1. Introduction
Hydrazine N
2H
4 is used as a rocket propellant, antioxidant, a pesticide precursor, a corrosion inhibitor in boilers, etc. [
1,
2]. However, hydrazine is toxic and its exposure can injure the lungs, liver, kidney, and central nervous system of living organisms [
3]. Therefore, the study of the exact mechanism ofhydrazine oxidation is of permanent interest.
The stoichiometry of the decomposition of hydrazine depends on the relative concentrations of OH
-, c(OH-) and hydrazine, c(N
2H
4) [
4,
5].
where x = c(OH-)/c(N
2H
4). For x >> 1 is dc(NH
3)/dt → 0 and for x<< 1 is dc(H
2)/dt → 0. Depending on the reaction conditions, different stiochiometry is also possible [
6].
Mass spectroscopic analysis of nitrogen molecules obtained by oxidation of
15N-enriched hydrazine by various oxidizing agents in aqueous solutions produced
14N
2,
15N
14N and
15N
2 molecules [
7,
8]. The relative content of the
15N
2 and
15N
14N molecules depends on the oxidizing agent used. Thus, some of nitrogen molecules must be formed by a mechanism including a N-N fission and the formation of the nitrogen-containing radicals from two different hydrazine molecules.
According to Cahn and Powell [
8] the randomized
15N
14N composition is obtained by one-electron oxidation of
15N enriched hydrazines whereas four-electron oxidizing agents (acid iodate, alkaline ferricyanide) produce unrandomized N
2 molecules (all four hydrogen atoms are removed from a single hydrazine molecule). According to Petek and Bruckenstein [
6] the electrooxidation of
15N labeled hydrazine (96.7% enrichment) on the Pt electrode produces N
2 molecules with the ratio of
14N
15N/
15N
15N = 0.07 ± 0.01 while in Ce(IV) solutions it was 0.9 ± 0.2. Simultaneous electrooxidation and homogeneous oxidation with electrogenerated Ce(IV) produced both isotopic forms between these two limits.
Cahn and Powell [
8] mentioned an alternative way of oxidation of hydrazine through diimine HN=NH intermediate as well.
Polarographic and voltammetric studies of hydrazine in solutions of intermediate acidity (e.g., 0.025 – 0.25 M H
2SO
4) indicate that higher initial hydrazine concentrations cause proportionally increased concentrations of diimine N
2H
2 and therefore its dimerization rate to tetrazene N
4H
4 increases more rapidly than the rate of further oxidation according to the Equation (4) [
9]. Other authors [
10,
11] mentioned an alternative disproportionation mechanism as well.
The relative stability of 2-tetrazene H
2N-N=N-NH
2 in trans-conformation is confirmed by its X-ray structure determined at -90
° C [
12].
The randomized
15N
14N composition in the case of high concentrations of tetrazene N4H4 can be explained in analogy with Equations (2) and (3) as follows
Quantum-chemical studies of tetrazenes are very rare. Zhao and Gimarc [
13] investigated the strain energies of (NH
)n rings, n = 3 – 8, at the Hartree-Fock and MP2 levels of theory using the 6-31G** basis sets. They found the maximal ring strain for n = 4. The preferred ring conformations have nearly perpendicular lone electron pairs on adjacent N atoms (the gauche effect). Ball [
14] evaluated the vibrational heats of formation of four stereoisomers of cyclo-(NH)
4 at several post-Hartree-Fock theoretical levels. Feng et al. [
15] studied the structures, charges, isomerization and decomposition of four tripodal (unusable for our purposes) and ten linear (
anti-conformation) N
4H
4 structures at the CCSD(T)/cc-pVTZ level of theory using B3LYP/6-311++G(d,p) optimized geometries. Hu and Zhang [
16] performed periodic DFT molecular dynamics calculations of high-pressure N
4H
4 structures at zero temperature. In the pressure range 30 – 100 GPa, three stable P2
1/m structures were found, namely the (NH)
4 solid in trans-conformation and an ambient pressure
trans-2-tetrazene H
2N-N=N-NH
2 and the most stable ammonium azide. Two additional molecular N
4H
4 structures (H
2N-N-NH-NH in
trans-conformation and NH
3…HN
3) and three transition states were also found.
In our previous study [
17] we have investigated the structures, thermodynamics, and electron characteristics of various N
4H
6 isomers in aqueous solutions by means of quantum chemistry at the CCSD/cc-pVTZ level of theory. The aim of our previous study was to explain the existence of
14N
15N molecules obtained by the homogenous oxidation of a mixture of non-labeled (
14N
2H
4) and
15N-labeled hydrazine (
15N
2H
4) in an aqueous solution according to the Equations (1)–(3). We focused on the N – N fissions in N
4H
6 structures obtained by hydrogen rearrangements as a crucial part of the entire oxidation reaction. The dominant abundance of NH
3... N
2... NH
3 species according to Gibbs energies obtained by splitting the lateral N – N bonds is in full agreement with the
14N
15N molecules formation. Except for stable cyclo-(NH)
4. H
2 structure, the initial cyclic structures are split into hydrazine and N
2H
2 species in agreement with the
14N
15N molecules formation but their abundance in aqueous solutions is vanishing.
Above, we have shown that the oxidation of hydrazine in solutions of intermediate acidity into
15N
14N molecules can proceed through N
4H
4 intermediates according to Equations (6)–(8) as well [
9,
10,
11]. In analogy to [
17], the main aim of our manuscript is a quantum-chemical study of N
4H
4 isomers in aqueous solutions and to determine the sites of possible N – N fissions in these molecules. The Gibbs energies of the decomposed N
4H
4 products allow us to predict the formation of
14N
15N in real systems. Thus we are interested dominantly in the nitrogen backbone and its possible fissions. The electronic structures of the stable isomers will be compared as well.
2. Results
In analogy to our previous study [
17], we use the linear backbone notation N1-N2-N3-N4 and the composition notation of N1H
m-N2H
n-N3H
p-N4H
q, where subscripts m, n, p and q denote the number of H atoms bonded to individual Nj atoms with j = 1 → 4 and m + n + p + q = 4. Geometry optimizations started with planar
anti- and
syn-conformations of the N1-N2-N3-N4 backbone denoted Amnpq and Bmnpq, respectively. During geometry optimization some N - H bonds can be split and the new ones can be created. In such cases, the original notation is preserved only with new values of m, n, p, or q. Alternatively, some N – N bonds can split and new structures with destroyed N1-N2-N3-N4 backbones are denoted as D(mn)(pq) or D(m)(npq), where the individual components are enclosed in round brackets. If new N – N bonds are formed instead of the split ones, the obtained structures are denoted with the letter E such as Emn)(pq. This means that the N2 – N3 bond is split and a new N1 – N4 bond is created, i.e., the new composition N2H
n-N1H
m-N4H
q-N3H
p. In general, if N1 and N2 atoms are
15N labeled, while N3 and N4 correspond to
14N, then splitting the N1-N2 and N3-N4 bonds would lead to
14N
15N molecules, unlike N2-N3 fissions.
An analogous notation Cmnpq is used for cyclo-N4H4 isomers. In these structures, any N – N fission can lead to the formation of the 15N14N molecules as a consequence of suitable H transfers within the cycle.
The different structures with the same Xmnpq notation, X = A, B, C, D or E, are distinguished by additional letters a, b, c, d, etc. at the end of the Xmnpq symbol, such as D(mn)(pq)b. These structures differ in energies because of different hydrogen bonding patterns.
