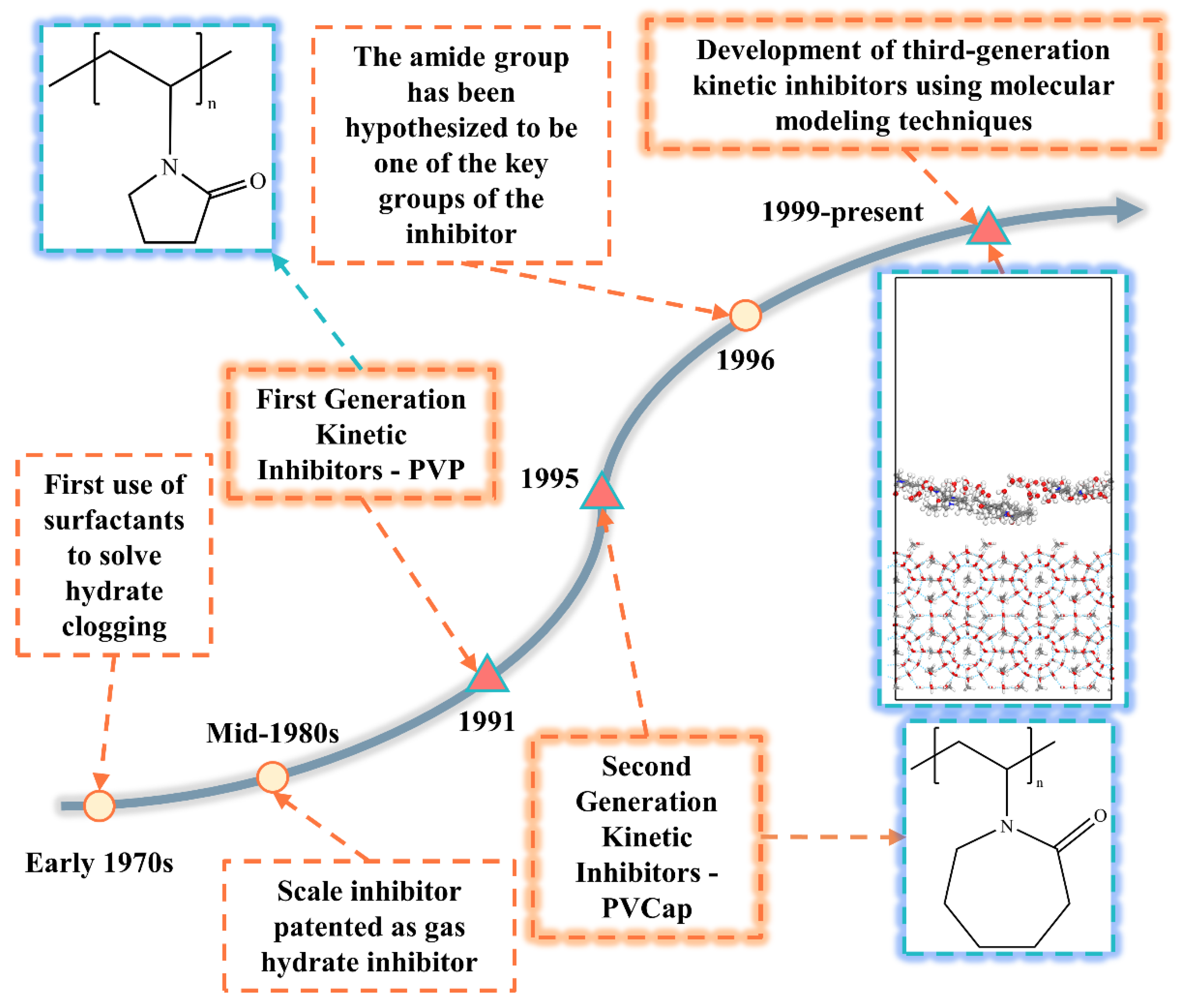
2. Natural Gas Hydrate Kinetic Inhibitors Research Development History
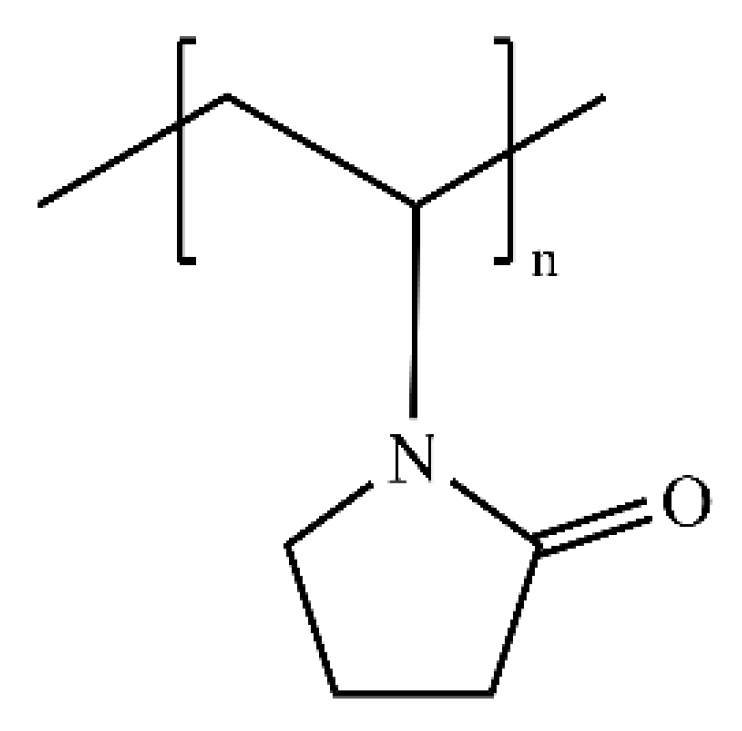
2.1. First-Generation Kinetic Inhibitors
2.2. Second-Generation Kinetic Inhibitors
2.3. Third-Generation Kinetic Inhibitors
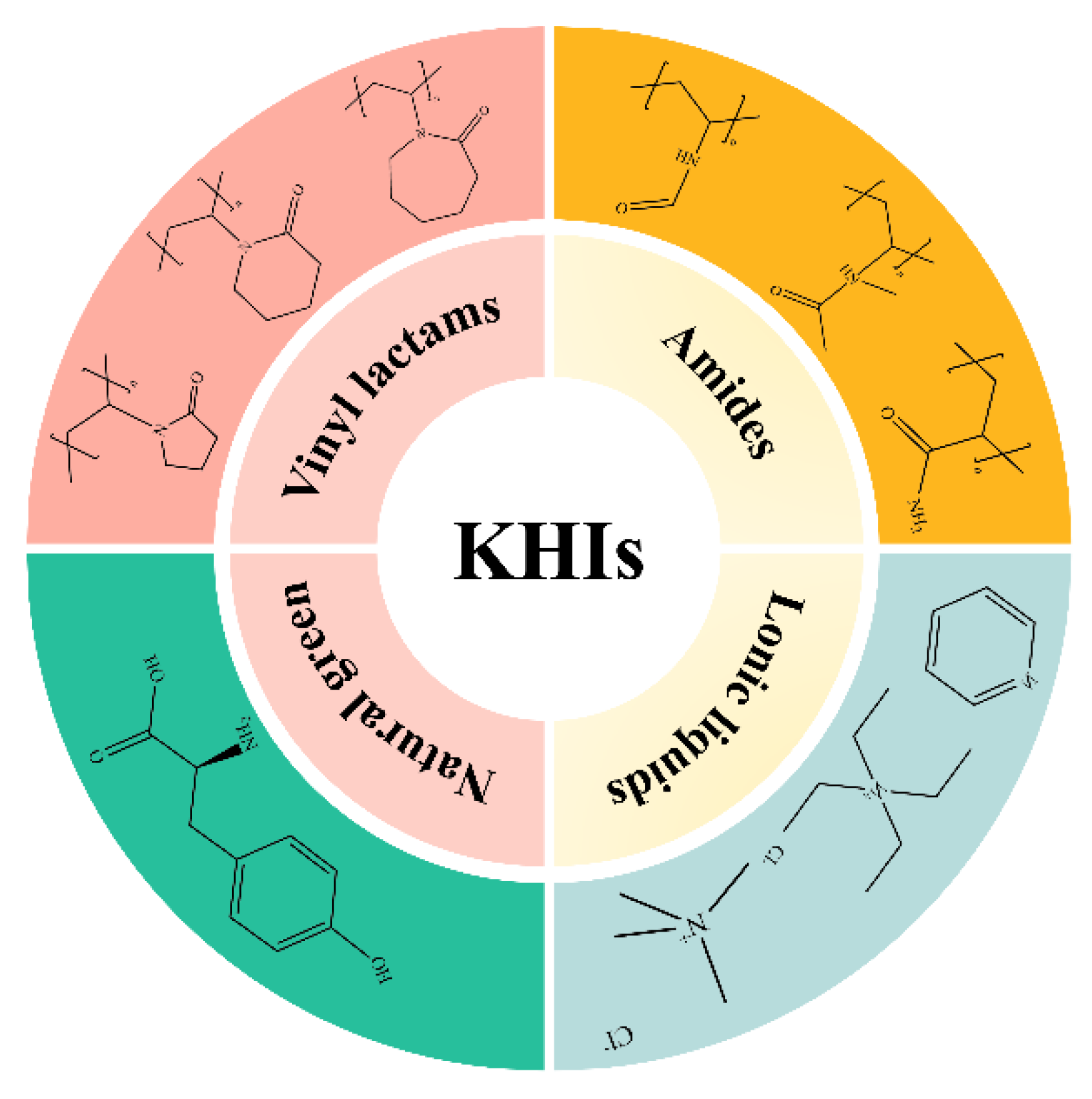
3. Structural Composition Classification of Natural Gas Hydrate Kinetic Inhibitors
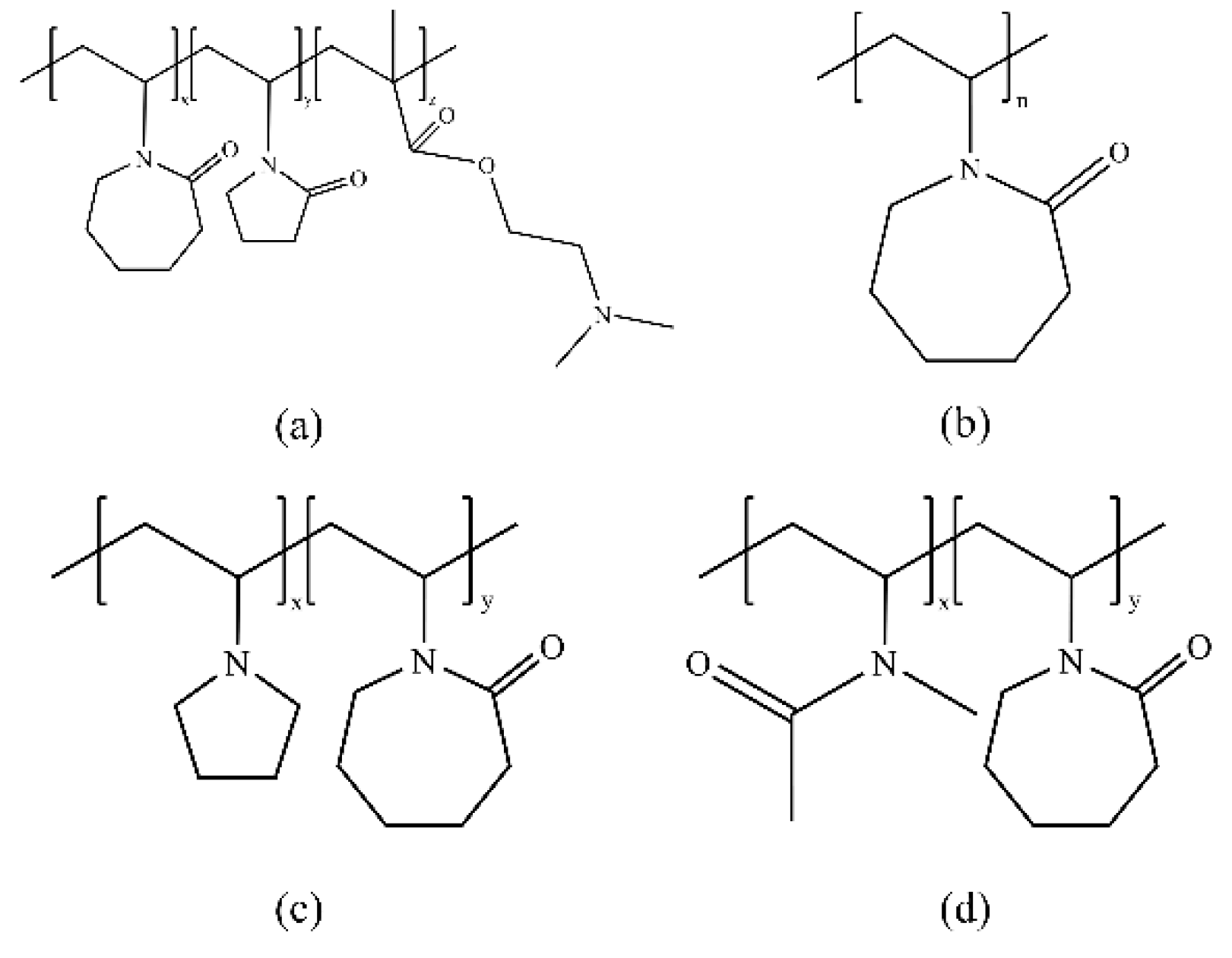
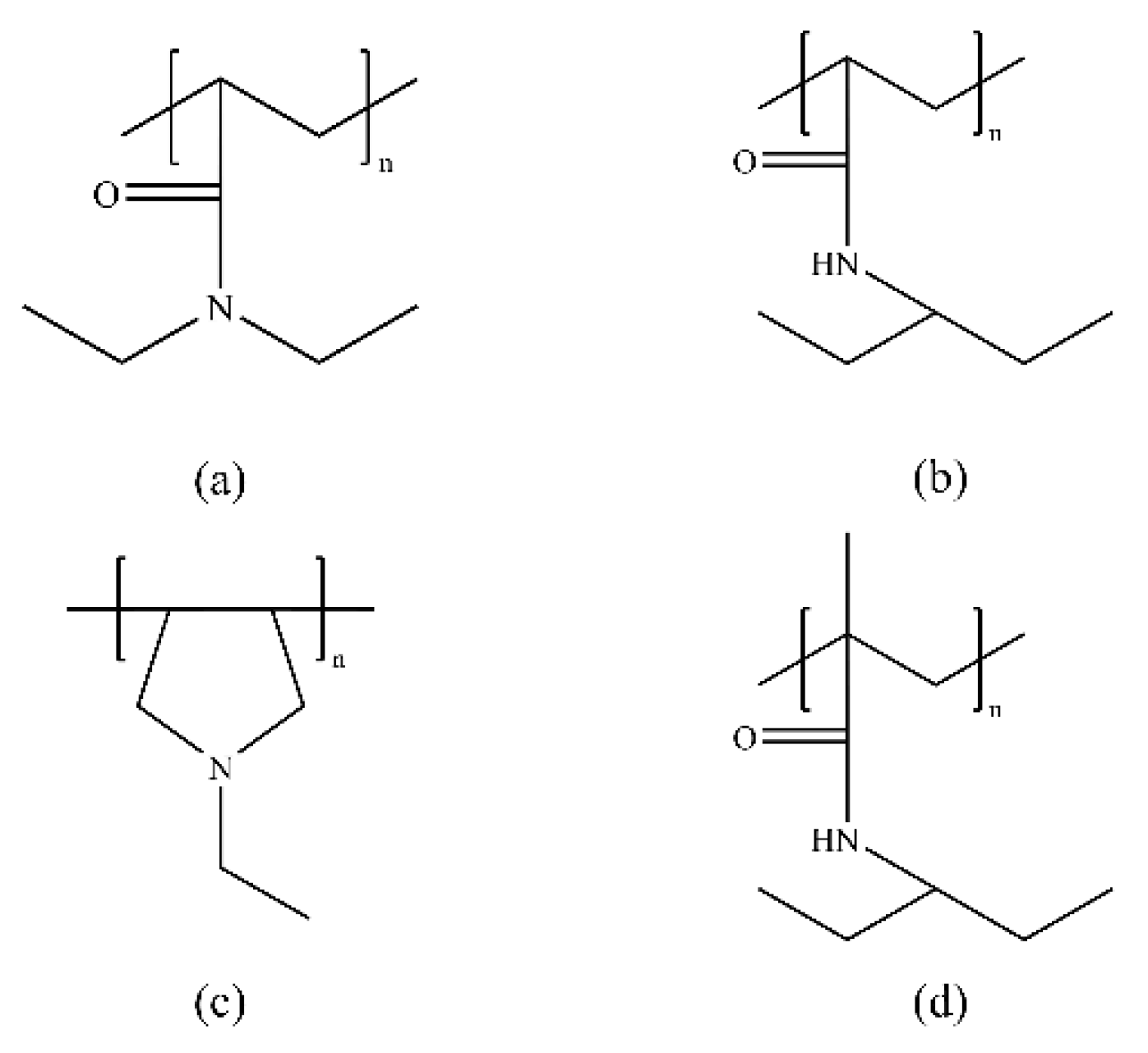
3.1. Vinyl Lactam Polymer Inhibitors
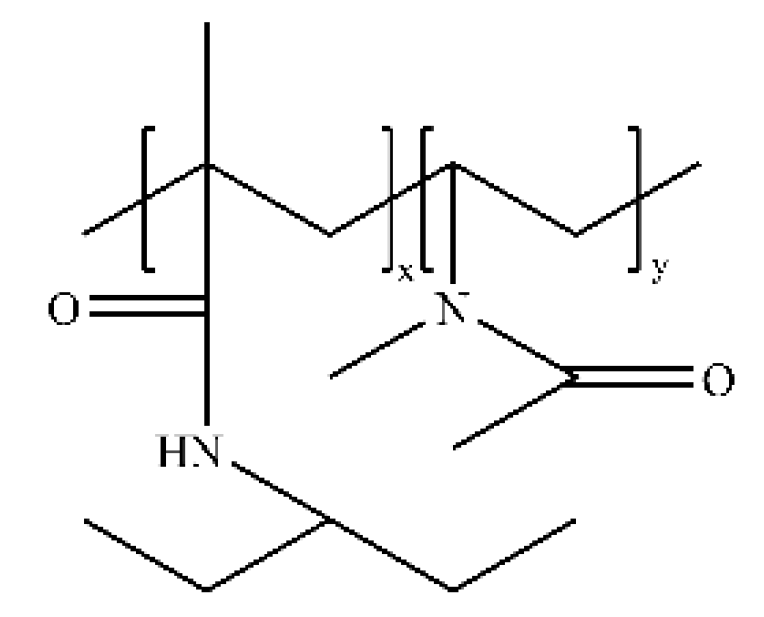
3.2. Amide Polymer Inhibitors
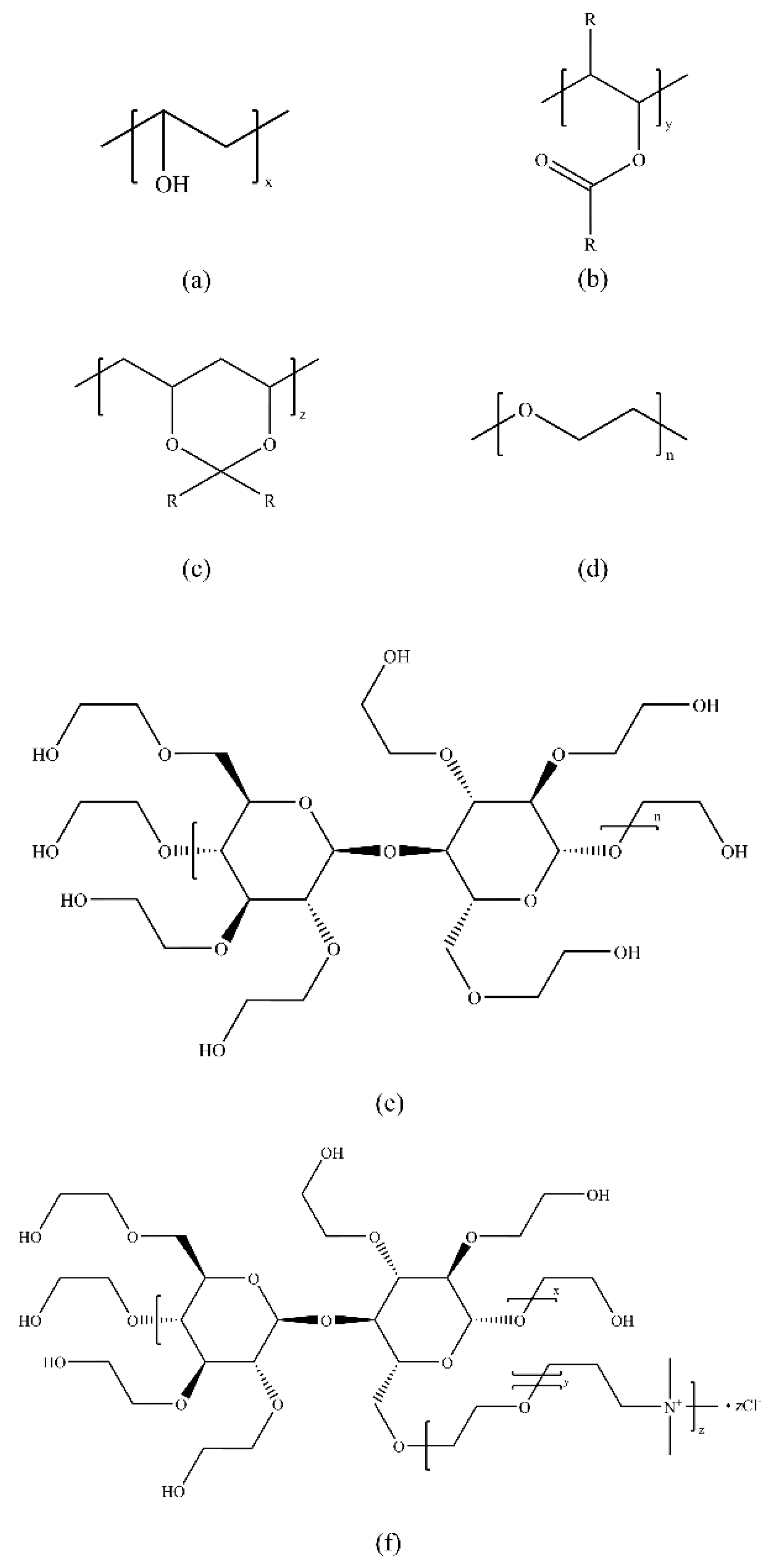
3.3. Natural Green Inhibitors
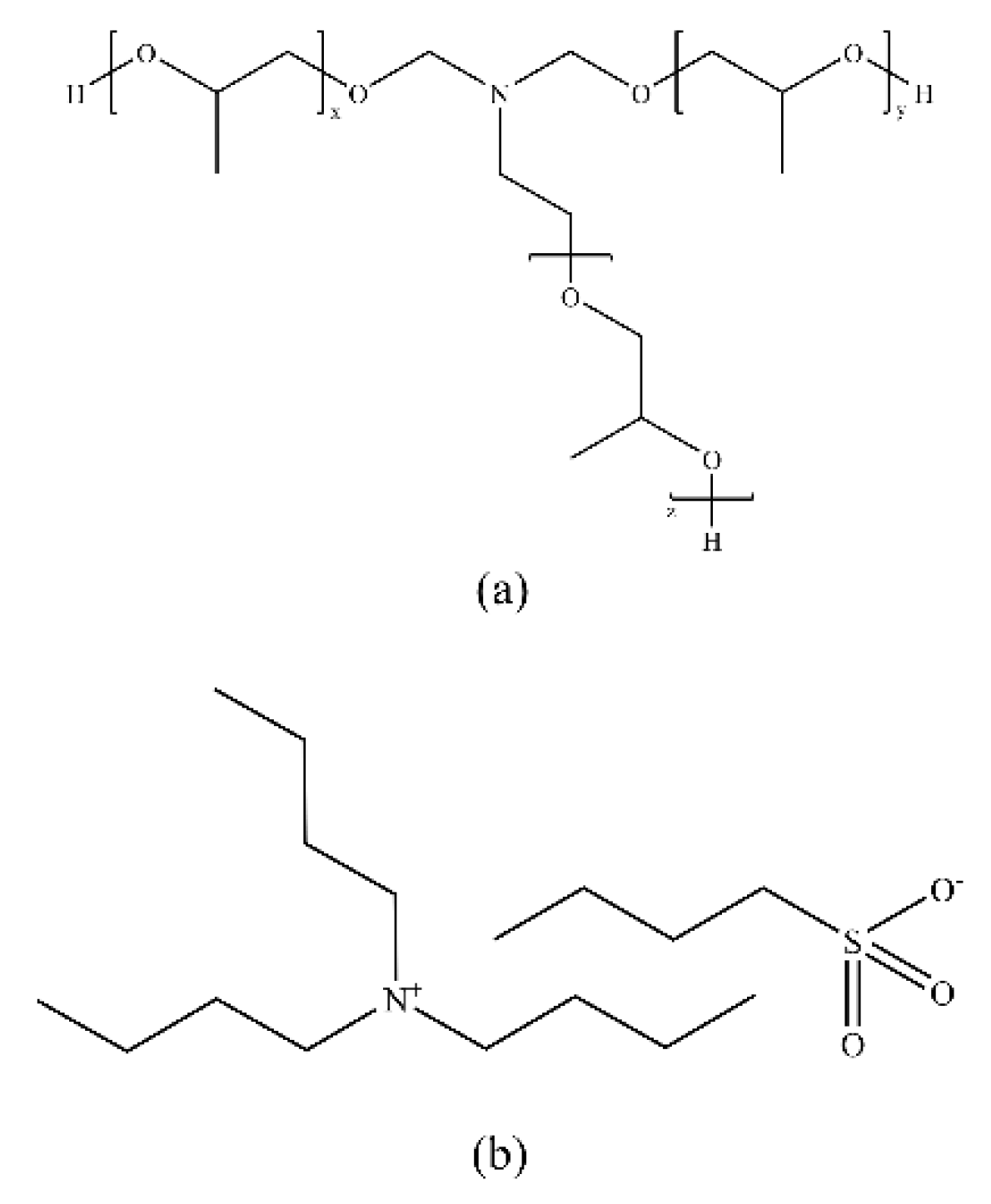
3.4. Ionic Liquid-Based Inhibitors
4. Mechanism of Inhibition of Hydrate Generation by Kinetic Inhibitors of Natural Gas Hydrates
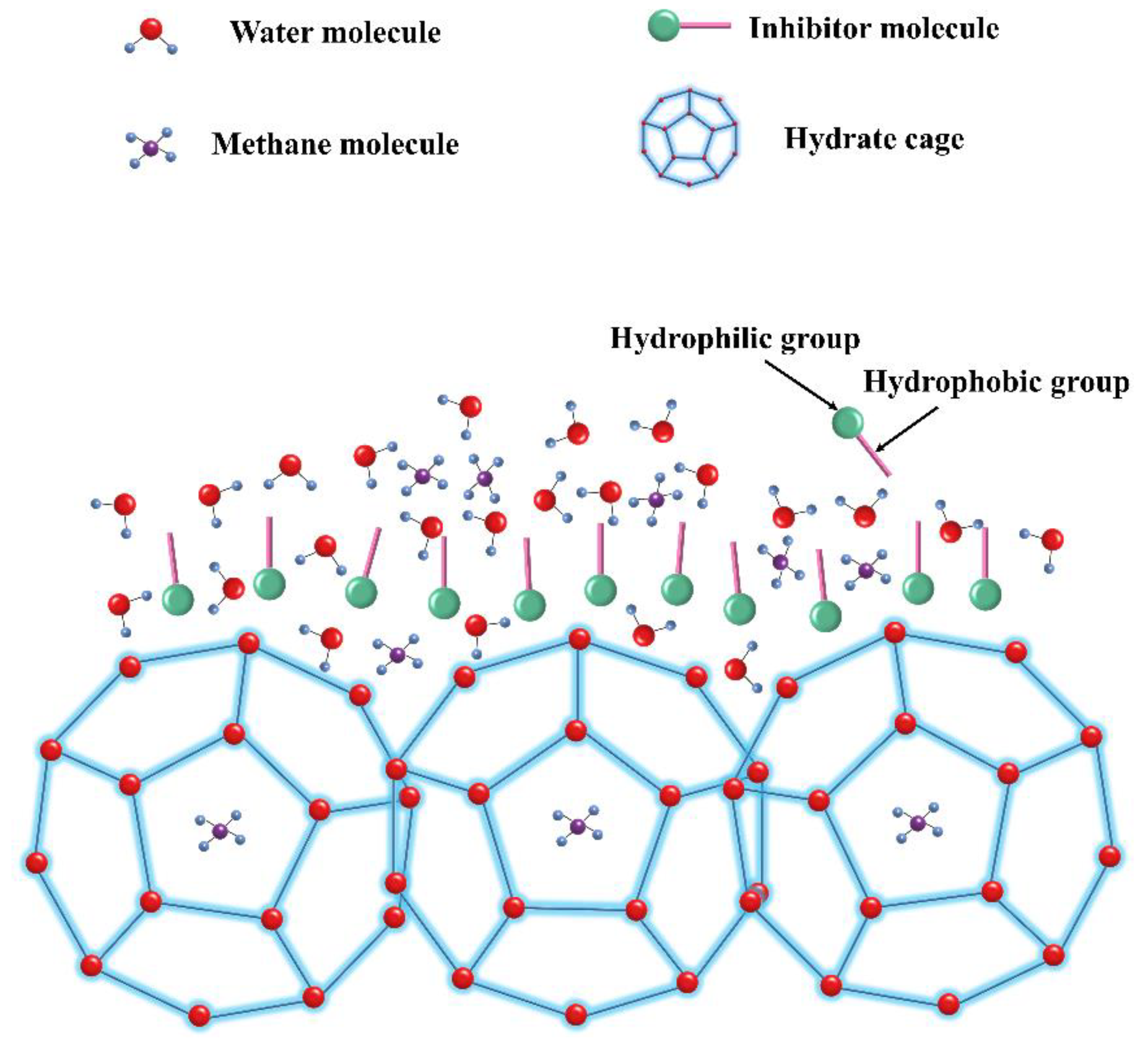
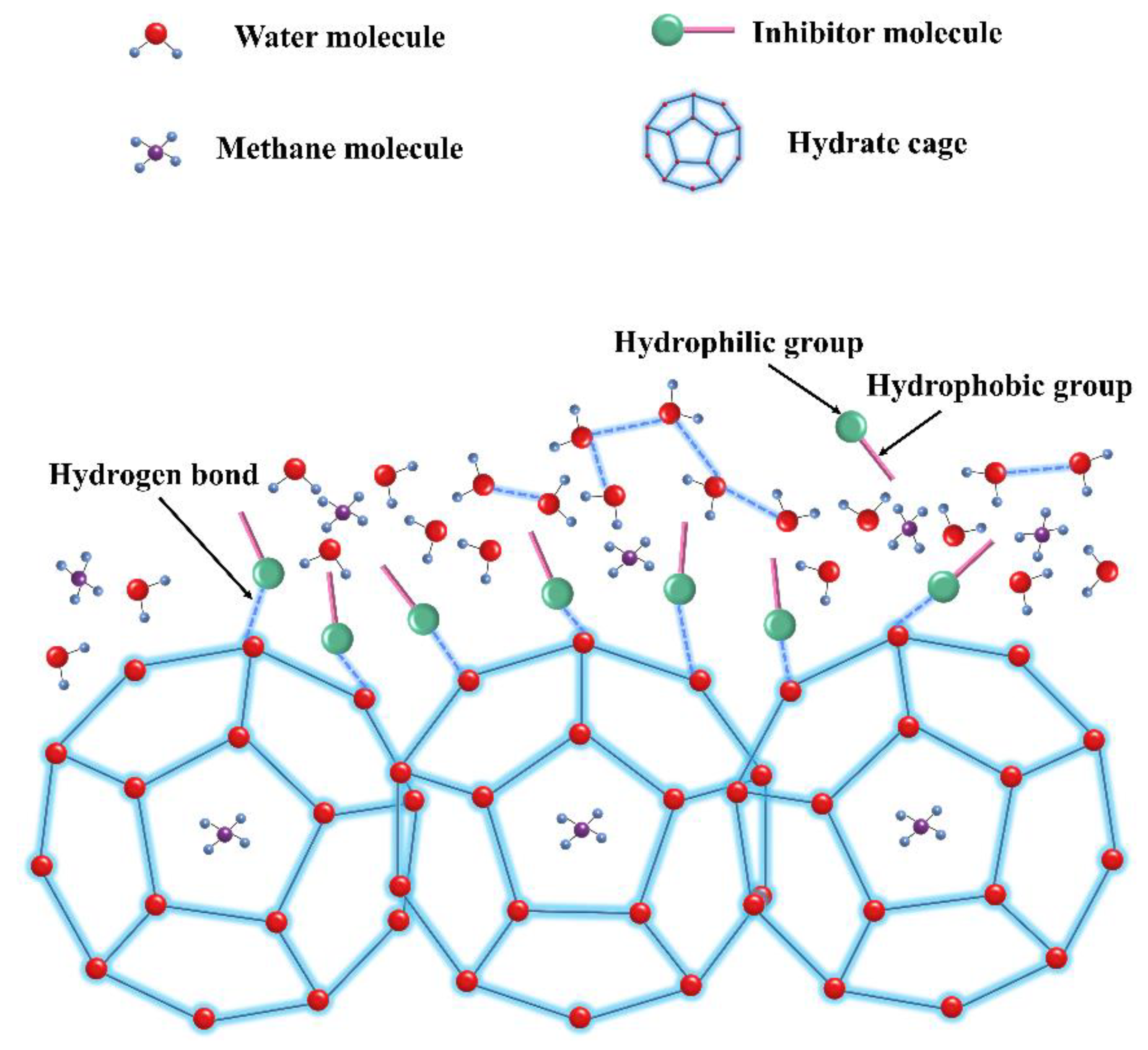
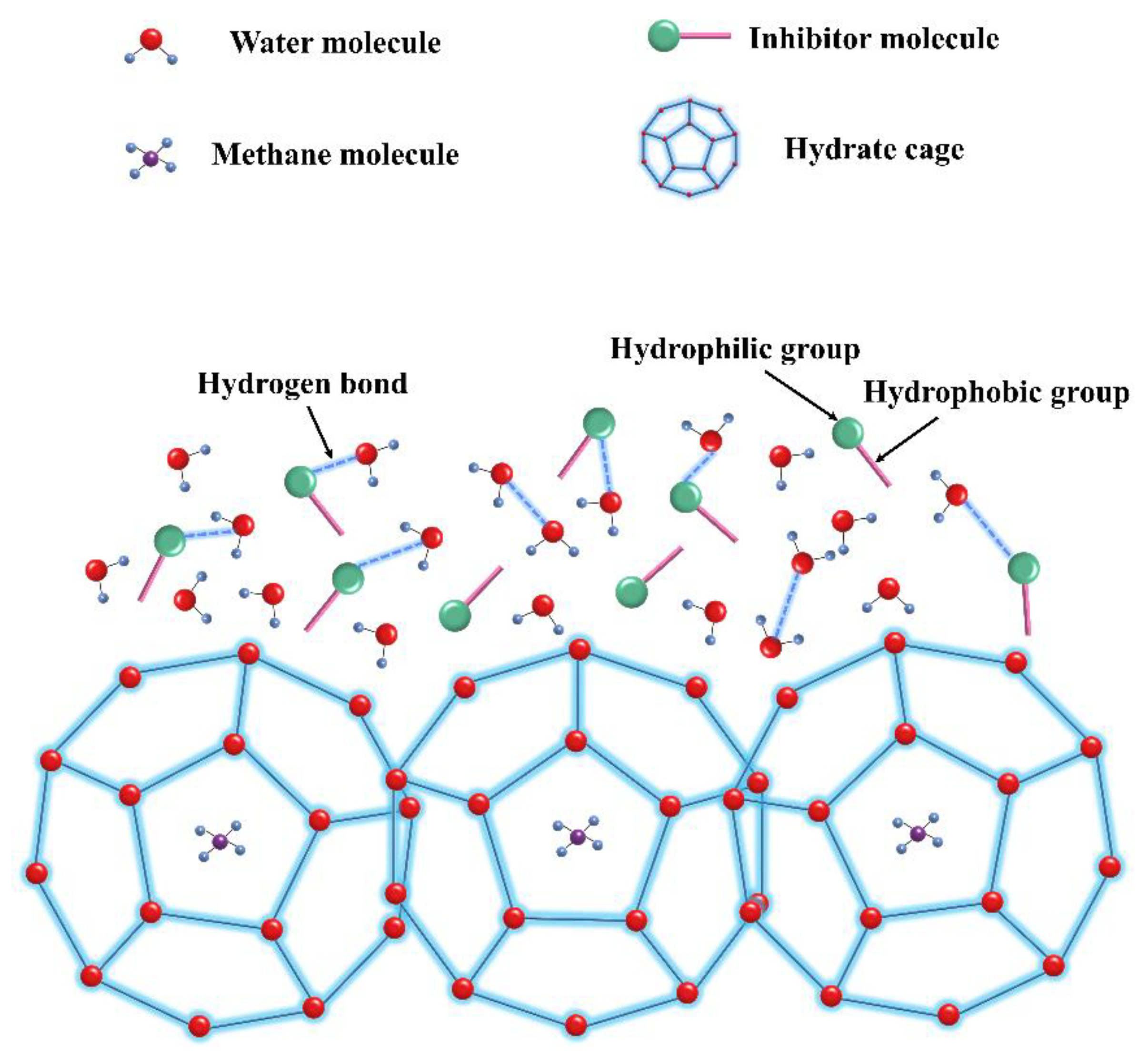
4.1. Perturbation Suppression Mechanism
