1. Introduction
The normal functions of the stomatognathic system are essential for the health and psychosocial well-being of every human being, and today there are increasing demands for aesthetic and financially accessible dental procedures to achieve health. Numerous and different artificial materials are used to restore lost, damaged or deformed oral structures under the common name dental materials. At one time, gold was the main dental material due to its high resistance to corrosion and suitable strength, but due to its high cost, it was pushed out of use. Today, the most commonly used are dental metal alloy materials composed of chromium (Cr), nickel (Ni), titanium (Ti), iron (Fe), molybdenum (Mo) and cobalt (Co) due to their good mechanical properties and corrosion resistance [
1,
2,
3]. Many studies indicate the interaction of dental materials with oral tissue, resulting in the evolution of high-performance dental materials to meet the different demands of the oral medium [
4]. Corrosion is considered the most important factor in the selection of metallic dental materials; therefore, it deserves special emphasis and must be evaluated in the oral environment that is constantly changing [
4]. Light alloys have an oxide layer that makes them resistant to further corrosion, however these biocompatible metallic materials tend to corrode locally under changing conditions in the oral medium and degrade over time, releasing metal ions into the oral cavity [
5,
6] and thus contributing to general toxicity. Also, due to the frequent mechanical loads to which dental materials are exposed in the oral cavity, the protective oxide layer is constantly damaged, causing wear and permanent electrochemical corrosion [
7]. Furthermore, saliva with variable PH, bacterial biofilm products, high fluoride concentrations, or gastric acid reflux, as well as masticatory and orthodontic forces affect the structural and chemical stability of dental materials [
8].
Orthodontic fixed devices as well as partial metal skeleton prostheses and bridges usually remain exposed to degradation processes in the oral cavity for several years. The process of degradation of dental metal alloys is the subject of in vitro research in which various parameters and their possible synergistic effects are analyzed, because a complete simulation of conditions inside the oral cavity is difficult to achieve due to complex intraoral processes [
9]. Almost all fixed orthodontic devices, as well as metal parts of partial dentures and bridges, are made of metal alloys, among the most used materials are alloys of stainless steel (SS), cobalt-chromium (CoCr), nickel-titanium (NiTi) and titanium-molybdenum alloy (TiMo) [
10]. At the same time, for each of these groups of materials, versions are offered on the market, which are advertised as hypoallergenic, but at the same time are more expensive.
A large number of studies conducted on allergic skin tests indicate evidence related to an allergy to dental alloys, and the metal that plays a leading role is nickel. This is of special interest for dental medicine, because nickel-containing metals are widely used in orthodontics and prosthetics [
11]. Nickel released from dental alloys can cause an allergic reaction in certain cases, but it is not clear whether it can also cause hypersensitivity. Schmalz and Garhammer [
11] believe that the relatively high incidence of nickel allergy should provide an incentive to replace cast Ni-based alloys with an available suitable alternative alloy. Previous research has shown that some of the new products on the market do not reduce surface or general corrosion but increase it [
12]. Also, the prevalence of oral allergies from metals that are part of various dental materials (from dental and root canal fillings, to orthodontic appliances and prosthetic crowns, dentures and implants) is increasing [
13] Hypersensitivity, allergic reactions and disease symptoms were described, manifesting complex interactions between various components of dental materials, which came as a result of the nanoparticles intake, electromagnetic radiation, galvanic corrosion and particular genetic individual factors [
12,
14]. For example, current European Council regulation ban the use of dental amalgam for treating teeth in children under 15 years old, and pregnant or breastfeeding women [
15]. Furthermore, the European Union (EU) legislative framework addresses the metal alloys’ corrosion issues, demanding the restriction of use of the probable carcinogenic, mutagenic and toxic for reproduction at humans (CMG) metals, like nickel, cobalt and chromium, and demand to develop new biomaterials [
16]. The findings so far confirm the need for independent testing of new materials on the market and the justification of using one or another version of the offered dental materials in clinical work and the non-/-justification of increased treatment costs [
17,
18].
Therefore, the aim of this research is to evaluate and compare ion release of standard dental alloys and their hypoallergenic equivalents. The hypothesis is that the hypoallergenic alloys will release less of the metallic ions in both acidic and neutral artificial saliva, compared to standard dental alloys.
2. Materials and Methods
Six types of preformed orthodontic archwire alloys were used:
1. BioForce Sentalloy (Dentsply GAC, NY, USA) - NiTi alloy (Ni=50,4 %; Ti = 49,6 %);
2. High Aesthetic (Dentsply GAC, NY, USA) – NiTi-coated alloy (Ni=50,4 %; Ti = 49,6 %), with a < 0.5 µm thin coating of gold and rhodium [
19];
3. Remanium (Dentaurum, Ispringen, Germany) - noble steel (SS) alloy (Cr 18-20%, Ni 8-10,5%, rest Fe);
4. Noninium (Dentaurum, Ispringen, Germany) - noble steel (SS Ni Free) alloy (Mn 16-20%, Cr 16-20%, Mo 1,8-2,5%, rest Fe);
5. Elgiloy (Dentaurum, Ispringen, Germany) - alloy CoCr (Ni 14-16%, Cr 19-21%, Mo 6-8%, Co 38-42%);
6. Rematitan Special (Dentaurum, Ispringen, Germany) — TiMo alloy (TMA) (Mo 11,5%, Ti 78%).
Artificial saliva was prepared from: 1.5 g/l KCl, 1.5 g/l NaHC03, 0.5 g/l 0.5 g/l KSCN, 0.9 g/l lactic acid. The pH values were adjusted with lactic acid imitating neutral oral environment (pH 6.6) and oral environment in persons with poor oral hygiene (pH 5.5) [
17].
Samples of alloys of dental materials were prepared in laboratory conditions at the University of Tetovo. Alloy samples were immersed in sterile artificial saliva (1 cm of sample alloy / 1 mL of artificial saliva) for a period of 3, 7, 14 and 28 days, at 37 ̊C and with moderate shaking on a mechanical shaker (100 r/min). Samples containing saliva without alloys served as negative control.
All measurements were made on 5 samples of each type of examined dental material in combination with different pH of artificial saliva, according to the power analysis of previous research [
20].
Ion release was recorded using inductively coupled plasma-mass-spectrometry (ICP-MS) (Agilent Technologies 7800, Santa Clara, United States), all specimens were acidified with 1 drop of concentrated HNO4, before injected into spectrometer. The release of metals was expressed as µg/cm2, to enable better evaluation of the aspect of biocompatibility by translating experimental results into clinical conditions, as described by Arndt et al. [
21]. Data presented in this way enable to calculate average daily release for, e. g. two wires (for upper and lower dental arch, total length 28 cm), as expected in clinical conditions, for persons with good (pH 6.6) or bad oral hygiene (pH 5.5); or any other clinical situation regarding type and dimensions of wires.
Statistical analysis for all obtained quantitative data was performed using statistical software R 4.3.1. (R Core Team, 2023) [
22]. The Shapiro-Wilk test was used as the basic test of normality of distributions. In cases of deviations from the normal distribution, the skewness and kurtosis of the distributions were calculated, and 3 for flatness and 7 for curvature were taken as reference values according to Kline's recommendations [
23]. Data analyses were done using analysis of variance, which allows testing for differences. Results with P<0.05 were considered significant.