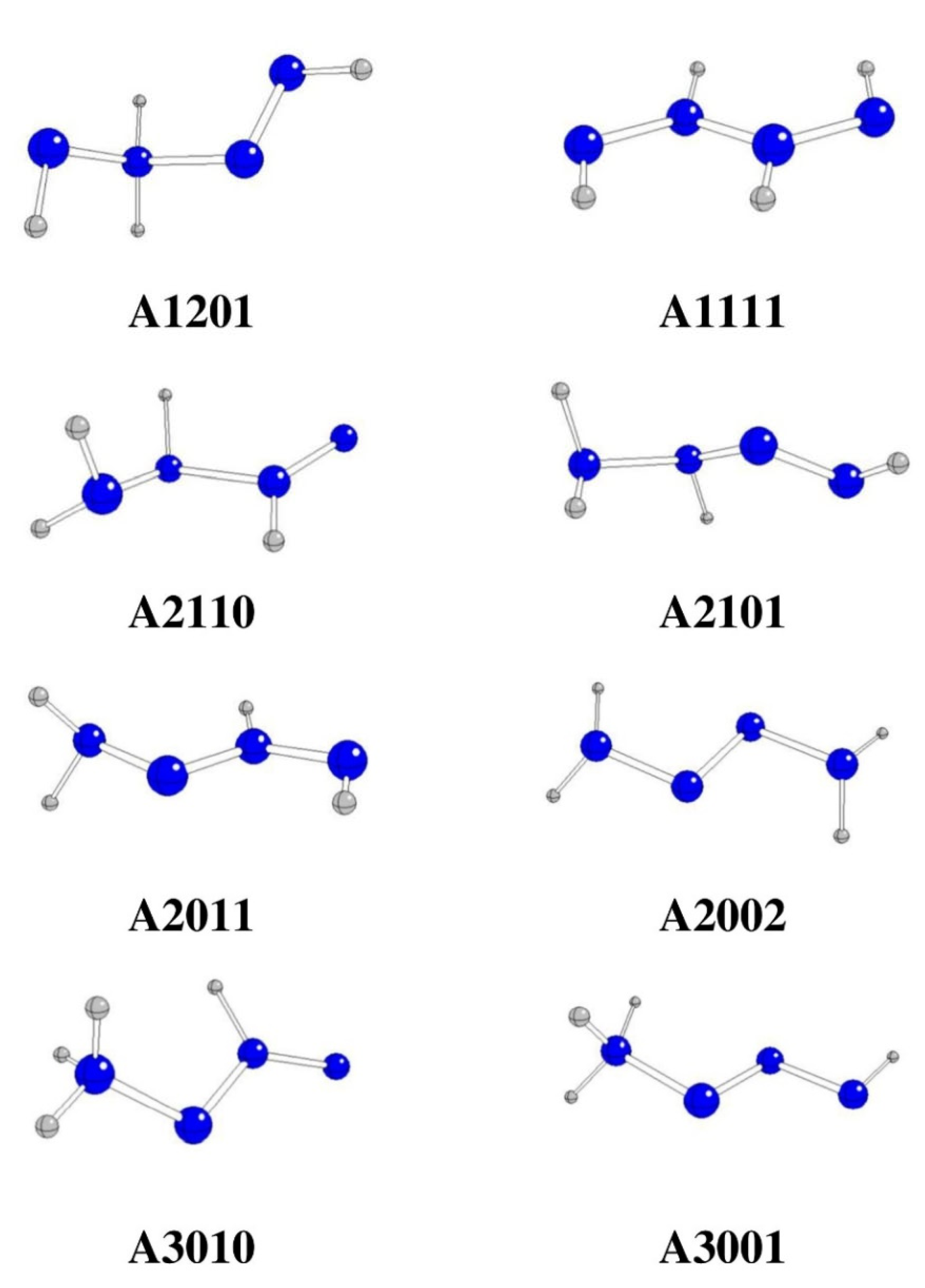
Among the starting structures in
anti-conformations, A2020 is transformed into A2011 and A4000 into A3010 due to H transfers from N3 to N4 and from N1 to N3 (see
Table 1 and
Figure 1). Both structures are practically identical to those obtained by geometry optimizations of original A2011 and A3010, respectively. A0220 is split into two H
2N=N species, A1210 and A2200 are split into H
2N-NH
2 and N
2, A1120 into HN=NH and H2N = N, while A3100 is split into NH
3-NH and N
2. In general, N2-N3 fissions cannot finally lead to
14N
15N molecules. According to Gibbs energy data (
Table 1), three of these split structures belong to the most stable intermediates of hydrazine oxidation reactions. The remaining anti-conformers preserve their original structures with only small changes (
Table 1,
Figure 1).
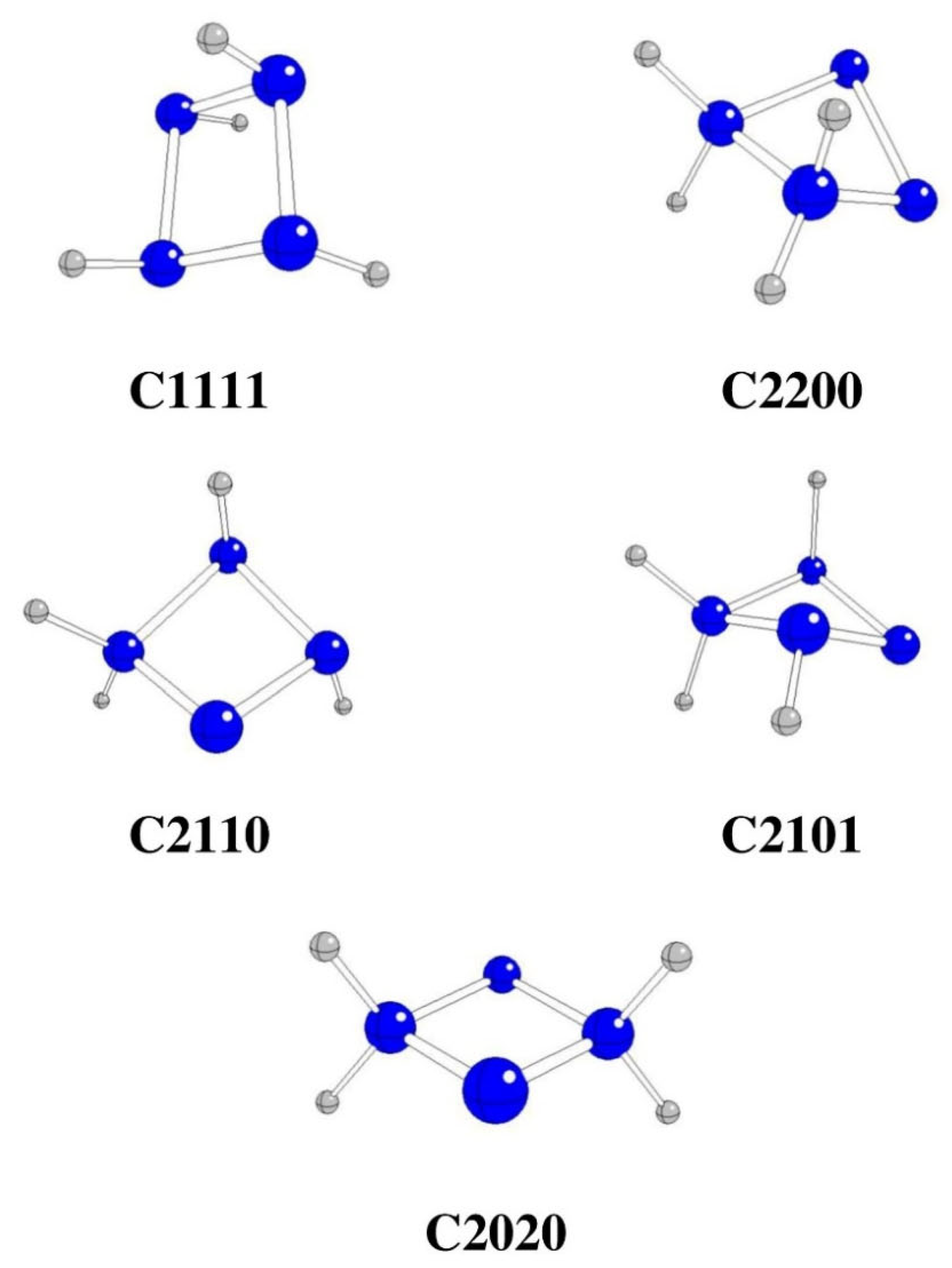
All starting cyclo-N
4H
4 structures preserve their (nearly) original conformations without any H transfers or N-N fissions (
Table 1,
Figure 3). However, their Gibbs energies are relatively high (in agreement with [
13]) and therefore it is highly unlikely (maybe except C1111) that they can serve as intermediates of hydrazine oxidation.