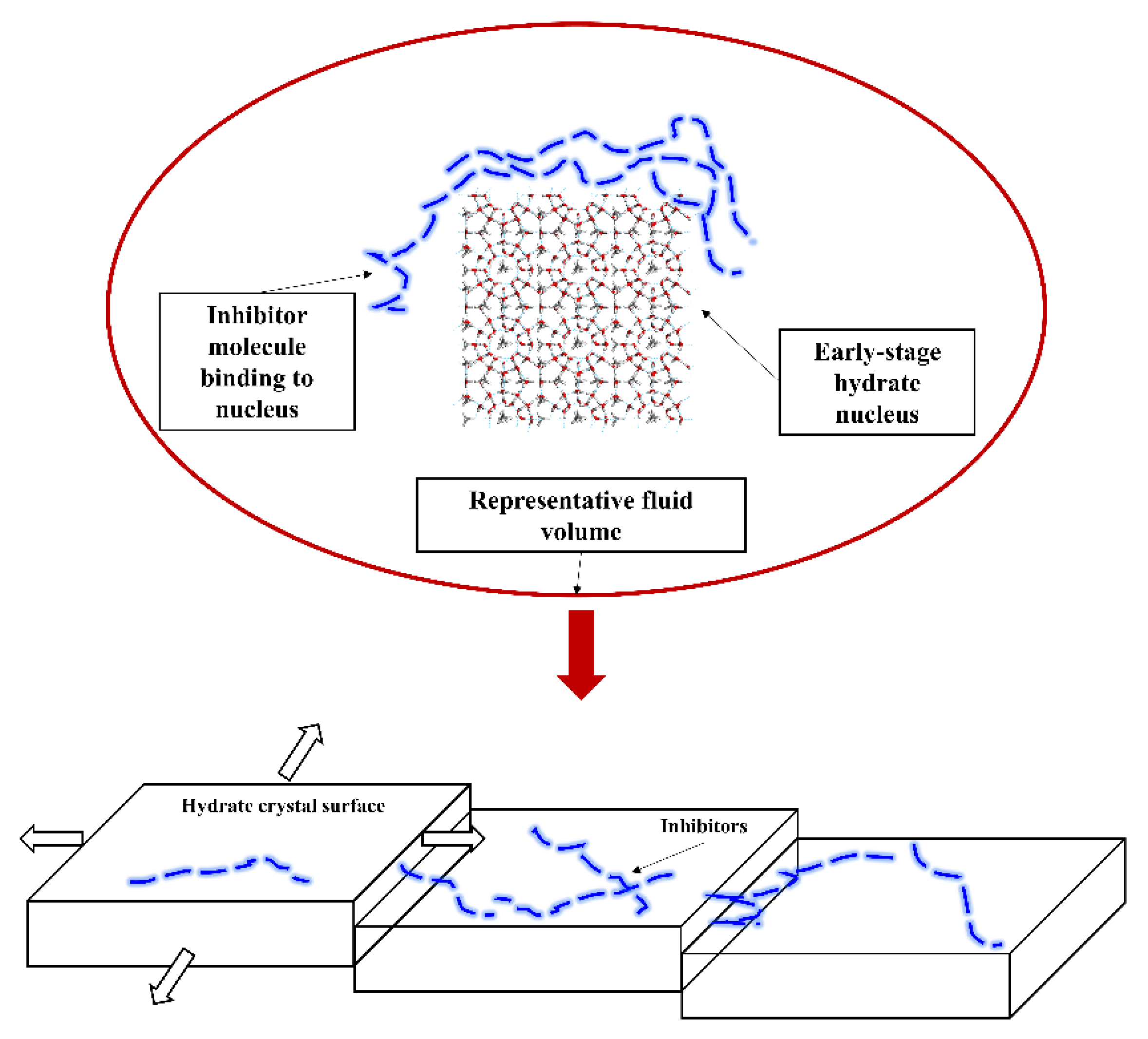
4.2. Layer Mass Transfer Obstruction Mechanism
4.3. Adsorption and Spatial Obstruction Mechanism
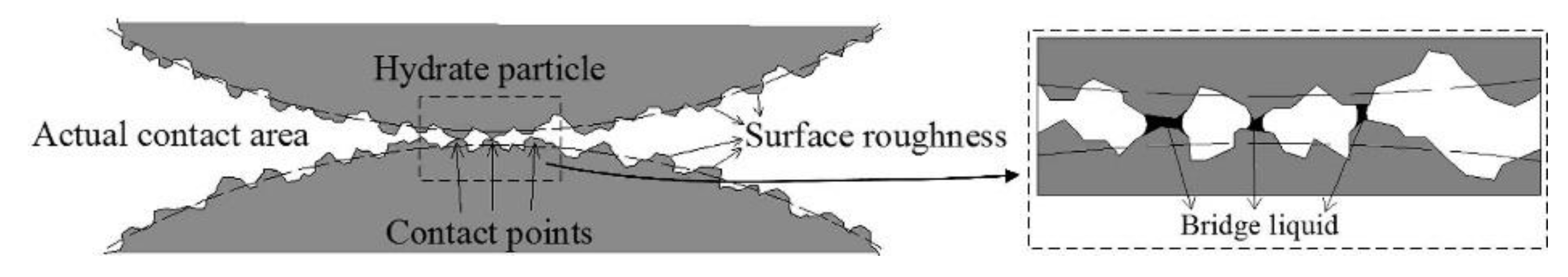
4.4. Particle Adhesion Mechanism
4.5. Other Mechanism
5. Conclusions and Outlook
Data availability
Declaration of Competing Interest
Acknowledgments
References
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef]
- Sun, C.; Li, W.; Yang, X.; Li, F.; Yuan, Q.; Mu, L.; Chen, J.; Liu, B.; Chen, G. Progress in Research of Gas Hydrate. Chin. J. Chem. Eng. 2011, 19, 151–162. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, N.; Liu, C.; Hao, X.; Zhang, Y.; Gao, K.; Peng, B.; Zheng, C.; Tang, W.; Guo, G. Molecular simulation studies on natural gas hydrates nucleation and growth: A review. China Geol. 2022, 5, 330–344. [Google Scholar] [CrossRef]
- Lei, Q.; Wang, H.; Zhao, Q.; Liu, D. Status analysis and advices on exploration and development of unconventional hydrocarbon resources. Nat. Gas Ind. 2008, 28, 7–10. [Google Scholar]