3. Results
3.1. Nickel Release
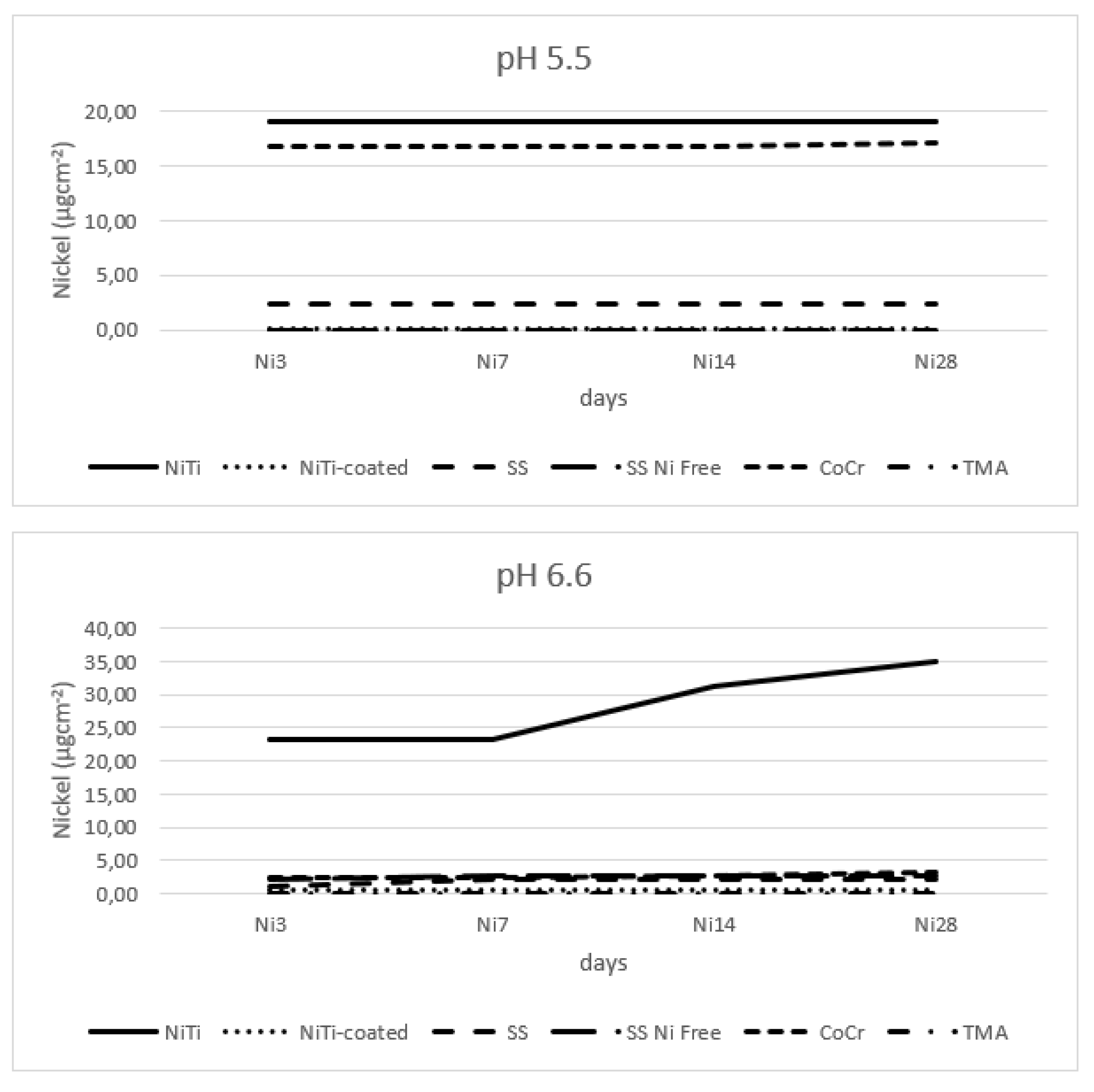
Results for the nickel release are presented in
Figure 1. There was no nickel detected for the TMA and SS Ni Free (at pH 5.5); at lower pH, NiTi and SS released most nickel, while at pH 6.6, again, NiTi released most nickel, while all other alloys showed minimum release. At pH 5.5, release was highest at the first time point, and later it was minimal. At pH 6.6, release was initially high, and again increased after 14 days of immersion. There were no values recorded for TMA and SS Ni Free, and no variability by time points; for this reasons, further analyses could not be performed.
3.2. Titanium Release
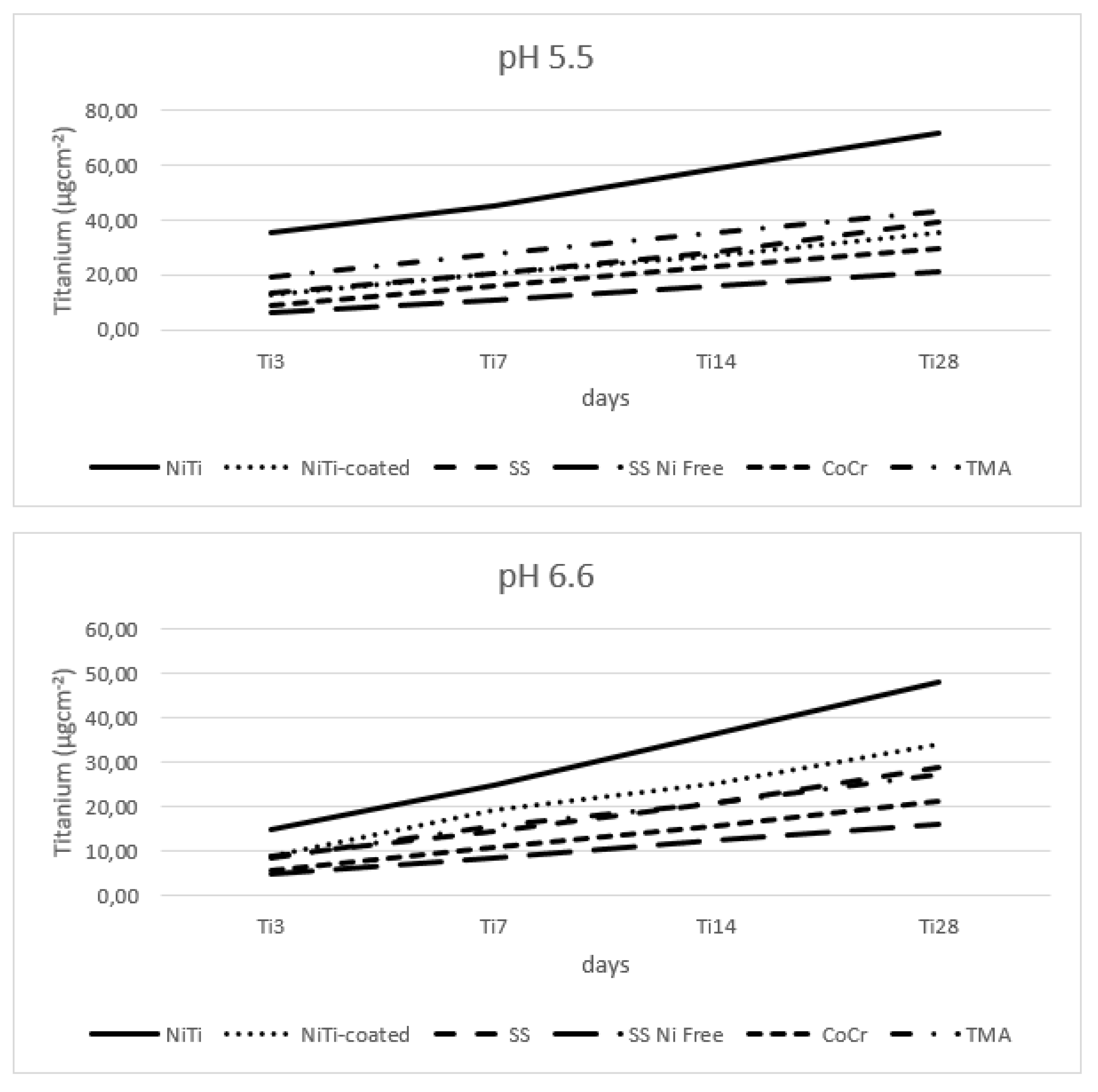
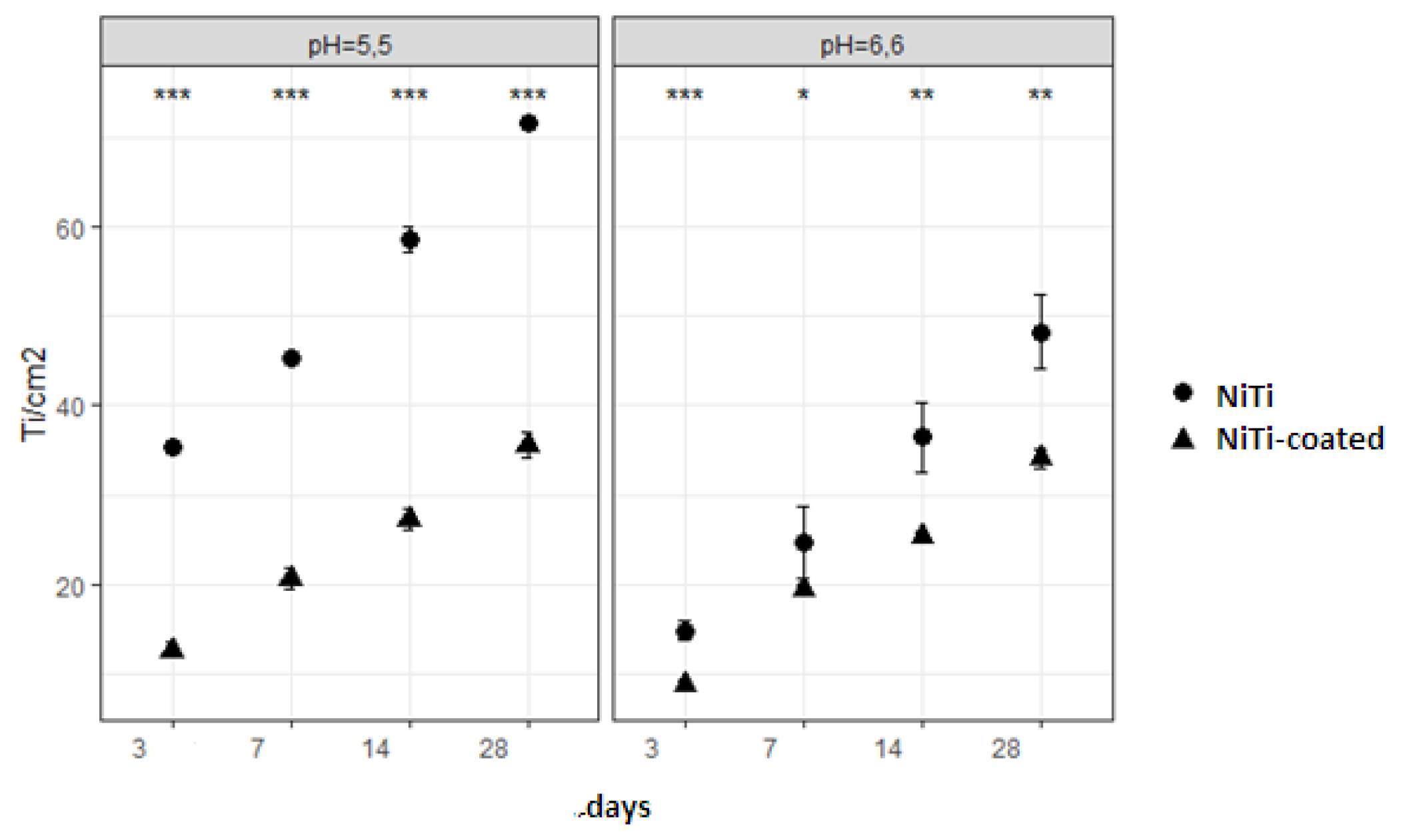