The last group of the systems under study is the D(mn)(pq) one. Unlike our previous study of N
4H
6 isomers [
17], N
4H
4 isomers are spontaneously split at N2-N3 bonds only. Geometry optimization of possible N
4H
4 isomers indicates that only the N1H
m-N2H
n and N3H
p-N4H
q subsystems can be obtained by possible hydrogen rearrangements (
Table 1,
Figure 4).
The bonding properties of N
4H
4 isomers are described in terms of individual bond lengths (
Table 2 and
Table 3) and in terms of Quantum Theory of Atoms-in-Molecules (QTAIM) [
18] such as the electron density ρ
BCP (
Table 4 and
Table 5) and ellipticity ε
BCP (
Table 6 and
Table 7) at their bond critical points (BCP). Molecular graphs consist of critical points and bond paths between individual atoms. The bond strengths decrease with bond lengths and increase with their BCP electron densities ρ
BCP. Their double bond character in acyclic structures increases with their BCP ellipticities ε
BCP. However, in cyclic structures, increased ε
BCP values may reflect mechanical bond strain. According to our previous study of N
4H
6 isomers [
17] single, double and triple N-N bonds exhibit BCP electron densities of around 0.2, 0.5 and 0.7 e/Bohr
3, respectively, with BCP ellipticities of ca 0.0, 0.2 and 0.0, respectively. Our results for N
4H
4 isomers are in agreement with these values despite the N1H
3-N2 bond lengths in A3010, A3001, B3010 and B3001a-b of ca 1.5 Å indicate weaker bonding. Except for A1201, the BCP ellipticity of the central N1 – N3 bonds is higher than that of the lateral N1 – N2 and N3 – N4 bonds. The significant deviations from N1-N2-N3-N4 planarity (such as in A1201, A2110, A2101, B2110, B2101) in agreement with the gauche effect [
14] decrease the BCP ellipticity (i.e. the π character) of N2-N3 bonds. The BCP electron density of N-N bonds decreases with the number of H atoms bonded to these N atoms. In cyclic structures, this dependence is less evident. The characteristics of N-H bonds exhibit high similarities as well. Higher ε
BCP(N-H) values can be ascribed mainly to the double-bond character of neighboring N-N bonds, as in [
17]. The hydrogen bonds N…H and non-bonding interactions N...N (see
Table 4,
Table 5,
Table 6 and
Table 7 and
Figures S1–S4 in the
Supplementary Materials) affect the above bonding properties as well.
The D systems consist of two independent molecules such as HN=NH, H
2N=N, H
2N-NH
2, H
3N-NH or N
2, which mutually interact through weak N…H hydrogen bonds and/or N…N non-bonding interactions only. These molecules were investigated in our previous study on N
4H
6 [
17], so their properties are not discussed here in more detail.
Similarly to [
17], the charges (
Table 8) on the lateral N1 and N4 atoms in the A and B structures are more negative than on the central N2 and N3 atoms. Negative N charges in the A, B and C systems (
Table 8 and
Table 9) increase with the number of bonded H atoms. Analogously, the positive hydrogen charges increase with the number of H atoms bonded to the same nitrogen. The charges of the H atoms bonded to the central N2 and N3 atoms in the A and B systems are more positive than their lateral N1 and N4 analogs. The D systems contain practically neutral N atoms in the N
2 subsystems, and their negative charges in other subsystems increase with the number of bonded H atoms (
Table 9). N…H hydrogen bonds and N…N non-bonding interactions (
Table 9 and
Figure S4 in
Supplementary Materials) affect the N and H charges as well.
4. Conclusions
Unlike our previous study of tetrazanes N
4H
6 decomposition [
17], we have not found any spontaneous splitting the bonds between lateral nitrogen atoms (i.e., N1-N2 and N3-N4 fissions) in tetrazenes N
4H
4 during geometry optimization. It implies that no hydrogen rearrangements in N
4H
4 species can cause that the original
14N-
14N-
15N-
15N backbone obtained by dimerization of either
15N labelled or unlabelled HN=NH would produce
14N
15N molecules. This finding might explain the observation of Cahn and Powell [
8] that the randomized
14N
15N composition is obtained by one-electron oxidation of
15N enriched hydrazines whereas four-electron oxidizing agents produce unrandomized N
2 molecules (they supposed that all four hydrogen atoms are removed from a single hydrazine molecule). It can be expected that the formation of the diamine HN=NH intermediate is more probable by four-electron oxidation as well.