- Zhou, S.; Li, Q.; Lyu, X.; Pang, W.; Fu, Q. Thinking and suggestions on research direction of natural gas hydrate development. China Offshore Oil Gas 2019, 31, 1–8. [Google Scholar]
- Liao, B.; Wang, J.; Han, X.; Wang, R.; Lv, K.; Bai, Y.; Jiang, H.; Shao, Z.; Wang, Y.; Sun, J. Microscopic molecular insights into clathrate methane hydrates dissociation in a flowing system. Chem. Eng. J. 2022, 430, 133098. [Google Scholar] [CrossRef]
- Liao, B.; Wang, J.; Sun, J.; Lv, K.; Liu, L.; Wang, Q.; Wang, R.; Lv, X.; Wang, Y.; Chen, Z. Microscopic insights into synergism effect of different hydrate inhibitors on methane hydrate formation: Experiments and molecular dynamics simulations. Fuel 2023, 340, 127488. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Liao, B.; Bai, Y.; Li, W.; Xu, J.; Wang, J. Development and performance evaluation of bioenzyme-responsive temporary plugging materials. Adv. Geo-Energy Res. 2024, 11, 20–28. [Google Scholar] [CrossRef]
- Wang, J.; Liao, B.; Liu, L.; Chen, L.; Huang, Y.; Zhao, K.; Sun, X.; Lv, K.; Zheng, Y.; Sun, J. The effect of multi-component Inhibitor systems on hydrate formation. Gas Sci. Eng. 2024, 122, 205214. [Google Scholar] [CrossRef]
- Wang, J.; He, Z.; Yan, Y.; Liu, L.; Yan, J.; Liao, B.; Zhao, K.; Li, Y.; Chen, L. Development of a dual-functional inhibitor for natural gas hydrates and construction of drilling fluid system. Gas Sci. Eng. 2024, 122, 205218. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Liu, J.-W.; Li, X.-S. Recent Advances on Natural Gas Hydrate Exploration and Development in the South China Sea. Energy Fuels 2021, 35, 7528–7552. [Google Scholar] [CrossRef]
- Liu, L.; Sun, Z.; Zhang, L.; Wu, N.; Yichao, Q.; Jiang, Z.; Geng, W.; Cao, H.; Zhang, X.; Zhai, B.; et al. Progress in Global Gas Hydrate Development and Production as a New Energy Resource. Acta Geol. Sin. - Engl. Ed. 2019, 93, 731–755. [Google Scholar] [CrossRef]
- Stewart, D. Well engineering concepts to make methane gas hydrate exploitation affordable. 2011.