Figure 2 shows release of titanium. Highest release was noted for the NiTi in both pH; the TMA had second highest release in pH 5.5, while in pH 6.6, it was the NiTi-coated alloy. Titanium showed increase in release over time in both pH. Analysis of the main effects for NiTi and NiTi-coated alloys, regarding release of titanium, showed significant differences for all time points in both pH (
Table 1 and
Figure 3).
3.3. Iron Release
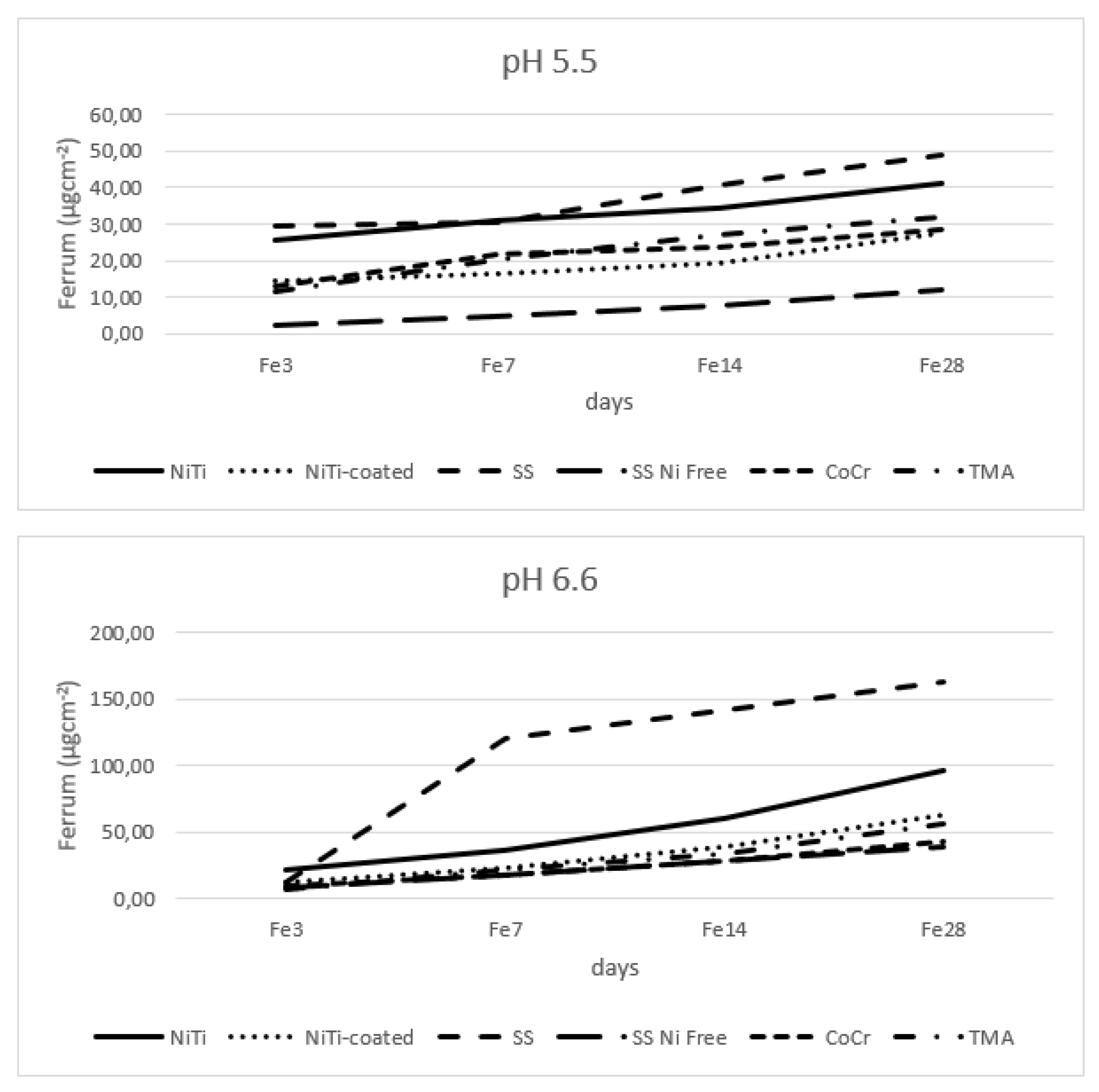
In
Figure 4 there are graphs showing iron release in both pH values. The SS alloy had highest, and nickel free SS alloy lowest release of iron in both pH values. The amounts released in higher pH were three times higher than those released in lower pH.