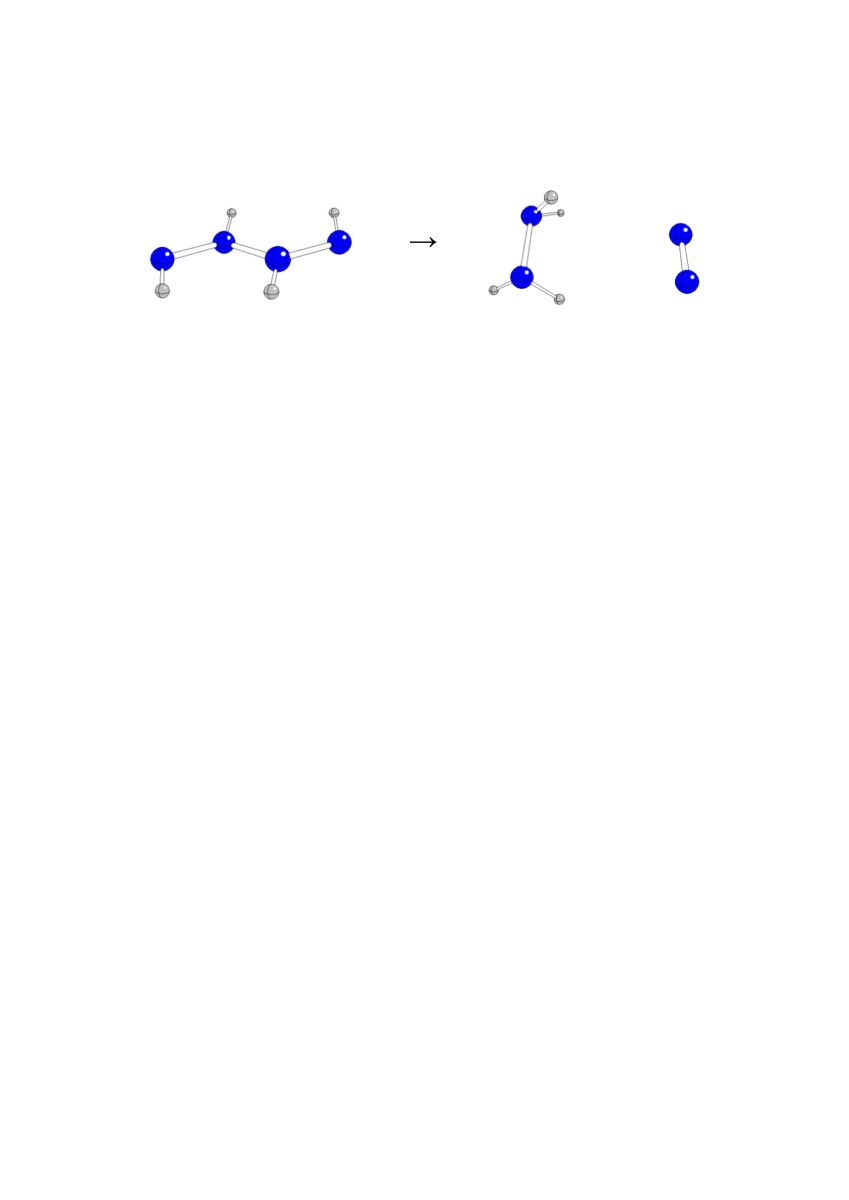
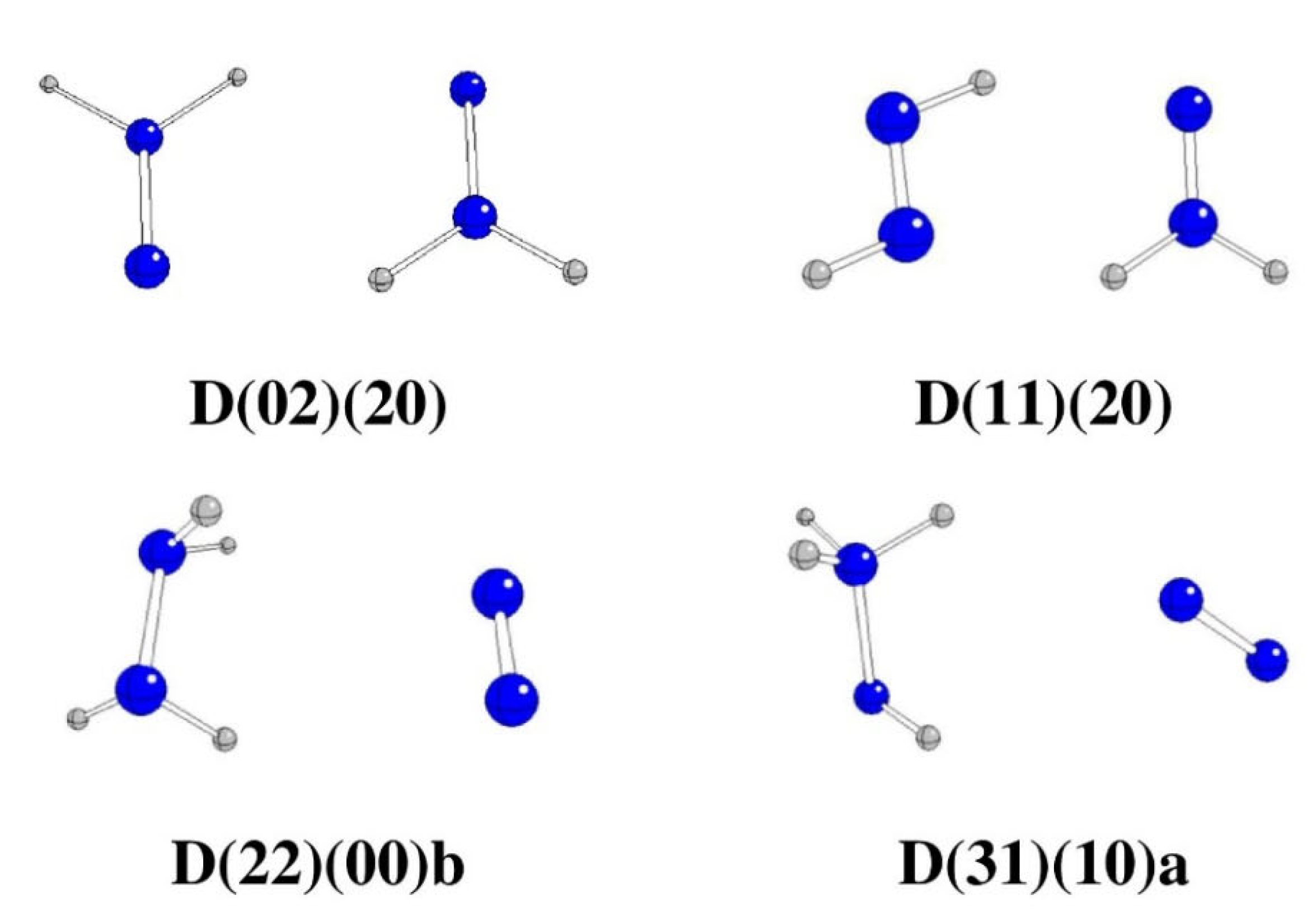
The most probable N
4H
4 split products are H
2N-NH
2 and N
2 (see
Table 1 for Gibbs energies), denoted as D(22)(00), which are obtained by splitting the bond between central nitrogen atoms (i.e., N2-N3 fissions) and so only
15N
2 and
14N
2 molecules are formed. Additionally, the formation of H
3N-NH and N
2 oxidation products, denoted as D(31)(00), is preferred over structures without any N-N fissions as well (see
Table 1 for Gibbs energies). The formation of H
2N=N and HN=NH oxidation products (such as D(02)(20) and D(11)(20), see
Table 1) is energetically less advantageous.
Hydrogen transfers in aqueous solutions are mediated by H
2O, H
3O
+ and/or OH
- species. Similarly as in our previous study of N
4H
6 decomposition [
17], the lateral N and H charges in N
4H
4 have very high charges that should support such H transfers. Nevertheless, the transfer of the third hydrogen to the lateral nitrogen from the central one without splitting the bond between both central nitrogens is energetically much less advantageous in comparison with most N
4H
4 isomers as indicated by Gibbs energies of the structures NH
3-N=NH=N, denoted as A3010 and B3010 (see
Table 1).
Unlike cyclo-N
4H
6 structures [
17], their cyclo-N
4H
4 analogs are stable, without any N-N fissions. Nevertheless, their very high Gibbs energies (
Table 1) indicate, that their relative abundance in aqueous solution is vanishing, so their involvement in hydrazine oxidation is highly improbable.
In agreement with our previous study [
17], the QTAIM analysis confirmed that single, double and triple N-N bonds exhibit BCP electron densities of ca. 0.2, 0.5 and 0.7 e/Bohr
3, respectively, with BCP ellipticities of ca 0.0, 0.2 and 0.0, respectively. Nevertheless, hydrogen bonds can cause significant deviations from these values.
Our study deals only with some thermodynamics aspects of hydrazine oxidation in aqueous solutions. We have not deal with transition states related to hydrogen transfers and the role of water molecules/ions by these transfers. For such model calculations the individual solvent molecules/ions must be included as well. Also the differences between reaction mechanisms of two- and four-electron oxidation [
8] of hydrazine are worth of study. Further theoretical and experimental studies in these fields are necessary.
Figure 1.
Optimized stable N4H4 structures in anti-conformation (N – blue, H – gray).
Figure 1.