- Max, M.D.; Johnson, A.H. Energy Resource Risk Factors. In Exploration and Production of Oceanic Natural Gas Hydrate: Critical Factors for Commercialization; Max, M.D., Johnson, A.H., Eds.; Springer International Publishing: Cham, 2019; pp. 347–417. ISBN 978-3-030-00401-9. [Google Scholar]
- Liu, Z.; Li, Y.; Wang, W.; Song, G.; Lu, Z.; Ning, Y. Wax and Wax–Hydrate Deposition Characteristics in Single-, Two-, and Three-Phase Pipelines: A Review. Energy Fuels 2020, 34, 13350–13368. [Google Scholar] [CrossRef]
- Yu, Y.-S.; Zhang, X.; Liu, J.-W.; Lee, Y.; Li, X.-S. Natural gas hydrate resources and hydrate technologies: a review and analysis of the associated energy and global warming challenges. Energy Environ. Sci. 2021, 14, 5611–5668. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, X. Current status and developing trend of deepwater drilling fluid technology. Spec. Oil Gas Reserv. 2013, 20, 1–7. [Google Scholar]
- Liu, T.; Hu, Y.; Gao, M.; Yi, D.; Bao, W.; Li, H. Evaluation and application of a combined natural gas hydrate inhibitor. Chem. Eng. Oil Gas 2019, 48, 39–41. [Google Scholar]
- Rossi, F.; Gambelli, A.M. Thermodynamic phase equilibrium of single-guest hydrate and formation data of hydrate in presence of chemical additives: a review. Fluid Phase Equilibria 2021, 536, 112958. [Google Scholar] [CrossRef]
- Masoudi, R.; Tohidi, B.; Danesh, A.; Todd, A.C.; Anderson, R.; Burgass, R.W.; Yang, J. Measurement and prediction of gas hydrate and hydrated salt equilibria in aqueous ethylene glycol and electrolyte solutions. Chem. Eng. Sci. 2005, 60, 4213–4224. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; Almomani, F.; Al-Ghouti, M.A.; Hassan, M.K.; Al-Muhtaseb, A. Towards Gas Hydrate-Free Pipelines: A Comprehensive Review of Gas Hydrate Inhibition Techniques. Energies 2022, 15, 8551. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/ inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Shen, Y.; Cheng, L.; Liu, B.; Yan, K.; Chen, G.; Li, T. Molecular dynamics simulation to explore the synergistic inhibition effect of kinetic and thermodynamic hydrate inhibitors. Energy 2022, 238, 121697. [Google Scholar] [CrossRef]
- Yang, C.; Zi, M.; Wu, G.; Zou, X.; Liu, K.; Chen, D. Concentration effect of kinetic hydrate inhibitor on hydrate formation and inhibition. Fuel 2022, 323, 124448. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, C.V.; Nguyen, T.A.H.; Nguyen, A.V. Surface Science in the Research and Development of Hydrate-Based Sustainable Technologies. ACS Sustain. Chem. Eng. 2022, 10, 4041–4058. [Google Scholar] [CrossRef]
- Wilson, I.; Saini, S.; Sreenivasan, H.; Sahu, C.; Krishna, S.; Gupta, P. Review and Perspectives of Energy-Efficient Methane Production from Natural Gas Hydrate Reservoirs Using Carbon Dioxide Exchange Technology. Energy Fuels 2023, 37, 9841–9872. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; Aman, Z.M. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Guo, K.; Wang, Y.; Lang, X. Present situation and prospect of performance evaluation methods for kinetic hydrate inhibitors (KHIs). Nat. Gas Ind. 2018, 38, 103–113. [Google Scholar]
- Zhou, S.; Li, Q.; Li, L.; Yu, X. Synthesis and properties of novel kinetic inhibit ors for natural gas hydrate. Petrochem. Technol. 2017, 46, 467–470. [Google Scholar]
- Zhao, K.; Liu, Y.; Zhang, P.; Han, Q.; Jiang, H. Synthesis and performance of new hydrate kinetic inhibitors. Nat. Gas Chem. Ind. 2013, 38, 51–55. [Google Scholar]
- Kelland, M.A. History of the development of low dosage hydrate inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; da Silveira, K.C.; Sheng, Q.; Postma, A.; Wood, C.D.; Seo, Y. Experimental evaluation of RAFT-based Poly(N-isopropylacrylamide) (PNIPAM) kinetic hydrate inhibitors. Fuel 2019, 235, 1266–1274. [Google Scholar] [CrossRef]
- Gnezdilov, D.; Varfolomeev, M.; Farhadian, A.; Pavelyev, R.; Semenov, M.; Chirkova, Y.; Nazarychev, S.; Balachina, E.; Semenov, A.; Stoporev, A. Effective prevention of structure II gas hydrate formation using the newly synthesized kinetic inhibitors. Chem. Eng. Sci. 2024, 292, 119986. [Google Scholar] [CrossRef]
- Yaqub, S.; Murtaza, M.; Lal, B. Towards a fundamental understanding of biopolymers and their role in gas hydrates: A review. J. Nat. Gas Sci. Eng. 2021, 91, 103892. [Google Scholar] [CrossRef]
- Storr, M.T.; Taylor, P.C.; Monfort, J.-P.; Rodger, P.M. Kinetic Inhibitor of Hydrate Crystallization. J. Am. Chem. Soc. 2004, 126, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Suri, A. Review of kinetic hydrate inhibitors based on cyclic amides and effect of various synergists. Energy Fuels 2021, 35, 15301–15338. [Google Scholar] [CrossRef]
- Roostaei, M.; Javanmardi, J.; Rasoolzadeh, A.; Mohammadi, A.H. Experimental determinations of the complete inhibition, the slow growth, and the rapid failure regions of methane hydrate formation in the presence of polyvinylpyrrolidone and polyvinylcaprolactam aqueous solutions. Energy Fuels 2021, 35, 3780–3787. [Google Scholar] [CrossRef]
- Lederhos, J.P.; Long, J.P.; Sum, A.; Christiansen, R.L.; Sloan, E.D. Effective kinetic inhibitors for natural gas hydrates. Chem. Eng. Sci. 1996, 51, 1221–1229. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Y.; Liang, D. Investigation into the Inhibition of Methane Hydrate Formation in the Presence of Hydroxy-and Esteryl-Terminated Poly (N-vinylcaprolactam). Energy Fuels 2022, 36, 3848–3856. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, B.; Wang, Z.; Zhang, J.; Wang, X. Prediction and management of hydrate reformation risk in pipelines during offshore gas hydrate development by depressurization. Fuel 2021, 291, 120116. [Google Scholar] [CrossRef]
- Pal, S.; Kundu, T.K. Theoretical study of methanol as inhibitor and cyclopentane as stabilizer of dodecahedron methane hydrate cage. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012081. [Google Scholar] [CrossRef]
- Yagasaki, T.; Matsumoto, M.; Tanaka, H. Effects of thermodynamic inhibitors on the dissociation of methane hydrate: a molecular dynamics study. Phys. Chem. Chem. Phys. 2015, 17, 32347–32357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yi, W.; Mu, J.; Qiu, Z.; Kang, Y.; Wang, Z. Development and performance evaluation of chitosan-graft-poly (N-vinyl pyrrolidone) as a dual-function inhibitor for hydrate decomposition and reformation. J. Mol. Liq. 2024, 401, 124625. [Google Scholar] [CrossRef]
- Mohsenzade, H.; Foroutan, S.; Dashti, A.; Ramezanian, N.; Roosta, H. Vinyl lactam-based copolymers and terpolymers as high cloud point kinetic hydrate inhibitors in methane-THF-water system. J. Mol. Liq. 2020, 308, 113068. [Google Scholar] [CrossRef]
- Zou, X.; Zi, M.; Wu, T.; Yao, Y.; Yang, C.; Chen, D. Synthesis and evaluation investigation of novel kinetic hydrate inhibitors at high subcooling conditions. Fuel 2023, 341, 127014. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Sun, H.; Zhang, L.; Shi, X.; Wang, J.; Guo, D.; Zhang, J. Effects of modified starch,CMC and XC on hydrate formation under different driving forces. J. China Univ. Pet. Ed. Natrual Sci. 2021, 45, 127–136. [Google Scholar]
- Lou, X.; Ding, A.; Maeda, N.; Wang, S.; Kozielski, K.; Hartley, P.G. Synthesis of effective kinetic inhibitors for natural gas hydrates. Energy Fuels 2012, 26, 1037–1043. [Google Scholar] [CrossRef]
- Gulbrandsen, A.C.; Svartås, T.M. Effects of PVCap on gas hydrate dissociation kinetics and the thermodynamic stability of the hydrates. Energy Fuels 2017, 31, 9863–9873. [Google Scholar] [CrossRef]
- Bruusgaard, H.; Lessard, L.D.; Servio, P. Morphology Study of Structure I Methane Hydrate Formation and Decomposition of Water Droplets in the Presence of Biological and Polymeric Kinetic Inhibitors. Cryst. Growth Des. 2009, 9, 3014–3023. [Google Scholar] [CrossRef]
- Talley, L.D.; Mitchell, G.F. Application of proprietary kinetic hydrate inhibitors in gas flowlines. In Proceedings of the Offshore Technology Conference; OTC, 1999; p. OTC-11036-MS.