Table 2 shows that all main effects and all interactions are statistically significant.
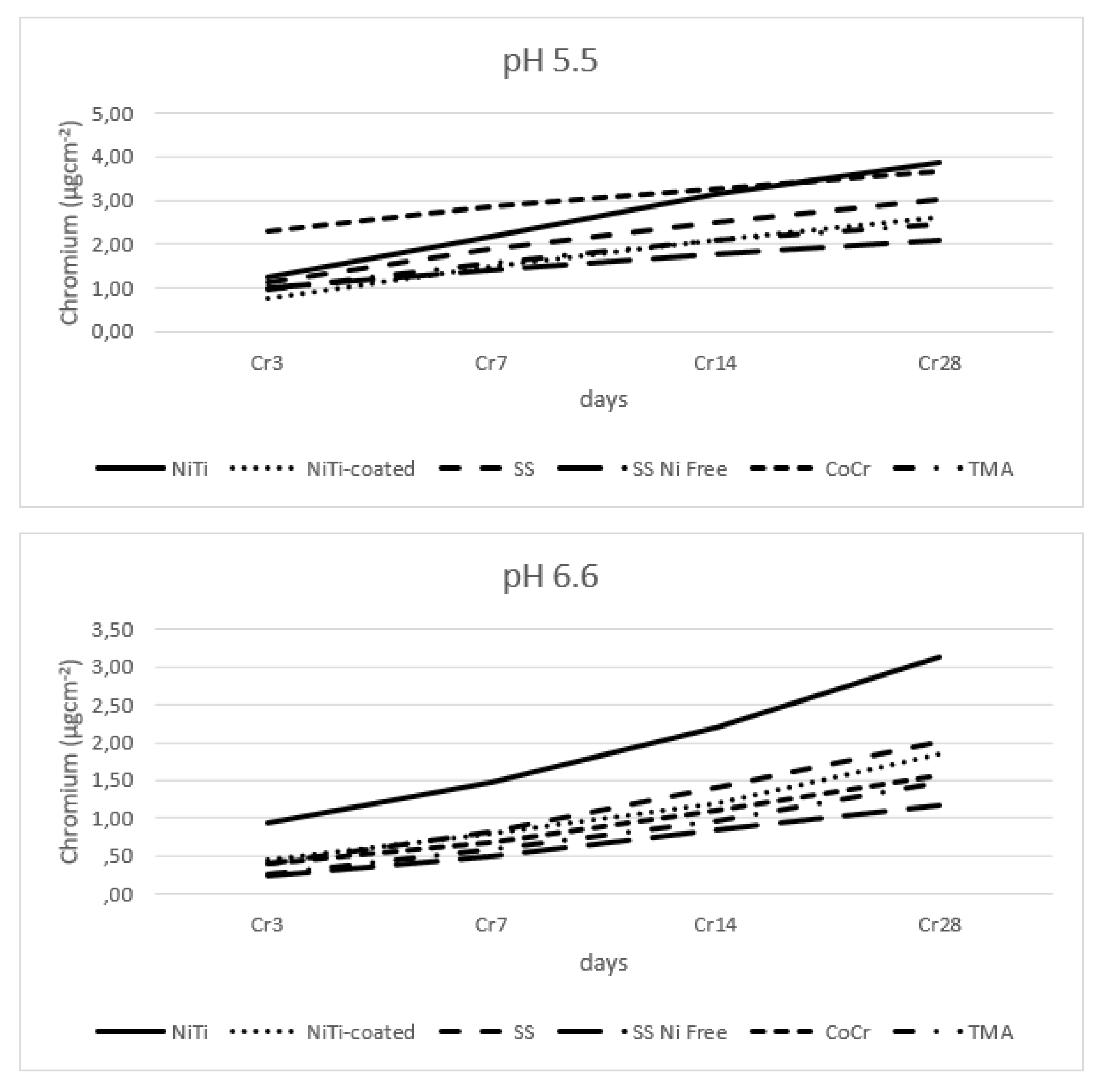
3.4. Chromium Release
Figure 5 shows results of the release of chromium in all tested alloys. In lower pH, most chromium was released from CoCr < SS < SS Ni Free (for alloys with declared Cr), and every other alloy also had recorded release of chromium in similar low amount, probably due to contamination during manufacturing processes.
Table 3 shows all the main effects for the SS and SS Ni Free alloys are significant; of the interactions, only the interaction of alloy type and pH value is insignificant.
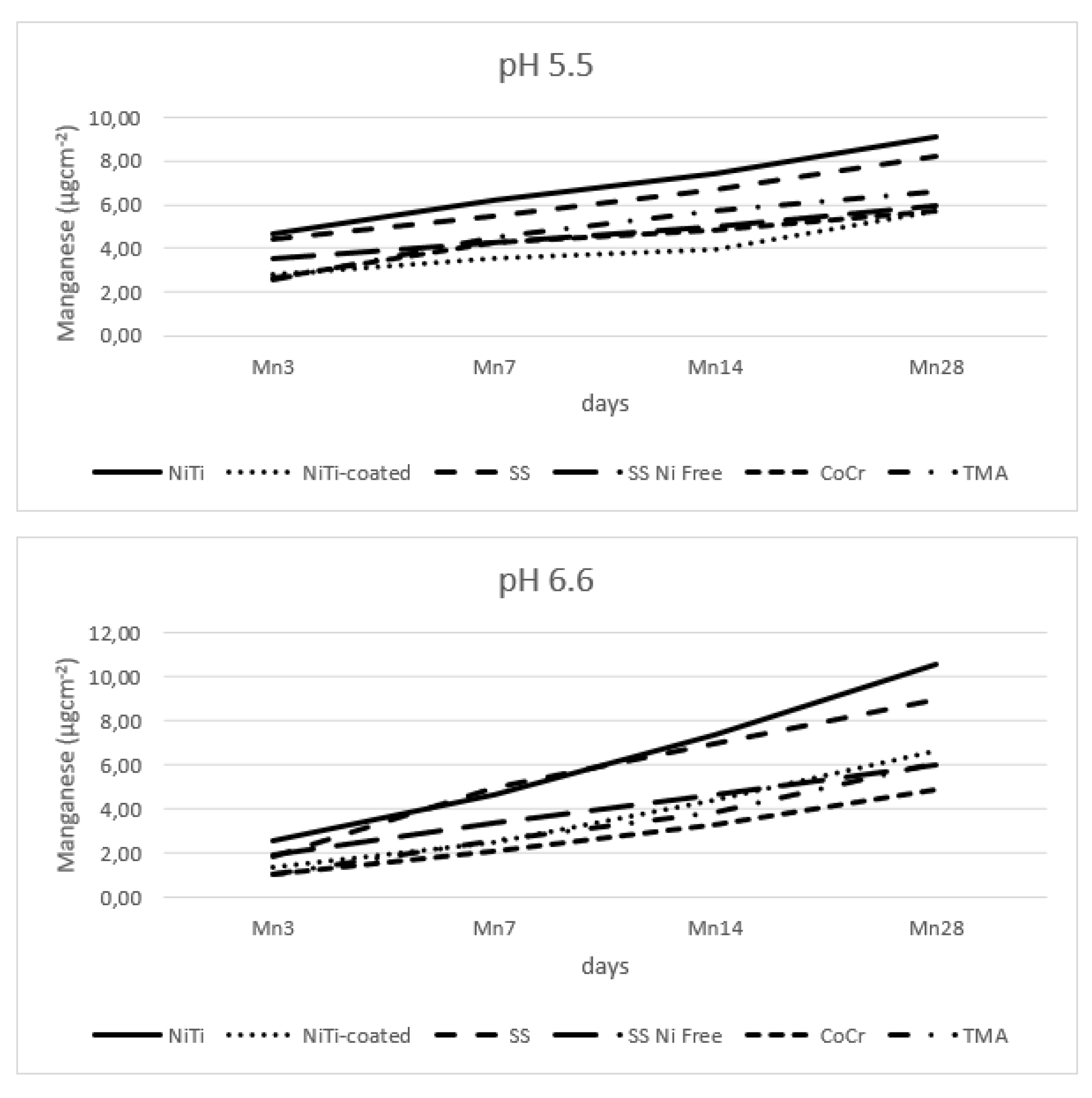
3.5. Manganese Release
Figure 6 shows release of the manganese from the tested alloys; recorded release was overall low, but present in all samples, not just the declared ones.