Optimized stable N4H4 structures in anti-conformation (N – blue, H – gray).
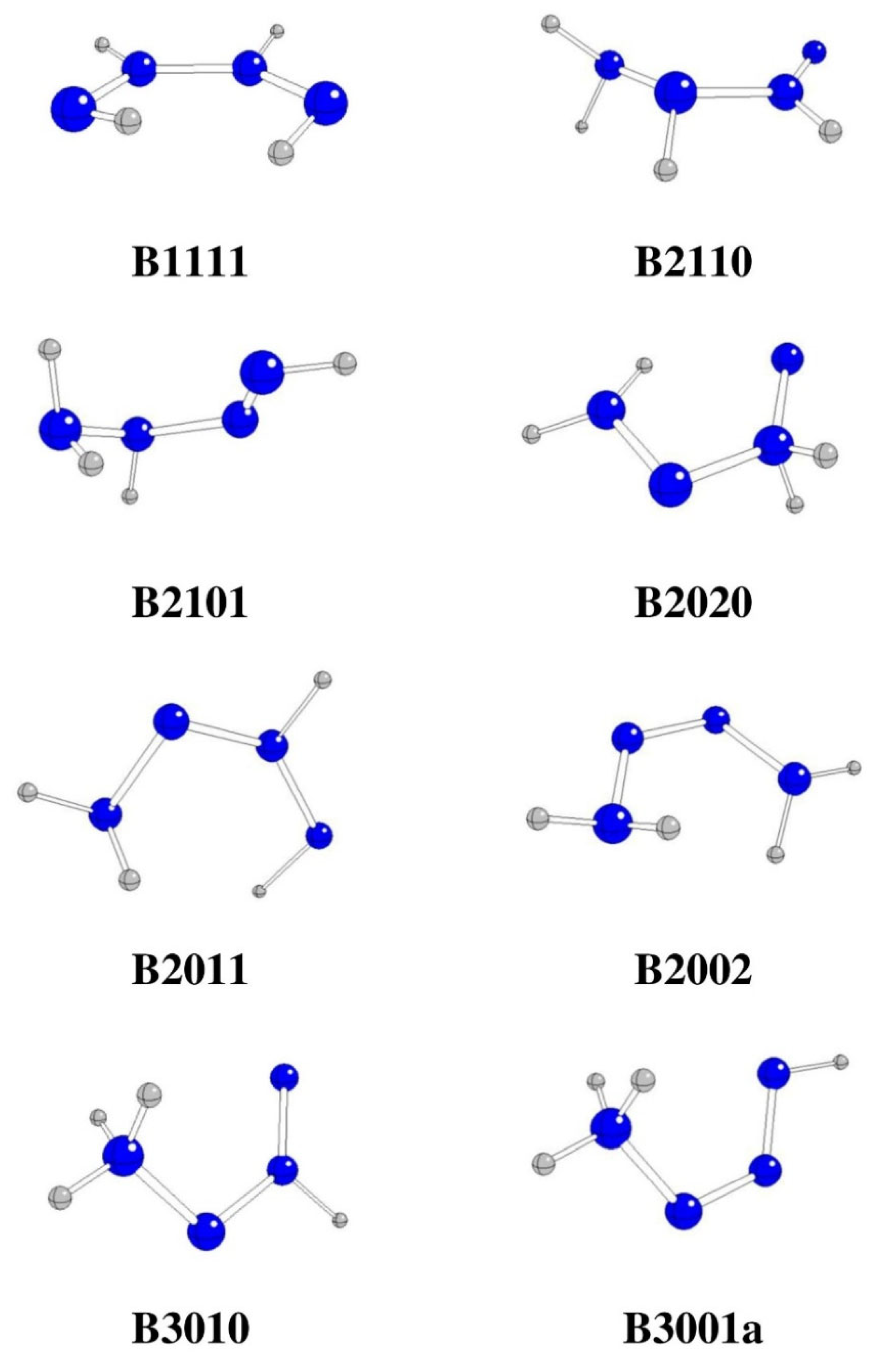
Figure 2.
Optimized stable N4H4 structures in syn-conformation (N – blue, H – gray).
Figure 2.
Optimized stable N4H4 structures in syn-conformation (N – blue, H – gray).
Figure 3.
Optimized stable cyclo-N4H4 structures (N – blue, H – gray).
Figure 3.
Optimized stable cyclo-N4H4 structures (N – blue, H – gray).
Figure 4.
Optimized stable N4H4 structures after N – N bond fission (N – blue, H – gray).
Figure 4.
Optimized stable N4H4 structures after N – N bond fission (N – blue, H – gray).
Table 1.
N1-N2-N3-N4 dihedral angles (Θ1234), absolute (G298) and relative (ΔG298) Gibbs free energies at 298.15 K of the optimized N4H4 structures obtained from the starting ones. The different structures with the same notation are distinguished by additional letters a, b, c, or d. The most stable structure is highlighted in bold.
Table 1.
N1-N2-N3-N4 dihedral angles (Θ1234), absolute (G298) and relative (ΔG298) Gibbs free energies at 298.15 K of the optimized N4H4 structures obtained from the starting ones. The different structures with the same notation are distinguished by additional letters a, b, c, or d. The most stable structure is highlighted in bold.
| Starting |
Optimized |
Θ1234 [o] |
G298 [Hartree] |
ΔG298 [kJ/mol] |
Remarks |
| A0220 |
D(02)(20) |
-180.0 |
-220.84657 |
-251.26 |
N=NH2 + H2N=N |
| A1210 |
D(22)(00)a |
31.8 |
-221.02068 |
-4822.61 |
H2N-NH2 + N2
|
| A1201 |
A1201 |
-106.4 |
-220.84934 |
-323.99 |
|
| A1120 |
D(11)(20)a |
-179.1 |
-220.87553 |
-1011.62 |
HN=NH + H2N=N |
| A1111 |
A1111 |
180.0 |
-220.83700 |
0.00 |
|
| A2200 |
D(22)(00)b |
-176.2 |
-221.02115 |
-4834.95 |
H2N-NH2 + N2 |
| A2110 |
A2110 |
-152.4 |
-220.87955 |
-1117.17 |
|
| A2101 |
A2101 |
153.9 |
-220.91446 |
-2033.75 |
|
| A2020 |
A2011 |
180.0 |
-220.89237 |
-1453.77 |
3→4 H rearrangement |
| A2011 |
A2011 |
-180.0 |
-220.89237 |
-1453.77 |
|
| A2002 |
A2002 |
173.0 |
-220.93100 |
-2468.02 |
|
| A3100 |
D(31)(00)a |
176.3 |
-220.96823 |
-3445.51 |
H3N-NH + N2
|
| A3010 |
A3010 |
-180.0 |
-220.84234 |
-140.20 |
|
| A3001 |
A3001 |
180.0 |
-220.89414 |
-1500.24 |
|
| A4000 |
A3010 |
180.0 |
-220.84232 |
-139.87 |
1→3 H rearrangement |
| B0220 |
D(02)(20) |
0.0 |
-220.84657 |
-251.26 |
N=NH2 +H2N=N |
| B1210 |
E12)(10 |
-9.9 |
-220.91726 |
-2107.27 |
N2-N3 fission, N1-N4 bonding, → B2101 |
| B1201 |
D(22)(00)c |
-2.3 |
-221.02062 |
-4821.03 |
H2N-NH2 + N2
|
| B1120 |
D(11)(20)b |
-180.0 |
-220.87565 |
-1014.78 |
HN=NH + H2N=N |
| B1111 |
B1111 |
-2.7 |
-220.84994 |
-339.75 |
|
| B2200 |
D(22)(00)d |
17.8 |
-221.02057 |
-4819.72 |
H2N-NH2 + N2
|
| B2110 |
B2110 |
17.9 |
-220.87548 |
-1010.31 |
|
| B2101 |
B2101 |
-18.7 |
-220.91727 |
-2107.53 |
|
| B2020 |
B2020 |
-0.2 |
-220.74152 |
2506.88 |
|
| B2011 |
B2011 |
-2.4 |
-220.89749 |
-1588.20 |
|
| B2002 |
B2002 |
-4.2 |
-220.93084 |
-2463.82 |
|
| B3100 |
D(31)(00)b |
29.0 |
-220.96692 |
-3411.11 |
H3N=NH + N2
|
| B3010 |
B3010 |
0.0 |
-220.85489 |
-469.71 |
|
| B3001 |
B3001a |
0.0 |
-220.90266 |
-1723.94 |
|
| B4000 |
B3001b |
0.0 |
-220.89253 |
-1457.97 |
1→4 H rearrangement |
| C1111 |
C1111 |
11.8 |
-220.84534 |
-218.97 |
|
| C2200 |
C2200 |
-31.2 |
-220.71366 |
3238.35 |
|
| C2110 |
C2110 |
-18.7 |
-220.79133 |
1199.09 |
|
| C2101 |
C2101 |
27.8 |
-220.77308 |
1678.25 |
|
| C2020 |
C2020 |
0.0 |
-220.77314 |
1676.68 |
|
Table 2.