- Yang, C.; Ke, W.; Zhao, C.; Chen, D. Experimental evaluation of kinetic hydrate inhibitors and mixed formulations with monoethylene glycol for hydrate prevention in pure water and brine–oil systems. Energy Fuels 2020, 34, 12274–12290. [Google Scholar] [CrossRef]
- Perrin, A.; Musa, O.M.; Steed, J.W. The chemistry of low dosage clathrate hydrate inhibitors. Chem. Soc. Rev. 2013, 42, 1996–2015. [Google Scholar] [CrossRef]
- Sloan, E.D.; Subramanian, S.; Matthews, P.N.; Lederhos, J.P.; Khokhar, A.A. Quantifying hydrate formation and kinetic inhibition. Ind. Eng. Chem. Res. 1998, 37, 3124–3132. [Google Scholar] [CrossRef]
- Foroutan, S.; Mohsenzade, H.; Dashti, A.; Roosta, H. Comparison of kinetic inhibition of ethylene and methane hydrate formation when PEGs with low and high molecular weights meet common KHIs. Fuel 2020, 276, 118029. [Google Scholar] [CrossRef]
- Roosta, H.; Dashti, A.; Mazloumi, S.H.; Varaminian, F. Effects of chemical modification of PVA by acrylamide, methacrylamide and acrylonitrile on the growth rate of gas hydrate in methane-propane-water system. J. Mol. Liq. 2018, 253, 259–269. [Google Scholar] [CrossRef]
- Chung, J.S.; Triantafyllou, M.S.; Ellinas, C.P. Proceedings of the First (1991) International Offshore and Polar Engineering Conference; International Society of Offshore & Polar Engineers, 1991. ISBN 0-9626104-5-3.
- Tang, C.; Zhang, Y.; Liang, D.; Li, X. Inhibition effect of chain end modified polyvinyl caprolactam on methane hydrate formation. CIESC J. 2022, 73, 2130. [Google Scholar] [CrossRef]
- Liang, S.; Hall, K.W.; Laaksonen, A.; Zhang, Z.; Kusalik, P.G. Characterizing key features in the formation of ice and gas hydrate systems. Philos. Trans. R. Soc. A 2019, 377, 20180167. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Wells, D.; Hartley, P.G.; Kozielski, K.A. Statistical analysis of supercooling in fuel gas hydrate systems. Energy Fuels 2012, 26, 1820–1827. [Google Scholar] [CrossRef]
- Vatamanu, J.; Kusalik, P.G. Observation of two-step nucleation in methane hydrates. Phys. Chem. Chem. Phys. 2010, 12, 15065–15072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Wang, L.; Bai, J.; Yuan, L.-F.; Yang, J.; Zeng, X.C. Highly confined water: Two-dimensional ice, amorphous ice, and clathrate hydrates. Acc. Chem. Res. 2014, 47, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Khurana, M.; Yin, Z.; Linga, P. A review of clathrate hydrate nucleation. ACS Sustain. Chem. Eng. 2017, 5, 11176–11203. [Google Scholar] [CrossRef]
- Jinhao, S.U.I.; Zhi, W.; Xuanji, L.; Xuanwei, Z.; Yumo, Z.H.U.; Shangfei, S.; Bohui, S.H.I.; Jing, G.; Xia, L.O.U. Paired KHI-MEG for synergistic inhibition of methane hydrate reformation. Chem. Ind. Eng. Prog. 2022, 41, 5373. [Google Scholar] [CrossRef]
- Shahnazar, S.; Bagheri, S.; TermehYousefi, A.; Mehrmashhadi, J.; Abd Karim, M.S.; Kadri, N.A. Structure, mechanism, and performance evaluation of natural gas hydrate kinetic inhibitors. Rev. Inorg. Chem. 2018, 38, 1–19. [Google Scholar] [CrossRef]
- Li, D.; Laroui, A.; Ma, S.; Wang, J.; Wang, D.; Kelland, M.A.; Dong, J. Dependence of the kinetic hydrate inhibition effect of poly (N-vinylpyrrolidone) upon the molecular weight is influenced by water mobility in millisecond dynamics. Energy Fuels 2020, 34, 13664–13672. [Google Scholar] [CrossRef]
- Xinyu, Y.I.N.; Pihui, P.I.; Xiufang, W.E.N.; Yu, Q. Application of special wettability materials for anti-hydrate-nucleation and anti-hydrate-adhesion in oil and gas pipelines. Chem. Ind. Eng. Prog. 2023, 42, 4076. [Google Scholar] [CrossRef]
- Ghosh, R.; Kelland, M.A. Pushing the known performance envelope of kinetic hydrate Inhibitors─ powerful synergy of trialkylamine oxides with acrylamide-based polymers. Energy Fuels 2021, 36, 341–349. [Google Scholar] [CrossRef]
- Del Villano, L.; Kelland, M.A.; Miyake, G.M.; Chen, E.Y.-X. Effect of polymer tacticity on the performance of poly (N, N-dialkylacrylamide) s as kinetic hydrate inhibitors. Energy Fuels 2010, 24, 2554–2562. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Y.; Feng, Y.; Liu, S. Molecular mechanisms of Poly (N-alkyl methacrylamides) s as Kinetic hydrate inhibitors. Chem. Eng. Sci. 2022, 258, 117775. [Google Scholar] [CrossRef]
- Muraoka, M.; Kelland, M.A.; Yamamoto, Y.; Suzuki, K. Critical growth rate of hydrate crystal growth inhibitors in the low growth rate region. Cryst. Growth Des. 2021, 21, 4979–4985. [Google Scholar] [CrossRef]
- Chin, Y.D.; Srivastava, A. Advances in LDHIs and applications. In Proceedings of the Offshore Technology Conference; OTC, 2018; p. D041S052R001.
- Dann, K. Surfactant effect on hydrate crystallization mechanism. 2017.
- Liu, J.; Liang, D.; Li, J.; Lin, D.; Wu, S.; Lu, F. A review of flow assurance studies on hydrate slurry in oil-water system. Chem. Ind. Eng. Prog. 2023, 42, 1739–1759. [Google Scholar]
- Ke, W.; Kelland, M.A. Kinetic hydrate inhibitor studies for gas hydrate systems: a review of experimental equipment and test methods. Energy Fuels 2016, 30, 10015–10028. [Google Scholar] [CrossRef]
- Chua, P.C.; Kelland, M.A. Tetra (iso-hexyl) ammonium Bromide The Most Powerful Quaternary Ammonium-Based Tetrahydrofuran Crystal Growth Inhibitor and Synergist with Polyvinylcaprolactam Kinetic Gas Hydrate Inhibitor. Energy Fuels 2012, 26, 1160–1168. [Google Scholar] [CrossRef]
- Kelland, M.A.; Kvæstad, A.H.; Astad, E.L. Tetrahydrofuran hydrate crystal growth inhibition by trialkylamine oxides and synergism with the gas kinetic hydrate inhibitor poly (N-vinyl caprolactam). Energy Fuels 2012, 26, 4454–4464. [Google Scholar] [CrossRef]
- Guangchun, S.; Yuxing, L.I.; Wuchang, W.; Kai, J.; Zhengzhuo, S.H.I.; Pengfei, Z. A review on hydrate deposition in oil and gas transmission pipelines. Chem. Ind. Eng. Prog. 2017, 36, 3164. [Google Scholar] [CrossRef]
- Liu, C.; Hao, X.; Meng, Q.; Li, C.; Sun, J. RESEARCH PROGRESS IN BASIC CHARACTERISTICS OF GAS HYDRATE. Mar. Geol. Lett. 2020, 36, 1–10. [Google Scholar]
- Kelland, M.A. Tailored Amine Oxides─ Synergists, Surfactants, and Polymers for Gas Hydrate Management, a Minireview. Energy Fuels 2023, 37, 8919–8934. [Google Scholar] [CrossRef]
- G. T. Rivers; D. L. Crosby. Method and compositions for inhibiting formation of hydrocarbon hydrates 2005.
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly (ionic liquid) s: syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Yuan, C.; Sun, J.; Chen, C.; Liang, X.; von Solms, N.; Song, Y. Molecular insights into the wettability of antifreeze protein-adsorbed hydrate surface: Application to hydrate anti-agglomeration. Chem. Eng. J. 2024, 481, 148387. [Google Scholar] [CrossRef]
- Reyes, F.T.; Kelland, M.A.; Sun, L.; Dong, J. Kinetic Hydrate Inhibitors: Structure–Activity Relationship Studies on a Series of Branched Poly (ethylene citramide) s with Varying Lipophilic Groups. Energy Fuels 2015, 29, 4774–4782. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Musa, O.M.; Steed, J.W. Problems associated with sour gas in the oilfield industry and their solutions. Energy Fuels 2015, 29, 4667–4682. [Google Scholar] [CrossRef]
- Karaaslan, U.; Parlaktuna, M. PEOA New Hydrate Inhibitor Polymer. Energy Fuels 2002, 16, 1387–1391. [Google Scholar] [CrossRef]
- Jokandan, E.F.; Naeiji, P.; Varaminian, F. The synergism of the binary and ternary solutions of polyethylene glycol, polyacrylamide and Hydroxyethyl cellulose to methane hydrate kinetic inhibitor. J. Nat. Gas Sci. Eng. 2016, 29, 15–20. [Google Scholar] [CrossRef]
- Klomp, U.C. Method for inhibiting the plugging of conduits by gas hydrates 2005.
- Ghosh, R.; Pomicpic, J.; Kelland, M.A. High-Performance Kinetic Hydrate Inhibition with Poly (N-isopropyl methacrylamide) and Triisopentylamine Oxide─ Surprising Concentration-Dependent Results. Energy Fuels 2023, 37, 4928–4936. [Google Scholar] [CrossRef]
- Anderson, B.J.; Tester, J.W.; Borghi, G.P.; Trout, B.L. Properties of inhibitors of methane hydrate formation via molecular dynamics simulations. J. Am. Chem. Soc. 2005, 127, 17852–17862. [Google Scholar] [CrossRef] [PubMed]
- Storr, M.T.; Taylor, P.C.; Monfort, J.-P.; Rodger, P.M. Kinetic inhibitor of hydrate crystallization. J. Am. Chem. Soc. 2004, 126, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Storr, M.T.; Rodger, P.M. A molecular dynamics study of the mechanism of kinetic inhibition. Ann. N. Y. Acad. Sci. 2000, 912, 669–677. [Google Scholar] [CrossRef]
- Ree, L.; Kelland, M.A.; Haddleton, D.; Alsubaie, F. Comparison of the Kinetic Hydrate Inhibition Performance of Block and Statistical N-Alkylacrylamide Copolymers. Energy Fuels 2017, 31, 1355–1361. [Google Scholar] [CrossRef]
- Zeng, H. Inhibition of clathrate hydrates by antifreeze proteins; 2004. ISBN 978-0-494-00011-3.