The results showed that all main effects were significant except for pH. Of the interactions, only the interaction of alloy type and pH value were insignificant, for the SS and SS Ni Free alloys (
Table 4).
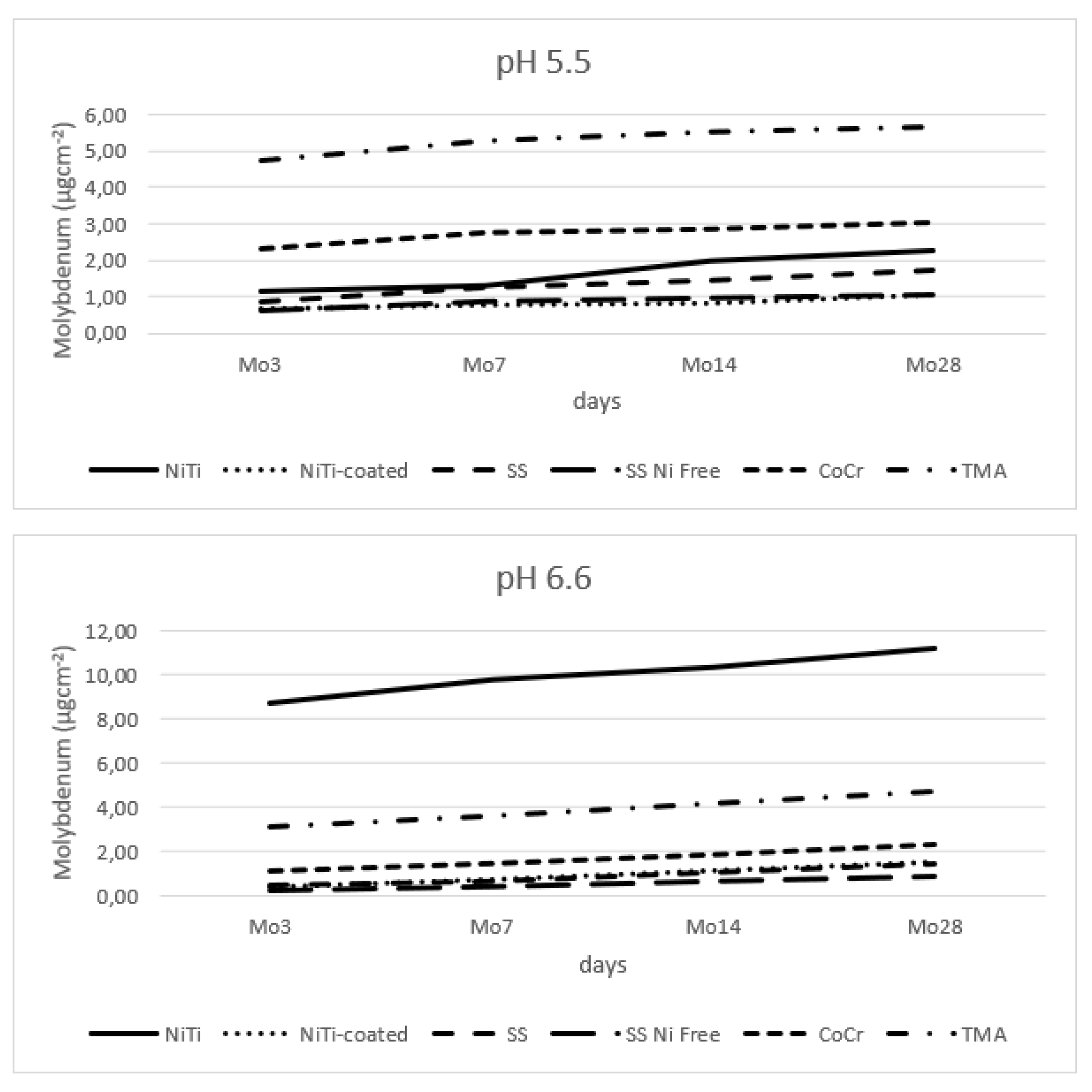
3.6. Molybdenum Release
Figure 7 shows release of molybdenum from the tested alloys. Within the lower pH, the release was: TMA > CoCr > NiTi > SS > SS Ni Free > NiTi-coated.
The results from
Table 5 show that all main effects are statistically significant. Of the interactions, the interaction of type and pH value and the interaction of type of alloy, pH value and time are not significant.