Interatomic distances (in Å) in the optimized Amnpq and Bmnpq structures. The different structures with the same notation are distinguished by additional letters a or b.
Table 2.
Interatomic distances (in Å) in the optimized Amnpq and Bmnpq structures. The different structures with the same notation are distinguished by additional letters a or b.
| Structure |
N1-N2 |
N2-N3 |
N3-N4 |
N1-H |
N2-H |
N3-H |
N4-H |
| A1201 |
1.409 |
1.501 |
1.225 |
1.017 |
1.021(2×) |
- |
1.025 |
| A1111 |
1.324 |
1.288 |
1.324 |
1.019 |
1.015 |
1.019 |
1.015 |
| A2110 |
1.414 |
1.463 |
1.190 |
1.009
1.012 |
1.016 |
1.035 |
- |
| A2101 |
1.410 |
1.370 |
1.243 |
1.011
1.014 |
1.013 |
- |
1.020 |
| A2011 |
1.437 |
1.280 |
1.276 |
1.015(2×) |
- |
1.023 |
1.022 |
| A2002 |
1.389 |
1.236 |
1.389 |
1.010
1.015 |
- |
- |
1.010
1.015 |
| A3010 |
1.459 |
1.396 |
1.210 |
1.016
1.022(2×) |
- |
1.040 |
- |
| A3001 |
1.458 |
1.306 |
1.278 |
1.018(2×)
1.022 |
- |
- |
1.016 |
| B1111 |
1.311 |
1.294 |
1.311 |
1.018 |
1.012 |
1.012 |
1.018 |
| B2110 |
1.405 |
1.485 |
1.187 |
1.009
1.012 |
1.017 |
1.031 |
- |
| B2101 |
1.408 |
1.359 |
1.246 |
1.013
1.018 |
1.007 |
- |
1.019 |
| B2020 |
1.352 |
1.433 |
1.342 |
1.004
1.025 |
- |
1.026
1.038 |
- |
| B2011 |
1.400 |
1.276 |
1.294 |
1.010
1.021 |
- |
1.014 |
1.017 |
| B2002 |
1.388 |
1.243 |
1.388 |
1.009
1.016 |
- |
- |
1.009
1.016 |
| B3010 |
1.437 |
1.382 |
1.218 |
1.015
1.023(2×) |
- |
1.030 |
- |
| B3001a |
1.485 |
1.288 |
1.286 |
1.014
1.021(2×) |
- |
- |
1.015 |
| B3001b |
1.507 |
1.289 |
1.277 |
1.015
1.021(2×) |
- |
- |
1.025 |
Table 3.
Interatomic distances (in Å) in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a, b, c, or d.
Table 3.
Interatomic distances (in Å) in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a, b, c, or d.
| Structure |
N1-N2 |
N2-N3 |
N3-N4 |
N1-N4 |
N1-H |
N2-H |
N3-H |
N4-H |
| C1111 |
1.469 |
1.469 |
1.469 |
1.469 |
1.018 |
1.018 |
1.018 |
1.018 |
| C2200 |
1.434 |
1.491 |
1.511 |
1.495 |
1.032
1.023 |
1.032
1.023 |
- |
- |
| C2110 |
1.459 |
1.467 |
1.488 |
1.484 |
1.019
1.024 |
1.020 |
1.023 |
- |
| C2101 |
1.457 |
1.479 |
1.530 |
1.456 |
1.022
1.023 |
1.023 |
- |
1.020 |
| C2020 |
1.468 |
1.468 |
1.468 |
1.468 |
1.019(2×) |
- |
1.019(2×) |
- |
| D(02)(20) |
1.226 |
3.133 |
1.226 |
3.375 |
- |
1.028
1.032 |
1.028
1.032 |
- |
| D(11)(20)a |
1.245 |
3.386 |
1.224 |
3.314 |
1.027 |
1.029 |
1.028(2×) |
- |
| D(11)(20)b |
1.245 |
3.064 |
1.223 |
3.168 |
1.029 |
1.027 |
1.028(2×) |
- |
| D(22)(00)a |
1.445 |
3.709 |
1.096 |
3.492 |
1.011
1.014 |
1.011
1.014 |
- |
- |
| D(22)(00)b |
1.445 |
3.756 |
1.096 |
3.893 |
1.011
1.014 |
1.011
1.014 |
- |
- |
| D(22)(00)c |
1.445 |
3.758 |
1.096 |
3.461 |
1.011
1.014 |
1.011
1.014 |
- |
- |
| D(22)(00)d |
1.445 |
3.705 |
1.096 |
3.706 |
1.011
1.014 |
1.011
1.014 |
- |
- |
| D(31)(00)a |
1.466 |
3.418 |
1.096 |
4.353 |
1.014
1.020(2×) |
1.018 |
- |
- |
| D(31)(00)b |
1.466 |
3.545 |
1.096 |
3.742 |
1.014
1.020(2×) |
1.018 |
- |
- |
Table 4.
BCP electron density (in e/Bohr3) of N-N and N-H bonds in the optimized Amnpq and Bmnpq structures. The different structures with the same notation are distinguished by additional letters a or b. Data for additional N…H hydrogen bonds are presented in parentheses.
Table 4.