- Walker, V.K.; Zeng, H.; Ohno, H.; Daraboina, N.; Sharifi, H.; Bagherzadeh, S.A.; Alavi, S.; Englezos, P. Antifreeze proteins as gas hydrate inhibitors. Can. J. Chem. 2015, 93, 839–849. [Google Scholar] [CrossRef]
- Ohno, H.; Susilo, R.; Gordienko, R.; Ripmeester, J.; Walker, V.K. Interaction of antifreeze proteins with hydrocarbon hydrates. Chem. Eur. J. 2010, 16, 10409–10417. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wilson, L.D.; Walker, V.K.; Ripmeester, J.A. Effect of antifreeze proteins on the nucleation, growth, and the memory effect during tetrahydrofuran clathrate hydrate formation. J. Am. Chem. Soc. 2006, 128, 2844–2850. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Sun, J.; Liu, Y.; Qin, Y.; Ling, Z.; Liu, W.; Li, W. The roles of functional groups of antifreeze protein in inhibition of hydrate growth. Fuel 2022, 327, 125060. [Google Scholar] [CrossRef]
- Neugroschl, A. Investigating the effects of kinetic hydrate inhibitors and antifreeze glycoproteins in inhibiting the growth of THF hydrates, Yeshiva University, 2022.
- Han, S.; Maruthamuthu, M.K.; Lee, W.; Hong, S.H.; Kang, S.-P. Efficacy of antifreeze proteins from Clupea harangues and Anarhichas minor on gas hydrate inhibition via cell surface display. Chem. Eng. Sci. 2020, 215, 115470. [Google Scholar] [CrossRef]
- Bagherzadeh, S.A.; Alavi, S.; Ripmeester, J.A.; Englezos, P. Why ice-binding type I antifreeze protein acts as a gas hydrate crystal inhibitor. Phys. Chem. Chem. Phys. 2015, 17, 9984–9990. [Google Scholar] [CrossRef]
- Tang, C.; Liang, D. Inhibitory effects of novel green inhibitors on gas hydrate formation. Chin. J. Chem. Eng. 2019, 27, 2107–2117. [Google Scholar] [CrossRef]
- Maddah, M.; Maddah, M.; Peyvandi, K. Investigation on structural properties of winter flounder antifreeze protein in interaction with clathrate hydrate by molecular dynamics simulation. J. Chem. Thermodyn. 2021, 152, 106267. [Google Scholar] [CrossRef]
- Bourgmayer, P.; Sugier, A.; Behar, E. A New Proposal for Solving Hydrates Problem in Multiphase Flow.; 1989; pp. 19–21.
- O’Reilly, R.; Ieong, N.S.; Chua, P.C.; Kelland, M.A. Missing Poly(N-vinyl lactam) Kinetic Hydrate Inhibitor: High-Pressure Kinetic Hydrate Inhibition of Structure II Gas Hydrates with Poly(N-vinyl piperidone) and Other Poly(N-vinyl lactam) Homopolymers. Energy Fuels 2011, 25, 4595–4599. [Google Scholar] [CrossRef]
- Chua, P.C.; Kelland, M.A. Poly(N-vinyl azacyclooctanone): A More Powerful Structure II Kinetic Hydrate Inhibitor than Poly(N-vinyl caprolactam). Energy Fuels 2012, 26, 4481–4485. [Google Scholar] [CrossRef]
- Ke, W.; Chen, D. A short review on natural gas hydrate, kinetic hydrate inhibitors and inhibitor synergists. Chin. J. Chem. Eng. 2019, 27, 2049–2061. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Guo, J.; Chen, G.; Zhong, J.; Yan, Y.; Zhang, J. Molecular insights into the kinetic hydrate inhibition performance of Poly (N-vinyl lactam) polymers. J. Nat. Gas Sci. Eng. 2020, 83, 103504. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Zhou, G.; Huang, W. Synergism of thermodynamic hydrate inhibitors on the performance of poly (vinyl pyrrolidone) in deepwater drilling fluid. J. Nat. Gas Sci. Eng. 2015, 23, 47–54. [Google Scholar] [CrossRef]
- Suri, A.; Singh, A. Synergistic Hydrate Inhibition by Iota-Carrageenan with Kinetic Hydrate Inhibitors. In Proceedings of the SPE Middle East Oil and Gas Show and Conference; SPE, 2023; p. D021S081R007.
- Makogon, T.Y. Experimental and computer study of the effect of kinetic inhibitors on clathrate hydrates. 1990-1999-Mines Theses Diss. 1997.
- Mozaffar, H. Development and application of a novel crystal growth inhibition (CGI) method for evaluation of kinetic hydrate inhibitors, Heriot-Watt University, 2013.
- Singh, A.; Suri, A. A review on gas hydrates and kinetic hydrate inhibitors based on acrylamides. J. Nat. GAS Sci. Eng. 2020, 83. [Google Scholar] [CrossRef]
- Chua, P.C.; Kelland, M.A.; Ishitake, K.; Satoh, K.; Kamigaito, M.; Okamoto, Y. Kinetic Hydrate Inhibition of Poly(N-isopropylmethacrylamide)s with Different Tacticities. Energy Fuels 2012, 26, 3577–3585. [Google Scholar] [CrossRef]
- Farhadian, A.; Varfolomeev, M.A.; Shaabani, A.; Zaripova, Y.F.; Yarkovoi, V.V.; Khayarov, K.R. Inhibition performance of chitosan-graft-polyacrylamide as an environmentally friendly and high-cloud-point inhibitor of nucleation and growth of methane hydrate. Cryst. Growth Des. 2020, 20, 1771–1778. [Google Scholar] [CrossRef]
- Colle, K.; Talley, L.D.; Longo, J.M. World Patent Application WO 2005/005567, 2005. Google Sch. There No Corresp. Rec. This Ref.
- Ajiro, H.; Takemoto, Y.; Akashi, M.; Chua, P.C.; Kelland, M.A. Study of the Kinetic Hydrate Inhibitor Performance of a Series of Poly (N-alkyl-N-vinylacetamide) s. Energy Fuels 2010, 24, 6400–6410. [Google Scholar] [CrossRef]
- Zeng, H.; Wilson, L.D.; Walker, V.K.; Ripmeester, J.A. The inhibition of tetrahydrofuran clathrate-hydrate formation with antifreeze protein. Can. J. Phys. 2003, 81, 17–24. [Google Scholar] [CrossRef]
- Maddah, M.; Maddah, M.; Peyvandi, K. Molecular dynamics simulation of methane hydrate formation in presence and absence of amino acid inhibitors. J. Mol. Liq. 2018, 269, 721–732. [Google Scholar] [CrossRef]
- Xu, P.; Lang, X.; Fan, S.; Wang, Y.; Chen, J. Molecular Dynamics Simulation of Methane Hydrate Growth in the Presence of the Natural Product Pectin. J. Phys. Chem. C 2016, 120, 5392–5397. [Google Scholar] [CrossRef]
- Farkhadian, A.; Varfolomeev, M.A.; Zaripova, Yu.F.; Yarkovoi, V.V. Synthesis and Testing of New Kinetic Inhibitor of Methane Hydrates Based on Amphiphilic Polyurethane. Chem. Technol. Fuels Oils 2019, 55, 159–164. [Google Scholar] [CrossRef]
- Dong Lee, J.; Wu, H.; Englezos, P. Cationic starches as gas hydrate kinetic inhibitors. Chem. Eng. Sci. 2007, 62, 6548–6555. [Google Scholar] [CrossRef]
- Farhadian, A.; Kudbanov, A.; Varfolomeev, M.A.; Dalmazzone, D. Waterborne Polyurethanes as a New and Promising Class of Kinetic Inhibitors for Methane Hydrate Formation. Sci. Rep. 2019, 9, 9797. [Google Scholar] [CrossRef]
- Li, S.; Lv, R.; Yan, Z.; Huang, F.; Zhang, X.; Chen, G.-J.; Yue, T. Design of Alanine-Rich Short Peptides as a Green Alternative of Gas Hydrate Inhibitors: Dual Methyl Group Docking for Efficient Adsorption on the Surface of Gas Hydrates. ACS Sustain. Chem. Eng. 2020, 8, 4256–4266. [Google Scholar] [CrossRef]
- Xiao, C.; Adidharma, H. Dual function inhibitors for methane hydrate. Chem. Eng. Sci. 2009, 64, 1522–1527. [Google Scholar] [CrossRef]
- Tariq, M.; Rooney, D.; Othman, E.; Aparicio, S.; Atilhan, M.; Khraisheh, M. Gas Hydrate Inhibition: A Review of the Role of Ionic Liquids. Ind. Eng. Chem. Res. 2014, 53, 17855–17868. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Keong, L.K.; Ahmed, I. Tetramethyl ammonium chloride as dual functional inhibitor for methane and carbon dioxide hydrates. Fuel 2019, 236, 251–263. [Google Scholar] [CrossRef]
- Nashed, O.; Sabil, K.M.; Ismail, L.; Japper-Jaafar, A.; Lal, B. Mean induction time and isothermal kinetic analysis of methane hydrate formation in water and imidazolium based ionic liquid solutions. J. Chem. Thermodyn. 2018, 117, 147–154. [Google Scholar] [CrossRef]
- Zare, M.; Haghtalab, A.; Ahmadi, A.N.; Nazari, K. Experiment and thermodynamic modeling of methane hydrate equilibria in the presence of aqueous imidazolium-based ionic liquid solutions using electrolyte cubic square well equation of state. Fluid Phase Equilibria 2013, 341, 61–69. [Google Scholar] [CrossRef]
- Del Villano, L.; Kelland, M.A. An investigation into the kinetic hydrate inhibitor properties of two imidazolium-based ionic liquids on Structure II gas hydrate. Chem. Eng. Sci. 2010, 65, 5366–5372. [Google Scholar] [CrossRef]
- Kim, K.; Kang, S.-P. Investigation of pyrrolidinium-and morpholinium-based ionic liquids into kinetic hydrate inhibitors on structure I methane hydrate.; 2011; pp. 17–21.