3.7. Cobalt Release
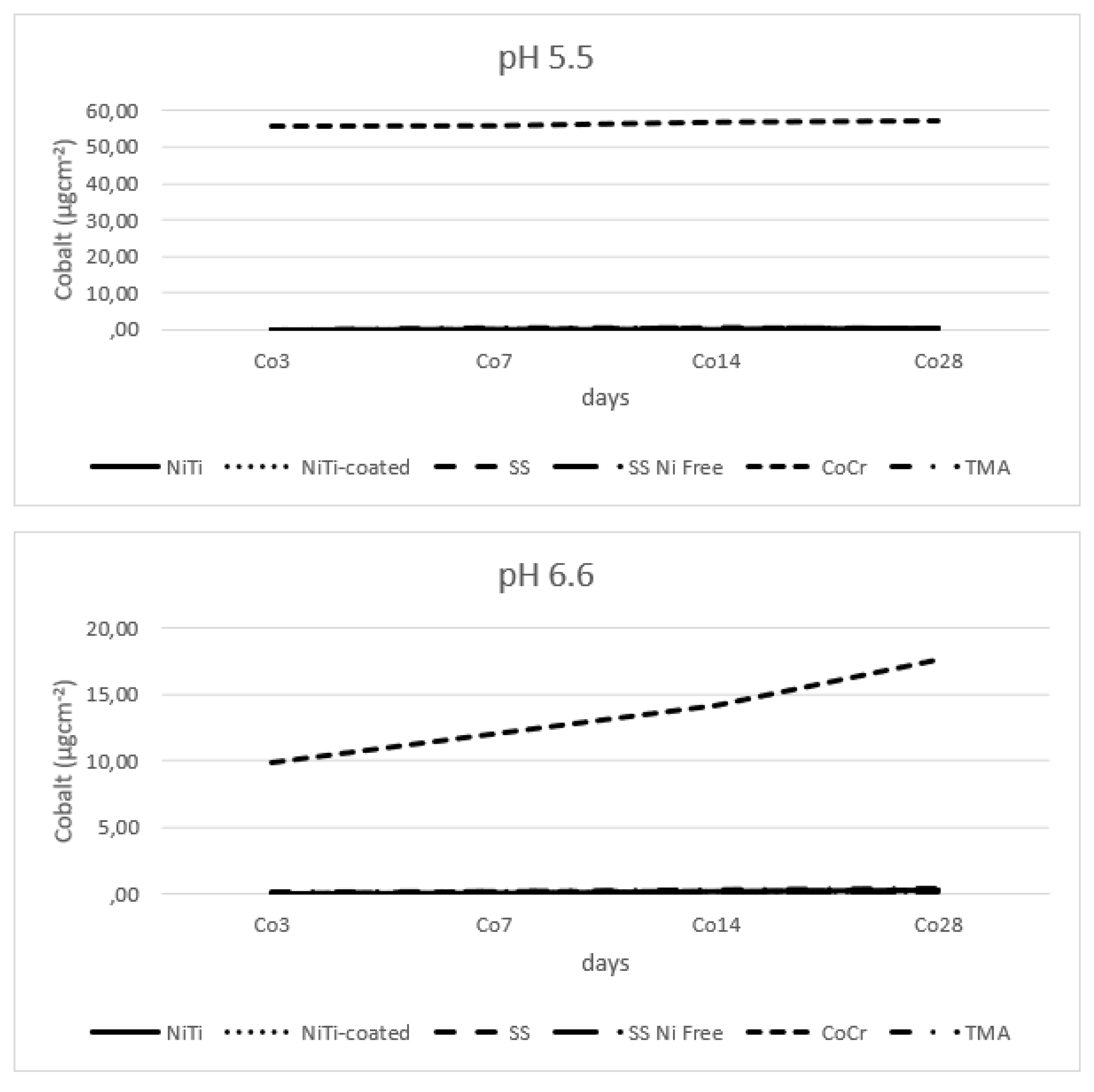
Figure 8 shows minimum release of cobalt from all the alloys, except from CoCr.
The results in
Table 6 show that all main effects and all interactions were statistically significant.
4. Discussion
The ion release from the tested dental alloys showed lower release from the proposed hypoallergenic variants, when compared to the standard materials. The most of the nickel was released from the uncoated NiTi wires and the stainless-steel wires, while their counterparts had no nickel released. Previous research by Laird et al. also found that hypoallergenic alloys release less metallic ions, when compared to the standard alloys. Research by Edward Cho et al. showed that even thickened rhodium coating on NiTi wires leaches nickel, but the overall amount is low, and almost undetectable in subcutaneous tissue [
25]. Previous research showed that Ni is the most common cause of the allergy, and it also acts as a catalyst increasing risk to sensitization and allergy to cobalt [
26]. The interactions and even catalytic effects are the reason more to keep the overall leaching from dental alloys as low as possible.
The titanium release was also significantly bigger in the classic NiTi, when compared to the coated NiTi. Recent research shows more and more cases of the adverse reactions in dental patients, caused by the various combinations of galvanic coupling, modified by a number of extrinsic (various radiation sources) and intrinsic hereditary factors [
12]. The research showed that accumulation of the titanium (and iron) increased the sensitivity of gingival epithelial cells to microorganisms in the oral cavity, and involved in the lymphocytes, macrophages, monocytes and osteoclasts, deteriorating both soft and hard tissue in peri-implantitis cases [
27,
28]. Therefrom, the release of iron should be also kept at minimum, to decrease inflammation and susceptibility to infections of oral mucosa and alveolar bone. In our research, the release of iron was significantly lower in Ni-free SS, when compared to the classic SS alloy. Noteworthy, the higher release of iron was also recorded from classical NiTi alloy, probably due to contamination during the manufacturing process. Also, the iron release from the coated NiTi was significant, in favour of the coated wire alloy.
There is a continuous trend which tends to keep the uptake of the metals, metallic ions and nanoparticles as low as possible, based on the recommendations and legislative of the EU, International Agency for Research on Cancer (IARC) of the World Health Organization (WHO), and the US National Toxicology Program (NTP) and Food and Drug Administration (FDA) [
16,
29,
30,
31]. With the new labelling and consumer warning becoming mandatory, for alloys containing a CMR substance, like nickel, cobalt and chromium, it seems advisable to use alloys with lower quantities of the implicated materials. Our research showed four times higher concentrations of the cobalt are being released from the CoCr alloy, in comparison to the counterpart wire made mainly from titanium and molybdenum, which makes it less recommendable for use.
Manganese is an essential element involved in several physiological functions of the human body, but ongoing Mn accumulation leads to the increased formation of reactive oxygen species (ROS), which contributes to mitochondrial dysfunction, DNA fragmentation, and apoptosis [
32]. Overall, the released manganese levels are low, but still higher for the standard SS alloy, when compared to the hypoallergenic counterpart, with the time of immersion as the most significant factor.
Recommended Dietary Allowances (RDAs) described daily dietary levels of uptake of molybdenum for different age groups, suggesting that children of different age groups are rather sensitive to the uptake levels, although the evidence on influence on human health is limited [
33]. Data on immunotoxicity of the molybdenum is also limited, with reported approximately 3% of patients with stainless steel stents, who also had delayed contact hypersensitivity to molybdenum. Further studies on influence of molybdenum on human health are necessary [
34].
Mineral sources and elements such as chromium, iron, and manganese, among others, are essential for humans because they are involved in a variety of metabolic functions, enzyme activities, receptor sites, hormonal function, and protein transport at specific concentrations, can be regulated to some extent, but excessive overload can result in a number of unfavourable cellular responses from the production of ROS to DNA and protein damage, cell apoptosis and carcinogenesis [
35,
36,
37]. Therefrom, the overall intake of the various elements should be kept as low as possible, as all interactions are still to be investigated.
5. Conclusions
Release of metals from dental orthodontic alloys in vitro is overall lower for the hypoallergenic equivalents, when compared to standard dental alloys.
NiTi released more of the titanium and nickel metal ions, when compared to the coated NiTi.
Stainless steel released more of the iron, chromium and nickel, when compared to the nickel free SS.
CoCr released cobalt in four times higher concentrations than that of the molybdenum released by the TMA.
Author Contributions
Conceptualization, Z.J.O. and V.K..; methodology, V.K.; software, P.T.K. and V.K..; validation, J.A.J. and A.A.R.; formal analysis, M.C. and K.K.; investigation, Z.J.O. and V.K.; resources, Z.J.O.; data curation, V.K.; writing—original draft preparation, Z.J.O., P.T.K. and V.K.; writing—review and editing, J.A.J., M.C. and K.K.; visualization, K.K. and D.V.; supervision, V.K.; project administration, D.V.; funding acquisition, P.T.K. All authors have read and agreed to the published version of the manuscript.
Funding
“This research was funded by Faculty of Dental Medicine, University of Rijeka, grant number CroRIS ID 8071”. “The APC was funded by Faculty of Dental Medicine, University of Rijeka, grant number CroRIS ID 8071”.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study. Requests to access the datasets should be directed to visnja.katic@uniri.hr
Acknowledgments
Thanks to Miroslav Rajter for the statistical analysis.