BCP electron density (in e/Bohr3) of N-N and N-H bonds in the optimized Amnpq and Bmnpq structures. The different structures with the same notation are distinguished by additional letters a or b. Data for additional N…H hydrogen bonds are presented in parentheses.
| Structure |
N1-N2 |
N2-N3 |
N3-N4 |
N1-H |
N2-H |
N3-H |
N4-H |
| A1201 |
0.3088 |
0.5150 |
0.3426 |
0.3426 |
0.3505(2×) |
- |
0.3441 |
| A1111 |
0.3805 |
0.4345 |
0.3805 |
0.3415 |
0.3491 |
0.3491 |
0.3415 |
| A2110 |
0.3203 |
0.2944 |
0.5366 |
0.3535
0.3503 |
0.3504 |
0.3350 |
- |
| A2101 |
0.3233 |
0.3662 |
0.4891 |
0.3525
0.3495 |
0.3506 |
- |
0.3494 |
| A2011 |
0.3054 |
0.4439 |
0.4393 |
0.3489(2×) |
- |
0.3461 |
0.3428 |
| A2002 |
0.3442 |
0.5015 |
0.3442 |
0.3514
0.3462 |
- |
- |
0.3514
0.3462 |
| A3010 |
0.2815 |
0.3389 |
0.5083 |
0.3478
0.3442(2×) |
- |
0.3325 |
- |
| A3001 |
0.2866 |
0.4237 |
0.4459 |
0.3447(2×)
0.3421 |
- |
- |
0.3504 |
| B1111 |
0.3959 |
0.4279 |
0.3959 |
0.3429 |
0.3506 |
0.3506 |
0.3429 |
| B2110 |
0.3267 |
0.2784 |
0.5386 |
0.3530
0.3505 |
0.3497 |
0.3388 |
- |
| B2101 |
0.3236 |
0.3726 |
0.4848 |
0.3511
0.3456 |
0.3564 |
- |
0.3498 |
| B2020 |
0.3717 |
0.3104 |
0.3743 |
0.3536
0.3307 |
- |
0.3359
0.3168 |
(0.0466) |
| B2011 |
0.3338 |
0.4460 |
0.4172 |
0.3524
0.3401 |
- |
0.3548 |
0.3469 |
| B2002 |
0.3435 |
0.4883 |
0.3435 |
0.3531
0.3435 |
- |
- |
0.3531
0.3435 |
| B3010 |
0.2990 |
0.3503 |
0.4972 |
0.3496
0.3430(2×) |
- |
0.3424 |
- |
| B3001a |
0.2670 |
0.4397 |
0.4356 |
0.3504
0.3432(2×) |
- |
- |
0.3506 |
| B3001b |
0.2517 |
0.4336 |
0.4395 |
0.3484
0.3422(2×) |
- |
- |
0.3395 |
Table 5.
BCP electron density (in e/Bohr3) of N-N and N-H bonds in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a, b, c, or d. Data for additional N…H hydrogen bonds are presented in parentheses.
Table 5.
BCP electron density (in e/Bohr3) of N-N and N-H bonds in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a, b, c, or d. Data for additional N…H hydrogen bonds are presented in parentheses.
| Structure |
N1-N2 |
N2-N3 |
N3-N4 |
N1-N4 |
N1-H |
N2-H |
N3-H |
N4-H |
| C1111 |
0.2916 |
0.2916 |
0.2916 |
0.2916 |
1.018 |
1.018 |
1.018 |
1.018 |
| C2200 |
0.3175 |
0.2566 |
0.2620 |
0.2542 |
0.3384
0.3450 |
0.3383
0.3451 |
- |
- |
| C2110 |
0.2964 |
0.2917 |
0.2759 |
0.2665 |
0.3498
0.3467 |
0.3522 |
0.3496 |
- |
| C2101 |
0.2940 |
0.2946 |
0.2470 |
0.2785 |
0.3465
0.3464 |
0.3513 |
- |
0.3522 |
| C2020 |
0.2815 |
0.2808 |
0.2819 |
0.2817 |
0.3530
0.3532 |
- |
0.3536(2×) |
- |
| D(02)(20)a |
0.4964 |
- |
04964 |
- |
(0.0233) |
0.3422
0.3383 |
0.3422
0.3383 |
(0.0233) |
| D(11)(20)a |
0.4863 |
- |
0.4986 |
- |
0.3484
(0.0158) |
0.3471 |
0.3426(2×) |
(0.0194) |
| D(11)(20)b |
0.4864 |
- |
0.4986 |
- |
0.3471 |
0.3484
(0.0157) |
0.3426(2×) |
(0.0193) |
| D(22)(00)a |
0.2955 |
- |
0.7139 |
0.0036 |
0.3530
0.3500 |
0.3530
0.3503 |
(0.0027) |
- |
| D(22)(00)b |
0.2957 |
-(a)
|
0.7138 |
- |
0.3530
0.3502 |
0.3530
0.3500 |
(0.0038) |
(0.0027) |
| D(22)(00)c |
0.2957 |
- |
0.7139 |
0.0039 |
0.3530
0.3503 |
0.3530
0.3500 |
(0.0026) |
- |
| D(22)(00)d |
0.2958 |
0.0023 |
0.7140 |
- |
0.3530
0.3503 |
0.3531
0.3503 |
- |
(0.0027) |
| D(31)(00)a |
0.2685 |
0.0048 |
0.7139 |
- |
0.3525
0.3491
0.3486 |
0.3408 |
(0.0057) |
- |
| D(31)(00)b |
0.2681 |
- |
0.7139 |
- |
0.3525
0.3492
0.3483 |
0.3412 |
(0.0043) |
(0.0024) |
Table 6.
BCP ellipticity of N-N and N-H bonds in the optimized Amnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a or b. Data for additional N…H hydrogen bonds are presented in parentheses.
Table 6.
BCP ellipticity of N-N and N-H bonds in the optimized Amnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a or b. Data for additional N…H hydrogen bonds are presented in parentheses.
| Structure |
N1-N2 |
N2-N3 |
N3-N4 |
N1-H |
N2-H |
N3-H |
N4-H |
| A1201 |
0.184 |
0.023 |
0.176 |
0.063 |
0.006(2×) |
- |
0.009 |
| A1111 |
0.216 |
0.354 |
0.216 |
0.030 |
0.032 |
0.032 |
0.030 |
| A2101 |
0.011 |
0.134 |
0.187 |
0.045
0.049 |
0.052 |
- |
0.002 |
| A2110 |
0.034 |
0.172 |
0.067 |
0.046
0.050 |
0.041 |
0.032 |
- |
| A2011 |
0.022 |
0.300 |
0.223 |
0.041(2×) |
- |
0.026 |
0.013 |
| A2002 |
0.101 |
0.238 |
0.105 |
0.041
0.045 |
- |
- |
0.041
0.045 |
| A3010 |
0.146 |
0.277 |
0.036 |
0.007
0.012(2×) |
- |
0.030 |
- |
| A3001 |
0.094 |
0.214 |
0.189 |
0.006
0.010(2×) |
- |
- |
0.021 |
| B1111 |
0.200 |
0.343 |
0.200 |
0.028 |
0.036 |
0.036 |
0.028 |
| B2110 |
0.038 |
0.177 |
0.079 |
0.047
0.051 |
0.041 |
0.031 |
- |
| B2101 |
0.016 |
0.149 |
0.182 |
0.039
0.043 |
0.057 |
- |
0.003 |
| B2020 |
0.173 |
0.129 |
0.181 |
0.041(2×) |
- |
0.007
0.022 |
(0.209) |
| B2011 |
0.094 |
0.292 |
0.211 |
0.039(2×) |
- |
0.031 |
0.019 |
| B2002 |
0.095 |
0.241 |
0.102 |
0.040(2×) |
- |
- |
0.040(2×) |
| B3010 |
0.130 |
0.272 |
0.028 |
0.005(3×) |
- |
0.033 |
- |
| B3001a |
0.073 |
0.211 |
0.174 |
0.002(2×)
0.004 |
- |
- |
0.021 |
| B3001b |
0.069 |
0.205 |
0.185 |
0.005(3×) |
- |
- |
0.012 |
Table 7.
BCP ellipticity of N-N and N-H bonds in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a, b, c, or d. Data for additional N…H hydrogen bonds are presented in parentheses.
Table 7.