- Kim, K.-S.; Kang, J.W.; Kang, S.-P. Tuning ionic liquids for hydrate inhibition. Chem. Commun. 2011, 47, 6341–6343. [Google Scholar] [CrossRef]
- Norland, A.K.; Kelland, M.A. Crystal growth inhibition of tetrahydrofuran hydrate with bis- and polyquaternary ammonium salts. Chem. Eng. Sci. 2012, 69, 483–491. [Google Scholar] [CrossRef]
- Tariq, M.; Atilhan, M.; Khraisheh, M.; Othman, E.; Castier, M.; García, G.; Aparicio, S.; Tohidi, B. Experimental and DFT Approach on the Determination of Natural Gas Hydrate Equilibrium with the Use of Excess N2 and Choline Chloride Ionic Liquid as an Inhibitor. Energy Fuels 2016, 30, 2821–2832. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lim, J.I.; Kim, J.H.; Lee, J.D. Kinetic Studies on Methane Hydrate Formation in the Presence of Kinetic Inhibitor via in Situ Raman Spectroscopy. Energy Fuels 2012, 26, 7045–7050. [Google Scholar] [CrossRef]
- Tumba, K.; Reddy, P.; Naidoo, P.; Ramjugernath, D.; Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Phase Equilibria of Methane and Carbon Dioxide Clathrate Hydrates in the Presence of Aqueous Solutions of Tributylmethylphosphonium Methylsulfate Ionic Liquid. J. Chem. Eng. Data 2011, 56, 3620–3629. [Google Scholar] [CrossRef]
- Tariq, M.; Connor, E.; Thompson, J.; Khraisheh, M.; Atilhan, M.; Rooney, D. Doubly dual nature of ammonium-based ionic liquids for methane hydrates probed by rocking-rig assembly. RSC Adv. 2016, 6, 23827–23836. [Google Scholar] [CrossRef]
- Li, X.-S.; Liu, Y.-J.; Zeng, Z.-Y.; Chen, Z.-Y.; Li, G.; Wu, H.-J. Equilibrium Hydrate Formation Conditions for the Mixtures of Methane + Ionic Liquids + Water. J. Chem. Eng. Data 2011, 56, 119–123. [Google Scholar] [CrossRef]
- Keshavarz, L.; Javanmardi, J.; Eslamimanesh, A.; Mohammadi, A.H. Experimental measurement and thermodynamic modeling of methane hydrate dissociation conditions in the presence of aqueous solution of ionic liquid. Fluid Phase Equilibria 2013, 354, 312–318. [Google Scholar] [CrossRef]
- English, N.J.; MacElroy, J.M.D. Perspectives on molecular simulation of clathrate hydrates: Progress, prospects and challenges. Chem. Eng. Sci. 2015, 121, 133–156. [Google Scholar] [CrossRef]
- Sa, J.-H.; Kwak, G.-H.; Han, K.; Ahn, D.; Lee, K.-H. Gas hydrate inhibition by perturbation of liquid water structure. Sci. Rep. 2015, 5, 11526. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.; Sapronova, A.; Kvamme, B.; Johannsen, K.; Haug, J. Impact of Low-Dosage Inhibitors on Clathrate Hydrate Stability. Macromol. Symp. 2010, 287, 168–176. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; He, J.; Ge, K. Research progress and prospects of performance of kinetic hydrate inhibitor and effect of functional group. J. Cent. South Univ. Sci. Technol. 2022, 53, 772–798. [Google Scholar]
- Erfani, A.; Varaminian, F.; Muhammadi, M. Gas hydrate formation inhibition using low dosage hydrate inhibitors. In Proceedings of the 2nd National Iranian conference on gas hydrate (NICGH); 2013.
- Zhang, J.S.; Lo, C.; Couzis, A.; Somasundaran, P.; Wu, J.; Lee, J.W. Adsorption of Kinetic Inhibitors on Clathrate Hydrates. J. Phys. Chem. C 2009, 113, 17418–17420. [Google Scholar] [CrossRef]
- Farhadian, A.; Naeiji, P.; Varfolomeev, M.A.; Peyvandi, K.; Kiiamov, A.G. Reconsideration of the micellization theory: Promotion or inhibition of gas hydrate formation for gas storage and flow assurance applications. Chem. Eng. J. 2022, 427, 131852. [Google Scholar] [CrossRef]
- Farhadian, A.; Shadloo, A.; Zhao, X.; Pavelyev, R.S.; Peyvandi, K.; Qiu, Z.; Varfolomeev, M.A. Challenges and advantages of using environmentally friendly kinetic gas hydrate inhibitors for flow assurance application: A comprehensive review. Fuel 2023, 336, 127055. [Google Scholar] [CrossRef]
- Khurana, M.; Yin, Z.; Linga, P. A review of clathrate hydrate nucleation. ACS Sustain. Chem. Eng. 2017, 5, 11176–11203. [Google Scholar] [CrossRef]
- Kvamme, B. Molecular Dynamics Simulations As a Tool For the Selection of Candidates For Kinetic Hydrate Inhibitors.; OnePetro, 2001.
- Kvamme, B.; Kuznetsova, T.; Aasoldsen, K. Molecular dynamics simulations for selection of kinetic hydrate inhibitors. J. Mol. Graph. Model. 2005, 23, 524–536. [Google Scholar] [CrossRef]
- Larsen, R.; Knight, C.A.; Sloan, E.D. Clathrate hydrate growth and inhibition. Fluid Phase Equilibria 1998, 150–151, 353–360. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Y.; Chen, G.; Zhang, J.; Liu, S. Kinetic hydrate inhibitor performance and adsorption characteristics of poly (N-alkyl-N-vinyl acetamide) s: A first-principles study. Colloids Surf. Physicochem. Eng. Asp. 2022, 635, 128097. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Y.; Chen, G.; Zhang, J.; Liu, S. Adsorption behavior of kinetic inhibitors on hydrate surfaces and its relation to the inhibition performance. Chem. Phys. Lett. 2021, 784, 139108. [Google Scholar] [CrossRef]
- King, H.E., Jr.; Hutter, J.L.; Lin, M.Y.; Sun, T. Polymer conformations of gas-hydrate kinetic inhibitors: A small-angle neutron scattering study. J. Chem. Phys. 2000, 112, 2523–2532. [Google Scholar] [CrossRef]
- Yang, J.; Tohidi, B. Characterization of inhibition mechanisms of kinetic hydrate inhibitors using ultrasonic test technique. Chem. Eng. Sci. 2011, 66, 278–283. [Google Scholar] [CrossRef]
- Zeng, H.; Lu, H.; Huva, E.; Walker, V.K.; Ripmeester, J.A. Differences in nucleator adsorption may explain distinct inhibition activities of two gas hydrate kinetic inhibitors. Chem. Eng. Sci. 2008, 63, 4026–4029. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Liu, J.; Han, D.; Rohani, S.; Gao, Z.; Gong, J. Inhibition of Crystal Nucleation and Growth: A Review. Cryst. Growth Des. 2024, 24, 2645–2665. [Google Scholar] [CrossRef]
- Zeng, H.; Walker, V.; Ripmeester, J. Approaches to the design of better low-dosage gas hydrate inhibitors. Angew. Chem.-Int. Ed. 2007, 46, 5402–5404. [Google Scholar] [CrossRef] [PubMed]
- Urdahl, O.; Lund, A.; Mørk, P.; Nilsen, T.-N. Inhibition of gas hydrate formation by means of chemical additives—I. Development of an experimental set-up for characterization of gas hydrate inhibitor efficiency with respect to flow properties and deposition. Chem. Eng. Sci. 1995, 50, 863–870. [Google Scholar] [CrossRef]
- Makogon, Y.F.; Makogon, T.Y.; Holditch, S.A. Kinetics and Mechanisms of Gas Hydrate Formation and Dissociation with Inhibitors. Ann. N. Y. Acad. Sci. 2000, 912, 777–796. [Google Scholar] [CrossRef]
- Makwashi, N.; Zhao, D.; Ismaila, T.A.; Paiko, I. Pipeline gas hydrate formation and treatment: a review. In Proceedings of the 3rd national engineering conference on building the gap between academia and industry, Faculty of Engineering, Bayero University, Kano; 2018; pp. 2450–2454.
- Wang, J.; Meng, Y.; Han, B.; Liu, Z.; Zhang, L.; Yao, H.; Wu, Z.; Chu, J.; Yang, L.; Zhao, J. Hydrate blockage in subsea oil/gas flowlines: Prediction, prevention, and remediation. Chem. Eng. J. 2023, 461, 142020. [Google Scholar] [CrossRef]
- Fidel-Dufour, A.; Gruy, F.; Herri, J.-M. Rheology of methane hydrate slurries during their crystallization in a water in dodecane emulsion under flowing. Chem. Eng. Sci. 2006, 61, 505–515. [Google Scholar] [CrossRef]
- Camargo, R.; Palermo, T. Rheological Properties of Hydrate Suspensions in an Asphaltenic Crude Oil.; 2002.
- Camargo, R.; Palermo, T.; Sinquin, A.; Glenat, P. Rheological Characterization of Hydrate Suspensions in Oil Dominated Systems. Ann. N. Y. Acad. Sci. 2000, 912, 906–916. [Google Scholar] [CrossRef]
- Aspenes, G.; Dieker, L.E.; Aman, Z.M.; Høiland, S.; Sum, A.K.; Koh, C.A.; Sloan, E.D. Adhesion force between cyclopentane hydrates and solid surface materials. J. Colloid Interface Sci. 2010, 343, 529–536. [Google Scholar] [CrossRef]
- Erstad, K.; Høiland, S.; Fotland, P.; Barth, T. Influence of Petroleum Acids on Gas Hydrate Wettability. Energy Fuels 2009, 23, 2213–2219. [Google Scholar] [CrossRef]
- Aspenes, G.; Høiland, S.; Barth, T.; Askvik, K.M. The influence of petroleum acids and solid surface energy on pipeline wettability in relation to hydrate deposition. J. Colloid Interface Sci. 2009, 333, 533–539. [Google Scholar] [CrossRef]
- Høiland, S.; Askvik, K.M.; Fotland, P.; Alagic, E.; Barth, T.; Fadnes, F. Wettability of Freon hydrates in crude oil/brine emulsions. J. Colloid Interface Sci. 2005, 287, 217–225. [Google Scholar] [CrossRef]
- Sjöblom, J.; Øvrevoll, B.; Jentoft, G.; Lesaint, C.; Palermo, T.; Sinquin, A.; Gateau, P.; Barré, L.; Subramanian, S.; Boxall, J.; et al. Investigation of the Hydrate Plugging and Non-Plugging Properties of Oils. J. Dispers. Sci. Technol. 2010, 31, 1100–1119. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Lang, X.; Fan, S. Effects of Polyvinyl Alcohol on the Adhesion Force of Tetrahydrofuran Hydrate Particles. Energy Fuels 2011, 25, 3204–3211. [Google Scholar] [CrossRef]
- Moon, C.; Hawtin, R.W.; Rodger, P.M. Nucleation and control of clathrate hydrates: insights from simulation. Faraday Discuss. 2007, 136, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Yagasaki, T.; Matsumoto, M.; Tanaka, H. Adsorption Mechanism of Inhibitor and Guest Molecules on the Surface of Gas Hydrates. J. Am. Chem. Soc. 2015, 137, 12079–12085. [Google Scholar] [CrossRef]
- Thakre, N.; Jana, A.K. Physical and molecular insights to Clathrate hydrate thermodynamics. Renew. Sustain. Energy Rev. 2021, 135, 110150. [Google Scholar] [CrossRef]
| Time | Research phase | Thrust | Representative inhibitors |
|---|---|---|---|
| 1991-1995 | Phase I | Discovery of kinetic inhibitors and screening | Polyvinylpyrrolidone (PVP) |
| 1995-1999 | Phase Ⅱ | Improvements based on studies of first-generation kinetic inhibitors | Polyvinyl caprolactam (PVCap) and PVCap copolymer (PVPNC) |
| 1999-present | Phase Ⅲ | Design of kinetic inhibitor molecules using molecular simulation methods to develop kinetic inhibitors with improved performance | Vinyl lactam-based polymer inhibitors, ionic liquid-based inhibitors, and complex inhibitors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).