Conflicts of Interest
“The authors declare no conflicts of interest.” “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Upadhyay D, Panchal MA, Dubey RS, Srivastava VK. Corrosion of alloys used in dentistry: A review. Mat Sci Eng. 2006;432(1-2):1-11.
- Westphalen GH, Menezes LM, Prá D, Garcia GG, Schmitt VM, Henriques JA, Medina-Silva R. In vivo determination of genotoxicity induced by metals from orthodontic appliances using micronucleus and comet assays. Genet Mol Res. 2008;7(4):1259-66. PMID: 19065761. [CrossRef]
- Wichelhaus, A.; Geserick, M.; Hibst, R.; Sander, F.G. The effect of surface treatment and clinical use on friction in NiTi orthodontic wires. Dent. Mater. 2005, 21, 938–945. PMID: 15923033. [CrossRef]
- Quadras DD, Nayak USK, Kumari NS, Priyadarshini HR, Gowda S, Fernandes B. In vivo study on the release of nickel, chromium, and zinc in saliva and serum from patients treated with fixed orthodontic appliances. Dent Res J (Isfahan). 2019;16(4):209-215. PMID: 31303873; PMCID: PMC6596177.
- Prokisch H, Scharfe C, Camp DG 2nd, Xiao W, David L, Andreoli C, Monroe ME, Moore RJ, Gritsenko MA, Kozany C, Hixson KK, Mottaz HM, Zischka H, Ueffing M, Herman ZS, Davis RW, Meitinger T, Oefner PJ, Smith RD, Steinmetz LM. Integrative analysis of the mitochondrial proteome in yeast. PLoS Biol. 2004;2(6):e160. . Epub 2004 Jun 15. PMID: 15208715; PMCID: PMC423137. [CrossRef]
- Chikhale, R., Akhare, P., Umre, U., Jawlekar, R., Kalokhe, S., Badole, N., & Beri, A. In Vitro Comparison to Evaluate Metal Ion Release: Nickel-Titanium vs. Titanium-Molybdenum Orthodontic Archwires. Cureus, 2024 16(3), e56595. [CrossRef]
- Hosiner D, Gerber S, Lichtenberg-Fraté H, Glaser W, Schüller C, Klipp E. Impact of acute metal stress in Saccharomyces cerevisiae. PLoS ONE. 2014 Jan 9;9(1):e83330. PMID: 24416162; PMCID: PMC3886979. [CrossRef]
- Shemtov-Yona K, Rittel D, Levin L; et al. The effect of oral-like environment on dental implants’ fatigue performance. Clin Oral Implants Res. 2014;25:e166–70.
- Noguti J, de Oliveira F, Peres RC; et al. The role of fluoride on the process of Ti corrosion in oral cavity. Biometals. 2012;25:859–62.
- R. Messer, J. Wataha, Dental Materials: Biocompatibility, Editor(s): K.H. Jürgen Buschow, Robert W. Cahn, Merton C. Flemings, Bernhard Ilschner, Edward J. Kramer, Subhash Mahajan, Patrick Veyssière, Encyclopedia of Materials: Science and Technology, Elsevier, 2002, Pages 1-10, ISBN 9780080431529. [CrossRef]
- Schmalz G, Garhammer P. Biological interactions of dental cast alloys with oral tissues. Dent Mater. 2002 Jul;18(5):396-406. PMID: 12175579. [CrossRef]
- Tibau AV, Grube BD, Velez BJ, Vega VM, Mutter J. Titanium exposure and human health. Oral Sci Int. 2019;16:15–24. [CrossRef]
- Evrard L, Waroquier D, Parent D. Allergies to dental metals. Ti: A new allergen. Rev Med Brux. 2010;31:44–9.
- Lee J-J, Song K-Y, Ahn S-G; et al. Evaluation of effect of galvanic corrosion between nickel-chromium metal and Ti on ion release and cell toxicity. J Adv Prosthodont. 2015;7:172–7.
- REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending Regulation (EU) 2017/852 on mercury as regards dental amalgam and other mercury-added products subject to export, import and manufacturing restrictions. Accessed on June 3, 2024: https://data.consilium.europa.eu/doc/document/PE-53-2024-INIT/en/pdf.
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 2020, 10, 1151. [CrossRef]
- Katić V, Curković HO, Semenski D, Baršić G, Marušić K, Spalj S. Influence of surface layer on mechanical and corrosion properties of nickel-titanium orthodontic wires. Angle Orthod. 2014;84(6):1041-8. Epub 2014 Mar 21. PMID: 24654939; PMCID: PMC8638488. [CrossRef]
- Jusufi Osmani, Z.; Poljšak, B.; Zelenika, S.; Kamenar, E.; Marković, K.; Perčić, M.; Katić, V. Ion Release and Surface Changes of Nickel–Titanium Archwires Induced by Changes in the pH Value of the Saliva—Significance for Human Health Risk Assessment. Materials 2022, 15, 1994. [CrossRef]
- Iijima M, Muguruma T, Brantley W, Choe HC, Nakagaki S, Alapati SB, Mizoguchi I. Effect of coating on properties of esthetic orthodontic nickel-titanium wires. Angle Orthod. 2012;82(2):319-25. Epub 2011 Aug 9. PMID: 21827235; PMCID: PMC8867949. [CrossRef]
- Jamilian A, Moghaddas O, Toopchi S, Perillo L. Comparison of nickel and chromium ions released from stainless steel and NiTi wires after immersion in Oral B®, Orthokin® and artificial saliva. J Contemp Dent Pract 2014;15(4):403-6.
- Arndt M, Brück A, Scully T, Jäger A, Bourauel C. Nickel ion release from orthodontic Ni-Ti wires under simulation of realistic in-situ conditions. J Mater Sci. 2005;40:3659–67.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, 2013; Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.
- Kline RB. Principles and practice of structural equation modelling. 2016; Guilford Press, New York, USA.
- Laird C, Xu X, Yu Q, Armbruster P, Ballard R. Nickel and chromium ion release from coated and uncoated orthodontic archwires under different pH levels and exposure times. J Oral Biosci. 2021;63(4):450-454. PMID: 34740833. [CrossRef]
- Edward Cho, Zuisei Kanno, Ikuo Yonemitsu, Hajime Kiyokawa, Nobutaka Ohira, Takashi Ono, Motohiro Uo, Effect of rhodium plating on the ion dissolution from nickel-titanium and pure nickel wires, Asian Pacific Journal of Dentistry, 2022, Volume 22, Issue 2, Pages 21-28. [CrossRef]
- Bonefeld CM, Nielsen MM, Vennegaard MT, Johansen JD, Geisler C, Thyssen JP. Nickel acts as an adjuvant during cobalt sensitization. Exp Dermatol. 2015;24(3):229-31. PMID: 25580744. [CrossRef]
- Fretwurst T, Buzanich G, Nahles S; et al. Metal elements in tissue with dental peri-implantitis: A pilot study. Clin Oral Implants Res. 2016;9:1178–86.
- Wachi T, Shuto T, Shinohara Y; et al. Release of Ti ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology. 2015;327:1–9.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Chromium, Nickel and Welding. Volume 49. Available online: https://publications.iarc.fr/67 (accessed on 27 June 2024).
- National Toxicology Program. Available online: https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html (accessed on 27 June 2024).
- Biological Responses to Metal Implants. FDA Report Published on September 2019. Available online: https://www.fda.gov/media/132446/download (accessed on 28 June 2024).