BCP ellipticity of N-N and N-H bonds in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a, b, c, or d. Data for additional N…H hydrogen bonds are presented in parentheses.
| Structure |
N1-N2 |
N2-N3 |
N3-N4 |
N1-N4 |
N1-H |
N2-H |
N3-H |
N4-H |
| C1111 |
0.059 |
0.059 |
0.059 |
0.059 |
0.039 |
0.039 |
0.039 |
0.039 |
| C2200 |
0.022 |
0.209 |
0.270 |
0.209 |
0.005
0.004 |
0.005
0.004 |
- |
- |
| C2110 |
0.052 |
0.052 |
0.163 |
0.232 |
0.002
0.005 |
0.029 |
0.038 |
- |
| C2101 |
0.072 |
0.181 |
0.186 |
0.067 |
0.001
0.005 |
0.031 |
- |
0.026 |
| C2020 |
0.222 |
0.222 |
0.222 |
0.222 |
0.003(2×) |
- |
0.003(2×) |
- |
| D(02)(20)a |
0.008 |
- |
0.008 |
- |
(0.006) |
0.030(2×) |
0.030(2×) |
(0.006) |
| D(11)(20)a |
0.161 |
- |
0.018 |
- |
0.049
(0.022) |
0.005 |
0.032(2×) |
(0.005) |
| D(11)(20)b |
0.161 |
- |
0.018 |
- |
0.005 |
0.004
(0.020) |
0.032(2×) |
(0.005) |
| D(22)(00)a |
- |
0.005 |
0.832 |
- |
0.049
0.045 |
0.049
0.045 |
(0.676) |
- |
| D(22)(00)b |
0.011 |
-(a)
|
0.003 |
- |
0.045
0.049 |
0.044
0.049 |
(0.637) |
(0.621) |
| D(22)(00)c |
0.010 |
- |
0.003 |
0.653 |
0.049
0.045 |
0.049
0.045 |
(0.629) |
- |
| D(22)(00)d |
0.010 |
0.330 |
0.005 |
- |
0.049
0.045 |
0.049
0.045 |
- |
(0.354) |
| D(31)(00)a |
0.151 |
0.316 |
0.003 |
- |
0.011(2×)
0.009 |
0.076 |
(0.220) |
- |
| D(31)(00)b |
0.145 |
- |
0.004 |
- |
0.009
0.011(2×) |
0.078 |
(0.266) |
(1.271) |
Table 8.
Atomic charges of N and H (bonded to N in brackets) in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a or b.
Table 8.
Atomic charges of N and H (bonded to N in brackets) in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a or b.
| Structure |
N1 |
N2 |
N3 |
N4 |
H(N1) |
H(N2) |
H(N3) |
H(N4) |
| A1201 |
-0.77 |
-0.42 |
-0.03 |
-0.35 |
0.32 |
0.47(2×) |
- |
0.44 |
| A1111 |
-0.55 |
-0.21 |
-0.21 |
-0.55 |
0.37 |
0.51 |
0.51 |
0.37 |
| A2110 |
-0.72 |
-0.37 |
-0.21 |
-0.19 |
0.42(2×) |
0.42 |
0.44 |
- |
| A2101 |
-0.68 |
-0.35 |
-0.02 |
-0.39 |
0.41(2×) |
0.44 |
- |
0.41 |
| A2011 |
-0.71 |
-0.14 |
-0.19 |
-0.45 |
0.41(2×) |
- |
0.50 |
0.39 |
| A2002 |
-0.68 |
-0.07 |
-0.07 |
-0.68 |
0.42(2×) |
- |
- |
0.42(2×) |
| A3010 |
-0.74 |
-0.33 |
-0.21 |
-0.35 |
0.48(2×)
0.50 |
- |
0.38 |
- |
| A3001 |
-0.73 |
-0.24 |
-0.11 |
-0.54 |
0.49(3×) |
- |
- |
0.45 |
| B1111 |
-0.59 |
-0.28 |
-0.28 |
-0.59 |
0.36 |
0.53 |
0.53 |
0.36 |
| B2110 |
-0.70 |
-0.37 |
-0.23 |
-0.19 |
0.41(2×) |
0.42 |
0.44 |
- |
| B2101 |
-0.68 |
-0.34 |
-0.04 |
-0.42 |
0.40(2×) |
0.46 |
- |
0.40 |
| B2020 |
-0.74 |
-0.19 |
-0.48 |
-0.39 |
0.46(2×) |
- |
0.53(2×) |
- |
| B2011 |
-0.69 |
-0.09 |
-0.21 |
-0.53 |
0.42(2×) |
- |
0.51 |
0.38 |
| B2002 |
-0.71 |
-0.05 |
-0.05 |
-0.071 |
0.43(2×) |
- |
- |
0.43(2×) |
| B3010 |
-0.72 |
-0.31 |
-0.22 |
-0.41 |
0.47(2×)
0.500 |
- |
0.41 |
- |
| B3001a |
-0.76 |
-0.20 |
-0.09 |
-0.59 |
0.49(3×) |
- |
- |
0.36 |
| B3001b |
-0.79 |
-0.19 |
-0.08 |
-0.53 |
0.50(3×) |
- |
- |
0.31 |
Table 9.
Atomic charges of N and H (bonded to N in brackets) in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a, b, c, or d.
Table 9.
Atomic charges of N and H (bonded to N in brackets) in the optimized Cmnpq and Dmnpq structures. The different structures with the same notation are distinguished by additional letters a, b, c, or d.
| Structure |
N1 |
N2 |
N3 |
N4 |
H(N1) |
H(N2) |
H(N3) |
H(N4) |
| C1111 |
-0.36 |
-0.36 |
-0.36 |
-0.36 |
0.40 |
0.40 |
0.40 |
0.40 |
| C2200 |
-0.43 |
-0.43 |
-0.44 |
-0.44 |
0.47
0.49 |
0.47
0.49 |
- |
- |
| C2110 |
-0.45 |
-0.33 |
-0.38 |
-0.39 |
0.46
0.49 |
0.42 |
0.37 |
- |
| C2101 |
-0.44 |
-0.33 |
-0.44 |
-0.37 |
0.50(2×) |
0.37 |
- |
0.40 |
| C2020 |
-0.43 |
-0.35 |
-0.43 |
-0.35 |
0.46
0.49 |
- |
0.46
0.49 |
- |
| D(02)(20) |
-0.28 |
-0.52 |
-0.52 |
-0.27 |
- |
0.46
0.42 |
0.46
0.42 |
- |
| D(11)(20)a |
-0.36 |
-0.36 |
-0.51 |
-0.26 |
0.48 |
0.40 |
0.43(2×) |
- |
| D(11)(20)b |
-0.36 |
-0.36 |
-0.51 |
-0.26 |
0.40 |
0.38 |
0.43(2×) |
- |
| D(22)(00)a |
-0.70 |
-0.69 |
0.01 |
-0.02 |
0.38(2×) |
0.38(2×) |
- |
- |
| D(22)(00)b |
-0.71 |
-0.70 |
0.06 |
0.05 |
0.38(2×) |
0.37
0.39 |
- |
- |
| D(22)(00)c |
-0.71 |
-0.70 |
0.06 |
0.07 |
0.38(2×) |
0.38(2×) |
- |
- |
| D(22)(00)d |
-0.70 |
-0.71 |
0.01 |
0.03 |
0.38(2×) |
0.38(2×) |
- |
- |
| D(31)(00)a |
-0.71 |
-0.84 |
0.05 |
0.10 |
0.44(2×)
0.47 |
0.289 |
- |
- |
| D(31)(00)b |
-0.72 |
-0.84 |
-0.01 |
0.02 |
0.45(2×)
0.47 |
0.28 |
- |
- |