- Harischandra DS, Ghaisas S, Zenitsky G, Jin H, Kanthasamy A, Anantharam V, Kanthasamy AG. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front Neurosci. 2019;13:654. PMID: 31293375; PMCID: PMC6606738. [CrossRef]
- Agency for Toxic Substances and Disease Registry. Division of Toxicology and Human Health Sciences. Environmental Toxicology Branch. U.S. Department of Health and Human Services. Toxicological profile for molybdenum: Draft for public comment.external link disclaimer April 2017.
- Köster R, Vieluf D, Kiehn M, Sommerauer M, Kähler J, Baldus S, Meinertz T, Hamm CW. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet. 2000;356(9245):1895-7. Erratum in: Lancet 2001 Jan 27;357(9252):316. PMID: 11130387. [CrossRef]
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014; 7(2):60-72. PMID: 26109881; PMCID: PMC4427717. [CrossRef]
- Kim KT, Eo MY, Nguyen TTH, Kim SM. General review of titanium toxicity. Int J Implant Dent. 2019;5(1):10. PMID: 30854575; PMCID: PMC6409289. [CrossRef]
- Grosgogeat, B.; Vaicelyte, A.; Gauthier, R.; Janssen, C.; Le Borgne, M. Toxicological Risks of the Cobalt–Chromium Alloys in Dentistry: A Systematic Review. Materials 2022, 15, 5801. [CrossRef]
Figure 1.
Nickel release from the tested alloys.
Figure 1.
Nickel release from the tested alloys.
Figure 2.
Titanium release from the tested alloys.
Figure 2.
Titanium release from the tested alloys.
Figure 3.
Comparison of titanium release from NiTi and NiTi-coated alloys for every time point and pH showed significant differences.
Figure 3.
Comparison of titanium release from NiTi and NiTi-coated alloys for every time point and pH showed significant differences.
Figure 4.
Iron (ferrum) released from dental alloys.
Figure 4.
Iron (ferrum) released from dental alloys.
Figure 5.
Release of chromium from wire alloys.
Figure 5.
Release of chromium from wire alloys.
Figure 6.
Release of manganese from the alloy wires.
Figure 6.
Release of manganese from the alloy wires.
Figure 7.
Release of molybdenum from alloy wires.
Figure 7.
Release of molybdenum from alloy wires.
Figure 8.
Release of cobalt from alloy wires.
Figure 8.
Release of cobalt from alloy wires.
Table 1.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the titanium released from NiTi and NiTi-coated alloys.
Table 1.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the titanium released from NiTi and NiTi-coated alloys.
| Effect |
DFn1
|
DFd2
|
F3
|
p4
|
ges5
|
| alloy |
1.0 |
16.00 |
703.27 |
0.000 |
0.967 |
| pH |
1.0 |
16.00 |
277.97 |
0.000 |
0.921 |
| time |
1.5 |
23.93 |
1946.00 |
0.000 |
0.975 |
| alloy:pH |
1.0 |
16.00 |
188.09 |
0.000 |
0.888 |
| alloy:time |
1.5 |
23.93 |
79.59 |
0.000 |
0.619 |
| pH:time |
1.5 |
23.93 |
1.31 |
0.280 |
0.026 |
| alloy:pH:time |
1.5 |
23.93 |
3.79 |
0.048 |
0.072 |
Table 2.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the iron released from SS and SS Ni Free alloys.
Table 2.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the iron released from SS and SS Ni Free alloys.
| Effect |
DFn1
|
DFd2
|
F3
|
p4
|
ges5
|
| alloy |
1.00 |
16.00 |
116.44 |
0.000 |
0.852 |
| pH |
1.00 |
16.00 |
66.34 |
0.000 |
0.767 |
| time |
1.04 |
16.72 |
198.19 |
0.000 |
0.720 |
| alloy:pH |
1.00 |
16.00 |
26.27 |
0.000 |
0.566 |
| alloy:time |
1.04 |
16.72 |
83.05 |
0.000 |
0.518 |
| pH:time |
1.04 |
16.72 |
111.83 |
0.000 |
0.592 |
| alloy:pH:time |
1.04 |
16.72 |
66.41 |
0.000 |
0.462 |
Table 3.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the chromium released from SS and SS Ni Free alloys.
Table 3.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the chromium released from SS and SS Ni Free alloys.
| Effect |
DFn1
|
DFd2
|
F3
|
p4
|
ges5
|
| alloy |
1.00 |
16.00 |
105.94 |
0.000 |
0.864 |
| pH |
1.00 |
16.00 |
338.63 |
0.000 |
0.953 |
| time |
1.78 |
28.46 |
10382.57 |
0.000 |
0.962 |
| alloy:pH |
1.00 |
16.00 |
0.91 |
0.355 |
0.052 |
| alloy:time |
1.78 |
28.46 |
783.81 |
0.000 |
0.657 |
| pH:time |
1.78 |
28.46 |
123.36 |
0.000 |
0.232 |
| alloy:pH:time |
1.78 |
28.46 |
18.75 |
0.000 |
0.044 |
Table 4.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the manganese released from SS and SS Ni Free alloys.
Table 4.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the manganese released from SS and SS Ni Free alloys.
| Effect |
DFn1
|
DFd2
|
F3
|
p4
|
ges5
|
| alloy |
1.00 |
16.00 |
19.68 |
0.000 |
0.534 |
| pH |
1.00 |
16.00 |
2.73 |
0.118 |
0.137 |
| time |
1.17 |
18.74 |
1062.00 |
0.000 |
0.820 |
| alloy:pH |
1.00 |
16.00 |
0.08 |
0.785 |
0.004 |
| alloy:time |
1.17 |
18.74 |
67.33 |
0.000 |
0.224 |
| pH:time |
1.17 |
18.74 |
92.44 |
0.000 |
0.284 |
| alloy:pH:time |
1.17 |
18.74 |
11.91 |
0.002 |
0.049 |
Table 5.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the molybdenum released from CoCr and TMA alloys.
Table 5.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the molybdenum released from CoCr and TMA alloys.
| Effect |
DFn1
|
DFd2
|
F3
|
p4
|
ges5
|
| alloy |
1.00 |
16.0 |
448.72 |
0.000 |
0.963 |
| pH |
1.00 |
16.0 |
115.28 |
0.000 |
0.869 |
| time |
1.07 |
17.2 |
656.49 |
0.000 |
0.760 |
| alloy:pH |
1.00 |
16.0 |
2.30 |
0.149 |
0.117 |
| alloy:time |
1.07 |
17.2 |
18.04 |
0.000 |
0.080 |
| pH:time |
1.07 |
17.2 |
58.63 |
0.000 |
0.221 |
| alloy:pH:time |
1.07 |
17.2 |
2.11 |
0.164 |
0.010 |
Table 6.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the cobalt released from CoCr and TMA alloys.
Table 6.
Analysis of the main effects using analysis of variance for repeated measurements with testing of the effects of pH, type of alloy and time (mixed ANOVA), for the cobalt released from CoCr and TMA alloys.
| Effect |
DFn1
|
DFd2
|
F3
|
p4
|
ges5
|
| alloy |
1.00 |
16.0 |
2952.04 |
0.000 |
0.994 |
| pH |
1.00 |
16.0 |
1143.87 |
0.000 |
0.986 |
| time |
1.28 |
20.4 |
188.40 |
0.000 |
0.321 |
| alloy:pH |
1.00 |
16.0 |
1129.63 |
0.000 |
0.985 |
| alloy:time |
1.28 |
20.4 |
160.17 |
0.000 |
0.286 |
| pH:time |
1.28 |
20.4 |
83.23 |
0.000 |
0.173 |
| alloy:pH:time |
1.28 |
20.4 |
76.17 |
0.000 |
0.160 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).